Abstract
The aging process appears to be a precursor to many age-related diseases, perhaps the most impactful of which is cardiovascular disease (CVD). Heart disease, a manifestation of CVD, is the leading cause of death in the USA, and heart failure (HF), a syndrome that develops as a consequence of heart disease, now affects almost six million American. Importantly, as this is an age-related disease, this number is likely to grow along with the ever-increasing elderly population. Hallmarks of the aging process and HF patients with a reduced ejection fraction (HFrEF) include exercise intolerance, premature fatigue, and limited oxygen delivery and utilization, perhaps as a consequence of diminished peripheral vascular function. Free radicals and oxidative stress have been implicated in this peripheral vascular dysfunction, as a redox imbalance may directly impact the function of the vascular endothelium. This review aims to bring together studies that have examined the impact of oxidative stress on peripheral vascular function and oxygen delivery and utilization with both healthy aging and HFrEF.
Keywords: Cardiovascular disease, Endothelial function, Free radicals, O2, Blood flow, Oxygen transport
Linking vascular function, O2 delivery and utilization, and exercise intolerance with advancing age and heart failure
A hallmark of the aging process and many age-related diseases, such as HF, is a fall in exercise capacity [1–3]. Importantly, limited blood flow and the subsequent fall in O2 delivery to the active skeletal muscles has been implicated as a key factor that contributes to this functional decline [1]. With advancing age and HF, central and peripheral hemodynamic changes occur that can affect compliance in arteries and arterioles, arterial blood pressure, and ultimately alter the vascular response to exercise [2]. In the limited number of studies that have examined muscle blood flow in the legs during conventional cycle ergometry, aging was typically associated with a 20–30 % reduction in blood flow during submaximal work when compared to younger subjects [1, 4]. However, the cardiac output to oxygen consumption (VO2) relationship appears to be well preserved in older subjects [5]. These data, coupled with previous work in the literature, suggest a possible mechanism for age-related attenuation in muscle blood flow could be the maldistribution of submaximal cardiac output.
During whole-body exercise, it is possible that due to free radically mediated vascular dysfunction in older subjects, blood is being directed inappropriately toward respiratory muscles and other viscera instead of toward the active muscle mass. However, when we minimized central limitations to maximal exercise capacity, by employing the isolated single-leg knee extensor (KE) model, the age-related attenuation in blood flow was still apparent [6]. This finding strongly suggests an age-related decline in peripheral vascular function [6], though the exact mechanism behind this deficit was not unveiled. Again, free radicals and oxidative stress have been implicated in this peripheral vascular dysfunction as a redox imbalance may directly impact the function of the vascular endothelium [7]. There are two equally plausible, but starkly contrasting theories regarding free radicals and skeletal muscle blood flow: Free radicals have been proposed to both limit vasodilation and blood flow by reducing NO bioavailability [7] and increase blood flow and vasodilation via their direct vasoactive properties [8, 9]. Although there is evidence that free radicals are more prevalent with age [10] and HF [11, 12], the role of free radicals in vascular function, O2 delivery and utilization, and exercise intolerance in these populations is not well understood.
Vascular function with advancing age
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in the USA, and advancing age remains the primary risk factor in the development of CVD [13]. Though there are many factors that contribute to the age-related increase in CVD risk, vascular dysfunction has been identified as a key player in this process [14]. While this dysfunction may manifest at several points in the circulatory system, age-related changes in the vascular endothelium appear to be particularly detrimental in the progression toward age-related CVD. Once believed to be simply a physical barrier between the blood and arterial wall, the vascular endothelium now is understood to synthesize and release a wide array of biologically active molecules that influence the function and health of the arteries and surrounding tissues. Among the most important of the endothelial-derived molecules is nitric oxide (NO), a substance that produces marked regional vasodilation in a paracrine fashion. Thus, endothelial dysfunction has come to be broadly defined as any deviation from a healthy endothelial phenotype, and is often associated with a reduction in NO bioavailability [15].
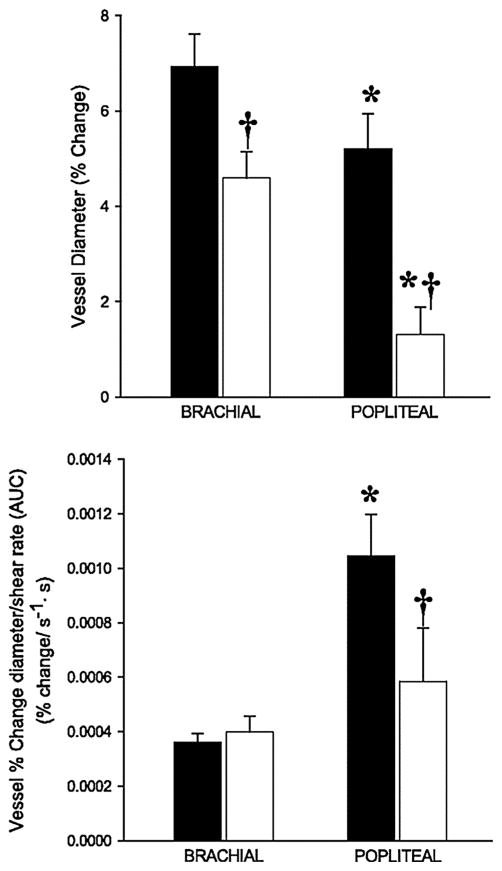
Considering the apparent role of vascular aging in the sequelae of many CVDs, it is not surprising that the detection and treatment of age-related vascular dysfunction has emerged as an area of significant research interest. Over the past decade, our group has undertaken a series of studies aimed at refining the experimental assessment [16] and treatment of vascular function in various populations, including the elderly [17–23]. One of the most widely utilized approaches for the determination of endothelium-dependent vasodilation is the flow-mediated dilation (FMD) test. Developed by Celermajer et al. [24], the ultrasonic assessment of conduit vessel FMD in response to occlusion-induced hyperemia has been established as a reliable, noninvasive measurement of endothelial function that has been documented to correlate with invasively assessed endothelial function in the coronary arteries [25]. Using this methodology, we undertook a study to evaluate FMD in the arm (brachial artery, BA) and leg (popliteal artery, PA) of both young and old healthy subjects in an effort to comprehensively evaluate the limb-specific effects of age on vascular structure and function [26]. The rationale for examining endothelium-dependent vasodilation in a limb-specific manner is based on previous observations that the upper extremities do not always parallel both the structural and functional alterations of the lower extremities that are associated with aging and disease [27, 28]. In this study, we identified a greater FMD in the young compared with the old in both the BA and PA when expressed in traditional terms (% dilation) (Fig. 1, top), a finding that is consistent with the literature in suggesting attenuated vascular endothelial-dependent vasodilation in older individuals [20, 29]. However, when the limb FMD responses were normalized for the shear rate, the primary stimulus from vasodilation during FMD testing, the old group revealed a preserved FMD in the BA, but not the PA (Fig. 1, bottom), revealing attenuated endothelial-dependent vasodilation only in the legs with age.
Fig. 1.
Brachial artery (BA) and popliteal artery (PA) flow-mediated dilation (FMD) (top panel) and FMD normalized for shear rate area under the curve (AUC) (bottom panel) in young and old subjects. *Significant difference between BA and PA (P < 0.05). *Significant difference between young and old groups (P < 0.05). Note the similar BA FMD normalized for shear rate between young and old groups, whereas the same normalization for shear in the PA does not correct the attenuated dilation. Modified from [26]
These data in the upper and lower extremities suggest a greater predisposition for leg-specific vascular dysfunction and, therefore, differing susceptibility to vascular disease in anatomically distinct locations. Indeed, the study of Angerer et al. [27], which investigated the effects of coronary artery disease on FMD in the BA and PA, found an attenuated PA FMD compared with the BA in both patient and age-matched controls, with the greatest reduction in vascular function in the diseased patients. Unfortunately, this clinical study did not evaluate the shear stimulus, thus limiting inference in the context of our limb-specific data [26]. Nevertheless, the present data reveal an intriguing scenario, where FMD in the arm is preserved with age, while the leg reveals a significant age-related attenuation in endothelial-derived vasodilation. It is tempting to speculate that the age-related and limb-specific progression of vascular disease described elsewhere [27, 28] is the consequence of physical stresses over the life span, which are exclusive to the leg vasculature, such as larger hydrostatic and transmural forces, as well as the continued stresses associated with daily locomotion [30, 31].
While traditional FMD testing has become a gold standard for noninvasive assessment of endothelium-dependent vasodilation, our group has also utilized a progressive handgrip (HG) exercise paradigm to examine BA vasodilatory capacity. Compared with conventional FMD testing, which relies upon a single vasodilatory response to a somewhat complex and dynamically changing shear rate, progressive handgrip exercise evokes multiple stepwise increases in shear rate, resulting in a linear BA vasodilatory response [32] that provides a robust method with which to assess vascular function. Using this approach, we demonstrated a severe age-related impairment in BA vasodilation during rhythmic handgrip exercise, with a >50 % reduction in BA vasodilation in older subjects at the highest exercise intensity (Fig. 2, black symbols) [19]. These data are comparable to data collected in our laboratory and by others who have documented similar age-related reductions in brachial artery FMD [7, 21, 29].
Fig. 2.
