Abstract
Converging experimental evidence indicates that CD4+ regulatory T cells control progression of autoimmune insulitis in nonobese diabetic (NOD) mice. Here, we studied the nature of these regulatory T cells and their mode of action in diabetes-prone NOD Rag-/- or severe combined immunodeficient (SCID) mice harboring a transgenic T cell receptor derived from the diabetogenic T cell clone BDC2.5. We first show that diabetes onset is prevented in such mice by infusion of polyclonal CD4+ T cells expressing l-selectin (CD62L) but not prevented or only marginally prevented by CD4+CD25+ T cells. Similarly, we found with a cotransfer model that CD4+CD62L+ T cells but not CD4+CD25+ T cells inhibited diabetes transfer into NOD SCID recipients by transgenic NOD BDC2.5 SCID cells. Unexpectedly, cotransfer of transgenic NOD BDC2.5 SCID cells and spleen cells from WT diabetic NOD mice did not induce diabetes, whereas each individual population did so. Data are presented arguing for the role of CD4+CD62L+ T cells present within the polyclonal diabetogenic population in mediating this apparently paradoxical effect. Collectively, these data confirm the central role of CD4+CD62L+ regulatory T cells in controlling disease onset in a well defined transgenic model of autoimmune diabetes and suggest the intervention of homeostatic mechanisms as part of their mode of action.
The role of T cell-mediated immunoregulation in autoimmune diseases has been a matter of debate for the last three decades. The existence and the role of regulatory T cells in the control of organ-specific autoimmune diseases has regained credibility with the emergence of the T helper 1/T helper 2 paradigm and the demonstration of the occurrence of a polyautoimmune syndrome after day 3 thymectomy in nonautoimmune prone BALB/c mice and its prevention by the infusion of CD4+CD25+ T cells from normal syngeneic donors (1–9). Simultaneous data were reported in the nonobese diabetic (NOD) mouse model suggesting that the delayed appearance of the disease after a long prodromal phase of nonagressive insulitis was mediated by CD4+ regulatory T cells (4, 5). Thus, disease onset is accelerated by thymectomy at 3 weeks (10) and cyclophosphamide treatment (11). Disease transfer in prediabetic adult mice requires either sublethal irradiation (12) or CD4+ T cell depletion (13). Additionally, disease transfer into immunoincompetent recipients by spleen cells from diabetic NOD mice is prevented by concomitant injection of CD4+ T cells derived from young prediabetic NOD mice (14).
There has recently been much interest in the characterization of the CD4+ T cell subsets mediating this regulation. Using the cotransfer model just described, we have shown that regulatory T cells selectively expressed l-selectin (CD62L) (15) and to a lesser extent CD25 (refs. 16 and 17 and unpublished data). These results were at variance with the phenotype of regulatory cells characterized in other models such as autoimmune gastritis or colitis where the role of CD4+CD25+ T cells or CD4+CD45RBlow T cells, respectively, was highlighted (8, 18, 19). Recently, we presented evidence by using a single test model [NOD severe combined immunodeficient (SCID) mice] that depletion of CD25+, CD45RBlow, or CD62L+ T cells induced distinct autoimmune diseases, namely gastritis, colitis, or diabetes (17).
The WT NOD model suffers from the weakness of the still-elusive knowledge of the functional hierarchy of the various target β cell antigens involved and of the obvious complexity of the polyclonal autoimmune T cell response mediating the disease. This complexity is at the origin of the major interest given to the BDC2.5 transgenic model in which diabetes is mediated by clonotypic T cells (20–22). The model was further improved by back-crossing the NOD BDC2.5 mouse to various mutant mice unable to rearrange endogenous T cell receptors (SCID, Rag-/-, and Cα-/-). At variance with NOD BDC2.5 mice, mice back-crossed into the NOD SCID, NOD Rag-/-, or NOD Cα-/- backgrounds showed fulminant diabetes, appearing by 3–5 weeks of age, strongly arguing for the regulatory role of endogenously rearranged T cells lacking the transgenic T cell receptor (TCR) (22–24).
Here, we report additional results indicating that in this well defined model of autoimmune diabetes T cell regulation can still be demonstrated directly (protection from diabetes onset by infusion of regulatory T cells into NOD BDC2.5 Rag-/- mice) or indirectly by using the cotransfer model described above. We found that in both of these experimental systems regulation is mediated by CD4+CD62L+ T cells with a minimal or no role for CD4+CD25+ T cells. We also present data indicating a possible role for homeostasis mechanisms in the regulatory function exerted by CD4+CD62L+ T cells.
