Abstract
The utility of vaccine strategies to treat neurodegenerative diseases such as Alzheimer's disease (AD) may still hold promise. Both active and passive immunization strategies reduced AD-like pathology and restored cognitive deficits in transgenic mice. These results were initially met with considerable optimism; however, phase IIa clinical trials were halted because of a small but significant occurrence of meningoencephalitis. Knowledge gained from studies on amyloid-β peptide (Aβ) immunotherapy will allow optimization of new-generation vaccines, targeting highly specific epitopes while reducing undesired side effects. In harnessing and steering the immune system, an effective response can be generated against Aβ. If this proves successful, Aβ vaccination could provide the first definitive treatment for AD.
Alzheimer's disease (AD) is the most common cause of age-related cognitive decline, affecting >12 million people worldwide (1). The disease is characterized in its earlier stages by progressive memory impairment and cognitive decline, altered behavior, and language deficits. Later, patients present with global amnesia and slowing of motor functions, with death typically occurring within 9 years after diagnosis (2). Current drug therapy aims at slowing cognitive decline and ameliorating the affective and behavioral symptoms associated with disease progression. However, these drugs provide limited symptomatic treatment, without targeting the underlying cause of AD. Immunization of AD patients provides a novel means of specifically targeting the neurotoxic effects of amyloid-β peptide (Aβ) and thereby targeting disease progression.
The Amyloid Cascade Hypothesis
The main constituent of amyloid consists of a 40- to 43-aa peptide, Aβ, and is derived from the proteolytic cleavage of a family of ubiquitously expressed membrane-spanning proteins, termed the amyloid precursor proteins (APP) (3). Under normal conditions, the most abundant species in the brain is the Aβ (1–40) peptide (Aβ40); however, much of the fibrillar Aβ is composed of the longer, more fibrillogenic Aβ (1–42) peptide (Aβ42) (3). These normally soluble peptides undergo conformational change and polymerize into an aggregated and toxic form, rich in β-structure (4). Initially, Aβ42 is deposited in an immature, diffuse (nonfibrillar) plaque, with little or no detectable neuritic dystrophy.
Early studies have shown that synthetic fibrillar forms of Aβ are toxic to cultured neurons (5–7). Several mechanisms of Aβ-induced neurotoxicity have been proposed, including oxidative stress, free-radical formation, disrupted calcium homeostasis, induction of apoptosis, chronic inflammation, and activation of complement (8). Although it has been shown that increased levels of Aβ in the brain correlate with cognitive decline (9), relatively weak correlations exist between fibrillar amyloid plaque density and severity of dementia (10–12). Recent studies point to other forms of Aβ, namely, small oligomers as the neurotoxic species (13, 14).
Recent reports using antibodies raised against synthetic Aβ oligomers detected a 70-fold increase in oligomeric species in AD patients over control brains (15). Moreover, Kayed et al. (16) found that soluble oligomers display a common conformation-dependent structure common to all oligomers independent of their sequence, which suggests a shared mechanism of toxicity. Functionally, it has been found that naturally secreted oligomers inhibit hippocampal long-term potentiation in vivo (17). Taken together, these results suggest that strategies aimed at treating amyloid disorders should target oligomers of Aβ. In doing so, the equilibrium between monomers and higher-order aggregates can be disrupted, resulting in neutralization of soluble, toxic species.
Immunization Against Aβ in Transgenic Mouse Models of AD
Since Schenk et al. (18) first reported that immunization of PDAPP mice with synthetic, preaggregated Aβ42 reduced the extent and progression of AD pathology (Fig. 1), much progress has been made in designing a vaccine appropriate for human use. Several strategies, including active and passive immunization, have been explored (Table 1), which not only hold promise as potential therapeutics but also address both the cognitive dysfunction and Aβ accumulation.
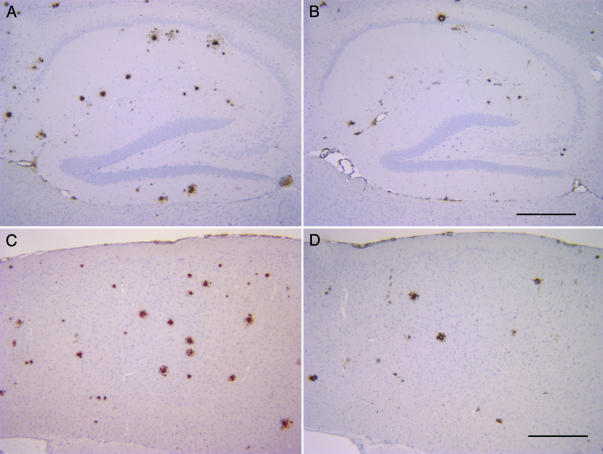
Fig. 1.
Aβ42-immunized TgCRND8 mice have a 50% reduction in plaque burden than untreated TgCRND8 mice. Representative pictures of the distribution of Aβ plaques labeled by Dako 6F/3D anti-Aβ antibody in the hippocampus (A and B) and cortex (C and D) of control peptide-immunized (A and C) and Aβ42-immunized TgCRND8 mice (B and D). (Scale bars = 100 μm.)