Change in brachial artery (BA) diameter in young and old subjects at rest and at 3 levels of handgrip exercise after ingestion of the antioxidant cocktail or placebo. Values for % change in BA diameter are not exact and are displayed solely for reference purposes. $Significant difference between young and old groups (P < 0.05). *Significant difference between PL and AO conditions (P < 0.05). Modified from [19]
To examine the mechanism(s) responsible for this observed decrement, a pair of studies were undertaken to explore the contribution of NO to BA vasodilation during progressive handgrip exercise in younger individuals [32] and healthy older subjects [33]. For these pharmacologic studies, subjects performed handgrip exercise before and after the infusion of NG-monomethyl-L-arginine (L-NMMA) directly into the BA for regional inhibition of nitric oxide synthase (NOS). In the young, we observed a 70 % decrement in BA vasodilation following L-NMMA despite similar exercise-induced increases in shear rate, providing clear evidence of NO-dependent vasodilation in this experimental model (Fig. 3, top) [32]. In contrast, an identical dose of L-NMMA only reduced BA vasodilation in the elderly cohort by 30 % (Fig. 3, bottom) [33]. Together, these findings documented a predominantly shear rate-mediated vasodilatory role for NO in the young, but not the old, suggesting that NO-independent mechanisms play a greater role in the vasodilatory response to handgrip-induced BA vasodilation with advancing age. Interestingly, when BA vasodilation was normalized for shear rate in the elderly cohort, a 40 % greater increase in shear rate was required to elicit the same vasodilation in the older subjects compared with the young, supporting the age-related decline in NO-mediated vascular function observed previously [7, 21, 29].
Fig. 3.
Brachial artery (BA) vasodilation during progressive handgrip exercise in the young (top panel) and old (bottom panel) during control and NG-monomethyl-L-arginine (l-NMMA) conditions. *Significant difference from rest (P < 0.05). *Significant difference from control (P < 0.05). Modified from [32, 33]
Building on these studies evaluating endothelium-dependent vasodilation via FMD and HG exercise, our group has developed the passive leg movement (PLM) paradigm as an additional, noninvasive assessment of vascular function in the leg. In contrast to FMD and HG measurements of conduit vessel vasodilation, the PLM test involves continuous movement of the knee joint through a 90-degree range of motion, eliciting an increase in limb blood flow. Using this “reductionist” approach, we have evaluated the relative contribution of central (i.e., cardiac output, CO) and peripheral (i.e., leg blood flow) factors contributing to PLM-induced hyperemia in a variety of cohorts. In the elderly, we have completed a series of studies [17, 18, 22] which have collectively identified a marked reduction in PLM-induced hyperemia due to an age-related decline in both central and peripheral responses. With respect to the peripheral vascular response, beat-to-beat assessment of leg blood flow during PLM revealed a reduction in both the peak change in blood flow (Fig. 4, top, black symbols) and the area under the curve (AUC) for the blood flow response (Fig. 4, bottom, black bars) across the first minute of PLM in the elderly compared to their younger counterparts. Taken together, these findings from FMD, HG, and PLM vascular testing modalities strongly support an age-related decline in peripheral vascular function in the elderly, which may ultimately contribute to the subsequent development of age-related cardiovascular disease, including the clinical syndrome known as heart failure (HF).
Fig. 4.
Passive limb movement (PLM)-induced changes in leg blood flow (top panel) and leg blood flow area under the curve (AUC) (bottom panel) in young and old with and without intra-arterial NG-monomethyl-L-arginine (l-NMMA) infusion. To increase clarity of the data presented, only the first 60 s of PLM are displayed. *Significant difference between control and l-NMMA (P < 0.05). Modified from [18]
Vascular function in HFrEF
Heart disease is the leading cause of death in the USA, accounting for one in every four deaths in 2010 and costing over $300 billion annually in health care, medication, and lost productivity [13]. HF, a syndrome that develops as a consequence of heart disease from multiple etiologies, now affects almost six million Americans [34], and as this is an age-related disease, it is estimated that this number will grow to almost 10 million in the next 15 years [35]. Though HFrEF patients present with a host of symptoms related to impaired cardiac contractile function, in recent years attention has turned toward disease-related changes in the periphery that may contribute to HFrEF pathophysiology. With respect to skeletal muscle, a variety of alterations specific to muscle structure and metabolic state, including muscle atrophy, alterations in fiber type, reduced mitochondrial enzymes, and decreased mitochondrial volume density, have all been associated with HFrEF [36–39]. Though an in-depth discussion of these skeletal muscle maladaptations in HFrEF is beyond the scope of the present discussion, this topic has been the subject of several recent reviews [40–42]. The skeletal muscle vasculature also appears to be altered as a consequence of the disease. Indeed, it is now well accepted that peripheral endothelial function is impaired in HFrEF patients [43, 44], which may represent the combined effects of an attenuated CO, reduced levels of physical activity, elevated peripheral vasoconstriction, and neurohormonal activation. Importantly, endothelial dysfunction has been documented in peripheral and coronary arteries in HFrEF patients and is associated with progression of the disease and increased mortality [45]. With the recognition that peripheral vascular dysfunction represents an aspect of HFrEF pathophysiology that is remediable [46], there is still significant interest in studies examining this component of HF progression.
Using several of the noninvasive vascular tests outlined above, our group has recently undertaken a series of studies to further examine the nature of peripheral endothelial dysfunction in HFrEF patients. Using the FMD test methodology, we documented a clear reduction in FMD in HFrEF patients compared to age-matched, healthy controls [47], which is in agreement with previous findings [43, 44] and confirms the presence of overt vascular dysfunction in this population. Building on this study, we sought to further characterize lower limb vascular function in this patient group using the PLM technique. As outlined above, PLM provokes an increase in limb blood flow and leg vascular conductance (LVC) that provides an index of NO-mediated vasodilation [48]. However, unlike FMD, PLM directly interrogates the leg, which plays a major role in human locomotion and exercise capacity, and thus this mode of assessment is very specific to the challenges of exercise intolerance faced by patients with HF. Furthermore, the PLM test also activates the muscle mechanoreflex, provoking an increase in heart rate (HR) and CO in an attempt to maintain perfusion pressure in the skeletal muscle circulation, and there is evidence from animal models of HF that this reflex may be exaggerated in HFrEF [49], contributing to exercise intolerance in these patients. Thus, building upon previous studies documenting vascular [43, 44, 47] and autonomic [50, 51] dysfunction in HFrEF, we sought to partition the central and peripheral contributors to movement-induced hyperemia in this patient group utilizing the PLM approach [52]. In this study, we found no evidence for an exaggerated mechanoreflex during PLM in optimally medicated patients with HFrEF. In fact, the HR and CO responses to continuous PLM in patients with HFrEF were attenuated compared to controls. Concomitantly, the patients exhibited a diminished movement-induced leg blood flow response, whether viewed in terms of the peak change in leg blood flow (Fig. 5, top) or blood flow AUC (Fig. 5, bottom) that could not be attributed to these central hemodynamic differences, because the rise in arterial blood pressure was similar in both groups in response to PLM. These findings highlight the lack of an exaggerated mechanically induced contribution to the exercise pressor reflex in medicated patients with HFrEF and, despite exhibiting central hemodynamic dysfunction, further demonstrate the presence of a profound peripheral vascular dysfunction in this patient group.
Fig. 5.
Passive limb movement (PLM)-induced changes in leg blood flow (top panel) and leg blood flow area under the curve (AUC) (bottom panel) in controls and heart failure patients with reduced ejection fraction (HFrEF). To increase clarity of the data presented, only the first 60 s of PLM are displayed *Significant difference between control and HFrEF (P < 0.05). Modified from [52]
The impact of oxidative stress on vascular function in aging and heart failure
In view of the weight of evidence for impaired vascular function in both normal aging and age-related diseases such as HF, it is not surprising that significant effort has been committed to identifying the pathways responsible for this pathophysiology. Though there are undoubtedly a host of potentially overlapping mechanisms that contribute to generalized vascular dysfunction, one area of ongoing interest in our group and others is oxidative stress. Defined as an excess production of free radicals relative to antioxidant defenses, oxidative stress has been documented to play an important role in both the normal vascular aging process [53] and in the pathogenesis [11] and prognosis [12] of HF. While the functional consequences of oxidative stress are widespread, the vascular endothelium represents an area that is particularly vulnerable to the harmful effects of free radicals due to the interaction with NO. Indeed, following formation and release from the endothelium, the fate of NO is dictated to a large degree by the presence of O2-centered free radicals such as superoxide (O2−) which bind readily to NO [54]. This “scavenging” process reduces the amount of NO available to provoke smooth muscle cell relaxation, resulting in an impairment in NO-dependent vasodilation. Inflammation and subsequently elevated oxidative stress has thus been implicated as a major contributor to the reduction in NO bioavailability and development of endothelial dysfunction, a deleterious phenotype that is independently associated with adverse long-term outcomes in both the elderly [14] and HF patients [45].
