Abstract
Sepsis refers to the presence of a serious infection that correlates with systemic and uncontrolled immune activation. Posttranslational histone modification plays an important role in chromatin decondensation, which is regulated by citrullination. Citrullinated histone H3 (H3cit) has been identified as a component of neutrophil extracellular traps (NETs), which are released into the extracellular space as part of the neutrophil response to infection. The conversion of arginine to citrulline residues on histones is catalyzed by peptidylarginine deiminase 4 (PAD4). This study's goals were to characterize the presence of PAD4-catalyzed H3cit and NET formation during the onset of sepsis and elucidate the effects on the immune response when this mechanism of action is blocked. Adult C57BL/6 male mice were treated with Cl-amidine, an inhibitor of PAD4, 1 h prior to sepsis induced by cecal ligation and puncture (CLP). Twenty-four hours after CLP, cytokine levels, H3cit protein expression, neutrophil counts, and NET production were evaluated in the peritoneal cavity. Survival studies were also performed. Here we demonstrate that Cl-amidine treatment prior to CLP improves overall survival in sepsis and the abrogation of PAD4 has minimal effects on the proinflammatory immune response to sepsis, while it has no effect on overall neutrophil migration to the peritoneum.
Keywords: Neutrophils, Cecal ligation and puncture, Inflammation, Posttranslational modification
Introduction
Sepsis is a life-threatening condition that elicits an exacerbated and damaging immune response with approximately 250,000 cases leading to death in the USA annually [1,2,3]. As of 2009, the CDC listed sepsis as the 11th leading cause of death in the USA, making sepsis a significant health care problem [4,5].
Neutrophils are considered one of the most crucial components of the innate immune system during septic infection [6]. Neutrophil extracellular traps (NETs) are complex structures made of nuclear chromatin, histones, granular antimicrobial proteins, and some cytoplasmic proteins [7]. NETs are capable of physically ensnaring bacteria and facilitating the interactions between bacteria and antimicrobial effectors, ultimately leading to enhanced bacterial killing [7]. Components of NETs have been shown to be elevated in septic and septic shock patients [8,9], suggesting that NETs may play an important part in the innate immune response to infection [10]. However, increasing evidence has linked NET formation to various disease states, such as autoimmune diseases, as well as sepsis, suggesting that they contribute to excessive inflammation and tissue damage [6,11,12].
Posttranslational histone modification plays an important role in chromatin decondensation, which is regulated by citrullination [13]. Citrullinated histone H3 (H3cit) has been identified as a component of NETs that is released into the extracellular space as part of the neutrophil response to infection [10,14,15,16]. Recently, it has been revealed that peptidylarginine deiminase 4 (PAD4) has a regulatory role in NET formation through mediating chromatin decondensation through hypercitrullination of the target histones H3, H4, and H2A [16,17,18]. This histone citrullination and NET formation are essential elements of host defense and have been shown to be necessary in innate immunity during bacterial infection [10].
In order to determine a putative role of NETs in sepsis, it is necessary to characterize the presence of PAD4 catalyzed H3cit and NET formation during the onset of sepsis as well as the effects on the immune response when this mechanism of action is blocked. While genetic PAD4 knockout mice have been used to study the role of NETs in various disease states, including sepsis [10,19,20,21,22], to date there is no inhibitor available that specifically targets NET formation. N-α-benzoyl-N5-(chloro-iminoethyl)-L-ornithine amide, or Cl-amidine, is a pharmaceutical inhibitor of peptidylarginine deiminases (PADs) including PAD4 [23,24]. It irreversibly inactivates PADs by covalently modifying an active site cysteine that is important for its catalytic activity [23]. It was previously found to repress the formation of NET-like structures in HL-60 cells [17,25], and has also been used in various studies to examine the mechanism of PAD4 and NET inhibition [10,21,24].
Here we first assessed the level of H3cit protein modification in a mouse cecal ligation and puncture (CLP) model of sepsis. We then suppressed H3cit in vivo using a dose of Cl-amidine prior to CLP and studied what effect this had on H3cit modification as well as its effect on the immune response and overall survival. We found that Cl-amidine treatment prior to surgery significantly improves overall survival in a CLP model of sepsis, but it seems to have little effect on the proinflammatory or anti-inflammatory cytokine response and no effects on overall neutrophil migration to the source of infection.
Materials and Methods
Mice
C57BL/6 male mice, aged 8–12 weeks, were obtained from the Jackson Laboratory (Bar Harbor, Maine, USA) and used in all experiments. All protocols carried out with animals were done according to the NIH Guide for Animal Use and Care guidelines, and were approved by the Rhode Island Hospital Institutional animal care and use committee (AWC 0110-13).
Sepsis Model Induced by CLP
Mice were anesthetized with isoflurane and a midline incision was made in the abdomen. The cecum was isolated and ligated at a point approximately 1 cm from the cecal tip, punctured twice with a 22-gauge needle, then gently squeezed to extrude a small amount of feces from the perforation sites. In the sham/CLP mice, the cecum was exposed but neither ligated nor punctured. The cecum was then placed back into the peritoneal cavity and the incision was sutured closed in two layers. Mice were resuscitated with 1 ml of Ringers lactate by subcutaneous injection [26].
Cl-Amidine Calculations and Dosing
Cl-amidine (Cayman Chemical, Ann Arbor, Mich., USA) was reconstituted in EtOH for a stock solution of 20 mg/ml and kept at −20°C. Working solutions were created by diluting Cl-amidine stock solution with PBS to a concentration of 2.0 mg/ml. Mice in the Cl-amidine treatment groups were given doses of 50 mg/kg [27,28] subcutaneously, 30–60 min prior to CLP (control animals received PBS).
Survival Study
Both Cl-amidine-treated and control mice (n = 12/group) were subjected to CLP, and received additional doses once a day for 7 days. Their survival was observed; log-rank statistical analysis was used to determine if a statistically significant difference in septic mortality was evident between the two groups at p < 0.05.
