Abstract
The ovarian follicle is the fundamental functional tissue unit of mammalian ovary. Each ovarian follicle contains one single oocyte. Isolation and in vitro culture of ovarian follicles to obtain fertilizable oocytes have been regarded as a promising strategy for women to combat infertility. The follicles from Peromyscus are considered as a better model than that from inbred mice for studying follicle culture. This is because Peromyscus mice are outbred (as with humans) with an increased life span. In this article, we reviewed studies on this subject conducted using Peromyscus follicles. These studies show that the conventional 2D micro-drop and 3D hanging-drop approaches established for in vitro culture of early preantral follicles from inbred mice are not directly applicable for cultivating the follicles from Peromyscus However, the efficiency could be significantly improved by culturing multiple early preantral follicles in one hanging drop of Peromyscus ovarian cell-conditioned medium. It is further revealed that the mechanical heterogeneity in the extracellular matrix of ovary is crucial for developing early preantral follicles to the antral stage and for the subsequent ovulation to release cumulus-oocyte complex. These findings may provide valuable guidance for furthering the technology of in vitro follicle culture to restore fertility in the clinic.
Keywords: hanging drop, microfluidics, microcapsule, ovulation, mechanobiology, alginate
1. Introduction
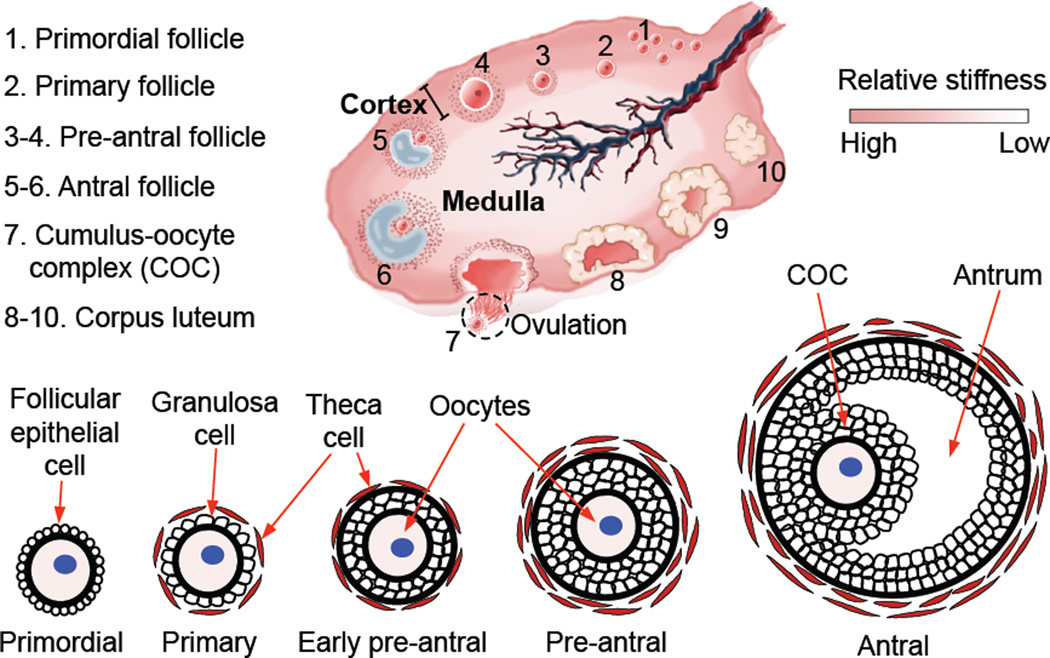
The ovary is a critical organ of the female reproductive system and the ovarian follicle is the fundamental functional tissue unit in ovaries of mammals including humans and Peromyscus. Each ovarian follicle contains one single oocyte. As schematically illustrated in Fig. 1, in a healthy ovary, the primordial (1) follicles continuously develop to the primary (2), secondary (early and late preantral, 3–4), and tertiary (early and late antral, 5–6) stages. The primordial follicle consists of one immature primary oocyte surrounded by one layer of follicular epithelial cells. Although the exact mechanism for the activation of primordial follicles and their development to the primary stage is still not well understood, it is observed that the follicular epithelial cells become more polygonal and are often called granulosa cells in the primary follicle. The primary follicles also contain some theca cells outside the single layer of granulosa cells. The secondary (or preantral) follicles differentiate them from the primary ones by the appearance of at least two layers of granulosa cells, and the tertiary (or antral) follicles have a unique fluid-filled antrum (also called antral cavity). However, few follicles (often one for humans) could develop through all the stages to ovulate and release a grown oocyte in the form of cumulus-oocyte complex (COC) during each estrous/menstrual cycle, leaving behind the corpus luteum. The follicles that could not develop to the stage of ovulation degenerate in the ovary, which often occurs in patients with ovarian disorders that are either genetic or acquired [1–8]. Therefore, isolation and in vitro culture of ovarian follicles to obtain fertilizable oocytes have been regarded as a promising strategy for women to combat infertility.
Figure 1.
A schematic illustration of the mammalian ovary showing the difference in mechanical properties between the ovarian cortex and medulla, the morphology of ovarian follicles at various developmental stages, and the ovulation of cumulus-oocyte complex (COC) from antral follicles leaving behind the corpus luteum. The schematic of mammalian ovary is reprinted and redrawn from reference [47] with permission from Elsevier.
In the past decades, studies have been reported to culture (mainly preantral) follicles on the 2D flat surface of culture dish [9,10], in homogeneous 3D hanging drop [11], and in homogeneous 3D hydrogel of alginate or an interpenetrating network of alginate and fibrin [12–17]. Moreover, results from some of the past studies demonstrate the potential of in vitro follicle culture for restoring or preserving fertility as live birth of offspring has been achieved using this approach [18–21]. However, such success has been limited to inbred mice and further research needs to be done using other biomedical model systems such as outbred animals including outbred mice and non-human primates, so that the technology can be further developed to eventually be applicable for human fertility preservation [16,22–31]. This is because the widely used inbred animals for biomedical research are unnatural (or man-made) with their haplotype structure and genetic make-up not resembling that of humans or even wild mice [32–37]. The Peromyscus (also called deer mice because their fur color resembles that of deer), outbred mice that are indigenous rodents in N. America, might be a better model than the widely used inbred mice for studying follicle culture in terms of the translational value. This is because the Peromyscus mice are outbred (as with humans) with a life span that is closer to humans than inbred mice. These mice have been proposed as a more appropriate animal model than inbred species for research on phylogeography, seciation, chromosomes, population genetics, aging, evolution, and haematology [38–45].
In this review, we performed a survey of the studies on in vitro culture of follicles from Peromyscus mice. Only early preantral follicles from Peromyscus have been studied in this field. The follicles have been cultured using the conventional 2D micro-drop and homogeneous 3D hanging-drop methods [46], and a novel biomimetic approach that recapitulates the mechanical heterogeneity (Fig. 1) experienced by the developing follicles in vivo [47,48]. These studies show that the paracrine interactions between multiple follicles and between follicles and ovarian stromal cells, together with the mechanical heterogeneity of the extracellular matrix in the ovary, are all important for the development of early preantral follicles of Peromyscus to the antral stage. Moreover, the mechanical heterogeneity is important in regulating the ovulation of the antral follicles to release cumulus-oocyte complex (COC). These findings may provide valuable guidance for furthering the technology of in vitro follicle culture to restore fertility in the clinic.
