Abstract
Total mercury (Hg) was measured in coastal fishes from Southern New England (RI, USA), and Hg exposure was estimated for anglers and family members that consumed these resources. Fish Hg was positively related to total length (n = 2028 across 7 fish species), and interspecies differences were evident among legally harvestable fish. Many recreational anglers and their families experienced excessively high Hg exposure rates, which was attributed to the enriched Hg content of frequently consumed fishes. Specifically, 51.5% of participants in this study had Hg exposures exceeding the US EPA reference dose, including 50.0% of women of childbearing years. These results are noteworthy given that Hg neurotoxicity occurs in adults and children from direct and prenatal low-dose exposure. Moreover, this study underscores the need for geographic-specific research that accounts for small-scale spatial variations in fish Hg and dietary habits of at-risk human populations.
Keywords: Fish consumption, Mercury exposure, Recreational anglers, US EPA threshold and reference dose values
1. Introduction
Methylmercury (MeHg) is a pervasive environmental contaminant that can cause systemic toxicity in humans, including neurological and cardiovascular disorders, immune deficiencies, and reproductive defects (Moszcynski et al., 1990; Salonen et al., 1995; Grandjean et al., 1997; Sorensen et al., 1999). Humans are exposed to MeHg principally through the consumption of contaminated fish (Hightower and Moore, 2003). This exposure pathway is a consequence of: (1) MeHg constituting the majority of total mercury in fish muscle tissue (e.g., > 95%; Bloom, 1992; Piraino and Taylor, 2009; Payne and Taylor, 2010), (2) fish absorbing and accumulating MeHg at concentrations that are potentially toxic to humans (US EPA, 1997), and (3) the high efficiency with which MeHg is absorbed through the human gastrointestinal tract (Aberg et al., 1969). Moreover, subsequent to dietary intake, MeHg is readily absorbed into the human blood, distributes to all tissues, and penetrates the blood-brain and placental barriers (Suzuki et al., 1984; Soria et al., 1992). The critical elements affecting MeHg exposure from fish include contaminant concentrations in edible tissues and the frequency of consumption (Burger, 2013).
Public health professionals affiliated with federal and state agencies respond to the threat of MeHg in fish by issuing consumption advisories to inform residents of possible health risks (RIDOH, 2016; US FDA, 2016b). Consumption advisories, however, are limited by several factors that potentially undermine their efficacy. Foremost, advisories are frequently predicated on nationally aggregated data that broadly estimate fish MeHg concentrations, as well as the rate of fish consumption by the human subpopulation of interest (US EPA, 1997; US FDA, 2016a). Therefore, advisories often lack the appropriate site-specific detail to accurately report contamination risks of fish collected from a particular body of water or human demographic. This concern is especially warranted for the northeastern US, a region that relies heavily on local fisheries and experiences elevated mercury contamination from both local point sources and long-range transport processes (Chen et al., 2008; Mahaffey et al., 2009; Taylor et al., 2012). Investigations focusing on site-specific assessments of fish Hg contamination and human exposure will therefore provide health officials with the data required to make informed decisions regarding the safe consumption of local fishery resources.
Relative to the general population, specific US subpopulations can be more susceptible to MeHg toxicity because of elevated rates of fish consumption. For example, economic status, ethnic and cultural identity and practices, and coastal residency are important factors governing fish consumption, and thus, MeHg exposure (McKelvey et al., 2007; Mahaffey et al., 2009; Lincoln et al., 2011). Recreational anglers in the US are also a subpopulation of concern, yet information on their dietary habits and MeHg intake are lacking. Of the few studies that have addressed this topic, recreational anglers often have elevated MeHg body burdens because of their higher consumption of local fish (Al-Majed and Preston, 2000; Kosatsky et al., 2000; Dellinger 2004; Gobeille et al., 2006; Knobeloch et al., 2007). It is important to note, that with few exceptions (Karouna-Reneir et al., 2008; Lincoln et al., 2011; Burger, 2013), these prior investigations focused almost exclusively on freshwater recreational anglers or were conducted internationally; whereas in the US, the majority of fish consumed by recreational anglers are of estuarine or marine origin (US EPA, 2002).
Southern New England (SNE), including Connecticut, Massachusetts, and Rhode Island, supports an extensive saltwater recreational fishery. From 2010–2014, the mean annual landing of marine fishes in SNE equaled ~ 3 million fish, which constitutes ~13.5% of the total recreational catch from the entire US Atlantic Coast (NMFS, 2016). During this same time period, residents of SNE participated in ~ 650,000 oceanic angler trips annually (NMFS, 2016). Despite the prominence of this recreational fishery, estimates of fish consumption of estuarine and marine species by SNE anglers and their families are not well documented. A regional analysis of adult women’s blood Hg also revealed that individuals in the northeastern US had higher contaminant levels relative to other regions, suggesting that SNE residents are a more highly exposed group relative to the national average (Mahaffey et al., 2009). Hence, acquiring data on MeHg exposure in SNE recreational anglers is particularly warranted and necessary to support public health risk assessments and risk management decisions related to the issuance of fish consumption advisories.
The main objectives of this study were twofold. First, recreationally-important finfish were collected from SNE coastal waters (Narragansett Bay Estuary and Rhode Island/Block Island Sound), and total mercury (Hg) concentrations were measured in their muscle tissues (Piraino and Taylor, 2009; 2013; Payne and Taylor, 2010; Szczebak and Taylor, 2011). Accordingly, this investigation provides an analysis of Hg contamination in several important fisheries at small spatial scales, and serves as a basis of comparison with other localized geographic areas and broader Atlantic coast-wide assessments. The second objective of this study was to estimate Hg exposure in SNE recreational anglers and their families owing to the dietary intake of fish during summer months (June to September). A food frequency questionnaire was disseminated to the human subpopulation of interest (anglers and family members), and inquired about a person’s fish eating habits. These geographically-specific data were then incorporated into a human exposure assessment model, which in turn assessed an individual’s daily exposure to Hg at a local scale. To evaluate the efficacy of the assessment model, results were compared to regional and national estimates of human exposure to Hg (Mahaffey et al., 2009), and the reference dose (RfD) established by the US Environmental Protection Agency (US EPA, 1997).
2. Methods
2.1. Fish collection, processing, and preservation
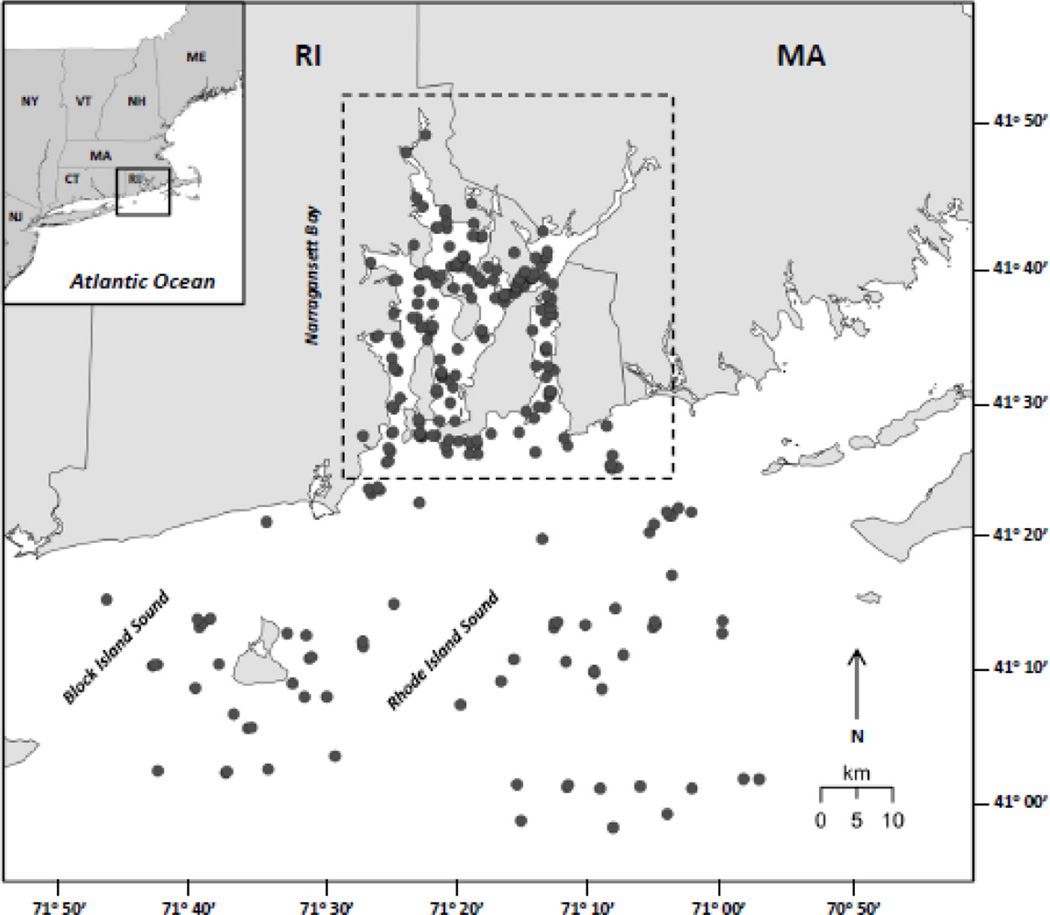
Target fishes in this study were black sea bass (Centropristis striata), bluefish (Pomatomus saltatrix), scup (Stenotomus chrysops), striped bass (Morone saxatilis), summer flounder (Paralichthys dentatus), tautog (Tautoga onitis), and winter flounder (Pseudopleuronectes americanus). These species were selected for analysis because they constitute > 92% of the SNE recreational fish harvest (NMFS, 2016). Target fishes were collected from the Narragansett Bay and Rhode Island Sound/Block Island Sound from May to September (2006–2015) using bottom trawls and hook & line (Fig. 1). Fishes collected in the field were immediately placed on ice for transportation and frozen at −20 °C after returning to the laboratory. In the laboratory, fishes were partially thawed and measured for whole-body wet weight (g) and total length (TL; cm) (Table 1). For each fish, a white muscle tissue sample (~ 2.5 g wet weight with skin and scales removed) was extracted from the dorsal region above the operculum on each side of the fish using stainless-steel surgical blades. This axial muscle tissue is accessible in all fishes, and its total Hg concentration is indicative of the whole-body fillet (Piraino and Taylor, 2009; Payne and Taylor, 2010; Szczebak and Taylor, 2011). For final preservation, muscle tissue samples were freeze-dried for 48 hr (Labconco FreeZone 4.5-L Benchtop Freeze-Dry System). Dried samples were then reweighed (g dry weight) to assess tissue water content, homogenized with a clean stainless-steel spatula, and stored at room temperature in 40-mL borosilicate vials.
Fig. 1.
Map of Narragansett Bay, Rhode Island Sound, and Block Island Sound with points denoting collection sites of target fish (black sea bass, bluefish, scup, striped bass, summer flounder, tautog, and winter flounder).
Table 1.
