Abstract
Glatiramer acetate (GA, Copaxone, Copolymer 1) is an approved drug for the treatment of multiple sclerosis and is highly effective in the suppression of experimental autoimmune encephalomyelitis in various species. The mode of action of GA is by initial strong promiscuous binding to MHC molecules and consequent competition with various myelin antigens for their presentation to T cells. A further aspect of its action is potent induction of specific suppressor cells of the T helper 2 (Th2) type that migrate to the brain and lead to in situ bystander suppression. Furthermore, the GA-specific cells in the brain express the antiinflammatory cytokines IL-10 and transforming growth factor β, in addition to brain-derived neurotrophic factor, whereas they do not express IFN-γ. Based on this immunomodulatory mode of action, we explored the potential of GA for two other applications: prevention of graft rejection and amelioration of inflammatory bowel diseases. GA was effective in amelioration of graft rejection in two systems by prolongation of skin graft survival and inhibition of functional deterioration of thyroid grafts, across minor and major histocompatibility barriers. In all transplantation systems GA treatment inhibited the detrimental secretion of Th1 inflammatory cytokines and induced beneficial Th2/3 antiinflammatory response. GA was effective also in combination with low-dose immunosuppressive drugs. Inflammatory bowel diseases are characterized by detrimental imbalanced proinflammatory immune reactivity in the gut. GA significantly suppressed the various manifestations of trinitrobenzene sulfonic acid-induced colitis, including mortality, weight loss, and macroscopic and microscopic colonic damage. GA suppressed local lymphocyte proliferations and tumor necrosis factor α detrimental secretion but induced transforming growth factor β, thus confirming the involvement of Th1 to Th2 shift in GA mode of action.
Glatiramer acetate (GA), known also as Copolymer 1 (Cop 1, Copaxone), is a synthetic amino acid copolymer that was developed in our laboratory (1, 2). GA is an approved drug for multiple sclerosis (MS) that slows the progression of disability and reduces relapse rate, and it exhibits a very high safety profile (3). MS and its animal model, experimental autoimmune encephalitis (EAE), are inflammatory autoimmune diseases of the central nervous system (CNS) characterized by myelin destruction and axonal damage. GA, composed of the amino acids l-alanine, l-lysine, l-glutamic acid, and l-tyrosine in a molar ratio of 4.2:3.4:1.4:1.0, was designed to simulate myelin basic protein (MBP), one of the major myelin autoantigens involved in EAE and MS, and its immunological cross-reactivity with MBP is essential for its activity (4). Indeed, GA suppresses very efficiently EAE in several species, including primates. Furthermore, the suppressive activity of GA is not restricted to MBP, because it ameliorates EAE induced by other encephalitogens such as protelipid protein and myelin oligodendrocyte glycoprotein (2).
The mechanism by which GA induces its beneficial effect in animals and patients was extensively investigated over the years by us and others. These studies demonstrated that GA exerts its therapeutic activity by immunomodulating various levels of the immune response, which differ in their degree of specificity. The prerequisite step is the binding of GA to MHC class II molecules. GA exhibited a very rapid, high, and efficient binding to various MHC class II molecules on murine and human antigen-presenting cells, and even displaced peptides from the MHC-binding site (5). This competition for binding to the MHC can consequently lead to inhibition of various pathological effector functions. Interestingly, it was recently demonstrated that GA promotes T helper 2 (Th2) cell development and increased IL-10 production through modulation of dendritic cells (6). This modulation on the level of antigen-presenting cells is the least specific step and can be beneficial for the modulation of detrimental immune responses to various antigens. However, in MS and EAE, in addition to the MHC blocking, GA was shown to inhibit the response to the immunodominant epitope of MBP peptide 82–100 in a strictly antigen-specific manner by acting as a T cell receptor antagonist (7).
