Abstract
The rising incidence of esophageal adenocarcinoma (EAC) is mirrored by the increasing prevalence of Barrett’s Esophagus (BE), a precursor lesion resulting in a large number of individuals “at risk” for this lethal malignancy. Among BE patients only ~0.3% annually will develop EAC. Since large numbers of patients are followed in endoscopic surveillance, there is a need for risk prediction among a growing population of BE patients.
We identified 4 potential biomarkers from an inflammation (IL-1β)-dependent mouse model of Barrett’s esophagus and tested them in 189 BE patients with and without HGD/early cancer (T1). The primary goal was to distinguish BE patients with no evidence of dysplasia from those with dysplasia.
Increasing stem cell marker LGR5 and niche cell marker DCLK1 and decreasing differentiation marker (secretory mucus cells, TFF2+ cells) correlated with elevated tumor score in the mouse. Having outlined the origin of those markers in the BE mouse model we showed the applicability for human BE: We compared 96 patients with non-dysplastic BE tissue to 97 patients with BE and HGD or early cancer. Low levels of TFF2 (AUC 87.2%) provided the best discrimination between non-dysplastic BE and BE with cancer, followed by high levels of DCLK1 (AUC 83.4%), low GC ratio (AUC 79.4%) and high LGR5 (AUC 71.4%). The GC ratio, rather than the presence of GCs per se, was found to be an important discriminator. These findings may be useful in developing future risk prediction models for BE patients and ultimately to improve EAC surveillance.
Keywords: Barrett esophagus, risk prediction, esophageal cancer, stem cells, goblet cells
Introduction
Barrett’s Esophagus (BE) is a premalignant condition defined by replacement of the squamous epithelium in the distal esophagus by specialized intestinal metaplasia (IM) (1). The development of BE is thought to represent the initial step in the histopathologic progression to low-grade as well as high-grade dysplasia (HGD) and esophageal adenocarcinoma (EAC) (2). The incidence of EAC has increased at a relative rate of 4 to 10% annually and about 460% in 30 years in regions of the Western World (3) and the cancer has still a very poor prognosis with a mean five-year survival rate of less than 20%. The prevalence of the precursor lesion, BE, has also concordantly increased greatly over the last decades, resulting in a large number of individuals “at risk” for this very lethal malignancy. Nevertheless, accurate historical assessments of BE rates are difficult to estimate and therefore one might speculate that factors other than the presence of intestinal metaplasia are correlating with risk assessment and increased EAC rates. While close monitoring of BE patients would in theory reduce EAC mortality, only about 0.2–0.3% per year will eventually develop EAC (4). A large number of patients are therefore kept under endoscopic surveillance for the detection of a relatively small number of cancers. It seems clear that there is a critical need to develop better preventive strategies, possibly by risk stratification and identification of high-risk BE subsets that would benefit from targeted intervention.
Regular endoscopic surveillance of BE patients does offer the opportunity for early intervention, as the neoplasm in theory can be detected and resected at a potentially curable stage. However, the development of an optimal surveillance strategy for BE patients has been restricted by the lack of a tractable preclinical model of BE and EAC.
We previously established a transgenic mouse model in which IL-1β expression was targeted to the esophageal and squamous forestomach mucosa. These mice exhibit spontaneous esophagitis, followed by progression to BE and EAC (5). In contrast to most human BE disease, the BE mouse model has a lower incidence of classic intestinal metaplasia but on the other hand a high rate rate of progression to EAC. In addition, consistent with the first description of this disease by N. Barrett himself and others (6,7), careful analysis of the BE mouse model revealed that the metaplastic lesions originate from the gastric cardia, particularly from LGR5+ progenitor cells (8–11). In response to esophageal inflammation, the LGR5+ epithelial progenitors appear to migrate from the gastric cardia into the squamous esophagus, and in both human and murine BE LGR5+ progenitors were strongly associated with the development of dysplasia (12,13). Furthermore, we could demonstrate that prior to the development of IM, a gut-like columnar-lined epithelium (CLE) from a regenerative cell lineage expressing TFF2 and CDX2 appears in the esophagus (14–18).
In this translational study we apply findings from the BE mouse model to human tissue and evaluate four specific biomarkers (GC ratio, TFF2, LGR5, DCLK1) to distinguish the characteristics of IM that is associated with cancer. We suggest that this novel risk prediction model shows great promise and might contribute to individually based risk stratification in BE surveillance programs.
Materials and Methods
Animal Studies
Mice were allowed a standard chow diet from birth until weaning, and water ad libitum in groups of less than five animals. Once per week the animals are transferred to a fresh cage under a transfer station. Water bottles are changed weekly, All animal experiments were approved by the District Government of Upper Bavaria and performed in accordance with the German Animal Welfare and Ethical Guidelines of the Klinikum rechts der Isar, TUM, Munich, Germany. The Dclk1-CreERT2 transgenic mice were crossed to Rosa26R-Tomato/GFP reporter strains as previously described (19). Human IL-1β transgenic mice (BE mouse model) generated in our laboratory by targeting expression of IL-1β to the esophagus using the Epstein Barr virus L2 promoter have been previously described. The mice show chronic esophagitis and progress over time (approximately 12 months) to metaplasia and dysplasia (5). All transgenic mice were on a pure C57/B6 background after 6 backcrosses. C57/B6, LgR5-CreTM-IRES-GFP, Rosa26R-LacZ mice were purchased from Jackson Laboratories Inc. (West Grove, PA).
Paraffin sections fixed in 10% formalin were incubated with primary antibodies: DCLK1 (Abcam 1:200), TFF2 as previously described (20), and control IgG2a. Biotinylated secondary antibodies (Jackson Immunoresearch Laboratories Inc., West Grove, PA) and ABC avidin-biotin-DAB detection kit (Vector Labs) were used for detection and visualization according to the manufacturer’s protocol.
The stomach and esophagus from transgenic and control mice were fixed in 10% formalin, imbedded in paraffin, cut into 5 μm sections, and stained with hematoxylin/eosin (H&E), Periodic acid Schiff reaction (PAS) as well as Alcian Blue. The area of mucus producing cells (i.e. goblet-like cells) versus the non mucus producing columnar cells were evaluated in the overall metaplastic region of the Barrett’s metaplasia at the SCJ semiquantitatively by adapting a previously established scoring system (5). The percentage of mucus producing cells within the whole epithelium was calculated.
Human study population
In order to analyze the four biomarkers in human BE, we collected a total of 189 specimens from a tertiary community hospital pathology department in Germany (Klinikum Bayreuth, Institut für Pathologie, Bayreuth, Germany). All samples were identified by a pathology database search that included patients from January 2008 to May 2013. The Ethics Committee of TU-Munich approved the study protocol. Inclusion criterion was diagnosed BE with IM including goblet cells (GCs) at any site on either biopsies or endoscopic mucosal resection (EMR)) and an EAC UICC stage < pT2; there was no limitation on age. Subjects with a history of additional malignancy or EAC treatment other than EMR were excluded, there was no limitation on age. Including only EMR specimens was done do reduce a tumor specific field effect on the remaining tissue, which we assumed would be minimized by choosing T1 EMR specimens only
The study used a case-control design with two groups of approximately equal numbers of patients:. The one (non-dysplastic BE) group included endoscopic biopsies from 94 patients that according to records never showed no signs of dysplasia/EAC at any time point. The other (BE associated with EAC) group consisted of 95 primarily EMR samples with BE and HGD or early cancer simultaneously. Patients with only low-grade dysplasia were excluded in advance in order to avoid issues of diagnostic accuracy (21). We were considering all available esophageal material for a patient available for analysis. In cases where only endoscopic biopsy material was used, all available patient material (at least 3 different biopsy sites) were taken into consideration to get comparable results with EMR specimens.