Total length (cm), whole-body wet weight (kg), and total mercury concentrations (Hg; ppm wet weight) of target fishes within their respective “sublegal” and “legal” harvestable size range. Sample sizes (n), means (± 1 SD), and ranges (in parentheses) are reported. The percent of fishes exceeding the US Environmental Protection Agency (% > EPA) Hg threshold of 0.3 ppm are also presented.
| Species | n | Length | Weight | Hg | % > EPA |
|---|---|---|---|---|---|
| Striped bass1 | |||||
| Sublegal | 89 | 49.5 ± 11.2 (26.2–69.5) |
1.40 ± 0.85 (0.18–4.03) |
0.163 ± 0.090 (0.031–0.410) |
7.9 |
| Legal | 60 | 82.5 ± 9.0 (71.2–104.5) |
5.48 ± 1.97 (2.97–11.3) |
0.337 ± 0.150 (0.131–0.751) |
55.0 |
| Total | 149 | 62.8 ± 19.2 | 2.90 ± 2.40 | 0.233 ± 0.146 | 26.8 |
| Bluefish1 | |||||
| Sublegal | 756 | 21.3 ± 15.2 (6.5–60.5) |
0.29 ± 0.55 (0.01–2.72) |
0.085 ± 0.094 (0.010–0.582) |
3.7 |
| Legal2 | 70 | 67.6 ± 4.9 (61.4–83.5) |
2.69 ± 0.63 (1.78–4.41) |
0.319 ± 0.156 (0.113–0.909) |
45.7 |
| Total | 826 | 25.2 ± 19.5 | 0.49 ± 0.87 | 0.104 ± 0.120 | 7.5 |
| Tautog1 | |||||
| Sublegal | 64 | 34.2 ± 7.0 (10.7–40.5) |
0.80 ± 0.46 (0.07–1.58) |
0.138 ± 0.099 (0.028–0.632) |
6.3 |
| Legal | 99 | 47.4 ± 5.1 (41.0–65.5) |
2.36 ± 0.88 (1.09–5.98) |
0.287 ± 0.201 (0.060–0.861) |
35.4 |
| Total | 163 | 42.2 ± 8.8 | 1.77 ± 1.07 | 0.227 ± 0.183 | 24.2 |
| Black sea bass1 | |||||
| Sublegal | 101 | 28.7 ± 5.1 (10.5–35.3) |
0.36 ± 0.17 (0.02–0.71) |
0.114 ± 0.056 (0.023–0.405) |
1.0 |
| Legal | 128 | 44.4 ± 6.4 (35.5–60.0) |
1.26 ± 0.49 (0.60–3.00) |
0.218 ± 0.118 (0.079–0.598) |
20.3 |
| Total | 229 | 37.5 ± 9.8 | 0.87 ± 0.59 | 0.172 ± 0.109 | 11.7 |
| Summer flounder1 |
|||||
| Sublegal | 136 | 36.9 ± 6.5 (14.0–45.5) |
0.56 ± 0.24 (0.03–1.18) |
0.098 ± 0.057 (0.018–0.353) |
3.7 |
| Legal | 109 | 52.1 ± 5.3 (45.6–68.5) |
1.54 ± 0.61 (0.90–3.74) |
0.192 ± 0.091 (0.043–0.423) |
12.8 |
| Total | 245 | 43.6 ± 9.7 | 0.94 ± 0.64 | 0.140 ± 0.088 | 7.7 |
| Scup | |||||
| Sublegal | 99 | 17.2 ± 5.1 (6.5– 25.2) |
0.13 ± 0.09 (0.01–0.41) |
0.051 ± 0.024 (0.014–0.131) |
0.0 |
| Legal | 121 | 30.8 ± 4.1 (25.4–42.0) |
0.51 ± 0.17 (0.24–0.97) |
0.175 ± 0.111 (0.040–0.553) |
9.9 |
| Total | 220 | 24.7 ± 8.2 | 0.34 ± 0.23 | 0.119 ± 0.104 | 5.5 |
| Winter flounder1 |
|||||
| Sublegal | 145 | 21.2 ± 4.7 (12.6–30.2) |
0.15 ± 0.11 (0.03–0.61) |
0.027 ± 0.012 (0.007–0.073) |
0.0 |
| Legal | 51 | 35.5 ± 4.0 (30.4–47.5) |
0.61 ± 0.27 (0.28–1.98) |
0.055 ± 0.026 (0.019–0.155) |
0.0 |
| Total | 196 | 24.9 ± 7.8 | 0.27 ± 0.26 | 0.035 ± 0.021 | 0.0 |
Portions of data sets have been previously published in Payne and Taylor (2010), Piraino and Taylor (2009; 2013), and Szczebak and Taylor (2011)
Rhode Island does not implement a minimum size limit for bluefish, and thus lengths > 61.0 cm TL were used for these analyses
2.2. Fish mercury analysis
MeHg typically accounts for the majority of total Hg in fish tissue (> 95%), and this has been verified for target fishes examined in this study (Piraino and Taylor, 2009; Payne and Taylor, 2010); hence, total Hg was determined to be a reliable measurement of fish MeHg content. Total Hg concentrations in mg/kg dry weight were measured in fish muscle tissue (~ 0.03 g dry weight) using automated combustion atomic absorption spectrophotometry (DMA-80 Direct Hg Analyzer, Milestone, Inc., Shelton, Connecticut, USA), with a detection limit of 0.01 ng Hg (US EPA, 1998b). The Hg analyzer was calibrated using certified reference materials (CRMs) of known Hg concentrations, and included solid standards (TORT-1: lobster hepatopancreas; DORM-2: dogfish muscle) and aqueous standards prepared by the National Research Council Canada, Institute of Environmental Chemistry (Ottawa, Canada) and the National Institute of Standards and Technology (Gaithersburg, Maryland, USA), respectively. Calibration curves were highly linear (R2 > 0.99; p < 0.0001), and the recovery of independently analyzed samples of TORT-1, DORM-2, and PACS-2 (marine sediment) CRMs ranged from ~ 92% to 108% (mean ~ 96%; median ~ 97%). All samples were analyzed as duplicates, and an acceptance criterion of 10% was implemented. Duplicate samples with < 10% error were averaged for subsequent analysis (mean and median absolute difference between duplicates ~ 3.6% and 2.9%, respectively). Samples with > 10% error were reanalyzed to achieve the acceptance criterion or were eliminated from further analysis. For additional quality control, blanks were analyzed every 10 samples to assess instrument accuracy and potential drift. Finally, total Hg data were converted to wet weight using known wet-dry ratios (water content range ~ 76–79%), and hereafter all units are presented as mg/kg wet weight (ppm).
Mean Hg concentrations in fish muscle tissue were compared across species using a one-way analysis of variance (ANOVA) model. Prior to the ANOVA analysis, Hg data were log(x)-transformed to meet assumptions of normality and homogeneity of variance. The post hoc examination of fish Hg across 7 levels of species was performed using a Ryan-Einot-Gabriel-Welsch (Ryan’s Q) multiple comparison test (Day and Quinn, 1989). One-sample t-tests were also used to compare intraspecific Hg concentrations to the US EPA Hg threshold level (0.3 ppm wet weight), noting that fishery resources with Hg concentrations exceeding this threshold may cause deleterious effects to human health (US EPA, 1997). For the abovementioned statistical analyses, Hg data were limited to legally harvestable fish, as determined by Rhode Island management regulations active at the time of publication (RIDEM, 2016): black sea bass (> 35.6 cm TL); scup (> 25.4 cm TL); striped bass (> 71.1 cm TL); summer flounder (> 45.7 cm TL); tautog (> 40.6 cm TL); and winter flounder (> 30.5 cm TL). Rhode Island does not implement a minimum size limit for bluefish, and thus lengths > 61.0 cm TL were used for these analyses because they represent sizes commonly caught and consumed by recreational anglers (Burger, 2009; 2013; Burger and Gochfeld, 2011). Moreover, for each target species, non-linear (exponential) regression models were used to examine the relationship between fish TL and the Hg content of their muscle tissue. These models were then used to predict the Hg concentration of each species at their minimum legal sizes, as defined above, and other pre-defined lengths that enabled direct comparisons with intraspecific Hg values reported in the literature (see below).
To assess spatial variations in contamination, total Hg concentrations of target fishes collected from SNE coastal waters (this study) were compared to other localized geographic areas and aggregated data for the Atlantic Coast (Florida to Maine). For the latter comparison, mean Hg values for SNE and Atlantic Coast fishes were statistically contrasted using one-sample t-tests. The Atlantic coast-wide data were available from the US EPA’s Mercury in Marine Life Project (US EPA, 2003), including the Environmental Monitoring and Assessment Program (EMAP) National Coastal Assessment (NCA) (US EPA, 2016). For a complete description of the survey design, sample processing, and analytical methods, refer to US EPA (2003), Harvey et al. (2008), and references therein.
2.3. Fish eating habits of anglers and families
The fish eating habits of SNE anglers and their families were evaluated using a food frequency questionnaire (FFQ), with pre-approval from the Roger Williams University Institutional Review Board (approved 6 September 2012). The FFQ was distributed using two methods: (1) an online survey tool (SurveyMonkey™) maintained by the Rhode Island Saltwater Anglers Association (September 2012), and (2) hard copy questionnaires presented to attendees of the Galilee Fishing Tournament and Seafood Festival (September 2012 and 2013; Narragansett, RI) and New England Saltwater Fishing Show (March 2013; Providence, RI). The FFQ was distributed to willing participants that self-identified as adults (≥ 18 years of age) and local saltwater anglers (or family members of anglers) (total participants = 371). Prior to completing the FFQ, participants signed a consent form, at which time they were made aware that the study was being conducted by the lead author who is affiliated with Roger Williams University, Department of Marine Biology. The FFQ required ~ 10 minutes to complete and consisted of ten questions. The first portion of the FFQ requested demographic information, including age, gender, ethnicity, race, and state of residence. The second section of the survey questioned a person’s fish eating habits, including the frequency of fish (finfish/shellfish) consumption during the previous summer (June to September), the source of fish consumed (locally caught from Narragansett Bay and/or Rhode Island/Block Island Sound versus store/restaurant bought), and the extent of fish consumption that was comprised of one or more of the seven target species in this study. For the latter, to assist with proper identification, common nomenclature (e.g., scup = porgy; summer flounder = fluke; tautog = blackfish) and visual images of each fish species were provided.
Mean differences in fish consumption rates (total fish meals/day and target fish meals/day) and percent of fish meals comprised of target fishes were examined using three-way ANOVA models, with the study participants’ age, gender, and state of residence as fixed factors. The post hoc examination of response variables across 4 levels of age (18–45, 46–55, 56–65, and 66+) and states (CT, MA, RI, and Other) were performed using Ryan’s Q multiple comparison tests. Two-way ANOVA models were also used to examine differences in the fish eating habits of participants as a function of their self-identified ethnicity and race. These demographic data were not included in the original ANOVA models because some participants did not report ethnic information or self-identified for more than one race designation. Moreover, prior to these analyses, fish eating habits data were log(x+1)-transformed to meet assumptions of normality and homogeneity of variance. Rates of fish consumption specific to SNE anglers and their families were also statistically compared to other human (sub)populations using one-sample t-tests. The alternative populations of interest varied by spatial scale and/or geographic location, and included the US national population (0.077 meals/day), coastal populations (Atlantic = 0.203 meals/day; Pacific = 0.173 meals/day; Gulf = 0.197 meals/day; Great Lakes = 0.120 meals/day) and New Jersey/New York anglers (0.267 meals/day) (May and Burger, 1996; US EPA, 2001; Mahaffey et al., 2009).