Table 1. Summary of vaccine studies in transgenic mouse models of AD.
| Model | Treatment (age) | Active/passive (route) | Treatment schedule | Effect | Ref(s). |
|---|---|---|---|---|---|
| TgCRND8 | Prophylactic (6 wk) | Active | Chronic (19 wk) | ↓ Aβ levels | 19, 20 |
| ↓ Pathology | |||||
| + Behavior | |||||
| PDAPP | Prophylactic (6 wk) | Active | Chronic (11 mo) | ↓ Aβ levels | 18 |
| ↓ Pathology | |||||
| Treatment (11 mo) | Active | Chronic (8 mo) | ↓ Aβ levels | 18 | |
| ↓ Pathology | |||||
| Prophylactic (5 mo) | Active (nasal) | Chronic (7 mo) | ↓ Aβ levels | 21, 22 | |
| ↓ Pathology | |||||
| Prophylactic (8–20 mo) | Passive | Chronic (6 mo) | ↓ Aβ levels | 23–25 | |
| ↓ Pathology | |||||
| Treatment (11–24 mo) | Passive | Acute | ↓ Aβ levels | 26–28 | |
| + Behavior | |||||
| Prophylactic (1 and 12 mo) | Active (viral) | Chronic | ↓ Aβ levels | 29 | |
| ↓ Pathology | |||||
| + Behavior | |||||
| Tg2576 | Prophylactic (7–8 mo) | Active | Chronic | ↓ Aβ levels | 30, 31 |
| ↓ Pathology | |||||
| + Behavior | |||||
| Prophylactic (10–11 mo) | Active | Chronic (4 mo) | ↓ Aβ levels | 31 | |
| ↓ Pathology | |||||
| Treatment (18 mo) | Active | Chronic (4 mo) | ↓ Aβ levels | 31 | |
| NC pathology | |||||
| Treatment (10–18 mo) | Passive (intracranial) | Acute | ↓ Aβ levels | 24, 26 | |
| ↓ Pathology | |||||
| Tg2576 (FcRγ-/-) | Treatment (11–15 mo) | Active (i.p.) | Chronic (3 mo) | ↓ Aβ levels | 32 |
| APP + PS1 | Treatment (7.5–14.5 mo) | Active | Chronic (5 injections) | ↓ Aβ levels | 33 |
| ↓ Pathology | |||||
| NC behavior | |||||
| PSAPP | Prophylactic (5 wk) | Active (i.p./nasal) | Chronic (8 wk) | ↓ Aβ levels | 34 |
| ↓ Pathology | |||||
| APP23 | Prophylactic (21 mo) | Passive (i.p.) | Chronic (5 mo) | ↓ Aβ levels | 35 |
| ↑ Cerebral hemorrhages | |||||
| C57BL/6 | 6–8 wk | Active | Acute | Autoimmune encephalomyelitis | 36 |
Pathology is defined as amyloid plaque load. NC, no change.
Mechanisms of Action
Although active and passive immunization strategies have proven efficacious in mouse models of AD, it remains unclear how antibodies elicit this effect. Several hypotheses have been put forth to explain results observed in vivo and in vitro, but it is important to note that these are not mutually exclusive. Any number of these mechanisms may act under a given set of circumstances, with factors including the epitope, isotype, and amyloid burden likely to influence the primary means of clearance or sequestration.
Microglial Mediated Phagocytosis. Bard et al. (23) presented a model whereby peripherally administered antibodies enter the CNS and bind Aβ fibrils, with subsequent recruitment of microglia to phagocytose the complex by means of Fc receptor ligation. Ex vivo assays confirm the presence of internalized Aβ within microglia upon incubation with anti-Aβ antibodies and tissue sections. Follow-up studies were consistent with these observations, because the most effective antibodies (examined in vivo and ex vivo) were of the IgG2a isotype, which exhibits high affinity for Fc receptors on microglia (25). Microglial activation was also found to accompany plaque clearance in vivo by both active and passive immunization strategies (24, 26) or in contrast to decrease activation in active immunization in the TgCRND8 mouse model (Fig. 2) (20). However, recent reports suggest that multiple clearance mechanisms may act in concert to clear amyloid plaques (28, 32). Immunization of Tg2576 mice crossed with Fc receptor gamma knockout mice were as efficient at clearing plaques as Tg2576 mice alone, further supporting the idea of multiple clearance pathways (32). Another possibility involves internalization of Aβ/antibody microaggregates by microglia through the type A scavenger receptor (37). Indeed, a recent report from Wilcock et al. (33) suggests a two-phase mechanism of anti-Aβ antibody action, both independent and associated with microglial activation. This report suggests that down-regulation of microglial activation either through the use of F(ab) fragments or antiinflammatory drugs severely reduces the clearance of compact fibrillar but not diffuse plaques (38). Therefore, microglial activation may be necessary for efficient clearance of senile plaques from the CNS. If this is indeed the case, then a fine balance must be struck to clear plaques without eliciting further damage to the surrounding CNS milieu.