One approach for ameliorating oxidative stress and restoring a more favorable oxidant balance is through antioxidant (AO) supplementation. While large-scale clinical trials have failed to demonstrate a beneficial effect of long-term AO consumption on cardiovascular disease morbidity and mortality [55], smaller, interventional studies focused on acute AO-mediated changes in vascular reactivity have provided more promising results. Infused, supraphysiological doses of vitamin C have been documented to transiently restore endothelium-dependent vasodilation [7, 56] and skeletal muscle blood flow [57–59] in the elderly, and BH4 administration (a cofactor for endothelial NO production) acutely improves endothelial function [60]. However, studies utilizing more practical interventions, such as oral vitamin C at over-the-counter doses, have failed to demonstrate an improvement in age-related endothelial dysfunction [7], and few studies have investigated the efficacy of combined AOs on vascular function. Thus, we performed a series of studies designed to fill a void in the clinical literature by administering an oral AO cocktail [vitamin C (1000 mg), vitamin E (600 IU), and α-lipoic acid (600 mg)] to acutely lower plasma free radical concentration and to assess the acute vascular responses to this attenuated oxidative stress in young and older subjects.
Two studies were undertaken to noninvasively evaluate the impact of AO administration on vascular function. In the first study, the HG exercise modality was employed to examine exercise-induced brachial artery vasodilation before and after acute AO administration, and we observed a reversal of the age-related attenuation in brachial artery vasodilation following acute AO consumption (Fig. 2, white symbols) [19]. Subsequent to this study, we utilized FMD testing to identify an age-related decline in vascular function in the elderly that was readily reversible following AO administration [20]. Indeed, AO consumption acutely improved brachial artery FMD in the elderly to that of their younger counterparts (Fig. 6), suggesting that an AO cocktail at modest, enteral doses was sufficient to acutely reverse age-related endothelial dysfunction. Interestingly, in both of these studies, AO administration in the young, healthy cohort proved detrimental to vascular function (Figs. 2, 6), revealing an important beneficial role for free radicals under conditions of low oxidative stress. Though a comprehensive discussion regarding the impact of pro-versus antioxidant influences on vascular function is beyond the scope of this article, readers are referred to several recent reviews on this topic [61–63]. Importantly, in each of these studies [19, 20], we utilized electron paramagnetic resonance (EPR) spectroscopy to directly measure plasma free radical concentration, and were thus able to document the efficacy of the AO treatment to acutely reduce blood-borne free radicals. Together, these studies, indicating a profound improvement in endothelial function, extend former investigations documenting the capacity of AOs infused at a high dose to acutely improve endothelium-dependent vasodilation [7, 60, 64] in the elderly, revealing a similar beneficial effect using a typical, over-the-counter combination of AOs available to the general public. These findings thus provided important mechanistic insight regarding the link between endothelial dysfunction and oxidative stress in the elderly, and demonstrate a striking restoration of endothelial function as the consequence of a simple, oral AO intervention.
Fig. 6.
Brachial artery flow-mediated vasodilation (FMD) in young and older subjects following placebo (PL) or antioxidant (AO) administration. In the PL trial, an age-associated decline in FMD was apparent. Following AO consumption, FMD was reduced in young, but improved in the elderly. *Significant difference between young and older groups (P < 0.05). *Significant difference between PL and AO (P < 0.05). Modified from [20]
More recently, we have built upon these studies in the elderly to examine the potential of this AO intervention to acutely disrupt oxidative stress in HFrEF patients. As discussed above, the sequelae of HFrEF includes overt vascular dysfunction, which appears to be the consequence of an attenuated L-arginine–nitric oxide pathway [65] and has been, at least partially, attributed to the increased destruction of NO by free radicals. [66] Indeed, as with aging, previous studies have revealed that elevated levels of free radicals, particularly O2−, contribute to decreased NO bioavailability [67, 68] and the subsequent attenuation in endothelial function [69]. Furthermore, AO supplementation has previously restored endothelial function in healthy aged individuals [19, 20] as well as HF patients [70], presumably by improving NO bioavailability. Thus, we completed a pair of studies to determine whether acute AO administration could attenuate circulating plasma free radical concentration and subsequently improve vascular function in HFrEF patients compared to healthy controls.
In the first study [47], participants underwent FMD testing before and after acute AO consumption in a protocol identical to the aforementioned studies we have performed in the elderly [20]. Following AO consumption, we documented a marked increase in circulating AO capacity, but the consequence of this intervention on vascular function was less clear, as administration of the AO did not alter the FMD or reactive hyperemia (RH, an index of microvascular function) responses compared with placebo in the HFrEF patients. This was followed up by a study employing HG exercise to more broadly examine the impact of oral AO consumption on arterial blood pressure and indices of both central (i.e., CO and systemic vascular resistance [SVR]) and peripheral (arm blood flow and vascular resistance) hemodynamics in HFrEF patients and age-matched controls [71]. Although AO ingestion significantly increased plasma ascorbate concentration and endogenous AO activity (catalase) in both the patients and controls, only in the patients with HF did this intervention result in a significant hemodynamic response. Specifically, at rest, AO administration resulted in a significant fall in SVR (−12 %) that provoked a significant decrease in mean arterial pressure (MAP) (−5 %) and an increase in CO, responses which were maintained throughout the three stages of HG exercise. The peripheral response to AO administration contrasted starkly with these changes, with no change in either arm blood flow or vascular resistance. These data provide evidence that in HF patients, SVR is, at least in part, mediated by oxidative stress. However, this did not appear to be the result of limb or skeletal muscle-specific changes in PVR. Together, these studies [47, 71] have identified a somewhat varied response to AO consumption in HFrEF, which likely reflects the complex and heterogeneous manner in which oxidative stress may impact the circulatory system in this patient group. Whether this AO intervention could prove efficacious in rescuing the decrement in vascular function identified using the PLM model has not been examined, but is certainly a worthy of future investigation.
O2 delivery and utilization during exercise with age
While the determination of vascular function is invaluable in terms of CVD risk assessment and prognosis in both healthy aging and patient populations such as HF, these resting measurements may not reflect the functional capacity of the vascular system during stressors such as exercise. Indeed, during exercise, numerous cardiovascular, metabolic, and autonomic adjustments take place to ensure the adequate matching of O2 supply and demand. As blood carries oxygen to the active muscle, skeletal muscle blood flow is a major determinant of O2 supply and can therefore impact O2 utilization. Indeed, limited blood flow to active skeletal muscles has been implicated as an important factor that contributes to the decline in exercise capacity associated with the aging process [72]. Such limitations promote a sedentary lifestyle with advancing age, and it is now well established that physical inactivity represents an independent risk factor for many cardiovascular diseases [73], including HF. Thus, studies seeking to determine the mechanisms responsible for exercise intolerance remain a significant public health priority.
With advancing age, changes occur in both the central and peripheral circulation that can affect compliance in arteries and arterioles, blood pressure, and ultimately alter the vascular response to exercise [74]. Thus, in keeping with the ongoing research interests of our group in the areas of cardiovascular and exercise physiology, we sought to investigate the vascular and metabolic response of the leg muscles during both submaximal and maximal upright cycle exercise in old, sedentary individuals compared to young controls [4]. Our primary hypotheses were that the metabolic cost of work as measured by leg muscle VO2 would be similar in both groups, but that this would be achieved by a lower sub-maximal leg blood flow and elevated arterial–venous (a–v) O2 difference in the old subjects. Results from this study identified a preservation of exercising leg blood flow in sedentary old subjects compared with similarly sedentary young subjects during lower exercise intensities, but a clear decrement as submaximal exercise intensity increased. Muscle VO2 at the more taxing submaximal exercise intensities was similar to the young subjects, but was achieved in the old subjects by an elevated a–v O2 difference. However, at maximal effort, O2 delivery and VO2max were significantly reduced compared to their younger counterparts. Therefore, it is likely that the limited perfusion of exercising skeletal muscle during moderate to heavy exercise in these old sedentary subjects was directly responsible for the lower VO2max associated with the aging process.
Though this previous study during upright cycling identified a clear impairment in O2 delivery, use of this large muscle mass exercise modality leaves open the possibility that both central and peripheral hemodynamics contributed to the age-related reduction in exercising leg blood flow. Thus, an additional protocol was undertaken using the KE exercise modality to study isolated, dynamically exercising skeletal muscle in both young and old subjects, thereby investigating the consequences of aging on leg blood flow without taxing central hemodynamics [75]. Across a range of exercise intensities, we observed a consistently attenuated blood flow in old sedentary subjects when compared with young sedentary subjects with similar quadriceps muscle mass (Fig. 7, top), which appeared to be dictated by a persistent elevation in leg vascular resistance in the old group (Fig. 7, bottom). Interestingly, given the initially high level of vascular resistance in the old, the overall reduction in resistance across exercise intensities was actually greater in old than in young, suggesting a preserved ability to vasodilate during exercise with advancing age.
Fig. 7.
Changes in exercising limb muscle blood flow (top panel) and leg vascular resistance (bottom panel) during incremental knee extensor (KE) exercise. *Significant difference between young and old (P < 0.05). *Significantly different slope and y-intercept between young and old (P < 0.05). Modified from [75]
If central hemodynamic limitations were solely responsible for reduced muscle perfusion during exercise in the old, it would be expected that when these central limitations are minimized (as in KE), perfusion in the old subjects should equal that of young subjects at the same work rate. Thus, the finding of a lower leg blood flow even during isolated small muscle mass exercise suggests that in the old subjects, peripheral vascular limitations are responsible for the attenuated muscle blood flow. Together, these studies [4, 75] also emphasize the importance of considering exercise intensity and modality, as the clearest evidence for a peripheral limitation with age was observed at submaximal intensities of KE exercise, while central limitations to O2 delivery became a significant factor during higher intensity cycling exercise. These findings are in agreement with a growing body of evidence [4, 59, 75–77] supporting an overall attenuation in skeletal muscle blood flow during exercise with advancing age.