2.4. Human exposure to mercury
Human exposure to Hg from fish consumption (finfish and shellfish) was calculated using the following assessment model:
| (1) |
where, E is a person’s daily exposure to Hg (mg Hg/kg human body weight/day), PT is the proportion of fish eaten represented by one or more of the seven “target” species in this study (i.e., black sea bass, bluefish, scup, striped bass, summer flounder, tautog, and winter flounder), PO is the proportion of “other” fish eaten that are not represented by the target fishes (i.e., non-target fish/shellfish locally caught or store/restaurant bought), HgT and HgO are the total Hg concentrations (ppm) of “target” fishes and “other” fish, respectively, M is the amount of fish eaten per meal (kg), C is the rate of fish consumption (meals/day), and W is human body weight (kg). Values for PT, PO, and C were derived from individual responses to the FFQ. The total Hg concentrations of target fishes (HgT) were equal to the mean Hg content of legal-size fish within a species (Table 1). When more than one target species was reportedly eaten, it was assumed that meals were evenly distributed across the different fishes. The total Hg concentration of other fish (HgO) was equal to 0.041 ppm, and was calculated using Hg exposure data (3.33×10−5 mg Hg/kg human body weight/day) and fish consumption data (0.203 fish meals/day) specific to the Atlantic Coast human subpopulation (Mahaffey et al., 2009). The amount of fish eaten per meal (M) was equal to 0.227 kg (8 ounces; US EPA, 2001; Skinner et al., 2009), and the body weight (W) of male and female participants was equal to 70 kg and 67 kg, respectively (US EPA, 1997; 2001; Burger, 2013).
Mean differences in Hg exposure rates for study participants as a function of their demographic characteristics were examined using three-way and two-way ANOVA models and Ryan’s Q multiple comparison tests, as described above. SNE angler and family Hg exposure rates were also compared to the US EPA reference dose (RfD) of 1.0×10−4 mg Hg/kg human body weight/day, as well as exposure estimates for other coastal populations (Atlantic = 3.33×10−5 mg/kg/d; Pacific = 2.53×10−5 mg/kg/d; Gulf = 2.30×10−5 mg/kg/d; Great Lakes = 1.57×10−5 mg/kg/d; New Jersey anglers = 1.1×10−4 mg/kg/d) (Mahaffey et al., 2009; Burger, 2013), using one-sample t-tests. For the New Jersey subpopulation, the Hg exposure rate used for this analysis represents the maximum monthly mean Hg intake resulting from the consumption of black sea bass, bluefish, striped bass, summer flounder, and tautog (Burger, 2013).
3. Results and discussion
3.1. Intra- and interspecific analyses of mercury contamination in target fishes
In this study, 2028 fish across seven target species were analyzed for total Hg content, of which 638 fish had lengths greater than their minimum size for legal recreational harvest (RIDEM, 2016; Table 1). Target fish Hg concentrations ranged between 0.007 to 0.909 ppm (Table 1; Fig. 2). For legal-sized fish, Hg levels differed across species (1-way ANOVA: F = 76.81, df = 6, 637, p < 0.0001). Specifically, Hg concentrations were significantly higher in striped bass and bluefish (mean Hg = 0.32–0.34 ppm), followed by tautog and black sea bass (mean Hg = 0.22–0.29 ppm), summer flounder and scup (mean Hg = 0.18–0.19), and winter flounder (mean Hg = 0.06) (Ryan’s Q multiple comparison test) (Table 1; Fig. 2). Hg concentrations also increased significantly with fish total length, irrespective of species-type (Table 2; Fig. 3). The Hg-TL exponential regression models revealed that striped bass and bluefish attained Hg concentrations comparable to the US EPA threshold level (0.3 ppm) at their minimum legal size (0.24 and 0.31 ppm at 71.1 and 61.0 cm TL, respectively) (Fig. 3AB), and the mean Hg concentrations of harvestable striped bass and bluefish did not statistically differ from 0.3 ppm (Table 3). For the other target species, the estimated Hg content of their muscle tissues only surpassed the US EPA threshold level at lengths moderately (tautog and black sea bass) or substantially (scup and flounder spp.) greater than their legal harvestable sizes (Fig. 3C–G); although the mean Hg concentration of harvestable tautog was statistically equivalent to this threshold value (Table 3).
Fig. 2.
Total mercury concentrations (Hg; ppm wet weight) of legal-size target fish collected from Narragansett Bay and Rhode Island/Block Island Sound (box plot illustrating median, 1st and 3rd quartiles, and maximum and minimum values), including striped bass (SB), bluefish (BF), tautog (TG), black sea bass (BS), summer flounder (SF), scup (SC), and winter flounder (WF). The horizontal dashed line demarcates the US EPA Hg threshold level of 0.3 ppm wet weight.
Table 2.
Summary statistics for non-linear (exponential) regression models used to examine the relationship between fish total length (TL; cm) and intraspecific total mercury concentrations (Hg; ppm wet weight).
| Species | Regression model | F (df) | p | R2 |
|---|---|---|---|---|
| Striped bass | log(Hg) = 1.15× 10−2 × TL – 1.439 | 210.1 (1, 148) | < 0.0001 | 0.588 |
| Bluefish | log(Hg) = 2.01× 10−2 × TL – 1.730 | 2327.6 (1, 825) | < 0.0001 | 0.739 |
| Tautog | log(Hg) = 2.38× 10−2 × TL – 1.763 | 137.0 (1, 162) | < 0.0001 | 0.460 |
| Black sea bass | log(Hg) = 1.90× 10−2 × TL – 1.548 | 278.0 (1, 228) | < 0.0001 | 0.550 |
| Scup | log(Hg) = 3.09× 10−2 × TL – 2.024 | 1099.5 (1, 219) | < 0.0001 | 0.835 |
| Summer flounder | log(Hg) = 2.17×10−2 × TL – 1.879 | 400.9 (1, 244) | < 0.0001 | 0.623 |
| Winter flounder | log(Hg) = 1.81× 10−2 × TL – 1.974 | 121.5 (1, 195) | < 0.0001 | 0.385 |
Fig. 3.
Total mercury concentrations (Hg; ppm wet weight) of target fish as a function of total length (cm), including striped bass (A), bluefish (B), tautog (C), black sea bass (D), summer flounder (E), scup (F), and winter flounder (G). Non-linear (exponential) regression models were fit to the data and represented by solid lines. The horizontal and vertical dashed lines demarcate the US EPA Hg threshold level (0.3 ppm weight) and intraspecific minimum legal harvestable sizes for Rhode Island waters (RIDEM, 2016), respectively.
Table 3.
Summary statistics for one-sample t-tests that compared mean total mercury concentrations (Hg; ppm wet weight) of target fishes from Southern New England (this study) relative to the “US EPA threshold” level (0.3 ppm wet weight) and species-specific data aggregated for the “Atlantic Coast” (Florida to Maine; US EPA, 2003), and mean fish consumption rates (meals/day) and Hg exposure rates (mg Hg/kg human body weight/day) of survey participants (this study) relative to estimates for the US national population, coastal populations (Atlantic, Pacific, Gulf, and Great Lakes) and New Jersey/New York anglers (May and Burger, 1996; Mahaffey et al., 2009; Burger, 2013). Participant Hg exposure rates were also compared to the US EPA reference dose (RfD; 1.0×10−4 mg Hg/kg human body weight/day).
| Target fish | US EPA threshold |
Atlantic Coast |
||||
|---|---|---|---|---|---|---|
| df | t-value | p-value | df | t-value | p-value | |
| Black sea bass | 127 | –7.75 | < 0.0001 | 127 | 6.50 | < 0.0001 |
| Bluefish | 69 | 1.03 | 0.306 | 69 | –4.40 | < 0.0001 |
| Scup | 120 | –12.38 | < 0.0001 | 120 | 13.99 | < 0.0001 |
| Striped bass | 59 | 1.93 | 0.059 | 59 | 9.49 | < 0.0001 |
| Summer flounder | 108 | –12.36 | < 0.0001 | 108 | 17.95 | < 0.0001 |
| Tautog | 98 | –0.65 | 0.515 | – | – | – |
| Winter flounder | 51 | –66.87 | < 0.0001 | 51 | 8.04 | < 0.0001 |
| Human population | Fish consumption rate |
Hg exposure rate |
||||
| df | t-value | p-value | df | t-value | p-value | |
| National | 370 | 18.26 | < 0.0001 | – | – | – |
| Atlantic Coast | 370 | 5.71 | < 0.0001 | 370 | 16.76 | < 0.0001 |
| Pacific Coast | 370 | 8.70 | < 0.0001 | 370 | 17.97 | < 0.0001 |
| Gulf Coast | 370 | 6.30 | < 0.0001 | 370 | 18.32 | < 0.0001 |
| Great Lakes | 370 | 13.98 | < 0.0001 | 370 | 19.42 | < 0.0001 |
| New Jersey/New York anglers | 370 | –0.67 | 0.503 | 370 | 5.12 | < 0.0001 |
| US EPA RfD | – | – | – | 370 | 6.64 | < 0.0001 |
The Rhode Island Department of Health (RIDOH) issues Hg consumption advisories to inform the public of potential health risks of eating fish (RIDOH, 2016). Their protocol for implementing advisories is based on the percentage of fish samples (legally harvestable fish within a species) that have Hg concentrations exceeding 0.3 ppm wet weight (R. Vanderslice, RIDOH, Office of Environmental Health Risk, Providence, RI, personal communication). This criterion was derived from the US EPA reference dose for Hg (mg Hg/kg body weight/day) and standard default values for adult body weight and for fish consumption. No advisories are issued when < 10% of the fish samples exceed 0.3 ppm. The general population is advised to limit fish consumption to 1 meal/week and 1 meal/month when 10–30% and 30–50% of the samples exceed 0.3 ppm, respectively, or to not consume any fish when > 50% of the samples exceed this criterion. More stringent guidelines are recommended for women of childbearing age (18–45 years of age) and young children (< 14 years of age), who are advised to avoid all fish consumption when > 10% of the samples exceed 0.3 ppm. According to RIDOH guidelines and the Hg data for legal-sized fish presented in this study, consumption advisories for the general population are warranted for summer flounder and black sea bass (12.8% and 20.3% of samples > 0.3 ppm, respectively; advisory = 1 meal/week), tautog and bluefish (35.4% and 45.7% of samples > 0.3 ppm, respectively; advisory = 1 meal/month), and striped bass (55.0% of samples > 0.3 ppm; advisory = do not eat) (Table 1). Alternatively, according to RIDOH guidelines, women of childbearing age and young children are advised to avoid consuming these fishes altogether. Hg advisories do not currently exist in Rhode Island (or Connecticut and Massachusetts) for the abovementioned species; thus existing advisories may be insufficiently protective in limiting Hg exposure, especially for high-end fish consumers. Conversely, for the other target species (scup and winter flounder), total Hg concentrations were relatively low (Table 1), which justifies their exclusion from state-specific consumption advisories.
Harvest regulations for recreational fisheries are necessary to protect the sustainability of the fish population. These management regulations are frequently based on fish body size (e.g., total length; RIDEM, 2016), whereby fish exceeding a pre-determined minimum length are acceptable for harvest, and ultimately, human consumption. Given the propensity for fish tissue Hg concentrations to increase with body size (Table 2; Fig. 3), regulations that promote the harvesting of the largest fishes may inadvertently expose human consumers to highly contaminated fish (Sackett et al., 2013). In this study, scup and flounder maintained relatively low Hg concentrations throughout their entire size range (Table 1; Fig. 3E–G), and thus, current harvest regulations aptly manage the fisheries without jeopardizing human health. Conversely, striped bass, bluefish, and tautog accumulated Hg at concentrations that are potentially toxic to humans, especially for high-end fish consumers (Table 1; Fig. 3A–C). Thus, existing regulations for these species inadequately protect against Hg exposure. Accordingly, for highly contaminated species, it is recommended that fishery management agencies adopt slot length limits (i.e., harvestable size range that includes a minimum and maximum length), which benefit anglers and recipients of their catches (e.g., family members) by preventing the harvesting and consumption of large-bodied, Hg-enriched fishes (Sackett et al., 2013). Using this study as an example, if a slot limit of 16–19 inches was implemented for tautog (40.6–48.3 cm TL), the percent of harvestable fishes exceeding the US EPA threshold of 0.3 ppm would decline to 19.4% (as opposed to 35.4%; Table 1), and correspondingly, the recommended consumption advisory would be revised to 1 meal/week (as opposed to 1 meal/month). An ancillary benefit of slot limits is that the largest, most productive female fish (with respect to egg production and larval quality) remain in the spawning population, which is critical for the sustainability of exploited species (Birkeland and Dayton, 2005 and references therein). Ideally, fishery management and public health agencies will work collaboratively in the future to more effectively meet their respective objectives of sustaining robust fish populations and reducing health risks associated with fish consumption (Sackett et al., 2013).
