Abstract
Schizophrenia (SZ) and Autism Spectrum Disorder (ASD) have been both described as disorders of the self. However, the manner in which the sense of self is impacted in these disorders is strikingly different. In the current review, we propose that SZ and ASD lay at opposite extremes of a particular component of the representation of self; namely, self-location and the construct of peripersonal space. We evaluate emerging literature suggesting that while SZ individuals possess an extremely weak or variable bodily boundary between self and other, ASD patients possess a sharper self-other boundary. Furthermore, based on recent behavioral and neural network modeling findings, we propose that multisensory training focused on either sharpening (for SZ) or making shallower (for ASD) the self-other boundary may hold promise as an interventional tool in the treatment of these disorders.
Keywords: Schizophrenia, Autism, Self, Location, Peripersonal, Multisensory
1. Schizophrenia and Autism Spectrum Disorder as Disturbances in “Minimal Selfhood”
Schizophrenia (SZ) and autism spectrum disorder (ASD) have long been characterized as disorders of the self (Bleuler 1911; Kanner 1943; Nasrallah, 2012). Both disorders are associated with the loss of a coherent sense of self, anomalous self-experience, and the blurring of the distinction between the self and other (Ferri et al., 2012; Parnas et al., 2002; Sass et al., 2003; Hobson & Meyer, 2005). Accordingly, it has been argued that the poor social functioning exhibited by patients with SZ or ASD may be partly a product of a more basic disturbance in the sense of self (Nelson et al., 2009b). Thus, elucidating the neurocognitive mechanisms underlying the altered sense of self in SZ and ASD could lead to a better understanding of the etiologies of these disorders.
Phenomenological descriptions identify three levels of selfhood (Parnas et al., 2000; 2003): (1) a pre-reflective selfhood involved in the bottom-up assembly of a sense of self (Legrand 2007); (2) an explicit awareness of the sense of self that is the invariant subject of experience and action, and (3) a social-narrative self containing personality traits and states, habits, and preferences (Northoff et al., 2006). While the latter conceptualizations of the self are reliant on higher order cognitive constructs and their associated cortical structures (Northoff & Bermpohl 2004), the pre-reflective sense of self is associated with lower-level sensory and multisensory processes and the responsible brain regions (Tsakiris, 2010). As a consequence of this, recent theoretical models of both SZ and ASD have emphasized that self-disturbances in SZ and ASD may be in part attributable to changes in sensory and multisensory function (Parnas et al., 1998; 2002; 2005; Nelson et al., 2009b).
Although SZ and ASD patients frequently show overlap in their symptomology (e.g., negative symptoms in SZ resemble ASD features), the manner in which ASD and SZ groups interact with their surrounding world is often strikingly different (Crespi & Badcock 2008). While an over-extended sense of presence (Blanke et al., 2014) and the loss of the self-other boundary (Nelson et al., 2009a; Thakkar, Nichols, McIntosh and Park, 2011; Michael and Park, 2016) are commonplace in SZ, ASD is most frequently associated with difficulties in establishing interpersonal relatedness and problems in adapting a fragile self-concept to changing environments (Asperger, 1974). In the current review we propose that despite these striking different characteristics, abnormalities of basic self-experience in SZ and ASD are closely related, and that self-location (primarily as indexed by the representation of peripersonal space) serves as a fundamental organizational principle placing SZ and ASD at opposite ends of a continuum that relates to the boundary between self and other. Such a model highlights the fact that SZ and ASD patients may interact with the world in very distinct manners, yet may share mechanistic similarities in regards to disruptions in the constructs of self and peripersonal space.
Specifically, we propose that the etiological and pathophysiological processes that define SZ and ASD may be founded, respectively, in an extreme shallowness or steepness in the manner in which these two neuropsychiatric conditions represent the boundary between the bodily ‘self’ and ‘other’. As indexed by the representation of peripersonal space (PPS), shallowness and steepness between self and other may be quantified as the spatial extent over which the interactions between touch and the major exteroceptive senses (i.e., vision and audition) transitions from being absent to being complete. In such a conceptualization, a long transition space would equate to a shallow PPS representation, while a short transition space would equate to a steep PPS representation. As indirectly indexed by the ease or difficulty with which patients disembody their own spatial location in order to locate their bodies at another location, a shallow self-other boundary gradient is indexed by the proneness to disembodiment, while a steep self-other boundary gradient is illustrated by an inflexibility in altering one's self-location. This conceptualization allows for a dimensional analysis of shared symptoms, and may represent a powerful framework from which to elucidate the complexity of abnormal social processes in these two disorders. Further, this framework naturally accommodates the observation that SZ and ASD exhibit similarities in their symptomology, while being clearly distinct pathologies.