O2 delivery and utilization during exercise in HFrEF
Debilitating dyspnea and fatigue triggered by exercise are hallmark symptoms of HFrEF, and may be so extreme that the ability to perform everyday tasks become impaired, leading to a significantly compromised quality of life in this patient group [78]. Although central cardiac limitations are the paramount characteristic of HFrEF, impaired cardiac function does not fully explain the degree of exercise intolerance and symptom status in this patient population [79–83]. This observation has steered studies focusing on exercise limitations in HFrEF toward potentially limiting factors in the periphery. Indeed, a variety of alterations specific to both skeletal muscle (muscle atrophy, fiber type changes, reduced mitochondrial enzymes, decreased mitochondrial volume density) [36–39] and the vascular–skeletal muscle interface (greater sympathetic vasoconstrictor tone, decreased capillarity, and smaller capillary diameter) [84–86] have all been associated with HF. However, the contribution of these skeletal muscle changes to the limited exercise capacity in HFrEF patients is currently not well understood. Thus, we recently performed a series of studies with the overall goal of examining disease-related changes in O2 delivery during exercise in HFrEF patients in order to better understand the central and peripheral hemodynamic contributions to their exercise intolerance.
The first in this series of studies utilized conventional cycle ergometry and single-leg KE exercise to greatly vary muscle mass recruitment in both HFrEF patients and healthy control subjects in both normoxia and hyperoxia (100 % O2) [87]. With this approach, peak leg VO2, normalized for the active muscle mass, was significantly greater during KE exercise (with cardiac reserve) than cycle exercise (without cardiac reserve) in both groups. Likewise, O2-enriched breathing increased O2 availability during both cycle and KE exercise, but this only increased peak leg VO2 during cycling in HFrEF patients (Fig. 8). Together, these observations imply that, during whole-body exercise such as cycling, CO and O2 delivery contributes significantly to the exercise limitation experienced by HFrEF patients. However, this multifaceted research also provides evidence that, despite improvements in peak leg VO2 afforded by increased O2 availability, the convective and diffusive components of O2 transport from blood to skeletal muscle in HFrEF patients are still compromised during KE exercise when compared with well-matched control subjects, suggestive of a significant role of the peripheral vasculature in this phenomenon.
Fig. 8.
Effect of hyperoxia (100 % oxygen) on peak leg VO2 assessed at maximal cycle (a) and knee extensor (KE) exercise (b) in heart failure patients with reduced ejection fraction (HFrEF) (n = 12) and control subjects (n = 8). Control data have been normalized to 100 % as a point of reference for the data collected in the HFrEF patients. Reproduced with permission from [87]
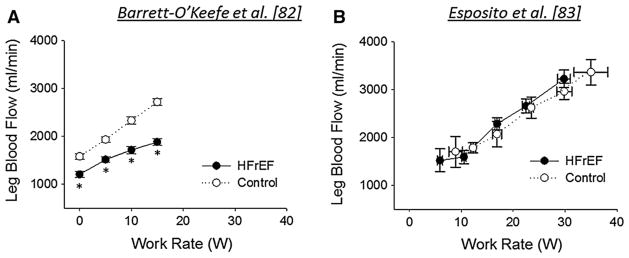
While this initial study provided new insight regarding disease-related impairments in O2 delivery during maximal whole-body exercise, it left unanswered the questions of how muscle blood flow is regulated during submaximal exercise intensities, and whether this regulation differs between small muscle mass exercise modalities. Thus, to comprehensively assess the hemodynamic response to small muscle mass exercise in HFrEF patients and healthy age-matched control subjects, a study was recently undertaken that employed exercise paradigms utilizing both upper (HG exercise) and lower (KE exercise) limbs across a wide range of intensities [88]. During HG exercise, both groups exhibited a similar forearm hyperemic and vasodilatory response during lower intensity [15 % maximal voluntary contraction (MVC)] HG exercise, but HFrEF patients exhibited a 15–25 % attenuation in forearm blood flow at higher intensities (30 and 45 % MVC), due to an impaired vasodilatory capacity. During KE exercise, HFrEF patients exhibited a 20–35 % lower leg blood flow and LVC compared to control subjects, with the most substantial decrements at the highest exercise intensity (15 W) (Fig. 9a). Together, these findings indicate that HFrEF patients exhibit a severely compromised ability to vasodilate vasculature of both the upper and lower limbs, restricting exercising skeletal muscle perfusion and likely limiting exercise capacity in this patient group. Interestingly, this observed reduction in leg blood flow during KE exercise in HFrEF patients appears to differ based on pharmacotherapy. Indeed, in a separate group of HFrEF patients with somewhat differing medications and in whom beta-blockers were withheld 48 h prior to study, we observed that exercising leg blood flow and VO2 were not diminished during multiple submaximal upright cycling and KE exercise intensities compared to healthy, age-matched controls [89] (Fig. 9b). The ability of beta-blockade to acutely reduce leg blood flow during KE exercise has been reported in young, healthy individuals [90], supporting the importance of this pathway in the regulation of muscle blood flow during exercise. These divergent results using almost identical methodologies not only highlight the importance of carefully considering background pharmacotherapy in this highly medicated patient group, but may also suggest that beta-blockade contributes to reduced O2 delivery during exercise in this patient group.
Fig. 9.
Leg blood flow during knee extensor (KE) exercise in heart failure patients with reduced ejection fraction (HFrEF) and healthy, age-matched controls. Interestingly, despite the use of identical exercise modalities, a disease-related reduction in leg blood flow was observed at submaximal work rates in the study from Barrett-O’Keefe et al. (a), while no decrement was present at submaximal or maximal exercise intensities in the study from Esposito et al. (b). *Significant difference between control and HFrEF (P < 0.05). Modified from [88] and [89]
Fortunately, potential disease-related impairments in skeletal muscle convective and diffusive O2 transport maybe remediable. Indeed, it is now widely recognized that regular exercise in HFrEF patients improves quality of life and reduces symptoms, hospitalization, disability, and even, perhaps, mortality [91–93]. Traditionally, this attenuated exercise capacity has been attributed predominantly to the central hemodynamic limitations associated with the failing cardiac pump, but it is now evident that peripheral factors also contribute to this exercise limitation, as first highlighted by the work of Poole and Musch in a rat model of HF [94] and later confirmed in humans [87]. However, the mechanisms responsible for training-induced adaptations are less well known, due, it could be argued, at least in part to the use of whole-body exercise training regimens. Specifically, while whole-body cycle training has consistently yielded significant improvements in exercise capacity in HFrEF patients [92], because whole-body exercise induces a complex interaction between central hemodynamic and peripheral responses, such an approach leaves doubt as to the role of central and peripheral hemodynamic adaptations in response to exercise training.
Thus, to isolate the peripheral training-induced effects, we performed a study that sought to determine the physiological mechanisms responsible for the anticipated improvement in whole-body exercise capacity following 8 weeks of small muscle mass KE exercise training (3 times/week, varied intensity, with overall intensity progressively increased based upon biweekly assessments, 50 min/session/leg) in HFrEF patients [95]. As anticipated, this small muscle mass exercise training had no effect on maximal CO, because this exercise training paradigm only minimally stresses the heart and was therefore appeared not to have stimulated central hemodynamic adaptations. In contrast, there were a multitude of significant peripheral structural and functional adaptations that contributed to improved patient exercise capacity, both during maximal small muscle mass and cycling exercise. Specifically, in addition to significant training-induced muscle morphometric changes, both convective and diffusive O2 transport were increased at maximal KE and cycle exercise, yielding a significant increase in VO2peak in each scenario (Fig. 10). These peripheral structural and O2 transport improvements, without a change in CO, provided evidence of significant peripheral vascular and metabolic plasticity in HFrEF patients that can be developed in isolation with small muscle mass training and then harnessed to the benefit of whole-body exercise capacity. Additionally, these findings highlight the importance of skeletal muscle-specific adaptations in patients with HF allowing the contributions of these peripheral factors to be partitioned, perhaps guiding future clinical interventions aimed at improving exercise intolerance.
Fig. 10.
A comparison of oxygen transport and utilization parameters assessed at maximal cycle (top panel) and knee extensor exercise (KE) (bottom panel) both before and after KE training in heart failure patients with reduced ejection fraction (HFrEF) (n = 5) normalized to values from healthy controls (n = 8). Leg V̇2, one-leg O2 uptake;Q̇2, one-leg O2 delivery; CaO2–CvO2, arterial–venous O2 content difference. Reproduced with permission from [95]
While compromised central hemodynamics are the hallmark of HF, the notion that peripheral dysfunction exacerbates these central hemodynamic abnormalities, the so-called muscle hypothesis of HF [96], has been increasingly recognized [49, 97–99]. Specifically, the exercise pressor reflex (EPR) has been reported to be upregulated in both animal models of HF [100] and in patients with HF [101, 102], leading to excessive increases in HR, ventilation (VE), sympathetic nerve activity, and arterial bloodpressure. The sensitization of this reflex has the potential to result in greater exertional dyspnea and increased cardiac afterload, exaggerating myocardial work, reducing exercise tolerance, and subsequently enhancing disease progression due to inactivity.