2. Isolation of ovarian follicles from Peromyscus
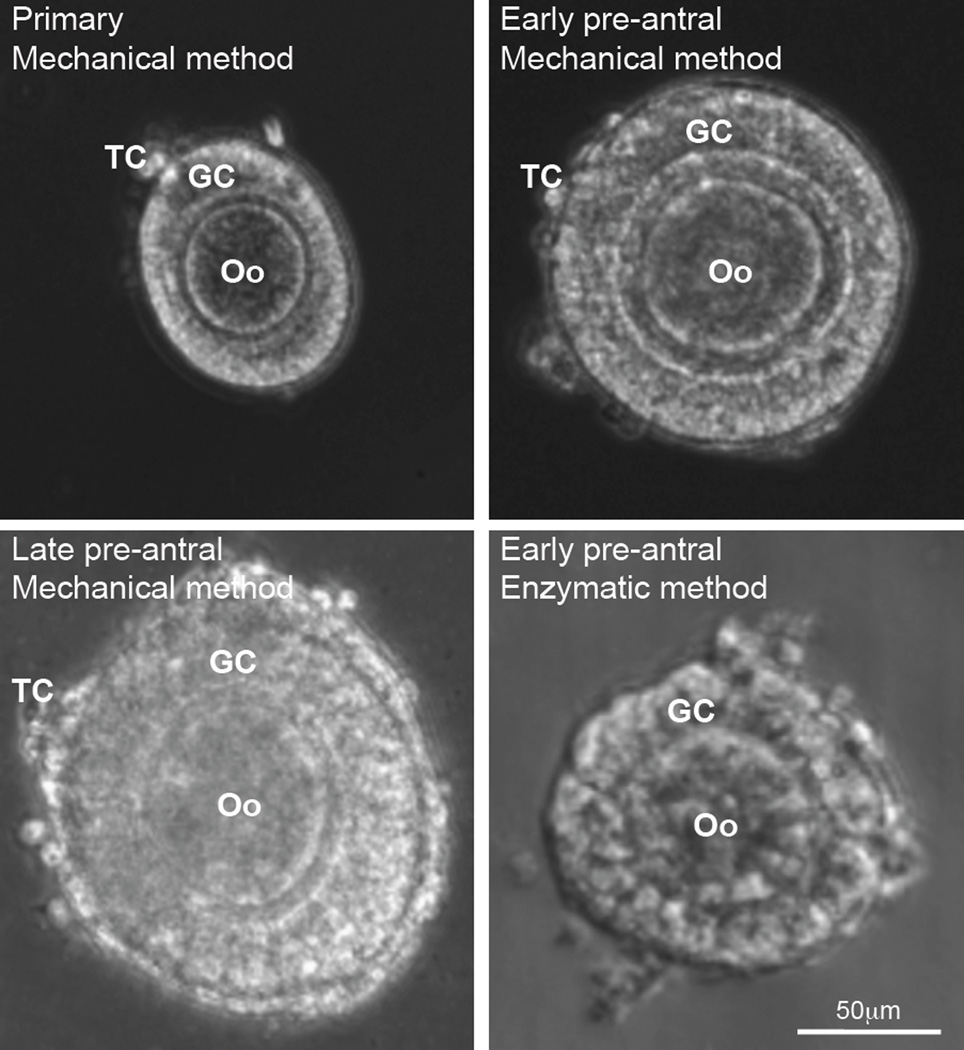
Both the mechanical and enzymatic methods have been used to isolate ovarian follicles from Peromyscus [46]. The former is achieved by using two 30-guage needles to mechanically break up the extracellular matrix between follicles in the ovarian tissue. Typical images showing the morphology of primary (75–99 µm), early preantral (100–125 µm), and late preantral (126–180 µm) follicles isolated with the mechanical method are given in Fig. 2. For the enzymatic method, the ovarian extracellular matrix between follicles is digested by using type I collagenase, which however, can also degrade the collagen within the follicles to damage the follicle integrity. Typical image of an early preantral follicles isolated using the enzymatic method showing compromised architecture is also shown in Fig. 2. Therefore, the studies on in vitro culture of follicles from Peromyscus have been focused on using follicles isolated with the mechanical method. In addition, only early preantral follicles from Peromyscus have been studied for in vitro culture so far using both the conventional and novel biomimetic methods, as detailed below.
Figure 2.
Images showing the typical morphology of primary (75–99 µm), early preantral (100–125 µm), and late preantral (126–180 µm) follicles retrieved from ovaries of Peromyscus using the mechanical method together with that of early preantral follicle obtained using the enzymatic method: The follicles retrieved by the mechanical method have an intact outer membrane of theca cells (TCs) and an intact layer(s) of granulosa cells (GCs) in the middle together with a primary oocyte (Oo) in the center. In contrast, the middle and particularly, the outer layer of the follicles retrieved by the enzymatic method were severely compromised. The figure is reprinted and redrawn from reference [46] with permission from Mary Ann Liebert Inc.
3. In vitro culture of early preantral follicles of Peromyscus with the 2D micro-drop and 3D hanging-drop methods
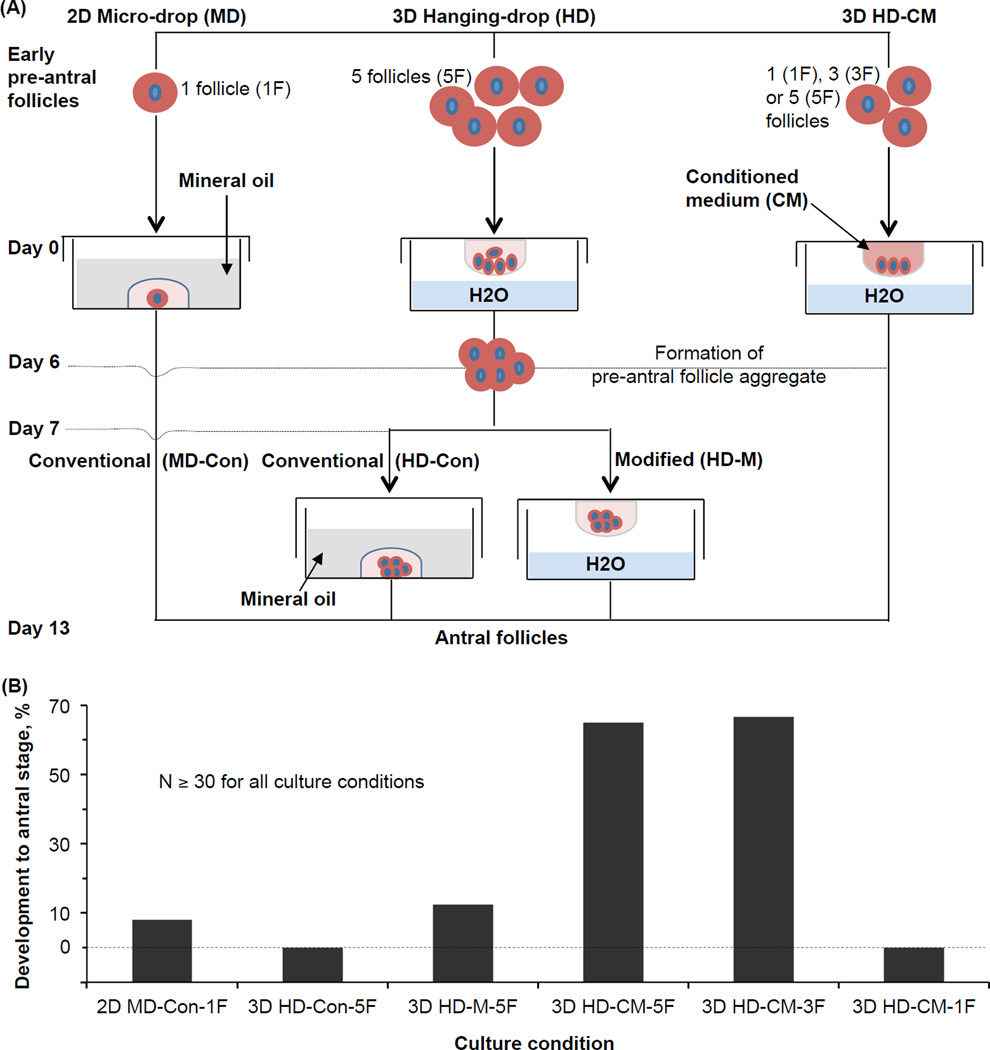
A comprehensive study was conducted to culture the early preantral follicle of Peromyscus using both the 2D micro-drop and 3D hanging-drop approaches [46]. As illustrated in Fig. 3A, for the conventional 2D micro-drop (2D MD-1F) method [9], one single follicle is cultured in a small (10–20 µl) drop of follicle culture medium (optimized for inbred mice) overlaid with mineral oil in culture dishes for 13 days. The follicle culture medium was α-MEM-glutamax medium, supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS), 5 µg/ml insulin, 5 µg/ml transferrin, 5 ng/ml selenium, 100 mIU/ml recombinant human follicle-stimulating hormone (FSH), and 1% (v/v) penicillin and streptomycin. For the conventional 3D hanging-drop (3D HD-Con-5F) method optimized for inbred mice [11], five single early preantral follicles were placed in each hanging drop of 25 µl of the aforementioned follicle culture medium. After six day’s culture to form a single aggregate of the five follicles in each hanging drop, the follicle aggregate was further cultured in a micro drop of 30 µl of the follicle culture medium overlaid with mineral oil till day 13.