2. The Representation of Peripersonal Space as a Proxy for Self-Other Differentiation
Blanke and Metzinger (2009) put forward that minimal selfhood is composed of a process of self-identification, a first-person perspective, and self-location. The latter component, self-location, is amenable to experimental manipulation, as a number of paradigms have been developed that elicit perceived shifts in the location of a particular limb, or even of the entire body (Blanke et al., 2015). For example, in the Rubber-Hand Illusion (RHI; Botvinick & Cohen, 1998), a visible fake rubber hand is either stroked synchronously or asynchronously with tactile stimulation given on the hidden real hand. After a short period of stimulation, healthy participants report feeling ownership over the fake hand, and demonstrate a bias in the localization of their real hand in the direction of the rubber hand. This so-called proprioceptive drift can be taken as an indirect measure for self- or limb-location, and hence as a proxy measure of the ease or difficulty (and magnitude) with which subjects will alter their bodily representation.
Importantly, proprioceptive drift does not take place if the real and fake hands are placed too far away from one another (Lloyd 2007), which suggests that localizing one's hands is not only influenced by processing on the limbs themselves, but also by the space occupied by the hands. Indeed, Makin and colleagues, 2008, suggest that alterations in the bottom-up multisensory processing of the PPS surrounding the hand (Rizzolati et al., 1997; Ferri et al., 2013) are crucial in engendering the RHI. Most recently it has been shown in that the delineation of one's PPS representation around the trunk (Noel et al., 2014; Galli et al., 2014; Serino et al., 2015) shifts toward the location of a synchronously stroked virtual avatar (Noel et al., 2015; Salomon et al., 2016). Collectively, there is a growing body of evidence suggestive that the integration of body-related multisensory signals within PPS is a fundamental component of the ability to localize oneself in space and differentiate self from other.
The close relationship between one's PPS representation and the differentiation between self and other is apparent in that sharing sensory experiences with another blurs self-other distinctions (Tajadura-Jimenez, 2012; Sforza et al., 2010) and causes a remapping of one's PPS representation onto the other (Maister et al., 2014). Additionally, the size and placement of one's PPS representation (that is, the location of its boundaries) has been shown to accommodate to the presence of others (Fini et al., 2014) and to do so depending on the quality of the social interaction (Teneggi et al., 2013). It must be noted, however, that prior work has largely focused on the location and absolute size of the PPS, as opposed to the shape of its boundaries, when examining bodily self-other differentiation. This is surprising, as the representation of PPS is undoubtedly one that takes the form of a gradient, with the differentiation between self and non-self blurring as a function of distance from the body (Canzoneri et al., 2012; Longo et al., 2007). In the next two sections, we review literature pertaining to the examination of bodily illusions and the representation of PPS with an emphasis on differences in the gradient by which SZ and ASD populations encode self-other bodily distinctions.
3. Schizophrenia as a Disorder of Self Due to a Shallow Self-Other Gradient
Clinical reports and empirical studies have highlighted the wide array of body-related neurobiological processing abnormalities that characterizes SZ (Chang et al., 2001; 2005; Murakami et al., 2010; Agorastos et al., 2011; Holt et al., 2015). More recently, it has been suggested that a fragile bodily self-representation may be a core component of the pathology, and that the weaknesses in this representation may be caused by inadequate body-related multisensory integration processes (Postmes et al., 2014), which manifests as a loss of implicit self-knowledge and self-other differentiation (Gallace & Ferri, 2014). Indeed, recent theories that frame SZ as a disorder characterized by a disembodiment of the self (Zahavi, 2005; Fusch et al., 2005; Stanghellini 2009; Nasrallah, 2012) echo the original viewpoint expressed by Bleuler (1911).
Thakkar and colleagues (2011) and Peled and colleagues (2000) found a stronger RHI in SZ patients than in controls. This increased proneness to the illusion was demonstrated both by self-report questionnaires and by proprioceptive drift. Importantly, the onset of the illusion took place earlier in SZ than in controls (Peled et al., 2000). Further, during the RHI there was also a significant alteration in the latency of evoked somatosensory responses (Peled et al., 2003), as well as a stimulation-dependent change in skin temperature (Thakkar et al., 2011) and most strikingly, an out-of-body experience in a patient with SZ (Thakkar et al., 2011). A weaker and more variable sense of body boundary has also been demonstrated in patients with SZ via the Pinocchio Illusion (PI), which engenders a sensation that one's nose is growing in response to a tactile-proprioceptive manipulation (Michael & Park, 2016). Interestingly, the PI was associated with social isolation regardless of diagnosis, suggesting that reduced self-other social interactions may contribute to disturbances of the bodily self. Collectively, these results suggest a weaker and more variable representation of the body in space in SZ – and hence, a reduced distinction between bodily self and other (shallower gradient from self to other). Ferri and colleagues (2013) recently corroborated the observation that the RHI can be induced in SZ simply by tactile expectation; however, they also showed that the effect itself was dampened in SZ as compared to controls. Overall the body of evidence appears to indicate an increased proneness to the RHI in the schizophrenia-spectrum (Peled et al., 2000; 2003; Thakkar et al., 2011; Germine et al., 2014), and thus a more flexible PPS representation.
