Abstract
Aberrant calcium influx is a common feature following ischemic reperfusion (I/R) in transient global cerebral ischemia (GCI) and causes delayed neuronal cell death in the CA1 region of the hippocampus. Activation of calcium-calmodulin (CaM) dependent protein kinase IIα (CaMKIIα) is a key event in calcium signaling in ischemic injury. The present study examined the effects of intracerebroventricular (icv) injection of tatCN21 in ischemic rats 3 hours after GCI reperfusion. Cresyl violet and NeuN staining revealed that tatCN21 exerted neuroprotective effects against delayed neuronal cell death of hippocampal CA1 pyramidal neurons 10 days post GCI. In addition, TatCN21 administration ameliorated GCI-induced spatial memory deficits in the Barnes maze task as well as anxiety-like behaviors and spontaneous motor activity in the elevated plus maze and open field test, respectively. Mechanistic studies showed that the administration of tatCN21 decreased GCI induced phosphorylation, translocation and membrane targeting of CaMKIIα. Treatment with tatCN21 also inhibited the level of CaMKIIα-NR2B interaction and NR2B phosphorylation. Our results revealed an important role of tatCN21 in inhibiting CaMKIIα activation and its beneficial effects in neuroprotection and memory preservation in an ischemic brain injury model.
Keywords: Global cerebral ischemia, Hippocampus, TatCN21, CaMKIIα, NR2B
Introduction
Global cerebral ischemia (GCI) most often occurs during cardiac arrest as a consequence of the transient failure of the cardiovascular system initiating a series of pathophysiological reactions that eventually results in the death of neurons in susceptible brain regions. Patients who successfully survive the initial ischemic event are still at a major risk of dying or developing delayed neuronal cell death leading to severe disabilities (Schneider et al., 2009; Mangus et al., 2014). The brain has limited ischemic tolerance due to high consumption of oxygen, restricted anaerobic metabolism, and glycogen stores. The CA1 region of the hippocampus has been reported as the most vulnerable region of the brain to delayed neuronal cell death following transient ischemia (Kirino et al., 1985; Schmidt-Kastner & Freund, 1991). Subsequent damage to the CA1 region is the root cause of persistent neurological symptoms, including cognitive and memory impairment, observed following GCI (Bothe et al., 1986).
Calcium-calmodulin (CaM) dependent protein kinase II (CaMKII), a multifunctional member of the calcium/calmodulin dependent kinase family, plays a significant role in synaptic plasticity, learning, and memory (Babcock et al., 2002; Lee et al., 2015). The α and β isoforms of CaMKII are abundant proteins in the brain, making up as much as 2% of the total protein content within the hippocampus (Babcock et al., 2002). Previous studies showed that excitotoxic stimulation initiated by Ca2+ influx through the NMDA receptor rapidly activated CaMKIIα and triggered its phosphorylation at Thr286 of CaMKIIα following GCI (Meng & Zhang, 2002; Matsumoto et al., 2004). The influx of Ca2+ into the postsynaptic densities promotes the translocation of CaMKIIα from cytosol to the membrane and increases neuronal firing and further calcium influx (Strack et al., 1997; Vest et al., 2010). Overwhelming evidence demonstrates that shifts in CaMKIIα activity associated with such conditions as cerebral stoke, epilepsy, and traumatic brain injuries are a result of excitotoxic calcium dysregulation (Hanson et al., 1994; Churn et al., 1995; Schwarzbach et al., 2006).
A previous study has shown that membrane-bound NR2B is a binding partner for Thr286 phosphorylated CaMKII α and that the CaMKII-NMDA complex is isolated from the post synaptic density (Strack & Colbran, 1998). NR2B can be phosphorylated and activated by phosphorylated CaMKIIα (Strack et al., 2000). The interaction between CaMKIIα and NR2B is largely believed to play a pivotal role in ischemic brain injury. This knowledge is critical because NMDA receptors are dominant ion channels and play a crucial role in calcium over-activation and neuronal damage during ischemic condition. Importantly, CaMKIIα is the most sensitive protein kinase to cellular calcium concentration fluctuations. Due to the current lack of effective neuroprotective drugs that can address GCI-induced neurological impairments, investigation of this interaction could open up new avenues of targeted treatments. In the current study, we tested the effects of tatCN21, a CaMKIIα inhibiting peptide, in inhibition of CaMKIIα phosphorylation and its binding to NR2B, as well as the efficacy of tatCN21 in CA1 neuroprotection and amelioration of behavioral deficits following GCI.
Materials and Methods
Global Cerebral Ischemia (GCI) Model
Adult male Sprague-Dawley rats weighing (200–250g, Charles River Laboratories) were used in this study. Animals were housed in propylene cages in an air conditioned room at an ambient temperature of 25 ± 2 °C and relative humidity 45–55% with a 12 h light/dark cycle and allowed ad libitum access to pellet diet and water. All the procedures were approved by the institutional animal care committee. The rats were randomly divided in to 3 groups (n=6–10 animals in each group): (a) Sham group without GCI and tatCN21; (b) Control group (GCI + Scrambled tatCN21); (c) GCI plus tatCN21 treated rats. The experimental design was shown in Figure 1. Four vessel occlusion (4VO) GCI model was developed as previously described (Zhou et al., 2011; Lu et al., 2015). Briefly, rats were anesthetized with sodium pentobarbital (50 mg/kg ip), then both vertebral arteries were electro-cauterized and the bilateral common carotid arteries (CCAs) were exposed and a silastic ligature was loosely secured around each artery. Wound clips were used to close the incision. On the following day, rats were anesthetized lightly with isofluorane and the CCAs were re-exposed and occluded with aneurysm clips for 15 min to induce GCI. Subsequently, the clips were released, restoring the common carotid artery blood flow. Rats that lost righting reflex within 30 s with accompanying pupil dilation were selected for experimental procedures. For sham-operated rats, the surgeries were performed identically as with ischemic animals with the exception that the CCAs were not occluded and the vertebral arteries were not cauterized. The rectal temperature was maintained at 37 °C using a thermal blanket
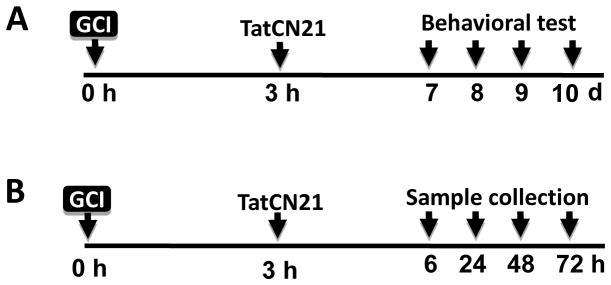
Fig. 1.