Figure 3.
In vitro culture of early preantral follicles of Peromyscus with 2D micro-drop and various versions of 3D hanging-drop methods: (A) A schematic illustration of the various methods used for in vitro culture of early preantral follicles, and (B) the quantitative data of development to the antral stage using the various methods. N: number of early preantral follicles. The figure is reprinted and redrawn from reference [46] with permission from Mary Ann Liebert Inc.
As shown in Fig. 3B, none of these two conventional method optimized for in vitro culture of the early preantral follicles from inbred mice could be applied for effectively culturing the early preantral follicles from Peromyscus, with a percentage of development to the antral stage less than 10%. This percentage could be improved to more than 10% by modifying the conventional 3D HD-Con-5F method to continuously culture the 5 follicles and their aggregate in hanging drops for 13 days (i.e., the 3D HD-M-5F method). This is probably because continuous culture in the hanging drop could prevent the detachment of theca and granulosa cells from the follicles to attach on the surface of the petri dishes [46].
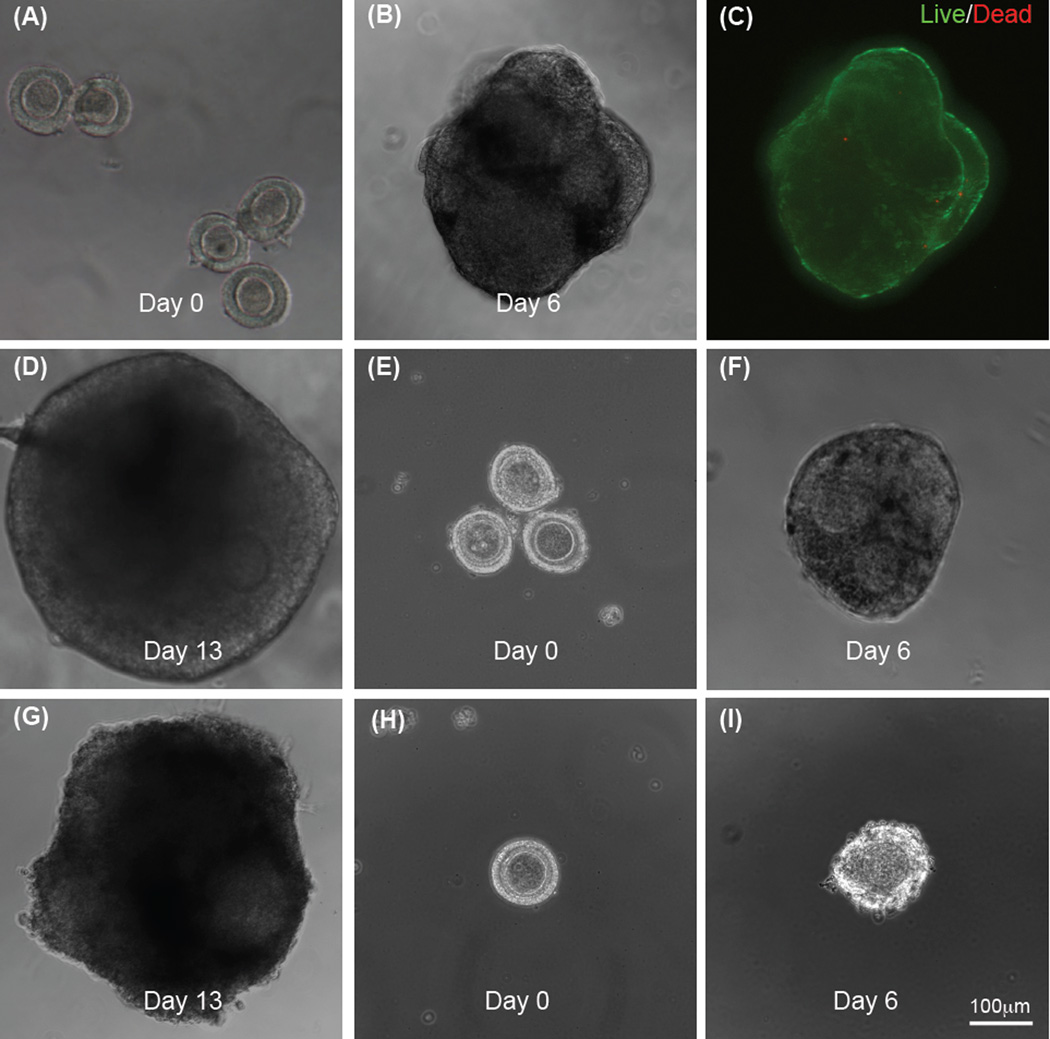
More importantly, when the conventional follicle culture medium optimized for inbred mice is replaced with the Peromyscus ovarian stromal cell-conditioned medium (CM) to modify the hanging-drop method (i.e., the 3D HD-CM-5F method), 65% of the early preantral follicles could develop to the antral stage. Typical images showing five single early preantral follicles in one hanging drop of the conditioned medium on day 0, the aggregate formed from the five follicles in the hanging drop on day 6, high viability of the follicles in the aggregate, and aggregated antral follicles on day 13 are shown in Fig. 4A, B, C, and D, respectively. The conditioned medium was made by incubating ovarian stromal cells isolated from one Peromyscus ovary with 5 ml of α-MEM–glutamax medium supplemented with 10% (v/v) heat-inactivated FBS and 1% (v/v) penicillin-streptomycin solution, in a 60 mm culture dish at 37 °C in 5% CO2 air for two days. The resultant medium was collected and the procedure repeated once to make a total of 10 ml of the medium, which is further supplemented with 5 µg/ml insulin, 5 µg/ml transferrin, 5 ng/ml selenium, and 100 mIU/mL recombinant human follicle stimulating hormone (FSH) to produce the eventual ovarian stromal cell-conditioned medium (CM) for further use. The ovarian stromal cells were isolated by dissociating the cells from the ovarian extracellular matrix using trypsin and type I collagenase at 37 °C, filtering through a 40 µm filter, centrifuging at 390 g, and culturing for 20 h in a 60 mm dish in 5 ml of DMEM supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C in 5% CO2 air.
Figure 4.
Typical micrographs showing development of early preantral follicles obtained mechanically from the Peromyscus ovaries under in vitro culture in hanging drop of ovarian cell-conditioned medium: Five single follicles per drop on day 0 (A), one single aggregate formed from the five follicles on day 6 (B), high viability (green) of the follicle aggregate on day 6 (C), and the aggregate of antral follicles developed from the five early preantral follicles after continuous culture in hanging drop for 13 days (D); three follicles per drop on days 0 (E) and 6 (F) and the aggregate of antral follicles developed from the three early preantral follicles after continuous culture in hanging drop for 13 days (G); and only one follicle per hanging drop on day 0 (H) and the degenerated follicle on day 6 (I). The figure is reprinted and redrawn from reference [46] with permission from Mary Ann Liebert Inc.