Methods
Mice. NOD mice (Kd, I-Ag7, and Db), NOD-SCID mice, BDC2.5 TCR transgenic NOD mice, NOD BDC2.5 Cα-/-, NOD BDC2.5 Rag-/-, NOD BDC2.5 SCID, and NOD-Lyt5.2 congenic mice were bred in our animal facility under specific pathogen-free conditions. Colorimetric strips were used to monitor glycosuria and glycemia (Glukotest and Haemoglukotest, Roche Molecular Biochemicals).
Cell Preparations Used in Transfer and Cotransfer Experiments. Thymocyte and total spleen cell suspensions were prepared either from 2- to 3-week-old NOD BDC2.5 Rag-/- and NOD BDC2.5 SCID mice or 6-week-old diabetic WT NOD mice. For some experiments, B lymphocytes were removed by magnetic bead-activated cell sorting (MACS, Miltenyi Biotec, Bergisch-Gladbach, Germany) using biotinylated B220 followed by streptavidin-coated paramagnetic beads. T cell splenocytes were then purified on the basis of the CD62L expression by using MACS. The sorting was performed as described (18). Cells were incubated for 20 min at 4°C with biotinylated CD62L antibodies, washed, and incubated with streptavidin-coated paramagnetic beads (10 μl per 10 × 106 cells) for 15 min at 4°C. After washing, the cells, resuspended in PBS plus 5% FCS, were passed through the column within the MACS device (Miltenyi Biotec). Purity of the sorted cells was 90–97%, and recovery ranged from 50% to 70%.
Adoptive Cell Transfers. CD4+CD62L+ T cells isolated from 6-week-old prediabetic NOD mice were infused i.p. into NOD BDC2.5 Rag-/- mice at 10 days of age. For cotransfer experiments, recipients were adult 6- to 8-week-old NOD SCID mice. Transgenic BDC2.5 cells were purified from the spleen or thymus of NOD BDC2.5 SCID mice by using CD4+-specific magnetic bead cell sorting. Animals were injected i.v. either with a single-cell population or a mixture of two distinct cell populations. The precise cell numbers used varied depending on the experiments and are detailed in Results.
Antibodies and Methods for Fluorescence-Activated Cell Sorting Analysis. Biotinylated and/or phycoerythrin (PE) and allophycocyanine (APC)-labeled MEL-14 (CD62-L), CD25 (7D4 or PC61), and CD4 antibodies were obtained from Pharmingen. PE- and APC-labeled streptavidin was from Pharmingen. Cells were stained in PBS containing 5% heat-inactivated FCS (Invitrogen) and 0.01% sodium azide and incubated for 15 min with the appropriate concentration of biotin-labeled antibodies. After washing, cells were incubated for another 15 min with FITC- and/or PE-labeled antibodies and PE- or APC-labeled streptavidin. After washing, the cells were fixed by using 0.7% formaldehyde (Sigma-Aldrich) containing PBS. Control staining was performed by using labeled isotype-matched irrelevant antibodies. cellquest software was used for acquisition and analysis (Becton Dickinson).
Histology and Insulitis Scoring. After diabetes onset or 16–26 weeks of age, mice were killed, and pancreas were removed for histology. Frozen pancreatic tissues were sectioned. Sections were stained with hematoxylin/eosin to detect islet-infiltrating lymphocytes. Peri-insulitis refers to the presence of few cells outside or at the closed periphery of intact islets. Invasive insulitis refers to the presence of inflammatory cells infiltrating the entire islets with clear cellular damages.
CD3 Antibody Treatment. The mAb to mouse CD3ε 145–2C11 was generated and purified as described (25). Neonates (1–3 days old) or 2- to 4-week-old prediabetic or overtly diabetic (glycemia, >3.5–4 g/liter) NOD BDC2.5 Cα-/- mice were treated with the antibody (days 1–5, 5–20 μg per day except for neonates, one single 250-μg i.p. injection).
