Abstract
Catalase (CAT, OMIM: 115500) is an endogenous antioxidant enzyme and genetic variations in the regulatory regions of the CAT gene may alter the CAT enzyme activity and subsequently may alter the risk of oxidative stress related disease. In this study, potential influence(s) of the A-21T (rs7943316) and C-262T (rs1001179) genetic polymorphisms in the CAT promoter region, using the ALGGEN-PROMO.v8.3 online software were analyzed. Our findings show that the A allele at the -21 position creates a new potential binding site for PAX-6 and the T allele at the -262 position changes the TFII-I binding site into STAT4 response element. The PAX-6 and STAT4 are the multifunctional and enhancing transcription factors.
Key Words: Catalase, PAX-6, STAT4, Transcription factors
INTRODUCTION
Oxidative stress may play a significant role in the risk of chronic diseases [1]. Although reactive oxygen species can cause oxidative damage to cellular macromolecules, such as DNA and lipids, multiple antioxidant defenses can neutralize reactive oxygen species [2].
Catalase (CAT, OMIM; 115500), an endogenous antioxidant enzyme, plays a major role in controlling hydrogen peroxide concentration in human cells. It decomposes H2O2 into H2O and O2, thereby protecting the cells from oxidative stress. It has been suggested that functional polymorphism in the gene encoding catalase enzyme affects the enzyme activity, thereby altering the protection against oxidative stress [3].
Several epidemiologic studies have suggested that single nucleotide polymorphism in CAT gene may be associated with many diseases, such as hypertension, cancers, diabetes, nephropathy, and other diseases accompanied by oxidative stress [3-9]. The polymorphisms such as C-262T (rs1001179) and A-21T (rs7943316) which are located in the promoter region of the CAT gene were found to be associated with altered catalase activity [10, 11]. In this study, we analyzed the A-21T and C-262T polymorphisms in the promoter region and their effects on transcription factor binding sites.
MATERIALS AND METHODS
First, the catalase gene promoter sequence was obtained from the NCBI. Then both polymorphic sites (rs1001179) and (rs7943316) were identified on sequence. For identification of transcription factor binding site the ALGGEN-PROMO.v8.3 online software (http://alggen.lsi.upc.es/cgibin/promo_v3/promo/promoinit.cgi?dirDB=TF_ 8.3) was used. To identify transcription factor area, wild type allele and variant alleles were analyzed separately. In all analysis the maximum matrix dissimilarity rate was assumed 3%.
RESULTS AND DISCUSSION
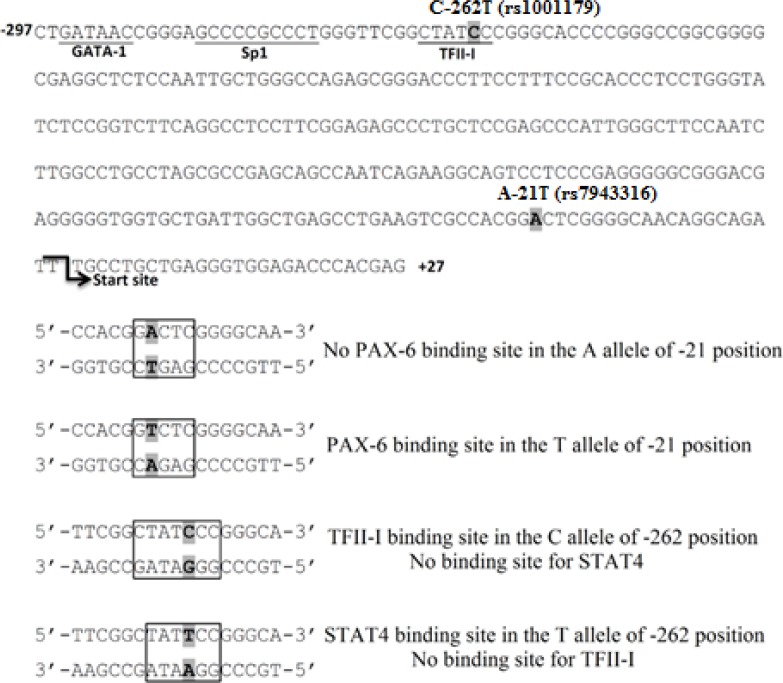
The result shows that the -21A>T substitution in the promoter region of CAT, creates a new potential PAX-6 responsive element (GACAC to GTCTC) (Fig. 1). Moreover, the C allele at the position -262 changes the TFII-I binding site (CTATCCC) into STAT4 (CTATTCC) responsive element (Fig. 1).
Figure 1.
Genetic polymorphisms in the promoter region of the human catalase (CAT) gene and its influence on transcription factor recognition sites
This is the first report showing that the A-21T and C-262T polymorphisms create new putative PAX-6 and STAT4 binding sites, respectively. The PAX-6 is a highly conserved multifunctional transcription factor and has been proposed to bind to promoter sequences of catalase gene. Also PAX-6 is a key regulatory gene of eye and brain development [12]. Previous study showed that the A allele of the CAT at the -844 position (rs769214) might create a binding site for PAX-6 [13]. The A allele on -844 position is the more frequent allele in Caucasian population and induced a higher transcriptional activity than the alternative allele (G allele) [13].