Weakened self-other boundary distinction in SZ is also supported by evidence suggesting a shallower PPS representation in SZ. Delevoye-Turrell and colleagues (2011) had patients with SZ and healthy controls perform judgments related to the location of the boundary of PPS by asking them to indicate when objects and people entered or exited their reaching space. Results demonstrated that judgments of the extension of PPS (as indexed by the measure of reaching space judgment) were on average, depending on the modality of response, either greater or similar for SZ than for healthy controls. Importantly for the current argument, however, results also revealed that patients with SZ were significantly more variable in their judgments, and this higher variability was selective for PPS judgments. Thus, it appears that the boundary between peri- and extra-personal space in SZ was either less well defined (i.e., shallower), or more variable on a trial-by-trial basis. Indeed, patients with SZ tend to demonstrate more variable responses than TD in an array of tasks. Furthermore, the variability observed by Delevoye-Turrell and colleagues (2011) was positively correlated to the general Positive and Negative Syndrome Scale (PANSS) score of the patients, indicating that the more severe the symptoms, the greater variability when judging the limits of PPS. Further, the magnitude of the errors made during judgments of reaching space correlated negatively on a subject-per-subject basis with their disorganization cluster score of the PANSS, indicating that the patients' capacity to repeatedly judge the limits of PPS decreased with increasing disorganized thoughts and behavior. The fact that variability in SZ's PPS representation correlated with both positive and negative symptoms of the disease points to a central role for the PPS representation in the psychopathology of the diseases. In addition, Ferri et al., 2015, have recently demonstrated that the variance in the PPS network (e.g., ventral Premotor Cortex), and not mean BOLD activity, predicts inter-individual variability in PPS size, reinforcing the importance of heightened PPS variability in SZ.
4. Autism Spectrum Disorder as a Disorder of Self Due to a Steep Self-Other Gradient
In ASD, the most direct examinations of the sharpness differentiating the bodily self and the bodily other come from studies of the RHI, as well as from studies of the more sociological-rooted concept of personal space, which, if “invaded”, causes considerable social distress (Sommer, 1959).
Cascio and colleagues (2012) report that individuals with ASD do experience the RHI (and thus, demonstrate some malleability in their body representation), but that the susceptibility to this illusion is delayed. While neurotypical individuals exhibited a proprioceptive drift both 3 and 6 minutes after the start of synchronous stroking, the shift in perceived hand location in the case of the ASD group was only apparent after 6 minutes of synchronous visuo-tactile stimulation. The reason behind the need for additional synchronous stroking in the ASD group is unknown, however, the authors proposed two possible explanations. First, it is well established that ASD individuals possess larger temporal “windows” (Stevenson et al., 2014; Noel et al., 2015; 2016a,b,c) within which they bind auditory and visual cues. In other words, ASD individuals will categorize two temporally disparate unisensory events (e.g., audio presentation followed by visual presentation) as happening synchronously at temporal disparities that TD individuals will clearly perceive as asynchronous (Foss-Feig 2010; Kwakye et al., 2011). The second possibility is that the delayed effect of the RHI in ASD may reflect a stronger tendency for these individuals to focus preferentially on tactile and proprioceptive signals and/or to dampen far exteroceptive signals (e.g., visual and auditory) in the presence of competing input from several modalities.
Regardless of mechanism, these findings strongly point to increased difficulty in disembodying the bodily self and embodying the bodily other in ASD, suggestive of a steeper and less flexible gradient from self-to-other. Furthermore, as the field of embodied cognition would predict, this bodily effect (i.e., less proneness to the RHI in ASD) appears to have social and cognitive ramifications. Cascio and colleagues, (2012) report that the children with ASD who displayed the least empathy were also those whom were least likely to experience the illusion. This finding fits well with the argument that an exceedingly sharp self-other boundary, and an anomalously inflexible self-localization representation, might be an important component of social and communication weaknesses in ASD.
A natural extension of these findings predicts that the PPS in ASD will show a steeper gradient between self and other. Stated more concretely, the prediction is that the spatial extent within which the far exteroceptive sensory modalities, such as audition and vision, transition from not influencing tactile processing on the body to influencing tactile processing is smaller than under typical circumstances. Instead of being a gradual transition, it is hypothesized that the PPS gradient may be almost a step function. This hypothesis, to the best of our knowledge, has not been tested from the framework of multisensory processing, but has received some examination from a social cognitive neuroscience and sociological perspective in the examination of personal space regulation in clinical populations (including ASD).
Kennedy & Adolphs, 2014, note that individuals with ASD are reported as having violated the personal space of others significantly more often as compared to their unaffected siblings. Further, these authors observed in a laboratory setting a complete absence of personal space in three individuals with ASD (Kennedy & Adolphs, 2014). For these three, when asked to either stop approaching another individual, or to indicate to the other individual to stop approaching them, individuals with ASD never stopped, and never indicated to the other individual to cease approaching them. An analogous finding was reported in a virtual environment by Parsons and colleagues (2004), who found that ASD participants were significantly more likely to be judged as bumping into or walking in between other people when compared with matched controls.