To further examine the role of the EPR in HF, our group has undertaken a series of studies utilizing intrathecal injection of the μ-opioid receptor agonist fentanyl to transiently and partially inhibit the afferent arm of this reflex, providing the opportunity to examine signaling via the thinly myelinated “mechanosensitive” group III afferents and/or the unmyelinated “metabosensitive” group IV afferents. Using this pharmacologic technique in combination with the KE exercise modality, we undertook a study to evaluate the impact of sympathoexcitation mediated by group III/IV afferents on O2 delivery and skeletal muscle fatigue in HFrEF patients and healthy, age-matched controls [103]. Following fentanyl injection, norepinephrine spillover was lower during exercise in the HFrEF patients, which was associated with an increase in leg blood flow (Fig. 11, top) and LVC (Fig. 11, bottom) across exercise intensities. Given the established relationship between O2 delivery and muscle fatigue [104], this improvement likely contributed to the observed 30 % attenuation in the end-exercise quadriceps fatigue and reduced effort perception in these conditions. Interestingly, this study also revealed that group III/IV muscle afferents contribute substantially to the exercise-induced cardiac response in patients with HF. Perhaps most importantly, however, this study documents that such feedback, previously determined to be overactive in HF, appears to result in an excessive sympathetically mediated restraint of leg blood flow during exercise in these patients. This attenuation of leg blood flow and the associated reduction in O2 delivery and uptake likely contribute to the compromised fatigue resistance and associated exercise intolerance which characterize this population.
Fig. 11.
Hemodynamic responses to inhibition of group III/IV skeletal muscle afferent feedback, achieved via intrathecal injection of the μ-opioid agonist fentanyl, at rest and during KE exercise in heart failure patients with reduced ejection fraction (HFrEF) (left panels) and healthy controls (right panels). *Significant difference between control and fentanyl trials, P < 0.05. Modified from [103]
While this study during volitional exercise provided an integrative physiological model in which to better understand the role of the EPR in HF, it was not possible to determine the independent contribution of mechanosensitive and metabosensitive afferent signals to the overall cardiovascular response. This may be an important distinction, particularly considering that the nature of disease-related changes in the afferent aspects of the EPR remains a topic of ongoing debate [105, 106]. Indeed, there is evidence for both exaggerated [107–110] and similar [106, 111–114] reflex increases in arterial blood pressure during metaboreflex activation in human HF, and a large body of literature from animal models of HF suggests that an enhanced mechanoreflex is the likely factor mediating the sensitization of the EPR in this population [49]. Thus, to elucidate the role of mechanosensitive muscle afferents, we performed the PLM test on HFrEF patients before and after fentanyl administration as a method by which to isolate the muscle mechanoreflex [115]. During PLM, pharmacological blunting of afferent feedback with fentanyl had no effect on central hemodynamics or pulmonary ventilation, but significantly improved the peripheral hemodynamic response. Indeed, the partial blockade of afferent signaling increased the peak PLM-induced change in leg blood flow (Fig. 12), while norepinephrine spillover and retrograde leg blood flow, two indicators of sympathetic nervous system activity, tended to be reduced (P < 0.10). Additionally, fentanyl administration resulted in greater PLM-induced increases in muscle oxygenation, suggestive of increased microvascular perfusion. These findings suggest that, under normal conditions, in patients with HF, a heightened mechanoreflex augments peripheral sympathetic vasoconstriction, a phenomenon that may confound existing central hemodynamic abnormalities, further contributing to exercise intolerance in this patient population.
Fig. 12.
Passive limb movement (PLM)-induced changes in leg blood flow during before and following intra-thecal fentanyl administration in heart failure patients with reduced ejection fraction (HFrEF). Dashed line indicates the start of leg movement. AUC area under the curve. *Significant difference between control and fentanyl trials, P < 0.05. Modified from [115]
The impact of oxidative stress on O2 delivery and utilization in aging and heart failure
In contrast to the documented impact of oxidative stress on endothelium-dependent vasodilation, much less is known about how redox balance influences the dynamic process ofO2 delivery and utilization during exercise. Muscle, itself, has been commonly implicated as the main site of free radical production during elevated muscle metabolism such as exercise. However, free radicals are also generated by various oxidases that are not directly linked to the muscle mitochondria (e.g., xanthine oxidase, NAD(P)H oxidases), but are tightly coupled with exercise and the concomitant increase in blood flow-induced shear stress. The degree to which each of these potential sources of free radicals contributes to the elevated oxidative stress associated with exercise is not yet well understood. What is well accepted is that the rapid changes in redox state of skeletal muscle associated with exercise offer a unique scenario that affords the opportunity to study the mechanisms and impact of elevated free radical generation. While some direct measurements in animals and the use of putative manifestations of oxidative stress have established much about the species, source, and compartmentalization of free radicals during muscle contraction [116–118], direct evidence of increased rates of free radical production during exercise in humans is still scarce. Thus, we utilized electron paramagnetic resonance spectroscopy to directly quantify plasma free radical concentrations in the skeletal muscle arterial and venous vasculature during multiple levels of KE exercise in young, healthy subjects [119]. With this approach, we observed a progressive increase in the net outflow of α-phenyl-tert-butylnitrone (PBN) adducts in addition to lipid hydroperoxides (LH) during increasing intensities of KE exercise, findings that are consistent with the generation of secondary, lipid-derived O2-centered alkoxyl radicals. This study thus provided direct evidence for an incremental increase in free radical outflow across a functionally isolated and energetically active skeletal muscle bed, providing important proof of concept that oxidative stress could potentially be an important player in the regulation of skeletal muscle blood flow during exercise.
This study, identifying elevated free radical formation during exercise, raised the question of whether this change in redox balance during exercise is actually detrimental to O2 delivery in populations associated with elevated oxidative stress, such as the elderly and patients with HF, and if so, whether acute AO consumption could mitigate the attenuated exercise-induced hyperemia in these populations. With respect to age, Dinenno et al. recently identified the ability of acute AO administration, accomplished through both intravenous [59] and oral [120] ascorbic acid consumption, to increase forearm blood flow and VO2 during HG exercise in the elderly. While these studies convincingly identified the ability of AO treatment to improve O2 delivery, it is noteworthy that indirect markers of oxidative stress remained unchanged following AO administration in these studies, leaving some uncertainty regarding the mechanisms responsible for the improved local vasodilation. Building on this previous work in the forearm, we have recently examined the impact of oral AOconsumption on leg blood flow and VO2 during KE exercise in COPD patients compared to healthy, age-matched controls [121]. Surprisingly, AO consumption did not improve O2 delivery or utilization during any level of KE exercise in the old control group, despite the known efficacy of this treatment to acutely reduce plasma free radical concentration at rest and during exercise in the elderly [122]. Additional studies coupling electron paramagnetic resonance determination of plasma free radical concentration with assessments of blood flow and VO2 across multiple exercise modalities are clearly needed to clarify the role of oxidative stress on O2 delivery and utilization withadvancing age.
Despite these divergent findings in the elderly, given the marked elevation in oxidative stress [70, 123] and accompanying reduction in O2 delivery [88] that has been reported during exercise in HFrEF, it seems plausible that this patient group would be well positioned to benefit from AO administration. Using L-NMMA to inhibit eNOS, Katz et al. [124] failed to identify a change in exercise hyperemia during handgrip exercise between eNOS and placebo trials in class II–III HF patients, suggesting that the NO-mediated regulation of exercising forearm blood flow is impaired in this cohort. As discussed above, based on these earlier observations [88], we examined forearm blood flow during multiple levels of handgrip exercise before and after AO consumption in HFrEF patients and age-matched controls to determine whether disruptions in oxidative stress could “rescue” impaired O2 delivery in the patient group. Contrary to our hypothesis, there were no differences in forearm blood flow as a consequence of AO administration, though it is noteworthy that the patient group did not exhibit a clear decrement in blood flow compared to age-matched controls in this study [71]. To our knowledge, there have been no studies to date that have examined the impact of acute AO consumption on O2 delivery during lower limb exercise in HFrEF patients, and this represents yet another area that is worthy of additional study.
Summary
As the single greatest risk factor for developing HF is advancing age, it is perhaps not surprising that studies in both healthy elderly individuals and HFrEF patients have separately identified impairments in peripheral vascular function, O2 delivery and utilization, and exercise tolerance, which in some instances appear to be a consequence of altered redox balance. However, as highlighted in this review, despite this potential synergistic effect of aging and HFrEF, there are still some clear physiological differences, again some related to free radicals and oxidative stress, that may help to guide our efforts to develop clinically relevant interventions that can benefit patients with HFrEF.
Acknowledgments
Funded in part by HL091830 (R.S.R.), I01RX000182 (R.S.R.), I01RX001697 (R.S.R.), I21RX001433 (R.S.R.), E9275-L (R.S.R.), I21RX001572 (M.A.), HL116579 (M.A.), HL103786 (M.A.), AHA14-17770016 (M.A.), I21RX001418 (D.W.W.), and HL118313 (D.W.W.).
