Abstract
Astrocytes respond to a variety of CNS injuries by cellular enlargement, process outgrowth, and upregulation of extracellular matrix proteins that function to prevent expansion of the injured region. This astrocytic response, though critical to the acute injury response, results in the formation of a glial scar that inhibits neural repair. Scar forming cells (fibroblasts) in the heart can undergo mesenchymal-endothelial transition into endothelial cell fates following cardiac injury in a process dependent on p53 that can be modulated to augment cardiac repair. Here, we sought to determine whether astrocytes, as the primary scar-forming cell of the CNS, are able to undergo a similar cellular phenotypic transition and adopt endothelial cell fates. Serum deprivation of differentiated astrocytes resulted in a change in cellular morphology and upregulation of endothelial cell marker genes. In a tube formation assay, serum deprived astrocytes showed a substantial increase in vessel-like morphology that was comparable to human umbilical vein endothelial cells and dependent on p53. RNA-sequencing of serum-deprived astrocytes demonstrated an expression profile that mimicked an endothelial rather than astrocyte transcriptome and identified p53 and angiogenic pathways as specifically up-regulated. Inhibition of p53 with genetic or pharmacologic strategies inhibited astrocyte-endothelial transition. Astrocyte-endothelial cell transition could also be modulated by miR-194, a microRNA downstream of p53 that affects expression of genes regulating angiogenesis. Together, these studies demonstrate that differentiated astrocytes retain a stimulus-dependent mechanism for cellular transition into an endothelial phenotype that may modulate formation of the glial scar and promote injury-induced angiogenesis.
Keywords: astrocyte, endothelia, p53, phenotype transition, neural repair, transdifferentiation
Introduction
Critical barriers to the recovery of function after central nervous system injury are the formation of a glial scar and insufficient vascular supply to recovering tissue. While a regenerative response replenishes lost parenchyma with new viable tissue and can lead to structural and functional recovery, a scarring response replaces damaged tissue with scar tissue and results in significant impairment of tissue structure and function. In the CNS, astrocytes play a central role in scar formation [1]. In the setting of injury, resident astrocytes become reactive, changing their cellular morphology, undergo process extension, increasing the expression of glial fibrillary acidic protein (GFAP) [2], and can proliferate [3]. They also notably increase the expression of various extracellular matrix proteins including chondroitin proteoglycans, laminin, tenascin C, and fibronectin that form a glial scar around the injury core. While such scarring is beneficial in limiting the expansion of injury, it ultimately limits axonal outgrowth and impairs recovery of function [4,5]. Ischemic injury in the brain also stimulates angiogenesis in adjacent peri-infarct tissue bringing additional blood flow and reparative neural precursors cells to the peri-infarct region [6,7]. Reactive gliosis and post-ischemic neovascularization occur early on after injury and a strong spatio-temporal interplay of these processes is thought to affect the final burden of glial scarring and functional recovery [8]. Not surprisingly then, decreasing astrocyte proliferation, reactive gliosis, and growth inhibitory signaling all improve recovery [9–11].
Recent data has suggested that scar formation in post-mitotic tissues might be amenable to modulation by manipulating the plasticity of scar forming cells. Ubil et al [12] showed that a population of resident cardiac fibroblasts that contribute to post-ischemic cardiac scar formation undergo a cellular phenotype change into functional endothelial cells. This process is mediated by cell stress and dependent on p53. Moreover, p53-agonists can be used to promote fibroblast-endothelial cell transitions to increase neovascularization, decrease scarring, and enhance cardiac function after ischemic injury. Using a paradigm of differentiated astroglial cells in culture, we examined whether astroglial cells could change their fate to an endothelial phenotype.
Here, we show that under conditions of cellular stress triggered by serum deprivation, differentiated astrocytes are capable of undergoing a phenotypic cellular transition into endothelial cells. Astrocytes exposed to serum deprivation upregulate endothelial gene markers including a number of brain specific endothelial cell markers, behave like endothelial cells in an angiogenic assay, and adopt an endothelial cell-like transcriptome. In addition, we show that this cellular transition is dependent on p53 and that the p53-responsive miR-194 can drive this cellular phenotype change. Strategies to promote astrocyte-to-endothelial transition after CNS injury may hold therapeutic promise by modulating glial scar formation and promoting neovascularization.
Materials & Methods
Animal care and use
All animal studies were approved by the Institutional Animal Care and Use Committee at the University of California, Los Angeles. Wild-type C57/Bl6 mice were purchased from Jackson Laboratories. p53-heterozygote knockout mice were purchased from Jackson Labs and bred to homozygous null.
NPC isolation
Neural progenitor cells (NPCs) were isolated from the subventricular zone (SVZ) of postnatal day 7 (P7) C57BL/6 or p53-heterozygote or p53-null mice. SVZ tissue was microdissected with a curette from 2 mm thick coronal sections in Hibernate medium (Brain Bits). Four SVZ regions from each P3 mouse were pooled and dissociated in digestion medium (2 mg/ml papain (Worthington) and 200 U/ml DNase I (Roche) in Hibernate medium without calcium), triturated, filtered through 40 um cell strainers, and resuspended in NPC medium (Dulbecco’s modified Eagle’s medium/F12 containing1X B27 supplement (Life Technologies), 20 ng/ml of epidermal growth factor (EGF), 10 ng/ml of basic fibroblast growth factor (bFGF), heparin (5 μg/mL), 100 units penicillin and 0.1 mg/ml streptomycin (Life Technologies)). NPCs were grown as neurosphere suspension cultures as previously described (1) and passaged every 7 days, with addition of 20 ng/ml EGF and 10 ng/ml bFGF every two days.
NPC to astrocyte differentiation & serum deprivation
At passage 2, neurospheres were dissociated to single cells with Accumax enzyme and plated at 1.4X104 cells/cm2 in T75 flasks coated with 80 ug/ml growth factor-reduced matrigel (Corning). At 3d after plating, NPCs were rinsed with PBS and switched to astrocyte differentiation medium (Neurobasal medium (Life Technologies) containing 1X B27 supplement, 1X Glutamatax, and 5% FBS) [13]. Cultures achieved high astrocytic purity after 3d in differentiation medium by visual morphology and astrocytic phenotyping via immunocytochemistry and qPCR for astrocytic markers (Supplemental Figure 1). All experimental manipulations (tube formation assays, serum deprivation and p53 gain- and loss-of-function) were started 3d after induction of astrocytic differentiation. To serum deprive cells, astrocyte differentiation media was removed and cells washed 2X in sterile PBS. Astrocyte differentiation media either containing 5% FBS or 0% FBS was added and cells cultured for varying time points (24 h, 48 h, or 72 h).