Our present findings also show that PAX-6 bind to the CAT promoter only if the T allele was present at the -21 positions. To our knowledge, the T allele at the -21 position may induce the CAT promoter activity. The signal transducer and activator of transcription (STAT) family of molecules is localized to the cytoplasm. STAT4 regulates various genes expression as a transcription factor and is involved in T helper cell (Th1) cell development [14].
The present results show that STAT4 binds to the CAT promoter only if the T allele is present at the -262 position; however the C allele is the putative binding site for TFII- 1 transcription factor. Oxidative stress induces catalase activity. In addition, studies have shown that reactive oxygen species activate the STAT family protein via serine/threonine kinases [15]. It may conclude that STAT4 may induce the CAT promoter activity. The influence of the CAT A-21T and C-262T polymorphisms on the CAT mRNA levels should be further researched.
Acknowledgments:
The author is indebted to Kunduz University for supporting of this study.
Conflict of interest statement:
The author declares he has no conflict of interest.
References
- 1.Cross CE, Halliwell B, Borish ET, Pryor WA, Ames BN, Saul RL, McCord JM, Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987;107:526–455. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- 2.Guyton KZ, Kensler TW. Oxidative mechanisms in carcinogenesis. Br Med Bull. 1993;49:523–544. doi: 10.1093/oxfordjournals.bmb.a072628. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Zhang L, Sun D, Li Z, Wang L, Liu P. Genetic polymorphisms of superoxide dismutases, catalase, and glutathione peroxidase in age-related cataract. Mol Vis. 2011;17:2325–2332. [PMC free article] [PubMed] [Google Scholar]
- 4.Saadat M, Saadat S. Genetic polymorphism of CAT C-262T and susceptibility to breast cancer, a case-control study and meta-analysis of the literatures. Pathol Oncol Res. 2015;21:433–437. doi: 10.1007/s12253-014-9840-4. [DOI] [PubMed] [Google Scholar]
- 5.Chang D, Hu ZL, Zhang L, Zhao YS, Meng QH, Guan QB, Zhou J, Pan HZ. Association of catalase genotype with oxidative stress in the predication of colorectal cancer: modification by epidemiological factors. Biomed Environ Sci. 2012;2:156–162. doi: 10.3967/0895-3988.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Ceriello A, Morocutti A, Mercuri F, Quagliaro L, Moro M, Damante G, Viberti GC. Defective intracellular antioxidant enzyme production in type 1 diabetic patients with nephropathy. Diabetes. 2000;49:2170–2177. doi: 10.2337/diabetes.49.12.2170. [DOI] [PubMed] [Google Scholar]
- 7.Hodgkinson AD, Bartlett T, Oates PJ, Millward BA, Demaine AG. The response of antioxidant genes to hyperglycemia is abnormal in patients with type 1 diabetes and diabetic nephropathy. Diabetes. 2003;52:846–851. doi: 10.2337/diabetes.52.3.846. [DOI] [PubMed] [Google Scholar]
- 8.Zarei N, Saadat I, Farvardin-Jahromi M. The relationship between NQO1 C609T and CAT C-262T genetic polymorphisms and the risk of age-related cataracts. Mol Biol Res Commun. 2015;3:143–149. [PMC free article] [PubMed] [Google Scholar]
- 9.Ebrahimpour S, Saadat I. Association of CAT C-262T and SOD1 A251G single nucleotide polymorphisms susceptible to gastric cancer. Mol Biol Res Commun. 2014;4:223–229. [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn J, NowellS , McCann SE, Yu J, Carter L, Lang NP, Kadlubar FF, Ratnasinghe LD, Ambrosone CB. Associations between catalase phenotype and genotype: modification by epidemiologic factors. Cancer Epidemiol Biomarkers Prev. 2006;15:1217–1222. doi: 10.1158/1055-9965.EPI-06-0104. [DOI] [PubMed] [Google Scholar]
- 11.Nadif R, Mintz M, Jedlicka A, Bertrand JP, Kleeberger SR, Kauffmann F. Association of CAT polymorphisms with catalase activity and exposure to environmental oxidative stimuli. Free Radic Res. 2005;39:1345–1350. doi: 10.1080/10715760500306711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra S, Maurya SK, Srivastava K, Shukla S, Mishra R. Pax6 influences expression patterns of genes involved in neuro-degeneration. Ann Neurosci. 2015;4:226–231. doi: 10.5214/ans.0972.7531.220407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hebert-Schuster M, Cottart CH, Laguillier-Morizot C, Raynaud-Simon A, Golmard JL, Cynober L, Beaudeux JL, Fabre EE, Nivet-Antoine V. Catalase rs769214 SNP in elderly malnutrition and during renutrition: is glucagon to blame? Free Radic Biol Med. 2011;51:1583–1588. doi: 10.1016/j.freeradbiomed.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Qu A, Wang H. Signal transducer and activator of transcription 4 in liver diseases. Int J BiolSci. 2015;11:448–855. doi: 10.7150/ijbs.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Andrade KQ, Moura FA, Dos Santos JM, de Araújo OR, de Farias Santos JC, Goulart MO. Oxidative stress and inflammation in hepatic diseases: therapeutic possibilities of N-acetylcysteine. Int J Mol Sci. 2015;16:30269–30308. doi: 10.3390/ijms161226225. [DOI] [PMC free article] [PubMed] [Google Scholar]