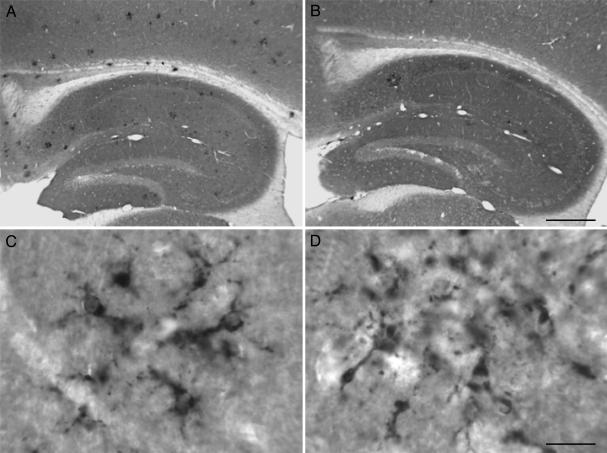
Fig. 2.
Aβ42-immnized TgCRND8 mice have reduced microglial activation in comparison with control peptide-immunized TgCRND8 mice. Representative pictures of the distribution of activated microglia as labeled by anti-CD68 IgG in the hippocampus (A and B) of control peptide-immunized (A and C) and Aβ42-immunized TgCRND8 mice (B and D). Higher magnification reveals that activated microglia have similar morphology under both immunization paradigms (C and D). [Scale bars = 75 μm (A and B) and 5 μm (C and D).]
Peripheral Sink Hypothesis. An alternate mechanism comes from the observation that long-term peripheral administration of a monoclonal antibody (m266) results in a rapid increase in plasma Aβ with subsequent reduction in amyloid burden, without binding of m266 to Aβ deposits in the brain (24). These results suggested that sequestration of plasma Aβ disrupts the Aβ equilibrium between the CNS and plasma, resulting in increased efflux of Aβ out of the brain, into the periphery where it is degraded. This hypothesis is supported by a number of observations. Aβ-peptides have been shown to be transported readily between the CNS and plasma (39–41). Moreover, in nondemented patients, i.v. infusion of anti-Aβ antibodies led to increased Aβ levels in the plasma, with concomitant decreases in Aβ within the cerebrospinal fluid (42). These findings are further supported by Lemere et al. (34), who observed a 28-fold increase in serum Aβ after chronic, active immunization of PSAPP mice.
Inhibition of Fibrillogenesis and Cytotoxic Aβ Species. Previous work by Solomon and colleagues (43–45) predicted that antibodies raised against the N terminus of Aβ could inhibit in vitro aggregation and could bind to preexisting Aβ fibrils, resulting in disaggregation and protection from their neurotoxic effects. We extended this line of evidence by demonstrating that antibodies directed against residues 4–10 of Aβ1–42 inhibit both fibrillogenesis and cytotoxicity, without eliciting a harmful cytotoxic T cell response in TgCRND8 mice (20). Consistent with these results are studies by Bard et al. (25) demonstrating that plaque clearance is only seen with antibodies directed against the N-terminal region of Aβ. Furthermore, a single administration of anti-Aβ3–6 IgG1 was effective at plaque clearance and resolution of neuritic lesions within 4 days and lasted up to 32 days in the PDAPP mouse (46). These results support the use of passive immunization strategies because, once plaques are cleared, neuronal morphology is restored and therefore may have a direct impact on cognitive function.
We also suggested that antibodies induced by immunization of TgCRND8 mice likely target only a subset of Aβ, namely soluble species such as protofibrils or oligomers (20). This suggestion seems likely because total Aβ brain levels do not decrease after immunization of TgCRND8 mice (19), and several independent studies have presented similar findings (27, 47, 48).
Aβ Vaccination in Humans
After promising preclinical results in several species (mice, rabbits, guinea pigs, and monkeys), clinical trials using Aβ42 (AN-1792) in conjunction with the T helper (Th) 1 adjuvant QS-21 were initiated (42, 49). Although results of phase I trials showed good tolerability, phase IIa trials were halted when 18 of 298 patients immunized with AN-1792 presented with symptoms consistent with meningoencephalitis (50, 51). Several reports have since been published regarding the neuropathology and clinical outcome of acute immunization, which should prove useful in designing new generation vaccines (51–55).