Schematic diagram depicting experimental design. (A) GCI model was developed at 0 h, and tatCN21 (50 μg/rat) was icv injected 3 h after GCI. On day 7 through day 10, behavioral tests were performed to check learning and memory, anxiety, and spontaneous motor activity. Rats were anesthetized with sodium pentobarbital, transcardially perfused and sacrificed on day 10 and brain sections were collected. (B) 3 h after GCI, animals were treated with tatCN21 and scrambled tat peptide (cont) and sacrificed at 6, 24, 48 and 72 h after reperfusion. Whole brains were quickly removed and hippocampal CA1 region were rapidly micro dissected, immediately frozen and preserved for different assay.
Administration of tatCN21 peptide
The tatCN21 peptide (GRKKRRQRRR-NH2-KRPPKLGQIGRSKRVVIEDDR-COOH) peptide was synthesized by 21st Century Biochemicals. The tatCN21 peptide is composed of 21 amino acids linked to the human immunodeficiency virus (HIV)-tat peptide that facilitates entry into the cell where the CN21 sequence binds to the catalytic domain of activated CaMKIIα and inhibits its autonomous activity (Chang et al., 1998; Vest et al., 2007). The tatCN21 peptide was administered (50 μg/rat) bilaterally via intracerebroventricular (icv) injection, at 3 h post GCI. The 50 μg dose of the inhibitor was chosen based on a dose response curve performed in our lab which showed it to be the most optimal and effective dose in inhibiting CaMKIIα activation in the hippocampal CA1 region following GCI. For icv injection, rats were placed on a stereotaxic instrument under deep anesthesia and the skull was exposed and cleaned. The tatCN21 and scrambled control tatCN21 peptide were dissolved in 0.9% filtered saline and injected into the lateral ventricles via a 10 μL Hamilton syringe (anteroposterior, 0.8 mm; lateral, 1.5 mm; depth, 3.5 mm; from bregma). The incision was closed using wound clips and rats were placed on a warming pad to recover from anesthesia.
Histological analysis and Immunofluorescence Staining
Histological examination was performed as previously described (Zhang et al., 2009a). All the animals were anesthetized with general anesthesia and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4. Brains were quickly removed and post-fixed in the same fixative for 12 h at 4 °C, cryoprotected with 30% sucrose at 4 °C until they sank. Coronal sections (25 μm) were cut on a Leica RM2155 microtome and collected throughout the entire dorsal hippocampus. To investigate the neuroprotective effect of tatCN21 peptide, brain sections were stained with 0.01% (w/v) cresyl violet for 10 min, followed by graded ethanol dehydration. The stained sections were examined, and images were captured using an AxioVision4Ac microscope system (Carl Zeiss, Germany). For immunofluorescence staining, the sections were washed for 20 min in 0.1% PBS-Triton X-100. After incubation with a blocking solution containing 10% donkey serum for 1 h at room temperature, sections were incubated with mouse anti-NeuN monoclonal antibody (1:500, EMD Millipore, Billerica, MA), rabbit anti-p-CaMKIIα (1:200, abcam, USA) and rabbit anti-p-NR2B ( 1:200, Life Technologies Corporation, USA) overnight at 4°C. The sections were then washed 3 times, followed by incubation with the appropriate combination of Alexa Fluor donkey anti-mouse/rabbit secondary antibody (1:500, Thermo Fisher Scientific) for 1 h at room temperature. Thereafter, sections were washed and mounted using Vectashield mounting medium with 4′, 6-diamidino-2-phenylindole (DAPI) (H-1200; Vector Laboratories, Inc., CA, USA), and a cover slip was added. The staining was imaged under a Zeiss LSM510 Meta confocal laser microscope (Carl Zeiss) using 40× objective lens with the image size set at 1024 × 1024 pixels. The captured images were then processed and analyzed by the LSM 510 META software. Cell counts from the right and left hippocampus on each of the sections were averaged to provide the mean value as described previously (Zhang et al., 2009b).
Duolink II In Situ PLA hybridization
Interaction of CaMKIIα and NR2B in CA1 region of hippocampus was investigated by Duolink II in situ PLA immunoassay was performed as described (Sareddy et al., 2015; Tu et al., 2015). Hippocampal sections taken 6 h after GCI were prepared as described above. Tissue sections were blocked in 10% donkey serum for 1 h and incubated overnight with appropriate primary antibodies at 4 °C. The slides were then incubated with Duolink PLA Rabbit MINUS and PLA Mouse PLUS proximity probes for 1 h at 37 °C. Ligation and amplification were carried out using the Duolink in situ detection reagent kit (Sigma-Aldrich) according to the manufacturer’s protocol. Images were captured under LSM510 confocal microscope and red spots representing the interactions were quantitatively analyzed using NIH ImageJ software.