The above activities, however, do not necessarily play an essential role in the modulation of MS and EAE in vivo, because GA is degraded in the periphery and not likely to reach the brain and compete with the relevant myelin antigens in situ. It is therefore likely that, in CNS diseases, additional immunomodulatory mechanisms/factors that can access the blood–brain barrier mediate the therapeutic activity of GA. We have previously demonstrated that GA-treated animals (either by s.c. injections or oral administration) develop GA-specific T cells in the peripheral immune system. These cells can adoptively transfer protection against EAE (8). Furthermore, T cell lines and hybridomas could be isolated from spleens of animals rendered unresponsive to EAE by GA (9). Both cell types act as regulatory suppressor cells, as they inhibited in vitro the response of MBP-specific effector cells and inhibited in vivo EAE induced by different CNS antigens. These GA-induced cells were indeed characterized as Th2/3 cells secreting high amounts of antiinflammatory cytokines such as IL-4, IL-10, and transforming growth factor (TGF)-β, but not Th1 cytokines, in response to both GA and MBP (10). Other myelin antigens such as proteolipid protein (PLP) and myelin oligodendrocyte glycoprotein (MOG) could not activate the GA-induced cells to secrete by Th2 cytokines. Yet, the disease induced by PLP and MOG can be suppressed by GA as well as by GA-induced cells, probably by “bystander mechanisms” (11). A shift from a Th1-biased cytokine profile toward a Th2-biased profile was also observed in GA-treated MS patients (12–14), indicating that such GA-specific cells are involved in the therapeutic effect brought about by GA in MS. However, until recently, GA-induced Th2 regulatory cells were demonstrated only in the periphery (spleens and lymph nodes of experimental animals or peripheral blood mononuclear cells in human) and not in the organ in which the pathological processes of EAE and MS occur. There was no information indicating whether they can reach the CNS and whether they actually function as suppressor cells in situ. In this article, we review our recent findings on the immunomodulatory activity of GA in the target organ of MS/EAE, namely the CNS. In addition, based on the immunomodulatory mode of action manifested by GA, two previously undescribed applications of GA for the suppression of graft rejection and inflammatory bowel diseases (IBDs) are described.
In Situ Immunomodulation by GA in the CNS
The ability of GA-specific T cells, induced in the periphery by either injection or oral treatment with GA, to pass the blood–brain barrier and accumulate in the CNS was demonstrated by their isolation from brains of actively sensitized GA-treated mice, as well as by localization of GA-specific cells in the brain after their passive transfer to the periphery. Thus, a specific ex vivo reactivity to GA, manifested by proliferation and by Th2 cytokine secretion, was found in whole lymphocyte population obtained from brains of EAE induced mice treated by GA parenterally (15) or orally (16). Moreover, as demonstrated in these studies, highly reactive GA-specific T cell lines that secrete in vitro IL-4, IL-5, IL-10, and TGF-β in response to GA and cross-react with MBP at the level of Th2 cytokine secretion, were obtained from both brains and spinal cords of GA-treated mice. In contrast, no reactivity to the control antigen lysozyme could be obtained in lymphocytes isolated from CNS of mice injected with lysozyme. Furthermore, an in situ immunomodulatory effect induced by GA treatment in the brains of EAE-induced mice was manifested by a decrease in the level of the inflammatory cytokine IFN-γ, and by the secretion of the antiinflammatory cytokine IL-10, in response to the encephalitogen MBP.
Adoptively transferred fluorescently labeled GA-specific cells were found in the brain 7 and 10 days after their injection to the periphery (15, 16), whereas lysozyme-specific cells were absent in the CNS, indicating that the GA cells induced in the periphery penetrate and persist in the CNS. It is noteworthy that the ability of GA-induced T cells to persist in the CNS, whereas cells with different specificities were completely absent, was previously shown and attributed to their cross-reactivity with the myelin antigen MBP. This enables their in situ activation, whereas T cells that are not able to recognize their specific antigen in the CNS decline to baseline level.
