Abstract
Autoimmune CD4+ T cells can mediate the ability to withstand neurodegenerative conditions. Here we show that the ability to spontaneously manifest a T cell-dependent protective response is restricted by naturally occurring CD4+CD25+ regulatory T cells (Treg); depletion of Treg was beneficial in two mouse strains (C57BL/6J and BALB/c/OLA) differing in their spontaneous T cell-dependent ability to withstand the consequences of optic nerve injury. Passive transfer of exogenous Treg was destructive in BALB/c/OLA mice (which can spontaneously manifest a T cell-dependent protective anti-self response to injury) but beneficial in C57BL/6J mice (which have only limited ability to manifest such a response). This dichotomy was resolved by the finding that, in severe combined immunodeficient mice, a beneficial effect is obtained by passive transfer of either Treg-free CD4+ T cells (Teff) or Treg alone, indicating that neuroprotection can be achieved by either Treg or Teff in the absence of the other. We attribute these disparate effects of Treg to their differential interaction (in part via IL-10 and transforming growth factor β) with local innate immune cells (microglia) in the presence and in the absence of effector T cells. Activation of microglia by pro- and antiinflammatory cytokines in suitably controlled amounts might trigger different signal transduction pathways, each of which induces a neuroprotective microglial phenotype. These results suggest that, under neurodegenerative conditions, the effects of Treg, and possibly also of other regulatory T cells, might not be uniform, and that their expression in different individuals might be genetically determined. Therefore, therapeutic intervention based on induction of regulatory T cells might have limitations.
Keywords: autoimmunity, neuroprotection, neurodegenerative conditions, hippocampal slice cultures, immune regulation
The systemic adaptive T cell-dependent immune response plays a key role in the ability of neurons in the central nervous system (CNS) to withstand injurious conditions (1–3). Studies from our laboratory have shown that, after a CNS injury, T cells nonselectively migrate to the site of injury (4), and suggest that homing T cells, which encounter their relevant antigens at the lesion site, are the ones that contribute to the repair. Such T cells become locally activated to produce neurotrophic factors (5) and cytokines, which are capable of affecting the activity of resident microglia and hence the fate of threatened neurons. We further showed that the T cell-dependent protection evoked after an axonal injury, being an anti-self-response (6), is constitutively limited by naturally occurring CD4+CD25+ regulatory T cells (Treg) (6). It is, however, amenable to therapeutic boosting, either by postinjury immunization with antigens derived from dominant proteins residing in the site of damage or by depletion of Treg (2).
Treg comprise ≈10% of the CD4+ T cell population, and have been widely viewed as safeguards against the pathogenic effects of autoimmune CD4+CD25- effector T cells (Teff) thought to have escaped from the thymus during clonal deletion (7–10). This view was challenged, however, as a result of findings by our group, which suggested that a T cell subpopulation such as Treg is needed, at least in the context of neurodegeneration, not as a way to avoid the occurrence of autoimmunity, but as a means of allowing autoimmune T cells to exist on permanent stand-by, ready for activation when the need arises (6). This view appears at first sight to contradict the finding that mucosally administered self-antigens, for example, myelin basic protein or myelin oligodendrocyte glycoprotein, can boost the ability of rodents to withstand the neurodegenerative aftermath of optic nerve crush (11) or stroke (12), respectively, via induction of T cells that produce the characteristic regulatory cytokine IL-10 (13, 14).
The hypothesis underlying the present study is that regulatory T cells (both naturally occurring and mucosally induced) exert differential effects in different strains, depending (at least in part) on the constitutive ability to regulate autoimmunity (15). To test this hypothesis, we used a model of glutamate toxicity in the mouse eye. The amino acid glutamate is a vital neurotransmitter (16) that, at high concentrations, is toxic to neurons (17). Normally, the concentration of glutamate in the CNS is extremely low (2 μM) compared to its concentration in peripheral blood (80 μM). Glutamate is inert at the periphery and exerts its effect only via interaction with its specific receptors, which are found mainly on neurons and glial cells (18). In neurons that suffer a mechanical injury, an increase in extracellular glutamate is a common cause of the secondary degenerative process that results in progressive neuronal death (19, 20). Glutamate-mediated neurotoxicity is also a factor in the etiology of chronic neurodegenerative diseases (such as glaucoma) (21) and mental disorders (such as schizophrenia) (22). Studies by our group have shown that the ability of retinal neurons to withstand glutamate toxicity is at its lowest in animals whose adaptive immunity is deficient (23).
The results of this study showed that, after glutamate intoxication, boosting of autoimmune T cells by vaccination with specific retinal autoantigens was neuroprotective in both BALB/c/OLA and C57BL/6J mice, i.e., in two strains that differ in their spontaneous T cell-dependent ability to withstand the consequences of a CNS injury (24). Similarly, elimination of Treg improved neuronal survival in both strains. In contrast, exogenously supplied Treg had opposite effects in the two strains. In BALB/c/OLA mice (shown to be capable of spontaneously manifesting a neuroprotective autoimmune response to injury; ref. 24), Treg significantly weakened the ability to fight off neurodegeneration, whereas in C57BL/6J mice (in which the physiological protective response is severely limited; ref. 24), Treg conferred neuroprotection. The observed effect of exogenous Treg, found to be detrimental in wild-type BALB/c/OLA mice, turned out to be beneficial in BALB/c/OLA mice with severe combined immune deficiency (SCID). These results suggest that the effect of Treg on postinjury neuronal survival is context dependent. Experiments in vitro showed that both effector T cells (activated CD4+ T cells free of Treg), acting partially via IFN-γ, and Treg (CD4+CD25+ T cells), acting partially via IL-10 and/or transforming growth factor β (TGF-β), can endow microglia with a phenotype that is protective for damaged CNS.