RNA isolation & qPCR
Following serum deprivation, cells were harvested by trypsinization and centrifugation or direct lysis. RNA isolation was performed using Nucleospin miRNA collection kit (Clontech) to collect large and small RNA. Fifty-500 ng of total RNA was used to generate first strand cDNA (Superscript VILO, ThermoFisher). Real-time qPCR was performed using a Roche Lightcycler 480 and SYBR green Master Mix (Roche). Primers for VECAD, eNOS, PECAM, Claudin 5 and six brain-specific endothelial genes identified based on comparative transcriptional analysis of the mouse blood-brain barrier were used [14]. Primer sequences are available in Supplemental Table 1. Fold expression values and 95% confidence intervals were calculated using established methodology. One-way ANOVA with post-hoc Student’s t-test and Bonferroni correction for multiple comparisons using delta-Ct values were used to determine statistical significance (p<0.005). Representative data from a single experiment are presented though cultures and qPCR analysis were performed in triplicate with similar results. For qPCR in p53-null mice and in miR-194 gain and loss of function studies, we utilized a custom qPCR array employing selected genes from above biologic replicates and technical triplicates. Raw Ct values were analyzed using SABiosciences array analysis software.
Immunocytochemistry
To determine culture purity and measure the upregulation of endothelial markers at the protein level, NPC were differentiated into astrocytes on matrigel coated glass coverslips. Following serum deprivation, cells were immediately fixed with 4% paraformaldehyde and stored at 4C until immunocytochemistry. Cells were washed twice with PBS pH 7.4, blocked and permeabilized with 5% normal donkey serum (Jackson Immunoresearch) and 0.3% TX-100 (Sigma) in 1X PBS for 45 min at RT. The following primary antibodies were used: rat anti-glial fibrillary acidic protein (GFAP) (Life Technologies), rabbit anti-ALDH1L1 (Abcam), rabbit anti-VECAD (Abcam), rabbit anti-CD31 (Bioss), rabbit anti-claudin (Abcam) and rabbit anti-eNOS (Abcam). Primary antibody incubations occurred at 4C for 24 h, after which cells were washed twice with PBS and appropriate donkey anti-rat or donkey anti-rabbit secondary antibodies (Jackson Immunoresearch) along with DAPI (Life Technology) were added for 45 min at RT. Cells were again washed twice with PBS and coverslips inverted onto glass slides. Cellular imaging was performed using a Nikon C2 confocal microscope. Quantitation was performed by measuring the total and VECAD-positive cells in each of five 60X fields per time point. One-way ANOVA with post-hoc Student’s t-test and Bonferroni correction for multiple comparisons were used to determine statistical significance.
Tube formation assay
This assay was performed generally as previously reported [15]. For each assay, 1.5 × 104 cells/well were plated on undiluted growth factor reduced matrigel and cultured in triplicate for 24 hr in astrocyte differentiation medium with (5%) or without (0%) serum. Pifithrin-α 100 μM (P4359, Sigma) or RITA 0.1 μM (506149, EMD Chemicals) were added to the cells cultured with the above culture medium. NPCs and HUVECs were used as controls. After 24 hrs of growth, cells were fixed and stained using Acti-Stain (Cytoskeleton, Inc.). Confocal z-stack images of each well were obtained at 4X magnification and maximum intensity projection images were analyzed using AngioTool [16] with statistical significance determined using a one-way ANOVA with post-hoc Student’s t-test and Bonferroni correction for multiple comparisons.
RNA-seq and bioinformatic analysis
Duplicate cultures of astrocytes were cultured from NPCs as above and serum deprived for 48 hr. RNA was isolated by direct lysis and purified as above. RINs ranged from 7.8–8.6. Serum deprived primary cardiac fibroblast cultures and RNA were isolated as previously reported [12]. RNA-sequencing of astrocytes was carried out by the UCLA Neuroscience Genomics Core using TrueSeq with RiboZero treatment. Samples were sequenced using paired end reads resulting in 50M reads per sample. RNA-sequencing of cardiac fibroblasts was performed using standard Illumina RNA-seq library construction protocols and sequenced on the Illumina HiSeq 2000. Reads were aligned to the latest mouse_mm10 reference genome using the STAR (ver 2.4.0) spliced read aligner [17]. Read counts for RefSeq genes were generated by HT-seq [18]. Differentially expressed genes (DEG) were analyzed using both the negative binomial R/Bioconductor EdgeR package and Limma-voom analysis packages to reduce the likelihood of a Type-I error. DEG were selected using an FDR of <0.01 (EdgeR package). Enrichr pathway analysis was performed using both up- and down-regulated gene lists [19]. For cell type enrichment analysis, expression values for different cells types were downloaded [20]. For each cell type, enrichment index was calculated log2([FPKM_one_cell_type]/[FPKM_avg_all_other_cell_types]). Top 500 cell specific genes were selected and plotted against log2 fold change from serum deprived astrocytes compared to control.
miR-194 qPCR & gain and loss of function
Astrocyte cultures were generated as above and cultured in 5% FBS or 0% FBS for 48h. RNA was isolated as above and small RNA fractions were generated. miR cDNA was generated using the NCode VILO cDNA synthesis protocol (ThermoFisher). Primers for miR-194, miR-103a, and snoRNA-202 were generated using miR primer software [21] (sequences available in Supplemental Table 1). To determine the effect of miR-194 gain and loss of function, astrocyte cultures were generated as above. After 3 days of astrocyte differentiation, cultures were transfected with miR-194 mimic or inhibitor siRNAs (Ambion/ThermoFisher) using RNAiMAX and standardized manufacturer protocol. Immediately following the initiation of transfection, cultures were changed to either 5% FBS or 0% FBS media and cultured for an additional 48 h. Transfection efficiency was measured by red fluorescent oligodT co-transfection. RNA was isolated as above. Duplicate cultures were plated on glass coverslips and fixed and stained as above.