Lastly, Gessaroli and colleagues (2013) showed that during social interactions, children with ASD felt comfortable at a greater distance compared to typically developing (TD) children. Moreover, in TD children, personal space shrunk after interaction with a confederate, but it failed to do so in ASD children (see Nechamkin et al., 2003, for a similar result in SZ). These findings converge on the general notion that the regulation of personal space size and flexibility is strongly affected in autism. While the results of Gessaroli and colleagues (2013) are in contrast to those of Kennedy and colleagues (2014) and Parsons and colleagues (2004) in terms of the size of the representation of peripersonal space (larger in ASD in the former, but smaller in the latter two), all reports are in agreement with regard to the inflexibility (i.e., rigidity) of PPS representation in ASD, as evidenced by its failure to reduce in size after social interaction with a confederate. We suggest that the body of work on PPS in ASD can be captured within the view of a steeper and less flexible gradient between self and other.
5. Self-Other Differentiation as a Continuum of Peripersonal Space Representation
As highlighted above, available evidence suggests that both SZ and ASD may be disorders of the self, but may manifest in this domain in opposing ways. Although a detailed delineation of PPS has not been systematically studied in both ASD and SZ, the collective evidence seems to indicate that while individuals with SZ have an anomalously shallow PPS representation characterized by heightened variability, those with ASD seem to exhibit a steeper PPS gradient. To capture these findings, we have formulated a working model that the boundary within which individuals differentiate between self and other lies along a continuum, where the gradient between the self and the other may be shallower or steeper. It is conceivable that possessing a shallow boundary between self and other may be beneficial in certain scenarios (i.e., empathizing with others), while possessing a steeper boundary may be advantageous in others (i.e., attributing self-experience appropriately). Extending into the pathophysiological ends of this continuum, SZ may represent a particular instance in which the distinction between self and other is exceptionally weak (i.e., too shallow of a PPS boundary), while ASD may represent a particular instance in which the distinction between self and other is exceptionally strong (i.e., too sharp of a PPS boundary). In both of these cases the experience with others is likely to be impaired, which may then cascade into socio-communicative deficits and difficulties interacting with the physical world. We do not mean to imply that this difference in the gradient between self and other is the only dimension over which these two populations differ, but rather emphasize it as an important and perhaps underappreciated dimension that may strongly relate to areas of clinical symptomology.
6. Toward body-centered intervention?
We argue here that the self-other boundary plays an important role in successful social interactions. By extension, interventions designed to improve or ‘normalize’ the PPS boundary may hold therapeutic potential for those living with SZ and ASD. Seminal electrophysiological studies in monkeys (Iriki et al., 1996), as well as behavioral studies in humans (Maravita & Iriki, 2004), have established that the representation of the space around the hand is malleable and can be enlarged after the utilization of tools. Additionally, recent neural network models (Magosso et al., 2010a; 2010b) have proposed that the remapping of PPS can be accounted for by Hebbian strengthening (Hebb, 1949) of visual or auditory synapses converging on multisensory areas, which functionally extend the visual and auditory receptive fields of individual neurons in these multisensory areas. Thus, a hypothesis emerging from this model is that the expansion of PPS due to tool-use may be independent of the usage of a tool per se, but may rather be simply the result of repetitive and synchronous stimulation provided by auditory and/or visual events in conjunction with coincident tactile stimulation. This hypothesis has recently been confirmed in a combined behavioral-computational study (Serino et al., 2015).
A corollary of this prediction is that the synchronous administration of spatially fixed exteroceptive (i.e., visual and/or auditory) sensory signals with tactile information should strengthen the PPS boundary representation. On the other hand, according to Hebbian rule, the administration of temporally synchronous yet spatially random audio- and/or visuo-tactile stimulation should weaken the strength of the connections between unisensory and multisensory areas that give rise to PPS boundary representation. Hence, if the steepness of the PPS boundary (steep or shallow) affects the capacity to discriminate between self and other, it would follow that exposure to particular patterns of multisensory stimulation could either sharpen or dampen this boundary between self and other. Capitalizing on neural plasticity, SZ patients may benefit from the exposure to repetitive temporally synchronous and spatially non-random multisensory stimulation (thus sharpening the PPS boundary), while ASD patients may benefit from exposure to temporally synchronous and spatially random multisensory stimulation (thus making the PPS boundary more shallow).
Figure 1.