Materials and Methods
Animals. The mice used in this study were handled according to the Association for Research in Vision and Opthalmology resolution on the use of animals in research. Wild-type male C57BL/6J mice and male BALB/c/OLA wild-type, nude, and SCID mice, all aged between 8 and 13 weeks, were supplied under germ-free conditions by the Animal Breeding Center of The Weizmann Institute of Science. The mice were housed in a light- and temperature-controlled room and matched for age in each experiment. Mice were anesthetized by i.p. administration of ketamine (80 mg/kg; Ketaset, Fort Dodge, IA) and xylazine (16 mg/kg; Vitamed, Ramat-Gan, Israel). Before tissue excision, the mice were killed with a lethal dose of pentobarbitone (170 mg/kg; CTS, Kiryat Malachi, Israel).
Antibodies and Reagents. Mouse recombinant IL-2, anti-mouse ζ-CD3, anti-mouse CTLA-4, murine recombinant (mr)-IFN-γ, mrIL-10, and mrTGF-β (R & D Systems), and rat anti-mouse phycoerythrin-conjugated CD25 antibody (Pharmingen, Becton Dickinson, Franklin Lakes, NJ) were used.
Labeling of Retinal Ganglion Cells. Mice were anesthetized as described above and placed in a stereotactic device. The skull was exposed and the bregma was identified and marked. The neurotracer dye FluoroGold (5% solution in saline, Fluorochrome, Denver) was stereotactically injected with a Hamilton syringe, and the skin over the wound was sutured (25).
Induction of Toxicity by Injection of Glutamate. The right eyes of anesthetized mice were punctured with a 27-gauge needle in the upper part of the sclera, and a Hamilton syringe with a 30-gauge needle was inserted as far as the vitreal body. Each mouse was injected with a total volume of 1 μl of PBS containing l-glutamate (400 nmol; Sigma).
Assessment of Retinal Ganglion Cell (RGC) Survival. At the end of the experimental period, the mice were given a lethal dose of pentobarbitone (170 mg/kg). Their eyes were enucleated, and the retinas were detached, prepared as flattened whole mounts in 4% paraformaldehyde in PBS, and labeled cells from four to six fields of identical size (0.076 mm2) were counted (25, 26). The average number of RGCs per field was calculated for each retina. The number of RGCs in the contralateral (uninjured) eye was also counted, and served as an internal control.
Vaccination with Retinal Antigens. The retina-specific proteins S antigen (S-Ag; retinal arrestin) and interphotoreceptor retinoid binding protein (IRBP) were purified from bovine retina. S-Ag was the kind gift of Paul Hargrave and Hugh McDowell (University of Florida, Gainesville). Bovine IRBP was purified from retinal extracts as described (27), by affinity chromatography on Con A followed by fast performance liquid chromatography. Bovine S-Ag was prepared from the Con A column flowthrough. The extract was dialyzed against 10 volumes of 10 mM Hepes/15 mM NaCl/1 mM EDTA/1 mM benzamidine, pH 7.0, with the buffer changed once. S-Ag was purified by the method of Buczylko and Palczewski (28) as modified by Puig et al. (29). The eluant in the heparin-agarose column was finally eluted via a column in which the NaCl gradient was from 10 to 400 mM. The vaccination consisted of 50 μg of antigen in complete Freund's adjuvant (Difco).
In Vitro Model of Hippocampal Slices. BALB/c/OLA mice, aged 8–10 days, were decapitated and their brains were rapidly removed under sterile conditions and placed in ice-cold preparation medium consisting of minimum essential medium (MEM; GIBCO) with 1% l-glutamine (GIBCO) at pH 7.35. The frontal pole was removed, and the brains were cut into 350-μm horizontal slices on a vibratome (Pelco, Redding, Germany), beginning at the ventral surface. Slices containing the hippocampi were cultured on Falcon cell culture inserts, pore size 0.4 μm (Becton Dickinson), in six-well plates. The cultivation medium contained 50% MEM, 25% HBSS (GIBCO), 25% normal horse serum, 2% glutamine, 10 μg/ml insulin-transferrin-sodium selenite supplement (Boehringer Mannheim), 2.64 mg/ml glucose (Braun, Melsungen, Germany), 0.1 mg/ml streptomycin, 100 units/ml penicillin, and 0.8 μg/ml vitamin C (all from Sigma). Organotypic hippocampal slice cultures (OHSCs) were incubated at 35°C in a humidified atmosphere with 5% CO2 for 24 h, during which time the slices were either left untreated or treated with 4 × 105 microglia cells per well. Tissue loss was assessed by addition of propidium iodide (PI) (5 μg/ml; Sigma) to the medium for 30 min at the end of the incubation period. Excess PI was then washed away with cultivation medium, and the slices were prepared for microscopy and visualized. To quantify neural cell death in the OHSCs, PI intensity in each slice was assessed by use of image-pro software (Media Cybernetics, Carlsbad, CA). PI staining intensity for a specific treatment was compared to the untreated control by using a two-tailed Student's t test.
Purification of Murine CD4+CD25+/CD4+CD25- T Cells. Lymph nodes (axillary, inguinal, superficial cervical, mandibular, and mesenteric) and spleens were harvested and mashed. T cells were purified (enriched by negative selection) on T cell columns (R & D Systems). The enriched T cells were incubated with anti-CD8 microbeads (Miltenyi Biotec), and negatively selected CD4+ T cells were incubated with phycoerythrin-conjugated anti-CD25 (30 μg per 108 cells) in PBS/2% FCS. They were then washed and incubated with anti-phycoerythrin microbeads and subjected to magnetic separation with AutoMACS. The retained cells were eluted from the column as purified CD4+CD25+ cells. The negative fraction consisted of CD4+CD25- T cells. Purified cells were cultured in 24-well plates (1 ml) with T cell-depleted spleen cells as accessory cells (irradiated with 3,000 rad; 1 rad = 0.01 Gy) and 0.5 μg/ml anti-CD3, supplemented with 100 units of mouse recombinant IL-2.
Microglial Cultures. Microglia were purified from the cerebral cortices of newborn (day 0) BALB/c/OLA mice, as described (30). IFN-γ (20 ng/ml), Il-10 (24 ng/ml), TGF-β (28 ng/ml), or activated effector (CD5+CD25-) or regulatory (CD4+CD25+) T cells (1.5 × 105 per well) were added to the culture medium for 12 h. After treatment, microglia were washed three times with PBS and prepared for application on hippocampal slices.