Results
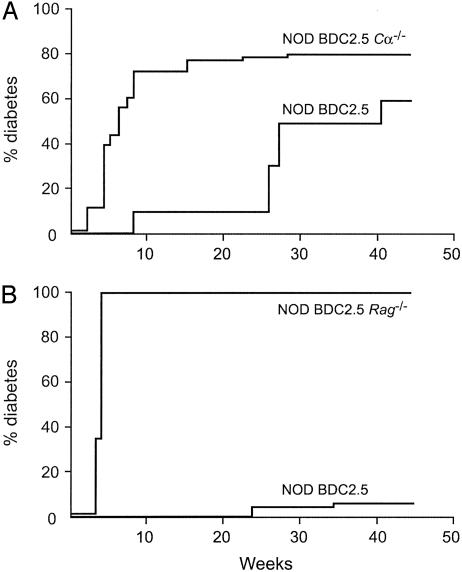
Diabetes Incidence Significantly Differs When the BDC2.5 Transgene Is Introduced into the NOD Mouse or NOD Immunodeficient Backgrounds. The initial manuscript describing the NOD BDC2.5 mouse line reported that at backcross (BC) 3 diabetes incidence in transgenic mice was higher than in littermate controls that showed a relatively low incidence rate as compared to conventional WT NOD mice (85% in NOD BDC2.5 versus 40% in littermate controls by 25 weeks of age) (21). When we introduced these NOD BDC 2.5 mice (after embryo transfer) in our animal facility in well controlled specific pathogen-free conditions, at BC7 diabetes incidence had already decreased as compared to the first report observed (10% and 60% at 25 and 40 weeks of age, respectively) (Fig. 1A). After further back-crossing into the NOD background up to BC12, the diabetic phenotype almost completely disappeared (Fig. 1B). Similar observations have been reported (24).
Fig. 1.
Incidence of diabetes in immunodeficient NOD mice expressing exclusively the BDC2.5 TCR transgene. Cumulative diabetes incidence in NOD BDC2.5 transgenic mice. NOD BDC2.5 Cα-/- mice or NOD BDC2.5 Rag-/- mice were backcrossed 7 (A)or >12 (B) times onto the NOD background. Introduction of the BDC2.5 TCR transgene into T cell-deficient NOD mice strongly accelerated the onset of diabetes. NOD BDC2.5 SCID mice showed a diabetes incidence identical to NOD BDC2.5 Rag-/- mice.
Conversely, rapid-onset diabetes was regularly observed in almost all animals when the BDC2.5 transgene was introduced into various immunodeficient NOD backgrounds. Thus, incidence of diabetes in NOD BDC2.5 Cα-/- mice (BC7) was 40% at 4 weeks and 80% at 15 weeks of age versus 0% and 10%, respectively, in NOD BDC2.5 littermates (Fig. 1 A). The difference in incidence was even more striking when studying NOD BDC2.5 SCID and NOD BDC2.5 Rag-/- mice at BC12 because in both cases fulminant diabetes appeared in 100% of the animals by days 15–25 (Fig. 1B).
Infusion of Purified CD4+CD62L+ T Cells Protects NOD BDC2.5 Rag-/- Mice from Diabetes. NOD BDC2.5 Rag-/- mice were injected i.p. at 10 days of age with 7 × 106 CD4+CD62L+ T cells purified by magnetic bead sorting from prediabetic (6–7 weeks old) WT Lyt5 (CD45.2) congenic NOD mice.
As already reported by others (24), we observed that mice injected with 5 × 106 purified CD4+ T cells were very efficiently protected from disease (0% diabetes at 6–7 weeks after infusion as compared with 100% at 3–4 weeks of age in control unmanipulated NOD BDC2.5 Rag-/- mice) (data not shown).
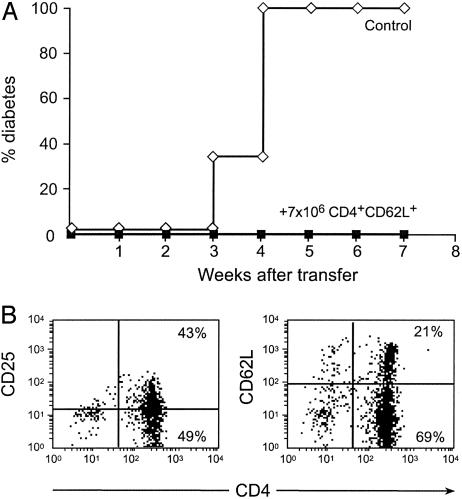
Moreover, as shown in Fig. 2A, mice injected with CD4+CD62L+ T cells were fully protected from diabetes. Conversely, only limited protection was observed when purified CD25+ T cells were used in identical experimental conditions (data not shown).
Fig. 2.
Infusion of CD4+CD62L+ T cells can protect NOD BDC2.5 Rag-/- mice from developing fulminant diabetes. (A) Prediabetic NOD BDC2.5 Rag-/- mice were infused at 10 days of age with CD4+CD62L+ T cells isolated from 6-week-old WT NOD Lyt5 congenic mice (CD45.2) (7 × 106 cells per recipient). CD4+CD62L+ T cells from young animals can afford significant protection against diabetes development (P < 0.0001). (B) Fluorescence-activated cell sorting analysis of donor-type cells, as assessed by the expression of the congenic marker CD45.2, recovered from diabetes-free protected mice 7 weeks after infusion of regulatory CD4+CD62L+ T cells.