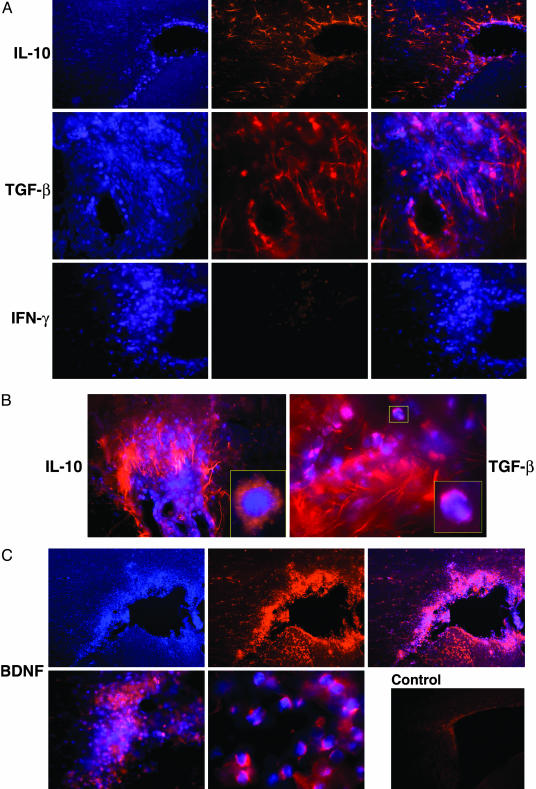
Once the presence of GA-specific Th2 cells in the CNS was confirmed, their ability to actually function as suppressor cells in the diseased organ and secrete antiinflammatory cytokines in situ had to be further verified. We therefore attempted to study the reactivity of GA-induced T cells in the brain (17). To trace the low amounts of cytokines secreted by a specific subset of T cells on the level of the whole CNS tissue, we used a double labeling approach in which prelabeled specific T cells were adoptively transferred and their expression in the brain was subsequently immunohistologically detected. Brain sections containing the penetrating Hoechst-labeled GA-specific T cells (stained blue), their immunohistological staining (red), as well as the merged images are demonstrated in Fig. 1. As depicted in Fig. 1 A, GA-specific cells in the brain manifested intensive expression of the two antiinflammatory cytokines, IL-10 and TGF-β, but no trace of the inflammatory cytokine, IFN-γ. Of special interest is the finding that IL-10 and TGF-β were expressed not only by the GA-labeled cells themselves, but also by unlabeled cells within their vicinity (Figs. 1B). These surrounding bystander cells had elongated astrocyte-like morphology consistent with activated microglia and their astrocyte nature was further corroborated by staining with antibodies to glial fibrillary acidic protein (GFAP), which bind to astrocyte filaments. The glial cells displayed similar or even higher intensity of IL-10 and TGF-β expression compared to the staining of the GA-specific population. Such spreading of positive staining could result either from genuine IL-10 and TGF-β secretion by the astrocytes that had been activated by the cytokines stemming from the GA-specific cells, or from the binding of IL-10 and TGF-β secreted by the GA-specific cells to their specific receptors on these astrocytes. In both cases, this Th2/3 spreading suggests a bystander therapeutic effect of GA on the CNS resident cells. IL-10 is a potent regulatory cytokine in autoimmunity that inhibits Th1 cells and macrophage activation in addition to its effector multifunctional therapeutic reactivity (18, 19). Moreover, IL-10 can modulate glial cell responses by inhibiting MHC class II, as well as nitric oxide and chemokine expression (20). TGF-β suppresses cytotoxic T cell response, production of tumor necrosis factor α and IFN-γ, as well as other factors that contribute to myelin damage such as lysosomal enzymes and nitrogen intermediates (21). Hence, the in situ ability of GA-specific infiltrating cells to express and induce the expression of these potent modulating cytokines and bystander CNS cells may contribute to its therapeutic activity.
Fig. 1.
Immunohistochemical analysis of cytokines and BDNF expression by GA-specific cells in the brain. Activated Hoechst-labeled GA-specific cells were injected into the peritoneum of EAE-induced mice. After 7 days, the mice were perfused, and brain sections (20 μm) were stained immunocytochemically for IL-10, TGF-β, INF-γ, and BDNF. (A)(Left) Staining for Hoechst labeling (blue). (Center) Immunohistological staining of the specific cytokines (red). (Right) Merged images. (B) Merged images of sections depicting perivascular infiltrations in the cortex. Enlarged regions accentuate the complete overlap on a single cell level. (C) Staining for BDNF. (Upper) An area surrounding the lateral ventricle. (Lower Left) Merged image of perivascular area. (Center) Enlarged section of a merged image of single cell staining. (Right) Brain section from a control EAE-induced mouse that did not receive GA-labeled cells and stained for BDNF. (Scale bar is 50 μm for A and C Lower Left, 20 μm for B, and 100 μm for Upper and Control.)
It is of special importance that, in addition to the secretion of Th2/3 cytokines, GA-specific cells express in situ the potent brain-derived neurotrophic factor (BDNF), as demonstrated in Fig. 1C. BDNF is a key regulator of neuronal development that supports neuronal survival and regulates neurotransmitter release and dendritic growth (22, 23). It can also rescue injured or degenerating neurons and induce axonal outgrowth, remyelination, and regeneration (24). The full-length BDNF receptor, tyrosine kinase receptor B, has been found in neurons in the vicinity of MS plaques, and in reactive astrocytes in MS lesions (25). It can therefore be postulated that BDNF secreted by the GA-infiltrating cells can actually function in the MS/EAE target tissue.