Results
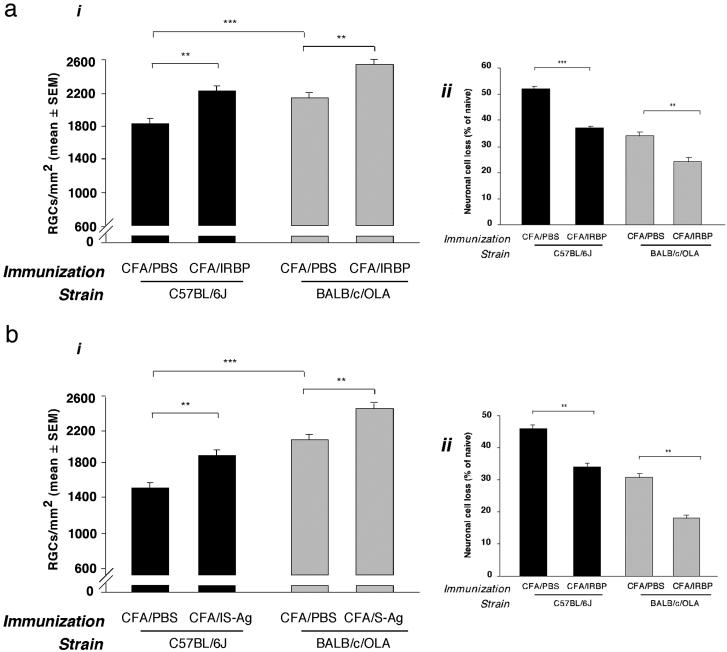
BALB/c/OLA and C57BL/6J Mice, Although Differing in Postinjury Neuronal Survival, Can Both Benefit from Vaccination with Self-Antigens. We have previously shown that postinjury survival of RCGs, measured by their retrograde labeling 1 week after intraocular injection of glutamate, is significantly better in BALB/c/OLA than in C57BL/6J mice (24). Here we examined whether mice of both strains can benefit from boosting of their autoimmune response by vaccination with self-antigens expressed in the retina (the site of glutamate intoxication). Mice of both strains showed a significant increase in surviving RGCs after immunization with IRBP (Fig. 1a) or with S-Ag (Fig. 1b). IRBP has been shown to cause experimental autoimmune uveoretinitis in C57BL/6J mice (albeit at higher doses than those used in this study), but not in BALB/c mice (31). S-Ag is not uveitogenic in either strain. With both self-antigens, the percentage of neurons that survived was significantly higher in the BALB/c/OLA than in the C57BL/6J mice. The protective effect, however, was more clearly apparent in the latter, possibly because the limited effectiveness of the endogenous protective mechanism in C57BL/6J mice allows more room for remedial intervention.
Fig. 1.
Neuroprotection induced in BALB/c/OLA and C57BL/6J mice by vaccination with retinal-specific antigens. BALB/c/OLA and C57BL/6J mice were immunized with 50 μg of IRBP (a) or S-Ag (b) or (as a control) with PBS, all emulsified in complete Freund's adjuvant. Significantly more neurons survived glutamate toxicity in PBS-immunized BALB/c/OLA mice than in PBS-immunized C57BL/6J mice. Immunization with each of the retinal antigens increased neuronal survival in both strains. Mean numbers of RGCs per mm2 are shown (ai and bi). Neuronal cell death is presented as a percentage of the mean total number of neurons in wild-type mice. (P values between groups, obtained by two-tailed Student's t test, are indicated by asterisks above the graph bars: **, P < 0.01; ***, P < 0.001; n = 6–8 in each group.)
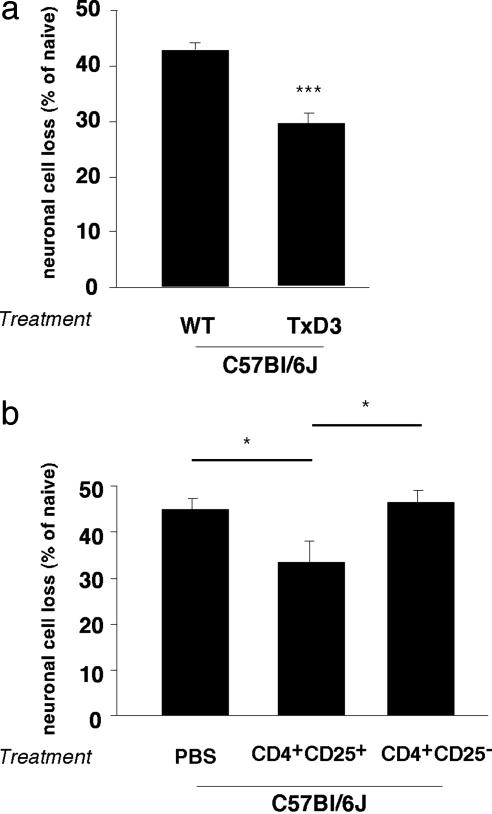
CD4+CD25+ T Cells Exert Opposing Effects in C57BL/6J Mice. The above results suggested that the inherent limitation of the ability of C57BL/6J mice to evoke a timely T cell-mediated protective response might be attributable to the constitutive presence of a suppressive mechanism. One such mechanism might, paradoxically, be provided by the naturally occurring CD4+CD25+ Treg (32, 33). Depletion of Treg was previously shown by our group to be an effective means of increasing neuronal survival after axonal injury in BALB/c/OLA mice (6), but has never been tested in C57BL/6J mice. Here we subjected C57BL/6J mice to thymectomy 3 days after birth, a protocol known to result in depletion of Treg in mice (9). As adults, these mice were subjected to glutamate intoxication. The loss of RGCs in the thymectomized C57BL/6J mice was significantly lower than in nonthymectomized matched controls that were similarly exposed to glutamate toxicity (expressed as a percentage of RGCs in normal nonintoxicated matched controls in each case; 29 ± 1.8% compared to 44 ± 2.0%, P < 0.001; Fig. 2a). These results suggested that the weak spontaneous T cell-dependent response to a CNS insult in wild-type C57BL/6J mice is due, at least in part, to Treg, which have a negative effect on the ability of the mice to withstand injurious conditions.