Sample Collection
At 24 or 48 h after the sham/CLP procedures, the mice were euthanized with a CO2 overdose. Blood was collected in a heparinized tube via cardiac puncture and centrifuged to obtain plasma. To collect peritoneal fluid for Western blot and cytokine analysis, 1 ml of 1× PBS was injected intraperitoneally, recollected, and centrifuged at 10,000 g for 10 min. The supernatant was collected. For the collection of peritoneal cells, 5 ml of 1× PBS was injected and recollected via IP, and centrifuged at 10,000 g for 10 min. The cell pellet was collected for NET analysis and morphological analysis. For Western blot analysis, cell pellets were lysed and spun down again at 10,000 g for 10 min and lysate was collected. The lung, liver, spleen, and kidney were also harvested for cytokine analysis. The protein concentration in lavage fluid and cell/tissue lysate was assessed by Bradford assay. All of the samples collected were stored at −80°C until needed.
H3cit Protein Modification
Samples were probed for H3cit via Western blot. Proteins (25 μg per lane) were separated by 16% SDS-page gels and transferred on to polyvinylidene fluoride membranes (Novex, San Diego, Calif., USA). The membranes were blocked with 5% milk in PBST and probed with anti-histone H3 (citrulline R2+R8+R17; ab5103) or anti-histone H3 (ab8898; Abcam, Cambridge, Mass., USA) at 1 μg/ml overnight at 4°C and subsequently incubated with a HRP-conjugated anti-rabbit IgG (1:10,000) at room temperature for 1 h. Chemiluminescence detection was performed using ECL reagent (GE Healthcare, Pittsburgh, Pa., USA), and films were developed using the standard procedure. The abundance of modified histone H3 protein was densitometrically assessed on an Alpha-Innotech image analyzer (San Leandro, Calif., USA) [29]. H3cit protein was then normalized to either β-actin for the cell lysates or to the Ponceau-stained membrane for secretory proteins in peritoneal fluid [30].
Measurement of Cytokines
Concentrations of interleukin (IL)-6 and IL-10 from the peritoneal fluid, plasma, and tissue samples were assessed via ELISA according to manufacturer's protocols (BD Bioscience) [31].
NET Visualization/Flow Cytometry
Cells collected from peritoneal lavage were resuspended (1:20) in 1× PBS and placed on tissue culture plates coated with 1% BSA and incubated at 37°C for 1–2 h. A total of 5 μM of Sytox green (Thermo Fischer Scientific, Waltham, Mass., USA) was added 10 min prior to imaging. The images were captured using a Nikon TE-2000U inverted microscope (Nikon, Melville, N.Y., USA) coupled to an iXonEM + 897E back illuminated EMCCD camera (Andor, Belfast, UK). Bright field images were captured using NIS-Elements software (Nikon). A xenon lamp illuminated cells through a 33-mm ND4 filter and 20× Nikon Plan Apochromat objective using a Nikon B2-A long-pass emission filter set cube.
For flow cytometry, all the antibodies used for analysis were purchased from eBioscience (San Diego, Calif., USA). LY6G+ cell populations in the peritoneal lavage were determined with a FACS Array flow cytometer (BD Bioscience, San Jose, Calif., USA) and analyzed with FlowJo software (Tree Star, Ashland, Oreg., USA) [31].
NET Quantification
Neutrophils were prepared as described above for NET visualization/flow cytometry. NETs were visualized with Sytox green and multiple images were obtained per well. Images were thresholded using the default thresholding algorithm in ImageJ (NIH, Bethesda, Md., USA) and gated to include extruded NETs and exclude stained nuclei. NET formation was quantified as the percent area of the totaled imaged field. Well averages were then ensemble averaged. Data represent 4–6 wells per condition.
Statistics
Results are expressed as the mean ± SEM. The statistical significance of the results presented were determined by one-way ANOVA (for multiple comparison) with all pairwise multiple comparison procedures (the Holm-Sidak or Dunn's method), unpaired two-tailed Student's t test (for normal distribution data), or log-rank test (for survival study) where appropriate. The statistical software used was SigmaPlot 11.0 (Systat Software Inc., San Jose, Calif., USA), and p ≤ 0.05 was used as a cutoff for significance. For NET quantification ANOVA analysis with Newman-Keuls post hoc analysis or unpaired-sample two-tailed Student's t test as appropriate was performed using MATLAB (Mathworks, Natick, Mass., USA) or Excel (Microsoft, Redmond, Wash., USA) running the statistiXL data package (statistiXL, Nedlands, W.A., Australia). The null hypothesis was rejected at p < 0.05.
Results
Citrullination of Histone H3 Is Increased 24 h after CLP
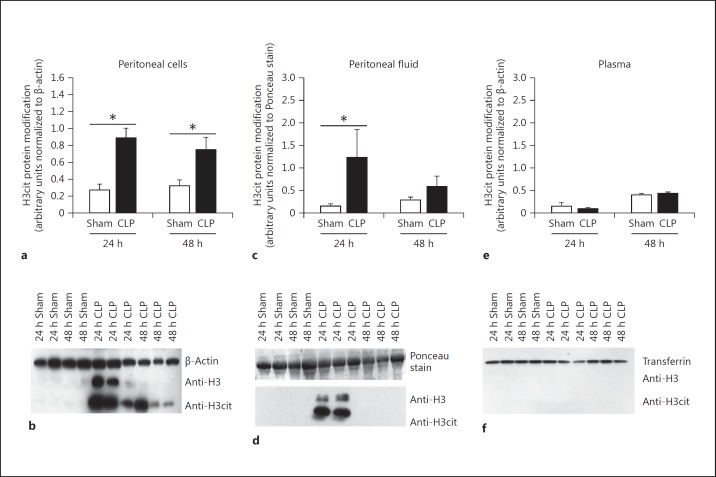
Histone H3 citrullination has been used as a marker for PAD4 activity and NET formation [10,17]. First, we wanted to determine if H3cit protein modification was evident in our CLP model of sepsis. The abundance of H3cit protein modification was measured in plasma, peritoneal fluid, and peritoneal cells 24 and 48 h after CLP via Western blot assay. Histone H3 was also measured in whole cell lysates and peritoneal fluid. To further ensure that H3cit modification was independent of histone H3, we blotted for histone H3 as well as H3cit in nuclear protein isolated from peritoneal cells and determined that H3cit levels were separate from histone H3 levels (online suppl. fig. 1; for all online suppl. material, see www.karger.com/doi/10.1159/000448808). At 24 h after CLP, H3cit protein abundance was significantly elevated in the peritoneal cells (fig. 1a, b) as well as in peritoneal fluid (fig. 1c, d) when compared to sham mice; however, H3cit was not detected in the plasma of sham or CLP mice (fig. 1e, f). At 48 h after CLP, a significant increase of H3cit was still detected in peritoneal cell lysates; however, H3cit was no longer present in the peritoneal fluid. Therefore, a 24-hour time point was chosen for later experiments.