Intense staining with BDNF, IL-10, and TGF-β antibodies was observed only in brains of mice that had been adoptively transferred with GA-specific cells. In control mice, only faint background staining was noted (Fig. 1C). The expression of IL-10, TGF-β, and BDNF was clearly verified on single cell level by cytoplasmatic as well as membranalic staining (Fig. 1 B and C). It is noteworthy that GA-specific cells expressing IL-10, TGF-β, and BDNF were visible in various regions all over the brain, including the cortex, the thalamus, the basal ganglia, and the hippocampus, mainly in the tissue surrounding the ventricles and in perivascular locations. They were observed in brains of normal as well as EAE-induced mice, after adoptive transfer of GA-specific cells. In both cases, staining intensity on a single cell level looked similar. However, the total expression on whole tissue level was significantly higher in EAE-induced mice, because many more GA-specific cells were present in the brains of those mice than in brains of normal mice. Hence, under pathological conditions, treatment with GA leads to a more extensive expression of modulator substances by GA-specific cells in the CNS. The expression of BDNF, as well as IL-10 and TGF-β, by the GA-specific cells in the CNS, draws a direct linkage between the therapeutic activity of GA in MS/EAE and its in situ immunomodulatory effect.
The Effect of GA on Manifestations of Graft Rejection
The pathological process of graft rejection is mediated by host T cells that recognize the graft alloantigens presented on self MHC molecules as nonself, proliferate, secrete cytokines, and recruit additional inflammatory and cytotoxic cells (26–28). To prevent graft rejection, it is therefore essential to inhibit alloantigen presentation and suppress the inflammatory processes. In view of the immunomodulating activities of GA and the immunopathological nature of immune rejection, the ability of GA to alleviate this detrimental reactivity was investigated. Indeed, in our previous studies (29), we showed that GA inhibited mixed lymphocyte reaction (MLR), an in vitro assay used clinically to assess immune rejection between donor and recipient. Subsequently, by using the B10D2 → BALB/c model of lethal graft versus host disease (GVHD), which is similar to the MHC-matched bone marrow transplantation in human, we demonstrated that GA was effective in vivo as well and prevented the immune rejection of graft against host. Thus, posttransplantation administration of GA over a limited time significantly reduced the incidence, onset, and severity of GVHD, resulting in improved long-term survival (29). However, a more prevalent obstruction in human transplantation is the response of the host against the graft. Immune-rejection is still the major barrier to successful transplantations. Current protocols to prevent rejection rely on the use of nonspecific immunosuppressive drugs, such as cyclosporin A (CyA) and FK506 (tacrolimus) (30, 31). These drugs, however, induce severe side effects and render patients vulnerable to infections. Consequently, they have a narrow therapeutic window, and any deviation from this range results in rejection of the transplanted organ or in intolerable toxicity, infections, and malignancy. It was therefore of interest to examine whether GA was also effective in preventing the response of host against graft. We investigated the ability of GA by itself as well as in combination with two immunosuppressive drugs, CyA and FK506, to suppress the rejection of strongly mismatched allografts. This was tested in three experimental systems: skin-graft transplantation and thyroid-graft assay in mice and heterotropic heart transplantation in rats.
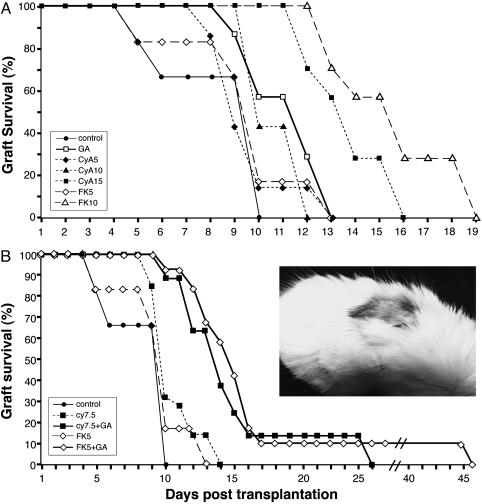
In the skin transplantation system, GA significantly prevented skin graft rejection, as demonstrated in four strain combinations across minor and major MHC barriers (32). The ability of GA, in comparison to CyA and FK506, to suppress the rejection of strongly mismatched allografts across MHC barriers (C57BL/6 → BALB/c) is demonstrated in Fig. 2A. The prolongation of skin graft survival by 100 mg/kg GA (27% increase in survival compared with untreated control) was longer than that obtained by 5 mg/kg CyA or FK506 (14% and 12% respectively), and similar to that of 10 mg/kg CyA. GA was less effective than the highest doses of these immunosuppressive drugs, 15 mg/kg CyA or 10 mg/kg FK506 (62% and 88% prolongation). However, these effective doses of CyA and FK506 had a toxic effect on the mice, inducing considerable weight loss, weakness, and mortality in 20% of the mice, whereas mice injected daily with a much larger dose (100 mg/kg) of GA looked completely healthy.