Fig. 2.
Decreased neuronal loss in C57BL/6J mice after depletion or exogenous supply of naturally occurring regulatory CD4+CD25+ T cells. (a) Adult C57BL/6J mice that had been thymectomized 3 days after birth and wild-type adult mice of the same strain were injected intravitreally with a toxic dose of glutamate (400 nmol). Significantly more RGCs survived in the thymectomized mice than in control (nonthymectomized) age-matched mice (P < 0.001; Student's t test; n = 5–6 in each group, indicated by ***). (b) Adult C67BL/6J mice were injected with 1.5 × 106 CD4+CD25+ T cells (Treg) or with PBS (control) and were then exposed to intravitreal glutamate toxicity (400 nmol). In mice that received an exogenous supply of Treg, RGC loss was significantly decreased relative to PBS- or Teff-injected control mice (P < 0.05; two-tailed Student's t test; n = 7–8 in each group, indicated by *). For ease of comparison between the two sets of experiments, graph bars indicate the percentage of RGC loss.
To verify that the beneficial effect of Treg depletion in wild-type C57BL/6J mice is indeed caused by their negative effect, we replenished glutamate-intoxicated wild-type C57BL/6J mice with passively transferred Treg. We anticipated that injection of these mice with Treg on top of existing endogenous Treg would further exacerbate neuronal survival. Surprisingly, however, the effect of this injection was beneficial (Fig. 2b), resulting in significantly decreased RGC loss relative to matched glutamate-intoxicated controls that were injected with PBS free of T cells or with naïve effector T cells (33 ± 3.1% compared to 43 ± 1.8% or 44.5 ± 2.9%, respectively; P < 0.05). These results thus suggested that the ability of wild-type C57BL/6J mice to withstand glutamate toxicity can be improved either by depletion or by exogenous supplementation of Treg.
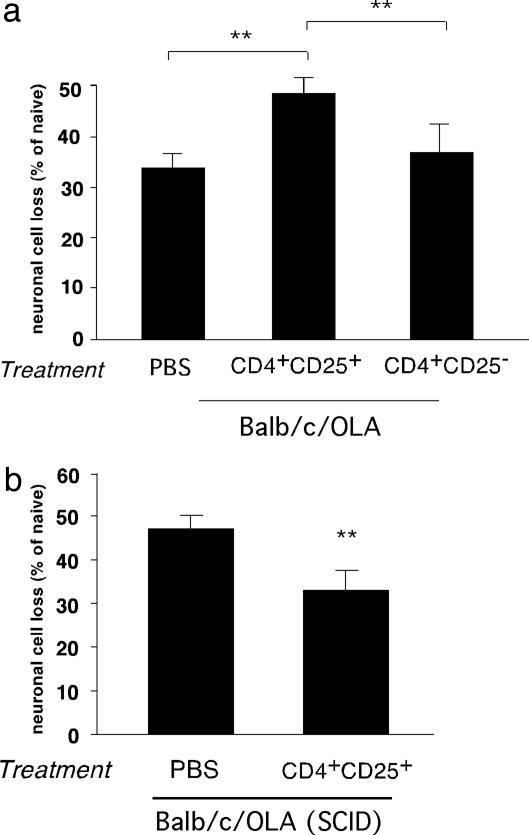
Differential Effect of CD4+CD25+ Tregs in BALB/c/OLA Mice Under Conditions of Immune Sufficiency and Immune Insufficiency. The effect of exogenously administered Treg in wild-type glutamate-intoxicated C57BL/6J mice was the opposite of their previously demonstrated suppressive effect after an optic nerve crush injury in BALB/c/OLA mice (6). This finding prompted us to examine the effect of Treg in BALB/c/OLA mice after glutamate intoxication. Here too, as in the earlier study (6), injection of activated Treg caused a significant decrease in RGC survival (Fig. 3a), confirming that the adverse effect of Treg on neuronal survival in the BALB/c/OLA strain occurs independently of the type of acute insult, and that injection of Treg in strains that differ in genetic control of the immune response might result in nonuniform results.
Fig. 3.
Differential effect of CD4+CD25+ regulatory T cells on neuronal survival in BALB/c/OLA and BALB/c/OLA SCID mice. (a) Naïve adult BALB/c/OLA mice injected with 1.5 × 106 CD4+CD25+ T cells (Treg) or 1.5 × 106 CD4+CD25- T cells (Teff) were exposed to intravitreal glutamate toxicity (400 nmol). Significantly fewer RGCs survived in mice that received Treg than in either PBS-treated control mice or mice treated with Teff (P < 0.01; two-tailed Student's t test; n = 8 in each group, indicated by **). (b) SCID mice of the BALB/c/OLA strain were injected with 1.5 × 106 CD4+CD25+ T cells (Treg) or with PBS (control) and were then exposed to intravitreal glutamate toxicity (400 nmol). Significantly more RGCs survived in the mice that had received Treg than in PBS-treated control mice (P < 0.01; two-tailed Student's t test; n = 6–7 in each group, indicated by **). For ease of comparison between the two sets of experiments, graph bars indicate percentage of RGC loss.
To determine whether the negative effect of the injected Treg on RGC survival in BALB/c/OLA mice is due to down-regulation of their spontaneous T cell-dependent protective mechanism, which in C57BL/6J mice is severely limited, we injected Treg into glutamate-intoxicated SCID mice of the BALB/c/OLA strain. We anticipated that if the adverse effect of Treg in BALB/c/OLA mice is exerted via their suppressive effect on the endogenous T cell-dependent mechanism, then their injection into SCID mice would result in no effect. Surprisingly, however, Treg injection in these immune-deficient BALB/c/OLA mice was protective, resulting in decreased RGC loss (47 ± 3.0% in PBS-treated SCID mice compared to 32 ± 3.5% in Treg-treated SCID mice, P < 0.01; Fig. 3b).