Evidence of reflux in adjacent squamous-cell epithelium was evaluated by scoring 0 to 3 according to no-, mild-, moderate- and severe- hyper-regenerating esophagopathia. As not all of the specimens obtained were in perfect condition, we only included those with sufficient non-lacerated tissue available. The numbers of analyzed samples were detailed in every figure.
H&E/Immunohistochemistry
All staining was performed on 5 μm paraffin sections. Standard H&E and Alcian Blue protocols, as well as (PAS)-reaction were used for evaluation of GC density. For immunohistochemical staining, incubation was performed using primary antibodies: DCLK1 (Abgent Inc., 1:200), TFF2 (Proteintech Group, 1:1000) and LGR5 (Abcam, 1:100). Biotinylated secondary antibodies (Jackson Immunoresearch, West Grove, PA) were diluted (1:200) in 2% bovine serum albumin/PBS and incubated one hour each at room temperature.
The mounted slides were deparaffinized in xylene, and rehydrated through descending concentrations of ethanol. Antigen retrieval was performed using a citrate buffer heated in a pressure cooker for 5 mins and then cooled to room temperature. Blocking of endogenous peroxidases was accomplished by incubating sections in 3 % hydrogen peroxide for 10 mins and in 5 % goat serum for 30 mins. The prepared primary antibodies were incubated with sections overnight at 4°C. Avidin/Biotin detection kit (Vector Lab. Inc., Burlingame, CA) was used according to the manufacturer’s protocol. Sections were counterstained with hematoxylin, dehydrated in ascending concentrations of ethanol followed by clearance with xylene, and cover slipped permanently for light microscopy. Negative controls were obtained by excluding the primary antibody.
Histopathologic analysis & IHC scoring
All human specimens were examined by R.S. and M.V. (specialist in GI pathology) without knowledge of the clinical report. Scoring of IHC was based on consensus opinion. Goblet Cell (GC) density was calculated separately by the two independent investigators and mean values were used to calculate the final GC ratio. Bright field microscopy was done on a Zeiss Axio microscope with low-power view; images were captured on an Axio-Cam HRc and analyzed with AxioVision Software for Windows (Carl Zeiss AG, Oberkochen, Germany).
As we did not intend to compare IM to EAC, we were selecting all areas of non dysplastic IM for both groups. The transition zone from IM to dysplasia/EAC was marked and dysplastic regions not taken into account. Additionally, in 10 EAC (T1) containing EMR specimens we performed a separate evaluation of histopathological marker and GC ratio in a very close area to dysplasia at a distance >3 mm and in a more distant area (>3mm) to evaluate tumor specific effects in the close neighboring tissue. The GC density was calculated by the following method: every lumen surrounded by columnar-lined cells was assumed to represent one Barrett crypt. Each crypt was scored as (+) or (−) for GCs. The GC ratio was then defined as the number of (+) crypts divided by the total number of crypts. Every investigator calculated mean data of 3 random low-power fields. In order to address the issue with pseudo-GCs (22), different staining was compared and submucosal Brunner-glands, as well as lumen open to the surface, were not taken into account.
For scoring of immunohistochemistry (IHC), a semiquantitative approach (i.e. modified immunoreactive-score (IRS) described by Remmele et al. (23)) was used. Again, only areas of non-malignant BE were evaluated; the highest scoring per patient specimen (i.e. whole EMR or all available patients biopsies) was used to define the final score. Staining in the submucosal glands and the epithelial surface was again ignored. Whenever a single Barrett crypt showed cells with positive staining, it was considered as being positive (+). The percentage of (+) crypts was used to generate the final score. For the stem cell marker LGR5 and the niche cell marker DCLK1, staining scores were adapted from prior BE analyses (24). The scale ranged from 0 to 3, where 0 represented < 10%, 1 represented 10% to 30%, 2 represented 30% to 60% and 3 represented > 60% of positive crypts within the BE area. The scale used for TFF2 scoring was based on a modified Allred-Score previously used in BE (25), with a scale from 0 to 4, where 0 meant < 1%, 1 < 25%, 2 < 50%, 3 < 75% and 4 > 75% of positive crypts within the BE area.
Statistical Analysis
In the mouse model, we used ANOVA to score the significance of mucus cell ratio, TFF2, DCLK1 and LGR5 in correlation with the tumor score (range 1 – 4). In human tissue, we used the t-test to formally evaluate whether the mean of the GC ratio from the non-dysplastic BE group differed significantly from the BE with EAC cohort. To test whether the distribution of TFF2, LGR5 or DCLK1 scores in humans differed significantly between both groups, we used the chi-squared test. A p-value of <0.05 was considered statistically significant. A logistic regression model was set up separately for each biomarker (GC ratio, TFF2, LGR5 and DCLK1) to determine the effect of each marker on the outcome of a subject (non-dysplastic BE vs. BE with EAC). Additional models that included combinations of the biomarkers were generated. For each model, we assessed its ability to discriminate between non-dysplastic BE and BE with EAC by estimating each model’s area under the receiver-operator characteristic curve (AUC). This measure ranges from 0.5 (no discriminative ability) to 1 (perfect discrimination). Further, we determined the true-positive rates (sensitivity) and true-negative rates (specificity) by calculating a receiver-operating characteristic (ROC) curve. The optimum cutoff value was determined by exploring the point of the ROC curve for which Youden’s index reaches its maximum. We used R v3.2.1 with package “pROC” for these calculations (26,27).
Results
A high GC ratio correlates with Barrett metaplasia but not Adenocarcinoma
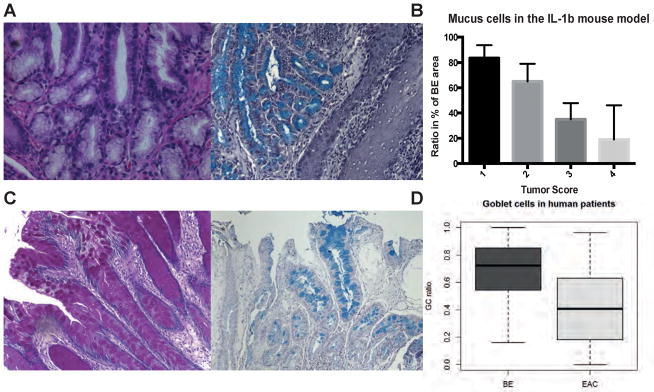
While GCs have primarily been used as a marker of intestinal metaplasia, we and others have proposed that GCs are terminally differentiated cells with reduced ability to transform into malignant cells (5,12,28,29). Here, we analyzed the abundance of mucus producing (i.e. goblet-like) cells in pL2-IL-1β mice with or without tumors as a surrogate for GC-like differentiation. True GC differentiation is rarely seen in mouse models of upper GI metaplasia/dysplasia, therefore we propose that extensive mucus differentiation could be assumed to represent the murine equivalent of intestinal metaplasia seen in human BE. PAS and Alcian Blue positive mucus producing cells were counted in regions of BE metaplasia at the SCJ in 9 and 12 months old pL2-IL-1β mice. Tumors were scored as described previously (5) and correlated with the amount (%) of mucus producing cells in the total BE region in the mouse model. Indeed, we could demonstrate that a decreased ratio of goblet-like cells within the area of BE tissue at the SCJ significantly (ANOVA p<0.0001) correlated with an increased tumor score (from 1 to 4, Figure 1A, 1B) at two different stages in the pL2-IL-1β mouse model. Based on this data from the BE mouse model, we hypothesized that an increased ratio of GCs within the intestinal metaplasia of human BE tissue might also correlate with a reduced cancer risk. Since dysplasia is associated with a loss of GCs, it was of interest to correlate the GC ratio with the differentiation status of the tissue. Using a case-control study design, we compared the GC ratio in patients with nondysplastic BE to the GC ratio in BE patients with T1 non-invasive cancer in endoscopic mucosal resection (EMR) material, focusing in the latter on nondysplastic BE tissue adjacent to the cancer site. Analysis of the cohorts’ characteristics revealed that both groups were predominantly male, with 69% and 84% men in the BE and EAC groups, respectively. There was no significant difference in age between the two groups (Table 1). No correlation of GC ratio with age or gender was found (data not shown). Notably, the GC ratio differed significantly (p<0.0001), with a mean GC ratio of 0.69 in the 94 non-dysplastic BE patients and 0.41 in the 95 patients with BE with and cancer group (Figure 1C, 1D). To exclude tumor specific effects in the tumor containing EMR specimens, a second evaluation in a more closely adjacent or more distant part of the EMR specimen was performed and did not show a difference in GC ratio (and all other markers as described below) between close (<3mm) and distant (>3mm) tissue locations (Supplementary Figure 1), indicating that the observed analysis is indeed reflecting a field and patient specific effect with the BE tissue rather than a localized phenomenon. Thus, in this human cohort, an elevated GC ratio could discriminate between BE with and without adjacent dysplasia.