Brain Homogenate and Subcellular Fractionations for Western blot analysis
The subcellular cytosolic fractions and crude membrane fractions were extracted via a protocol previously described (Zhang et al., 2008). Briefly, the hippocampal CA1 regions were homogenized in ice cold homogenization medium consisting of 50 mM HEPES (pH 7.4), 150 mM NaCl, 12 mM β-glycerophosphate, 3 mM dithiotheitol (DTT), 2 mM sodium orthovanadate (Na3VO4), 1 mM EGTA, 1 mM NaF, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1% Triton X-100 and inhibitors of proteases and enzymes (Thermo Scientific, Rockford, IL) with a Teflon-glass homogenizer. The homogenates were centrifuged at 12,000 × g for 15 min at 4°C (cytosolic fraction), and further centrifuged at 100,000 × g for 30 min to obtain the membrane-enriched fractions. The pellet membranes fractions were re-suspended in buffer A containing 0.1% Triton X-100 for 10 sec by sonication. The protein concentrations were determined by the Modified Lowry Protein Assay (Pierce, Rockford, ILL). All the samples were stored at −80°C until use. For western blot analysis, 50 μg of proteins were separated on 4–20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to PVDF membrane. The membranes were blocked and incubated with primary antibody at 4 °C overnight and probed by incubation with HRP-conjugated secondary antibodies for 1 h at room temperature. The following primary antibodies were used in this study: CaMKIIα (1:1500, Proteintech, IL), p-CaMKIIα (1:1500, Abcam), NR2B (1:1500, Proteintech, IL), p-NR2B (1:1500, Life Technologies Corporation, USA), Na/K ATPase (1:1000, Thermo Fisher) and HRP conjugate GADPH (1:1000, Proteintech, IL). Bound proteins were visualized using a CCD digital imaging system (HM3050A, Zhang lab). Band densities were normalized to the loading control of GAPDH signals, and were analyzed with the ImageJ analysis software (Version 1.49; NIH, USA). A Mean ± SE was calculated from the data for all animal groups for graphical presentation and statistical comparison.
Co-Immunoprecipitation (Co-IP) analysis
As described previously (Zhang et al., 2008), tissue homogenates were diluted with HEPES buffer containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 12 mM β-glycerophosphate, 3 mM dithiotheitol (DTT), 2 mM sodium orthovanadate (Na3VO4), 1 mM EGTA, 1 mM NaF, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1% Triton X-100 and inhibitors of proteases and enzymes (Thermo Scientific, Rockford, IL). Samples were incubated with anti-CaMKIIα or NR2B antibody overnight at 4 °C. After the addition of protein A/G-Sepharose beads (Santa Cruz Biotechnology), the mixture was incubated at 4°C for 2 h. The beads were isolated by centrifugation and washed three times with HEPES buffer and eluted by heating at 100 °C in Laemmli buffer. The associated proteins in immunoprecipitates were examined by Western blotting analysis with anti-NR2B or anti-CaMKIIα antibody to detect their interactions.
Barnes Maze
The Barnes maze test was performed to evaluate the spatial learning and memory in rats (Barnes, 1979; Ferguson et al., 2012). The basic function of Barnes maze is to measure the ability of a rat to learn and remember the location of a target zone. It was performed as previously described by our laboratory (Lu et al., 2015). The Barnes maze consists of a circular platform 122 cm in diameter on a 1.0 m stand with 18 evenly spaced 10 cm diameter holes around the circumference with an escape box (20×15×12 cm) placed underneath one of the holes. Several visual cues were placed around the table serving as visual cues to facilitate spatial orientation. Testing was performed with a bright flood incandescent light (500 W, 1000 lx) illuminating the maze surface. Rats were also exposed to noxious auditory stimulus with the use of digital metronome software and two computer speakers facing the platform. On day 7, 8 and 9 after ischemia, 3 days of training trials, one trial per day, were performed with a maximum trial length of 180 s. The time taken to enter into the target hole was recorded with an overhead camera. The probe trial was performed on day 10 after GCI. The escape box was replaced with a black plastic cover and the time spent in the target quadrant was recorded for 90 s. The platform and escape box were cleaned with 70% ethanol and dried with a blower fan between each test. The primary latency and the time spent in the target quadrant were quantified using ANY-maze video tracking software (Stoelting Co., Wood Dale, IL).
Elevated Plus Maze and Open Field tests
The elevated plus maze was used to assess anxiety-like behavior of rodents in a novel environment (Carobrez & Bertoglio, 2005). The maze consists of two opposing open arms (50 cm×10 cm×0.5 cm), and two opposing closed arms (50 cm×10 cm×40 cm), crossing perpendicularly with an open center area (10 cm×10 cm) elevated 50 cm from the floor as previously described by our lab (Lu et al., 2015). At the beginning of the test, the rat was placed on the central platform facing one of the open arms. Rats were allowed to move freely about the maze for 5 min during which time spent by rats in the open arms as well as risk assessment behaviors were measured. An open-arm entry was defined as all four of the paws being placed in the open arm. Risk assessment behavior was defined as a stretch-attend response in which the rat stretched its body forward with sniffing or an obvious scan. The maze was cleaned with 70 % ethanol between each test. Videos were recorded and analyzed using ANY maze video tracking system. The open field test is used to measure locomotor and exploratory activities in rodents (Prut & Belzung, 2003), rendering it a useful method to determine motor deficits and anxiety. This test measures aspects of locomotor (track length), exploratory (number of rearing) and anxiety-like behavior. The open field enclosure consists of a floor base (96 cm×96 cm) with 50-cm walls. The base was painted with white lines (6 mm) to form 16 equivalent squares. During a 5 min observation period, the number of lines crossed, time spent rearing, and time spent grooming were observed and recorded. The field was cleaned with 70% ethanol between each test and given time to dry completely. Videos were recorded and analyzed using ANY-maze video tracking system.
Statistical Analysis
Quantified data are presented as mean ± SE and analyzed by GraphPad PRISM 6.0 software. Statistical analysis was performed using one–way or two-way analysis of variance (ANOVA), with Bonferroni’s post hoc test (for multiple comparison all pairs of column) or Dunnett’s post hoc tests (for multiple comparisons versus a control). A level of P < 0.05 was considered to be statistically significant.