These results suggested that the adverse effect of Treg in the wild-type BALB/c/OLA mice, unlike their beneficial effect in the SCID mice, results from their suppression of the T cell-dependent protective mechanism, thus further supporting the contention that the ability to respond to immune manipulations based on regulatory T cells is strain-related (34).
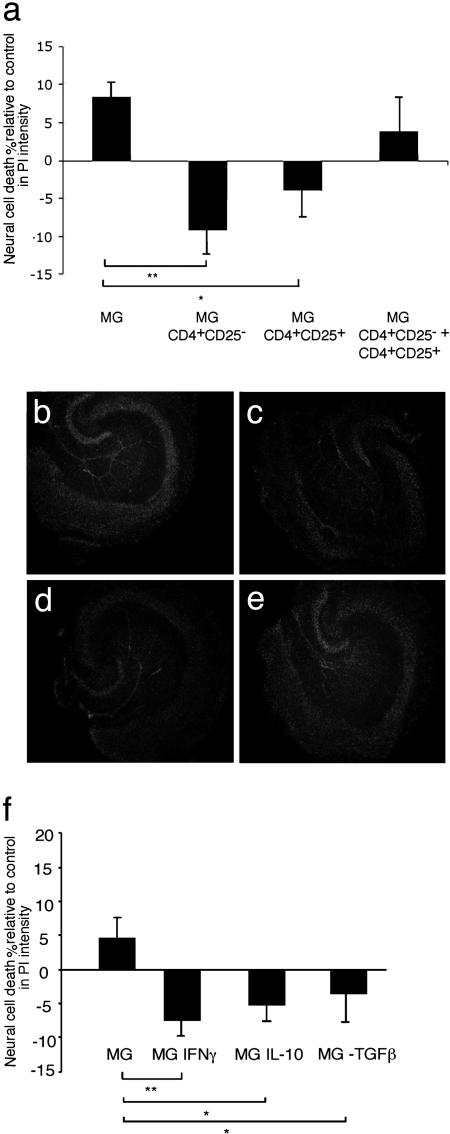
The Treg Neuroprotective Response Is Mediated in Part Through Microglial Activation by Treg-Derived IL-10 and/or TGF-β. We postulated that one way in which T cells confer neuroprotection occurs via their homing to the site of a lesion (35), where they influence the behavior of the local microglia (36). To gain some insight into the mechanisms underlying the beneficial and the inhibitory effects of Treg on neuronal survival, we carried out an in vitro experiment by using OHSCs. With this preparation, excision of the hippocampus corresponds to the primary insult, and the subsequent neural death in vitro is therefore a measure of secondary degeneration of the neural tissue (37, 38). Microglia were exposed to activated Teff, Treg, or both, and then washed well and added to OHSCs. Survival of neural tissue, assessed by the intensity of PI staining in the dentate gyrus within the hippocampal slices, was taken as a measure of the protective or the destructive activity of the microglia. Application of enriched untreated microglial cultures (80–85% pure) had a slight but nonsignificant effect on neuronal survival in the OHSCs (Fig. 4a). Microglia preincubated with either Treg or Teff significantly decreased neural death in these cultures (Fig. 4a). These results suggested that both Treg and Teff could endow microglia with a protective phenotype. However, treatment of microglia with a combination of Teff and Treg wiped out the protective effect observed upon treatment with each of these T cell populations separately (Fig. 4a). Representative photomicrographs are shown in Fig. 4 b–e.
Fig. 4.
Decreased neuronal death in mouse organotypic hippocampal slices treated with activated microglia. OHSCs were obtained from BALB/c/OLA mice. Immediately after sectioning, the slices were cocultured for 24 h with microglia that had been preincubated (12 h) with CD4+CD25+ T cells (Treg) or CD4+CD25- T cells (Teff), or with a combination of Treg and Teff, or with microglia preincubated with IFN-γ, IL-10, or TGF-β. In all experiments, control slices were treated with naïve microglia or were left untreated. Twenty-four hours after coculturing of brain slices and microglia, slices were stained with PI (a fluorescent dye that stains only dead cells) and analyzed by fluorescence microscopy. (a) Intensity of PI in the treated groups, calculated as a percentage of the intensity measured in untreated control OHSCs (*, P < 0.05; **, P < 0.01; two-tailed Student's t test; n = 6–8 slices per group). (b) Representative micrograph of slices treated with naïve microglia. (c) Slices treated with microglia preincubated with Treg. (d) Slices treated with microglia preincubated with Teff. (e) Slices treated with microglia preincubated with a combination of Treg and Teff. (f) Intensity of PI in slices treated with the indicated cytokines, calculated as percentage of the intensity measured in untreated control OHSCs.
It is commonly accepted that Treg exert their effect, at least in part, via production of TGF-β or IL-10 (14, 39, 40). Moreover, in a C57BL/6J mouse model of stroke, nasally administered myelin oligodendrocyte glycoprotein was recently shown to confer neuroprotection via IL-10 (12). We therefore considered the possibility that the Treg-induced effect on microglia is partially dependent on IL-10 or TGF-β, and that the neuroprotective effect of Teff is mediated, at least in part, through IFN-γ (41). Microglia exposed to IFN-γ were indeed found to be protective when added to OHSCs (O. Butovsky, A. Talpalar, K. Ben-Yaakov, and M.S., unpublished observations). Examination revealed that the protective effect of microglia preincubated with IL-10 or TGF-β was similar to that of microglia incubated with IFN-γ (Fig. 4f).
Discussion
In this study we demonstrate strain-related ability of naturally occurring Treg to act, under neurodegenerative conditions in the CNS, as effector cells, suppressor cells, or both. It seems that the opposing effects of this dual activity reflect the immune status of the tissue, as determined by genetic control of the immune response. We further showed that both the suppressor and the effector activities could be mediated through dialogue with microglia, and could be mimicked, at least in part, by IL-10 or TGF-β.