3.2. Spatial analysis of Hg contamination in target fishes
This study examined Hg concentrations in target fishes at relatively small spatial scales (i.e., site-specific analysis), and serves as a basis of comparison with other localized geographic areas and broader Atlantic coast-wide assessments (Table 4). For the latter, the Hg content of target fishes collected from SNE waters was significantly greater than contaminant levels reported for the Atlantic Coast aggregated data (on average, ~ 3.2 × higher in SNE vs. Atlantic; exception = bluefish; Table 3), which underscores the importance of measuring Hg at appropriate spatial scales. It is noteworthy, however, that fish lengths were not reported in the Atlantic coast-wide assessments (US EPA, 2003); thus confounding direct comparisons with the current investigation.
Table 4.
Literature review of target fish total mercury concentrations (Hg; ppm wet weight). The following information is provided for each source document: target fish species, location of sample, sample size (n), mean total length (TL; cm), and mean Hg concentrations reported in the “literature”. For comparative purposes, the total Hg content of target fishes (pre-defined size) were estimated from the exponential (Hg-TL) regression models presented in “this study” (Table 2). When the literature did not report length information (nr = not reported), this study’s total Hg represented the mean of the legal harvestable fishes (Table 1).
| Target fish / Location |
n | TL | Total Hg | Literature source |
|
|---|---|---|---|---|---|
| Literature | This study | ||||
| Black sea bass | |||||
| Atlantic (aggregated) |
nr | nr | 0.15 | 0.22 | US EPA (2003) |
| Gulf of Mexico (FL) |
33 | 20.0 | 0.14 | 0.07 | Tremain and Adams (2012) |
| Mid-Atlantic (NJ) |
19 | 48.0 | 0.16 | 0.23 | Burger and Gochfeld (2011) |
| New York Bight (NJ) |
14 | 27.9 | 0.05 | 0.10 | Deshpande et al. (2000)1 |
| Bluefish | |||||
| Atlantic (aggregated) |
nr | nr | 0.40 | 0.32 | US EPA (2003) |
| Long Island Sound (NY- CT) |
177 | 65.3 | 0.34 | 0.38 | Skinner et al. (2007)2 |
| Mid-Atlantic (NC) |
40 | 71.8 | 0.33 | 0.52 | Cross et al. (2015)3 |
| Mid-Atlantic (NJ) |
206 | 47.0 | 0.35 | 0.16 | Burger and Gochfeld (2011) |
| New York Bight Apex (NJ) |
14 | 55.4 | 0.10 | 0.24 | Deshpande et al. (2000)1 |
| North Atlantic (CT) |
46 | 40.6 | 0.14 | 0.12 | Hammerschmidt and Fitzgerald (2006)2 |
| North and Mid-Atlantic (NJ-MA) |
21 | 45.7 | 0.47 | 0.15 | Staudinger (2011) |
| Scup | |||||
| Atlantic Coast (aggregated) |
nr | nr | 0.03 | 0.18 | US EPA (2003) |
| Mid-Atlantic (NJ) |
27 | 26.0 | 0.09 | 0.06 | Burger and Gochfeld (2011) |
| North and Mid-Atlantic (NJ-RI) |
44 | 14.8 | 0.03 | 0.03 | US EPA (2016) |
| Striped bass | |||||
| Atlantic (aggregated) |
nr | nr | 0.15 | 0.34 | US EPA (2003) |
| Annapolis River (Nova Scotia, Canada) |
21 | 90.9 | 0.77 | 0.40 | Ray et al. (1984)3 |
| Apalachicola River (FL) |
8 | 74.2 | 0.19 | 0.26 | Brim et al. (2001)4 |
| Chesapeake Bay (MD) |
70 | 66.0 | 0.12 | 0.21 | Mason et al. (2006)5 |
| Chesapeake Bay (VA) |
20 | 54.9 | 0.11 | 0.16 | Xu et al. (2013) |
| Long Island Sound (NY- CT) |
132 | 78.2 | 0.37 | 0.29 | Skinner et al. (2007)2 |
| Mid-Atlantic (NJ) |
178 | 83.0 | 0.39 | 0.33 | Burger and Gochfeld (2011) |
| Summer flounder |
|||||
| Atlantic Coast (aggregated) |
nr | nr | 0.04 | 0.19 | US EPA (2003) |
| Chesapeake Bay (VA) |
46 | 42.8 | 0.07 | 0.11 | Xu et al. (2013) |
| Mid-Atlantic (NJ) |
260 | 52.0 | 0.14 | 0.18 | Burger and Gochfeld (2011) |
| New York Bight (NJ) |
14 | 36.0 | 0.04 | 0.08 | Deshpande et al. (2000)1 |
| North and Mid-Atlantic (DE-RI) |
53 | 36.4 | 0.07 | 0.08 | US EPA (2016) |
| North and Mid-Atlantic (NJ-MA) |
34 | 45.3 | 0.14 | 0.13 | Staudinger (2011) |
| Tautog | |||||
| Mid-Atlantic (NJ) |
47 | 42.0 | 0.20 | 0.17 | Burger and Gochfeld (2011) |
| New York Bight (NJ) |
14 | 31.0 | 0.08 | 0.09 | Deshpande et al. (2000)1 |
| North Atlantic (CT) |
32 | 41.4 | 0.19 | 0.17 | Hammerschmidt and Fitzgerald (2006)5 |
| Winter flounder |
|||||
| Atlantic (aggregated) |
nr | nr | 0.03 | 0.06 | US EPA (2003) |
| Long Island Sound (NY) |
14 | 26.9 | 0.04 | 0.03 | Rolfhus et al. (2008)4,5 |
| Mid-Atlantic (NJ) |
58 | 37.0 | 0.06 | 0.05 | Burger and Gochfeld (2011)6 |
| North Atlantic (CT) |
41 | 23.6 | 0.02 | 0.03 | Hammerschmidt and Fitzgerald (2006)5 |
| North Atlantic (CT-NH) |
22 | 16.4 | 0.04 | 0.02 | US EPA (2016) |
| North Atlantic (MA) |
50 | 16.4 | 0.07 | 0.06 | Schwartz et al. (1996)7 |
n based on composite samples (3 fish per composite)
TL and total Hg represent scaled means across years
Size reported as whole-body wet weight and converted to TL
Mercury units converted from dry weight to wet weight
Mercury reported as methylmercury
TL assumed to be the median of the reported size range
Winter flounder reported as “legal-size” with some flounder as small as 25 cm included in analysis; Total Hg for literature value represents average concentration in flounder from Boston Harbor, Salem Harbor, and coastal MA
A thorough review of the literature identified 30 studies that documented size-dependent mercury concentrations in the muscle tissue of target fishes collected from other Atlantic and Gulf Coast regions (Florida to New Hampshire; Table 4). Total Hg concentrations of SNE target fishes at pre-defined (literature-reported) lengths were estimated using the Hg-TL regression models in this study (Table 2), after which and values were compared to the Hg levels in conspecifics from other geographic locations (Table 4). Accordingly, target fish Hg concentrations in this study differed from literature values, although no discernible spatial patterns in Hg contamination were evident, i.e., SNE fishes demonstrated both depressed and elevated contamination levels relative to conspecifics from other locations (Table 4). Discrepancies in site-specific Hg concentrations were most pronounced in striped bass and bluefish (Table 4), such that mean (± 1 SD) Hg concentrations of SNE fish differed by ~ 0.135 ± 0.113 ppm (46.5 ± 29.5% of total Hg) relative to fishes from other regions. Spatial variations in Hg concentrations were also evident in the other target species, albeit not to the extent observed in striped bass and bluefish (mean Hg difference = 0.027 ± 0.021 ppm; 32.2 ± 22.4% of total Hg) (Table 4). These collective results indicate that total Hg concentrations in target fishes from SNE waters, in most instances, do not reflect other localized or coast-wide contamination patterns. This reiterates the need for research focused at sufficiently small spatial scales (i.e., local assessments of Hg contamination). Such a dedicated effort will provide health officials and risk assessors with data required to make informed decisions regarding the safe consumption of local fishery resources.
3.3. Methodological constraints of survey data
Studies that rely on FFQs to ascertain the fish eating habits of a human subpopulation are subject to methodological problems that potentially confound the results (Burger, 2013); including, (1) the biased recruitment of survey participants, (2) participants’ having recall bias and, (3) participants’ misinterpreting or failing to complete the questionnaire. First, a key objective of this study was to quantify the fish eating habits of recreational anglers and their families during summer months (June to September), and thus this investigation was intentionally biased toward a sensitive subpopulation and time period when fish consumption rates are presumably elevated (Burger, 2013), and concomitantly, Hg exposure rates are maximal. Second, the ability of participants to accurately recall their fish eating habits during the previous summer may be constrained by this study’s design, especially for participants completing the survey in the spring (March, n = 99; recall = 6–9 months) relative to the fall (September, n = 272; recall = 0–3 months). However, FFQs that rely on self-reported information are the primary method used by investigators to determine the fish eating behavior of a human demographic (Burger, 2013), and it has been verified that consumption recall studies are reproducible, acceptable, and generate accurate data within the timeframe implemented in this investigation (Järvinen et al., 1993; US EPA, 1998a; Burger, 2013). Moreover, in this study, there were no statistical differences in the fish eating habits of participants completing the survey in the spring relative to the fall (Standard t-test: Fish consumption rate: t-value = –0.324, df = 369, p = 0.746; Percent target fish consumption: t-value = – 1.207, df = 369, p = 0.228), suggesting that the month of survey completion did not affect a person’s recollection of their fish eating behavior. Third, the FFQ disseminated in this study was intentionally simplified to facilitate a person’s willingness to participate and improve their comprehension of the questionnaire (10 questions; ~ 10-minute completion time), recognizing that extensive, complex surveys are often problematic with respect to low recruitment and failed interpretation (Burger, 2013). In this study, there was no evidence that participants misinterpreted the questionnaire (D. Taylor, personal observation), and the overwhelming majority of surveys were completed in their entirety (i.e., 98.4% of the surveys initiated were fully completed and used for subsequent analysis).
3.4. Demographics of survey participants
A total of 371 anglers (or family members) completed the FFQ (Table 5), of which 70.6% were male. The mean age of the participants was 53.0 ± 13.3 years (range = 18–81 years), with males being slightly older than females (males = 54.6 ± 13.6 years; females = 49.3 ± 12.0 years). Additionally, 34.9% of female participants were women of childbearing age (18–45 years). The overwhelming majority of participants self-identified as non-Hispanic (98.4%) and white (96.3%), while the remaining races included African American (1.3%), American Indian (1.3%), and Asian (1.1%). Survey participants almost exclusively resided in SNE states (97.6%), with the majority living in Rhode Island (72.8%), followed by Massachusetts (17.5%) and Connecticut (7.3%). Participants living in non-SNE (“other”) states resided in New Jersey, New York, New Hampshire, and Maine.
Table 5.