Several findings provide hope for AD patients despite the termination of clinical trials. Postmortem examination of two AD patients who received injections of AN-1792 revealed absent or sparse plaques in the neocortex, lacking dystrophic neurites or reactive astrocytes (as compared with unimmunized controls); reactive microglia in association with areas devoid of plaques; and decoration of plaques by IgG and C3 complement. Taken together, these results suggest that an effective immune response was generated that resulted in clearance of Aβ from the patient (53). Moreover, Hock et al. (54) showed that patients who generate antibodies exhibit slower rates of cognitive decline; this effect was even observed in patients who experienced transient episodes of meningoencephalitis. Neither case altered cerebro-vascular amyloid; in the latter case, multiple small hemorrhages, including acute lesions and lesions with macrophages filled with hemosyderin, were detected in the cerebral cortex. It is unclear whether these lesions have a direct link to immunization; however, a report of increased incidence of microhemorrhages in APP23 mice after passive Aβ immunotherapy was reported (35). These studies suggested that AD patients may need to be screened for the presence and severity of cerebral amyloid angiopathy before initiating Aβ immunotherapy.
Neuropathological analysis also revealed infiltration of T lymphocytes, predominantly of the CD4+ type in one patient and the CD8+ type in the other, and abnormalities of cerebral white matter, including extensive macrophage infiltration and a reduction in the density of myelinated fibers (53). Although no inflammatory reaction was observed in preclinical trials, a recently published report demonstrates that vaccination of C57BL/6 mice with Aβ and pertussis toxin induces autoimmune encephalomyelitis, with characteristics (inflammatory foci in the CNS containing macrophages, B and T cells, circulating anti-Aβ antibodies, and a predominantly CD4+-mediated Th1 response) similar to those observed in humans (36).
The inflammatory response observed in human subjects is probably attributable to a T cell-mediated event (9, 56). Infiltration of activated T cells had been predicted well before commencement of clinical trials (57). T cell epitopes have been mapped to the mid- to carboxy-terminal region (residues 15–42) of Aβ (Fig. 3) (58). We subsequently showed that antibodies directed toward the N terminus (residues 4–10) were sufficient to inhibit cytotoxicity and fibrillogenesis without eliciting an inflammatory response (20). Thus, immunization with the full-length Aβ1–42 peptide, containing both B and T cell epitopes, would be expected to result in extensive T cell activation. Recent reports of increased T cell reactivity in AD patients and older humans, predominantly against amino acids 16–33 of Aβ, seem in line with these predictions. These results become difficult to interpret, however, in light of contradictory reports indicating that lymphocytes from AD patients exhibit weak proliferative responses to Aβ and other synthetic peptides corresponding to parts of the APP sequence, as compared with young and aged healthy individuals (59). Moreover, APP transgenic mice were found to be hyporesponsive to human Aβ, in terms of humoral and cellular immune responses (60), suggesting that increased production of Aβ from such a young age may induce a form of central and peripheral T cell tolerance.
Fig. 3.
The sequence of B and T cell epitopes within the Aβ1–42 amino acid sequence as determined by mathematical algorithms.
Future Perspectives
The AN-1792 trials highlighted the importance of a directed immune response. Many factors, such as antigen, adjuvant, and delivery systems, can be modified to elicit specific cellular and humoral responses. Given the distinct location of B and T cell epitopes within Aβ, new immunogens can be designed which lack the irrelevant C terminus but retain those residues (4–10) required for binding to Aβ (Fig. 3) (20). Sigurdsson et al. (61) have demonstrated that immunization with a synthetic nontoxic/nonfibrillar Aβ homologous peptide could reduce AD pathology and potentially offer a safer alternative than immunization with Aβ1–42. Lemere and colleagues (62) have shown that when administered nasally Aβ1–15 was not as efficient as Aβ1–42 at priming an immune response but was equally efficient at boosting titers. Elan's experimental vaccine has been further examined, and it has been found that patients who developed the inflammatory response recognized the tail end of Aβ, exactly the same as patients who do not (63). These results suggest that modifying the antigen alone may not be sufficient to avoid encephalitogenic responses in all patients.
Immune responses will also need to be directed toward a Th2 response, which promotes antibody production, down-regulates proinflammatory Th1 responses, and results in the release of antiinflammatory cytokines that have the potential to mitigate chronic inflammatory conditions already present in AD patients (64). This is particularly important given recent findings that microglia-mediated release of nitric oxide by Aβ-reactive Th1 cells can contribute to AD neurotoxicity; Th2 cells, however, were found to counterbalance the toxic effects of NO (65). Nasal immunization paradigms have illustrated the potential to decrease AD pathology while inducing the expression of the antiinflammatory cytokines IL-4, IL-10, and transforming growth factor beta β (TGF-β) (21, 22). Wyss-Coray et al. (66) have demonstrated that modest increases in astroglial production of TGF-β1 results in a marked reduction in plaque burden and overall Aβ load, presumably through promotion of microglia. The choice of adjuvant used in a vaccine protocol will also have modulatory effects on the Th1 versus Th2 responses. Nontransgenic mice immunized with Aβ1–42 mixed with Alum induce primarily a Th2 response, whereas TitreMax Gold, complete Freund's adjuvant, and QS21 induce predominantly a Th1 response (67). Furthermore the use of costimulatory molecules or chemokines to target effective antigen presentation by dendritic cells in the lymph nodes have been successful in in vivo models of AIDS and cancer (68, 69).