The remarkable improvement of the follicle development to the antral stage with the use of ovarian cell-conditioned medium suggest that the importance of paracrine interactions between the ovarian stromal cells and the follicles in facilitating follicle growth. In addition, these paracrine interactions are largely unidirectional (i.e., from the stromal cells to follicles) as only the stromal cells were used to make the conditioned medium.
The effect of the number of follicles in each hanging drop on the follicle development was further examined by putting 3 (i.e., 3D HD-CM-3F method) and 1 (i.e., 3D HD-CM-1F method) follicle in the hanging drop of ovarian cell-conditioned medium. As shown in Figs. 3B and 4A–G, the development with 3 early preantral follicles in the hanging drop of ovarian cell-conditioned medium is similar to that with five follicles. However, if only one follicle is used, the outcome of development is dismal (Fig. 3B) and the follicles were observed to degenerate in six days of culture (Fig. 4H–I). These results suggest that in addition to the stromal cells-follicle paracrine interactions, the follicle-follicle paracrine interactions are crucial for developing the early preantral follicles form Peromyscus into the antral stage.
4. In vitro culture of early preantral follicles of Peromyscus in biomimetic ovarian microtissue
As shown in Fig. 1, the extracellular matrix in the ovary is actually heterogeneous with a harder outer layer and softer inner layer that are known as the ovarian cortex and medulla, respectively [49,50]. To mimic this two-layered configuration with mechanical heterogeneity in the ovary, a non-planar microfluidic device was designed and fabricated to encapsulate early preantral follicle in microcapsules with a softer core (medulla) and a harder shell (cortex) [47,48]. Due to this biomimetic nature, this follicle-laden core-shell microcapsule is called biomimetic ovarian microtissue. More details on how to fabricate the microfluidic device together with the mechanisms in using the device to generate the biomimetic ovarian microtissue can be found elsewhere [47,48,51–54]. This review is focused on the outcome of culturing early preantral follicles from Peromyscus in the biomimetic ovarian microtissue.
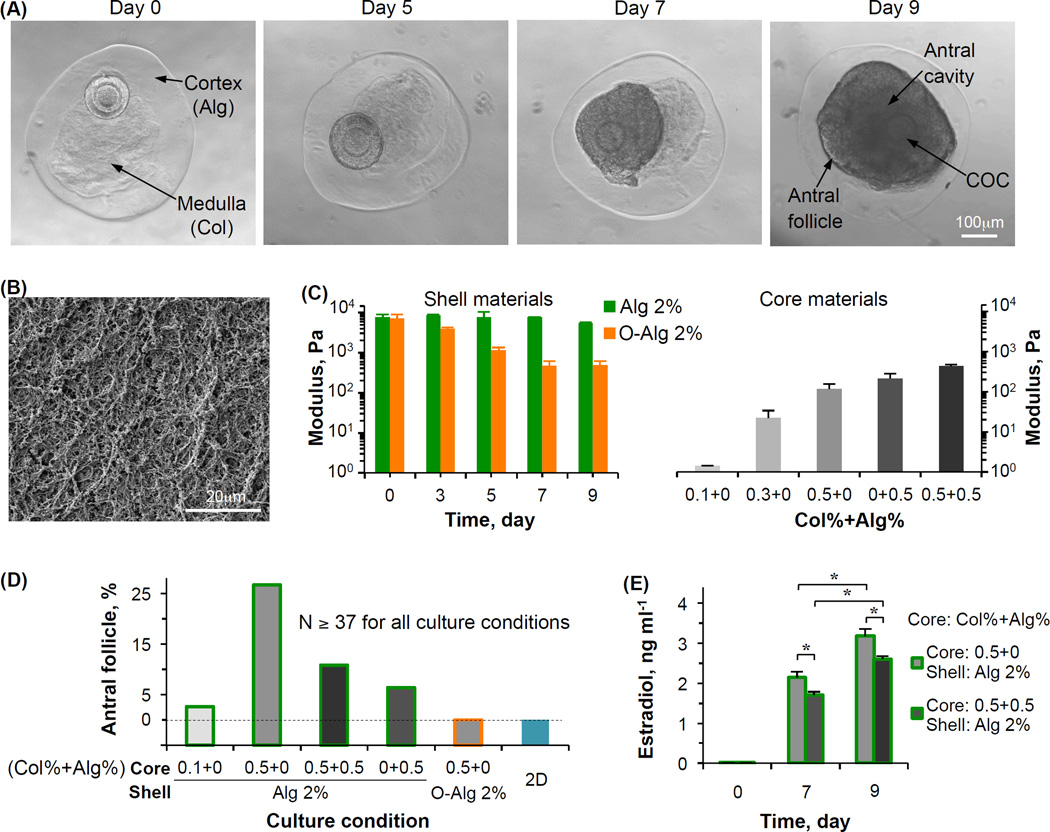
Typical images showing an early preantral follicle of Peromyscus encapsulated in the biomimetic ovarian microtissue with a 0.5% collagen (Col) core and 2% alginate (Alg) shell on day 0 and its development to the antral stage are given in Fig. 5A. The fibrous collagen core enclosed in the alginate hydrogel shell is visible, which is further confirmed by scanning electron microscopy (Fig. 5B) and confocal reflectance microscopy [47,48]. The defining feature of an antral follicle is that it contains a cumulus-oocyte complex (COC) inside a fluid-filled antrum or antral cavity (day 9, Fig. 5A). To study the mechanical heterogeneity on the follicle development, various core and shell materials were used to generate in the ovarian microtissues for culturing the early preantral follicles and the mechanical properties (elastic modulus) of the various materials are shown in Fig. 5C. The elastic modulus of regular alginate as the shell material was constantly more than 10 times higher than that of all core materials. The modulus of oxidized alginate (O-Alg) decreases with culture time and after ~7 days, it is similar to the highest modulus of the core materials. It is worth noting that alginate was used to fabricate the biomimetic ovarian microtissue because of its excellent biocompatibility and mild and reversible gelation conditions, which are not harmful to living cells [54–57]. Collagen is used because it is abundant in the extracellular matrix of ovarian tissue.
Figure 5.
Development of early preantral follicles under miniaturized biomimetic 3D culture in core-shell microcapsules showing the crucial role of mechanical heterogeneity in regulating the follicle development: (A) Typical images showing the growth of an early preantral follicle in the biomimetic microtissue with a 2% alginate shell and 0.5% collagen core, (B) a typical scanning electron microscopy (SEM) image showing the collagen fiber in the 0.5% collagen core, (C) elastic modulus of various shell and core materials used for fabricate the ovarian microtissue, (D) quantitative data of the percentage of development to the antral stage of early preantral follicles cultured under various conditions collected from references [47,48], and (E) production of estradiol by the growing follicles under the two best culture conditions. Alg: alginate; Col: collagen; O-Alg: oxidized alginate; N: number of early preantral follicles; and *: p < 0.05. The figure is reprinted and redrawn from reference [47] with permission from Elsevier (for panel A) and from reference [48] with permission from John Wiley & Sons, Inc. (for panels B, C, and E).
Figure 5D shows the quantitative data on the development of the early preantral follicles to the antral stage, both under miniaturized 3D culture in the various ovarian microtissues made of the different core (gray) and shell (green or orange for regular and oxidized alginate, respectively) materials and under 2D culture. The aforementioned ovarian stromal cell-conditioned medium was used for all the culture conditions. First of all, the development under 2D culture is dismal. The development under the miniaturized 3D culture was observed to be strongly dependent on the core and shell materials. The 0.5% collagen (0% alginate) core together with the 2% alginate shell leads to the best development. Moreover, the culture condition with the same core (i.e., 0.5% collagen and 0% alginate) but with a 2% oxidized alginate shell results in 0% development. These data indicate the crucial role of mechanical heterogeneity in regulating the follicle growth since the only difference between the regular and oxidized alginate is that the modulus of the latter decreases to that similar to the core materials in ~7 days (Fig. 5C). This is further supported by the reduced development for the culture condition with the 2% alginate but with a harder core (0.5% collagen and 0.5% alginate) where the adhesion molecule (i.e., collagen) is the same as that in the core for the optimal culture condition. In addition, the development in the microtissues (2% alginate shell) with 0.1% collagen alone or 0.5% alginate alone in the core is not as good as that in microtissue with 0.5% collagen alone in the core (2% alginate shell), suggesting that sufficient cell adhesion in the extracellular matrix is also important for follicle development.