Number and percent (in parentheses) of survey participants that self-identified a given age, ethnicity, race, and state of residence.
| Male | Female | Combined | |
|---|---|---|---|
| Age | |||
| 18–45 | 58 (22.1) | 38 (34.9) | 96 (25.9) |
| 46–55 | 76 (29.0) | 40 (36.7) | 116 (31.3) |
| 56–65 | 67 (25.6) | 24 (22.0) | 91 (24.5) |
| 66+ | 61 (23.3) | 7 (6.4) | 68 (18.3) |
| Total | 262 | 109 | 371 |
| Ethnicity1 | |||
| Hispanic | 3 (1.2) | 3 (2.8) | 6 (1.6) |
| Not Hispanic | 257 (98.8) | 106 (97.2) | 363 (98.4) |
| Total | 260 | 109 | 369 |
| Race1 | |||
| American Indian | 4 (1.5) | 1 (0.9) | 5 (1.3) |
| Asian | 3 (1.1) | 1 (0.9) | 4 (1.1) |
| Black | 2 (0.8) | 3 (2.7) | 5 (1.3) |
| White | 257 (96.6) | 105 (95.5) | 362 (96.3) |
| Total | 266 | 110 | 376 |
| State of residence | |||
| Connecticut | 20 (7.6) | 7 (6.4) | 27 (7.3) |
| Massachusetts | 53 (20.2) | 12 (11.0) | 65 (17.5) |
| Rhode Island | 182 (69.5) | 88 (80.7) | 270 (72.8) |
| Other2 | 7 (2.7) | 2 (1.8) | 9 (2.4) |
| Total | 262 | 109 | 371 |
Ethnicity and race “totals” vary because some participants did not report ethnic information, or conversely, self-identified for more than one race designation.
“Other” states include New Jersey, New York, New Hampshire, and Maine
3.5. Fish eating habits of survey participants
The mean fish consumption rate of survey participants during summer months was 0.26 ± 0.19 meal/day (Table 6), with meals including all types of finfish and shellfish. The majority of these participants were high-end fish consumers (78.4% eating ≥ 1 fish meal/week), whereas a very low percentage of individuals infrequently or avoided eating fish (5.4% eating ≤ 1 fish meal/3 months) (Fig. 4A). Rates of fish consumption did not statistically differ as a function of the participant’s state of residency or ethnicity and race (Table 7). The latter contradicts previous findings that recreational and subsistence anglers of certain ethnic and racial groups have exceedingly high fish consumption rates, including Hispanics (Burger and Gochfeld, 1991; Burger et al., 1992), African Americans (Burger et al., 1999a; 1999b), American Indians (Burger, 1999), and Asians (Burger et al., 1999a). The absence of an ethnic-race effect on fish consumption patterns in this study is likely the result of low samples sizes for the minority groupings, thereby precluding robust statistical analyses. In contrast, fish consumption rates of participants did vary across age and gender categories (Table 7). Specifically, participants ≥ 66 years of age consumed significantly more fish meals than the 18–45 age-class (66+ = 0.29 ± 0.20 meal/day; 18–45 = 0.22 ± 0.19 meal/day), and females ate more fish meals than males (female = 0.29 ± 0.21 meal/day; male = 0.25 ± 0.18 meal/day). The mean fish consumption rate of participants in this study was significantly greater than literature values reported for national and regional coastal populations, and equivalent to the high-end fish eating habits of New York and New Jersey harbor anglers (Table 3). Therefore, the FFQ results presented herein categorize SNE anglers (and family members) as an “at-risk” subpopulation that may be highly exposed to Hg due to their elevated rates of fish consumption.
Table 6.
Mean (± 1 SD) fish consumption rates (meals/day) and mercury exposure (mg Hg/kg human body weight/day) of survey participants as a function of gender, age, ethnicity, race, and state of residence. The percent of participants in a demographic category with Hg exposure rates exceeding the US EPA reference dose (1.0×10−4 mg Hg/kg body weight/day) are also presented in parentheses. Sample sizes for each demographic category are presented in Table 5.
| Fish consumption rate | Hg exposure rate | |||||
|---|---|---|---|---|---|---|
| Male | Female | Combined | Male | Female | Combined | |
| Age | ||||||
| 18–45 | 0.21 ± 0.17 |
0.25 ± 0.22 |
0.22 ± 0.19 |
1.28×10−4 ± 1.21 ×10−4 (41.4) |
1.49×10−4 ± 1.61×10−4 (50.0) |
1.36×10−4 ± 1.38×10−4 (44.8) |
| 46–55 | 0.24 ± 0.17 |
0.27 ± 0.19 |
0.25 ± 0.18 |
1.38×10−4 ± 1.31×10−4 (47.4) |
1.40×10−4 ± 1.37×10−4 (50.0) |
1.39×10−4 ± 1.32×10−4 (48.8) |
| 56–65 | 0.27 ± 0.20 |
0.35 ± 0.22 |
0.29 ± 0.20 |
1.49×10−4 ± 1.24×10−4 (58.2) |
1.54×10−4 ± 1.22×10−4 (54.2) |
1.50×10−4 ± 1.23×10−4 (57.1) |
| 66+ | 0.28 ± 0.20 |
0.37 ± 0.26 |
0.29 ± 0.20 |
1.56×10−4 ± 1.07×10−4 (60.7) |
1.36×10−4 ± 1.26×10−4 (42.9) |
1.54×10−4 ± 1.08×10 −4 (58.8) |
| Ethnicity | ||||||
| Hispanic | 0.36 ± 0.00 |
0.06 ± 0.08 |
0.21 ± 0.17 |
2.53×10−4 ± 5.71 ×10−5 (100 ) |
2.17×10−5 ± 2.97×10−5 (0.0) |
1.37×10−4 ± 1.33×10−4 (50.0) |
| Not Hispanic | 0.25 ± 0.18 |
0.29 ± 0.21 |
0.26 ± 0.19 |
1.42×10−4 ± 1.21×10−4 (51.8) |
1.50×10−4 ± 1.41×10−4 (51.9) |
1.44×10−4 ± 1.27×10−4 (51.8) |
| Race | ||||||
| American Indian |
0.24 ± 0.14 |
0.14 | 0.22 ± 0.13 |
1.80×10−4 ± 1.04 ×10−4 (75.0) |
1.97×10−5 (0.0) | 1.48×10−4 ± 1.15×10−4 (60.0) |
| Asian | 0.29 ± 0.12 |
0.36 | 0.30 ± 0.11 |
1.43×10−4 ± 1.22×10−4 (33.3) |
2.60×10−4 (100) | 1.72×10−4 ± 1.15×10−4 (50.0) |
| African American |
0.36 ± 0.00 |
0.19 ± 0.14 |
0.26 ± 0.14 |
2.55×10−4 ± 3.96×10−5 (100) |
1.08×10−4 ± 8.32×10−5 (33.3) |
1.67×10−4 ± 1.02×10−4 (60.0) |
| White | 0.25 ± 0.18 |
0.29 ± 0.22 |
0.26 ± 0.19 |
1.43×10−4 ± 1.22×10−4 (52.1) |
1.46×10−4 ± 1.42×10−4 (50.5) |
1.44×10−4 ± 1.28×10−4 (51.7) |
| State | ||||||
| Connecticut | 0.29 ± 0.23 |
0.45 ± 0.26 |
0.33 ± 0.25 |
1.43×10−4 ± 9.82×10−5 (52.7) |
2.47×10−4 ± 1.59×10−4 (48.9) |
1.70×10−4 ± 1.23×10−4 (51.5) |
| Massachusetts | 0.23 ± 0.18 |
0.25 ± 0.20 |
0.24 ± 0.18 |
1.28×10−4 ± 1.18×10−4 (47.2) |
1.45×10−4 ± 1.23×10−4 (58.3) |
1.31×10−4 ± 1.18×10−4 (49.2) |
| Rhode Island | 0.25 ± 0.18 |
0.28 ± 0.21 |
0.26 ± 0.19 |
1.46×10−4 ± 1.25×10−4 (55.0) |
1.41×10−4 ± 1.40×10−4 (71.4) |
1.44×10−4 ± 1.30×10−4 (59.3) |
| Other | 0.33 ± 0 08 |
0.08 ± 0 09 |
0.27 ± 014 |
1.77×10−4 ± 106×10−4 (57 1) |
2.40×10−5 ± 2 37×10−5 (0 0) |
1.43×10−4 ± 1.14×10−4 (44 4) |
Fig. 4.
Percent of male and female survey participants that reportedly consumed finfish/shellfish at a particular frequency (A), percent of fish meals comprised of one or more target fishes (B), and percent of participants consuming a target species at least once during the summer (C). Target fishes include summer flounder (SF), striped bass (SB), black sea bass (BS), bluefish (BF), scup (SC), tautog (TG), and winter flounder (WF).
Table 7.
Summary statistics for analysis of variance (ANOVA) models used to examine the effects of age, gender, and state of residence (3-way ANOVA) and ethnicity and race (2-way ANOVA) on survey participants’ fish consumption rates (“total” fish meals/day and “target” fish meals/day), percent of fish meals comprised of target fishes, and Hg exposure rates (mg Hg/kg body weight/day). Significant contrasts are also presented.
| Factor | Age | Gender | State | Contrasts | |||
|---|---|---|---|---|---|---|---|
| F (df) | p | F (df) | p | F (df) | p | ||
| Fish eating rate (total) |
3.48 (3) | < 0.05 | 4.64 (1) | < 0.05 | 1.10 (3) | 0.347 | 66+ > 18- 45; Female > Male |
| Fish eating rate (target) |
0.79 (3) | 0.501 | 0.11 (1) | 0.736 | 0.68 (3) | 0.565 | – |
| Percent target fish |
1.04 (3) | 0.374 | 5.00 (1) | < 0.05 | 1.01 (3) | 0.386 | Male > Female |
| Factor | Ethnicity | Race | Contrasts | ||||
| F (df) | p | F (df) | p | ||||
| Fish eating rate (total) |
0.51 (1) | 0.475 | 0.18 (3) | 0.907 | – | ||
| Fish eating rate (target) |
0.03 (1) | 0.856 | 0.76 (3) | 0.516 | – | ||
| Percent target fish |
0.39 (1) | 0.534 | 1.78 (3) | 0.150 | – | ||
| Hg exposure rate |
0.02 (1) | 0.895 | 0.12 (3) | 0.951 | – |
Among all survey participants, the mean percent of fish meals represented by target species was 66.7 ± 32.1%. Moreover, 76.8% of participants indicated that the majority of their fish meals were comprised of one or more of the target species (≥ 50%), and 34.8% reported that their fish meals were exclusively these species (100%) (Fig. 4B). Conversely, 6.5% of participants recounted having not eaten a target fish during the previous summer (Fig. 4B), even if finfish/shellfish constituted a portion of their overall diet. The mean percent of fish meals represented by target fishes did not statistically differ by the participant’s demographic characteristics, with the exception of gender (Table 7; Fig. 4B), whereby males ate a higher percentage of target fishes as compared to females (68.8 ± 31.7% and 61.7 ± 32.4%, respectively). The rate of target fish consumption (target fish meals/day) was calculated as the product of total fish consumption per day and the proportion of fish meals comprised of target species. Accordingly, the mean rate of target fish consumption across participants equaled 0.18 ± 0.17 meal/day. This rate of preferred fish consumption did not statistically differ across the demographic features analyzed in this study (Table 7).