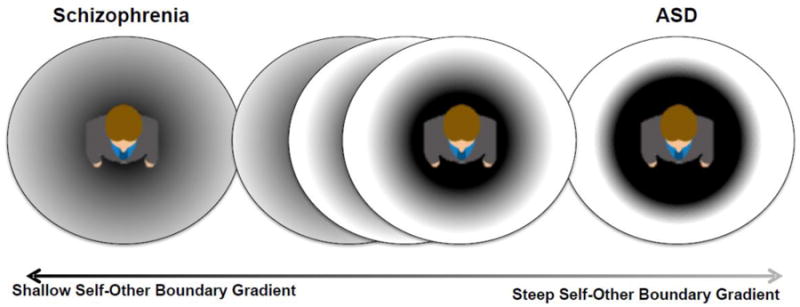
Schematic representation of the working hypothesis that self-other differentiation may lie on a continuum, and that although individuals with SZ and ASD exhibit distinct symptomatologies, these psychopathological conditions may index extreme cases of difficulties in self-other differentiation. The solid line circles represent the overall size of PPS (not necessarily changing in size as we move from SZ to healthy to ASD), while the color gradient represents the shallowness (SZ)/steepness (ASD) of the gradient with which self and other are differentiated throughout PPS.
Acknowledgments
Funding: This work was supported in part by NIH CA183492 and HD083211 and by the Simons Foundation Autism Research Initiative and the Wallace Research Foundation. No funding agency influenced any aspect of the current work nor in the decision to publish.
Footnotes
Author Contributions: Author JPN drafted the manuscript. All authors edited, contributed, and have approved the final manuscript.
Conflict of Interest: All authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agorastos A, Huber CG, Dunker S, Wiedemann K. Reduced pain perception in schizophrenia: A case of an undetected intrathoracicpencil. Am J Psychiatry. 2011;168:854–855. doi: 10.1176/appi.ajp.2011.11040581. [DOI] [PubMed] [Google Scholar]
- Asperger H. Fruhkindlicher Autismus. Medizinische Klinik, 1974. 2008;69(49):2024–2027. [PubMed] [Google Scholar]; Behav Brain Res. 191:1–10. doi: 10.1016/j.bbr.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Blanke O, Metzinger T. Full-body illusions and minimal phenomenal selfhood. Trends Cogn Sci. 2009;13:7–13. doi: 10.1016/j.tics.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Blanke O, Slater M, Serino A. Behavioral, neural, and computational principles of bodily self-consciousness. Neuron. 2015;88:145–66. doi: 10.1016/j.neuron.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Blanke O, Pozeg P, Hara M, Hendrich L, Serino A, Yamamoto T, et al. Neurological and robot-controlled induction of an apparition. Current Biology. 2014;24(22):2681–2686. doi: 10.1016/j.cub.2014.09.049. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia Praecox oder Gruppe der Schizophrenien. Deuticke; Leipzig, Germany: 1911. [Google Scholar]
- Bonini L, Maranesi M, Livi A, Fogassi L, Rizzolatti G. Space-dependent representation of objects and other's action in monkey ventral premotor grasping neurons. J Neurosci. 2014;34:4108–4119. doi: 10.1523/JNEUROSCI.4187-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Cohen J. Rubber hands ‘feel’ touch that eyes see. Nature. 1998;391(6669):756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- Brozzoli C, Gentile G, Bergouignan L, Ehrsson HH. A shared representation of the space near oneself and others in the human prefrontal cortex. Current Biology. 2013;23(18):1764–8. doi: 10.1016/j.cub.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Canzoneri E, Ubaldi S, Rastelli V, Finisguerra A, Bassolino M, Serino A. Tool-use reshapes the boundaries of body and peripersonal space representations. Exp Brain Res. 2013;228:25–42. doi: 10.1007/s00221-013-3532-2. [DOI] [PubMed] [Google Scholar]
- Cascio CJ, Foss-Feig JH, Burnette CP, Heacock JL, Cosby AA. The rubber hand illusion in children with autism spectrum disorders: delayed influence of combined tactile and visual input on proprioception. Autism. 2012;16(4):406–19. doi: 10.1177/1362361311430404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BP, Lenzenweger MF. Somatosensory processing and schizophrenia liability: proprioception, exteroceptive sensitivity, and graphesthesia performance in the biological relatives of schizophrenia patients. J Abnorm Psychol. 2005;114:85–95. doi: 10.1037/0021-843X.114.1.85. [DOI] [PubMed] [Google Scholar]
- Chang BP, Lenzenweger MF. Somatosensory processing in the biological relatives of schizophrenia patients: a signal detection analysis of two-point discrimination. J Abnorm Psychol 2001. 2003;110:433–442. doi: 10.1037//0021-843x.110.3.433. [DOI] [PubMed] [Google Scholar]; Comprehensive Psychiatry. 44(2):121–134. doi: 10.1053/comp.2003.50017. [DOI] [PubMed] [Google Scholar]
- Crespi B, Badcock C. Psychosis and autism as diametrical disorders of the social brain. Behav Brain Sci. 2008;31:241e320. doi: 10.1017/S0140525X08004214. [DOI] [PubMed] [Google Scholar]
- Delevoye-Turrell Y, Vienne C, Coello Y. Space boundaries in schizophrenia: Voluntary action for improved judgments of social distance. Social Psychology. 2011;42(3):193–204. [Google Scholar]