Production of estradiol by the granulosa cells is an important molecular event accompanying follicle growth to the antral stage. As shown in Fig. 5E, culturing early preantral follicles in the optimal microtissue with a 0.5% collagen core and 2% alginate shell also produces significantly more estradiol than the second best (judged by the percentage of follicle development to the antral stage, Fig. 5D) culture condition with a 0.5% collagen+0.5% alginate core and 2% alginate shell. These data further show the crucial role of mechanical heterogeneity in regulating the follicle function and growth at the molecular level.
5. In vitro ovulation to release COCs from antral follicles obtained by in vitro follicle culture
As illustrated in Fig. 1, the event following the development to the antral stage in vivo is ovulation, a delicate reproductive process that results in the release of a cumulus-oocyte complex (COC) from each antral follicle. Although the exact mechanisms that regulate the ovulation process are still not fully understood, it is well accepted in contemporary literature that ovulation is triggered by the surge of luteinizing hormone (LH) from the pituitary gland. This LH surge activates a cascade of epidermal growth factor (EGF) mediated signaling pathways to induce differentiation of granulosa cells into cumulus cells and expansion of granulosa cells in the follicles, resulting in the escape of the COC out of the follicle and ovary [58–63].
However, no ovulation was observed for the antral follicles obtained by culturing early preantral follicles of Peromyscus with the aforementioned 2D micro-drop and 3D hanging-drop approaches [46], by adding the well-established doses of LH (2.5 IU/ml) and EGF (5 ng/ml) into the medium to induce ovulation for up to 18 hours [9,58–63]. As a result, the cumulus-oocyte complex (COC) in the antral follicles has to be isolated manually by puncturing the follicles using syringe needles [64].
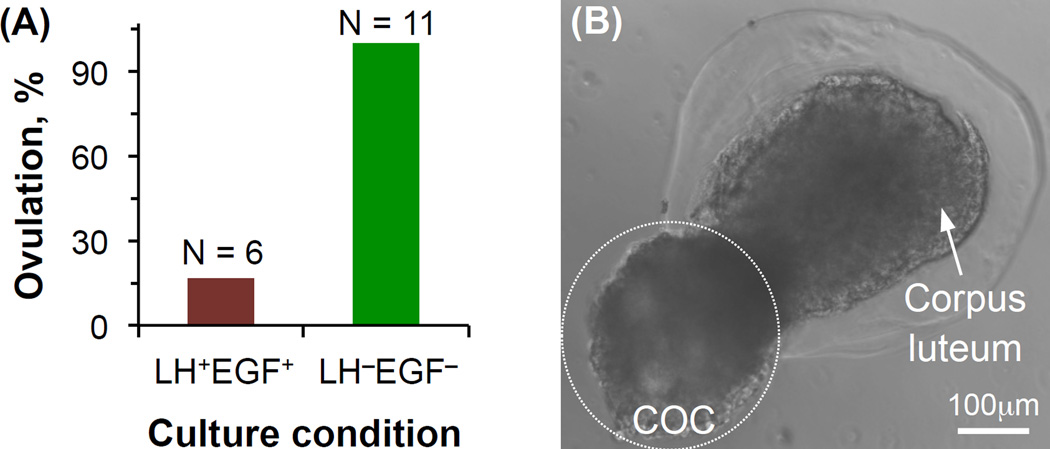
Importantly, ovulation was indeed observed for the antral follicles obtained by culturing the early preantral follicles of Peromyscus in the aforementioned optimal biomimetic ovarian microtissue with a 0.5% collagen core and 2% alginate shell (Fig. 5D). To study the role of LH and EGF in initiating ovulation, 6 antral follicles in the biomimetic ovarian microtissue were cultured in the conditioned medium with LH (2.5 IU/ml) and EGF (5 ng/ml) for up to 18 hours (LH+EGF+) and 11 antral follicles in the biomimetic ovarian tissue were observed as control without LH or EGF treatment (LH−EGF−). Less antral follicles were used for the LH+EGF+ group because according to the contemporary literature, the probability of ovulation from this group would be much higher than that for the LH−EGF− group.
In contrast to our anticipation, only 1 out of the 6 antral follicles in the biomimetic ovarian tissue treated with LH and EGF ovulated while ovulation was observed for all the 11 antral follicles in the biomimetic ovarian microtissue without LH or EGF treatment (Fig. 6A). A typical image showing the biomimetic ovulation via breaking apart the cortex (i.e., the alginate hydrogel shell) to release a COC and leaving behind a corpus luteum-like tissue complex is given in Fig. 6B. It is worth noting that this is the first study achieving ovulation in vitro in a biomimetic fashion via breaking apart the cortex. Interestingly, only 1 out of 3 antral follicles obtained from culturing early preantral follicles in the sub-optimal ovarian microtissue with a 0.5% alginate core and 2% alginate shell ovulated under the LH−EGF− culture.
Figure 6.
Biomimetic ovulation in vitro via breaking apart the cortex (i.e., the alginate hydrogel shell) to release cumulus-oocyte complex (COC): (A) The effect of the combination of luteinizing hormone (LH) and epidermal growth factor (EGF) on the biomimetic ovulation, and (B) a typical image showing the ovulation of cumulus-oocyte complex (COC) from the biomimetic ovarian microtissue leaving behind a corpus luteum-like structure. N: number of antral follicles. The figure is reprinted and redrawn from reference [47] with permission from Elsevier
All these data suggest the crucial role of mechanical heterogeneity in the ovary in regulating follicle ovulation and the LH surge and EGF signaling might even suppress the ovulation of some antral follicles to allow few of them being ovulated during each estrous/menstrual cycle, as occurs naturally. These results are consistent with the literature suggesting disruption of the normal physical microenvironment in the ovary may cause ovarian disorders such as premature ovarian failure (POF) and polycystic ovary syndrome (PCOS) with compromised function of ovulating and releasing oocytes from the ovary [50,65].
6. In vitro maturation (IVM) of COCs to obtain MII oocytes
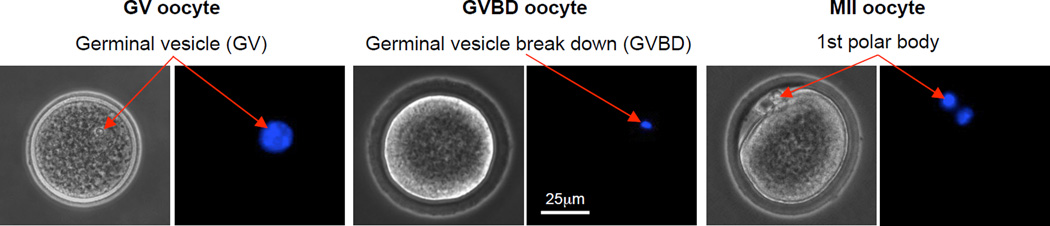
Although the oocytes in the COCs ovulated in vivo from a healthy ovary are usually at the stage of metaphase II (MII) that can be further fertilized with sperm for embryonic development to induce pregnancy, most of the oocytes in the COCs either manually isolated or spontaneously ovulated from the antral follicles obtained by in vitro culture are in the early immature stages [46–48]. These oocytes with an apparent germinal vesicle (GV) and undergone germinal vesicle break down (GVBD) are called GV and GVHD oocytes, respectively. Typical images showing the morphology of GV, GVBD, and MII oocytes are given in Fig. 7. The MII oocytes are featured by the appearance of a first polar body as a result of the meiotic development from the prophase I to metaphase I, anaphase I, telaphase I, prophase II, and MII.