Figure 1.
A) PAS and Alcian Blue staining of murine BE metaplasia in the cardia showing mucus producing cells; B) Correlation of mucus cell ratio (%) with the tumor score; (%) of area containing mucus producing cells was counted in the metaplastic and dysplastic area of the mouse forestomach (ANOVA p<0.0001, R square 0.6951, F 39.52) C) PAS and Alcian Blue staining of human BE metaplasia showing specialized intestinal metaplasia with GCs; D) Boxplot of the GC ratio in human biopsies from BE or adjacent to EAC tissue with bars marking mean values (0.69 vs. 0.41 in BE and EAC resp., p<0.0001) and whiskers defining range from Min to Max.
Table 1.
Age distribution did not vary and was conform with literature; both groups were associated with male gender (OR 0.318, p =0.0013)
| Number of patients | Mean age (±stdw) | Male sex (%) | |
|---|---|---|---|
| BE | 94 | 62 (±12) | 69% |
| EAC | 95 | 61 (±12) | 84% |
Trefoil factor family 2 expression correlates with decreased risk of dysplasia
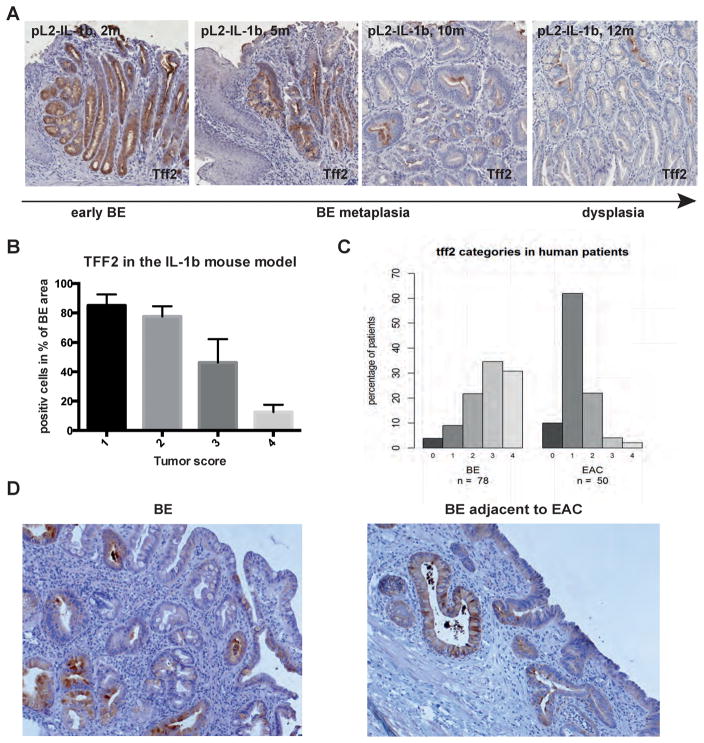
TFF2, previously known as spasmolytic polypeptide, is a small peptide normally expressed in the stomach and duodenum but upregulated throughout the gastrointestinal tract in the setting of injury and inflammation (18). While TFF2 is not expressed in the normal esophageal squamous epithelium, the presence in TFF2-expressing cells at the base of Barrett’s glands has been reported by several groups (12,13). In the BE mouse model, TFF2 is concordantly absent in esophageal squamous epithelium but abundant in the gastric cardia of IL-1β mice (Figure 2A) and WT mice (data not shown). Interestingly, TFF2 was found to be highly upregulated (ANOVA p<0.0001) in the lower esophagus of L2-IL-12 mice with Barrett’s-like metaplasia, but was downregulated (ANOVA p<0.0001) in older (9 mo and 12 mo) pL2-IL-1β mice with dysplasia and tumors (Figure 2A, 2B). Therefore, we hypothesized that TFF2 functions as a marker of non-dysplastic Barrett metaplasia, with decreased TFF2 expression representing an increased risk of malignant progression.
Figure 2.
A) TFF2 immunohistochemistry of BE metaplasia in the pL2-IL-1β mouse model with increasing status of early (2 months) to metaplastic (5 and 10 months) BE and dysplasia (12 months). B) Histopathologcial score in correlation with TFF2 staining in the pL2-IL-1β mouse model (ANOVA p<0.0001, R square 0.8728, F 54.91). C) TFF2 staining in human biopsies from BE or adjacent to EAC tissue was scored from 0 to 4, percentage of each score in both groups is shown (4/9/22/35/31% in BE as well as 10/62/22/4/2% in EAC). D) Representative TFF2 staining of human biopsies from BE or adjacent to EAC tissue.
Indeed, in our human cohort, a significant upregulation of TFF2 was present in the non-malignant BE compared to the EAC group (p<0.0001). In non-dysplastic BE patients, 87% had a score of 2 or more, whereas in BE with cancer patients 72% had values of 0 or 1 (Figure 2C). Immunohistochemistry showed that TFF2 was specifically expressed in epithelial cells mainly at the base of the Barrett’s glands; the surface layer or upper part of the glands did not show any TFF2 positivity (Figure 2D). Among non-dysplastic Barrett’s glands, few showed a complete absence of TFF2, indicating the nearly ubiquitous presence of TFF2 in non-dysplastic BE glands (18). TFF2 staining could also be found in adjacent gastric cardia or corpus tissue, but was not detected in esophageal squamous epithelium and there was no difference between close (<3mm) and distant (>3mm) tissue locations (Supplementary Figure 1).