- Dworkin RH. Pain insensitivity in schizophrenia: a neglected phenomenon and some implications. Schizophr Bull. 1994;20:235–248. doi: 10.1093/schbul/20.2.235. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH. The experimental induction of out-of-body experiences. Science, 2007. 2005;317:1048. doi: 10.1126/science.1142175. [DOI] [PubMed] [Google Scholar]; exteroceptive sensitivity, and graphesthesia performance in the biological relatives of schizophrenia patients. J Abnorm Psychol. 114:85–95. doi: 10.1037/0021-843X.114.1.85. [DOI] [PubMed] [Google Scholar]
- Ferri F, Ardizzi M, Ambrosecchia M, Gallese V. Closing the Gap between the Inside and the Outside: Interoceptive Sensitivity and Social Distances. PLoS ONE. 2013;8(10):e75758. doi: 10.1371/journal.pone.0075758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri F, Chiarelli AM, Merla A, Gallese V, Costantini M. The body beyond the body: expectation of a sensory event is enough to induce ownership over a fake hand. Proc R Soc B Biol Sci. 2013;280:20131140. doi: 10.1098/rspb.2013.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri F, Costantini M, Salone A, Di Iorio G, Martinotti G, Chiarelli A, Merla A, Di Giannantonio M, Gallese V. Upcoming tactile events and body ownership in schizophrenia. Schizophr Res. 2014;152:51–57. doi: 10.1016/j.schres.2013.06.026. [DOI] [PubMed] [Google Scholar]
- Ferri F, Frassinetti F, Mastrangelo F, Salone A, Ferro FM, Gallese V. Bodily self and schizophrenia: the loss of implicit self-body knowledge. Conscious Cogn. 2012;21:1365–1374. doi: 10.1016/j.concog.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Fini C, Constantini M, Committeri G. Sharing Space: the Presence of Other Bodies Etends the Space Judged as Near. PLoS One. 2014;9(12):e114719. doi: 10.1371/journal.pone.0114719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss-Feig JH, Kwakye L, Cascio CJ, Burnette CP, Kadivar H, Stone WL, Wallace MT. An extended multisensory temporal binding window in autism spectrum disorders. Exp Brain Res. 2010;203:381–389. doi: 10.1007/s00221-010-2240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T. Corporealized and disembodied minds: A phenomenological view of the body in melancholia and schizophrenia. Philosophy, Psychiatry and Psychology. 2005;12:95–107. [Google Scholar]
- Gallase A, Spence C. Visual capture of apparent limb position influences tactile temporal order judgments. Neurosci Lett. 2005;379:63–68. doi: 10.1016/j.neulet.2004.12.052. [DOI] [PubMed] [Google Scholar]
- Gallese V, Ferri F. Psychopathology of the bodily self and the brain: the case of schizophrenia. Psychopathology. 2014;47(6):357–364. doi: 10.1159/000365638. [DOI] [PubMed] [Google Scholar]
- Galli G, Noel JP, Canzoneri E, Blanke O, Serino A. The wheelchair as a full-body tool extending the peripersonal space. Front Psychol. 2015;6:639. doi: 10.3389/fpsyg.2015.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessaroli E, Santelli E, di Pellegrino G, Frassinetti F. Personal space regulation in childhood autism spectrum disorders. PloS One. 2013;8:e74959. doi: 10.1371/journal.pone.0074959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior. New York: Wiley & Sons; 1949. [Google Scholar]
- Hobson RP, Meyer JA. Foundations for self and other: a study in autism. Dev Sci. 2015;8:281–91. doi: 10.1111/j.1467-7687.2005.00439.x. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Boeke EA, Coombs G, 3rd, DeCross SN, Cassidy BS, Stufflebeam S, Rauch SL, Tootell RB. Abnormalities in personal space and parietal-frontal function in schizophrenia. Neuroimage Clin. 2015;9:233–43. doi: 10.1016/j.nicl.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriki A, Tanaka M, Iwamura Y. Coding of modified body schema during tool use by macaque postcentral neurones. Neuroreport. 1996;7:2325–2330. doi: 10.1097/00001756-199610020-00010. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic Disturbances of Affective Contact. Nervous Child. 1943;2:217–50. [PubMed] [Google Scholar]
- Kennedy DP, Adolphs R. Violations of Personal Space by Individuals with Autism Spectrum Disorder. PLoS ONE. 2014;9(8):e103369. doi: 10.1371/journal.pone.0103369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakye LD, Foss-Feig JH, Cascio CJ, Stone WL, Wallace MT. Altered auditory and multisensory temporal processing in autism spectrum disorders. Front Integr Neurosci. 2011;4:129. doi: 10.3389/fnint.2010.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand D. Pre-reflective self-as-subject from experiential and empirical perspectives. Consciousness and Cognition. 2007;16(3):583–599. doi: 10.1016/j.concog.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Lenggenhager B, Tadi T, Metzinger T, Blanke O. Video ergo sum: manipulating bodily self-consciousness. Science. 2007;317:1096–1099. doi: 10.1126/science.1143439. [DOI] [PubMed] [Google Scholar]