We have previously shown that injury to the CNS evokes a protective autoimmune response directed against dominant proteins presented by antigen-presenting cells at the lesion site (6, 42). After an acute injury, BALB/c/OLA mice are able to manifest a protective T cell-dependent response spontaneously, whereas C57BL/6J mice are not, or at least not within the therapeutic time window (24). The ability to spontaneously manifest a neuroprotective response apparently depends on the genetically determined ability to activate autoimmune T cells (Teff) (6, 43), which normally are kept under permanent Tregmediated control (44).
Recent studies in our laboratory have shown that depletion of Treg in BALB/c/OLA mice significantly improves neuronal survival after mechanical injury of the optic nerve (6, 45, 46), presumably because it allows more efficient activation of Teff. However, neuronal survival can also be significantly improved by myelin antigens administered mucosally (11, 12), via a mechanism that was suggested to be IL-10 dependent (12). We undertook the present study with the aim of clarifying contradictory findings in connection with T-cell regulation of immune-mediated neuroprotection in general, and of IL-10 in particular. In all published studies of oral or nasal tolerance, the reported experiments have been restricted to rats or mice that are susceptible to CNS-related experimental autoimmune diseases (Lewis rats and C57BL/6J mice) and, in correlation, whose ability to spontaneously recruit a T cell-dependent protection after acute CNS insult is limited (24). In contrast, the studies in which we showed that passive transfer of Treg decreases the ability to withstand the consequences of CNS injuries were carried out in BALB/c/OLA mice, a strain endowed with the spontaneous ability to resist the consequences of acute optic nerve injury (23, 24).
In attempting to resolve these apparently contradictory results, we discovered that, under certain conditions, even Treg can display a protective response. Thus, whereas Treg inhibited the ability of wild-type BALB/c/OLA mice to cope with the neurodegenerative consequences of injury, in SCID mice on the same background they were protective. Moreover, our present findings that, in C57BL/6J mice, depletion of Treg and passive administration of Treg both resulted in increased neuronal survival are in line with the apparently contradictory observations in this strain that active vaccination with self-antigens in complete Freund's adjuvant is protective (present study) and that oral immunization with myelin oligodendrocyte glycoprotein leads to neuronal rescue after stroke (12). On the basis of the above findings, we suggest that, in the absence of any intervention in BALB/c mice, Treg interfere only partially with the spontaneous response to injurious conditions, whereas in C57BL/6J mice they block it.
Taken together, the above results suggest that the inherent inability to manifest a T cell-dependent protective autoimmunity after an acute CNS insult (as, for example, in C57BL/6J mice) is a reflection not of a T cell-dependent destructive mechanism but of the failure of Teff to spontaneously overcome the normal suppression imposed by Treg. However, in the absence of the ability to spontaneously harness Teff (as in wild-type C57BL/6J mice) or in immune-deficient mice, Treg can induce the microglia to adopt a favorable phenotype. The effects of Treg and Teff on microglia are evidently expressed via different and even mutually antagonistic cytokines and therefore probably via different intracellular pathways (47, 48). Nevertheless, both T cell populations are capable of ensuring that the innate response represented by the microglia will be properly regulated.
Among the effector T cells that can be locally activated for the benefit of injured tissue are T helper 1 (Th1) and Th2 cells directed to self-antigens (2, 38). The characteristic cytokine of these T cells is IFN-γ. IL-10 is a characteristic cytokine of induced regulatory T cells (14, 39). The mucosally induced protective effect in C57BL/6J mice has also been attributed to IL-10 (12). In a rat model of spinal cord injury, homing T cells were colocalized with microglia expressing class II major histo-compatibility complex proteins at the lesion site (30). Therefore, it seems reasonable to assume that the beneficial effect of such T cells is achieved, at least in part, through their proper activation of microglia. Interestingly, however, although each of these T-cell populations was able to endow microglia with a neuroprotective phenotype when administered on its own, when Teff and Treg were administered together not only there was no additive effect, but the effect of each was wiped out by the other. Studies have provided evidence indicating that Teff home to the site(s) of injury in the CNS (49). As to Treg, data from studies of autoimmune diseases suggest that these cells are well endowed with the ability to migrate to tissues (15).
Acquisition by microglia of a particular phenotype after CNS injury can have a profound effect on the tissue (50, 51). Studies from other laboratories as well as ours have shown that microglia can acquire a phenotype that is protective, destructive, or merely ineffective, depending on the stimulus (48, 52–54). In a strain (such as BALB/c/OLA) that is genetically equipped to regulate autoimmunity, the balance between the need for autoimmunity and the need for Treg (to prevent autoimmune disease) seems to be properly met. In contrast, in C57BL/6J mice the ratio between Teff and Treg appears not to be optimal, with consequent failure to manifest the prompt autoimmune response needed to fight off neurodegenerative disorders, to shut off the immune response once turned on, or both (36, 55).
Many therapeutic strategies for autoimmune diseases and neurodegenerative disorders are based on induction of immune regulation (by strengthening both naturally occurring and antigen-induced regulatory T cells), with the aim of altering or down-regulating the phenotype of the T cell-mediated response (56–58). According to the present results, clinical treatment protocols based on IL-10 or any other regulatory cytokine for neurodegenerative disorders would have to be individually suited to particular patients depending on their natural susceptibility to autoimmune disease development. Therefore, regulation-based therapies for CNS-related pathologies should be designed and administered with extreme caution.
Acknowledgments
We thank S. R. Smith for editing the manuscript and A. Shapira for animal maintenance. M.S. holds the Maurice and Ilse Katz Professorial Chair in Neuroimmunology. The work was supported by Proneuron Ltd., Industrial Park, Ness-Ziona, Israel.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Therapeutic Vaccines: Realities of Today and Hopes for Tomorrow,” held April 1–3, 2004, at the National Academy of Sciences in Washington, DC.
Abbreviations: Treg, naturally occurring CD4+CD25+ regulatory T cells; Teff, effector T cells; SCID, severe combined immunodeficiency; RGC, retinal ganglion cell; S-Ag, S antigen; IRBP, interphotoreceptor retinoid binding protein; OHSC, organotypic hippocampal slice culture; PI, propidium iodide; TGF-β, transforming growth factor β.