Results
Serum deprivation in cultured astrocytes increases endothelial gene expression
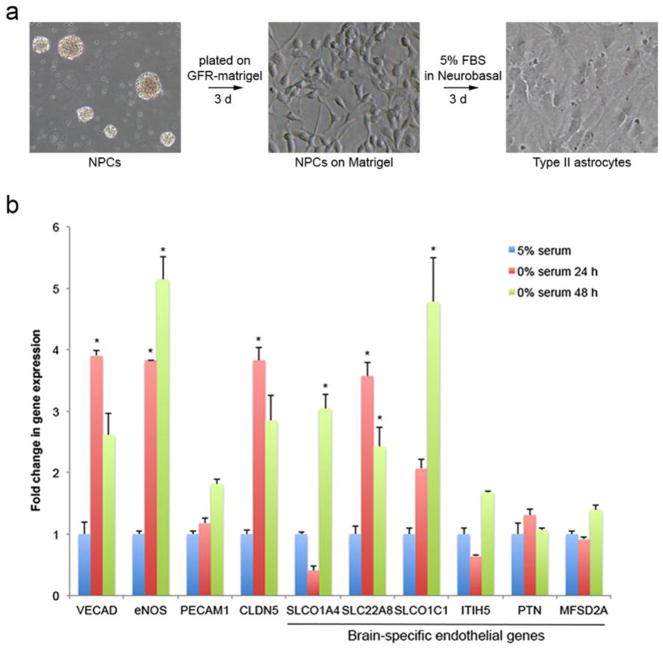
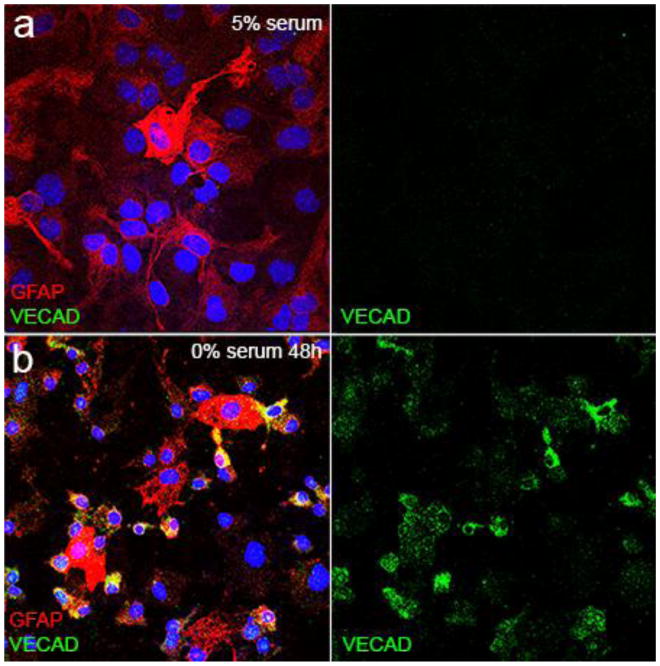
To determine if astrocytes were capable of cellular transition towards an endothelial phenotype, we established a culture paradigm with induced differentiation of subventricular zone derived neural precursor cells (NPCs) from post-natal day 7 mice [22] into Type II astrocytes (Figure 1a). This resulted in a highly pure culture of primary astrocytes showing ubiquitous GFAP staining by immunofluorescence (Supplemental Figure 1a) and by qPCR, a 15-fold increase in ALDH1L1 and 6-fold increase in GFAP with no measureable expression of neuronal or oligodendrocytic markers (Supplemental Figure 1b). As serum deprivation induced stress has been shown to induce endothelial transition of cardiac fibroblasts, scar-forming cells that modulate extracellular matrix and provide tissue barrier protection after injury (similar to astrocytes), we subjected NPC derived type II astrocytes to serum deprivation to determine if they were capable of adoption of an endothelial phenotype. Serum deprivation resulted in significant increased expression of endothelial-specific genes including VECAD and eNOS as well as several brain-specific endothelial genes [14] (Figure 1b). After serum deprivation, the number of VECAD-positive cells increase from zero in control and after 24 h of serum deprivation to 23.5% (+/− 0.34; p=0.002) at 48 h and 35.3% (+/−1.03; p=0.000002) at 72 h (Figure 2). Serum deprivation also produced an increase in GLUT-1 expression (Supplemental Figure 2). Notably, we also cultured astrocytes and NPCs under conditions of serum deprivation with 1% O2, but hypoxia did not have any additive effect on the expression of endothelial markers (Supplemental Figure 3). These indicate that serum deprivation in vitro induces the beginning of an endothelial phenotype in astrocytes by increasing the expression endothelial specific genes in astrocytes.
Figure 1.
Cultured astrocytes were generated from subventricular neural precursors grown as neurospheres by culturing on growth-factor reduced matrigel for 3 days followed by media change to defined media with 5% fetal bovine serum (FBS) for 3 days. This generated a highly pure culture of Type II astrocytes (a). After differentiation as in (a), 24 h and 48 h of serum deprivation resulted in significant increases in a number of endothelial genes by qPCR, including a number of brain-specific endothelial genes [14](b). Asterisk indicates p<0.005, n=3.
Figure 2.
Serum-deprivation increases expression of vascular endothelial cell adhesion molecule (VECAD) in astrocytes. Compared to astrocytes grown in serum with robust and ubiquitous GFAP expression (red) (a), VECAD-immunoreactive cells (green) increase to 23.5% (+/− 0.34; p=0.002) at 48 h (b) and 35.3% (+/−1.03; p=0.000002) at 72 h with notable cellular morphology changes including a small nucleus and consolidation of cellular processes in GFAP-immunoreactive cells compared to those without VECAD-immunoreactivity. Scale bar = 10 μm.
Astrocytes form tubes in an angiogenic tube formation assay
To determine whether serum deprived astrocytes would exhibit functional characteristics of endothelial cells, we performed a tube formation assay [15]. This is a well-characterized in vitro assay that has been extensively used to study angiogenesis, involves cell migration within a three-dimensional extracellular matrix gel, and measures a unique angiogenic function of endothelial cells [15]. NPC derived astrocytes were seeded onto Matrigel coated tissue culture dishes and subjected to control or serum deprived conditions. Twenty-four hours after seeding, astrocytes grown in serum containing media showed no evidence of tube formation and tended to cluster into small spheroids (Figure 3a, c). In contrast, astrocytes subjected to serum deprivation exhibited robust tube formation (Figure 3b, d). Tube formation was associated with an increase in VECAD immunoreactivity (Figure 3e, f) that often coincided with decreased GFAP immunoreactivity within the tube formation assay. Taken together, these observations strongly suggest that astrocytes following transition to an endothelial cell phenotype exhibit functional characteristics of endothelial cells. p53-mediated stress pathways have been shown to regulate fibroblast endothelial cellular transitions in the myocardium [12]. Serum deprived astrocytes show dramatic increases in both the number of junctions (Figure 3g; p=0.27 × 10−6) and the average vessel length (Figure 3h; p=0.18 × 10−6). Inhibition of p53 signaling using the small molecule p53 antagonist, pifithrin-α (100 μM) [23] dramatically decreased tube formation (p=0.14 × 10−6 (junctions); p=0.24 × 10−6 (average vessel length)). In contrast, addition of the small molecule p53 agonist, RITA (100 nM) [24] did not further augment tube formation. Serum deprived astrocytes behaved similarly to HUVECs with respect to average vessel length (Figure 3c) but formed tubes with a higher overall complexity than HUVECs, reflected in a greater number of junctions seen with serum deprivation in astrocytes compared to HUVECs (p=1.0 × 10−4).
Figure 3.