Fig. 2.
The effect of GA, CyA, and FK506 on the survival of strongly mismatched skin grafts. BALB/c mice were transplanted with skin grafts originated in C57BL/6 donors. (A) The effect of GA in comparison to various doses of immunosuppressive drugs: 100 mg/kg GA starting 2 weeks before transplantation; 5, 10, or 15 mg/kg CyA starting 6 days before transplantation; and 5 or 10 mg/kg FK506 starting 6 days before transplantation. (B) The effect of GA in combination with immunosuppressive drugs: 100 mg/kg GA was administered with either 7.5 mg/kg CyA or 5 mg/kg FK506. The effect of the combined treatment in comparison to the immunosuppressive drugs alone is demonstrated. Grafts were considered rejected when no viable donor epidermis remained. At least seven mice were tested in each group. Statistical significance for graft survival over untreated control (P < 0.05 by Kaplan–Meier test) was obtained for GA, CyA (15 mg/kg), and FK506 (10 mg/kg) (A), and for the two combination treatments (B). (Inset) One mouse treated by combination of GA and FK506 in which engraftment was sustained for 45 days even though treatment was discontinued 20 days after transplantation.
In an attempt to reduce the dosage and toxicity of the current immunosuppressive regimens and to improve their efficacy, we investigated the ability of GA to inhibit graft rejection in a combined treatment with suboptimal and less toxic doses of these suppressants. We found that combined treatments of GA with various doses of CyA or FK506 significantly extended skin graft survival compared with the effect of each drug alone. Representative results (Fig. 2B) demonstrate the synergistic effect induced by the adding of GA to either 7.5 mg/kg CyA or 5 mg/kg FK506, resulting in a combination effect (the ratio between the effect obtained with and without GA) of 5.4 and 3.1, respectively. Moreover, in each of these combination groups, one mouse survived for a prolonged period (25 and 45 days for GA with CyA and FK506, respectively), even though treatment was discontinued 20 days after transplantation. These skin grafts showed hair growth (Fig. 2B), suggesting that blood supply and engraftment have taken place in this fully disparate system by the combination treatment with GA. These results are of significance, because skin is one of the most stubborn tissues in transplantation, and even a modest prolongation in survival is considered meaningful.
In the thyroid transplantation system, donor's thyroid glands were transplanted in the kidney capsule of recipient mice, and the iodine absorbency of the transplanted kidney served as a quantitative measure not only for the graft survival but also for the function of the transplanted thyroid. The effect of the treatment was expressed numerically as the mean function index (MFI), which is the ratio between the net iodine absorbance in treated versus untreated mice. In this system as well, GA induced a significant beneficial effect and consistently improved the function of grafted thyroid from various origins, in different strain combinations (32). For example, in the C57BL/6 → BALB/c strain combination GA treatment resulted in functional increase of 3.5- and 2.2-fold over control untreated transplanted mice (P < 0.05). Likewise, the various doses of the two immunosuppressive drugs induced beneficial effects: the MFI ranged between 1.2–6.5 and 3.1–4.1 with increased doses of CyA and FK506, respectively. However, the combined treatments of GA with either FK506 or CyA improved the grafted thyroid function to a greater extent, and resulted in much higher functionality values of 12.5–24.1, whereas the MFI of either immunosuppressant alone never exceeded the value of 10. Thus, significant net effects of the combination treatment with GA, ranging between 2.8- and 20.1-fold for CyA and 2.2-3.9-fold for FK506, were obtained. Furthermore, in both skin and thyroid transplantation, the beneficial effect in the mice treated by combination of each drug, CyA or FK506, with GA was higher than that obtained with double dose of the immunosuppressive drug alone. An even more prominent effect was achieved in a heterotropic heart transplantation model in rats that allowed the study of vascularized and perfused organs, which is relevant to human transplantation (33). In this system, the combination of GA with 2.5 mg/kg CyA resulted in 28.7 days of survival, longer than that obtained by a 4-fold higher dose of CyA alone.