Rangan et al. (70) have identified recombinant antibody light-chain fragments with proteolytic activity, capable of hydrolyzing Aβ in vitro. Although these fragments currently demonstrate broad substrate specificity, they may prove therapeutically useful if the antibody could be engineered to specifically target pathogenic forms of Aβ, such as oligomers or protofibrils. Also, Frenkel et al. (71) suggested a novel approach, where intracellular expression of a site-directed single-chain antibody, which has been shown to inhibit fibrillogenesis and cytotoxicity in vitro, could target Aβ before it is released from the cell.
DNA vaccines or viral vectors may also be developed to enhance antigen expression and ease of vaccine administration. One report of a recombinant adeno-associated virus vaccine, consisting of Aβ1–42 fused to cholera toxin B subunit, cleared plaques and improved behavior in the PDAPP mouse (29). Cholera toxin B is the nontoxic subunit and has been shown to act as a non-Th1-inducing adjuvant. DNA vaccines also contain immunostimulatory sequences, such as cytosine-phosphate-guanosine repeats, that act as adjuvants. Alternatively, DNA vaccines can be constructed to express the antigen of choice along with an immunomodulatory protein. The chimeric DNA minigene encoding Aβ fused to mouse IL-4 generated a strong humoral response in wild-type mice (72). The antibodies generated were primarily to the N terminus, of the IgG1 and IgG2a subtype and recognize human amyloid plaques, suggesting not only a Th2 response but also therapeutic potential. To date clinical trials using DNA have required relatively high doses of DNA to elicit immune responses. The development of micro-particles and nanoparticles for DNA delivery systems may circumvent this limitation (73). Alternatively, oral vaccines consisting of adeno-associated viral vectors have been shown to induce antibody production for 6 months after a single dose (63). This approach may be safer than intramuscular injections because epithelia lining the gut have a rapid turnover and therefore will decrease the half-life of the vector within the body.
Conclusions
Insights gained from the pathology, biochemistry, and genetics of AD have allowed identification of a target for therapy, Aβ, and the generation of transgenic mouse models that recapitulate pathological and behavioral aspects of the disease in which to test hypotheses. The first set of evidence that immunization with Aβ could reduce AD pathology and restore cognitive deficits in transgenic mice was met with considerable optimism; this optimism was short-lived, however, because clinical trials of a vaccine were canceled because of a small but significant occurrence of meningoencephalitis. Despite numerous adverse events associated with clinical trials of AN-1792, preliminary data demonstrate that vaccination can reduce AD pathology and mitigate progressive cognitive decline associated with the disease. Knowledge gained from studies on Aβ immunotherapy will allow optimization of the vaccine to avoid side effects, while generating a highly specific and effective immune response against what is now believed to be the causative agent of synaptic loss and cognitive decline, Aβ. If this proves successful, Aβ vaccination could provide the first definitive treatment for AD.
Acknowledgments
This work was supported by the Ontario Alzheimer's Society (P.S.G.-H. and J.M.), Canadian Institutes of Health Research (P.S.G.-H. and J.M.), the Natural Sciences and Engineering Research Council of Canada (J.M.), the Scottish Rite Charitable Foundation (J.M.), the Howard Hughes Foundation (P.S.G.-H.), and the Canadian Genetic Diseases Network (P.S.G.-H.).
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Therapeutic Vaccines: Realities of Today and Hopes for Tomorrow,” held April 1–3, 2004, at the National Academy of Sciences in Washington, DC.
Abbreviations: AD, Alzheimer's disease; Aβ, amyloid-β peptide; APP, amyloid precursor proteins; Th, T helper.
References
- 1.Citron, M. (2002) Nat. Neurosci. 5, Suppl., 1055-1057. [DOI] [PubMed] [Google Scholar]
- 2.Davis, K. L. & Samuels, S. C. (1998) in Pharmacological Management of Neurological and Psychiatric Disorders, eds. Enna, S. J. & Coyle, J. T. (McGraw–Hill, New York), pp. 267-316.