Fig. 1.
H3cit protein modification is present after CLP. a, b The H3cit protein modification is highly abundant in cells collected from the peritoneal cell lysates collected 24 h after CLP. At 48 h after CLP there is still an increase in H3cit protein modification. c, d Fluid collected from the peritoneal cavity is also increased in H3cit protein modification at 24 h after CLP, while levels decrease after 48 h. e, f At both time points, the H3cit protein modification was undetectable in the bloodstream. H3cit protein was then normalized to either β-actin for the cell lysates or to the Ponceau-stained membrane that demonstrated equal loading in the peritoneal fluid. Sham, n = 4; CLP, n = 5–6 per group. * p < 0.05, one-way ANOVA.
Cl-Amidine Reduces the Abundance of the H3cit Protein Modification in Peritoneal Fluid and Peritoneal Cells after CLP
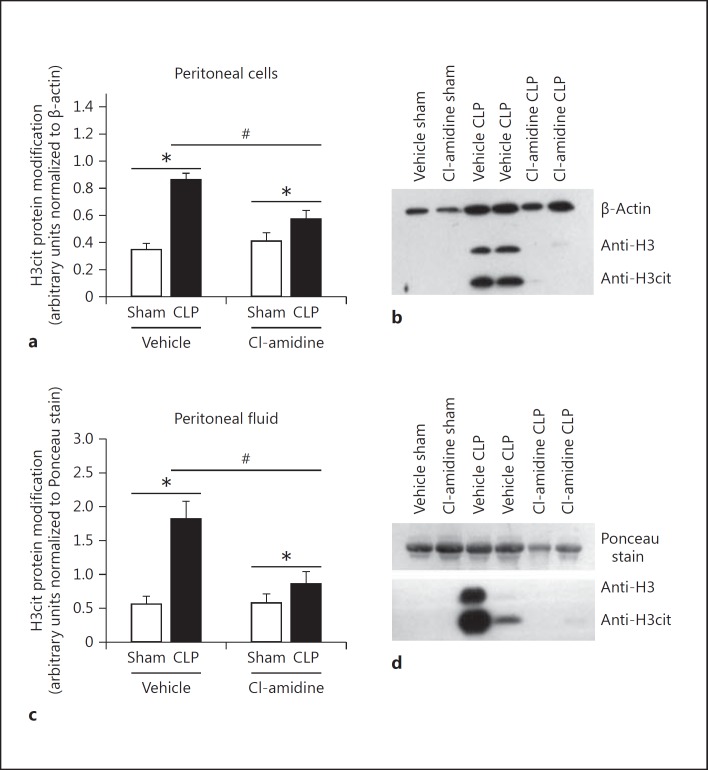
We wanted to determine whether inhibition of PAD4 activity would eliminate or significantly decrease H3cit protein modification in mice subjected to CLP. Cl-amidine was administered at a dose of 50 mg/kg given subcutaneously 30–60 min prior to CLP. Overall, in vivo Cl-amidine treatment significantly reduced H3cit protein modification at 24 h after CLP in peritoneal cells (fig. 2a, b) as well as in peritoneal fluid (fig. 2c, d). While elimination of an H3cit protein band was not seen in every treated animal, there was still a consistent reduction in comparison to the vehicle-treated mice.
Fig. 2.
H3cit protein modification is significantly diminished in the peritoneal cavity in mice treated with Cl-amidine 24 h after CLP. Mice were either treated with Cl-amidine given at 50 mg/kg by subcutaneous injection or vehicle control (1× PBS) 30–60 min prior to CLP. Peritoneal cell lysates and peritoneal fluid were then collected from both groups of mice 24 h after CLP. a, b The abundance of H3cit protein modification was significantly diminished in the peritoneal cell lysates collected from treated mice as compared to vehicle control mice. c, d The levels of H3cit protein were also significantly diminished in peritoneal fluid after treatment. Overall, treatment with Cl-amidine did not fully eliminate the H3cit protein modification in every treated animal; however, there was still a consistent reduction in comparison to the vehicle-treated mice. H3cit protein was then normalized to either β-actin for the cell lysates or a nonspecific 64-kD band located on the Ponceau-stained membrane that demonstrated equal loading in the peritoneal fluid gels. Sham, n = 4; CLP, n = 6–8 per group. * p < 0.05 sham versus CLP, # p < 0.05 CLP vehicle versus CLP Cl-amidine, one-way ANOVA.
Cl-Amidine Treatment Reduces NET Formation in Peritoneal Cells after CLP
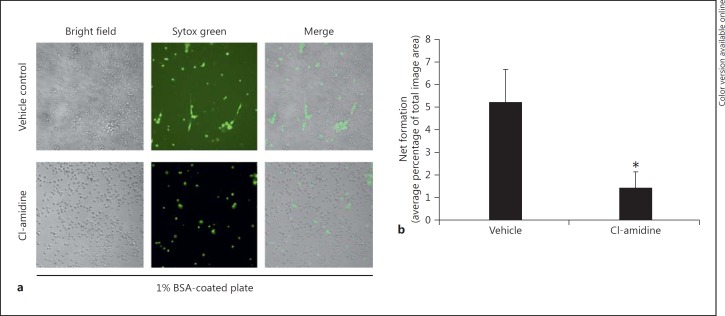
To support the observation that Cl-amidine treatment reduces the H3cit protein modification implicated in NET formation after CLP, the capacity for NET formation by peritoneal neutrophils was assessed ex vivo. Peritoneal content was collected via lavage (which is a compilation of mostly CLP-activated neutrophils, and macrophages) from mice 24 h after CLP. Peritoneal cells were cultured on BSA-coated plates with no ex vivo stimulation and stained with Sytox green for extracellular nucleic acid visualization, which has been used to identify NET formation in stimulated cells [32]. After 1–2 h of incubation, there was a marked reduction of NET formation in mouse cells taken from the Cl-amidine-treated mice as compared to the vehicle-treated CLP mice (fig. 3a, b).