All target fishes in this study were consumed by SNE anglers and their families, with summer flounder, striped bass, and black sea bass representing the most frequently eaten species (68.7%, 59.8%, and 46.6% of participants consumed these species, respectively), followed by bluefish (34.0%), scup (28.0%), tautog (24.5%), and winter flounder (13.5%) (Fig. 4C). Patterns of preferred fish consumption were consistent across the demographic characteristics of the participants, including gender; although males reportedly ate a higher percentage of target fishes relative to females, irrespective of species-type (Fig. 4C). Further, males, on average, consumed a greater number of target species throughout the summer (mean number of species eaten = 3.1 ± 1.8) compared to females (2.0 ± 1.7) (Standard t-test: t-value = 4.98, df = 369, p < 0.0001). The preferred consumption of target species in this study also closely corresponded to the fish eating habits of New Jersey saltwater anglers (n = 321); such that the percent of these anglers eating a given (self-caught) fish was reported as: summer flounder (75%), striped bass (57%), black sea bass (42%), bluefish (36%), and tautog (27%) (Burger, 2013).
3.6. Mercury exposure analysis of Southern New England anglers and their families
Geographic-specific data from this study, including fish Hg levels and human fish eating habits, were incorporated into an exposure assessment model (equation 1), which in turn evaluated a person’s daily exposure to Hg. Patterns of fish consumption, as determined from self-reported survey data, are routinely used to estimate Hg exposure rates in a human demographic of interest (Burger, 2013). Moreover, numerous studies assert that human Hg body burdens are highly correlated with fish dietary intake (Airey, 1983; Burger, 2000 and references therein), including significant linkages between fish consumption rates and Hg levels in human blood (Knobeloch et al., 2005; 2007), hair (Johnsoon et al., 2004; Schoeman et al., 2010), and umbilical cord blood and tissue (Jedrychowski et al., 2007; Sakamoto et al., 2007). Accordingly, the modeling exercise employed in this study ostensibly approximates Hg exposure rates in SNE anglers and their families, and these data, in turn, support initial risk assessment and management decisions related to the issuance of fish consumption advisories.
The mean Hg exposure rate of participants in this study, derived from fish consumption, equaled 1.44×10−4 ± 1.27×10−4 mg Hg/kg body weight/day (range = 0.00–7.84×10−4 mg Hg/kg body weight/day) (Table 6; Fig. 5), and exposure rates did not differ by demographic characteristics (Tables 6 and 7). Participant Hg exposure rates, however, were significantly higher (~ 23–89%) than values reported for other coastal populations, including the Atlantic, Pacific, Gulf of Mexico, Great Lakes, and New Jersey saltwater anglers (Table 3). Further, mean Hg exposure rates from this study were significantly greater than the US EPA RfD of 1.0×10−4 mg Hg/kg body weight/day (Table 3). More specifically, 51.5% of all participants had exposure rates exceeding the RfD, including 50.0% of women of childbearing years (18–45) (Table 6; Fig. 5). These results are noteworthy given that numerous epidemiology studies have demonstrated Hg neurotoxicity in adults and children from low-dose exposure (Myers et al., 2000; Yokoo et al., 2003). Adults exposed to low levels of Hg, for example, are at risk for deficits in neuropsychological function and possible increased risk of cardiovascular disease (Yokoo et al., 2003; Virtanen et al., 2005; Choi et al., 2009). Chronic, low-dose prenatal Hg exposure from maternal consumption of fish also causes notable end points of neurotoxicity in children, including poor performance on neurobehavioral tests, particularly tests of attention, fine-motor function, language, visual-spatial abilities, and verbal memory (Kjellstrom et al., 1986; Davidson et al., 1995; Grandjean et al., 1997; Myers et al., 2000).
Fig. 5.
Mercury exposure rates (mg Hg/kg body weight/day) for survey participants as a function of gender and age class (box plot illustrating median, 1st and 3rd quartiles, and maximum and minimum values). The horizontal dashed line demarcates the US EPA Hg reference dose of 1.0×10−4 mg Hg/kg body weight/day.
The elevated Hg exposure rates of SNE anglers and family members are attributed to the high Hg content of several target species (e.g., striped bass, bluefish, and tautog; Table 1; Figs. 2 and 3A–C) and the frequent dietary intake of fish (Tables 3 and 6), and similar causations have been reported for anglers in other geographic areas (Al-Majed and Preston, 2000; Kosatsky et al., 2000; Dellinger, 2004; Gobeille et al., 2006; Knobeloch et al., 2007). It is important to note, however, that exposure assessments in this study may overestimate the annual risk due to artifacts of the FFQ design. For example, the FFQ implemented in this study evaluated the fish eating habits of survey participants during the peak angling season (June to September), when dietary intake of self-caught fishes and corresponding Hg exposure rates are presumably maximal. Conversely, if recreationally-caught fishes are routinely frozen and consumed throughout the year (or if consumption of purchased fish increases during non-angling periods), seasonal variations in Hg exposure rates will be less pronounced or absent. Burger (2013), however, determined that New Jersey saltwater anglers limited their consumption of self-caught fishes to 2–6 months per year, and the frequency of other fish consumption (i.e., purchased commercially or from restaurants) did not exceed 10 months per year. Consequently, Hg exposure rates in New Jersey anglers were not consistent throughout the year (highest in summer; Burger, 2013). Comparable intra-annual variations in Hg exposure likely occur for SNE anglers and their families; thus studies conducted exclusively during the summer may overestimate contaminant exposure when rates are projected for the entire year.
Alternatively, several assumptions of the assessment model used in this study may underestimate the extent of Hg exposure in survey participants. First, the assumed Hg content of “other” fish (non-target finfish/shellfish; HgO = 0.041 ppm) was estimated using human Hg exposure and fish consumption data documented for the broader Atlantic coastal population (Mahaffey et al., 2009). This presumed level of Hg contamination is consistent with values reported for some commonly-consumed finfish and shellfish species in the northeastern US, including gadids (Atlantic haddock, Melanogrammus aeglefinus = 0.06 ppm; pollock, Pollachius spp. = 0.03 ppm; US FDA, 2016a), salmon (Salmo and Oncorhynchus spp. = 0.01–0.03 ppm; Burger, 2013; US FDA, 2016a), crustaceans (mixed spp.: shrimp = 0.01 ppm; crab = 0.07 ppm; US FDA, 2016a), and bivalves (blue mussel, Mytilus edulis = 0.06 ppm; American oyster, Crassostrea virginica = 0.04 ppm; US EPA, 2003). However, other frequently eaten fishes have Hg concentrations far exceeding the default HgO value, including tuna (Thunnus spp. = 0.5–1.2 ppm; US EPA, 2003; Burger, 2013; Teffer et al., 2014), swordfish (Xiphias gladius = 1.0–1.4 ppm; Burger, 2013; US FDA, 2016a), and sharks (mixed spp. = 0.5–1.8 ppm; US EPA, 2003; Burger, 2013; Teffer et al., 2014; US FDA, 2016a); thus, preferential consumption of these highly contaminated species would result in vastly elevated Hg exposure rates. Second, it is customary for exposure assessment studies to use a default meal size of 8 ounces (M = 0.227 kg; US EPA, 2001; Skinner et al., 2007). The mean meal size for New Jersey saltwater anglers consuming flounder, striped bass, black sea bass, bluefish, and tautog, however, was reportedly equal to 9.5 ± 0.5 ounces (Burger, 2013). At this larger meal size, as opposed to the more conservative 8 ounce estimate, SNE anglers and their families would experience a 15.1% increase in their estimated Hg exposure rate (mean = 1.69×10−4 ± 1.51×10−4 mg Hg/kg body weight/day).
4. Conclusions: Developing and implementing more effective consumption advisories
Federal and state agencies provide dietary advice and public health recommendations for human fish consumption in an effort to limit exposure to environmental toxicants [e.g., Hg, polycyclic aromatic hydrocarbons (PAHs), and polychlorinated biphenyls (PCBs); RIDOH, 2016; US FDA, 2016b]. The successful development and implementation of consumption advisories for marine and estuarine fishes, however, are constrained by several factors. State authorities, for example, often develop advisories by deferring to federal monitoring programs that integrate measurements of fish Hg contamination over large spatial scales (e.g., broadly coastal or nationally aggregated data) (RIDOH, 2016; US FDA, 2016a). Further, federal agencies routinely report Hg concentrations in marine and estuarine fishes using broad taxonomic units, which include ambiguous groupings such as “flounder”, “shark”, and “tuna” (Payne and Taylor, 2010; US FDA, 2016a). Generalized taxonomic units, however, may be comprised of several fish species with unique life history characteristics (e.g., growth patterns, longevity, and trophic ecology), which in turn affect intraspecific Hg concentrations (Piraino and Taylor, 2009; Payne and Taylor, 2010; Szczebak and Taylor, 2011). Results from this study affirmed that total Hg concentrations of recreationally-important fishes from SNE waters were not indicative of other localized or coast-wide contamination patterns (Tables 3 and 4). Further, Hg contamination differed significantly across species (e.g., flounder spp.; Table 1; Fig. 2). Correspondingly, state consumption advisories for marine and estuarine fishes must be predicated on spatially-explicit data (i.e., local versus regional/national scales) and species-specific data (i.e., disentangling ambiguously defined taxonomic groups) in order to account for small-scale spatial variations in intraspecific Hg concentrations.
State consumption advisories based on the fish eating habits of the general population fail to recognize that sensitive subpopulations frequently have high dietary intake of fishery resources. Economic status, ethnic and cultural identity, coastal residency, and fishing practices (recreational or subsistence), for example, are causative factors affecting the fish eating behavior of specific demographic groups, and thus their rates of Hg exposure (McKelvey et al., 2007; Mahaffey et al., 2009; Lincoln et al., 2011; this study). Moreover, for many of the abovementioned subpopulations, consumption advisories are not effectively reported. Survey research shows that awareness of advisories was lowest among women, African Americans, senior citizens, people age 15–19, and people with less than a high school education (Katner et al., 2011). Therefore, reporting methods for advisories must be improved, especially for high-risk populations, e.g., informational material for women should be provided to health clinics, pediatricians and gynecologists. For recreational anglers, advisories should be effectively displayed on signs at bait shops, landings, boat launches, and angling locations and fishing licenses should include contact information for advisory reports (Katner et al., 2011).
Finally, consumption advisories focused exclusively on fish Hg contamination (or PAHs and PCBs) are limited because they neglect to account for the co-occurrence of other elements and nutrients in fish tissue that counteract Hg toxicity. For example, fish are an excellent source of dietary fatty acids (e.g., omega-3 fatty acids) that provide numerous health benefits to the human consumer, including lowering of blood cholesterol, reducing the incidence of heart disease, stroke, and pre-term delivery, and improving cognitive development (Daviglus et al., 2002; Bouzan et al., 2005; Cohen et al., 2005; Konig et al., 2005; McMichael and Butler, 2005; Willett, 2005). Problematically, consumption advisories and associated reports routinely emphasize fish species that are identified as high-risk for Hg contamination, and thus, there is sparse information for presumed low-risk species. This undermines the health benefits provided by fish that pose little threat to the health of fish-consuming citizens (e.g., scup and winter flounder; Table 3; Figs. 2 and 3). Measurements of intraspecific fatty acid concentrations will provide a synoptic examination of the benefits provided by fish consumption. Acquiring these data, in turn, will support public health risk-benefit assessments and management decisions related to the issuance of fish consumption advisories and guidelines.
Highlights.
Mercury of harvestable fish varied by species and often exceeded advisory values.
Mercury in target fish muscle tissue increased with total length.
Recreational anglers and families self-reported high levels of fish consumption.
Anglers and families often had mercury exposure exceeding reference dose values.
Geographic-specific data are needed to develop meaningful consumption advisories.