Results
Treatment with tatCN21 peptide significantly attenuated learning and memory deficits after GCI
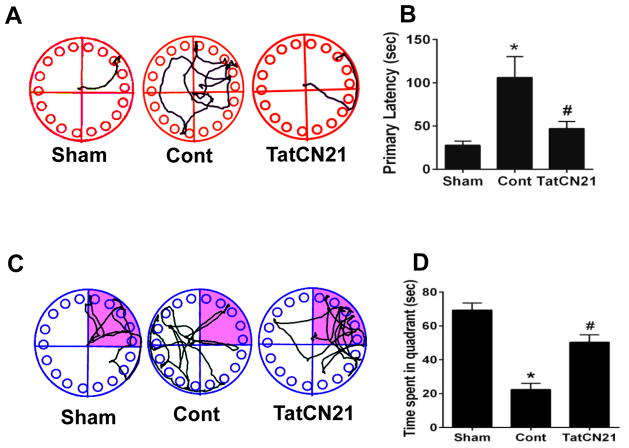
The Barnes maze test was used to check the spatial learning and memory in rats, testing the ability of an animal to recall the position of a previously learned escape chamber on a circular platform using environmental spatial cues (Barnes, 1979). The hippocampal CA1 region plays a very important role in processing of spatial location and spatial reference memory (Goodrich-Hunsaker et al., 2008). To investigate the effect of tatCN21 peptide on maintaining learning and memory following GCI, the Barnes maze task was performed on day 7, 8, 9 and 10 after GCI. Representative tracking plots demonstrate example paths on both training and probe days in treatment and control groups Fig. 2A, C. Ischemic control animals demonstrated increased latency to find the escape box compared to sham group animals. Animals treated with tatCN21, however, showed significantly decreased latency to find the escape box on the last training trial compared to control on day 9 after GCI Fig. 2B. In the probe trial, day 10 after GCI, the tatCN21 treated animals and sham-operated animals spent significantly more time in the target quadrant compared with control group Fig. 2D.
Fig. 2.
Icv infusion of tatCN21 peptide 3 h post GCI significantly attenuates the hippocampal dependent learning and memory deficits following GCI. (A, B) Barnes maze test was performed to check the spatial learning and memory ability of sham, control (cont) and tatCN21 group animals, as shown by latency to find the black escape box on day 9 following GCI. (C, D) Probe trials measure the time spent in the quadrant where the escape box was previously located but removed on day 10 after GCI. The representative tracks are shown and data were statistically compared. Data are represented as mean ±SE (n=10) per group, *P <0.05 vs. sham, #P < 0.05 vs. cont group.
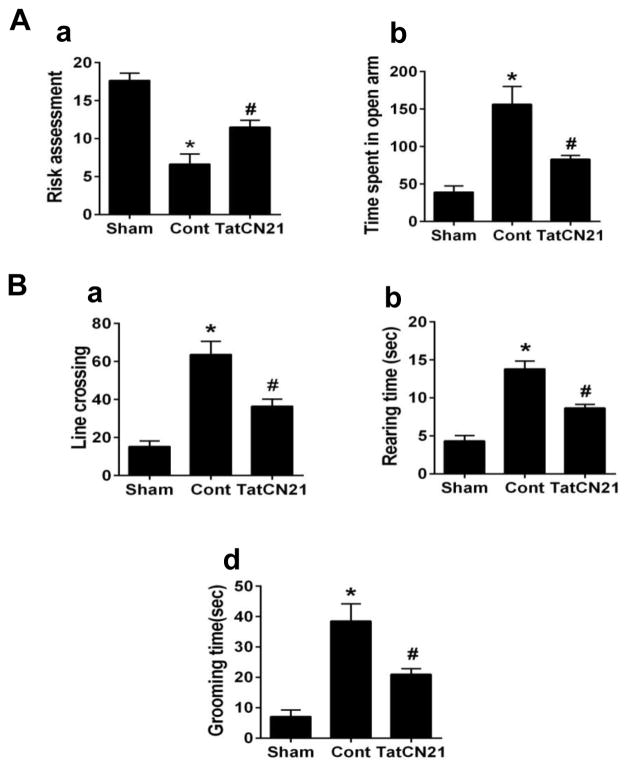
Treatment with tatCN21 peptide recovers anxiety related deficits and hyperactivity following GCI
The elevated plus maze was used to assess the exploratory and anxiety-like behavior in rats following GCI. Results shows that ischemic control animals displayed decreased risk assessment behavior compared with sham animals Fig. 3Aa. However, ischemic animals treated with tatCN21 exhibited increased risk assessment behavior relative to control group. While open arm entry assessment reveals that ischemic control animals spent more time in the open arms than sham animals Fig. 3Bb, treatment with tatCN21 reduced the time compared to control. Spontaneous locomotor and anxiety activity was assessed in the open field test by measuring the time spent rearing and grooming, as well as the total lines crossed. As shown in Fig. 3B, ischemic control animals exhibited increased line crossing, rearing time and grooming time compared to sham. By contrast, GCI induced hyperactivities were significantly ameliorated by the administration of tatCN21.
Fig. 3.
TatCN21 infusion 3 h after GCI reduced anxiety and locomotor activity behaviors in the elevated plus maze and open field test. (A) In the elevated plus maze test, the number of risk assessment and time spent in open arm were calculated and statistically compared. (B) In the open field test, number of line crossed, rearing, grooming were recorded and statistically analyzed. Data are represented as mean ±SE (n=10) per group, *P <0.05 vs. sham, #P < 0.05 vs. cont group.