Fig. 3.
Cl-amidine treatment in vivo reduces NET formation in murine neutrophils ex vivo. Septic mouse lavage contents were placed on tissue culture plates coated with 1% BSA (with no additional stimulation) incubated at 37°C for 1–2 h, and stained with Sytox green for extracellular nucleic acid visualization. a Peritoneal cells from Cl-amidine-treated mice display a decrease in extracellular nuclear material (NET formation) compared to control cells at 1–2 h. Original magnification ×20. b This reduction in NET formation is statistically significant when quantified as the percent area of the totaled imaged field. (Data represent 4–6 wells per condition.) * p < 0.05, ANOVA analysis with Newman-Keuls post hoc analysis or unpaired-sample two-tailed Student's t test as appropriate.
Cl-Amidine Treatment Does Not Alter Neutrophil Recruitment to the Peritoneum after CLP
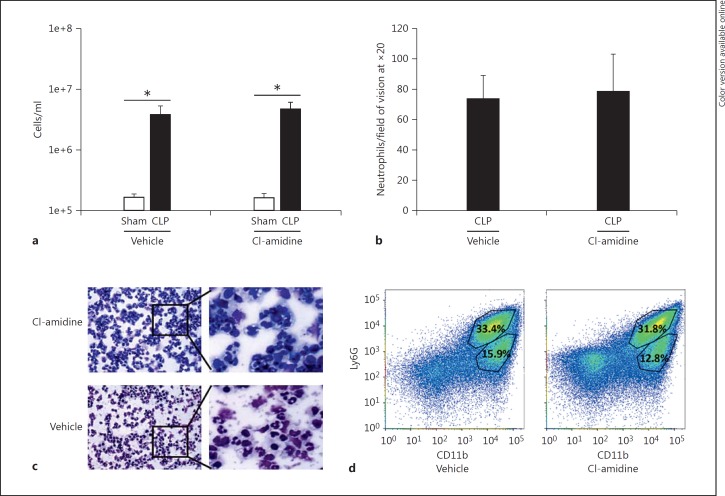
To determine whether the reduction of H3cit protein modification in the peritoneal cavity was due to PAD4 inhibition, as opposed to a possible off-target effect of Cl-amidine that may hamper neutrophil migration to the site of infection, we compared the number of neutrophils in the peritoneal cavity in the Cl-amidine-treated and vehicle-treated groups after CLP. Cell numbers were significantly increased in both treated groups subjected to CLP as compared to sham animals (fig. 4a). However, there was no difference in cell numbers between the Cl-amidine and vehicle-treated CLP groups. As the peritoneum after CLP is inundated with not just neutrophils, but also other innate immune cells [33,34,35,36], we further examined the cell heterogeneity in the peritoneum by performing a cytospin and Wright's stain on cells collected from the peritoneum after CLP. Once again there were comparable cell numbers as well as cell subtypes in both groups (fig. 4b, c). Cells from the peritoneum were also stained for CD11b and Ly6G to compare the percentages of macrophages (CD11bhigh/Ly6Glow) and neutrophils (CD11bhigh/Ly6Ghigh) present at the site of infection (fig. 4d). There was no difference in the percentage of CD11bhigh/Ly6Glow or CD11bhigh/Ly6Ghigh cells in the peritoneum in the treated CLP mice as compared to the vehicle-treated CLP mice.
Fig. 4.
Cl-amidine treatment has no effect on neutrophil migration to the peritoneum after CLP. Cells were collected from the peritoneum by injecting 5 ml of 1× PBS into the abdomen and then harvesting an equal volume. a The total number of cells as determined by a hemocytometer was not different between the Cl-amidine- and vehicle-treated groups. b, c Cytospins of peritoneal cells were Wright-stained and analyzed for neutrophil counts at ×20 magnification using an RGB, DIC N1 filter (0.33 µM/pixel). The number of neutrophils did not significantly differ between the vehicle control and the Cl-amidine-treated animals. d The percentage of Ly6G+ cells in the peritoneum in both groups at 24 h after CLP demonstrated no significant difference between the vehicle- and CL-amidine-treated animals, consistent with neutrophil counts. Sham, n = 4; CLP, n = 12 per group. * p < 0.05, one-way ANOVA.
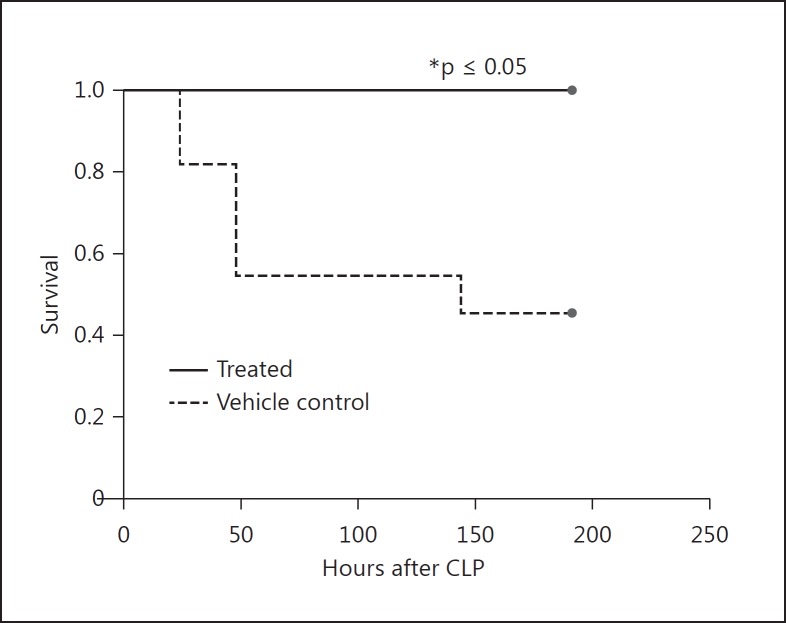
Cl-Amidine Treatment Improves Survival in the CLP Sepsis Model
Extracellular histones as well as circulating free DNA (cf-DNA) have been implicated in increased organ injury and mortality in the CLP model [8,11]. To better understand the role of PAD4 in sepsis mortality, we performed CLP in two groups: the first group was treated with Cl-amidine 30–60 min prior to the procedure, and the second with vehicle control. Mice were then given subsequent doses of Cl-amidine or vehicle, respectively, every day for 7 days. We observed that the Cl-amidine-treated animals were significantly protected from CLP-induced mortality, with a 100% survival rate at 7 days, as compared to the vehicle-treated group which had a 45% survival rate (fig. 5). Animals undergoing sham surgery had a 100% survival rate.