Serum-deprived astrocytes form tubes in a tube formation assay. Astrocytes grown in 5% FBS did not form tubes (a, c), while astrocytes grown in 0% FBS during the tube formation assay showed robust tube formation (b, d; upper panels = 10X brightfield; middle panels = 10X fluorescence after staining with DAPI (white) and Alexa Fluor 488 phallodin (green); scale bar = 200 μm). Tube formation is associated with an increase in VECAD immunoreactivity (f) compared to control (e) (10X confocal fluorescence overlay; scale bar = 100 μm). Number of junctions (g), and average vessel length (h), were measured in Angiotool. Serum deprivation resulted in a significant increase in both measurements (* = p<0.01). Addition of 100 nM RITA together with serum deprivation did not result in further increases in tube formation, while serum deprivation in the presence of pifithrin-α (100 μM) completely blocked tube formation (** = p<0.01). Average vessel length of serum-deprived astrocytes was similar to that of human umbilical vein endothelial cells (HUVECs) though the number of junctions was substantially higher in serum-deprived astrocytes (g; p<0.01).
Transcriptional analysis of astrocyte-endothelial cell transition
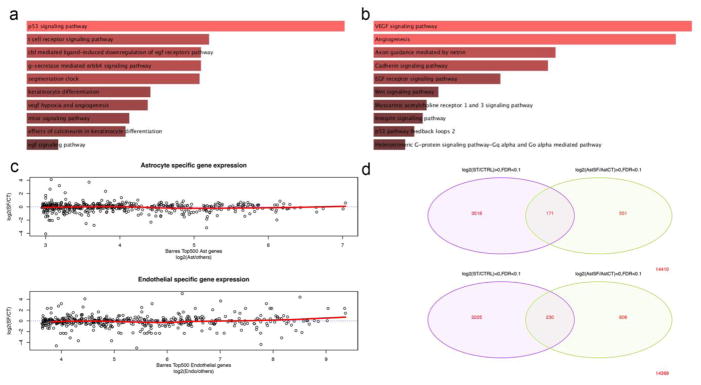
To better understand the molecular pathways triggered by serum-deprivation in cultured astroglial cells, we performed RNA-seq and comparative bioinformatics on serum-deprived astrocytes. Figure 4 illustrates the top 100 differentially expressed genes (DEG) with the highest read counts (FDR<0.01). In total, 376 genes were up-regulated in serum-deprived astrocytes while 614 genes were down-regulated (FDR<0.01) (Supplemental Table 2). We first performed gene ontology and pathway analysis on significantly upregulated genes using the Enrichr [19] database. Gene ontology of biological processes demonstrates genes/pathways regulating cell-cell adhesion via the plasma membrane, cell-cell adhesion, and the regulation of response to wounding (Supplemental Figure 4). Pathway analysis directly implicates the p53 signaling pathway (Figure 5a; Biocarta 2015; p=0.0039) as well as VEGF and angiogenesis pathways (Figure 5b; Panther 2015; p=0.0046 and p=0.0049, respectively). A similar analysis with the significantly down-regulated gene set implicates genes/pathways regulating μ-calpain (p=0.00058), m-calpain (p=0.0067), vascular endothelial ion channels (p=0.00336), integrin signaling (p=0.0015), and cholesterol biosynthesis (p=0.00119) (Supplemental Figure 5). To determine how closely the transcriptional profile of serum deprived astrocytes correlates with existing cell-type specific databases, we compared the DEG in our serum-deprived astrocyte cultures with existing brain cell-specific RNA-seq data sets [25] by plotting the top 500 cell-type specific genes characterizing astrocytes (upper panel) and endothelia (lower panel) from the Zhang et al database against log2 fold-change of each of those genes in our data set (Figure 5c). Only six of the highly expressed astrocyte genes showed >2 log2 fold-change in serum-deprived astrocytes (far right, upper panel) with a flat trendline (red line; upper panel) when comparing all genes. In contrast, 19 of the brain endothelial-specific genes showed >2 log2 fold-change in serum-deprived astrocytes, resulting in a shift of the trendline (red line; lower panel) towards an endothelial phenotype. Finally, we compared the DEGs found in our serum-deprived astrocytes with DEGs from serum-deprived primary cardiac fibroblasts that are known to under similar cellular phenotype plasticity towards an endothelial phenotype [12]. Despite the large difference in cellular background between cardiac fibroblasts and astrocytes, we found 171 commonly up-regulated genes (pHyper=0.12117) including as well as 230 commonly down-regulated genes (pHyper=0.0832) (Figure 5d). Common up-regulated genes represented 4.86% of the total up-regulated genes in fibroblasts and 31.0% in astrocytes. Common down-regulated genes represented 7.13% of the total down-regulated genes in fibroblasts and 28.5% in astrocytes. A total of 49 differentially expressed transcription factors were common between the two serum-deprived cell types (Supplemental Figure 6a & b; Supplemental Table 3). Enrichr pathway analysis of both the up- (logFC>0 for both cell populations) and down-regulated (logFC<0) shared transcription factors (FDR<0.1) implicates the p300/CBP signaling system (Supplemental Figure 6c & d). Together, these data indicate that astrocytes do harbor intrinsic cellular plasticity towards an endothelial phenotype with a gene expression profile that shares features with both known endothelial transcriptional databases and other cell types that have a similar cellular plasticity potential.
Figure 4.
Cluster dendrogram of the top 100 differentially expressed genes (DEG) using false discovery rate of <0.01 by EdgeR analysis from RNA-seq comparing serum-deprived astrocytes (Astrocyte SF) vs. astrocytes cultured in 5% FBS (Astrocyte Control). Red cells are increased expression; blue cells are decreased expression.
Figure 5.
Enrichr [19] pathway analysis of up-regulated DEG from serum-deprived astrocytes using Biocarta 2015 (a) and Panther 2015 (b) databases. Comparative analysis of cell-type genes from an existing CNS cell-type specific RNA-seq database [25] (log2-fold change) (x-axis) plotted against the log2-fold change in serum-deprived astrocytes (y-axis) with each circle representing individual genes (c). Upper panel compares database-defined astrocyte-specific genes, while lower panel shows endothelial-specific genes. The red trendline shows the average of expression in serum-deprived astrocytes across all genes, which favors an endothelial phenotype over astrocytes. Venn diagram of RNA-seq DEG from serum-deprived cardiac fibroblasts compared to serum-deprived astrocytes (d) showing the degree of overlap in up-regulated genes (upper panel) vs. down-regulated genes (lower panel).