Figure 7.
Typical phase contrast and fluorescence images of primary and secondary oocytes showing their morphology and nuclei distribution, respectively: The primary oocyte includes both GV (germinal vesicle) and GVBD (germinal vesicle breakdown) oocytes, while the MII (metaphase II) oocyte is secondary. A germinal vesicle containing all nuclear materials can be clearly seen in the GV oocyte, the germinal vesicle breaks down with the nuclear stain not as clearly identifiable in the GVBD oocyte, and the nuclear materials condense at two different locations with one being in the first polar body and the other in the cytoplasm of the MII oocyte. The figure is reprinted and redrawn from reference [46] with permission from Mary Ann Liebert Inc.
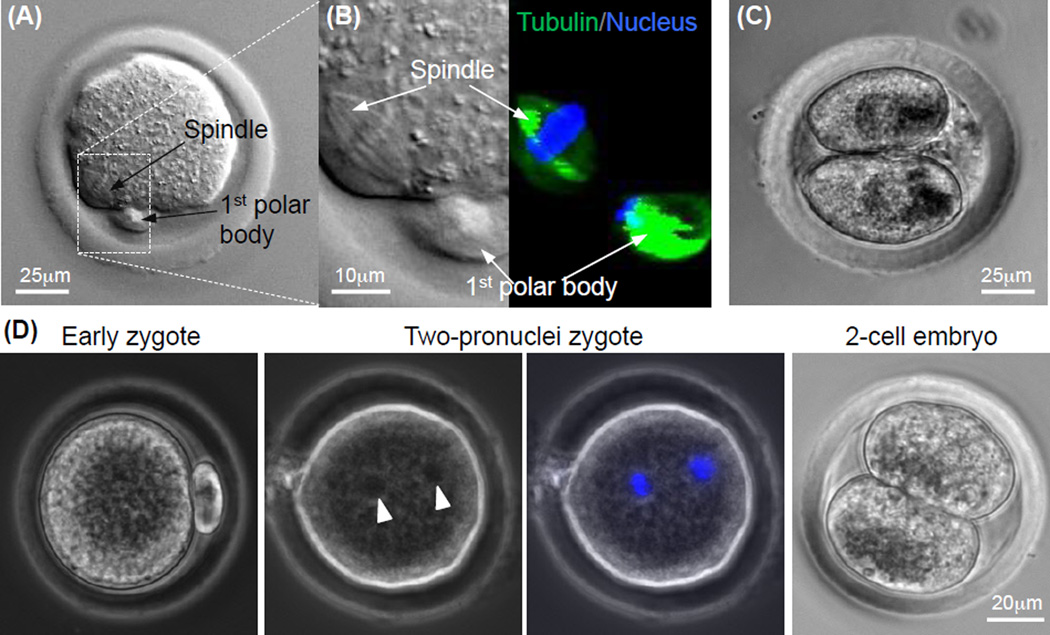
To obtain fertilizable MII oocytes of Peromyscus, in vitro maturation (IVM) of the COCs from the Peromyscus antral follicles obtained by in vitro culture using a protocol optimized by adding both leukemia inhibition factor (LIF) and epidermal growth factor (EGF) into the conventional medium for the IVM of the COCs of inbred mice [64,66]. Although none of the oocytes in the few COCs obtained from the sub-optimal ovarian microtissue developed to the metaphase II (MII) stage, 5 MII oocytes out of 11 COCs (45.5%) were obtained by culturing early preantral follicles of Peromyscus in the optimal biomimetic ovarian microtissue with a 0.5% collagen core and 2% alginate hydrogel shell. A typical image of the MII oocytes obtained by IVM is shown in Fig. 8A. To further confirm the developmental stage of the MII oocytes, the meiotic spindles and nuclei were stained by tubulin (in microtubules of the spindles) antibody and Hoechst, respectively. Figure 8B shows the ordered attachment of meiotic spindles (green) to the chromosomes (blue) in the cytoplasm while the arrangement of tubulin and chromosomes is chaotic in the first polar body, which is characteristic of MII oocytes.
Figure 8.
Quality of MII oocytes obtained by In vitro maturation of cumulus-oocyte complex from antral follicles developed from early preantral follicles by in vitro culture: (A) Typical image of a MII oocyte obtained from the antral follicles showing the characteristic 1st polar body and mitotic spindle, (B) nuclear and tubulin stains for visualizing the 1st polar body and mitotic spindle in the MII oocyte, (C) image of a two-cell embryo developed from the MII oocyte after parthenogenetic activation, and (D) typical micrographs showing development of MII oocyte after in vitro fertilization (early zygote) to the two-pronuclei and two-cell stages. The two pronuclei visible in the phase contrast image (arrow head) were further confirmed using fluorescence stain of the pronuclei (blue stains). The figure is reprinted and redrawn from reference [48] with permission from John Wiley & Sons, Inc. (for panels A–C) and from reference [46] with permission from Mary Ann Liebert Inc. (for panel D).
To further confirm the quality of the MII oocytes, their capability of being parthenogenetically activated using chemicals and fertilized with Peromyscus sperm in vitro was studied. Parthenogenetic activation of the MII oocytes was done by incubating the oocytes with Ca2+-free KSOM medium supplemented with 10 mM SrCl2 and 5 µg ml−1 cytochalasin B for 3.5 h [48,66]. Typical image of a 2-cell embryo developed from the parthenogenetically activated oocytes is shown in Fig. 8C. For in vitro fertilization (IVF) [46,64], 4 MII oocytes obtained by in vitro culture of early secondary preantral follicles were inseminated with 1.6 × 104 sperms in a 160 µl drop of TYH medium for 4.5 h. The fertilized oocytes were subsequently cultured in a drop of 50 µl of Hoppe and Pitts medium modified by removing sodium lactate and increasing the sodium chloride concentration to 5.97 g/ml at 37 °C in 5% CO2 air. Typical images showing a MII oocyte right after IVF (i.e., early zygote) and its development to the two-pronuclei and two-cell stages are shown in Fig. 8D. These data indicate fertilizable MII oocytes can be obtained by culturing early preantral follicles of Peromyscus in vitro. However, further studies are warranted to overcome the 8-cell block [64,66] of developing the early Peromyscus embryos to the blastocyst stage for implantation in vivo to produce offspring of Peromyscus.
7. Conclusions and outlook
In summary, the aforementioned studies demonstrate that the paracrine interactions between ovarian stromal cells and follicles, the paracrine interactions between follicles, and mechanical heterogeneity in the ovarian extracellular matrix (i.e., the harder cortex and softer medulla) all play important role in regulating the development of early preantral follicles of Peromyscus to the antral stage. Therefore, future studies to encapsulate multiple follicles in the core-shell microcapsule for culturing in ovarian stromal cell-conditioned medium may further improve the efficiency of developing early preantral follicles of Peromyscus to the antral stage. This multiple follicles-laden system that recapitulates the mechanical heterogeneity in the extracellular matrix of ovary may be efficient for in vitro ovulation of COCs from the multiple antral follicles developed from the early preantral follicles, as it has been demonstrated with the one follicle-laden ovarian microtissue in the aforementioned studies. In addition, the biomimetic ovarian microtissue system may be further applied to culture primary follicles of Peromyscus for their development to the antral stage and further ovulation to obtained COCs. Another important direction in this field is to search for the cues that are either chemical or mechanical to mature the oocytes in the follicles during in vitro culture so that COCs with MII oocytes could be ovulated from the antral follicles obtained by in vitro culture of early preantral or primary follicles. Further studies are also warranted to delineate the role of mechanical heterogeneity versus chemical cues (e.g., LH and EGF) in regulating the delicate ovulation process. Moreover, the design of the ovarian microtissue could be further optimized. For example, cell adhesion in the shell of the ovarian microtissue could be important because there is collagen in the native ovarian cortex for cell adhesion. This aspect of the cortical tissue was not considered in the ovarian microtissue in the aforementioned pioneering studies. Moreover, the effect of the percentage of the surface area of the follicles in the core versus shell could have important effect on the follicle development and ovulation, because this area percentage determines the mechanical signaling between the follicles and their heterogeneous environment in the microtissue. Lastly, alginate hydrogel microencapsulation has been shown to be exceptional in inhibiting ice formation during cooling and suppressing devitrification (i.e., forming ice in vitrified solutions) and the growth of ice crystals during warming cryopreserved samples [67,68]. This is attributed to the ultrahigh viscosity of the alginate hydrogel and its possible capability of binding to ice nuclei to prevent their growth. Therefore, the follicle-laden biomimetic microtissue with an alginate hydrogel shell could improve the outcome of follicle cryopreservation because ice formation (particularly inside cells) is the major cause of cell injury during cryopreservation. It is worth noting that the MII oocytes are more difficult to cryopreserve than the immature (prophase I) oocytes in the primary and early preantral follicles because the latter do not have the delicate arrangement of tubulin as that (Fig. 8B) in the MII oocytes arrested at the metaphase II stage of meiosis [28,69]. Further studies are warranted in this direction because cryopreservation of primary or early preantral follicles for future in vitro culture is important to the long-term preservation of fertility for women.