Fig. 5.
Cl-amidine treatment improves survival in the CLP sepsis model. Mice were divided into randomized groups and treated with a 50 mg/kg dose of Cl-amidine or 1× PBS (vehicle control) by subcutaneous injection 30–60 min prior to CLP and then once a day for 7 days after the procedure. The Cl-amidine-treated group had a 100% survival rate compared to the control group, which had a 45% survival rate. Sham animals had a 100% survival rate (data not shown). Sham, n = 3; CLP, n = 12 per group. * p < 0.05, log-rank test.
Cl-Amidine Treatment Leads to a Minimally Decreased Proinflammatory Response
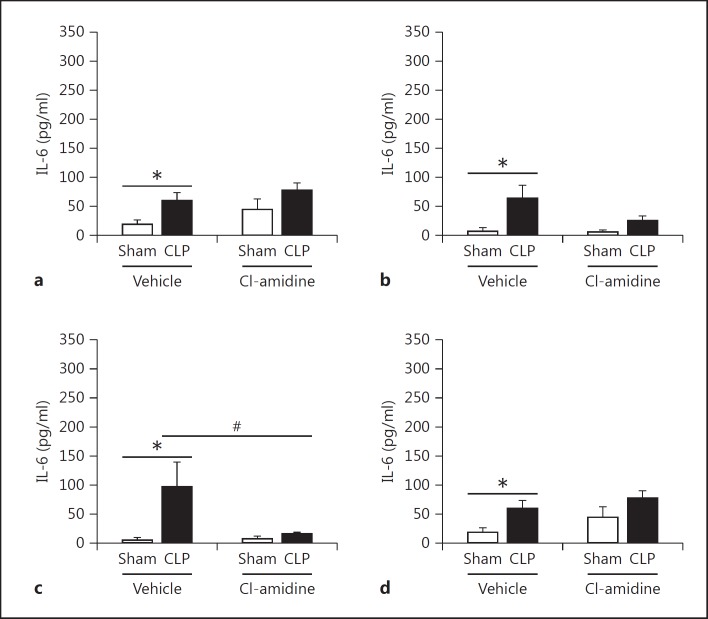
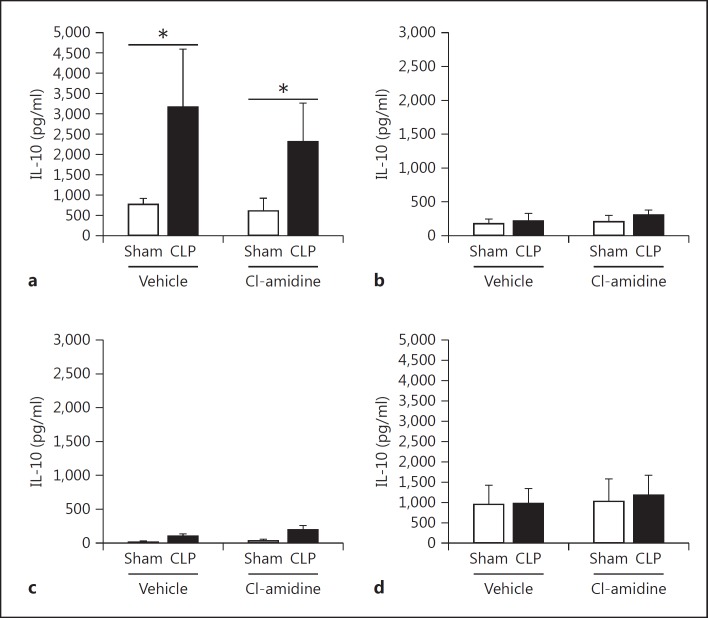
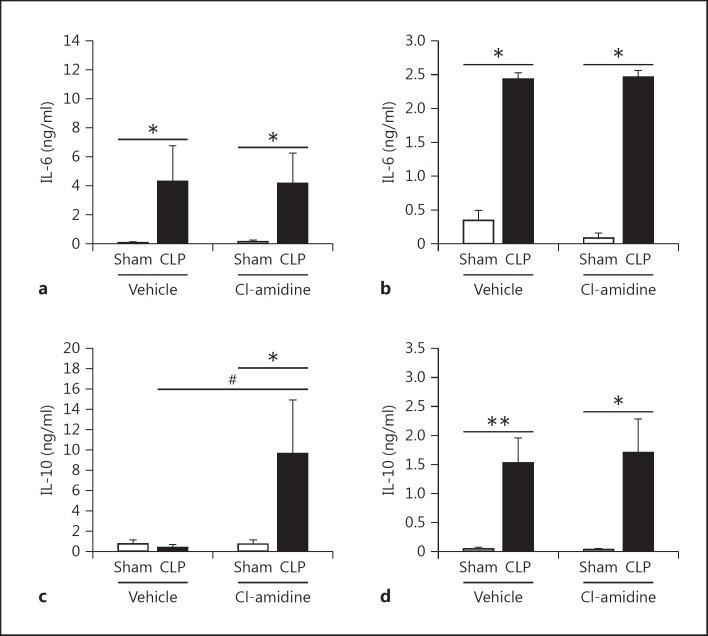
To further investigate how Cl-amidine increases survival, we measured alterations in the proinflammatory cytokine IL-6 as well as anti-inflammatory cytokine IL-10 in tissues known to be affected by sepsis [37,38,39], in the plasma, and in peritoneal fluid [40]. Extracellular histones and NETs have been attributed to causing excessive inflammation [12,41,42]. Here we found that there was a significant, marked reduction of IL-6 in the spleen. However, there was no significant reduction of IL-6 in the kidney, lung, and liver tissues (fig. 6). This may be attributed to the increase of other cell types, such as macrophages, which also secrete IL-6 after CLP as part of the initial innate immune response to infection [36]. In addition, there was also no difference in IL-10 levels found in tissues (fig. 7). When looking systemically (plasma) and at the site of infection (peritoneal fluid) there was no reduction of IL-6, but there was a significant increase of IL-10 levels in the bloodstream in the Cl-amidine-treated mice as compared to both the sham and vehicle control CLP mice (fig. 8).