Concerning the mechanism by which GA alleviates the immune rejection, our results indicate that, in the case of bone marrow transplantation, GA drastically reduced the cytotoxic activity toward host targets (34). In the graft rejection study, GA inhibited the responses of spleen and lymph node cells, as well as those of the T cell lines originated from them, against the graft (32). In both graft-versus-host and host-against-graft systems, GA treatment inhibited the secretion of Th1 inflammatory cytokines, IL-2 and IFN-γ and induced the secretion of beneficial Th2/3 antiinflammatory cytokines, IL-4, IL-5, IL-10, and TGF-β. This finding is in accord with the mechanism prevailing in the GA-induced suppression of EAE/MS. These data indicate that GA acts by immunomodulating the detrimental cellular response against the host/graft, suggesting that application of this well tolerated drug may result not only in reduction in the adverse effects but also in improvement of efficacy in the current immunosuppressive regimens in organ transplantation.
The Effect of GA on IBDs
IBDs are severe gastrointestinal disorders, characterized by detrimental immune reactivity in the gut that also involve mainly CD4+ Th1 cells, and an imbalance between pro- and antiinflammatory reactivity (35). Current medical treatments for IBD rely on the use of nonspecific antiinflammatory, as well as immunosuppressive, drugs (36, 37). However, these treatments do not modify the disease course, but only alleviate the symptoms while inducing severe side effects that limit their use. Moreover, a significant percentage of the patients is steroid-resistant. In view of the immunopathological “autoimmune-like” nature of IBD and the proposed mechanism for GA activity, it was of interest to test whether GA can also immunomodulate IBD. Toward this end, we tested the effect of GA on trinitrobenzene sulfonic acid (TNBS)-induced colitis, a murine model that resembles human Crohn's disease in terms of its histopathological features and cytokine reactivity (38).
The results obtained in three strains of mice clearly indicate that GA significantly ameliorates the various pathological manifestations of TNBS-induced colitis (R. Aharoni, B. Kayhan, and R. Arnon, unpublished data). The macroscopic colonic damage characteristic to the disease, severe ulceration and/or inflammation, adhesion to adjacent organs, diarrhea, and bowel wall thickening were all drastically reduced by GA treatment (Table 1). The prevention of colonic damage by GA treatment was also confirmed histologically. Whereas colonic specimens from the mice with TNBS-induced colitis revealed transmural inflammation, extensive ulceration, and severe disruption of the normal architecture, colons of TNBS-induced mice treated with GA demonstrated significantly less damaged intestine architecture and well conserved glandular structure. GA abrogated the weight loss characteristic to this disease, as manifested also in a faster subsequent weight regain. The beneficial suppression of all of the disease manifestations by GA treatment resulted in improved long-term survival (0–20% mortality in the different strains) in comparison to the untreated mice with TNBS-induced colitis, in which 40–60% mortality was observed. It is of significance that, although treatment was more effective when started 7 days before disease induction, considerable beneficial effect was achieved also when GA treatment started together with, and even several days after, disease induction. Daily injection of GA was the most effective treatment, but oral treatment also resulted in disease suppression. The effect of GA was corroborated in three highly susceptible strains of mice, including the SJL/J strain in which an exceptional aggressive disease was manifested, indicating that the ability of GA to ameliorate TNBS-induced colitis represents a general phenomenon.
Table 1. The effect of GA treatment on the macroscopic manifestations of TNBS colitis in different mouse strains.
| Macroscopic scoring of colon damage
|
|||||||
|---|---|---|---|---|---|---|---|
| Mouse strain | Treatment | n | Ulceration (max 10) | Adhesion (max 2) | Diarrhea (max 1) | Thickness (max 1) | Total score (max 14) |
| BALB/c | Control | 16 | 7.5 | 1.6 | 0.7 | 0.4 | 10.2 |
| GA oral | 13 | 2.1 | 0.4 | 0.2 | 0.0 | 2.7* | |
| SJL/JxBALB/c | Control | 8 | 6.8 | 1.6 | 0.7 | 0.5 | 9.6 |
| GA oral | 8 | 3.8 | 1.0 | 0.3 | 0.3 | 5.4 | |
| GA injected | 7 | 1.4 | 0.8 | 0.4 | 0.1 | 2.7* | |
| SJL/J | Control | 10 | 8.4 | 2.0 | 1.0 | 1.0 | 12.4 |
| GA oral | 11 | 7.1 | 1.8 | 0.9 | 0.9 | 10.7 | |
| GA injected | 11 | 3.5 | 1.2 | 0.5 | 0.5 | 5.7* | |
Colitis was induced by TNBS in 50% ethanol. GA was administered either orally every other day (0.25 mg, eight feedings starting 7 days before disease induction) or by daily injections (2.5 mg per mouse s.c. starting 7 days before induction). Colonic damage was examined 5–7 days after disease induction. Asterisks indicate statistically significant decrease in macroscopic manifestation in GA-treated versus untreated mice (P<0.05). max, maximal score.