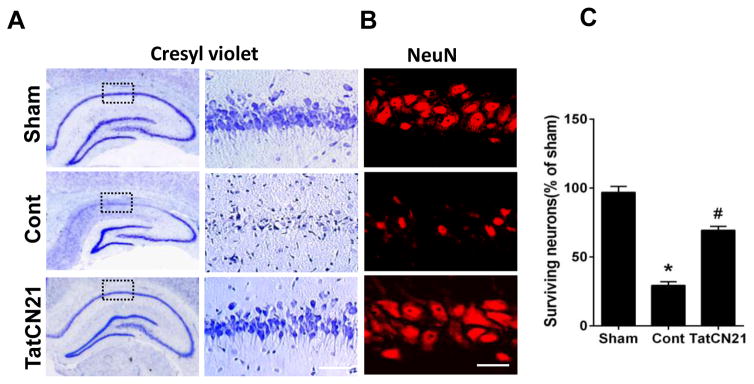
Treatment with tatCN21 significantly reduced the delayed neuronal cell death in hippocampal CA1 region following GCI
We next examined the potential neuroprotective effect of tatCN21 peptide against delayed neuronal cell death in the hippocampal CA1 region. Hippocampal sections were collected 10 days after GCI and were subjected to cresyl violet and immunofluorescence staining for the neuronal marker, NeuN. Representative sections of cresyl violet staining are presented in Fig. 4A. Ischemic control group animals displayed profound decreases in the number of surviving neurons in the CA1 region as compared with sham group animals. Treatment with tatCN21 apparently preserved the CA1 pyramidal neurons. Representative sections of NeuN staining results, presented in Fig. 4B, revealed that tatCN21 treatment significantly protected the hippocampal CA1 neurons compared to ischemic control. Quantification of the number of NeuN-positive cells were presented in Fig. 4C, showing treatment with tatCN21 significantly increases the number of surviving neurons in CA1 after GCI. These results demonstrate the novel neuroprotective efficacy of tatCN21 against delayed neuronal cell death in the sensitive CA1 region following GCI.
Fig. 4.
TatCN21 confers neuroprotection in hippocampal CA1 region on day 10 after GCI. (A) Cresyl violet staining shows the whole hippocampus overview and the CA1 region. Bar scale: 50 μm. (B) High magnification immunofluorescence staining of NeuN shows pyramidal neurons in the medial hippocampal CA1 region. Bar scale: 20 μm. (C) The number of surviving neurons was quantified and statistically compared. Data were represented as mean ± SE (n=6) per group, *P <0.05 vs. sham, #P <0.05 vs. cont group.
Effects of tatCN21 on the phosphorylation of CaMKIIα and the phosphorylation of NR2B in cytoplasm
To examine the effects of tatCN21 peptide on the phosphorylation of both CaMKIIα and NR2B, protein samples from hippocampal CA1 region were collected at different time points after reperfusion and subjected to Western blot analysis. We found that the level of phosphorylation of CaMKIIα and NR2B reached a maximum 6 h after reperfusion, and subsequently decreased over the observed time course of 72 h as shown in Fig. 5A and B. Therefore, the 6 h time point was selected to determine the effect of tatCN21 administration on CaMKIIα and NR2B phosphorylation. As shown in Fig 5C and D, treatment with tatCN21 remarkably reversed the GCI-increased level of phosphorylation compared with control group. We further investigated the level of p-CaMKII and p-NR2B in hippocampal sections by immunofluorescence staining. As seen in the representative images shown in Fig. 5E, treatment with tatCN21 apparently decreased the phosphorylation levels of both CaMKIIα and NR2B compared with control group.
Fig. 5.
Effect of tatCN21 on the phosphorylation of CaMKIIα, NR2B and their interactions in the hippocampal CA1 region 6 h after GCI. (A, B) Time course study of the cytosolic phosphorylation levels of CaMKIIα and NR2B at 6, 24, 48 and 72 h post GCI. Bands were scanned and the levels of p-CaMKIIα/CaMKIIα and p-NR2B/NR2B were determined. (C, D) Immunoblotting analysis of the p-CaMKIIα/CaMKIIα in the cytosolic fraction 6 h after GCI. (E) Representative immunofluorescence staining of p-CaMKIIα and p-NR2B in the CA1 region of hippocampus. Bar scale: 20 μm. Data are represented as mean ± SE (n=6) per group, *P <0.05 vs. sham, #P < 0.05 vs. cont group.
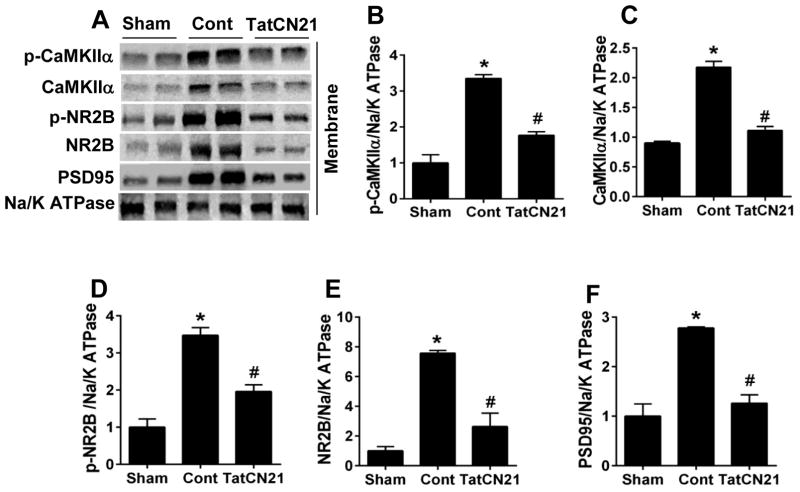
Effects of tatCN21 peptide on the membrane targeting of CaMKIIα, NR2B and PSD95 in hippocampal CA1 folowing GCI
It is known that, once activated, CaMKIIα translocates to the membranes, binding to and phosphorylating NR2B (Meng et al., 2003). As indicated in Fig. 6A–C, the levels of both total and phosphorylated CaMKIIα and NR2B were significantly increased 6 h post GCI in compared with sham animal, suggesting GCI induced membrane translocation of CaMKIIα and NR2B after their phosphorylation. By contrast, administration of tatCN21 effectively attenuated the membrane bound levels of CaMKIIα and NR2B compared with control animals. Interestingly, the membrane aggregation of PSD95, the scaffolding protein that interacts with NR2B via PDZ domains, was also effectively inhibited by tatCN21 peptide Fig. 6A and F.
Fig. 6.
TatCN21 reduced the membrane translocation of CaMKIIα, NR2B and PSD95 in hippocampal CA1 region 6 h after GCI. (A) Representative western blots of the indicated proteins using membrane proteins at 6 h after GCI. (B–F) Normalized protein expression levels of p-CaMKIIα, CaMKIIα, p-NR2B, NR2B and PSD95 were quantified and statistically compared. Data are represented as mean ± SE (n=6) per group, *P <0.05 vs. sham, #P < 0.05 vs. cont group.