Fig. 6.
Cl-amidine treatment significantly alters proinflammatory IL-6 cytokine levels in the spleen after CLP, but not in other tissues. Tissue homogenate lysates were normalized using a Bradford protein assay and were then assessed for IL-6 levels by ELISA. The tissues assessed were kidney (a), lung (b), spleen (c), and (d) liver 24 h after CLP. IL-6 levels were not significantly altered between the vehicle control and Cl-amidine-treated mice in all tissues except in the spleen, where Cl-amidine treatment significantly reduced IL-6 levels as compared to vehicle control CLP mice. Sham, n = 6; CLP, n = 12–16 per group. * p < 0.05 sham versus CLP, # p < 0.05 CLP vehicle versus CLP Cl-amidine, one-way ANOVA.
Fig. 7.
Cl-amidine treatment does not significantly alter anti-inflammatory IL-10 cytokine levels in tissues. The same tissue homogenate lysates used for IL-6 level assessment were also assessed for IL-10 levels as a measurement of alteration in the anti-inflammatory response. The tissues assessed were kidney (a), lung (b), spleen (c), and liver (d) 24 h after CLP. There was a significant increase in IL-10 levels in the kidneys in both the vehicle and Cl-amidine CLP groups compared to shams. However, IL-10 levels were not significantly different between the vehicle control and Cl-amidine-treated mice in all tissues. Sham, n = 6; CLP, n = 12–16 per group. * p < 0.05 sham versus CLP, one-way ANOVA.
Fig. 8.
Cl-amidine treatment increases systemic levels of IL-10 but has no impact on the systemic proinflammatory response after CLP. Plasma was collected from heparinized whole blood via cardiac puncture, and peritoneal fluid was collected from the abdomen by injecting 1 ml of 1× PBS intraperitoneally and then recollected. Both samples were analyzed for IL-6 and IL-10 levels. a, b IL-6 levels were significantly increased in the plasma and peritoneal fluid of both the vehicle and Cl-amidine-treated groups after CLP. c Systemically, IL-10 levels were significantly increased in the Cl-amidine-treated mice as compared to the vehicle control mice after CLP. d IL-10 levels were comparable in both groups at the site of infection in the peritoneum. Sham, n = 6; CLP, n = 12–16 per group. * p < 0.05, ** p < 0.01 sham versus CLP, # p < 0.05 CLP vehicle versus CLP Cl-amidine, one-way ANOVA.
Discussion
NETs are complex structures comprised of nuclear chromatin, histones, granular antimicrobial proteins, and cytoplasmic proteins [7]. While NET formation physically ensnares bacteria and facilitates the interactions between bacteria and antimicrobial effectors, ultimately leading to enhanced bacterial killing, they also contribute to detrimental effects such as excessive inflammation and tissue damage in the host [6,11,12].
PAD4 activity is an important component of neutrophil activation, NET formation, and the innate immune response [10,13]. Alternatively, PAD4 activity and histone citrullination has also been shown to be a contributor to various autoimmune diseases, such as rheumatoid arthritis, lupus, and IBD, and serves as a convergence point for many inflammatory signals that prompt the response of neutrophils to infections [27,41,42,43]. In the case of sepsis, these extracellular histones have been linked to tissue damage as well as increased mortality [11]. PAD4 gene deletion studies have been variable. PAD4−/− mice have been shown to be susceptible to bacterial infections and their neutrophils display a significant reduction in bacterial killing in vitro [10]. However, when additional in vivo studies of sepsis utilizing these PAD4−/− mice using the CLP model were performed, PAD4 gene deletion appeared to have no real effect on survival [19]. This discrepancy in survival results as compared to our findings may be attributed to the differences in the model procedure as well as differences in control mortality rates. Moreover, PAD inhibition as compared to PAD4 gene deletion may also account for the differences as the use of Cl-amidine or neutralization of circulating citrullinated histones using anti-H3cit antibody as a therapeutic strategy has been shown to increase survival in septic mice [44]. Our results confirm this increase in survival in the CLP model using a different dosing strategy, which included a smaller subcutaneous dose of Cl-amidine given prior to CLP. Thus, this demonstrates that chemical inhibition of PADs, and subsequently histone citrullination and NET formation, leads to increased survival against septic insult. Protein expression analysis demonstrated that the PAD inhibitor, Cl-amidine, did not fully negate all histone citrullination, but greatly suppresses it as compared to vehicle-treated CLP mice. Furthermore, the reduction of H3cit protein modification after Cl-amidine treatment is likely due to PAD4 inhibition and not a reduction of neutrophils emigrating into the site of infection.
With extracellular cf-DNA and citrullinated histones having been reported to be detected within the bloodstream during a septic infection [19,45] and circulating H3cit reported as a potential biomarker for the early diagnosis of septic shock [9], H3cit has been proposed as a target considered for improving survival in the CLP model [44]. In our model we were unable to detect H3cit within the bloodstream using protein expression by Western immunoblot as our method of detection at 24 or 48 h after CLP. Furthermore, others have suggested that the elevated cf-DNA levels reported during the early phase are not derived from neutrophils or NETs, but from other factors such as necrotic tissue or apoptotic cells [46]. The H3cit protein modification was detected in the peritoneal cavity after CLP. As neutrophils are one of the first immune cells to respond to infection [47], it is possible that the increase in citrullination is due to PAD4 activity. Collectively, our data suggest that NETosis is occurring within the peritoneum 24 h after CLP [11,16,17] and that the H3cit protein modification, and by extension NET formation, is reduced in the peritoneal cavity with Cl-amidine treatment.