Interestingly, when lymphocyte phenotypes were studied in spleen and lymph nodes of these reconstituted protected mice, it was observed that CD4+CD62L+ T cells (traced by using the congenic marker CD45.2) lost expression of CD62L and acquired that of CD25 (which was only initially expressed in a minority of donor cells) (Fig. 2B). In addition, transferred cells concentrated preferentially in mesenteric and peripancreatic lymph nodes rather than in the spleen.
Protection from Diabetes Transfer by BDC2.5 Transgenic T Cells into NOD SCID Recipients. Compared ability of transgenic BDC 2.5 and WT NOD diabetogenic T cells to transfer disease. We previously reported in extensive studies that diabetes can be induced, after transfer of polyclonal diabetogenic T cells recovered from the spleen of diabetic NOD mice, into immunodeficient NOD mice (i.e., sublethally irradiated, SCID, or neonates) (15, 26, 27). The transfer required the concomitant presence of CD4+ and CD8+ T cells. Diabetes was observed 4–10 weeks from transfer depending on the number of cells injected (28). Transfer was obtained with splenocytes but not with mature thymocytes. The diabetogenic potential of total splenocytes was detectable in cell donors by 15–20 weeks of age, i.e., 4–6 weeks before presumed diabetes onset (28). However, it was possible to find diabetogenic T cells at a much younger age when purified CD62L- spleen cells were used (ref. 17 and unpublished data).
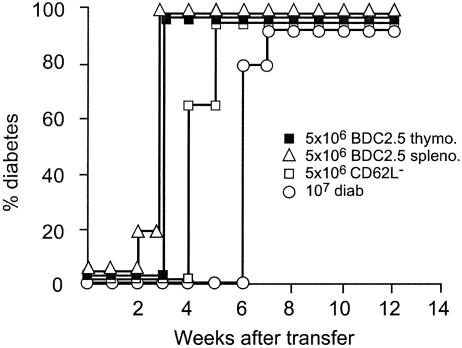
Results obtained with NOD BDC2.5 SCID mice in similar transfer studies provided very different results. Effective transfer was obtained upon injection of only transgenic T cells, which are CD4+, and did not require the presence of CD8+ T cells. Transfer was obtained with both transgenic splenocytes and thymocytes with similar efficiency (Fig. 3). Lastly, on a per-cell basis, transgenic cells appeared to be significantly more efficient at transferring diabetes than spleen cells from WT diabetic mice even after depletion of CD62L+ cells. Diabetes onset was much more rapid (100% diabetes at 3 weeks of age for NOD BDC 2.5 SCID cells versus 7 weeks for total spleen cells from WT diabetic mice and 5 weeks of age for CD62L- WT diabetic mice spleen cells) (Fig. 3).
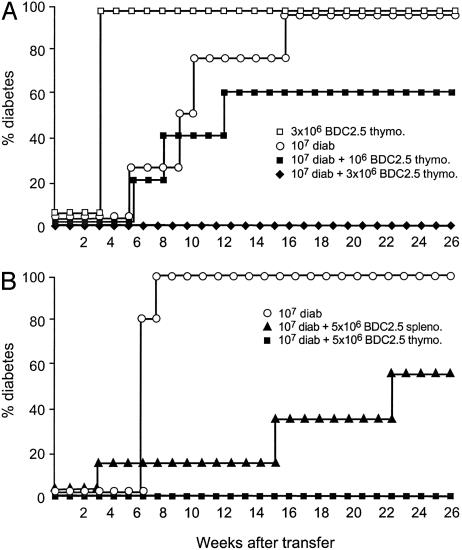
Fig. 3.
Thymocytes and splenocytes from BDC2.5 NOD SCID mice induce fulminant diabetes. NOD SCID recipients received diabetogenic cells (10 × 106 total spleen cells from overtly diabetic mice, diab), purified CD62L- T cells from diabetic donors, or thymocytes or splenocytes from 2- to 3-week-old NOD BDC2.5 SCID mice (5 × 106 thymo./spleno.). Fulminant diabetes was induced in recipients injected with BDC2.5 T cells.