Footnotes
Compliance with ethical standards
Conflicts of interest Drs. Wray, Amann, and Richardson have no conflicts of interest or financial ties to disclose.
References
- 1.Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol. 1998;85:68–75. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- 2.Beere P, Russell S, Morey M, Kitzman D, Higginbotham M. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation. 1999;100:1085–1094. doi: 10.1161/01.cir.100.10.1085. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan MJ, Hawthorne MH. Exercise intolerance in patients with chronic heart failure. Prog Cardiovasc Dis. 1995;38(1):1–22. doi: 10.1016/s0033-0620(05)80011-8. [DOI] [PubMed] [Google Scholar]
- 4.Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol. 2003;284(4):H1251–H1259. doi: 10.1152/ajpheart.00790.2002. [DOI] [PubMed] [Google Scholar]
- 5.Proctor D, Beck K, Shen P, Eickhoff T, Halliwill J, Joyner M. Influence of age and gender on cardiac output-VO2 relationships during submaximal cycle ergometry. J Appl Physiol. 1998;84(2):599–605. doi: 10.1152/jappl.1998.84.2.599. [DOI] [PubMed] [Google Scholar]
- 6.Lawrenson L, Poole JG, Kim J, Brown CF, Patel PM, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: the effect of age. Am J Physiol Heart Circ Physiol. 2003;285:H1023–H1031. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- 7.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556(1):315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res. 2003;93(6):573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 9.Richardson RS, Donato AJ, Uberoi A, Wray DW, Lawrenson L, Nishiyama S, Bailey DM. Exercise-induced brachial artery vasodilation: role of free radicals. Am J Physiol Heart Circ Physiol. 2007;292(3):H1516–H1522. doi: 10.1152/ajpheart.01045.2006. [DOI] [PubMed] [Google Scholar]
- 10.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273(5271):59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belch JJ, Bridges AB, Scott N, Chopra M. Oxygen free radicals and congestive heart failure. Br Heart J. 1991;65(5):245–248. doi: 10.1136/hrt.65.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104(22):2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 13.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American C Heart Association Statistics, S. Stroke Statistics. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 14.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107(3):490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 15.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci. 2011;120(9):357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55(5):1075–1085. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Richardson RS. Perfusion pressure and movement-induced hyperemia: evidence of limited vascular function and vasodilatory reserve with age. Am J Physiol Heart Circ Physiol. 2013;304(4):H610–H619. doi: 10.1152/ajpheart.00656.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS. Passive leg movement and nitric oxide-mediated vascular function: the impact of age. Am J Physiol Heart Circ Physiol. 2015;308(6):H672–H679. doi: 10.1152/ajpheart.00806.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donato AJ, Uberoi A, Bailey DM, Wray DW, Richardson RS. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am J Physiol Heart Circ Physiol. 2010;298(2):H671–H678. doi: 10.1152/ajpheart.00761.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, Witman MA, Ives SJ, Barrett-O’Keefe Z, Richardson RS. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension. 2012;59(4):818–824. doi: 10.1161/HYPERTENSIONAHA.111.189456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Evidence of preserved endothelial function and vascular plasticity with age. Am J Physiol Heart Circ Physiol. 2006;290(3):H1271–H1277. doi: 10.1152/ajpheart.00883.2005. [DOI] [PubMed] [Google Scholar]
- 22.McDaniel J, Hayman MA, Ives S, Fjeldstad AS, Trinity JD, Wray DW, Richardson RS. Attenuated exercise induced hyperaemia with age: mechanistic insight from passive limb movement. J Physiol. 2010;588(Pt 22):4507–4517. doi: 10.1113/jphysiol.2010.198770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidhu SK, Weavil JC, Venturelli M, Rossman MJ, Gmelch BS, Bledsoe AD, Richardson RS, Amann M. Aging alters muscle reflex control of autonomic cardiovascular responses to rhythmic contractions in humans. Am J Physiol Heart Circ Physiol. 2015;309(9):H1479–H1489. doi: 10.1152/ajpheart.00433.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celermajer D, Sorensen K, Gooch V, Spiegelhalter D, Miller O, Sullivan I, Lloyd J, Deanfield J. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340(8828):1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 25.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26(5):1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 26.Nishiyama SK, Wray DW, Richardson RS. Aging affects vascular structure and function in a limb-specific manner. J Appl Physiol. 2008;105(5):1661–1670. doi: 10.1152/japplphysiol.90612.2008. [DOI] [PubMed] [Google Scholar]
- 27.Angerer P, Negut C, Stork S, von Schacky C. Endothelial function of the popliteal artery in patients with coronary artery disease. Atherosclerosis. 2001;155(1):187–193. doi: 10.1016/s0021-9150(00)00536-0. [DOI] [PubMed] [Google Scholar]
- 28.Debasso R, Astrand H, Bjarnegard N, Ahlgren AR, Sandgren T, Lanne T. The popliteal artery, an unusual muscular artery with wall properties similar to the aorta: implications for susceptibility to aneurysm formation? J Vasc Surg. 2004;39(4):836–842. doi: 10.1016/j.jvs.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Celermajer D, Sorensen K, Spiegelhalter D, Georgakopoulos D, Robinson J, Deanfield J. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24(2):471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 30.Eiken O, Kolegard R. Comparison of vascular distensibility in the upper and lower extremity. Acta Physiol Scand. 2004;181(3):281–287. doi: 10.1111/j.1365-201X.2004.01291.x. [DOI] [PubMed] [Google Scholar]
- 31.Malhotra A, Cohen D, Syms C, Townsend RR. Blood pressure changes in the leg on standing. J Clin Hypertens. 2002;4(5):350–354. doi: 10.1111/j.1524-6175.2002.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wray DW, Witman MA, Ives SJ, McDaniel J, Fjeldstad AS, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Progressive handgrip exercise: evidence of nitric oxide-dependent vasodilation and blood flow regulation in humans. Am J Physiol Heart Circ Physiol. 2011;300(3):H1101–H1107. doi: 10.1152/ajpheart.01115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trinity JD, Wray DW, Witman MA, Layec G, Barrett-O’Keefe Z, Ives SJ, Conklin JD, Reese V, Richardson RS. Contribution of nitric oxide to brachial artery vasodilation during progressive handgrip exercise in the elderly. Am J Physiol Regul Integr Comp Physiol. 2013;305(8):R893–R899. doi: 10.1152/ajpregu.00311.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 35.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 36.Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85(5):1751–1759. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 37.Harrington D, Anker SD, Chua TP, Webb-Peploe KM, Ponikowski PP, Poole-Wilson PA, Coats AJ. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997;30(7):1758–1764. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- 38.Massie BM, Simonini A, Sahgal P, Wells L, Dudley GA. Relation of systemic and local muscle exercise capacity to skeletal muscle characteristics in men with congestive heart failure. J Am Coll Cardiol. 1996;27(1):140–145. doi: 10.1016/0735-1097(95)00416-5. [DOI] [PubMed] [Google Scholar]
- 39.Mancini DM, Walter G, Reichek N, Lenkinski R, McCully KK, Mullen JL, Wilson JR. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85(4):1364–1373. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- 40.Zizola C, Schulze PC. Metabolic and structural impairment of skeletal muscle in heart failure. Heart Fail Rev. 2013;18(5):623–630. doi: 10.1007/s10741-012-9353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Georgiadou P, Adamopoulos S. Skeletal muscle abnormalities in chronic heart failure. Curr Heart Fail Rep. 2012;9(2):128–132. doi: 10.1007/s11897-012-0090-z. [DOI] [PubMed] [Google Scholar]
- 42.Kinugawa S, Takada S, Matsushima S, Okita K, Tsutsui H. Skeletal muscle abnormalities in heart failure. Int Heart J. 2015;56(5):475–484. doi: 10.1536/ihj.15-108. [DOI] [PubMed] [Google Scholar]
- 43.Katz SD, Biasucci L, Sabba C, Strom JA, Jondeau G, Galvao M, Solomon S, Nikolic SD, Forman R, LeJemtel TH. Impaired endothelium-mediated vasodilation in the peripheral vasculature of patients with congestive heart failure. J Am Coll Cardiol. 1992;19(5):918–925. doi: 10.1016/0735-1097(92)90271-n. [DOI] [PubMed] [Google Scholar]
- 44.Drexler H, Hayoz D, Munzel T, Hornig B, Just H, Brunner HR, Zelis R. Endothelial function in chronic congestive heart failure. Am J Cardiol. 1992;69(19):1596–1601. doi: 10.1016/0002-9149(92)90710-g. [DOI] [PubMed] [Google Scholar]