In accord with the mechanism demonstrated in EAE/MS and in the transplantation systems, GA treatment resulted in significant reduction in the overall secretion of the proinflammatory cytokine tumor necrosis factor α and in beneficial elevation in the secretion of the Th3 essential cytokine TGF-β. These results suggest that GA can modulate the detrimental immune response involved in the pathogenesis of experimental colitis, which resembles Crohn's disease.
Conclusions
GA is an immunomodulator that affects different levels of the immune response, as an MHC blocker, T cell receptor antagonist, and as a potent inducer of regulatory T cells. These immunomodulatory activities, as well as the high safety profile of GA, support its application for various pathological autoimmune disorders such as MS, immune rejection, and IBD.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Therapeutic Vaccines: Realities of Today and Hopes for Tomorrow,” held April 1–3, 2004, at the National Academy of Sciences in Washington, DC.
Abbreviations: GA, glatiramer acetate; MS, multiple sclerosis; EAE, experimental autoimmune encephalitis; MBP, myelin basic protein; Th, T helper; IBD, inflammatory bowel disease; BDNF, brain-derived neurotrophic factor; TGF, transforming growth factor; TNBS, trinitrobenzene sulfonic acid; CyAm, cyclosporin A.
References
- 1.Teitelbaum, D., Meshorer, M., Hirshfeld, T., Sela, M. & Arnon, R. (1971) Eur. J. Immunol. 1, 242-248. [DOI] [PubMed] [Google Scholar]
- 2.Arnon, R. (1996) Immunol. Lett. 50, 1-15. [DOI] [PubMed] [Google Scholar]
- 3.Simson, D., Noble, S. & Perry, C. (2002) CNS Drugs 16, 825-850. [DOI] [PubMed] [Google Scholar]
- 4.Webb, C., Teitelbaum, D., Arnon, R. & Sela, M. (1973) Eur. J. Immunol. 3, 279-286. [DOI] [PubMed] [Google Scholar]
- 5.Fridkis-Hareli, M., Teitelbaum, D., Gurevich, E., Pecht, I., Brautbar, C., Kwon, O. J., Brenner, T., Arnon, R. & Sela, M. (1994) Proc. Natl. Acad. Sci. USA 9, 4872-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vieira, P. L., Heystek, H. C., Wormmeester, J., Wierenga, E. A. & Kapsenberg, M. L. (2003) J. Immunol. 170, 4483-4484. [DOI] [PubMed] [Google Scholar]
- 7.Aharoni, R., Teitelbaum, D., Arnon, R. & Sela, M. (1999) Proc. Natl. Acad. Sci. USA 96, 634-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lando, Z., Teitelbaum, D. & Arnon, R. (1979) J. Immunol. 132, 2156-2160. [PubMed] [Google Scholar]
- 9.Aharoni, R., Teitelbaum, D. & Arnon, R. (1993) Eur. J. Immunol. 23, 17-25. [DOI] [PubMed] [Google Scholar]
- 10.Aharoni, R., Teitelbaum, D. & Sela, M. (1996) Proc. Natl. Acad. Sci. USA 94, 10821-10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aharoni, R., Teitelbaum, D., Sela, M. & Arnon, R. (1998) J. Neuroimmunol. 91, 135-146. [DOI] [PubMed] [Google Scholar]
- 12.Miller, A., Shapiro, S., Gershtein, R., Kinarti, A., Rawashdeh, H., Honigman, S. & Lahat, N. (1988) J. Neuroimmunol. 92, 113-121. [DOI] [PubMed] [Google Scholar]