References
- 1.Hauben, E., Butovsky, O., Nevo, U., Yoles, E., Moalem, G., Agranov, G., Mor, F., Leibowitz-Amit, R., Pevsner, E., Akselrod, S., et al. (2000) J. Neurosci. 20, 6421-6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kipnis, J., Yoles, E., Mizrahi, T., Ben-Nun, A. & Schwartz, M. (2002) J. Neuroimmunol. 130, 78-85. [DOI] [PubMed] [Google Scholar]
- 3.Moalem, G., Leibowitz-Amit, R., Yoles, E., Mor, F., Cohen, I. R. & Schwartz, M. (1999) Nat. Med. 5, 49-55. [DOI] [PubMed] [Google Scholar]
- 4.Moalem, G., Monsonego, A., Shani, Y., Cohen, I. R. & Schwartz, M. (1999) FASEB J. 13, 1207-1217. [DOI] [PubMed] [Google Scholar]
- 5.Moalem, G., Gdalyahu, A., Shani, Y., Otten, U., Lazarovici, P., Cohen, I. R. & Schwartz, M. (2000) J. Autoimmun. 15, 331-345. [DOI] [PubMed] [Google Scholar]
- 6.Kipnis, J., Mizrahi, T., Hauben, E., Shaked, I., Shevach, E. & Schwartz, M. (2002) Proc. Natl. Acad. Sci. USA 99, 15620-15625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shevach, E. M., Thornton, A. & Suri-Payer, E. (1998) Novartis Found. Symp. 215, 200-211. [DOI] [PubMed] [Google Scholar]
- 8.Suri-Payer, E., Amar, A. Z., Thornton, A. M. & Shevach, E. M. (1998) J. Immunol. 160, 1212-1218. [PubMed] [Google Scholar]
- 9.Thornton, A. M. & Shevach, E. M. (1998) J. Exp. Med. 188, 287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McHugh, R. S., Whitters, M. J., Piccirillo, C. A., Young, D. A., Shevach, E. M., Collins, M. & Byrne, M. C. (2002) Immunity 16, 311-323. [DOI] [PubMed] [Google Scholar]
- 11.Monsonego, A., Beserman, Z. P., Kipnis, J., Yoles, E., Weiner, H. L. & Schwartz, M. (2003) J. Autoimmun. 21, 131-138. [DOI] [PubMed] [Google Scholar]
- 12.Frenkel, D., Huang, Z., Maron, R., Koldzic, D. N., Hancock, W. W., Moskowitz, M. A. & Weiner, H. L. (2003) J. Immunol. 171, 6549-6555. [DOI] [PubMed] [Google Scholar]
- 13.Faria, A. M., Maron, R., Ficker, S. M., Slavin, A. J., Spahn, T. & Weiner, H. L. (2003) J. Autoimmun. 20, 135-145. [DOI] [PubMed] [Google Scholar]
- 14.Zhang, X., Izikson, L., Liu, L. & Weiner, H. L. (2001) J. Immunol. 167, 4245-4253. [DOI] [PubMed] [Google Scholar]
- 15.Kohm, A. P., Carpentier, P. A., Anger, H. A. & Miller, S. D. (2002) J. Immunol. 169, 4712-4716. [DOI] [PubMed] [Google Scholar]
- 16.McBain, C. J. & Fisahn, A. (2001) Nat. Rev. Neurosci. 2, 11-23. [DOI] [PubMed] [Google Scholar]
- 17.Gillessen, T., Budd, S. L. & Lipton, S. A. (2002) Adv. Exp. Med. Biol. 513, 3-40. [DOI] [PubMed] [Google Scholar]
- 18.Rusakov, D. A. & Lehre, K. P. (2002) Trends Neurosci. 25, 492-494. [DOI] [PubMed] [Google Scholar]
- 19.Dickerson, L. W., Bonthius, D. J., Schutte, B. C., Yang, B., Barna, T. J., Bailey, M. C., Nehrke, K., Williamson, R. A. & Lamb, F. S. (2002) Brain Res. 958, 227-250. [DOI] [PubMed] [Google Scholar]
- 20.Misu, Y., Furukawa, N., Arai, N., Miyamae, T., Goshima, Y. & Fujita, K. (2002) Neurotoxicol. Teratol. 24, 629-638. [DOI] [PubMed] [Google Scholar]
- 21.Yoneda, S., Tanaka, E., Goto, W., Ota, T. & Hara, H. (2003) Brain Res. 967, 257-266. [DOI] [PubMed] [Google Scholar]
- 22.Goff, D. C. & Wine, L. (1997) Schizophr. Res. 27, 157-168. [DOI] [PubMed] [Google Scholar]
- 23.Schori, H., Yoles, E. & Schwartz, M. (2001) J. Neuroimmunol. 119, 199-204. [DOI] [PubMed] [Google Scholar]
- 24.Kipnis, J., Yoles, E., Schori, H., Hauben, E., Shaked, I. & Schwartz, M. (2001) J. Neurosci. 21, 4564-4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher, J., Levkovitch-Verbin, H., Schori, H., Yoles, E., Butovsky, O., Kaye, J. F., Ben-Nun, A. & Schwartz, M. (2001) J. Neurosci. 21, 136-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schori, H., Kipnis, J., Yoles, E., WoldeMussie, E., Ruiz, G., Wheeler, L. A. & Schwartz, M. (2001) Proc. Natl. Acad. Sci. USA 98, 3398-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pepperberg, D. R., Okajima, T. L., Ripps, H., Chader, G. J. & Wiggert, B. (1991) Photochem. Photobiol. 54, 1057-1060. [DOI] [PubMed] [Google Scholar]
- 28.Buczylko, J. & Palczewski, K. (1993) Photoreceptor Cells, ed. Hargrave, P. A. (Academic, Orlando, FL), pp. 226-236.