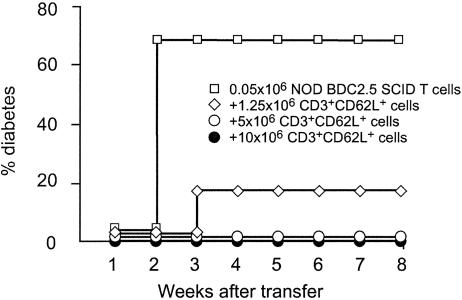
It is also interesting to note that diabetogenic T cells could be found in NOD BDC2.5 SCID mice as early as 15–20 days of age, much earlier than what was observed in WT NOD mice (i.e., diabetogenic CD62L- T cells appear by 5 weeks of age). In vivo interaction between polyclonal diabetogenic lymphocytes from WT NOD mice and BDC2.5 transgenic T cells. CD62L+ T cells inhibit diabetes transfer by BDC2.5 transgenic T cells. Using the cotransfer model we tested the capacity of purified CD62L+ T cells derived from 6-week-old prediabetic WT female NOD mice to inhibit disease transfer induced after infusion of 0.05 × 106 transgenic BDC2.5 spleen cells into NOD SCID recipients. Results showed that CD62L+ T cells totally prevented diabetes transfer in a long-lasting fashion and a dose-dependent manner (Fig. 4).
Fig. 4.
CD62L+ T cells from young prediabetic NOD mice protect from BDC2.5 T cell-induced diabetes. Two- to 3-week-old NOD BDC2.5 SCID splenocytes (0.05 × 106 cells) were injected i.v. either alone or with increasing numbers of CD62L+ T cells isolated from 6-week-old prediabetic NOD mice. Diabetes was completely abrogated upon transfer of CD62L+ T cells.
When cotransferred transgenic BDC2.5 cells and polyclonal spleen cells from overtly diabetic WT NOD mice are unable to transfer disease. As described above, both BDC2.5 transgenic thymocytes (1–5 × 106) and total spleen cells from diabetic mice (10 × 106) induced rapid onset of diabetes when injected into NOD SCID mice. Unexpectedly, NOD SCID recipients injected concomitantly with these two diabetogenic T cell populations remained disease-free (Fig. 5). Interestingly, the effect appeared to be dose-dependent, i.e., the proportion of disease-free animals among recipients increased when higher numbers of transgenic BCD2.5 cells were injected (Fig. 5).
Fig. 5.
Inhibition of diabetes development after cotransfer of BDC2.5 T cells and splenocytes from diabetic NOD mice. NOD SCID mice received total spleen cells from diabetic NOD mice (10 × 106) either alone or with increasing numbers of thymocytes from NOD BDC2.5 SCID mice (1–5 × 106). BDC2.5 thymocytes alone were used as the control population. Recipient mice transferred with the two potentially diabetogenic populations were protected from diabetes; the effect depended on the dose of transgenic BDC2.5 thymocytes injected.
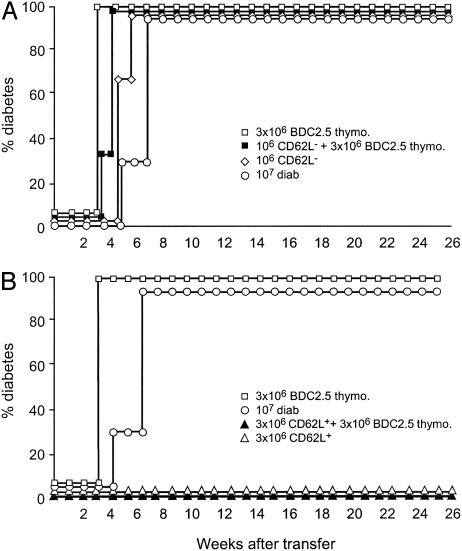
To dissect the possible cellular mechanisms explaining this paradoxical result, we separated WT diabetic mouse spleen cells based on their CD62L expression and coinjected these purified subsets with transgenic BDC 2.5 cells. As shown in Fig. 6A, when polyclonal diabetogenic CD62L- T cells were used in these cotransfers, diabetes appeared in all recipients with similar speed to that observed when transgenic BDC2.5 cells were injected alone (21–28 days after transfer). In contrast to this, coinjection of the transgenic cells with polyclonal CD62L+ T cells derived from overtly diabetic mice completely abrogated diabetes transfer in an identical fashion to that which was described above for CD62L+ T cells derived from 6-week-old prediabetic donors (Fig. 6B).
Fig. 6.
Coinjection of BDC2.5 thymocytes and CD62L+ T cells from diabetic mice prevents diabetes onset. T cells from overtly diabetic NOD mice were separated according to their CD62L expression and transferred alone or with 3 × 106 thymocytes from 2- to 3-week-old NOD BDC2.5 SCID mice. Total spleen cells from diabetic animals and transgenic BDC2.5 thymocytes cells were used as controls. In contrast to CD62L- T cells (A), CD62L+ T cells (B) were protected from diabetes.