The stem cell marker LGR5 can discriminate between BE and EAC associated BE
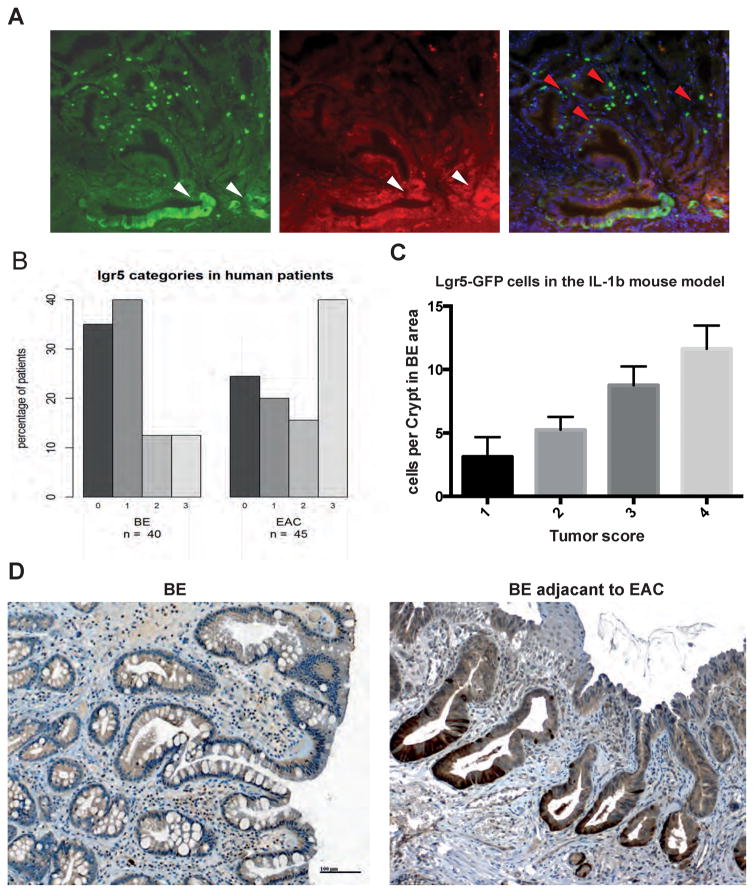
LGR5/GPR49, a leucine-rich orphan G protein-coupled receptor, was shown to specifically label stem cells in the intestine, the so-called crypt based columnar (CBC) cells (30,31). In the L2-IL-1β mouse model, we previously demonstrated that LGR5-expressing cells also function as stem cells in the gastric cardia, and can serve as potential cells of origin for BE and dysplasia (5). Here, we show through lineage tracing that LGR5+ cells can give rise to polyclonal metaplastic and dysplastic BE crypts at the SCJ in IL-1β mice crossed to Lgr5-CreERT2 mice and Confetti reporter mice (Figure 3A). L2-IL-1β; Lgr5-CreERT2;Confetti mice were induced with tamoxifen at 3 months of age, a time point where BE metaplasia containing increased LGR5 cells was present. Analysis of lineage tracing at 12 mo of age, 9 mo after Cre induction, confirmed the strong lineage relationship between LGR5+ cells in the cardia and the development of metaplastic and dysplastic tissue at the SJC (Figure 3A). In particular, the use of Confetti mice allowed us to demonstrate that multiple different LGR5+ cells within the BE tissue contribute to BE and EAC, since we observed at 9 mo post induction the presence of crypts with at least two different colors (Figure 3A). While we were not able to analyze a detailed time course of the development of BE clonal heterogeneity, the data were clear that multiple LGR5 stem cells present early on sustain BE crypts over time. Additionally, we were able to demonstrate significant correlation (ANOVA p<0.0001) between the number of LGR5+ cells and the tumor score in the BE mice, suggesting a possible direct contribution of LGR5+ cells to accelerated dysplasia (Figure 3B).
Figure 3.
A) Lineage tracing of BE metaplasia at the SCJ in pL-IL-1β mice crossed to Lgr5CreTM mice and Confetti reporter mice at the age of 12 months, 9 months after Tamoxifen treatment; white arrows show lineage of red and green clones in BE tissue and red arrows show single Lgr5-GFP positive cells in BE tissue. B) Histopathologcial score in correlation with Lgr5-GFP positive cells at the SCJ in the pL2-IL-1β mouse model (ANOVA p<0.0001, R square 0.8418, F 49.65). C) Representative LGR5 staining of human biopsies from BE or adjacent to EAC tissue. D) LGR5 staining in human biopsies from BE or adjacent to EAC tissue was scored from 0 to 3; percentage of each score in both groups is shown (35/41/12/12% in BE as well as 25/18/16/41% in EAC).
In our human patient cohort, immunohistochemistry demonstrated LGR5 expression near the base of the glands (Figure 3D). Almost no staining was visible at the surface. The LGR5+ cells were clustered and not spread singularly over the whole gland, concordant with the mouse data, suggesting the presence of a stem cell niche present in the lower third of the glands (12,13). LGR5+ cells were found in both non-dysplastic BE and in BE tissue adjacent to EAC. However, the distribution of LGR5 staining categories in non-dysplastic BE samples was significantly lower compared to those of the BE with EAC group (p=0.02). The LGR5 score showed a significant increase in BE tissue adjacent to EAC, i.e. 41% of the 44 EAC specimens had a LGR5 score of 3, compared with only 13% from the 40 BE specimens (Figure 3C). LGR5 was not expressed in any esophageal tissues other than columnar-epithelial cells, and in particular was absent in squamous epithelial cells (Figure 3B) and there was no difference between close (<3mm) and distant (>3mm) tissue locations (Supplementary Figure 1)..
DCLK1 identifies pre-dysplastic tissue
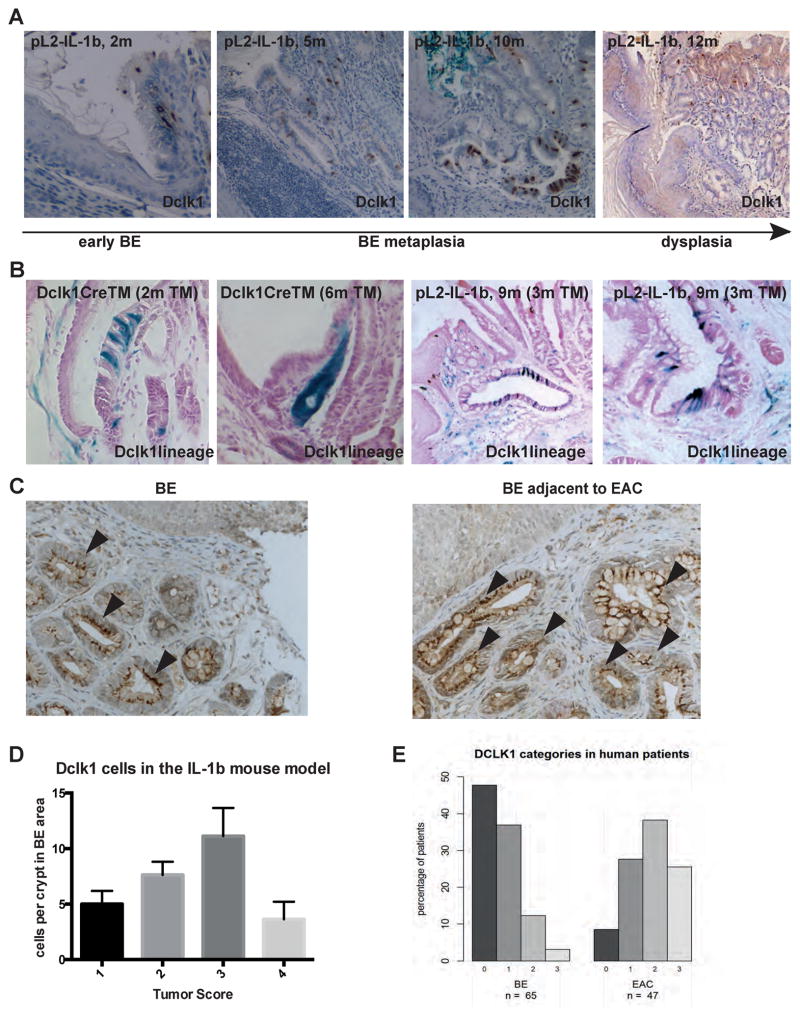
Doublecortin and CaM Kinase-Like-1 (DCLK1) is a microtubule-associated-kinase expressed in isolated cells in the stomach, intestine and colon (19). Using Dclk1-CreERT2 transgenic mice, we recently demonstrated that a subpopulation of intestinal DCLK1+ tuft-cells is exceptionally long-lived and appeared to regulate intestinal stem cell function. These long-lived DCLK1+ cells are expanded in chronic inflammation and in the setting of preneoplasia, and can also function as cancer initiating cells in colon cancer (19). Similar to observations in the inflamed colon, DCLK1+ cells were significantly (ANOVA p<0.0001) amplified in regions of metaplasia in our mouse model of BE and EAC (Figure 4A). We observed a further increase in more advanced BE with low-grade dysplasia (Figure 4A, 4D). However, as seen in other cancers, DCLK1+ cells disappeared with the development of severe dysplasia or a tumor score of 4 (Figure 4D), and therefore seem to serve as a marker for preneoplasia. In particular, we confirmed that long-lived DCLK1+ stem cells accumulate in the inflamed gastric cardia of the mouse (Figure 4B) and expand throughout the BE glands, as shown using L2-IL-1β; Dclk1-CreERT2; Rosa26r-LacZ mice induced at 3 mo and examined at 9 mo (Figure 4B). Of note, in this setting no lineage tracing of the crypts from Dclk1+ cells was detected under these conditions.