- 3.Selkoe, D. (2001) Physiol. Rev. 81, 741-766. [DOI] [PubMed] [Google Scholar]
- 4.McLaurin, J., Yang, D. S., Yip, C. M. & Fraser, P. E. (2000) J. Struct. Biol. 130, 259-270. [DOI] [PubMed] [Google Scholar]
- 5.Pike, C. J., Walencewicz, A. J., Glabe, C. G. & Cotman, C. W. (1991) Brain Res. 573, 311-314. [DOI] [PubMed] [Google Scholar]
- 6.Lorenzo, A. & Yankner, B. (1994) Proc. Natl. Acad. Sci. USA 91, 12243-12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iversen, L. L., Mortishire-Sith, R. J., Pollack, S. J. & Shearman, M. S. (1995) Biochem. J. 311, 1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodel, R. C., Hampel, H., Depboylu, C., Lin, S., Gao, F., Schock, S., Jackel, S., Wei, X., Buerger, K., Hoft, C., et al. (2002) Ann. Neurol. 52, 253-256. [DOI] [PubMed] [Google Scholar]
- 9.Näslund, J., Haroutunian, V., Mohs, R., Davis, K. L., Davies, P., Greengard, P. & Buxbaum, J. D. (2000) J. Am. Med. Assoc. 283, 1571-1577. [DOI] [PubMed] [Google Scholar]
- 10.Terry, R. D., Peck, A., DeTeresa, R., Schechter, R. & Horoupian, D. S. (1981) Ann. Neurol. 10, 184-192. [DOI] [PubMed] [Google Scholar]
- 11.Braak, H. & Braak, E. (1991) Acta Neuropathol. 82, 239-259. [DOI] [PubMed] [Google Scholar]
- 12.Dickson, D. W., Crystal, H. A., Bevona, C., Honer, W., Vincent, I. & Davies, P. (1995) Neurobiol. Aging 16, 285-298. [DOI] [PubMed] [Google Scholar]
- 13.McLean, C. A., Cherny, R. A., Fraser, F. W., Fuller, S. J., Smith, M. J., Beyreuther, K., Bush, A. I. & Masters, C. L. (1999) Ann. Neurol. 46, 860-666. [DOI] [PubMed] [Google Scholar]
- 14.Klein, W. L., Krafft, G. A. & Finch, C. E. (2001) Trends Neurosci. 24, 219-224. [DOI] [PubMed] [Google Scholar]
- 15.Gong, Y., Chang, L., Viola, K. L., Lacor, P. N., Lambert, M. P., Finch, C. E., Krafft, G. A. & Klein, W. L. (2003) Proc. Natl. Acad. Sci. USA 100, 10417-10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kayed, R., Head, E., Thompson, J. L., McIntire, T. M., Milton, S. C., Cotman, C. W. & Glabe, C. G. (2003) Science 300, 486-489. [DOI] [PubMed] [Google Scholar]
- 17.Walsh, D. N., Klyubin, I., Fadeeva, J. V., Cullen, W. K., Anwyl, R., Wolfe, M. S., Rowan, M. J. & Selkoe, D. J. (2002) Nature 416, 535-539. [DOI] [PubMed] [Google Scholar]
- 18.Schenk, D., Barbour, R., Dunn, W., Gordon, G., Grajeda, H., Guido, T., Hu, K., Huang, J., Johnson-Wood, K., Khan, K., et al. (1999) Nature 400, 173-177. [DOI] [PubMed] [Google Scholar]
- 19.Janus, C., Pearson, J., McLaurin, J., Matthews, P. M., Jiang, Y., Schmidt, S. D., Chishti, M. A., Horne, P., Heslin, D., French, J., et al. (2000) Nature 408, 979-982. [DOI] [PubMed] [Google Scholar]
- 20.McLaurin, J., Cecal, R., Kierstead, M. E., Tian, X., Phinney, A. L., Manea, M., French, J. E., Lambermon, M. H. L., Darabie, A. A., Brown, M. E., et al. (2002) Nat. Med. 8, 1263-1269. [DOI] [PubMed] [Google Scholar]
- 21.Lemere, C. A., Maron, R., Spooner, E. T., Grenfell, T. J., Mori, C., Desai, R., Hancock, W. W., Weiner, H. L. & Selkoe, D. J. (2000) Ann. N. Y. Acad. Sci. 920, 328-331. [DOI] [PubMed] [Google Scholar]
- 22.Weiner, H. L., Lemere, C. A., Maron, R., Spooner, E. T., Grenfell, T. J., Mori, C., Issazadeh, S., Hancock, W. W. & Selkoe, D. J. (2000) Ann. Neurol. 48, 567-579. [PubMed] [Google Scholar]
- 23.Bard, F., Cannon, C., Barbour, R, Burke, R., Games, D., Grajeda, H., Guido, T., Hu, K., Huang, J., Johnson-Wood, K., et al. (2000) Nat. Med. 6, 916-919. [DOI] [PubMed] [Google Scholar]
- 24.DeMattos, R. B., Bales, K. R., Cummins, D. J., Dodart, J., Paul, S. M. & Holtzman, D. M. (2001) Proc. Natl. Acad. Sci. USA 98, 8850-8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bard, F., Barbour, R., Cannon, C., Carretto, R., Fox, M., Games, D., Guido, T., Hoenow, K., Hu, K., Johnson-Wood, K., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 2023-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bacskai, B. J., Kajdasz, S. T., Christie, R. H., Carter, C., Games, D., Seubert, P, Schenk, D. & Hyman, B. T. (2001) Nat. Med. 7, 369-372. [DOI] [PubMed] [Google Scholar]