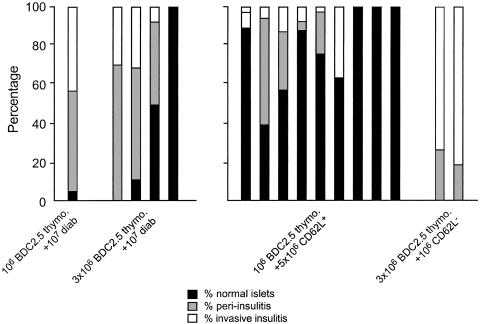
The histological analysis of pancreata in protected animals showed that some islets were free of infiltration, whereas others exhibited peripheral insulitis (Fig. 7). Such patterns significantly differed from the massive destructive insulitis observed in recipients injected with transgenic BDC2.5 thymocytes and CD62L- T cells in which diabetes uniformly appeared by 4 weeks after transfer (Figs. 6A and 7).
Fig. 7.
Protected mice exhibit intact islets and moderate peri-insulitis. The severity of insulitis was evaluated after diabetes onset or in diabetes-free recipient mice (16–26 weeks after transfer). Extensive and invasive insulitis was observed in NOD SCID recipient mice that became diabetic, like in the ones that were coinjected with CD62L- T cells and NOD BDC2.5 SCID thymocytes. Conversely, mice that remained free of diabetes showed only moderate peripheral insulitis. Mice cotransferred with polyclonal CD62L+ T cells showed a significant proportion of normal noninfiltrated islets.
Treatment with Antibodies to CD3. We previously reported that CD3 antibody treatment of recently diagnosed diabetic NOD mice induced long-term remission of the disease and that this effect is mediated by transforming growth factor-β-dependent CD4+ regulatory T cells (29). It was thus worthwhile to explore whether CD3 antibody treatment could also be efficient in mice expressing the BDC2.5 transgene in an immunodeficient background.
The CD3 antibody was injected into recently diabetic NOD BDC2.5 Cα-/- mice and 1- to 3-day-old (neonates) or 2- to 4-week-old prediabetic mice. The doses used were identical to those used in WT NOD mice at each of these points in time (i.e., 5–20 μg per day i.v. for 5 days in prediabetic or diabetic mice and a single 250-μg i.p injection in neonates). None of the CD3 antibody-treated NOD BDC2.5 Cα-/- mice showed long-term remission and/or prevention of the disease (Table 1).
Table 1. CD3 antibody treatment is ineffective to prevent diabetes or treat established disease in NOD BDC2.5 Cα-/- mice.
| Mice | CD3 treatment | No. of mice | Outcome |
|---|---|---|---|
| Neonates, 1–3 days of age | 250 μg i.p. | 14 | 9 diabetic 7 weeks after treatment, 4 diabetic 8 weeks after treatment, 1 diabetic 9 weeks after treatment |
| Prediabetic | |||
| 10 days of age | 20 μg for 5 consecutive days i.v. | 6 | 4–7 weeks after treatment |
| 21–28 days of age | 20 μg for 5 consecutive days i.v. | 2 | 8 weeks after treatment |
| Diabetic | 20 μg for 5 consecutive days i.v. | 13 | No disease remission |
Discussion
The occurrence of fulminant diabetes in immunodeficient NOD BDC2.5 mice (NOD BDC2.5 Cα-/-, NOD BDC2.5 SCID, or NOD BDC2.5 Rag-/-), contrasting with the absence of diabetes in NOD BDC2.5 transgenic mice, confirms the regulatory capacity of T cells bearing a endogenously rearranged TCRα chain (22–24). Similar findings have been reported in myelin basic protein-specific TCR transgenic mice (30).
The model used in this study allows the analysis of the sensitivity of clonotypic diabetogenic BDC2.5 CD4+ T lymphocytes to polyclonal regulatory T cells. It should be realized, however, that the experimental design cannot distinguish between the effect of a putative subset of antigen-specific regulatory T cells sharing the same specificity as effector cells from a nonantigen-specific effect.