Effect of tatCN21 on the interactions of CaMKIIα and NR2B in hippocampal CA1 at 6 h after GCI
We next performed Co-IP studies to assess the effect of tatCN21 peptide on the interaction of CaMKIIα and NR2B 6 h after GCI in the hippocampal CA1 region. As shown in Fig. 7A–C, the results clearly demonstrate that the interaction of CaMKIIα with NR2B was increased 6 h after GCI in the control group compared with sham group. Treatment with tatCN21 effectively decreased the CaMKIIα-NR2B interaction compared with control group. Duolink II in situ proximity ligation assay was performed to further investigate this phenomenon. As shown in Fig. 7D and E, the number of Duolink puncta observed was higher in the control group compared with the sham group. Treatment with tatCN21 peptide, however, diminished the association between CaMKIIα and NR2B, as evidenced by the decreased number of Duolink puncta compared with control group.
Fig. 7.
TatCN21 peptide decreased the interaction of CaMKIIα with NR2B following 6 h I/R. (A) Representative Co-IP of CaMKIIα and NR2B protein sample were immunoprecipated with NR2B antibody and WB with anti-CaMKIIα antibody. A reversal of Co-IP was also performed. (B, C) The binding level of CaMKIIα and NR2B were analyzed and shown as fold increased vs. Sham. (D) Duolink II in situ Proximity ligation assay using CaMKII α and NR2B antibodies in CA1 region of hippocampus 6 h after GCI. For clarity, both color and black and white images of Duolink puncta are provided. Bar scale: 50 μm. (E) Quantification of the Duolink puncta confirms that tatCN21 peptide treatment significantly reduces interaction of CaMKIIα and NR2B in the hippocampal CA1 region 6 h after GCI, compared with cont group. Data are represented as mean ± SE (n=6) per group, *P <0.05 vs. sham, #P < 0.05 vs. cont group, magnification 40x.
Discussion
The current study demonstrated that a cell permeable specific CaMKIIα peptide inhibitor, tatCN21, has efficacy as a potential treatment for neuroprotection and prevention of behavioral deficits accompanying GCI. This neuroprotection was illustrated vividly in the vulnerable hippocampal CA1 region. We also demonstrated the potential mechanism of action via inhibition of CaMKIIα phosphorylation and the membrane targeting of CaMKIIα. This is followed by the inhibition of CaMKIIα-NR2B complex formation and NR2B phosphorylation as well as its membrane translocation accompanied by aggregation of PSD95. The neuroprotective and behavioral improvement effects of tatCN21 peptide in our study appeared to be specific, as the control peptide (scrambled tat-peptide) offered no significant protection in ischemic control animals.
The inhibitory domain of the TatCN21 peptide is derived from the naturally occurring CaMKII inhibitory protein CaMKIIN (Chang et al., 1998; Vest et al., 2007). This peptide takes part in specific cellular events involved in inhibiting CaMKIIα activity, though its physiological function is still uncertain. The regulation of CaMKIIα activity is known to be required for controlling synaptic plasticity underlying higher brain function, such as learning and memory, long term potentiation (LTP), and glutamate induced cell death (Lisman et al., 2002; Lee & Silva, 2009; Buard et al., 2010; Ashpole & Hudmon, 2011). A recent study indicated that CaMKIIα inhibitor tatCN21 interferes with both aspects of CaMKIIα regulation, kinase activity and redistribution (Sanhueza et al., 2011). In the present study, we found the potent effects of tatCN21 peptide in inhibiting CaMKIIα phosphorylation and preventing aggregation on the membrane of hippocampal CA1 neurons following GCI.
It is generally believed that excitotoxic stimulation of NMDA receptor triggers Ca2+ influx and the activation of CaMKIIα (Hudmon & Schulman, 2002). The CaMKIIα is a major mediator of physiological excitatory glutamate signals underlying neuronal plasticity, learning, and memory. Over-influx of Ca2+ triggers phosphorylation of CaMKIIα, over elevating kinase activity (Coultrap et al., 2011). The elevated level of CaMKIIα activity appears to be essential for both calcium dysregulation and glutamatergic stimulation. A previous study showed that CaMKIIα was phosphorylated and translocated towards the membrane during ischemic events (Aronowski et al., 1992). The present study demonstrated that ischemia induced phosphorylation and membranous translocation of CaMKIIα, which is down regulated by tatCN21 peptide. This is in agreement with previous findings that revealed CaMKIIα targeted and phosphorylated NR2B following calcium influx (Strack et al., 2000).
The phosphorylation of CaMKIIα and NR2B were inhibited by post-GCI administration of tatCN21. The time course study clearly showed that the phosphorylation of CaMKIIα and NR2B both reached their peak at 6 h after I/R. This study clearly demonstrates the close relationship between CaMKIIα and NR2B and their phosphorylation following GCI. There is a line of evidence which reveals an important role of CaMKIIα contributing to NMDA receptor dependent long term potentiation in the hippocampus (Liu et al., 2001). In vitro observations have shown that CaMKIIα associates with the NR2B subunit of the NMDA receptor (Omkumar et al., 1996; Strack et al., 2000) upon calcium influx. Our present results further confirmed the binding of CaMKIIα with NR2B and increased NR2B phosphorylation after GCI. Moreover, the fact that preventing the CaMKIIα activation and subsequent NR2B phosphorylation mitigated neuronal damage corroborates with other studies investigating NR2B inhibition and the attenuated ischemic cell death (Chen et al., 2008). Several PSD proteins have been implicated in mediation synaptic events following CaMKIIα activation. (Lisman et al., 2002). Our results showed that tatCN21 mediated neuroprotection was accompanied by decreased membrane PSD95 aggregation, implying that decreased PSD95 membrane localization after GCI could contribute to the neuroprotective properties of tatCN21. Indeed, previous study showed that disruption of membrane aggregation of PSD95 and PSD95-NR2B binding following ischemic stroke conferred neuroprotection (Chen et al., 2015). Further studies should investigate whether tatCN21 affects the association of NR2B and PSD95 at the synaptic membrane, as well as other PSD95 associated contributors to ischemic injury.