Acknowledgments
This work was partially supported by a grant from NIH (R01EB012108). The authors would like to thank Mr. Pranay Agarwal for preparing the sketches of ovarian follicles at various stages in Fig. 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384:1302–1310. doi: 10.1016/S0140-6736(14)60834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Telfer EE, Zelinski MB. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil Steril. 2013;99:1523–1533. doi: 10.1016/j.fertnstert.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen CY, Kristensen SG, Greve T, Schmidt KT. Cryopreservation of ovarian tissue for fertility preservation in young female oncological patients. Future Oncol. 2012;8:595–608. doi: 10.2217/fon.12.47. [DOI] [PubMed] [Google Scholar]
- 4.Jin M, Yu Y, Huang H. An update on primary ovarian insufficiency. Sci China Life Sci. 2012;55:677–686. doi: 10.1007/s11427-012-4355-2. [DOI] [PubMed] [Google Scholar]
- 5.Donnez J, Dolmans MM. Cryopreservation and transplantation of ovarian tissue. Clin Obstet Gynecol. 2010;53:787–796. doi: 10.1097/GRF.0b013e3181f97a55. [DOI] [PubMed] [Google Scholar]
- 6.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–911. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update. 2005;11:391–410. doi: 10.1093/humupd/dmi012. [DOI] [PubMed] [Google Scholar]
- 8.Santos RR, Amorim C, Cecconi S, Fassbender M, Imhof M, Lornage J, et al. Cryopreservation of ovarian tissue: an emerging technology for female germline preservation of endangered species and breeds. Animal reproduction science. 2010;122:151–163. doi: 10.1016/j.anireprosci.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Kim IW, Gong SP, Yoo CR, Choi JH, Kim DY, Lim JM. Derivation of developmentally competent oocytes by the culture of preantral follicles retrieved from adult ovaries: maturation, blastocyst formation, and embryonic stem cell transformation. Fertil Steril. 2009;92:1716–1724. doi: 10.1016/j.fertnstert.2008.08.084. [DOI] [PubMed] [Google Scholar]
- 10.Desai N, Alex A, AbdelHafez F, Calabro A, Goldfarb J, Fleischman A, et al. Three-dimensional in vitro follicle growth: overview of culture models, biomaterials, design parameters and future directions. Reprod Biol Endocrinol. 2010;8:119. doi: 10.1186/1477-7827-8-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Tang Y, Ni L, Jongwutiwes T, Liu H, Z R. A modified protocol for in vitro maturation of mouse oocytes from secondary preantral follicles. Adv Biosci Biotechnol. 2012;3:57–74. [Google Scholar]
- 12.Xu M, Banc A, Woodruff TK, Shea LD. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng. 2009;103:378–386. doi: 10.1002/bit.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587–594. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30:5476–5485. doi: 10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril. 2010;93:2633–2639. doi: 10.1016/j.fertnstert.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu M, Fazleabas AT, Shikanov A, Jackson E, Barrett SL, Hirshfeld-Cytron J, et al. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol Reprod. 2011;84:689–697. doi: 10.1095/biolreprod.110.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shikanov A, Xu M, Woodruff T, Shea L. A Method for Ovarian Follicle Encapsulation and Culture in a Proteolytically Degradable 3 Dimensional System. J Vis Exp. 2011;49:e2695. doi: 10.3791/2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Van der Elst J, Van den Broecke R, Dhont M. Live offspring by in vitro fertilization of oocytes from cryopreserved primordial mouse follicles after sequential in vivo transplantation and in vitro maturation. Biol Reprod. 2001;64:171–178. doi: 10.1095/biolreprod64.1.171. [DOI] [PubMed] [Google Scholar]
- 19.Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in-vitro. Biol Reprod. 1989;41:268–276. doi: 10.1095/biolreprod41.2.268. [DOI] [PubMed] [Google Scholar]
- 21.Nayudu PL, Osborn SM. Factors influencing the rate of preantral and antral growth of mouse ovarian follicles in vitro. J Reprod Fertil. 1992;95:349–362. doi: 10.1530/jrf.0.0950349. [DOI] [PubMed] [Google Scholar]
- 22.Roy SK, Treacy BJ. Isolation and long-term culture of human preantral follicles. Fertil Steril. 1993;59:783–790. [PubMed] [Google Scholar]
- 23.Oktay K, Nugent D, Newton H, Salha O, Chatterjee P, Gosden RG. Isolation and characterization of primordial follicles from fresh and cryopreserved human ovarian tissue. Fertil Steril. 1997;67:481–486. doi: 10.1016/s0015-0282(97)80073-8. [DOI] [PubMed] [Google Scholar]
- 24.Abir R, Fisch B, Nitke S, Okon E, Raz A, Ben Rafael Z. Morphological study of fully and partially isolated early human follicles. Fertil Steril. 2001;75:141–146. doi: 10.1016/s0015-0282(00)01668-x. [DOI] [PubMed] [Google Scholar]
- 25.Gorman KB, Flint PL, Esler D, Williams TD. Ovarian follicle dynamics of female Greater Scaup during egg production. J Field Ornithol. 2007;78:64–73. [Google Scholar]
- 26.Guimaraes DA, de Garcia SC, Ferreira MA, da Silva Sdo S, de Albuquerque NI, Le Pendu Y. Ovarian folliculogenesis in collared peccary, Pecari tajacu (Artiodactyla: Tayassuidae) Revista de biologia tropical. 2012;60:437–445. [PubMed] [Google Scholar]
- 27.Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16:395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ting AY, Yeoman RR, Lawson MS, Zelinski MB. In vitro development of secondary follicles from cryopreserved rhesus macaque ovarian tissue after slow-rate freeze or vitrification. Hum Reprod. 2011;26:2461–2472. doi: 10.1093/humrep/der196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Telfer EE, McLaughlin M. In vitro development of ovarian follicles. Semin Reprod Med. 2011;29:15–23. doi: 10.1055/s-0030-1268700. [DOI] [PubMed] [Google Scholar]
- 30.Hovatta O, Wright C, Krausz T, Hardy K, Winston RML. Human primordial, primary and secondary ovarian follicles in long-term culture: effect of partial isolation. Hum Reprod. 1999;14:2519–2524. doi: 10.1093/humrep/14.10.2519. [DOI] [PubMed] [Google Scholar]
- 31.Hreinsson JG, Scott JE, Rasmussen C, Swahn ML, Hsueh AJW, Hovatta O. Growth differentiation factor-9 promotes the growth, development, and survival of human ovarian follicles in organ culture. J Clin Endocr Metab. 2002;87:316–321. doi: 10.1210/jcem.87.1.8185. [DOI] [PubMed] [Google Scholar]
- 32.Niu Y, Liang S. Genetic differentiation within the inbred C57BL/6J mouse strain. J Zool. 2009;278:42–47. [Google Scholar]
- 33.Mott R. A haplotype map for the laboratory mouse. Nature genetics. 2007;39:1054–1056. doi: 10.1038/ng0907-1054. [DOI] [PubMed] [Google Scholar]
- 34.Wade CM, Kulbokas EJ, 3rd, Kirby AW, Zody MC, Mullikin JC, Lander ES, et al. The mosaic structure of variation in the laboratory mouse genome. Nature. 2002;420:574–578. doi: 10.1038/nature01252. [DOI] [PubMed] [Google Scholar]