- 29.Puig, J., Arendt, A., Tomson, F. L., Abdulaeva, G., Miller, R., Hargrave, P. A. & McDowell, J. H. (1995) FEBS Lett. 362, 185-188. [DOI] [PubMed] [Google Scholar]
- 30.Butovsky, O., Hauben, E. & Schwartz, M. (2001) FASEB J. 15, 1065-1067. [DOI] [PubMed] [Google Scholar]
- 31.Sun, B., Rizzo, L. V., Sun, S. H., Chan, C. C., Wiggert, B., Wilder, R. L. & Caspi, R. R. (1997) J. Immunol. 159, 1004-1011. [PubMed] [Google Scholar]
- 32.Sakaguchi, S., Sakaguchi, N., Shimizu, J., Yamazaki, S., Sakihama, T., Itoh, M., Kuniyasu, Y., Nomura, T., Toda, M. & Takahashi, T. (2001) Immunol. Rev. 182, 18-32. [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi, S., Takahashi, T., Yamazaki, S., Kuniyasu, Y., Itoh, M., Sakaguchi, N. & Shimizu, J. (2001) Microbes Infect. 3, 911-918. [DOI] [PubMed] [Google Scholar]
- 34.Lundberg, C., Lidman, O., Holmdahl, R., Olsson, T. & Piehl, F. (2001) J. Comp. Neurol. 431, 75-87. [PubMed] [Google Scholar]
- 35.Hirschberg, D. L., Moalem, G., He, J., Mor, F., Cohen, I. R. & Schwartz, M. (1998) J. Neuroimmunol. 89, 88-96. [DOI] [PubMed] [Google Scholar]
- 36.Shaked, I., Porat, Z., Gersner, R., Kipnis, J. & Schwartz, M. (2004) J. Neuroimmunol. 146, 84-93. [DOI] [PubMed] [Google Scholar]
- 37.Kluge, A., Hailer, N. P., Horvath, T. L., Bechmann, I. & Nitsch, R. (1998) Hippocampus 8, 57-68. [DOI] [PubMed] [Google Scholar]
- 38.Wolf, S. A., Fisher, J., Bechmann, I., Steiner, B., Kwidzinski, E. & Nitsch, R. (2002) J. Neuroimmunol. 133, 72-80. [DOI] [PubMed] [Google Scholar]
- 39.Sundstedt, A., O'Neill, E., Nicolson, K. & Wraith, D. (2003) J. Immunol. 170, 1240-1248. [DOI] [PubMed] [Google Scholar]
- 40.Horwitz, D. A., Zheng, S. G. & Gray, J. D. (2003) J. Leukocyte Biol. 74, 471-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swanson, M. A., Lee, W. T. & Sanders, V. M. (2001) J. Immunol. 166, 232-240. [DOI] [PubMed] [Google Scholar]
- 42.Yoles, E., Hauben, E., Palgi, O., Agranov, E., Gothilf, A., Cohen, A., Kuchroo, V., Cohen, I. R., Weiner, H. & Schwartz, M. (2001) J. Neurosci. 21, 3740-3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz, M. & Kipnis, J. (2002) Trends Immunol. 23, 530-534. [DOI] [PubMed] [Google Scholar]
- 44.Thornton, A. M. & Shevach, E. M. (2000) J. Immunol. 164, 183-190. [DOI] [PubMed] [Google Scholar]
- 45.Kipnis, J., Cardon, M., Avidan, H., Lewitus, G. M., Mordechay, S., Rolls, A., Shani, Y. & Schwartz, M. (2004) J. Neurosci. 24, 6133-6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kipnis, J., Avidan, H., Markovich, Y., Mizrahi, T., Hauben, E., Prigozhina, T. B., Slavin, S. & Schwartz, M. (2004) Eur. J. Neurosci. 19, 1-8. [DOI] [PubMed] [Google Scholar]
- 47.Lee, S. J. & Benveniste, E. N. (1999) J. Neuroimmunol. 98, 77-88. [DOI] [PubMed] [Google Scholar]
- 48.Gehrmann, J., Matsumoto, Y. & Kreutzberg, G. W. (1995) Brain Res. Brain Res. Rev. 20, 269-287. [DOI] [PubMed] [Google Scholar]
- 49.Flugel, A., Berkowicz, T., Ritter, T., Labeur, M., Jenne, D. E., Li, Z., Ellwart, J. W., Willem, M., Lassmann, H. & Wekerle, H. (2001) Immunity 14, 547-560. [DOI] [PubMed] [Google Scholar]
- 50.Taylor, D. L., Diemel, L. T., Cuzner, M. L. & Pocock, J. M. (2002) J. Neurochem. 82, 1179-1191. [DOI] [PubMed] [Google Scholar]
- 51.Bruccoleri, A. & Harry, G. J. (2000) J. Neurosci. Res. 62, 146-155. [DOI] [PubMed] [Google Scholar]
- 52.Nakajima, K. & Kohsaka, S. (2001) J. Biochem. (Tokyo) 130, 169-175. [DOI] [PubMed] [Google Scholar]
- 53.Persidsky, Y., Ghorpade, A., Rasmussen, J., Limoges, J., Liu, X. J., Stins, M., Fiala, M., Way, D., Kim, K. S., Witte, M. H., et al. (1999) Am. J. Pathol. 155, 1599-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwartz, M., Shaked, I., Fisher, J., Mizrahi, T. & Schori, H. (2003) Trends Neurosci. 26, 297-302. [DOI] [PubMed] [Google Scholar]
- 55.Nevo, U., Kipnis, J., Golding, I., Shaked, I., Neumann, A., Akselrod, S. & Schwartz, M. (2003) Trends Mol. Med. 9, 88-93. [DOI] [PubMed] [Google Scholar]
- 56.Koh, D. R. (1998) Ann. Acad. Med. Singapore 27, 47-53. [PubMed] [Google Scholar]
- 57.Kagnoff, M. F. (1996) Baillieres Clin. Rheumatol. 10, 41-54. [DOI] [PubMed] [Google Scholar]
- 58.Weiner, H. L., Friedman, A., Miller, A., Khoury, S. J., al-Sabbagh, A., Santos, L., Sayegh, M., Nussenblatt, R. B., Trentham, D. E. & Hafler, D. A. (1994) Annu. Rev. Immunol. 12, 809-837. [DOI] [PubMed] [Google Scholar]