- 13.Neuhaus, O., Farina, C., Yassouridis, A., Wiendl, H., Bergh, F., Dose, T., Wekerle, H. & Hohlfeld, R. (2000) Proc. Natl. Acad. Sci. USA 97, 7452-7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duda, P. W., Schmied, M. C., Cook, S. L., Krieger, J. I. & Hafler, D. A. (2000) J. Clin. Invest. 105, 967-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aharoni, R., Teitelbaum, D., Leitner, O., Meshorer, A., Sela, M. & Arnon, R. (2000) Proc. Natl. Acad. Sci. USA 97, 11472-11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aharoni, R., Meshorer, A., Sela, M. & Arnon, R. (2002) J. Neuroimmunol. 126, 58-68. [DOI] [PubMed] [Google Scholar]
- 17.Aharoni, R., Kayhan, B., Eilam, R., Sela, M. & Arnon, R. (2003) Proc. Natl. Acad. Sci. USA 100, 14157-14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy, M. K., Torrance, D. S., Picha, K. S. & Mohler, K. M. (1992) J. Immunol. 149, 2496-2505. [PubMed] [Google Scholar]
- 19.Bettelli, E., Nicholson, L. B. & Kuchroo, V. (2003) J. Autoimmun. 20, 265-267. [DOI] [PubMed] [Google Scholar]
- 20.Ledeboer, A., Breve, J. P., Poole, S., Tilders, F. J. & Van Dam, A. M. (2000) Glia 30, 134-142. [DOI] [PubMed] [Google Scholar]
- 21.Morris, M. M., Dyson, H., Baker, D., Harbige, L. S., Fazakerley, J. K. & Amor, S. (1997) J. Neuroimmunol. 74, 185-197. [DOI] [PubMed] [Google Scholar]
- 22.Thoenen, H. (1995) Science 270, 593-598. [DOI] [PubMed] [Google Scholar]
- 23.Barde, Y. A. (1997) Nature 385, 391-393. [DOI] [PubMed] [Google Scholar]
- 24.Gravel, C., Gotz, R., Lorrain, A. & Sendtner, M. (1997) Nat. Med. 3, 765-770. [DOI] [PubMed] [Google Scholar]
- 25.Stadelmann, C., Kerschensteiner, M., Misgeld, T., Bruck, W., Hohlfeld, R. & Lassman, R. (2002) Brain 125, 75-85. [DOI] [PubMed] [Google Scholar]
- 26.Sykes, M. (1996) FASEB J. 10, 721-730. [DOI] [PubMed] [Google Scholar]
- 27.Auchincloss, H., Jr., & Sultan, H. (1996) Curr. Opin. Immunol. 8, 681-687. [DOI] [PubMed] [Google Scholar]
- 28.Nickerson, P., Steurer, W., Steiger, J., Zheng, X., Steele, A. W. & Strom, T. B. (1994) Curr. Opin. Immunol. 6, 757-764. [DOI] [PubMed] [Google Scholar]
- 29.Schlegel, P. G., Aharoni, R., Chen, Y., Chen, J., Teitelbaum, D., Arnon, R., Sela, M. & Chao, N. J. (1996) Proc. Natl. Acad. Sci. USA 93, 5061-5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masri, M. A. (2003) Mol. Immunol. 39, 1073-1077. [DOI] [PubMed] [Google Scholar]
- 31.Ma, A. & Chen, H. (2002) Curr. Drug Targets Cardiovasc. Haematol. Discord. 2, 57-71. [DOI] [PubMed] [Google Scholar]
- 32.Aharoni, R., Teitelbaum, D., Arnon, R. & Sela, M. (2001) Transplantation 27, 598-605. [DOI] [PubMed] [Google Scholar]
- 33.Aharoni, R., Yussim, A., Sela, M. & Arnon, R. (2004) Int. Immunopharmacol., in press. [DOI] [PubMed]
- 34.Aharoni, R., Schlegel, P. G., Teitelbaum, D., Roikhel-Karpov, O., Chen, Y., Arnon, R., Sela, M. & Chao, N. J. (1997) Immunol. Lett. 58, 79-87. [DOI] [PubMed] [Google Scholar]
- 35.Shanahan, F. (2001) Gastroenterology 120, 622-635. [DOI] [PubMed] [Google Scholar]
- 36.Sandborn, W. J. & Targan, S. R. (2002) Gastroenterology 122, 1592-1608. [DOI] [PubMed] [Google Scholar]
- 37.Van Deventer, S. J. H. (2000) Scand. J. Immunol. 51, 18-22. [DOI] [PubMed] [Google Scholar]
- 38.Witz, S. & Neurath, M. F. (2000) Int. J. Colorectal Dis. 15, 144-160. [DOI] [PubMed] [Google Scholar]