- Lloyd DM. Spatial limits on referred touch to an alien limb may reflect boundaries of visuo-tactile peripersonal space surrounding the hand. Brain Cogn. 2007;64:104–109. doi: 10.1016/j.bandc.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Longo MR, Lourenco SF. Space perception and body morphology: extent of near space scales with arm length. Exp Brain Res. 2007;177(2):285–290. doi: 10.1007/s00221-007-0855-x. [DOI] [PubMed] [Google Scholar]
- Magosso E, Ursino M, di Pellegrino G, Làdavas E, Serino A. Neural bases of peri-hand space plasticity through tool-use: insights from a combined computational-experimental approach. Neuropsychologia. 2010;48:812–830. doi: 10.1016/j.neuropsychologia.2009.09.037. [DOI] [PubMed] [Google Scholar]
- Magosso E, Zavaglia M, Serino A, di Pellegrino G, Ursino M. Visuotactile representation of peripersonal space: a neural network study. Neural Comput. 2010;22:190–243. doi: 10.1162/neco.2009.01-08-694. [DOI] [PubMed] [Google Scholar]
- Maister L, Cardini F, Zamariola G, Serino A, Tsakiris M. Your place or mine: Shared sensory experiences elicit a remapping of peripersonal space. Neuropsychologia. 2014;70:455–461. doi: 10.1016/j.neuropsychologia.2014.10.027. [DOI] [PubMed] [Google Scholar]
- Makin TR, Holmes NP, Ehrsson HH. On the other hand: dummy hands and peripersonal space. Behav Brain Res. 2008;191:1–10. doi: 10.1016/j.bbr.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Maravita A, Iriki A. Tools for the body (schema) Trends Cogn Sci. 2004;8:79–86. doi: 10.1016/j.tics.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Michael JC, Park S. Anomalous bodily experiences and perceived social isolation in schizophrenia: An extension of the Social Deafferentation Hypothesis. Schizophrenia Research. 2016;S0920-9964(16):30280–8. doi: 10.1016/j.schres.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H, Tamasawa N, Yamashita M, Takayasu S, Nigawara T, Matsui J, Suda T. Altered pain perception in schizophrenia. Lancet. 2010;375:864. doi: 10.1016/S0140-6736(09)62061-4. [DOI] [PubMed] [Google Scholar]
- Nasrallah HA. Impaired mental proprioception in schizophrenia. Current Psychiatry. 2012;11(8):4–5. [Google Scholar]
- Nechamkin Y, Salganik I, Modai I, Ponizovsky AM. Interpersonal distance in schizophrenic patients: Relationship to negative syndrome. International Journal of Social Psychiatry. 2003;49:166–174. doi: 10.1177/00207640030493002. [DOI] [PubMed] [Google Scholar]
- Nelson N, Fornito A, Harrison BJ, Yücel M, Sass LA, et al. A disturbed sense of self in the psychosis prodrome: linking phenomenology and neurobiology. Neurosci Behav Rev. 2009a;33:807–17. doi: 10.1016/j.neubiorev.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Nelson B, Sass LA, Thompson A, Yung AR, Francey SM, Amminger GP, et al. Does disturbance of self underlie social cognition deficits in schizophrenia and other psychotic disorders? Early Intervention in Psychiatry. 2009b;3(2):83–93. doi: 10.1111/j.1751-7893.2009.00112.x. [DOI] [PubMed] [Google Scholar]
- Noel JP, Grivaz P, Marmaroli P, Lissek H, Blanke O, Serino A. Full body action remapping of peripersonal space: the case of walking. Neuropsychologia. 2014;70:375–384. doi: 10.1016/j.neuropsychologia.2014.08.030. [DOI] [PubMed] [Google Scholar]
- Noel JP, Pfeiffer C, Blanke O, Serino A. Full body peripersonal space as the sphere of the bodily self? Cognition. 2015;144:49–57. doi: 10.1016/j.cognition.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel JP, Wallace MT, Orchard-Mills E, Alais D, Van der Burg True and perceived synchrony are preferentially associated with particular sensory pairings. Sci Rep. 2015;5:17467. doi: 10.1038/srep17467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel JP, Lukowska M, Wallace MT, Serino A. Multisensory simultaneity judgment and distance from the body. Journal of Vision. 2016a;16(3):21. doi: 10.1167/16.3.21. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel JP, De Niear M, Stevenson R, Alais D, Wallace M. Atypical rapid audio-visual temporal recalibration in Autism Spectrum Disorders. Autism Research. 2016b doi: 10.1002/aur.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel JP, De Niear M, Van der Burg E, Wallace MT. Audiovisual Simultaneity Judgment and Rapid Recalibration throughout the Lifespan. PLoS ONE. 2016c;11(8):e0161698. doi: 10.1371/journal.pone.0161698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain - a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Parnas J, Bovet P, Zahavi D. Schizophrenic autism: clinical phenomenology and pathogenetic implications. World Psychiatry. 2002;1(3):131–136. [PMC free article] [PubMed] [Google Scholar]