Cl-amidine treatment has been demonstrated to improve various inflammatory disease phenotypes in autoimmune disorders [27,28,43]. Interestingly, Cl-amidine treatment has also been shown to have an effect on dendritic cell maturation induced by TLR agonists to reduce proinflammatory cytokine levels as well as impair the proliferation of naïve CD4+ and CD8+ T cells both in vitro and in vivo [48]. A reduction in proinflammatory markers was also seen in our study where there was a reduction in IL-6 levels in the spleen after CLP. These data, taken together, suggest that Cl-amidine treatment may have a hand in reducing inflammation that is caused by extracellular histones and NETs. Surprisingly, IL-10 levels were significantly increased in the bloodstream in the Cl-amidine-treated mice compared to vehicle-treated mice after CLP, indicating that Cl-amidine treatment may be having an effect on the systemic anti-inflammatory response after CLP. However, IL-10 levels were not significantly affected by Cl-amidine treatment in the peritoneal cavity or in the various organ tissues analyzed. Therefore, it is unclear if PAD4 inhibition has any type of effect on the compensatory anti-inflammatory response that occurs concomitantly with the proinflammatory response [49]. In our model we cannot say definitively that these alterations in pro- and anti-inflammatory signaling are a direct result of NET inhibition, as Cl-amidine is a nonselective PAD inhibitor (not specific to PAD4 and NET formation alone). It would be interesting to further explore the effect of a selective inhibitor of PAD4 on the inflammatory response as it becomes available.
Overall, the results here demonstrate that histone H3 citrullination is present in the peritoneal cavity as well as within cells collected from the site of infection 24 h after being subjected to the CLP procedure. This citrullination is modulated when mice are treated with the PAD inhibitor Cl-amidine. Daily treatment significantly improved survival after CLP, suggesting that chemical inhibition of PAD4 is beneficial against polymicrobial septic mortality. Treatment did not affect overall neutrophil emigration into the site of infection. Nonetheless, Cl-amidine did diminish the ability of activated neutrophils to release NETs. While Cl-amidine has been implicated in improving inflammation in various autoimmune disease states, its direct mechanism of action is not clear [24,27,43]. Cl-amidine does not seem to have a significant impact on proinflammatory markers of infection, but does alter systemic IL-10 levels, thus having a possible effect on the immunosuppressive response in our model. It is possible that the increase in survival is due to secondary effects caused by Cl-amidine, such as an increase in apoptosis of other inflammatory cells, like that seen in a murine colitis model [43]. While PAD4 inhibitors are under development [50], there is still no specific inhibitor available to study the direct correlation between NET inhibition and subsequent host improvement during a septic infection. Our observations suggest that NET inhibition using Cl-amidine should be further explored as a possible therapeutic maneuver against the damaging proinflammatory response seen in polymicrobial sepsis.
Supplementary Material
Supplementary data
Supplementary data
Acknowledgements
This work was funded by NIH R01-GM46354 and R35 GM118097 (A.A.), and GM066194 (J.S.R). The authors would also like to thank Ms. Lauren Watts for her assistance with flow cytometry.
References
- 1.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 4.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 5.Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung H-C. Deaths: preliminary data for 2009. Natl Vital Stat Rep. 2011;59:1–51. [PubMed] [Google Scholar]
- 6.Kovach MA, Standiford TJ. The function of neutrophils in sepsis. Curr Opin Infect Dis. 2012;25:321–327. doi: 10.1097/QCO.0b013e3283528c9b. [DOI] [PubMed] [Google Scholar]
- 7.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 8.Margraf S, Lögters T, Reipen J, Altrichter J, Scholz M, Windolf J. Neutrophil-derived circulating free DNA (cf-DNA/NETs): a potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis. Shock. 2008;30:352–358. doi: 10.1097/SHK.0b013e31816a6bb1. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Liu B, Fukudome EY, Lu J, Chong W, Jin G, et al. Identification of citrullinated histone H3 as a potential serum protein biomarker in a lethal model of lipopolysaccharide-induced shock. Surgery. 2011;150:442–451. doi: 10.1016/j.surg.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207:1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:1777–1783. doi: 10.1161/ATVBAHA.111.242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leshner M, Wang S, Lewis C, Zheng H, Chen XA, Santy L, et al. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front Immunol. 2012;3:307. doi: 10.3389/fimmu.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones JE, Causey CP, Knuckley B, Slack-Noyes JL, Thompson PR. Protein arginine deiminase 4 (PAD4): current understanding and future therapeutic potential. Curr Opin Drug Discov Devel. 2009;12:616–627. [PMC free article] [PubMed] [Google Scholar]
- 15.Rohrbach AS, Slade DJ, Thompson PR, Mowen KA. Activation of PAD4 in NET formation. Front Immunol. 2012;3:360. doi: 10.3389/fimmu.2012.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol. 2008;180:1895–1902. doi: 10.4049/jimmunol.180.3.1895. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Wysocka J, Sayegh J, Lee Y-H, Perlin JR, Leonelli L, et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004;306:279–283. doi: 10.1126/science.1101400. [DOI] [PubMed] [Google Scholar]