Figure 4.
A) Representative DCLK1 immunohistochemistry of BE tissue in the pL2-IL-1β mouse model with increasing status of early (2 months) to metaplastic (5 and 10 months) BE and dysplasia (12 months). B) Lineage tracing of the SCJ of Dclk1CreTM mice crossed to LacZ reporter mice showing DCLK1+ cells at the murine forestomach and a typical lineage tracing event 6 months after Tamoxifen administration. In BE metaplasia at the SCJ in pL-IL-1β mice crossed to Dclk1CreTM mice and LacZ reporter mice at the age of 9 months, 6 months after Tamoxifen treatment, increasing numbers of DCLK1+ cells in BE tissue are shown (ANOVA p<0.0001, R square 0.7600, F 29.55). C) Representative DCLK1 staining of human biopsies from BE or adjacent to EAC tissue; arrowheads pointing at the cytoplasmatic location of DCLK1. D) Histopathologcial score in correlation with Dclk1-CreTm Lac Z positive cells at the SCJ in the pL2-IL-1β mouse model. E) DCLK1 staining in human biopsies from BE or adjacent to EAC tissue was scored from 0 to 3, percent of each score in both groups is shown (48/37/12/3% in BE as well as 9/28/38/26% in EAC).
In the human cohort, DCLK1+ cells were located primarely in clusters of epithelial cells in the lower third of the crypts; however, we could also find crypts where DCLK1+ cells were scattered among the entire crypt length (Figure 4C). The epithelial DCLK1+ cells showed a characteristic staining pattern in the cytoplasm. Differentiated cells like GCs showed no positivity for DCLK1. We could show a significant up regulation of DCLK1+ cells in BE associated with EAC (p<0.0001), 64% of the 47 EAC specimens had a DCLK1 score of 2/3 compared to 15% of the 65 non-dysplastic BE specimens (Figure 4E) and there was no difference between close (<3mm) and distant (>3mm) tissue locations (Supplementary Figure 1)..
Diagnostic model for detecting adjacent EAC
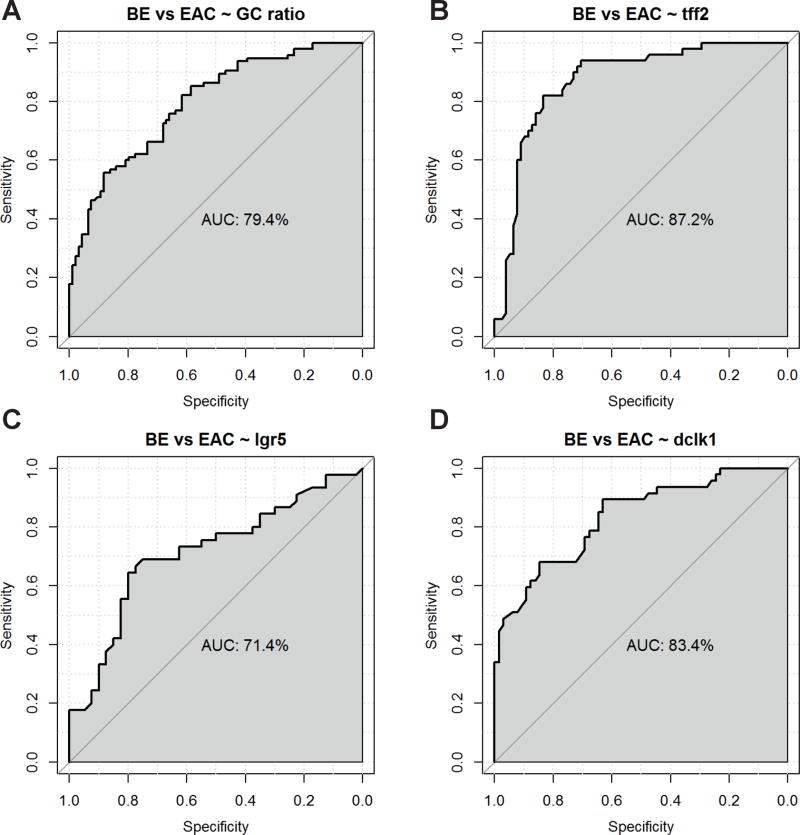
To determine whether the above markers may have utility in the identification of BE harboring occult neoplasia, or if they represent potential markers of high-risk BE, we evaluated how well each biomarker discriminated between the non-dysplastic BE and BE with cancer group. Figure 5 depicts the results of the logistic regression models for every biomarker. The ROC curves indicate that TFF2 showed the best discrimination between the BE and EAC group with an AUC of 87.2%, followed by DCLK1 (AUC of 83.4%), GC ratio (AUC of 79.4%) and LGR5 (AUC of 71.4%). The discrimination between the BE and EAC group was improved when combining the various marker proteins. Table 2 shows the results of ROC curves of logistic regression models for all possible marker combinations. The combination of GC ratio + TFF2 + DCLK1 attained an almost perfect discrimination between the EAC and BE group, with an AUC of 99.3% (sensitivity of 1 and specificity of 0.93), closely followed by the combination of all four marker proteins, which also revealed an almost perfect discrimination of 98.6%. However, even if information on only two of the IHC marker proteins was available, the discrimination was excellent (Table 2).
Figure 5.
Logistic regression models for all four biomarkers. The ROC plots indicate that TFF2 shows the best discrimination between BE and EAC, with an AUC of 87.2%, followed by DCLK1 (AUC: 83.4%), GC ratio (AUC: 79.4%) and LGR5 (AUC: 71.4%).
Table 2.
Results of ROC curves built on logistic regression models for possible marker combinations. All models were adjusted for age and sex. Sensitivity and specificity are obtained by applying the cutoff value that corresponds to the point of the ROC curve for which Youden’s index reaches its maximum.
| Marker(s) in model | Sensitivity | Specificity | AUC |
|---|---|---|---|
| GC ratio | 0.558 | 0.883 | 79.4% |
| TFF2 | 0.820 | 0.833 | 87.2% |
| LGR5 | 0.644 | 0.800 | 71.4% |
| DCLK1 | 0.681 | 0.846 | 83.4% |
|
| |||
| GC ratio + TFF2 | 0.939 | 0.789 | 92.5% |
| GC ratio + LGR5 | 0.512 | 0.923 | 76.7% |
| GC ratio + DCLK1 | 0.761 | 0.952 | 92.7% |
| TFF2 + LGR5 | 0.868 | 0.886 | 89.0% |
| TFF2 + DCLK1 | 1.000 | 0.814 | 95.0% |
| DCLK1 + LGR5 | 0.903 | 0.778 | 89.0% |
|
| |||
| GC ratio + TFF2 + LGR5 | 0.892 | 0.853 | 91.3% |
| GC ratio + TFF2 + DCLK1 | 1.000 | 0.930 | 99.3% |
| GC ratio + LGR5 + DCLK1 | 0.900 | 0.923 | 94.2% |
| TFF2 + LGR5 + DCLK1 | 0.963 | 0.917 | 96.6% |
|
| |||
| GC ratio + TFF2 + LGR5 + DCLK1 | 0.889 | 1.000 | 98.6% |
Discussion
Patients with BE are more likely than the general population to develop EAC, but risk factors that lead to BE progression and molecular biomarkers are not well understood. In a translational study that includes both mouse model data and human tissue samples, we provide evidence to discriminate dysplastic tissue among BE patients based on a low GC ratio, increased LGR5+ cells, increased DCLK1 cells, and decreased TFF2 cells. Based on results from the L2-IL-1β mouse model for esophageal carcinogenesis, we propose a model in which LGR5+ stem cells from the gastric cardia are recruited into the esophagus where they can differentiate into columnar epithelium, comprised of either GCs or TFF2+ progenitor cells, cell types that are normally absent from the squamous esophagus.