- 45.Katz SD, Hryniewicz K, Hriljac I, Balidemaj K, Dimayuga C, Hudaihed A, Yasskiy A. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation. 2005;111(3):310–314. doi: 10.1161/01.CIR.0000153349.77489.CF. [DOI] [PubMed] [Google Scholar]
- 46.Tousoulis D, Charakida M, Stefanadis C. Inflammation and endothelial dysfunction as therapeutic targets in patients with heart failure. Int J Cardiol. 2005;100(3):347–353. doi: 10.1016/j.ijcard.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 47.Witman MA, Fjeldstad AS, McDaniel J, Ives SJ, Zhao J, Barrett-O’Keefe Z, Nativi JN, Stehlik J, Wray DW, Richardson RS. Vascular function and the role of oxidative stress in heart failure, heart transplant, and beyond. Hypertension. 2012;60(3):659–668. doi: 10.1161/HYPERTENSIONAHA.112.193318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol. 2012;590(Pt 6):1413–1425. doi: 10.1113/jphysiol.2011.224741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol. 2012;302(5):H1050–H1063. doi: 10.1152/ajpheart.00943.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kienzle MG, Ferguson DW, Birkett CL, Myers GA, Berg WJ, Mariano DJ. Clinical, hemodynamic and sympathetic neural correlates of heart rate variability in congestive heart failure. Am J Cardiol. 1992;69(8):761–767. doi: 10.1016/0002-9149(92)90502-p. [DOI] [PubMed] [Google Scholar]
- 51.Floras JS, Ponikowski P. The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur Heart J. 2015;36(30):1974–1982b. doi: 10.1093/eurheartj/ehv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Witman MA, Ives SJ, Trinity JD, Groot HJ, Stehlik J, Richardson RS. Heart failure and movement-induced hemodynamics: partitioning the impact of central and peripheral dysfunction. Int J Cardiol. 2015;178:232–238. doi: 10.1016/j.ijcard.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Donato AJ, Morgan RG, Walker AE, Lesniewski LA. Cellular and molecular biology of aging endothelial cells. J Mol Cell Cardiol. 2015;89(Pt B):122–135. doi: 10.1016/j.yjmcc.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic Res Commun. 1993;18(4):195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 55.Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL. Antioxidant vitamin supplements and cardiovascular disease. Circulation. 2004;110(5):637–641. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]
- 56.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38(2):274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 57.Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol. 2007;103(5):1715–1721. doi: 10.1152/japplphysiol.00533.2007. [DOI] [PubMed] [Google Scholar]
- 58.Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide but not vasodilating prostaglandins contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol. 2010;299(5):H1633–H1641. doi: 10.1152/ajpheart.00614.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol. 2009;587(Pt 9):1989–2003. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568(Pt 3):1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trinity JD, Broxterman RM, Richardson RS. Regulation of exercise blood flow: role of free radicals. Free Radic Biol Med. 2016 doi: 10.1016/j.freeradbiomed.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Widlansky ME, Gutterman DD. Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxid Redox Signal. 2011;15(6):1517–1530. doi: 10.1089/ars.2010.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Craige SM, Kant S, Keaney JF., Jr Reactive oxygen species in endothelial function—from disease to adaptation. Circ J. 2015;79(6):1145–1155. doi: 10.1253/circj.CJ-15-0464. [DOI] [PubMed] [Google Scholar]
- 64.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101(25):2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- 65.Katz SD, Khan T, Zeballos GA, Mathew L, Potharlanka P, Knecht M, Whelan J. Decreased activity of the L-arginine-nitric oxide metabolic pathway in patients with congestive heart failure. Circulation. 1999;99(16):2113–2117. doi: 10.1161/01.cir.99.16.2113. [DOI] [PubMed] [Google Scholar]
- 66.Bauersachs J, Bouloumie A, Fraccarollo D, Hu K, Busse R, Ertl G. Endothelial dysfunction in chronic myocardial infarction despite increased vascular endothelial nitric oxide synthase and soluble guanylate cyclase expression: role of enhanced vascular superoxide production. Circulation. 1999;100(3):292–298. doi: 10.1161/01.cir.100.3.292. [DOI] [PubMed] [Google Scholar]
- 67.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 68.Wei EP, Kontos HA, Christman CW, DeWitt DS, Povlishock JT. Superoxide generation and reversal of acetylcholine-induced cerebral arteriolar dilation after acute hypertension. Circ Res. 1985;57(5):781–787. doi: 10.1161/01.res.57.5.781. [DOI] [PubMed] [Google Scholar]
- 69.Kubo SH, Rector TS, Bank AJ, Williams RE, Heifetz SM. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation. 1991;84(4):1589–1596. doi: 10.1161/01.cir.84.4.1589. [DOI] [PubMed] [Google Scholar]
- 70.Hornig B, Arakawa N, Kohler C, Drexler H. Vitamin C improves endothelial function of conduit arteries in patients with chronic heart failure. Circulation. 1998;97(4):363–368. doi: 10.1161/01.cir.97.4.363. [DOI] [PubMed] [Google Scholar]
- 71.Witman MA, McDaniel J, Fjeldstad AS, Ives SJ, Zhao J, Nativi JN, Stehlik J, Wray DW, Richardson RS. A differing role of oxidative stress in the regulation of central and peripheral hemodynamics during exercise in heart failure. Am J Physiol Heart Circ Physiol. 2012;303(10):H1237–H1244. doi: 10.1152/ajpheart.00568.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hearon CM, Jr, Dinenno FA. Regulation of skeletal muscle blood flow during exercise in ageing humans. J Physiol. 2015;594(8):2261–2273. doi: 10.1113/JP270593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2(2):1143–1211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wahren J, Saltin B, Jorfeldt L, Pernow B. Influence of age on the local circulatory adaptation to leg exercise. Scand J Clin Lab Investig. 1974;33(1):79–86. doi: 10.3109/00365517409114201. [DOI] [PubMed] [Google Scholar]
- 75.Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol. 2003;285(3):H1023–H1031. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- 76.Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol. 1998;85(1):68–75. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- 77.Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol. 2003;95(5):1963–1970. doi: 10.1152/japplphysiol.00472.2003. [DOI] [PubMed] [Google Scholar]
- 78.Sullivan MJ, Hawthorne MH. Exercise intolerance in patients with chronic heart failure. Prog Cardiovasc Dis. 1995;38(1):1–22. doi: 10.1016/s0033-0620(05)80011-8. [DOI] [PubMed] [Google Scholar]
- 79.Benge W, Litchfield RL, Marcus ML. Exercise capacity in patients with severe left ventricular dysfunction. Circulation. 1980;61(5):955–959. doi: 10.1161/01.cir.61.5.955. [DOI] [PubMed] [Google Scholar]
- 80.Higginbotham MB, Morris KG, Conn EH, Coleman RE, Cobb FR. Determinants of variable exercise performance among patients with severe left ventricular dysfunction. Am J Cardiol. 1983;51(1):52–60. doi: 10.1016/s0002-9149(83)80010-1. [DOI] [PubMed] [Google Scholar]
- 81.Weber KT, Kinasewitz GT, Janicki JS, Fishman AP. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation. 1982;65(6):1213–1223. doi: 10.1161/01.cir.65.6.1213. [DOI] [PubMed] [Google Scholar]
- 82.Szlachcic J, Massie BM, Kramer BL, Topic N, Tubau J. Correlates and prognostic implication of exercise capacity in chronic congestive heart failure. Am J Cardiol. 1985;55(8):1037–1042. doi: 10.1016/0002-9149(85)90742-8. [DOI] [PubMed] [Google Scholar]
- 83.Metra M, Raddino R, Dei Cas L, Visioli O. Assessment of peak oxygen consumption, lactate and ventilatory thresholds and correlation with resting and exercise hemodynamic data in chronic congestive heart failure. Am J Cardiol. 1990;65(16):1127–1133. doi: 10.1016/0002-9149(90)90326-v. [DOI] [PubMed] [Google Scholar]
- 84.Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation. 1989;80(4):769–781. doi: 10.1161/01.cir.80.4.769. [DOI] [PubMed] [Google Scholar]
- 85.LeJemtel TH, Maskin CS, Lucido D, Chadwick BJ. Failure to augment maximal limb blood flow in response to one-leg versus two-leg exercise in patients with severe heart failure. Circulation. 1986;74(2):245–251. doi: 10.1161/01.cir.74.2.245. [DOI] [PubMed] [Google Scholar]
- 86.Duscha BD, Kraus WE, Keteyian SJ, Sullivan MJ, Green HJ, Schachat FH, Pippen AM, Brawner CA, Blank JM, Annex BH. Capillary density of skeletal muscle: a contributing mechanism for exercise intolerance in class II–III chronic heart failure independent of other peripheral alterations. J Am Coll Cardiol. 1999;33(7):1956–1963. doi: 10.1016/s0735-1097(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 87.Esposito F, Mathieu-Costello O, Shabetai R, Wagner PD, Richardson RS. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol. 2010;55(18):1945–1954. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barrett-O’Keefe Z, Lee JF, Berbert A, Witman MA, Nativi-Nicolau J, Stehlik J, Richardson RS, Wray DW. Hemo-dynamic responses to small muscle mass exercise in heart failure patients with reduced ejection fraction. Am J Physiol Heart Circ Physiol. 2014;307(10):H1512–H1520. doi: 10.1152/ajpheart.00527.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Esposito F, Wagner PD, Richardson RS. Incremental large and small muscle mass exercise in patients with heart failure: evidence of preserved peripheral haemodynamics and metabolism. Acta Physiol. 2015;213(3):688–689. doi: 10.1111/apha.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gullestad L, Hallen J, Sejersted OM. K+ balance of the quadriceps muscle during dynamic exercise with and without beta-adrenoceptor blockade. J Appl Physiol. 1995;78(2):513–523. doi: 10.1152/jappl.1995.78.2.513. [DOI] [PubMed] [Google Scholar]