In conclusion, the current study demonstrates that tatCN21 peptide can enhance neuroprotection, preserve learning and memory, and attenuate GCI-induced anxiety and spontaneous motor activity. TatCN21 owes these neuroprotective properties due to its role as a specific inhibitor of CaMKIIα, which prevents CaMKIIα activation and targeting to NR2B that leads to detrimental NMDA receptor mediated cellular toxicity. Treatment with tatCN21 prevents the association of CaMKIIα with the NMDA receptor, preventing the phosphorylation of NR2B and membrane PSD95 aggregation. This study provides evidence of an important targetable mechanism of calcium toxicity in GCI as well as one potential therapeutic option. Furthermore, this study provides important evidence that inhibition of CaMKIIα over-activation in GCI exerts protective effects on hippocampal-dependent spatial learning.
Acknowledgments
This study was supported by Research Grant NS086929 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, USA; and the American Heart Association Grant-in-Aid 15GRNT25240004.
Footnotes
Conflict of interest
The author declares that there is no conflict of interest.
References
- Aronowski J, Grotta JC, Waxham MN. Ischemia-induced translocation of Ca2+/calmodulin-dependent protein kinase II: potential role in neuronal damage. Journal of neurochemistry. 1992;58:1743–1753. doi: 10.1111/j.1471-4159.1992.tb10049.x. [DOI] [PubMed] [Google Scholar]
- Ashpole NM, Hudmon A. Excitotoxic neuroprotection and vulnerability with CaMKII inhibition. Molecular and cellular neurosciences. 2011;46:720–730. doi: 10.1016/j.mcn.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Babcock AM, Everingham A, Paden CM, Kimura M. Baclofen is neuroprotective and prevents loss of calcium/calmodulin-dependent protein kinase II immunoreactivity in the ischemic gerbil hippocampus. Journal of neuroscience research. 2002;67:804–811. doi: 10.1002/jnr.10169. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. Journal of comparative and physiological psychology. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Bothe HW, Bosma HJ, Hofer H, Hossmann KA, Angermeier WF. Selective vulnerability of hippocampus and disturbances of memory storage after mild unilateral ischemia of gerbil brain. Stroke; a journal of cerebral circulation. 1986;17:1160–1163. doi: 10.1161/01.str.17.6.1160. [DOI] [PubMed] [Google Scholar]
- Buard I, Coultrap SJ, Freund RK, Lee YS, Dell’Acqua ML, Silva AJ, Bayer KU. CaMKII “autonomy” is required for initiating but not for maintaining neuronal long-term information storage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:8214–8220. doi: 10.1523/JNEUROSCI.1469-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neuroscience and biobehavioral reviews. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Chang BH, Mukherji S, Soderling TR. Characterization of a calmodulin kinase II inhibitor protein in brain. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:10890–10895. doi: 10.1073/pnas.95.18.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Lu TJ, Chen XJ, Zhou Y, Chen Q, Feng XY, Xu L, Duan WH, Xiong ZQ. Differential roles of NMDA receptor subtypes in ischemic neuronal cell death and ischemic tolerance. Stroke; a journal of cerebral circulation. 2008;39:3042–3048. doi: 10.1161/STROKEAHA.108.521898. [DOI] [PubMed] [Google Scholar]
- Chen Y, Brennan-Minnella AM, Sheth S, El-Benna J, Swanson RA. Tat-NR2B9c prevents excitotoxic neuronal superoxide production. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015;35:739–742. doi: 10.1038/jcbfm.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churn SB, Limbrick D, Sombati S, DeLorenzo RJ. Excitotoxic activation of the NMDA receptor results in inhibition of calcium/calmodulin kinase II activity in cultured hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:3200–3214. doi: 10.1523/JNEUROSCI.15-04-03200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap SJ, Vest RS, Ashpole NM, Hudmon A, Bayer KU. CaMKII in cerebral ischemia. Acta pharmacologica Sinica. 2011;32:861–872. doi: 10.1038/aps.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SA, Law CD, Abshire JS. Developmental treatment with bisphenol A causes few alterations on measures of postweaning activity and learning. Neurotoxicology and teratology. 2012;34:598–606. doi: 10.1016/j.ntt.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behavioral neuroscience. 2008;122:16–26. doi: 10.1037/0735-7044.122.1.16. [DOI] [PubMed] [Google Scholar]
- Hanson SK, Grotta JC, Waxham MN, Aronowski J, Ostrow P. Calcium/calmodulin-dependent protein kinase II activity in focal ischemia with reperfusion in rats. Stroke; a journal of cerebral circulation. 1994;25:466–473. doi: 10.1161/01.str.25.2.466. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H. Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annual review of biochemistry. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- Kirino T, Tamura A, Sano K. Selective vulnerability of the hippocampus to ischemia--reversible and irreversible types of ischemic cell damage. Progress in brain research. 1985;63:39–58. doi: 10.1016/S0079-6123(08)61974-3. [DOI] [PubMed] [Google Scholar]
- Lee JC, Tae HJ, Cho GS, Kim IH, Ahn JH, Park JH, Chen BH, Cho JH, Shin BN, Bae EJ, Park J, Kim YM, Choi SY, Won MH. Ischemic preconditioning protects neurons from damage and maintains the immunoreactivity of kynurenic acid in the gerbil hippocampal CA1 region following transient cerebral ischemia. International journal of molecular medicine. 2015;35:1537–1544. doi: 10.3892/ijmm.2015.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Silva AJ. The molecular and cellular biology of enhanced cognition. Nature reviews Neuroscience. 2009;10:126–140. doi: 10.1038/nrn2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nature reviews Neuroscience. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang G, Gao C, Hou X. NMDA receptor activation results in tyrosine phosphorylation of NMDA receptor subunit 2A(NR2A) and interaction of Pyk2 and Src with NR2A after transient cerebral ischemia and reperfusion. Brain research. 2001;909:51–58. doi: 10.1016/s0006-8993(01)02619-1. [DOI] [PubMed] [Google Scholar]