Our data suggest that the GC ratio, rather than the simple presence of GCs, is better at distinguishing non-dysplastic BE and BE associated with cancer in humans. Surprisingly, but consistent with previous findings (28,32), we noted an inverse correlation between the GC ratio and EAC. In combination with immunohistochemistry for our other biomarkers, we developed a diagnostic model for malignant progression in BE that can be tested further in prospective trials. Each IHC marker (TFF2, DCKL1 and LGR5) alone showed a strong ability to discriminate non-dysplastic BE associated with a patient with early cancer (T1) in an EMR specimen from non-dysplastic BE in a patient without any dysplasia in areas of otherwise identical-appearing intestinal metaplasia. Combining these markers in a logistic regression model attained a near perfect (>98%) discrimination between the BE correlated with EAC and the non-malignant BE group. Important limitations of these data are the retrospective, single-time-point nature of the analysis, and thus request the need for confirmation with a pre-defined cutoff value in independent prospective trials. However, patients with non-dysplastic BE that progress to HGD or EAC are quite infrequent, thus making validation of this model a challenging proposition. Of note, our data compare metaplastic tissue in esophagi with or without EAC and therefore represent a field or niche effect typical for malignant transformation that could be secondary to cancer development. Nevertheless, the graduate alterations in the mouse model and the fact that we only chose early non invasive T1 cancers in our EMR specimens would argue in favor of a continuous change during carcinogenesis and not a purely secondary effect do to progredient cancer growth. Moreover a separate analysis taking the distance to the dysplasia in the EAC bearing EMR specimens into account revealed no difference between close and distant tissue, arguing for a broader field effect. Future prospective studies need to evaluate if these changes can be used to predict the prospective risk of dysplasia development. The definition of a niche of high risk rather than a specific region of dysplastic growth might be important to estimate the malignant potential in patients with even short segment BE or only metaplastic changes at the GEJ or cardia.
Our data suggest that GCs represent a surrogate for a well-differentiated tissue type that is likely a stable and well-adapted sort of metaplasia, with limited potential for malignant transformation. In many recent GI society guidelines (e.g. AGA, BSG, DGVS), the role of GCs in BE has been much debated, with most guidelines suggesting that only patients with specialized IM (i.e. abundant GCs) should be diagnosed with BE and included in screening programs for EAC. While IM is present in the vast majority of BE cases (33,34), and some GCs can almost always be identified when a sufficient number of biopsies was taken, it is clear that EAC can be found in patients without classical BE with intestinal metaplasia, limiting the utility of intestinal metaplasia as the sole risk factor for EAC. Consistent with our surprising findings, a recent study demonstrated that the GC count in BE shows an inverse relationship with the presence of DNA content abnormalities by flow cytometry. This may suggest that a loss of GCs, which are terminally differentiated with low proliferative ability, may represent a biological mechanism necessary for the development of adenocarcinoma (28). Indeed, GCs are responsible for secreting components for a mucosal barrier and represent a major cellular component of the innate defense system and might serve mucosa protective in metaplasia (35). We showed a similar inverse correlation with TFF 2 protein expression, which has been shown to mark differentiated mucus neck cells in the gastric epithelium, which like GCs are a secretory cell type commonly found in BE. Unexpectedly however, TFF2+ cells are suppressed with progression to EAC, consistent with the loss of other differentiated cell types. TFF2 has been demonstrated to label intestinal metaplasia a long time ago (25,36) and to be present in BE epithelium in correlation with a secretory phenotype (37) but was never inversely associated with the risk to develop EAC in favor of a more proliferative and less differentiated cell lineage.
In the L2-IL-1β mouse model of BE, inhibition of mucus cell differentiation correlates with malignant progression (5). In human BE, columnar-like epithelium seems to precede the development of IM, and progression to IM is associated with the extent and the duration of the disease (33). In histopathologic studies of esophageal cancer resection specimens, gastric cardia-type columnar epithelium was found in the esophagus in 100% of cases, but GC metaplasia was found in only 21% (38). Additionally, a recent analysis of esophageal adenocarcinoma resection specimen using a combination of histopathologic spatial mapping and clonal ordering demonstrated that EAC cancer developed from a pre-malignant clonal expansion in columnar metaplasia lacking GCs, underlining the pre-malignant potential of metaplastic columnar epithelium without GCs in the context of BE (29). In our analysis a low ratio of GCs in adjacent tissue was significantly associated with the presence of dysplasia or cancer in mouse and human tissue, indicating that a more differentiated IM tissue might be even protective. Thus, we propose here that the increasing density of GCs within BE tissue might be a negative predictor for cancer development in EAC. Additionally, one might speculate that a similar marker panel can be used in patients with “carditis” and/or with intestinal metaplasia of the cardia. Indeed, we have hypothesized that GEJ cancer and EAC are really the same disease (8,39), and that EAC may actually originate from gastric cardia stem cells. Our mouse model of Barrett’s actually begins with carditis and metaplasia of the cardia, and does show increased Lgr5 and decreased Tff2 expression during progression to cancer. However, from a clinical perspective, patients with only carditis and lacking clear Barrett’s are typically not well diagnosed placed into surveillance programs, particularly if they show few goblet cells. We would argue that greater attention should be paid to carditis and goblet-cell poor metaplasia (e.g. columnar-like epithelium) at the GE junction.
The very proximal stomach, or gastric cardia, represents a zone of 4–5 gland units just below the squamous columnar junction (SCJ). The murine model of BE and EAC suggests that metaplastic lesions originate from stem cells in the gastric cardia (5,6,40), which over time appear to migrate proximally into the squamous esophagus leading to the development of dysplasia. In this study, we show in murine (L2-IL-1β) and human models of BE that stem/progenitor cells (LGR5+) are expanded in both the gastric cardia and BE tissue at the SCJ. Using LgR5-Cre-ERT2 mice, we were able to demonstrate here that polyclonal metaplasia and dysplasia can arise from a LGR5+ cardia stem cell. This clearly occurs first in the murine cardia, presumably in response to inflammation extending down from the SCJ. As a consequence, multiple clonal populations with highly variable patterns of genomic aberrations may arise (41), and prospective studies have shown that the diversity of clonal neoplastic populations arising within the BE segment is a strong, objective predictor of progression to EAC (42). While the presence of LGR5+ cells in BE has been reported previously (43), we have shown here that in BE an increase in LGR5 expression (a surrogate for undifferentiated cell types) in combination with other differentiation markers seems to predict progression towards EAC. It seems reasonable to assume that EAC arises from such same progenitors that give rise to BE. While some consideration is given to the possibility of “metaplasia of the most distal esophageal squamous epithelium” (44,45), or submucosal glands in the distal esophagus (44), a proximal extension of original cardiac mucosa is favored by our lineage tracing studies. The finding that Barrett’s heterogeneity results from multiple independent stem/progenitor clones, would argue against a transdifferentiation model. Although origins of BE in humans is still open to debate, recent data from a number of independent groups have demonstrated that BE is more similar histologically to gastric tissue (12). Nevertheless, lineage tracing cannot be performed in humans therefore such descriptive studies will for now be the only prove of such a concept in humans.