The results obtained confirmed those previously reported by Gonzalez et al. (24) showing that the pathogenic ability of BDC2.5 T cells can be down-regulated in vivo by polyclonal CD4+ T cells recovered from young (4 weeks old) prediabetic WT NOD mice. The present results are also in keeping with this previous report as far as CD4+CD25+ T cells are concerned. In fact, in both studies, CD4+CD25+ T cells showed only marginal ability to protect from diabetes transfer (24). However, at variance with these previous reported data, our study allowed the identification of a T cell subset defined by the expression of l-selectin (CD62L+) including regulatory T cells capable of totally inhibiting the in vivo diabetogenic potential of transgenic BDC2.5 T cells. One possible explanation for this apparent discrepancy between the two studies may relate to the fact that Gonzalez et al. (24) used very young (4 weeks old) prediabetic NOD mice as donors for regulatory T cells. There is, in fact, compelling evidence to show that in NOD mice regulatory T cells are exported from the thymus and start to colonize peripheral lymphoid organs by only 3 weeks of age (14). This is why in our present study older animals were used (i.e., either 6-week-old prediabetic or 15- to 25-week-old diabetic donors).
The mode of action of such regulatory cells is still ill defined. The acceleration of diabetes onset observed in BDC2.5 mice after the administration of an antibody to CTLA-4 could suggest a role for the CTLA-4 molecule, although one cannot exclude an agonistic effect of the antibody on effector T cells (31). One should also mention the possible role of cytokines, notably transforming growth factor β, by reference to other results obtained in our laboratory pointing to the central role of this cytokine in the regulatory function exerted by CD4+CD62L+ T cells (unpublished results). Another hypothesis involves an interaction, possibly of the homeostatic type, between polyclonal regulatory CD62L+ T cells and transgenic BDC2.5 T cells. It is striking to note that CD62L+ T cells, which are not in a sufficient amount or an adequate state of differentiation to inhibit the diabetogenic potential of CD62L- effector cells among spleen cells from diabetic mice, acquire this capacity after in vivo cohabitation with transgenic BDC2.5 T cells, a quite unexpected finding because of the potent intrinsic diabetogenic capacity of the transgenic population. These data are reminiscent of the report by Barthlott et al. (32) in the colitis model induced after injection in SCID mice of normal sorted CD45RBhigh T cells (18). Surprisingly enough increasing the number of CD45RBhigh T cells used to reconstitute SCID mice did not induce colitis as lower doses do (32). It was also shown in this study that high (but not low) proliferating TCR transgenic cells of an unrelated specificity could also inhibit colitis induction by pathogenic doses of CD45RBhigh T cells (32). Finally, these results confirm our previous report showing a unique role of CD62L+ in the regulation of autoimmune diabetes contrasting with apparent inability to control the onset of gastritis or colitis (17). The reasons for this unique effect on diabetes, but not on other autoimmune or immune-mediated diseases, remains to be determined, as do the precise phenotypic characteristics and mode of action of the T cell subset among CD62L+ T cells involved.
Lastly, one should note that antibodies to CD3 that have been previously shown to provide indefinite prevention of diabetes when injected into NOD neonates (33) or cure recently established diabetes in adult NOD mice (25, 34) had no effect in NOD BDC2.5 immunodeficient mice. This observation confirms other recently reported data indicating that the mode of action of this antibody does not involve depletion or inactivation of effector cells, but rather stimulation of transforming growth factor-β-dependent regulatory cells that are obviously missing in these mice (29).
As a whole, these results outline the remarkable capacity of polyclonal regulatory T cells to suppress the effector function of monoclonal pathogenic T cells and confirm the apparent diversity of regulatory T cell subsets that exhibit distinct phenotypes, organ specificity, and putative modes of action.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Therapeutic Vaccines: Realities of Today and Hopes for Tomorrow,” held April 1–3, 2004, at the National Academy of Sciences in Washington, DC.
Abbreviations: NOD, nonobese diabetic; SCID, severe combined immunodeficient; TCR, T cell receptor; BC, backcross.