- 91.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 92.Piepoli MF, Davos C, Francis DP, Coats AJ, ExTra MC. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) BMJ. 2004;328(7433):189. doi: 10.1136/bmj.37938.645220.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Tol BA, Huijsmans RJ, Kroon DW, Schothorst M, Kwakkel G. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: a meta-analysis. Eur J Heart Fail. 2006;8(8):841–850. doi: 10.1016/j.ejheart.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 94.Richardson TE, Kindig CA, Musch TI, Poole DC. Effects of chronic heart failure on skeletal muscle capillary hemodynamics at rest and during contractions. J Appl Physiol. 2003;95(3):1055–1062. doi: 10.1152/japplphysiol.00308.2003. [DOI] [PubMed] [Google Scholar]
- 95.Esposito F, Reese V, Shabetai R, Wagner PD, Richardson RS. Isolated quadriceps training increases maximal exercise capacity in chronic heart failure: the role of skeletal muscle convective and diffusive oxygen transport. J Am Coll Cardiol. 2011;58(13):1353–1362. doi: 10.1016/j.jacc.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coats AJ, Clark AL, Piepoli M, Volterrani M, Poole-Wilson PA. Symptoms and quality of life in heart failure: the muscle hypothesis. Br Heart J. 1994;72(2 Suppl):S36–S39. doi: 10.1136/hrt.72.2_suppl.s36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Esposito F, Mathieu-Costello O, Shabetai R, Wagner PD, Richardson RS. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol. 2010;55(18):1945–1954. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD. Exaggerated renal vasoconstriction during exercise in heart failure patients. Circulation. 2000;101(7):784–789. doi: 10.1161/01.cir.101.7.784. [DOI] [PubMed] [Google Scholar]
- 99.Wilson JR, Martin JL, Schwartz D, Ferraro N. Exercise intolerance in patients with chronic heart failure: role of impaired nutritive flow to skeletal muscle. Circulation. 1984;69(6):1079–1087. doi: 10.1161/01.cir.69.6.1079. [DOI] [PubMed] [Google Scholar]
- 100.Smith SA, Mammen PP, Mitchell JH, Garry MG. Role of the exercise pressor reflex in rats with dilated cardiomyopathy. Circulation. 2003;108(9):1126–1132. doi: 10.1161/01.CIR.0000084538.40542.56. [DOI] [PubMed] [Google Scholar]
- 101.Piepoli MF, Kaczmarek A, Francis DP, Davies LC, Rauchhaus M, Jankowska EA, Anker SD, Capucci A, Banasiak W, Ponikowski P. Reduced peripheral skeletal muscle mass and abnormal reflex physiology in chronic heart failure. Circulation. 2006;114(2):126–134. doi: 10.1161/CIRCULATIONAHA.105.605980. [DOI] [PubMed] [Google Scholar]
- 102.Ponikowski PP, Chua TP, Francis DP, Capucci A, Coats AJ, Piepoli MF. Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation. 2001;104(19):2324–2330. doi: 10.1161/hc4401.098491. [DOI] [PubMed] [Google Scholar]
- 103.Amann M, Venturelli M, Ives SJ, Morgan DE, Gmelch B, Witman MA, Groot HJ, Wray DW, Stehlik J, Richardson RS. Group III/IV muscle afferents impair limb blood in patients with chronic heart failure. Int J Cardiol. 2014;174(2):368–375. doi: 10.1016/j.ijcard.2014.04.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Amann M, Calbet JA. Convective oxygen transport and fatigue. J Appl Physiol. 2008;104(3):861–870. doi: 10.1152/japplphysiol.01008.2007. [DOI] [PubMed] [Google Scholar]
- 105.Middlekauff HR, Sinoway LI. Increased mechanoreceptor stimulation explains the exaggerated exercise pressor reflex seen in heart failure. J Appl Physiol. 2007;102(1):492–494. doi: 10.1152/japplphysiol.00994.2006. discussion 496. [DOI] [PubMed] [Google Scholar]
- 106.Piepoli MF, Coats AJ. Increased metaboreceptor stimulation explains the exaggerated exercise pressor reflex seen in heart failure. J Appl Physiol. 2007;102(1):494–496. doi: 10.1152/japplphysiol.00994a.2006. discussion 496-7. [DOI] [PubMed] [Google Scholar]
- 107.Carrington CA, Fisher WJ, Davies MK, White MJ. Muscle afferent and central command contributions to the cardiovascular response to isometric exercise of postural muscle in patients with mild chronic heart failure. Clin Sci. 2001;100(6):643–651. [PubMed] [Google Scholar]
- 108.Kon H, Nakamura M, Arakawa N, Hiramori K. Muscle metaboreflex is blunted with reduced vascular resistance response of nonexercised limb in patients with chronic heart failure. J Card Fail. 2004;10(6):503–510. doi: 10.1016/j.cardfail.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 109.Notarius CF, Atchison DJ, Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol. 2001;280(3):H969–H976. doi: 10.1152/ajpheart.2001.280.3.H969. [DOI] [PubMed] [Google Scholar]
- 110.Sterns DA, Ettinger SM, Gray KS, Whisler SK, Mosher TJ, Smith MB, Sinoway LI. Skeletal muscle metaboreceptor exercise responses are attenuated in heart failure. Circulation. 1991;84(5):2034–2039. doi: 10.1161/01.cir.84.5.2034. [DOI] [PubMed] [Google Scholar]
- 111.Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJ. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation. 1996;93(5):940–952. doi: 10.1161/01.cir.93.5.940. [DOI] [PubMed] [Google Scholar]
- 112.Piepoli M, Ponikowski P, Clark AL, Banasiak W, Capucci A, Coats AJ. A neural link to explain the “muscle hypothesis” of exercise intolerance in chronic heart failure. Am Heart J. 1999;137(6):1050–1056. doi: 10.1016/s0002-8703(99)70361-3. [DOI] [PubMed] [Google Scholar]
- 113.Shoemaker JK, Kunselman AR, Silber DH, Sinoway LI. Maintained exercise pressor response in heart failure. J Appl Physiol. 1998;85(5):1793–1799. doi: 10.1152/jappl.1998.85.5.1793. [DOI] [PubMed] [Google Scholar]
- 114.Silber DH, Sutliff G, Yang QX, Smith MB, Sinoway LI, Leuenberger UA. Altered mechanisms of sympathetic activation during rhythmic forearm exercise in heart failure. J Appl Physiol. 1998;84(5):1551–1559. doi: 10.1152/jappl.1998.84.5.1551. [DOI] [PubMed] [Google Scholar]
- 115.Ives SJ, Amann M, Venturelli M, Witman MA, Groot HJ, Wray DW, Morgan DE, Stehlik J, Richardson RS. The mechanoreflex and hemodynamic response to passive leg movement in heart failure. Med Sci Sports Exerc. 2015;48(3):368–376. doi: 10.1249/MSS.0000000000000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107(4):1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 117.Jackson MJ, Edwards RH, Symons MC. Electron spin resonance studies of intact mammalian skeletal muscle. Biochim Biophys Acta. 1985;847(2):185–190. doi: 10.1016/0167-4889(85)90019-9. [DOI] [PubMed] [Google Scholar]
- 118.Reid MB. Invited review: redox modulation of skeletal muscle contraction: what we know and what we don’t. J Appl Physiol. 2001;90(2):724–731. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]
- 119.Bailey DM, Young IS, McEneny J, Lawrenson L, Kim J, Barden J, Richardson RS. Regulation of free radical outflow from an isolated muscle bed in exercising humans. Am J Physiol Heart Circ Physiol. 2004;2004:00148. doi: 10.1152/ajpheart.00148.2004. [DOI] [PubMed] [Google Scholar]
- 120.Richards JC, Crecelius AR, Larson DG, Dinenno FA. Acute ascorbic acid ingestion increases skeletal muscle blood flow and oxygen consumption via local vasodilation during graded handgrip exercise in older adults. Am J Physiol Heart Circ Physiol. 2015;309(2):H360–H368. doi: 10.1152/ajpheart.00209.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rossman MJ, Trinity JD, Garten RS, Ives SJ, Conklin JD, Barrett-O’Keefe Z, Witman MA, Bledsoe AD, Morgan DE, Runnels S, Reese VR, Zhao J, Amann M, Wray DW, Richardson RS. Oral antioxidants improve leg blood flow during exercise in patients with chronic obstructive pulmonary disease. Am J Physiol Heart Circ Physiol. 2015;309(5):H977–H985. doi: 10.1152/ajpheart.00184.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wray DW, Uberoi A, Lawrenson L, Bailey DM, Richardson RS. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin Sci. 2009;116(5):433–441. doi: 10.1042/CS20080337. [DOI] [PubMed] [Google Scholar]
- 123.Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, Harrison DG, Hornig B, Drexler H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106(24):3073–3078. doi: 10.1161/01.cir.0000041431.57222.af. [DOI] [PubMed] [Google Scholar]
- 124.Katz SD, Krum H, Khan T, Knecht M. Exercise-induced vasodilation in forearm circulation of normal subjects and patients with congestive heart failure: role of endothelium-derived nitric oxide. J Am Coll Cardiol. 1996;28(3):585–590. doi: 10.1016/0735-1097(96)00204-5. [DOI] [PubMed] [Google Scholar]