Dclk1+ marks tuft cells in the GI tract, and tuft cells were amplified in regions of metaplasia and pre dysplasia in the IL-1β mouse model as well as in human samples. We recently demonstrated in the colon and intestine that Dclk1+ tuft cells play an important role in modulating the intestinal stem cell niche, since loss of DCLK1+ cells impairs the ability of stem cells to proliferate and expand following injury such as DSS colitis (19). Furthermore, we have previously shown that Dclk1+ cells are expanded in preneoplastic conditions (19) and in BE, Dclk1+ cells were markedly increased in the cardia and metaplastic tissue over time. Taken together, the data suggest that Dclk1+ cells are highly important niche cells supporting the proliferation of stem cells in response to injury or in the setting of carcinogenesis, and thus the expansion of Lgr5+ stem cells may occur in part due to the expanded niche, although Dclk1+ cells are often decreased with progression to cancer (46,47). The notion that tuft cells are niche cells responsible for maintaining mucosal homeostasis and communicating to other cell types has recently been demonstrated (48). It is therefore tempting to speculate that tuft cells are important not only during infection but also during carcinogenesis. Here, we show that increasing numbers of these potential niche cells are highly correlated with malignant transformation of the Barrett tissue and could be used as a good predictive biomarker for surveillance, as also proposed by C. Houchen and colleagues with the expression of DCLK1 in the epithelium, stroma and plasma of patients with BE/EAC (9).
In conclusion, this translation of mouse biomarkers to a study of BE patients highlights the importance of preclinical models for the understanding of human disease. The combination of high LGR5 or DCLK1, and low TFF2 or GC ratio into a diagnostic model appears promising in discriminating between non-dysplastic BE and BE associated with EAC. The novel integration of differentiation-, niche- and stem cell markers into a combined score allows a better discrimination of patients who developed EAC. We utilized Youden’s index for logistic regression in which all parameters are taken into account to define an ideal score to determine the power of a combined use of distinct markers. Nevertheless, its clinical benefit remains to be determined in further prospective studies. Overall, the data presented here highlight the potential for combining functionally relevant biomarkers, particularly those reflecting a lesser differentiating and stem cell expansion, to address our need for risk prediction tools to better stratify our BE patients.
Supplementary Material
Acknowledgments
Grant support: Deutsche Krebshilfe Max Eder Program to M. Quante and NIH U54 CA163004, NIH UO1 CA143056 to T.C. Wang
Footnotes
No conflict of interest exists
References
- 1.Quante M, Abrams JA, Lee Y, Wang TC. Barrett esophagus: what a mouse model can teach us about human disease. Cell Cycle. 2012;11(23):4328–38. doi: 10.4161/cc.2248522485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spechler SJ, Fitzgerald RC, Prasad GA, Wang KK. History, molecular mechanisms, and endoscopic treatment of Barrett’s esophagus. Gastroenterology. 2010;138(3):854–69. doi: 10.1053/j.gastro.2010.01.002. S0016-5085(10)00018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100(16):1184–7. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hvid-Jensen F, Pedersen L, Drewes AM, Sorensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. The New England journal of medicine. 2011;365(15):1375–83. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 5.Quante M, Bhagat G, Abrams JA, Marrache F, Good P, Lee MD, et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell. 2012 doi: 10.1016/j.ccr.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett NR. Chronic peptic ulcer of the oesophagus and ‘oesophagitis’. Br J Surg. 1950;38(150):175–82. doi: 10.1002/bjs.18003815005. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton SR, Smith RR. The relationship between columnar epithelial dysplasia and invasive adenocarcinoma arising in Barrett’s esophagus. Am J Clin Pathol. 1987;87(3):301–12. doi: 10.1093/ajcp/87.3.301. [DOI] [PubMed] [Google Scholar]
- 8.Quante M, Bhagat G, Abrams JA, Marache F, Good P, Lee MD, et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell. 2012;21(1):36–51. doi: 10.1016/j.ccr.2011.12.004. S1535-6108(11)00474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whorton J, Sureban SM, May R, Qu D, Lightfoot SA, Madhoun M, et al. DCLK1 is detectable in plasma of patients with Barrett’s esophagus and esophageal adenocarcinoma. Dig Dis Sci. 2015;60(2):509–13. doi: 10.1007/s10620-014-3347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Souza RF, Krishnan K, Spechler SJ. Acid, bile, and CDX: the ABCs of making Barrett’s metaplasia. Am J Physiol Gastrointest Liver Physiol. 2008;295(2):G211–8. doi: 10.1152/ajpgi.90250.2008. ajpgi.90250.2008. [DOI] [PubMed] [Google Scholar]
- 11.Vega KJ, May R, Sureban SM, Lightfoot SA, Qu D, Reed A, et al. Identification of the putative intestinal stem cell marker doublecortin and CaM kinase-like-1 in Barrett’s esophagus and esophageal adenocarcinoma. J Gastroenterol Hepatol. 2012;27(4):773–80. doi: 10.1111/j.1440-1746.2011.06928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald SA, Lavery D, Wright NA, Jansen M. Barrett oesophagus: lessons on its origins from the lesion itself. Nat Rev Gastroenterol Hepatol. 2015;12(1):50–60. doi: 10.1038/nrgastro.2014.181. [DOI] [PubMed] [Google Scholar]
- 13.Lavery DL, Nicholson AM, Poulsom R, Jeffery R, Hussain A, Gay LJ, et al. The stem cell organisation, and the proliferative and gene expression profile of Barrett’s epithelium, replicates pyloric-type gastric glands. Gut. 2014;63(12):1854–63. doi: 10.1136/gutjnl-2013-306508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatsuta T, Mukaisho K, Sugihara H, Miwa K, Tani T, Hattori T. Expression of Cdx2 in early GRCL of Barrett’s esophagus induced in rats by duodenal reflux. Dig Dis Sci. 2005;50(3):425–31. doi: 10.1007/s10620-005-2452-9. [DOI] [PubMed] [Google Scholar]
- 15.Hanby AM, Jankowski JA, Elia G, Poulsom R, Wright NA. Expression of the trefoil peptides pS2 and human spasmolytic polypeptide (hSP) in Barrett’s metaplasia and the native oesophageal epithelium: delineation of epithelial phenotype. J Pathol. 1994;173(3):213–9. doi: 10.1002/path.1711730303. [DOI] [PubMed] [Google Scholar]
- 16.Menke V, van Es JH, de Lau W, van den Born M, Kuipers EJ, Siersema PD, et al. Conversion of metaplastic Barrett’s epithelium into post-mitotic goblet cells by gamma-secretase inhibition. Dis Model Mech. 2010;3(1–2):104–10. doi: 10.1242/dmm.003012. 3/1-2/104. [DOI] [PubMed] [Google Scholar]