References
- 1.Sakaguchi, S. (2000) Cell 101, 455-458. [DOI] [PubMed] [Google Scholar]
- 2.Seddon, B. & Mason, D. (2000) Immunol. Today 21, 95-99. [DOI] [PubMed] [Google Scholar]
- 3.Shevach, E. M. (2002) Nat. Rev. Immunol. 2, 389-400. [DOI] [PubMed] [Google Scholar]
- 4.Bach, J. F. & Chatenoud, L. (2001) Annu. Rev. Immunol. 19, 131-161. [DOI] [PubMed] [Google Scholar]
- 5.Chatenoud, L., Salomon, B. & Bluestone, J. A. (2001) Immunol. Rev. 182, 149-163. [DOI] [PubMed] [Google Scholar]
- 6.Maloy, K. J. & Powrie, F. (2001) Nat. Immunol. 2, 816-822. [DOI] [PubMed] [Google Scholar]
- 7.Bach, J. F. & Francois Bach, J. (2003) Nat. Rev. Immunol. 3, 189-198. [DOI] [PubMed] [Google Scholar]
- 8.Asano, M., Toda, M., Sakaguchi, N. & Sakaguchi, S. (1996) J. Exp. Med. 184, 387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. (1995) J. Immunol. 155, 1151-1164. [PubMed] [Google Scholar]
- 10.Dardenne, M., Lepault, F., Bendelac, A. & Bach, J. F. (1989) Eur. J. Immunol. 19, 889-895. [DOI] [PubMed] [Google Scholar]
- 11.Yasunami, R. & Bach, J. F. (1988) Eur. J. Immunol. 18, 481-484. [DOI] [PubMed] [Google Scholar]
- 12.Wicker, L. S., Miller, B. J. & Mullen, Y. (1986) Diabetes 35, 855-860. [DOI] [PubMed] [Google Scholar]
- 13.Sempe, P., Richard, M. F., Bach, J. F. & Boitard, C. (1994) Diabetologia 37, 337-343. [DOI] [PubMed] [Google Scholar]
- 14.Boitard, C., Yasunami, R., Dardenne, M. & Bach, J. F. (1989) J. Exp. Med. 169, 1669-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbelin, A., Gombert, J. M., Lepault, F., Bach, J. F. & Chatenoud, L. (1998) J. Immunol. 161, 2620-2628. [PubMed] [Google Scholar]
- 16.Shimada, A., Rohane, P., Fathman, C. G. & Charlton, B. (1996) Diabetes 45, 71-78. [DOI] [PubMed] [Google Scholar]
- 17.Alyanakian, M. A., You, S., Damotte, D., Gouarin, C., Esling, A., Garcia, C., Havouis, S., Chatenoud, L. & Bach, J. F. (2003) Proc. Natl. Acad. Sci. USA 100, 15806-15811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powrie, F., Correa-Oliveira, R., Mauze, S. & Coffman, R. L. (1994) J. Exp. Med. 179, 589-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powrie, F. & Mason, D. (1990) J. Exp. Med. 172, 1701-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haskins, K., Portas, M., Bergman, B., Lafferty, K. & Bradley, B. (1989) Proc. Natl. Acad. Sci. USA 86, 8000-8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz, J. D., Wang, B., Haskins, K., Benoist, C. & Mathis, D. (1993) Cell 74, 1089-1100. [DOI] [PubMed] [Google Scholar]
- 22.Katz, J. D., Benoist, C. & Mathis, D. (1995) Science 268, 1185-1188. [DOI] [PubMed] [Google Scholar]
- 23.Kurrer, M. O., Pakala, S. V., Hanson, H. L. & Katz, J. D. (1997) Proc. Natl. Acad. Sci. USA 94, 213-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez, A., Andre-Schmutz, I., Carnaud, C., Mathis, D. & Benoist, C. (2001) Nat. Immunol. 2, 1117-1125. [DOI] [PubMed] [Google Scholar]
- 25.Chatenoud, L., Thervet, E., Primo, J. & Bach, J. F. (1994) Proc. Natl. Acad. Sci. USA 91, 123-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bendelac, A., Carnaud, C., Boitard, C. & Bach, J. F. (1987) J. Exp. Med. 166, 823-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larger, E., Becourt, C., Bach, J. F. & Boitard, C. (1995) J. Exp. Med. 181, 1635-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yasunami, R., Debray-Sachs, M. & Bach, J. F. (1990) in Frontiers in Diabetes Research: Lessons from Animal Diabetes III, ed. Shafrir, E. (Smith-Gordon, London), Vol. 19, pp. 88-93. [Google Scholar]
- 29.Belghith, M., Bluestone, J. A., Barriot, S., Megret, J., Bach, J. F. & Chatenoud, L. (2003) Nat. Med. 9, 1202-1208. [DOI] [PubMed] [Google Scholar]
- 30.Lafaille, J. J., Nagashima, K., Katsuki, M. & Tonegawa, S. (1994) Cell 78, 399-408. [DOI] [PubMed] [Google Scholar]
- 31.Luhder, F., Hoglund, P., Allison, J. P., Benoist, C. & Mathis, D. (1998) J. Exp. Med. 187, 427-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barthlott, T., Kassiotis, G. & Stockinger, B. (2003) J. Exp. Med. 197, 451-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayward, A. R. & Shreiber, M. (1989) J. Immunol. 143, 1555-1559. [PubMed] [Google Scholar]
- 34.Chatenoud, L., Primo, J. & Bach, J. F. (1997) J. Immunol. 158, 2947-2954. [PubMed] [Google Scholar]