p53 is required for astrocyte-endothelial cell transition
To confirm the role of p53 on astroglial cellular plasticity towards an endothelial phenotype, we generated NPC cultures from P7 p53-null and p53-heterozygote knockout mice [26]. We did not observe any significant differences in growth rate, cellular size or morphology, or astrocyte differentiation from NPCs in WT, p53-heterozygote, or p53-null cultures (data not shown). When seeded into a tube formation assay, both partial and complete lack of p53 significantly inhibited the formation of tubes (Figure 6a–c). Wild-type astrocytes from p53-null littermates formed robust tubes in conditions of serum-deprivation as we had observed in prior studies (Figure 6a). p53-heterozygote astrocytes appeared to form neighboring links and to partially change cellular morphology to a more linear morphology but did not generate substantial tubes (Figure 6b). p53-null astrocytes did not show the same degree of cellular morphology change nor exhibited any substantial tube formation (Figure 6c). The number of junctions (Figure 6d) and average vessel length (Figure 6e) were significantly reduced in both the p53-heterozygote and p53-null astrocyte cultures upon serum deprivation to levels that resemble astrocytes cultured in serum conditions. These observations suggest that p53 is required for this form of astrocyte-endothelial cellular transition. To confirm the effect of p53 deletion on endothelial gene expression, we developed a focused endothelial gene qPCR array comprising both canonical endothelial and brain-specific endothelial markers (VECAD, eNOS, claudin-5, slc22a8, slc1a4, and slc1c1), that were observed to be consistently upregulated in serum-deprived astrocytes. Compared to gene expression in wild-type serum-deprived astrocytes, p53-null serum-deprived astrocytes demonstrated a significant downregulation of these endothelial genes (Figure 6f). Inset cluster dendrogram (upper right, Figure 6f) indicates that a variable gene expression profile was observed in p53-heterozygote serum-deprived astrocytes (middle column), while global gene expression decreased for 6/7 genes in the array in p53-null astrocytes. Taken together, these data confirm the necessity of p53 on astroglial cellular plasticity towards an endothelial phenotype.
Figure 6.
p53 is required for astrocyte cellular plasticity. Wild-type, p53-heterozygote, and p53-null astrocyte cultures were serum-deprived during an angiogenic tube formation assay and stained with DAPI and Alexa Fluor 488-phallodin. Wild-type astrocytes robustly formed tubes (a) while p53-heterozygote (b), and p53-null astrocytes (c) did not. Both the number of junctions (d) and average vessel length (e) were significantly decreased in both p53-heterozygote and p53-null serum-deprived astrocytes (p<0.0167). Endothelial gene qPCR array demonstrates significantly decreased endothelial gene expression after 48 h of serum deprivation (log10 of 2− ΔCt) in p53-null astrocytes compared to wild-type astrocytes (f). Lines represent 2-fold regulation changes. Inset cluster dendrogram demonstrates gene-by-gene variation in wild-type (+/+), p53-heterozygote (+/−), and p53-null (−/−) astrocytes (red = high expression; green = low expression).
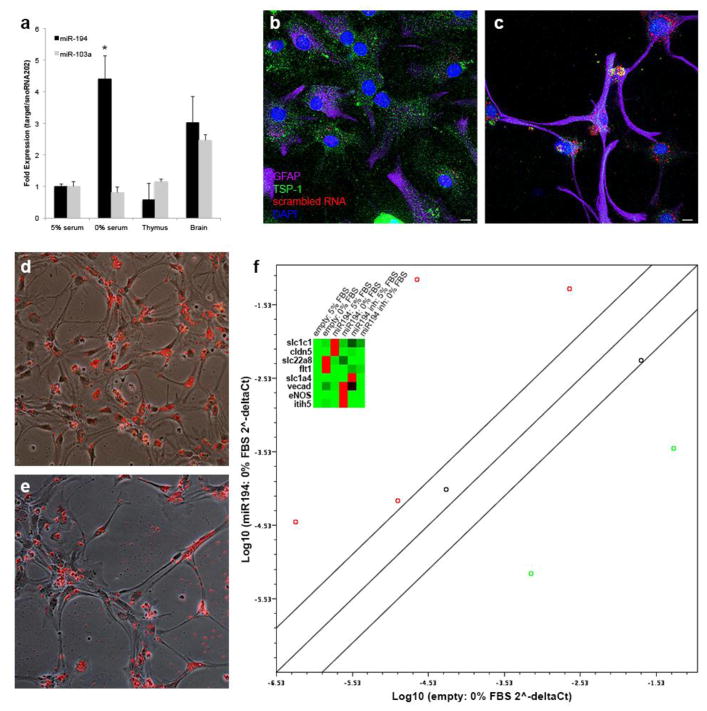
p53-responsive miR-194 can drive astroglial cellular plasticity
Next we investigated downstream mechanisms from p53, particularly the role of miR-194. miR-194 is a p53-dependent microRNA [27] that regulates expression of thrombospondin (TSP-1) [28], an anti-angiogenic factor that is partially secreted by astrocytes [29]. miR-194 is increased 4.4-fold with serum deprivation in astrocytes during astroglial-endothelial cellular transition (p = 0.05; CI 3.47–5.59) (Figure 7a). TSP-1 expression was robust in control cultures (Figure 7b) but decreased in serum-deprived astrocytes (Figure 7c). To determine if miR-194 could augment astroglial-endothelial cellular transition, we transfected differentiated astrocyte cultures with synthetic miR-194 mimic, miR-194 inhibitor or vehicle only (along with a red fluorescent indicator to determine transfection efficiency) at the time of serum deprivation. Vehicle only transfections demonstrated the typical morphology of serum-deprived astrocytes after 48 hrs with a high transfection efficiency (Figure 7d). miR-194 mimic cultures showed a greater degree of cellular elongation after 48 hrs of serum deprivation (Figure 7e). Gene expression analysis using a focused endothelial qPCR array, demonstrated that miR-194 further drives expression of endothelial specific genes compared to vehicle only transfected serum-deprived astrocytes (red circles, Figure 7f). Cluster dendrogram (inset, Figure 7f) illustrates the specific expression level of individual genes by culture and transfection condition. miR-194 inhibition reduces the expression of 4/8 of the endothelial specific genes compared to vehicle only transfected serum-deprived astrocytes (green circles, Supplemental Figure 7). Thus, miR-194, a p53-responsive microRNA, appears at least in part to mediate astroglial-endothelial cellular transition.
Figure 7.
miR-194 can drive astrocyte cellular plasticity. qPCR for miR-194 and miR-103a controlled for expression of snoRNA-202 in astrocytes grown in 5% FBS vs. 0% FBS for 48 hr. miR-194-regulated TSP-1 expression decreases with serum deprivation (c) compared to control cultures with robust TSP-1 expression (b). Mixed bright-field and fluorescent images of vehicle (d) and miR-194 mimic (e) showing a change in astrocyte morphology with miR-194 mimic transfection and serum-deprivation. Endothelial gene qPCR array demonstrates significantly increased endothelial gene expression after 48 h of serum deprivation in the presence of miR-194 mimic (log10 of 2− ΔCt) compared to vehicle only serum-deprived astrocytes (f). Lines represent 2-fold regulation changes. Inset cluster dendrogram demonstrates gene-by-gene variation in vehicle only, miR-194 mimic, and miR-194 inhibitor transfected conditions as well as serum conditions (red = high expression; green = low expression). Scale bar = 10 μm.