- 19.Martinod K, Fuchs TA, Zitomersky NL, Wong SL, Demers M, Gallant M, et al. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood. 2015;125:1948–1956. doi: 10.1182/blood-2014-07-587709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seri Y, Shoda H, Suzuki A, Matsumoto I, Sumida T, Fujio K, et al. Peptidylarginine deiminase type 4 deficiency reduced arthritis severity in a glucose-6-phosphate isomerase-induced arthritis model. Sci Rep. 2015;5:13041. doi: 10.1038/srep13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemmers S, Teijaro JR, Arandjelovic S, Mowen KA. PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS One. 2011;6:e22043. doi: 10.1371/journal.pone.0022043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinod K, Demers M, Fuchs TA, Wong SL, Brill A, Gallant M, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci USA. 2013;110:8674–8679. doi: 10.1073/pnas.1301059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo Y, Arita K, Bhatia M, Knuckley B, Lee Y-H, Stallcup MR, et al. Inhibitors and inactivators of protein arginine deiminase 4: functional and structural characterization. Biochemistry. 2006;45:11727–11736. doi: 10.1021/bi061180d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willis VC, Gizinski AM, Banda NK, Causey CP, Knuckley B, Cordova KN, et al. N-α-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol. 2011;186:4396–4404. doi: 10.4049/jimmunol.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slack JL, Causey CP, Thompson PR. Protein arginine deiminase 4: a target for an epigenetic cancer therapy. Cell Mol Life Sci. 2011;68:709–720. doi: 10.1007/s00018-010-0480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bai J, Tang L, Lomas-Neira J, Chen Y, McLeish KR, Uriarte SM, et al. TAT-SNAP-23 treatment inhibits the priming of neutrophil functions contributing to shock and/or sepsis- induced extra-pulmonary acute lung injury. Innate Immun. 2015;21:42–54. doi: 10.1177/1753425913516524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knight JS, Zhao W, Luo W, Subramanian V, O'Dell AA, Yalavarthi S, et al. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J Clin Invest. 2013;123:2981–2993. doi: 10.1172/JCI67390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knight JS, Luo W, O'Dell AA, Yalavarthi S, Zhao W, Subramanian V, et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ Res. 2014;114:947–956. doi: 10.1161/CIRCRESAHA.114.303312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perl M, Chung C-S, Perl U, Lomas-Neira J, de Paepe M, Cioffi WG, et al. Fas-induced pulmonary apoptosis and inflammation during indirect acute lung injury. Am J Respir Crit Care Med. 2007;176:591–601. doi: 10.1164/rccm.200611-1743OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero-Calvo I, Ocón B, Martínez-Moya P, Suárez MD, Zarzuelo A, Martínez-Augustin O, et al. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem. 2010;401:318–320. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 31.Shubin NJ, Chung CS, Heffernan DS, Irwin LR, Monaghan SF, Ayala A. BTLA expression contributes to septic morbidity and mortality by inducing innate inflammatory cell dysfunction. J Leukoc Biol. 2012;92:593–603. doi: 10.1189/jlb.1211641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byrd AS, O'Brien XM, Johnson CM, Lavigne LM, Reichner JS. An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J Immunol. 2013;190:4136–4148. doi: 10.4049/jimmunol.1202671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Braun OÖ, Zhang S, Norström E, Thorlacius H. Monocytes regulate systemic coagulation and inflammation in abdominal sepsis. Am J Physiol Heart Circ Physiol. 2015;308:H540–H547. doi: 10.1152/ajpheart.00336.2014. [DOI] [PubMed] [Google Scholar]
- 34.Kuethe JW, Midura EF, Rice TC, Caldwell CC. Peritoneal wash contents used to predict mortality in a murine sepsis model. J Surg Res. 2015;199:211–219. doi: 10.1016/j.jss.2015.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heffernan DS, Monaghan SF, Thakkar RK, Tran ML, Chung C-S, Gregory SH, et al. Inflammatory mechanisms in sepsis: elevated invariant natural killer T-cell numbers in mouse and their modulatory effect on macrophage function. Shock. 2013;40:122–128. doi: 10.1097/SHK.0b013e31829ca519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayala A, Perrin MM, Kisala JM, Ertel W, Chaudry IH. Polymicrobial sepsis selectively activates peritoneal but not alveolar macrophages to release inflammatory mediators (interleukins-1 and -6 and tumor necrosis factor) Circ Shock. 1992;36:191–199. [PubMed] [Google Scholar]
- 37.Dear JW, Yasuda H, Hu X, Hieny S, Yuen PST, Hewitt SM, et al. Sepsis-induced organ failure is mediated by different pathways in the kidney and liver: acute renal failure is dependent on MyD88 but not renal cell apoptosis. Kidney Int. 2006;69:832–836. doi: 10.1038/sj.ki.5000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doi K, Leelahavanichkul A, Yuen PST, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009;119:2868–2878. doi: 10.1172/JCI39421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiramatsu M, Hotchkiss RS, Karl IE, Buchman TG. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock. 1997;7:247–253. doi: 10.1097/00024382-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Hoesel LM, Neff TA, Neff SB, Younger JG, Olle EW, Gao H, et al. Harmful and protective roles of neutrophils in sepsis. Shock. 2005;24:40–47. doi: 10.1097/01.shk.0000170353.80318.d5. [DOI] [PubMed] [Google Scholar]
- 41.Raptopoulou A, Sidiropoulos P, Katsouraki M, Boumpas DT. Anti-citrulline antibodies in the diagnosis and prognosis of rheumatoid arthritis: evolving concepts. Crit Rev Clin Lab Sci. 2007;44:339–363. doi: 10.1080/10408360701295623. [DOI] [PubMed] [Google Scholar]
- 42.Wang S, Wang Y. Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis. Biochim Biophys Acta. 2013;1829:1126–1135. doi: 10.1016/j.bbagrm.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chumanevich AA, Causey CP, Knuckley BA, Jones JE, Poudyal D, Chumanevich AP, et al. Suppression of colitis in mice by Cl-amidine: a novel peptidylarginine deiminase inhibitor. Am J Physiol Gastrointest Liver Physiol. 2011;300:G929–G938. doi: 10.1152/ajpgi.00435.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Liu Z, Liu B, Zhao T, Chong W, Wang Y, et al. Citrullinated histone H3: a novel target for the treatment of sepsis. Surgery. 2014;156:229–234. doi: 10.1016/j.surg.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo L, Zhang S, Wang Y, Rahman M, Syk I, Zhang E, et al. Proinflammatory role of neutrophil extracellular traps in abdominal sepsis. Am J Physiol Lung Cell Mol Physiol. 2014;307:L586–L596. doi: 10.1152/ajplung.00365.2013. [DOI] [PubMed] [Google Scholar]
- 46.Hamaguchi S, Akeda Y, Yamamoto N, Seki M, Yamamoto K, Oishi K, et al. Origin of circulating free DNA in sepsis: analysis of the CLP mouse model. Mediators Inflamm. 2015;2015:614518. doi: 10.1155/2015/614518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jang B, Kim HW, Kim J-S, Kim WS, Lee BR, Kim S, et al. Peptidylarginine deiminase inhibition impairs Toll-like receptor agonist-induced functional maturation of dendritic cells, resulting in the loss of T cell-proliferative capacity: a partial mechanism with therapeutic potential in inflammatory settings. J Leukoc Biol. 2015;97:351–362. doi: 10.1189/jlb.3A0314-142RR. [DOI] [PubMed] [Google Scholar]
- 49.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis HD, Liddle J, Coote JE, Atkinson SJ, Barker MD, Bax BD, et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol. 2015;11:189–191. doi: 10.1038/nchembio.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data