Acknowledgments
We are grateful to S. Olszewski (Rhode Island Division of Fish and Wildlife, Jamestown, RI), B. Bourque, and numerous undergraduate research assistants (Roger Williams University, Bristol, RI) for their efforts in sample collection and preparation. We also thank P. Brown (Northeastern University, Boston, MA), L. Schaider (Silent Spring Institute, Newton, MA), and S. Medeiros (Rhode Island Saltwater Anglers Association, Coventry, RI) for assistance with the design and dissemination of the food frequency questionnaire. We are grateful to R. Vanderslice (Rhode Island Department of Health, Office of Environmental Health Risk, Providence, RI) for insights regarding state issuance of consumption advisories. Some of the data described in this manuscript were produced by the US Environmental Protection Agency through its Environmental Monitoring and Assessment Program (EMPA). The project described herein was supported by the Roger Williams University Foundation Fund Based Research Grant, the Rhode Island Recreational Fishing Advancement Grant, and by Award Number P20RR016457 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Human subjects: The food frequency questionnaire and survey consent form used in this study was pre-approved by the Roger Williams University Institutional Review Board (approved 6 September 2012). Further, all human participants in this study signed a consent form prior to completing the food frequency questionnaire.
Experimental animals: The collection and subsequent sacrificing of fishes used in this study were pre-approved by the Roger Williams University Institutional Animal Care and Use Committee (IACUC) (Animal Welfare Assurance No. = A3690-01).
References
- Aberg B, Ekman L, Falk R, Greitz U, Persson G, Snihs J. Metabolism of methyl mercury (203Hg) compounds in man: Excretion and distribution. Arch. Environ. Health. 1969;19:478–484. doi: 10.1080/00039896.1969.10666872. [DOI] [PubMed] [Google Scholar]
- Airey D. Total mercury concentrations in human hair from 13 countries in relation to fish consumption and location. Sci. Total Environ. 1983;180:157–180. doi: 10.1016/0048-9697(83)90067-0. [DOI] [PubMed] [Google Scholar]
- Al-Majed NB, Preston MR. Factors influencing the total mercury and methylmercury in the hair of the fishermen of Kuwait. Environ. Pollut. 2000;109:239–250. doi: 10.1016/s0269-7491(99)00261-4. [DOI] [PubMed] [Google Scholar]
- Birkeland C, Dayton PK. The importance in fishery management of leaving the big ones. Trends Ecol. Evolut. 2005;20:356–358. doi: 10.1016/j.tree.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Bloom NS. On the chemical form of mercury in edible fish and marine invertebrate tissue. Can. J. Fish. Aquat. Soc. 1992;49:1010–1017. [Google Scholar]
- Bouzan C, Cohen JT, Connor WE, Kris-Etherton PM, Gray GM, Konis A, Lawrence RS, Savitz DA, Teutsch SM. A quantitative analysis of fish consumption and stroke risk. Am. J. Prev. Med. 2005;29:347–352. doi: 10.1016/j.amepre.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Brim MS, Alam SK, Jenkins LG. Organochlorine pesticides and heavy metals in muscle and ovaries of Gulf coast striped bass (Morone saxatilis) from the Apalachicola River, Florida, USA. J. Environ. Sci. Health, Part B. 2001;36:15–27. doi: 10.1081/pfc-100000913. [DOI] [PubMed] [Google Scholar]
- Burger J. American Indians, hunting and fishing rates, risk and the Idaho National Engineering and Environmental Laboratory. Environ. Res. 1999;80:319–329. doi: 10.1006/enrs.1998.3923. [DOI] [PubMed] [Google Scholar]
- Burger J. Consumption advisories and compliance: The fishing public and the deamplification of risk. J. Environ. Plan. Manage. 2000;43:471–488. [Google Scholar]
- Burger J. Risk to consumers from mercury in bluefish (Pomatomus saltatrix) from New Jersey: Size, season and geographical effects. Envrion. Res. 2009;109:803–811. doi: 10.1016/j.envres.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J. Role of self-caught fish in total fish consumption rates for recreational fishermen: Average consumption for some species exceeds allowable intake. J. Risk Res. 2013;16:1057–1075. doi: 10.1080/13669877.2013.788546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Cooper K, Gochfeld M. Exposure assessment for heavy metal ingestion from a sport fish in Puerto Rico: Estimating risk for local fishermen. J. Toxicol. Environ. Health. 1992;36:355–365. doi: 10.1080/15287399209531644. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Fishing a superfund site: Dissonance and risk perception of environmental hazards by fishermen in Puerto Rico. Risk Anal. 1991;11:269–277. doi: 10.1111/j.1539-6924.1991.tb00603.x. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Mercury and selenium levels in 19 species of saltwater fish from New Jersey as a function of species, size, and season. Sci. Total Environ. 2011;409:1418–1429. doi: 10.1016/j.scitotenv.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Pflugh KK, Lurig L, Von Hagen LA, Von Hagen S. Fishing in urban New Jersey: Ethnicity affects information sources, perception, and compliance. Risk Anal. 1999a;19:217–229. doi: 10.1023/a:1006921610468. [DOI] [PubMed] [Google Scholar]
- Burger J, Stephens WL, Jr, Boring CS, Kuklinski M, Gibbons JW, Gochfeld M. Factors in exposure assessment: Ethnic and socioeconomic differences in fishing and consumption of fish caught along the Savannah River. Risk Anal. 1999b;19:427–438. doi: 10.1023/a:1007048628467. [DOI] [PubMed] [Google Scholar]
- Chen C, Amirbahman A, Fisher N, Harding G, Lamborg C, Nacci D, Taylor D. Methylmercury in marine ecosystems: Spatial patterns and processes of production, bioaccumulation, and biomagnification. EcoHealth. 2008;5:399–408. doi: 10.1007/s10393-008-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi AL, Weihe P, Budtz-Jørgensen E, Jørgensen PJ, Salonen JT, Tuomainen TP, Murata K, Nielsen HP, Petersen MS, Askham J, Grandjean P. Methylmercury exposure and adverse cardiovascular effects in Faroese whaling men. Environ. Health Perspect. 2009;117:367–372. doi: 10.1289/ehp.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JT, Bellinger DC, Connor WE, Shaywitz BA. A quantitative analysis of prenatal intake of n-3 polyunsaturated fatty acids and cognitive development. Am. J. Prev. Med. 2005;29:366–374. doi: 10.1016/j.amepre.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Cross FA, Evans DW, Barber RT. Decadal declines of mercury in adult bluefish (1972–2011) from the Mid-Atlantic Coast of the USA. Environ. Sci. Technol. 2015;49:9064–9072. doi: 10.1021/acs.est.5b01953. [DOI] [PubMed] [Google Scholar]
- Davidson P, Myers G, Cox C, Shamlaye C, Marsh D, Tanner M, Berlin M, Sloane-Reeves J, Cernichiari E, Choisy O, Choi A, Clarkson T. Longitudinal neurodevelopmental study of Seychellois children following in utero exposure to methylmercury from maternal fish ingestion: outcomes at 19 and 29 months. Neurotoxicology. 1995;16:677–688. [PubMed] [Google Scholar]
- Daviglus M, Sheeshka J, Murkin E. Health benefits from eating fish. Comments Toxicol. 2002;8:345–374. [Google Scholar]
- Day RW, Quinn GP. Comparisons of treatments after an analysis of variance in ecology. Ecol. Monogr. 1989;59:433–463. [Google Scholar]
- Dellinger JA. Exposure assessment and initial intervention regarding fish consumption of tribal members of the Upper Great Lakes Region in the United States. Environ. Res. 2004;95:325–340. doi: 10.1016/j.envres.2003.07.012. [DOI] [PubMed] [Google Scholar]
- Deshpande AD, Draxler AFJ, Zdanowicz VS, Schrock ME, Paulson AJ, Finneran TW, Scharack BL, Corbo K, Arlen L, Leimburg EA, Dockum BW, Pikanowski RA, May B, Rosman L. Contaminant levels in muscle of four species of recreation fish from the New York Bight Apex. Woods Hole, Massachusetts: NOAA Tech Mem NMFS-NE-157 National Marine Fisheries Service; 2000. [Google Scholar]
- Gobeille AK, Morland KB, Bopp RF, Godbold JH, Landrigan PJ. Body burdens of mercury in lower Hudson River area anglers. Environ. Res. 2006;101:205–212. doi: 10.1016/j.envres.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White R, Debes F, Arak S, Yokoyama K, Murata K, Sorensen N, Dahl R, Jorgensen P. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol. Teratol. 1997;20:1–12. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt CR, Fitzgerald WF. Bioaccumulation and trophic transfer of methylmercury in Long Island Sound. Arch. Environ. Contam. Toxicol. 2006;51:416–424. doi: 10.1007/s00244-005-0265-7. [DOI] [PubMed] [Google Scholar]
- Harvey J, Harwell L, Summers JK. Contaminant concentrations in whole-body fish and shellfish from US estuaries. Environ. Monit. Assess. 2008;137:403–412. doi: 10.1007/s10661-007-9776-1. [DOI] [PubMed] [Google Scholar]
- Hightower JM, Moore D. Mercury levels in high-end consumers of fish. Environ. Health Perspect. 2003;111:1–6. doi: 10.1289/ehp.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen R, Seppänen R, Knekt P. Short-term and long-term reproducibility of dietary history interview data. Int. J. Epidemiol. 1993;22:520–527. doi: 10.1093/ije/22.3.520. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, Perera F, Jankowski J, Rauh V, Flak E, Caldwell KL, Jones RL, Pac A, Lisowska-Miszczyk I. Fish consumption in pregnancy, cord blood mercury level and cognitive and psychomotor development of infants followed over the first three years of life: Krakow epidemiologic study. Environ. Int. 2007;33:1057–1062. doi: 10.1016/j.envint.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Johnsoon C, Sallsten G, Schutz A, Barregard I. Hair mercury levels versus freshwater consumption in household members of Swedish angling societies. Environ. Res. 2004;96:257–263. doi: 10.1016/j.envres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Karouna-Reneir NK, Rao KR, Lanza JJ, Rivers SD, Wilson PA, Hodges DK, Levine KE, Ross GT. Mercury levels and fish consumption practices in women of child-bearing age in the Florida Panhandle. Environ. Res. 2008;108:320–326. doi: 10.1016/j.envres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Katner A, Ogunyinka E, Sun MH, Soileau S, Lavergne D, Dugas D, Suffet M. Fishing, fish consumption and advisory awareness among Louisiana’s recreational fishers. Environ. Res. 2011;111:1037–1045. doi: 10.1016/j.envres.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Kjellstrom T, Kennedy P, Wallis S, Stewart A, Friberg L, Lind B, Wutherspoon T, Mantell C. Stage 2: Interviews and psychological tests at age 6. Solna. Sweden: National Swedish Environmental Protection Board, Report 3642; 1986. Physical and mental development of children with prenatal exposure to mercury from fish. [Google Scholar]