Discussion
The ability of differentiated cells to change their cellular phenotype (transdiffferentiation) is known to occur during wound healing [30]. Here, we demonstrate that differentiated astrocytes in vitro retain the intrinsic potential for transdifferentation towards an endothelial phenotype under the cellular stress of serum deprivation. This stimulus provokes a pro-angiogenic pathway that requires p53 but is not augmented by enhancing p53 activity and can be at least partially driven by the p53-responsive miR-194. These data suggest that p53-dependent mechanisms regulating injury-responsive fibroblast plasticity in the heart also regulate plasticity of glial cells and highlight the potential role for astrocyte cellular plasticity to modulate scar formation in the CNS after injury.
Artificial reprogramming of resident adult astrocytes in vivo has been previously reported with GFAP-promoter driven overexpression of Sox2 in the brain and spinal cord driving differentiated astrocytes into a functional neuronal phenotype [31,32]. Other reprogramming strategies have also demonstrated the ability of both transplanted human and intrinsic mouse astrocytes to differentiate into neuronal cell populations [33,31,34]. While the artificial transdifferentiation of astrocytes into neurons is intriguing and holds potential therapeutic promise, the intrinsic cellular plasticity of astrocytes to repair the brain by augmenting angiogenesis and potentially reducing glial scar formation is a novel and attractive translational target. The fact that astrocytes harbor this particular intrinsic transdifferentiation potential towards an endothelial phenotype is not necessarily surprising. Astrocytes have a wide variety of functions in both the resting and injured nervous system [35] and share a close functional relationship with endothelial cells both during development and into adulthood [36,29,37,38]. This study supports this close relationship and identifies a previously unreported form of astrocyte transdifferentiation. After stroke, reactive gliosis and angiogenesis share a tightly regulated spatio-temporal relationship suggesting that the microenvironment adjacent to a stroke may provide a fertile milieu to promote this transdifferentiation phenomenon. This study demonstrates that an injury stimulus already activates the cellular machinery to provoke astrocyte transdifferentiation and translational promise might be realized with partial augmentation of this intrinsic process. Moreover, shared molecular mechanisms in scar forming cells appear to be conserved in both the heart and brain, providing a single strategy with implications for repair in multiple organs.
In adult differentiated cells, the tumor-suppressor gene, p53, acts as a cellular injury response element known to react to a wide variety of cellular insults resulting in apoptosis, cell-cycle arrest and senescence [39,40]. The response to p53 is highly dependent on the cell type, the cellular environment, and the type of injury [41]. In astrocytes, p53 has been shown to promote cell cycle arrest [42–45], act as a nitric oxide (NO)-dependent signal to mitochrondia to promote cell death [46], promote astroglial hypertrophy after NMDA-mediated excitotoxicity [47], and induce astroglial proliferation and hypertrophy in the retina with modest overexpression [48]. Microarray analysis of hypoxic primary human astrocytes revealed selective alteration of p53 [49]. Thus, although well described to play a multi-faceted role in regulating the outcome of cell stress in a variety of cell types and tissues, this report highlights the importance of p53 in regulating astroglial cell fate following stress. The precise role of p53 in astrocytes and the astroglial response to injury remains unknown [50]. In this study, hypoxia had no additive effect on endothelial gene expression compared to serum deprivation alone suggesting that the key driver for astrocyte cellular plasticity is through a withdrawal of growth factors or cell surface energy detection that ultimately signal through p53. While we have shown that p53 is required for this astrocytic cellular plasticity, p53 agonism did not produce additive effects on either endothelial gene expression or functional angiogenic activity. This indicates that additional molecular pathways beyond p53 are needed to stimulate this cellular transition in astrocytes.
Gene expression analysis demonstrated that the expression profile of serum deprived astrocytes shares more similarity with a known endothelial cell profile than with a known astrocyte profile, though many of the cell-type specific genes do not vary. This is likely secondary to the stochastic nature of gene expression and cellular transition (i.e. all astrocytes subjected to serum deprivation do not transition into an endothelial phenotype, nor to the same degree). Indeed, comparative bioinformatics with serum-deprived cardiac fibroblasts that respond to myocardial ischemia with a similar p53-dependent cellular plasticity and become functional endothelial cells in vivo [12], illustrates that there is substantial overlap in the differentially expressed genes including 49 shared transcription factors (Figure 5d). One transcriptional system that provides a link between cell surface signaling, p53, and a regenerative program is the p300/CBP system (Supplemental Figure 6). This system is directly implicated by pathway analysis of both the up and down-regulated shared transcription factor list between serum-deprived astrocytes and cardiac fibroblasts (Supplemental Table 3). In the brain, the p300/CBP system has been shown to promote axonal regeneration [51], neurogenesis, and memory extension [52] suggesting that it has an active role in regenerative pathways in the CNS. Moreover, this system has been linked to determination of neural or glial fate in neural progenitors by its differential temporal association with neurogenin or STAT and the control of Smads and cell-surface BMP signaling [53]. This interesting transcriptional control system that helps to regulate glial cell fate in the CNS may also play a role in regulating intrinsic injury-induced astrocyte transdifferentiation.
In summary, this study identifies for the first time, an ability of astrocytes to adopt endothelial cell fates in a p53 dependent manner. Although currently unclear if astrocytes are capable of this injury-provoked cellular transition in vivo, the modulation of similar molecular mechanisms to regulate fibroblast endothelial transitions and post-injury scarring in vivo in the heart, illustrates the potential ability of this mechanism to regulate glial scarring. Future studies with fate mapping of astrocytes will demonstrate the physiological and therapeutic significance of astrocyte-endothelial cellular transitions in vivo. Any approach that reduces CNS scar formation and promotes recovery after injury holds therapeutic promise for neural repair.
Supplementary Material
Supplemental Figure 1. Culture purity of astrocyte differentiated neural precursors. After 3 days of culturing in astrocyte differentiation media, all cells demonstrate at least low level GFAP expression with most cells showing robust GFAP expression (a). qPCR analysis of phenotypic markers for astrocytes, neurons, and oligodendrocytes reveals a strong upregulation of astrocyte genes with down regulation of neuronal and oligodendrocyte marker genes compared to undifferentiated neural precursor cells (NPCs) (b).
Supplemental Figure 2. Serum-deprived astrocytes increase expression of GLUT-1. Astrocytes cultured in serum containing media show no significant GLUT-1 expression (a). After 48 hrs of serum-deprivation, astrocytes increase the expression of GLUT-1 (e). Individual confocal maximum intensity projection images are shown for each channel: DAPI (b, f), rabbit anti-GLUT-1 (c, g), and rat anti-GFAP (d, h), respectively. Scale bar = 10 μm.
Supplemental Figure 3. Hypoxia has no additive effect on endothelial gene expression in serum-deprived astrocytes. Culturing under 1% O2 had no additive effect on endothelial gene expression by qPCR in either astrocytes (a) or neural precursor cells (b). Baseline oxygen conditions were used a reference values for gene expression changes.
Supplemental Figure 4. Gene ontology of biological processes based on up-regulated genes (FDR<0.01).