- Parnas J. Clinical detection of schizophrenia-prone individuals: Critical appraisal. British Journal of Psychiatry. 2005;48:s111–112. doi: 10.1192/bjp.187.48.s111. [DOI] [PubMed] [Google Scholar]
- Parnas J. The self and intentionality in the pre-psychotic stages of schizophrenia: A phenomenological study. In: Zahavi D, editor. Exploring the self: Philosophical and psychopatological perspectives on self-experience. Amsterdam: John Benjamins; 2000. pp. 115–148. [Google Scholar]
- Parsons S, Mitchell P, Leonard A. The use and understanding of virtual environments by adolescents with autistic spectrum disorders. J Autism Dev Disord. 2004;34:449–466. doi: 10.1023/b:jadd.0000037421.98517.8d. [DOI] [PubMed] [Google Scholar]
- Peled A, Pressman A, Geva AB, Modai I. Somatosensory evoked potentials during a rubber-hand illusion in schizophrenia. Schizophr Res. 2003;64:157–163. doi: 10.1016/s0920-9964(03)00057-4. [DOI] [PubMed] [Google Scholar]
- Peled A, Ritsner M, Hirschmann S, Geva AB, Modai I. Touch feel illusion in schizophrenic patients. Biol Psychiatry. 2000;48:1105–1108. doi: 10.1016/s0006-3223(00)00947-1. [DOI] [PubMed] [Google Scholar]
- Postmes L, Sno HN, Goedhart S, van der Stel J, Heering HD, de Haan L. Schizophrenia as a self-disorder due to perceptual incoherence. Schizophr Res. 2014;152:41–50. doi: 10.1016/j.schres.2013.07.027. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Fogassi L, Gallese V. The space around us. Science. 1997;277:190–191. doi: 10.1126/science.277.5323.190. [DOI] [PubMed] [Google Scholar]
- Salomon R, Noel JP, Lukowska M, Faivre N, Metzinger T, Serino A, Blanke O. Unconscious of multisensory bodily inputs in the peripersonal space shapes bodily self-consciousness. BioRxiv. 2016 doi: 10.1016/j.cognition.2017.05.028. [DOI] [PubMed] [Google Scholar]
- Sass LA, Parnas J. Schizophrenia, consciousness, and the self. Schizophrenia Bulletin. 2003;29(3):427–444. doi: 10.1093/oxfordjournals.schbul.a007017. [DOI] [PubMed] [Google Scholar]
- Serino A, Canzoneri E, Marzolla M, di Pellegrino G, Magosso E. Extending peripersonal space representation without tool-use: evidence from a combined behavioral-computational approach. Frontiers in Behavioral Neuroscience. 2015;9(4):1–14. doi: 10.3389/fnbeh.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serino A, Noel JP, Galli G, Canzoneri E, Marmaroli P, Lissek H, Blanke O. Body part-centered and full body-centered peripersonal space representations. Sci Rep. 2015;5:18603. doi: 10.1038/srep18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sforza A, Bufalari I, Haggard P, Aglioti SM. My face in yours: Visuo-tactile facial stimulation influences sense of identity. Social neuroscience. 2010;5(2):148–162. doi: 10.1080/17470910903205503. [DOI] [PubMed] [Google Scholar]
- Sommer R. Studies in personal space. Sociometry. 1959;22:247–260. doi: 10.2307/2785668. [DOI] [Google Scholar]
- Stanghellini G. Embodiment and schizophrenia. World Psychiatry. 2009;8(1):56–59. doi: 10.1002/j.2051-5545.2009.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Siemann JK, Schneider BC, Eberly HE, Woynaroski TG, Camarata SM, Wallace MT. Multisensory temporal integration in autism spectrum disorders. J Neurosci. 2014;34(3):691–697. doi: 10.1523/JNEUROSCI.3615-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajadura-Jiménez A, Grehl S, Tsakiris M. The other in me: interpersonal multisensory stimulation changes the mental representation of the self. PloS one. 2012;7(7) doi: 10.1371/journal.pone.0040682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teneggi C, Canzoneri E, di Pellegrino G, Serino A. Social modulation of peripersonal space boundaries. Curr Biol. 2013;23:406–411. doi: 10.1016/j.cub.2013.01.043. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Nichols HS, McIntosh LG, Park S. Disturbances in body ownership in schizophrenia: Evidence from the rubber hand illusion and case study of a spontaneous out-of-body experience. PLoS ONE. 2011;6(10):e27089. doi: 10.1371/journal.pone.0027089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M, Haggard P. Experimenting with the acting self. Cogn Neuropsychol. 2005;22:387–407. doi: 10.1080/02643290442000158. [DOI] [PubMed] [Google Scholar]
- Tsakiris M. My body in the brain: a neurocognitive model of body-ownership. Neuropsychologia. 2010;48:703–712. doi: 10.1016/j.neuropsychologia.2009.09.034. [DOI] [PubMed] [Google Scholar]
- Zahavi D. Being someone. Psyche. 2005;11(5):1–20. [Google Scholar]