- Lu Q, Tucker D, Dong Y, Zhao N, Zhang Q. Neuroprotective and Functional Improvement Effects of Methylene Blue in Global Cerebral Ischemia. Molecular neurobiology. 2015 doi: 10.1007/s12035-015-9455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangus DB, Huang L, Applegate PM, Gatling JW, Zhang J, Applegate RL., 2nd A systematic review of neuroprotective strategies after cardiac arrest: from bench to bedside (Part I - Protection via specific pathways) Medical gas research. 2014;4:9. doi: 10.1186/2045-9912-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Shamloo M, Matsumoto E, Isshiki A, Wieloch T. Protein kinase C-gamma and calcium/calmodulin-dependent protein kinase II-alpha are persistently translocated to cell membranes of the rat brain during and after middle cerebral artery occlusion. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2004;24:54–61. doi: 10.1097/01.WCB.0000095920.70924.F5. [DOI] [PubMed] [Google Scholar]
- Meng F, Guo J, Zhang Q, Song B, Zhang G. Autophosphorylated calcium/calmodulin-dependent protein kinase II alpha (CaMKII alpha) reversibly targets to and phosphorylates N-methyl-D-aspartate receptor subunit 2B (NR2B) in cerebral ischemia and reperfusion in hippocampus of rats. Brain research. 2003;967:161–169. doi: 10.1016/s0006-8993(02)04267-1. [DOI] [PubMed] [Google Scholar]
- Meng F, Zhang G. Autophosphorylated calcium/calmodulin-dependent protein kinase II alpha induced by cerebral ischemia immediately targets and phosphorylates N-methyl-D-aspartate receptor subunit 2B (NR2B) in hippocampus of rats. Neuroscience letters. 2002;333:59–63. doi: 10.1016/s0304-3940(02)00961-8. [DOI] [PubMed] [Google Scholar]
- Omkumar RV, Kiely MJ, Rosenstein AJ, Min KT, Kennedy MB. Identification of a phosphorylation site for calcium/calmodulindependent protein kinase II in the NR2B subunit of the N-methyl-D-aspartate receptor. The Journal of biological chemistry. 1996;271:31670–31678. doi: 10.1074/jbc.271.49.31670. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European journal of pharmacology. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Sanhueza M, Fernandez-Villalobos G, Stein IS, Kasumova G, Zhang P, Bayer KU, Otmakhov N, Hell JW, Lisman J. Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:9170–9178. doi: 10.1523/JNEUROSCI.1250-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareddy GR, Zhang Q, Wang R, Scott E, Zou Y, O’Connor JC, Chen Y, Dong Y, Vadlamudi RK, Brann D. Proline-, glutamic acid-, and leucine-rich protein 1 mediates estrogen rapid signaling and neuroprotection in the brain. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E6673–6682. doi: 10.1073/pnas.1516729112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40:599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Schneider A, Bottiger BW, Popp E. Cerebral resuscitation after cardiocirculatory arrest. Anesthesia and analgesia. 2009;108:971–979. doi: 10.1213/ane.0b013e318193ca99. [DOI] [PubMed] [Google Scholar]
- Schwarzbach E, Bonislawski DP, Xiong G, Cohen AS. Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. Hippocampus. 2006;16:541–550. doi: 10.1002/hipo.20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack S, Choi S, Lovinger DM, Colbran RJ. Translocation of autophosphorylated calcium/calmodulin-dependent protein kinase II to the postsynaptic density. The Journal of biological chemistry. 1997;272:13467–13470. doi: 10.1074/jbc.272.21.13467. [DOI] [PubMed] [Google Scholar]
- Strack S, Colbran RJ. Autophosphorylation-dependent targeting of calcium/calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-D-aspartate receptor. The Journal of biological chemistry. 1998;273:20689–20692. doi: 10.1074/jbc.273.33.20689. [DOI] [PubMed] [Google Scholar]
- Strack S, McNeill RB, Colbran RJ. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. The Journal of biological chemistry. 2000;275:23798–23806. doi: 10.1074/jbc.M001471200. [DOI] [PubMed] [Google Scholar]
- Tu J, Zhang X, Zhu Y, Dai Y, Li N, Yang F, Zhang Q, Brann DW, Wang R. Cell-Permeable Peptide Targeting the Nrf2-Keap1 Interaction: A Potential Novel Therapy for Global Cerebral Ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:14727–14739. doi: 10.1523/JNEUROSCI.1304-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vest RS, Davies KD, O’Leary H, Port JD, Bayer KU. Dual mechanism of a natural CaMKII inhibitor. Molecular biology of the cell. 2007;18:5024–5033. doi: 10.1091/mbc.E07-02-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vest RS, O’Leary H, Coultrap SJ, Kindy MS, Bayer KU. Effective post-insult neuroprotection by a novel Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) inhibitor. The Journal of biological chemistry. 2010;285:20675–20682. doi: 10.1074/jbc.M109.088617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QG, Raz L, Wang R, Han D, De Sevilla L, Yang F, Vadlamudi RK, Brann DW. Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009a;29:13823–13836. doi: 10.1523/JNEUROSCI.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QG, Wang R, Han D, Dong Y, Brann DW. Role of Rac1 GTPase in JNK signaling and delayed neuronal cell death following global cerebral ischemia. Brain research. 2009b;1265:138–147. doi: 10.1016/j.brainres.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QG, Wang R, Khan M, Mahesh V, Brann DW. Role of Dickkopf-1, an antagonist of the Wnt/beta-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of tau phosphorylation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:8430–8441. doi: 10.1523/JNEUROSCI.2752-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Tu J, Zhang Q, Lu D, Zhu Y, Zhang W, Yang F, Brann DW, Wang R. Delayed ischemic postconditioning protects hippocampal CA1 neurons by preserving mitochondrial integrity via Akt/GSK3beta signaling. Neurochemistry international. 2011;59:749–758. doi: 10.1016/j.neuint.2011.08.008. [DOI] [PubMed] [Google Scholar]