Supplemental Figure 5. Gene ontology of biological processes based on down-regulated genes (FDR<0.01).
Supplemental Figure 6. Analysis of the common transcription factors between serum-deprived cardiac fibroblasts and astrocytes. Of the 49 shared transcription factors (FDR<0.1), 12 were up-regulated (logFC>0) in both cell populations (a, pHyper = 0.08596) and 10 were down-regulated (logFC<0) in both cell populations (b, pHyper = 0.18349). Enrichr pathway analysis using the PPI Hub Protein database of each gene list indicates that EP300 (p=0.00014 (up); p=0.0008 (down) and CREBBP (p=0.00002 (down) transcription regulatory system is common linker pathway in the both up- (b) and down-regulated (d) transcription factors. Notably, this system works to activate p53 further implicating this molecular system in the cellular plasticity observed in serum-deprived astrocytes.
Supplemental Figure 7. miR-194 inhibition reduces endothelial gene expression in serum-deprived astrocytes. Endothelial gene qPCR array demonstrates significantly decreased gene expression after 48 h of serum deprivation in the presence of miR-194 inhibitor (log10 of 2−ΔCt) compared to vehicle only serum-deprived astrocytes. Lines represent 2-fold regulation changes. Four of eight endothelial genes were down-regulated compared to empty transfected serum deprived astrocytes (green circles).
Primer sequences for qPCR
Acknowledgments
We acknowledge the support of the National Institute of Neurological Disorders and Stroke (NINDS) Informatics Center for Neurogenetics and Neurogenomics (P30 NS062691). JDH is supported by (NINDS) NS083740. AD is supported by National Heart, Lung, and Blood Institute (NHLBI) HL129178.
References
- 1.Gleichman AJ, Carmichael ST. Astrocytic therapies for neuronal repair in stroke. Neurosci Lett. 2014;565:47–52. doi: 10.1016/j.neulet.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 2.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50(4):427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 3.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182(2):399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 5.Wiese S, Karus M, Faissner A. Astrocytes as a source for extracellular matrix molecules and cytokines. Front Pharmacol. 2012;3:120. doi: 10.3389/fphar.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nih LR, Deroide N, Lere-Dean C, Lerouet D, Soustrat M, Levy BI, Silvestre JS, Merkulova-Rainon T, Pocard M, Margaill I, Kubis N. Neuroblast survival depends on mature vascular network formation after mouse stroke: role of endothelial and smooth muscle progenitor cell co-administration. Eur J Neurosci. 2012;35(8):1208–1217. doi: 10.1111/j.1460-9568.2012.08041.x. [DOI] [PubMed] [Google Scholar]
- 7.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26(50):13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayakawa K, Pham LD, Katusic ZS, Arai K, Lo EH. Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proc Natl Acad Sci U S A. 2012;109(19):7505–7510. doi: 10.1073/pnas.1121146109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Overman JJ, Clarkson AN, Wanner IB, Overman WT, Eckstein I, Maguire JL, Dinov ID, Toga AW, Carmichael ST. A role for ephrin-A5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke. Proc Natl Acad Sci U S A. 2012;109(33):E2230–2239. doi: 10.1073/pnas.1204386109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee B, Clarke D, Al Ahmad A, Kahle M, Parham C, Auckland L, Shaw C, Fidanboylu M, Orr AW, Ogunshola O, Fertala A, Thomas SA, Bix GJ. Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J Clin Invest. 2011;121(8):3005–3023. doi: 10.1172/JCI46358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adelson JD, Barreto GE, Xu L, Kim T, Brott BK, Ouyang YB, Naserke T, Djurisic M, Xiong X, Shatz CJ, Giffard RG. Neuroprotection from stroke in the absence of MHCI or PirB. Neuron. 2012;73(6):1100–1107. doi: 10.1016/j.neuron.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ubil E, Duan J, Pillai IC, Rosa-Garrido M, Wu Y, Bargiacchi F, Lu Y, Stanbouly S, Huang J, Rojas M, Vondriska TM, Stefani E, Deb A. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature. 2014;514(7524):585–590. doi: 10.1038/nature13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obayashi S, Tabunoki H, Kim SU, Satoh J. Gene expression profiling of human neural progenitor cells following the serum-induced astrocyte differentiation. Cell Mol Neurobiol. 2009;29(3):423–438. doi: 10.1007/s10571-008-9338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PloS one. 2010;5(10):e13741. doi: 10.1371/journal.pone.0013741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnaoutova I, Kleinman HK. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc. 2010;5(4):628–635. doi: 10.1038/nprot.2010.6. [DOI] [PubMed] [Google Scholar]
- 16.Zudaire E, Gambardella L, Kurcz C, Vermeren S. A computational tool for quantitative analysis of vascular networks. PLoS One. 2011;6(11):e27385. doi: 10.1371/journal.pone.0027385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31(2):166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma'ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics. 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Barres B. Brain RNAseq. 2014 http://web.stanford.edu/group/barres_lab/brain_rnaseq.html.