- 27.Dodart, J., Bales, K. R., Gannon, K. S., Greene, S. J., DeMattos, R. B., Mathis, C., DeLong, C. A., Wu, S., Wu, X., Holtzman, D. M., et al. (2002) Nat. Neurosci. 5, 452-457. [DOI] [PubMed] [Google Scholar]
- 28.Bacskai, B. J., Kajdasz, S. T., McLellan, M. E., Games, D., Seubert, P., Schenk, D. & Hyman, B. T. (2002) J. Neurosci. 22, 7873-7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang, J., Xu, S., Qin, J., Ma, S., Zhang, H., Kong, Q., Chen, D., Ba, D. & He, W. (2003) Neurobiol. Dis. 14, 365-379. [DOI] [PubMed] [Google Scholar]
- 30.Morgan, D., Diamon, D. M., Gottschall, P. E., Ugen, K. E., Dickey, C., Hardy, J., Duff, K., Jantzen, P., DiCarlo, G., Wilcock, D., et al. (2000) Nature 408, 982-985. [DOI] [PubMed] [Google Scholar]
- 31.Das, P., Murphy, M. P., Younkin, L. H., Younkin, S. G. & Golde, T. E. (2001) Neurobiol. Aging 22, 721-727. [DOI] [PubMed] [Google Scholar]
- 32.Das, P., Howard, V., Loosbrock, N., Dickson, D., Murphy, M. P. & Golde, T. E. (2003) J. Neurosci. 23, 8532-8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilcock, D. M., Gordon, M. N., Ugen, K. E., Gottschall, P. E., DiCarlo, G., Dickey, C., Boyett, K. W., Jantzen, P. T., Connor, K. E., Melachrino, J., et al. (2001) DNA Cell Biol. 20, 731-736. [DOI] [PubMed] [Google Scholar]
- 34.Lemere, C. A., Spooner, E. T., LaFrancois, J., Malester, B., Mori, C., Leverone, J. F., Matsuoka, Y., Taylor, J. W., DeMattos, R. B., Holtzman, D. M., et al. (2003) Neurobiol. Dis. 14, 10-18. [DOI] [PubMed] [Google Scholar]
- 35.Pfeifer, M., Boncristiano, S., Bondolfi, L., Stalder, A., Deller, T., Staufenbiel, M., Mathews, P. M. & Jucker, M. (2002) Science 298, 1379. [DOI] [PubMed] [Google Scholar]
- 36.Furlan, R., Brambilla, E., Sanvito, F., Roccatagliata, L., Olivieri, S., Bergami, A., Pluchino, S., Uccelli, A., Comi, G. & Martino, G. (2003) Brain 126, 285-291. [DOI] [PubMed] [Google Scholar]
- 37.Brazil, M. I., Chung, H. & Maxfield, F. R. (2000) J. Biol. Chem. 275, 16941-16947. [DOI] [PubMed] [Google Scholar]
- 38.Wilcox, D. M., Munireddy, S. K., Rosenthal, A., Ugen, K. E., Gordon, M. N. & Morgan, D. (2004) Neurobiol. Dis. 15, 11-20. [DOI] [PubMed] [Google Scholar]
- 39.Ghersi-Egea, J.-F., Gorevic, P. D., Ghiso, J., Frangione, B., Patlak, C. S. & Fensternacher, J. D. (1996) J. Neurochem. 67, 880-883. [DOI] [PubMed] [Google Scholar]
- 40.Poduslo, J. F., Curran, G. L., Sanyal, B. & Selkoe, D. J. (1999) Neurobiol. Dis. 6, 190-199. [DOI] [PubMed] [Google Scholar]
- 41.Shibata, M., Yamada, S., Kumar, S. R., Calero, M., Bading, J., Frangione, B., Holtzman, D. M., Miller, C. A., Strickland, D. K., Ghiso, J., et al. (2000) J. Clin. Invest. 106, 1489-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dodel, R. C., Hampel, H. & Du, Y. (2003) Lancet Neurol. 2, 215-220. [DOI] [PubMed] [Google Scholar]
- 43.Solomon, B., Koppel, R., Hanan, E. & Katzav, T. (1996) Proc. Natl. Acad. Sci. USA 93, 452-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solomon, B., Koppel, R., Frankel, D. & Hanan-Aharon, E. (1997) Proc. Natl. Acad. Sci. USA 94, 4109-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frenkel, D., Katz, O. & Solomon, B. (2000) Proc. Natl. Acad. Sci. USA 97, 11455-11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lombardo, J. A., Stern, E. A., McLellan, M. E., Kajdasz, S. T., Hickey, G. A., Bacskai, B. J. & Hyman, B. T. (2003) J. Neurosci. 23, 10879-10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kotilinek, A., Bacskai, B., Westerman, M., Kawarabayashi, T., Younkin, L., Hyman, B. T., Younkin, S. & Ashe, K. H. (2002) J. Neurosci. 22, 6331-6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lambert, M. P., Viola, K. L., Chromy, B. A., Chang, L., Morgan, T. E., Yu, J., Venton, D. L., Krafft, G. A., Finch, C. E. & Klein, W. L. (2001) J. Neurochem. 79, 595-605. [DOI] [PubMed] [Google Scholar]