- 35.Shorter K, Crossland J, Webb D, Szalai G, Felder M, Vrana P. Peromyscus as a Mammalian epigenetic model. Genet Res Int. 2012;2012(2012):1–11. doi: 10.1155/2012/179159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salcedo T, Geraldes A, Nachman MW. Nucleotide variation in wild and inbred mice. Genetics. 2007;177:2277–2291. doi: 10.1534/genetics.107.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein MS, Markert CL. Production of Chimeras in the Deer Mouse Peromyscus-Maniculatus-Bairdi. J Exp Zool. 1981;218:183–193. [Google Scholar]
- 38.Joyner C, Myrick L, Crossland J, Dawson W. Deer mice as laboratory animals. ILAR J. 1998;39(4):322–330. doi: 10.1093/ilar.39.4.322. [DOI] [PubMed] [Google Scholar]
- 39.Veres M, Duselis AR, Graft A, Pryor W, Crossland J, Vrana PB, et al. The biology and methodology of assisted reproduction in deer mice (Peromyscus maniculatus) Theriogenology. 2012;77:311–319. doi: 10.1016/j.theriogenology.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shorter KR, Crossland JP, Webb D, Szalai G, Felder MR, Vrana PB. Peromyscus as a Mammalian epigenetic model. Genet Res Int. 2012;2012:179–159. doi: 10.1155/2012/179159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duselis AR, Vrana PB. Aberrant growth and pattern formation in Peromyscus hybrid placental development. Biol Reprod. 2010;83:988–996. doi: 10.1095/biolreprod.110.085654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dewey MJ, Dawson WD. Deer mice: "The Drosophila of North American mammalogy". Genesis. 2001;29:105–109. doi: 10.1002/gene.1011. [DOI] [PubMed] [Google Scholar]
- 43.Chappell MA, Rezende EL, Hammond KA. Age and aerobic performance in deer mice. The Journal of experimental biology. 2003;206:1221–1231. doi: 10.1242/jeb.00255. [DOI] [PubMed] [Google Scholar]
- 44.Bedford NL, Hoekstra HE. Peromyscus mice as a model for studying natural variation. Elife. 2015;4 doi: 10.7554/eLife.06813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun Y, Desierto MJ, Ueda Y, Kajigaya S, Chen J, Young NS. Peromyscus leucopus mice: a potential animal model for haematological studies. Int J Exp Pathol. 2014;95:342–350. doi: 10.1111/iep.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi JK, Agarwal P, He X. In Vitro Culture of Early Secondary Preantral Follicles in Hanging Drop of Ovarian Cell-Conditioned Medium to Obtain MII Oocytes from Outbred Deer Mice. Tissue Eng Part A. 2013;19:2626–2637. doi: 10.1089/ten.tea.2013.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi JK, Agarwal P, Huang H, Zhao S, He X. The crucial role of mechanical heterogeneity in regulating follicle development and ovulation with engineered ovarian microtissue. Biomaterials. 2014;35:5122–5128. doi: 10.1016/j.biomaterials.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agarwal P, Choi JK, Huang H, Zhao S, Dumbleton J, Li J, et al. A biomimetic core-shell platform for miniaturized 3D cell and tissue engineering. Particle & Particle Systems Characterization. 2015;32:809–816. doi: 10.1002/ppsc.201500025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodruff TK, Shea LD. The role of the extracellular matrix in ovarian follicle development. Reprod Sci. 2007;14:6–10. doi: 10.1177/1933719107309818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodruff TK, Shea LD. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J Assist Reprod Gen. 2011;28:3–6. doi: 10.1007/s10815-010-9478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang H, He X. Interfacial tension based on-chip extraction of microparticles confined in microfluidic Stokes flows. Applied Physics Letters. 2014;105:143704. doi: 10.1063/1.4898040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang H, Sun M, Heisler-Taylor T, Kiourti A, Volakis J, Lafyatis G, et al. Stiffness-independent highly efficient on-chip extraction of cell-laden hydrogel microcapsules from oil emulsion into aqueous solution by dielectrophoresis. Small. 2015;11:5369–5374. doi: 10.1002/smll.201501388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang H, He X. Fluid displacement during droplet formation at microfluidic flow-focusing junction. Lab Chip. 2015;15:4197–4205. doi: 10.1039/c5lc00730e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agarwal P, Zhao S, Bielecki P, Rao W, Choi JK, Zhao Y, et al. One-step microfluidic generation of pre-hatching embryo-like core-shell microcapsules for miniaturized 3D culture of pluripotent stem cells. Lab Chip. 2013;13:4525–4533. doi: 10.1039/c3lc50678a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang W, He X. Microencapsulating and Banking Living Cells for Cell-Based Medicine. J Healthc Eng. 2011;2:427–446. doi: 10.1260/2040-2295.2.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromol Biosci. 2006;6:623–633. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 58.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JAS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: Key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20:1352–1365. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- 59.Fan HY, Liu ZL, Shimada M, Sterneck E, Johnson PF, Hedrick SM, et al. MAPK3/1 (ERK1/2) in Ovarian Granulosa Cells Are Essential for Female Fertility. Science. 2009;324:938–941. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilchrist RB, DM RB, Thompson JG. Oocyte maturation and ovulation- an orchestral symphony of signaling. Australian Biochemist. 2011;4 [Google Scholar]
- 61.Scaramuzzi RJ, Baird DT, Campbell BK, Driancourt MA, Dupont J, Fortune JE, et al. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod Fertil Dev. 2011;23:444–467. doi: 10.1071/RD09161. [DOI] [PubMed] [Google Scholar]
- 62.Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 63.Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330:366–369. doi: 10.1126/science.1193573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi JK, He X. In vitro maturation of cumulus-oocyte complexes for efficient isolation of oocytes from outbred deer mice. PLoS One. 2013;8:e56158. doi: 10.1371/journal.pone.0056158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma X, Fan L, Meng Y, Hou Z, Mao YDL, Wang W, et al. Proteomic analysis of human ovaries from normal and polycystic ovarian syndrome. Mol Hum Reprod. 2007;13:527–535. doi: 10.1093/molehr/gam036. [DOI] [PubMed] [Google Scholar]
- 66.Choi JK, He X. Improved Oocyte Isolation and Embryonic Development of Outbred Deer Mice. Scientific Reports. 2015;5:12232. doi: 10.1038/srep12232. (8pp) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang H, Choi JK, Rao W, Zhao S, Agarwal P, Zhao G, et al. Alginate hydrogel microencapsulation inhibits devitrification and enables large-volume low-CPA cell vitrification. Advanced Functional Materials. 2015;25:6839–6850. doi: 10.1002/adfm.201503047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang W, Yang G, Zhang A, Xu LX, He X. Preferential vitrification of water in small alginate microcapsules significantly augments cell cryopreservation by vitrification. Biomed Microdevices. 2010;12:89–96. doi: 10.1007/s10544-009-9363-z. [DOI] [PubMed] [Google Scholar]
- 69.Shaw JM, Oranratnachai A, Trounson AO. Fundamental cryobiology of mammalian oocytes and ovarian tissue. Theriogenology. 2000;53:59–72. doi: 10.1016/s0093-691x(99)00240-x. [DOI] [PubMed] [Google Scholar]