- 21.Busk PK. A tool for design of primers for microRNA-specific quantitative RT-qPCR. BMC Bioinformatics. 2014;15:29. doi: 10.1186/1471-2105-15-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294(5549):2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 23.Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285(5434):1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 24.Issaeva N, Bozko P, Enge M, Protopopova M, Verhoef LG, Masucci M, Pramanik A, Selivanova G. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med. 2004;10(12):1321–1328. doi: 10.1038/nm1146. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34(36):11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4(1):1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 27.Braun CJ, Zhang X, Savelyeva I, Wolff S, Moll UM, Schepeler T, Orntoft TF, Andersen CL, Dobbelstein M. p53-Responsive micrornas 192 and 215 are capable of inducing cell cycle arrest. Cancer Res. 2008;68(24):10094–10104. doi: 10.1158/0008-5472.CAN-08-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sundaram P, Hultine S, Smith LM, Dews M, Fox JL, Biyashev D, Schelter JM, Huang Q, Cleary MA, Volpert OV, Thomas-Tikhonenko A. p53-responsive miR-194 inhibits thrombospondin-1 and promotes angiogenesis in colon cancers. Cancer Res. 2011;71(24):7490–7501. doi: 10.1158/0008-5472.CAN-11-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120(3):421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Tosh D, Slack JM. How cells change their phenotype. Nat Rev Mol Cell Biol. 2002;3(3):187–194. doi: 10.1038/nrm761. [DOI] [PubMed] [Google Scholar]
- 31.Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, Zhang CL. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol. 2013;15(10):1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su Z, Niu W, Liu ML, Zou Y, Zhang CL. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun. 2014;5:3338. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torper O, Pfisterer U, Wolf DA, Pereira M, Lau S, Jakobsson J, Bjorklund A, Grealish S, Parmar M. Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci U S A. 2013;110(17):7038–7043. doi: 10.1073/pnas.1303829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rouaux C, Arlotta P. Direct lineage reprogramming of post-mitotic callosal neurons into corticofugal neurons in vivo. Nat Cell Biol. 2013;15(2):214–221. doi: 10.1038/ncb2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003;23(2):137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- 36.Mi H, Haeberle H, Barres BA. Induction of astrocyte differentiation by endothelial cells. J Neurosci. 2001;21(5):1538–1547. doi: 10.1523/JNEUROSCI.21-05-01538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma S, Kwon HJ, Huang Z. A functional requirement for astroglia in promoting blood vessel development in the early postnatal brain. PLoS One. 2012;7(10):e48001. doi: 10.1371/journal.pone.0048001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 39.Giaccia AJ, Kastan MB. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12(19):2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- 40.Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003;10(4):431–442. doi: 10.1038/sj.cdd.4401183. [DOI] [PubMed] [Google Scholar]
- 41.Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9(5):402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- 42.Cox LS, Lane DP. Tumour suppressors, kinases and clamps: how p53 regulates the cell cycle in response to DNA damage. Bioessays. 1995;17(6):501–508. doi: 10.1002/bies.950170606. [DOI] [PubMed] [Google Scholar]
- 43.Kippin TE, Martens DJ, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005;19(6):756–767. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meletis K, Wirta V, Hede SM, Nister M, Lundeberg J, Frisen J. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133(2):363–369. doi: 10.1242/dev.02208. [DOI] [PubMed] [Google Scholar]
- 45.Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, Stommel JM, Dunn KL, Wiedemeyer R, You MJ, Brennan C, Wang YA, Ligon KL, Wong WH, Chin L, DePinho RA. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455(7216):1129–1133. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yung HW, Bal-Price AK, Brown GC, Tolkovsky AM. Nitric oxide-induced cell death of cerebrocortical murine astrocytes is mediated through p53- and Bax-dependent pathways. J Neurochem. 2004;89(4):812–821. doi: 10.1111/j.1471-4159.2004.02395.x. [DOI] [PubMed] [Google Scholar]
- 47.Villapol S, Acarin L, Faiz M, Castellano B, Gonzalez B. Distinct spatial and temporal activation of caspase pathways in neurons and glial cells after excitotoxic damage to the immature rat brain. J Neurosci Res. 2007;85(16):3545–3556. doi: 10.1002/jnr.21450. [DOI] [PubMed] [Google Scholar]
- 48.Salazar JJ, Gallego-Pinazo R, de Hoz R, Pinazo-Duran MD, Rojas B, Ramirez AI, Serrano M, Ramirez JM. Super p53” mice display retinal astroglial changes. PLoS One. 2013;8(6):e65446. doi: 10.1371/journal.pone.0065446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mense SM, Sengupta A, Zhou M, Lan C, Bentsman G, Volsky DJ, Zhang L. Gene expression profiling reveals the profound upregulation of hypoxia-responsive genes in primary human astrocytes. Physiol Genomics. 2006;25(3):435–449. doi: 10.1152/physiolgenomics.00315.2005. [DOI] [PubMed] [Google Scholar]
- 50.Jebelli JD, Hooper C, Garden GA, Pocock JM. Emerging roles of p53 in glial cell function in health and disease. Glia. 2012;60(4):515–525. doi: 10.1002/glia.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaub P, Joshi Y, Wuttke A, Naumann U, Schnichels S, Heiduschka P, Di Giovanni S. The histone acetyltransferase p300 promotes intrinsic axonal regeneration. Brain. 2011;134(Pt 7):2134–2148. doi: 10.1093/brain/awr142. [DOI] [PubMed] [Google Scholar]
- 52.Chatterjee S, Mizar P, Cassel R, Neidl R, Selvi BR, Mohankrishna DV, Vedamurthy BM, Schneider A, Bousiges O, Mathis C, Cassel JC, Eswaramoorthy M, Kundu TK, Boutillier AL. A novel activator of CBP/p300 acetyltransferases promotes neurogenesis and extends memory duration in adult mice. J Neurosci. 2013;33(26):10698–10712. doi: 10.1523/JNEUROSCI.5772-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herdegen T, Delgado-García JM. Brain damage and repair : from molecular research to clinical therapy. Kluwer Academic Publishers; Dordrecht ; Boston: 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Culture purity of astrocyte differentiated neural precursors. After 3 days of culturing in astrocyte differentiation media, all cells demonstrate at least low level GFAP expression with most cells showing robust GFAP expression (a). qPCR analysis of phenotypic markers for astrocytes, neurons, and oligodendrocytes reveals a strong upregulation of astrocyte genes with down regulation of neuronal and oligodendrocyte marker genes compared to undifferentiated neural precursor cells (NPCs) (b).
Supplemental Figure 2. Serum-deprived astrocytes increase expression of GLUT-1. Astrocytes cultured in serum containing media show no significant GLUT-1 expression (a). After 48 hrs of serum-deprivation, astrocytes increase the expression of GLUT-1 (e). Individual confocal maximum intensity projection images are shown for each channel: DAPI (b, f), rabbit anti-GLUT-1 (c, g), and rat anti-GFAP (d, h), respectively. Scale bar = 10 μm.
Supplemental Figure 3. Hypoxia has no additive effect on endothelial gene expression in serum-deprived astrocytes. Culturing under 1% O2 had no additive effect on endothelial gene expression by qPCR in either astrocytes (a) or neural precursor cells (b). Baseline oxygen conditions were used a reference values for gene expression changes.
Supplemental Figure 4. Gene ontology of biological processes based on up-regulated genes (FDR<0.01).
Supplemental Figure 5. Gene ontology of biological processes based on down-regulated genes (FDR<0.01).
Supplemental Figure 6. Analysis of the common transcription factors between serum-deprived cardiac fibroblasts and astrocytes. Of the 49 shared transcription factors (FDR<0.1), 12 were up-regulated (logFC>0) in both cell populations (a, pHyper = 0.08596) and 10 were down-regulated (logFC<0) in both cell populations (b, pHyper = 0.18349). Enrichr pathway analysis using the PPI Hub Protein database of each gene list indicates that EP300 (p=0.00014 (up); p=0.0008 (down) and CREBBP (p=0.00002 (down) transcription regulatory system is common linker pathway in the both up- (b) and down-regulated (d) transcription factors. Notably, this system works to activate p53 further implicating this molecular system in the cellular plasticity observed in serum-deprived astrocytes.
Supplemental Figure 7. miR-194 inhibition reduces endothelial gene expression in serum-deprived astrocytes. Endothelial gene qPCR array demonstrates significantly decreased gene expression after 48 h of serum deprivation in the presence of miR-194 inhibitor (log10 of 2−ΔCt) compared to vehicle only serum-deprived astrocytes. Lines represent 2-fold regulation changes. Four of eight endothelial genes were down-regulated compared to empty transfected serum deprived astrocytes (green circles).
Primer sequences for qPCR