- 49.Schenk, D. (2002) Nat. Rev. Neurosci. 3, 824-828. [DOI] [PubMed] [Google Scholar]
- 50.Senior, K. (2002) Lancet Neurol. 1, 3 (lett.). [DOI] [PubMed] [Google Scholar]
- 51.Orgogozo, J. M., Gilman, S., Dartigues, J. F., Laurent, B., Puel, M., Kirby, L. C., Jouanny, P., Dubois, B., Eisner, L., Flitman, S., et al. (2003) Neurology 61, 46-54. [DOI] [PubMed] [Google Scholar]
- 52.Hock, C., Konietzko, U., Papassotiropoulos, A., Wollmer, A., Streffer, J., von Rotz, R. C., Davey, G., Moritz, E. & Nitsch, R. M. (2002) Nat. Med. 8, 1270-1275. [DOI] [PubMed] [Google Scholar]
- 53.Nicoll, J. A. R., Wilkinson, D., Holmes, C., Steart, P., Markham, H. & Weller, R. O. (2003) Nat. Med. 9, 448-452. [DOI] [PubMed] [Google Scholar]
- 54.Hock, C., Konietzko, U., Streffer, J. R., Tracy, J., Signorell, A., Müller-Tilmanns, B., Lemke, U., Henke, K., Moritz, E., Garcia, E., et al. (2003) Neuron 38, 547-554. [DOI] [PubMed] [Google Scholar]
- 55.Ferrer, I., Rovira, M. B., Guerra, M. L. S., Rey, M. J. & Costa-Jussa, F. (2004) Brain Pathol. 14, 11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiner, H. L. & Selkoe, D. J. (2002) Nature 420, 879-884. [DOI] [PubMed] [Google Scholar]
- 57.Grubeck-Loebenstein, B., Blasko, I., Marx, F. & Trieb, K. (2000) Trends Neurosci. 23, 114 (lett.). [DOI] [PubMed] [Google Scholar]
- 58.Monsonego, A., Zota, V., Karni, A., Krieger, J. I., Bar-Or, A., Bitan, G., Budson, A. E., Sperling, R., Selkoe, D. J. & Weiner, H. L. (2003) J. Clin. Invest. 112, 415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trieb, K., Ransmayr, G., Sgonc, R., Lassmann, H. & Grubeck-Loebenstein, B. (1996) J. Clin. Invest. 112, 415-422. [DOI] [PubMed] [Google Scholar]
- 60.Monsonego, A., Maron, R., Zota, V., Selkoe, D. J. & Weiner, H. L. (2001) Proc. Natl. Acad. Sci. USA 98, 10273-10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sigurdsson, E. M., Scholtzova, H., Mehta, P. D., Frangione, B. & Wisniewski, T. (2001) Am. J. Pathol. 159, 439-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leverone, J. F., Spooner, E. T., Lehman, H. K., Clements, J. D. & Lemere, C. A. (2003) Vaccine 21, 2197-2206. [DOI] [PubMed] [Google Scholar]
- 63.Spinney, L. (2004) Lancet Neurol. 3, 5 (lett.). [DOI] [PubMed] [Google Scholar]
- 64.Neuroinflammation Working Group (2000) Neurobiol. Aging 21, 383-421.10858586 [Google Scholar]
- 65.Monsonego, A., Imitola, J., Zota, V., Oida, T & Weiner, H. L. (2003) J. Immunol. 171, 2216-2224. [DOI] [PubMed] [Google Scholar]
- 66.Wyss-Coray, T., Lin, C., Yan, F., Yu, G., Rohde, M., McConlogue, L, Masliah, E. & Mucke, L. (2001) Nat. Med. 7, 612-618. [DOI] [PubMed] [Google Scholar]
- 67.Cribbs, D. H., Ghochikyan, A., Vasilevko, V., Tran, M., Petrushina, I., Sadzikava, N., Babikyan, D., Kesslak, P., Kieber-Emmons, T., Cotman, C. W., et al. (2003) Int. Immunol. 15, 505-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Puaux, A. L. & Michel, M. L. (2003) Comp. Immunol. Microbiol. Infect. Dis. 26, 357-372. [DOI] [PubMed] [Google Scholar]
- 69.Finn, O. J. (2003) Nat. Rev. Immunol. 3, 630-640. [DOI] [PubMed] [Google Scholar]
- 70.Rangan, S. K., Ruitian, L., Brune, D., Planque, S., Paul, S. & Sierks, M. R. (2003) Biochemistry 42, 14328-14334. [DOI] [PubMed] [Google Scholar]
- 71.Frenkel, D., Solomon, B. & Benhar, I. (2000) J. Neuroimmunol. 106, 23-31. [DOI] [PubMed] [Google Scholar]
- 72.Ghochikyan, A., Vasilevko, V., Petrushina, I., Movsesyan, N., Babikyan, D., Tian, W., Sadzikava, N., Ross, T. M., Head, E., Cribbs, D. H., et al. (2003) Eur. J. Immunol. 33, 3232-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cui, Z. & Mumper R. J. (2003) Rev. Ther. Drug Carrier Syst. 20, 103-137. [DOI] [PubMed] [Google Scholar]