- Knobeloch L, Anderson HA, Imm P, Peters D, Smith A. Fish consumption, advisory awareness, and hair mercury levels among women of childbearing age. Environ. Res. 2005;97:220–227. doi: 10.1016/j.envres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Knobeloch L, Gliori G, Anderson H. Assessment of methylmercury exposure in Wisconsin. Environ. Res. 2007;103:205–210. doi: 10.1016/j.envres.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Konig Q, Bouzan C, Cohen JT, Connor WE, Kris-Etherton PM, Gray GM, Lawrence RS, Savitz DA, Teutsch SM. A quantitative analysis of fish consumption and coronary heart disease mortality. Am. J. Prev. Med. 2005;29:335–346. doi: 10.1016/j.amepre.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kosatsky T, Przybysz R, Armstrong B. Mercury exposure in Montrealers who eat St. Lawrence River sportfish. Environ. Res. 2000;84:36–43. doi: 10.1006/enrs.2000.4073. [DOI] [PubMed] [Google Scholar]
- Lincoln RA, Shine JP, Chesney EJ, Vorhees DJ, Grandjean P, Senn DB. Fish consumption and mercury exposure among Louisiana recreational anglers. Environ. Health Perspect. 2011;119:245–251. doi: 10.1289/ehp.1002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey KR, Clickner RP, Jeffries RA. Adult women’s blood mercury concentrations vary regionally in the United States: Association with patterns of fish consumption (NHANES 1999–2004) Environ. Health Perspect. 2009;117:47–53. doi: 10.1289/ehp.11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RP, Heyes D, Sveinsdottir A. Methylmercury concentrations in fish from tidal waters of the Chesapeake Bay. Arch. Environ. Contam. Toxicol. 2006;51:425–437. doi: 10.1007/s00244-004-0230-x. [DOI] [PubMed] [Google Scholar]
- May H, Burger J. Fishing in a polluted estuary: Fishing behavior, fish consumption, and potential risk. Risk Anal. 1996;16:459–471. doi: 10.1111/j.1539-6924.1996.tb01093.x. [DOI] [PubMed] [Google Scholar]
- McKelvey W, Gwynn RC, Jeffery N, Kass D, Thorpe LE, Garg RK, Palmer CD, Parsons PJ. A biomonitoring study of lead, cadmium, and mercury in the blood of New York City adults. Environ. Health Perspect. 2007;115:1435–1441. doi: 10.1289/ehp.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ, Butler CD. Fish, health, and sustainability. Am. J. Prev. Med. 2005;29:322–323. doi: 10.1016/j.amepre.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Moszcynski P, Lisiewicz J, Bartus R. The serum immunoglobulins in workers after prolonged occupational exposure to the mercury vapors. Rev. Roum. Med. Intern. 1990;28:25–30. [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Palumbo D, Shamlaye C, Cox C, Chernichiari E, Clarkson TW. Secondary analysis from the Seychelles child development study: The child behavior checklist. Environ. Res. Section A. 2000;84:12–19. doi: 10.1006/enrs.2000.4085. [DOI] [PubMed] [Google Scholar]
- National Marine Fisheries Service (NMFS) [Accessed 20 April 2016];Fisheries Statistics Division. 2016 http://www.st.nmfs.noaa.gov/st1/
- Payne EJ, Taylor DL. Effects of diet composition and trophic structure on mercury bioaccumulation in temperate flatfishes. Arch. Environ. Contam. Toxicol. 2010;58:431–443. doi: 10.1007/s00244-009-9423-7. [DOI] [PubMed] [Google Scholar]
- Piraino MP, Taylor DL. Bioaccumulation and trophic transfer of mercury in striped bass (Morone saxatilis) and tautog (Tautoga onitis) in the Narragansett Bay (Rhode Island, USA) Mar. Environ. Res. 2009;67:117–128. doi: 10.1016/j.marenvres.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Piraino MP, Taylor DL. Assessment of nonlethal methods for predicting muscle tissue mercury concentrations in coastal marine fishes. Arch. Environ. Contam. Toxicol. 2013;65:715–723. doi: 10.1007/s00244-013-9946-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Jessop BM, Coffin J, Swetnam DA. Mercury and polychlorinated biphenyls in striped bass (Morons saxatilis) from two nova Scotia Rivers. Water Air Soil Pollut. 1984;21:15–23. [Google Scholar]
- Rhode Island Department of Environmental Management (RIDEM) [Accessed 20 April 2016];Marine fisheries minimum sizes & possession limits. 2016 http://www.dem.ri.gov/programs/bnatres/fishwild/mfsizes.htm.
- Rhode Island Department of Health (RIDOH) [Accessed 1 August];Mercury poisoning: About fish. 2016 http://www.health.ri.gov/healthrisks/poisoning/mercury/about/fish/
- Rolfhus K, Sandheinrich M, Wiener J, Bailey S, Thoreson K, Hammerschmidt C. Analysis of fin as a nonlethal method for monitoring mercury in fish. Environ. Sci. Tech. 2008;42:871–877. doi: 10.1021/es071427+. [DOI] [PubMed] [Google Scholar]
- Sackett DK, Cope WG, Rice JA, Aday DD. The influence of fish length on tissue mercury dynamics: Implications for natural resource management and human health risk. Int. J. Environ. Public Health. 2013;10:638–659. doi: 10.3390/ijerph10020638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M, Kaneoka T, Murata K, Nakai K, Satoh H, Akagi H. Correlations between mercury concentration in umbilical cord tissue and other biomarkers of fetal exposure to methylmercury in the Japanese populations. Environ. Res. 2007;103:106–111. doi: 10.1016/j.envres.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Salonen JT, Seppänen K, Nyyssçnen K, Korpela H, Kauhanen J, Kantola M, Tuomilehto J, Esterbauer H, Tatzber F, Salonen R. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation. 1995;91:645–655. doi: 10.1161/01.cir.91.3.645. [DOI] [PubMed] [Google Scholar]
- Schwartz JP, Duston NM, Batdorf CA. Metal concentrations in winter flounder, American lobster, and bivalve molluscs from Boston Harbor, Salem Harbor and coastal Massachusetts: A summary of data on tissues collected from 1986 to 1991: In: Sherman K, Jaworski NA, Smayda TJ, editors. The Northeast Shelf Ecosystem: Assessment, Sustainability, and Management. Cambridge, MA: Blackwell Science; 1996. pp. 285–312. [Google Scholar]
- Schoeman K, Tanaka T, Bend JR, Koren G. Hair mercury levels of women of reproductive age in Ontario, Canada: Implications to fetal safety and fish consumption. J Pediatr. 2010;157:127–131. doi: 10.1016/j.jpeds.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Skinner LC, Kane MW, Gottschall K, Simpson DA. Chemical residue concentrations in four species of fish and the American lobster from Long Island Sound, Connecticut and New York: 2006 and 2007. Albany, NY: New York State Department of Environmental Conservation; 2009. [Google Scholar]
- Sorensen N, Murata K, Budtz-Jorgensen E, Weihe P, Grandjean P. Prenatal methylmercury exposure as a cardiovascular risk factor at seven years of age. Epidemiology. 1999;10:370–375. [PubMed] [Google Scholar]
- Soria ML, Sanz P, Martinez D, Lopez-Artiguez M, Garrido R, Grilo A, Repetto M. Total mercury and methylmercury in hair, maternal and umbilical blood, and placenta from women in the Seville area. Bull. Environ. Contam. Toxicol. 1992;48:494–501. doi: 10.1007/BF00199063. [DOI] [PubMed] [Google Scholar]
- Staudinger MD. Species- and size-specific variability of mercury concentrations in four commercially important finfish and their prey from the northwest Atlantic. Marine Poll. Bull. 2011;62:734–740. doi: 10.1016/j.marpolbul.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yonemoto J, Satoh H, Naganuma A, Imura N, Kigawa T. Normal organic and inorganic mercury levels in the human feto-placental system. J. Appl. Toxicol. 1984;4:249–252. doi: 10.1002/jat.2550040507. [DOI] [PubMed] [Google Scholar]
- Szczebak JS, Taylor DL. Ontogenetic patterns in bluefish Pomatomus saltatrix feeding ecology and the effect on mercury biomagnification. Environ. Toxicol. Chem. 2011;30:1447–1458. doi: 10.1002/etc.516. [DOI] [PubMed] [Google Scholar]
- Taylor DL, Linehan JC, Murray DW, Prell WL. Indicators of sediment and biotic mercury contamination in a southern New England estuary. Marine Pollut. Bull. 2012;64:807–819. doi: 10.1016/j.marpolbul.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teffer AK, Staudinger MD, Taylor DL, Juanes F. Trophic influences on mercury accumulation in top pelagic predators of Southern New England. Mar. Environ. Res. 2014;101:124–134. doi: 10.1016/j.marenvres.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Tremain DM, Adams DH. Mercury in groupers and sea basses from the Gulf of Mexico: Relationships with size, age, and feeding ecology. T. Am. Fish. Soc. 2012;141:1274–1286. [Google Scholar]
- US Environmental Protection Agency (US EPA) Mercury Study Report to Congress. Volumes I-VII: Fate and Transport of Mercury in the Environment. EPA-452/R-97-005. Washington, D.C., USA: US Environmental Protection Agency; 1997. [Google Scholar]
- US Environmental Protection Agency (US EPA) Guidance for conducting fish and wildlife consumption surveys. EPA-823-B-98-007. Washington, D.C., USA: US Environmental Protection Agency; 1998a. [Google Scholar]
- US Environmental Protection Agency (US EPA) Mercury in solids and solutions by thermal decomposition, amalgamation, and atomic absorption spectrophotometry. EPA Method 7473 Report. Washington, D.C., USA: US Environmental Protection Agency; 1998b. [Google Scholar]
- US Environmental Protection Agency (US EPA) Water quality criterion for the protection of human health: Mercury. EPA-823-R-01-001. Washington, D.C., USA: US Environmental Protection Agency; 2001. [Google Scholar]
- US Environmental Protection Agency (US EPA) Estimated per capita fish consumption in the United States. EPA-821-C-02-003. Washington, D.C., USA: US Environmental Protection Agency; 2002. [Google Scholar]
- US Environmental Protection Agency (EPA) Mercury in marine life. Washington, D.C: US Environmental Protection Agency, Office of Wetlands, Watersheds, and Oceans, Oceans and Coastal Protection Division; 2003. Apr 30, p. 226. [Google Scholar]
- US Environmental Protection Agency (US EPA) [Accessed 20 April 2016];US EPA Environmental Monitoring and Assessment Program National Coastal Assessment, Northeast Region 2000–2002. 2016 http://www.epa.gov/emap/nca/
- US Food and Drug Administration (US FDA) Mercury levels in commercial fish and shellfish. Washington, D.C., USA: US Food and Drug Administration; 2016a. [Accessed 20 April 2016]. http://www.fda.gov/Food/FoodborneIllnessContaminants/Metals/ucm115644.htm. [Google Scholar]
- US Food and Drug Administration (US FDA) What you need to know about mercury in fish and shellfish. Washington, D.C., USA: US Food and Drug Administration; 2016b. [Accessed 1 August 2016]. http://www.fda.gov/food/resourcesforyou/consumers/ucm110591.htm. [Google Scholar]
- Virtanen JK, Voutilainen S, Rissanen TH, Mursu J, Tuomainen TP, Korhonen MJ, Valkonen VP, Seppänen K, Laukkanen JA, Salonen JT. Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arterioscler. Thromb. Vasc. Biol. 2005;25:228–233. doi: 10.1161/01.ATV.0000150040.20950.61. [DOI] [PubMed] [Google Scholar]
- Willett WC. Fish: Balancing health risks and benefits. Am. J. Prev. Med. 2005;29:320–321. doi: 10.1016/j.amepre.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Xu X, Newman MC, Fabrizio MC, Liang L. An ecologically framed mercury survey of finfish of the lower Chesapeake Bay. Arch. Environ. Contam. Toxicol. 2013;65:510–520. doi: 10.1007/s00244-013-9917-1. [DOI] [PubMed] [Google Scholar]
- Yokoo EM, Valente JG, Grattan L, Schmidt SL, Platt I, Silbergeld EK. Low level methylmercury exposure affects neuropsychological function in adults. Environ. Health. 2003;2:8. doi: 10.1186/1476-069X-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]