- 17.Stairs DB, Nakagawa H, Klein-Szanto A, Mitchell SD, Silberg DG, Tobias JW, et al. Cdx1 and c-Myc foster the initiation of transdifferentiation of the normal esophageal squamous epithelium toward Barrett’s esophagus. PLoS One. 2008;3(10):e3534. doi: 10.1371/journal.pone.0003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quante M, Marrache F, Goldenring JR, Wang TC. TFF2 mRNA transcript expression marks a gland progenitor cell of the gastric oxyntic mucosa. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.08.003. S0016-5085(10)01170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westphalen CB, Asfaha S, Hayakawa Y, Takemoto Y, Lukin DJ, Nuber AH, et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest. 2014;124(3):1283–95. doi: 10.1172/JCI73434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tu S, Chi AL, Lim S, Cui G, Dubeykovskaya Z, Ai W, et al. Gastrin regulates the TFF2 promoter through gastrin-responsive cis-acting elements and multiple signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2007;292(6):G1726–37. doi: 10.1152/ajpgi.00348.2006. 00348.2006. [DOI] [PubMed] [Google Scholar]
- 21.Wani S, Falk GW, Post J, Yerian L, Hall M, Wang A, et al. Risk factors for progression of low-grade dysplasia in patients with Barrett’s esophagus. Gastroenterology. 2011;141(4):1179–86. 86, e1. doi: 10.1053/j.gastro.2011.06.055. S0016-5085(11)00905-X. [DOI] [PubMed] [Google Scholar]
- 22.Riddell RH, Odze RD. Definition of Barrett’s esophagus: time for a rethink--is intestinal metaplasia dead? Am J Gastroenterol. 2009;104(10):2588–94. doi: 10.1038/ajg.2009.390. ajg2009390. [DOI] [PubMed] [Google Scholar]
- 23.Ellenrieder V, Beckh K, Muller D, Klatt S, Adler G. Intrahepatic high-grade malignant non-Hodgkin lymphoma in a patient with chronic hepatitis C infection. Z Gastroenterol. 1996;34(5):283–5. [PubMed] [Google Scholar]
- 24.Becker L, Huang Q, Mashimo H. Lgr5, an intestinal stem cell marker, is abnormally expressed in Barrett’s esophagus and esophageal adenocarcinoma. Dis Esophagus. 2010;23(2):168–74. doi: 10.1111/j.1442-2050.2009.00979.x. DES979. [DOI] [PubMed] [Google Scholar]
- 25.Warson C, Van De Bovenkamp JH, Korteland-Van Male AM, Buller HA, Einerhand AW, Ectors NL, et al. Barrett’s esophagus is characterized by expression of gastric-type mucins (MUC5AC, MUC6) and TFF peptides (TFF1 and TFF2), but the risk of carcinoma development may be indicated by the intestinal-type mucin, MUC2. Hum Pathol. 2002;33(6):660–8. doi: 10.1053/hupa.2002.124907. pii. [DOI] [PubMed] [Google Scholar]
- 26.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC bioinformatics. 2011;12(1):77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 28.Srivastava A, Golden KL, Sanchez CA, Liu K, Fong PY, Li X, et al. High Goblet Cell Count Is Inversely Associated with Ploidy Abnormalities and Risk of Adenocarcinoma in Barrett’s Esophagus. PLoS One. 2015;10(7):e0133403. doi: 10.1371/journal.pone.0133403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavery DL, Martinez P, Gay LJ, Cereser B, Novelli MR, Rodriguez-Justo M, et al. Evolution of oesophageal adenocarcinoma from metaplastic columnar epithelium without goblet cells in Barrett’s oesophagus. Gut. 2015 doi: 10.1136/gutjnl-2015-310748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 31.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457(7229):608–11. doi: 10.1038/nature07602. nature07602. [DOI] [PubMed] [Google Scholar]
- 32.Odze RD, Maley CC. Neoplasia without dysplasia: lessons from Barrett esophagus and other tubal gut neoplasms. Arch Pathol Lab Med. 2010;134(6):896–906. doi: 10.1043/1543-2165-134.6.896. [DOI] [PubMed] [Google Scholar]
- 33.Gatenby PA, Ramus JR, Caygill CP, Shepherd NA, Watson A. Relevance of the detection of intestinal metaplasia in non-dysplastic columnar-lined oesophagus. Scand J Gastroenterol. 2008;43(5):524–30. doi: 10.1080/00365520701879831. 790123634. [DOI] [PubMed] [Google Scholar]
- 34.Takubo K, Vieth M, Aida J, Sawabe M, Kumagai Y, Hoshihara Y, et al. Differences in the definitions used for esophageal and gastric diseases in different countries: endoscopic definition of the esophagogastric junction, the precursor of Barrett’s adenocarcinoma, the definition of Barrett’s esophagus, and histologic criteria for mucosal adenocarcinoma or high-grade dysplasia. Digestion. 2009;80(4):248–57. doi: 10.1159/000235923. 000235923. [DOI] [PubMed] [Google Scholar]
- 35.McCauley HA, Guasch G. Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia. Trends Mol Med. 2015 doi: 10.1016/j.molmed.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 36.van Baal JW, Rygiel AM, Milano F, Anderson M, Bergman JJ, Spek CA, et al. Gene expression profile comparison of Barrett’s esophagus epithelial cell cultures and biopsies. Dis Esophagus. 2008;21(7):628–33. doi: 10.1111/j.1442-2050.2008.00810.x. DES810. [DOI] [PubMed] [Google Scholar]
- 37.Van De Bovenkamp JH, Korteland-Van Male AM, Warson C, Buller HA, Einerhand AW, Ectors NL, et al. Gastric-type mucin and TFF-peptide expression in Barrett’s oesophagus is disturbed during increased expression of MUC2. Histopathology. 2003;42(6):555–65. doi: 10.1046/j.1365-2559.2003.01619.x. pii. [DOI] [PubMed] [Google Scholar]
- 38.Nakanishi Y, Saka M, Eguchi T, Sekine S, Taniguchi H, Shimoda T. Distribution and significance of the oesophageal and gastric cardiac mucosae: a study of 131 operation specimens. Histopathology. 2007;51(4):515–9. doi: 10.1111/j.1365-2559.2007.02793.x. HIS2793. [DOI] [PubMed] [Google Scholar]
- 39.Hayakawa Y, Sethi N, Sepulveda AR, Bass AJ, Wang TC. Oesophageal adenocarcinoma and gastric cancer: should we mind the gap? Nat Rev Cancer. 2016;16(5):305–18. doi: 10.1038/nrc.2016.24. [DOI] [PubMed] [Google Scholar]
- 40.Paull A, Trier JS, Dalton MD, Camp RC, Loeb P, Goyal RK. The histologic spectrum of Barrett’s esophagus. N Engl J Med. 1976;295(9):476–80. doi: 10.1056/NEJM197608262950904. [DOI] [PubMed] [Google Scholar]
- 41.Galipeau PC, Cowan DS, Sanchez CA, Barrett MT, Emond MJ, Levine DS, et al. 17p (p53) allelic losses, 4N (G2/tetraploid) populations, and progression to aneuploidy in Barrett’s esophagus. Proc Natl Acad Sci U S A. 1996;93(14):7081–4. doi: 10.1073/pnas.93.14.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maley CC, Galipeau PC, Finley JC, Wongsurawat VJ, Li X, Sanchez CA, et al. Genetic clonal diversity predicts progression to esophageal adenocarcinoma. Nat Genet. 2006;38(4):468–73. doi: 10.1038/ng1768. ng1768. [DOI] [PubMed] [Google Scholar]
- 43.von Rahden BH, Kircher S, Lazariotou M, Reiber C, Stuermer L, Otto C, et al. LgR5 expression and cancer stem cell hypothesis: clue to define the true origin of esophageal adenocarcinomas with and without Barrett’s Esophagus? J Exp Clin Cancer Res. 2011;30:23. doi: 10.1186/1756-9966-30-23. 1756-9966-30-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leedham SJ, Preston SL, McDonald SA, Elia G, Bhandari P, Poller D, et al. Individual crypt genetic heterogeneity and the origin of metaplastic glandular epithelium in human Barrett’s oesophagus. Gut. 2008;57(8):1041–8. doi: 10.1136/gut.2007.143339. gut.2007.143339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicholson AM, Graham TA, Simpson A, Humphries A, Burch N, Rodriguez-Justo M, et al. Barrett’s metaplasia glands are clonal, contain multiple stem cells and share a common squamous progenitor. Gut. 2011 doi: 10.1136/gutjnl-2011-301174. gutjnl-2011-301174. [DOI] [PubMed] [Google Scholar]
- 46.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–99. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 47.Delgiorno KE, Hall JC, Takeuchi KK, Pan FC, Halbrook CJ, Washington MK, et al. Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterology. 2014;146(1):233–44. e5. doi: 10.1053/j.gastro.2013.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2015 doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.