Abstract
With the aim to investigate the outcome of tandem high-dose chemotherapy and autologous stem cell transplantation (HDCT/auto-SCT) for high-grade gliomas (HGGs), we retrospectively reviewed the medical records of 30 patients with HGGs (16 glioblastomas, 7 anaplastic astrocytomas, and 7 other HGGs) between 2006 and 2015. Gross or near total resection was possible in 11 patients. Front-line treatment after surgery was radiotherapy (RT) in 14 patients and chemotherapy in the remaining 16 patients including 3 patients less than 3 years of age. Eight of 12 patients who remained progression free and 5 of the remaining 18 patients who experienced progression during induction treatment underwent the first HDCT/auto-SCT with carboplatin + thiotepa + etoposide (CTE) regimen and 11 of them proceeded to the second HDCT/auto-SCT with cyclophosphamide + melphalan (CyM) regimen. One patient died from hepatic veno-occlusive disease (VOD) during the second HDCT/auto-SCT; otherwise, toxicities were manageable. Four patients in complete response (CR) and 3 of 7 patients in partial response (PR) or second PR at the first HDCT/auto-SCT remained event free: however, 2 patients with progressive tumor experienced progression again. The probabilities of 3-year overall survival (OS) after the first HDCT/auto-SCT in 11 patients in CR, PR, or second PR was 58.2% ± 16.9%. Tumor status at the first HDCT/auto-SCT was the only significant factor for outcome after HDCT/auto-SCT. There was no difference in survival between glioblastoma and other HGGs. This study suggests that the outcome of HGGs in children and adolescents after HDCT/auto-SCT is encouraging if the patient could achieve CR or PR before HDCT/auto-SCT.
Keywords: High-grade Glioma, Brain Tumor, High-dose Chemotherapy, Autologous Stem Cell Transplantation, Children
Graphical Abstract
INTRODUCTION
While high-grade gliomas (HGGs) represent one of the most common central nervous system (CNS) tumors in adults, HGGs are less common in children and adolescents (1). HGGs include a variety of heterogeneous lesions with differing histologies, but the most common histologies are anaplastic astrocytoma (World Health Organization [WHO] grade III) and glioblastoma (WHO grade IV) (2). The prognosis of HGGs has been very poor and there is no universally accepted standard care for HGGs in children. Despite numerous treatment approaches, outcomes have remained dismal and the far majority of children are succumbing to their disease (3,4,5). Previous children's cancer group study showed the effectiveness of adjuvant chemotherapy but current conventional therapies are not yet sufficient for survival (4,6,7). In adults, concomitant temozolomide (TMZ) and radiotherapy (RT) prolonged survival duration and is now considered the standard of treatment (8); however, multiple studies failed to demonstrate the benefits of TMZ on long-term survival in children (4,9,10). In addition, prolongation of survival, not cure, is less meaningful in children than in adults.
A treatment strategy using high-dose chemotherapy and autologous stem cell transplantation (HDCT/auto-SCT) has shown clinical benefit in children with high-risk or recurrent solid tumors (11,12). HDCT/auto-SCT has also been used successfully in children with high-risk or recurrent brain tumors in children (13,14,15). Recently, several studies have suggested that further dose-escalation using tandem HDCT/auto-SCT might improve outcomes for patients with recurrent or high-risk solid tumors including brain tumors (16,17,18). In the present study, we reviewed retrospectively our experience with HDCT/auto-SCT for HGGs in children and adolescents to investigate the outcome and risk factors of the results after HDCT/auto-SCT.
MATERIALS AND METHODS
Patients
We reviewed the medical records of all patients younger than 18 years, who were diagnosed with HGGs at Samsung Medical Center between February 2006 and October 2015. Inclusion criteria were WHO grade 3 or 4 astrocytic tumors (glioblastoma, anaplastic astrocytoma, and gliomatosis cerebri) and other rare HGGs. Pontine glioma was not included in the analysis. During the study period, most patients were recommended to undergo HDCT/auto-SCT if they remained progression free during induction treatment. The subjects were retrospectively identified through survey of our institutional database. A detailed review of the clinical data was performed to ascertain the presenting features, degree of surgical resection, pathology, chemotherapy regimen, RT, and the response to treatment before and after HDCT/auto-SCT.
Response and toxicity criteria
We evaluated disease response using brain magnetic resonance imaging (MRI) with or without spine MRI. We estimated tumor size by MRI as the product of the greatest diameter and the longest perpendicular diameter. We categorized disease response as follows: 1) progressive disease (PD): greater than 25% increase in tumor size or the appearance of a new tumor; 2) stable disease (SD): less than 50% reduction in tumor size or less than 25% increase in tumor size; 3) partial response (PR): greater than 50% decrease in tumor size; and 4) complete response (CR): complete disappearance of all previously measurable tumors. Toxicities during tandem HDCT/auto-SCT were graded using the National Cancer Institute's Common Terminology Criteria (version 4.0).
Statistics
Event-free survival (EFS) was calculated from the date of diagnosis until the date of relapse, progression, or death, whichever occurred first. Overall survival (OS) was calculated from the date of diagnosis until death from any cause. Survival rates and standard errors were estimated using the Kaplan-Meier method. Differences in survival rates between groups were compared using the log-rank test. We performed multivariate analysis using Cox-regression analysis to find independent prognostic factors for survival. We analyzed differences in the frequency of toxicity between the first and second HDCT/auto-SCT using the χ2 test or Fisher's exact test. Differences in continuous variables between the first and second HDCT/auto-SCT were analyzed using the Mann-Whitney U test. P values less than 0.05 were considered significant.
Ethics statement
The study protocol was approved by the Institutional Review Board at Samsung Medical Center, Seoul, Korea (IRB No. 2016-05-009). The need for informed consent was waived by the board.
RESULTS
Patient characteristics
A total of 30 patients (21 boys and 9 girls) were diagnosed with HGGs during the study period. Patient characteristics are summarized in Table 1. The median age at diagnosis was 12.5 years (range 0.3–18.0), and 3 patients were younger than 3 years at diagnosis. Glioblastoma (n = 16) was the most frequent pathology, followed by anaplastic astrocytoma (n = 7), high-grade astroblastoma (n = 3), gliomatosis cerebri (n = 2) and other HGGs (n = 2). Primary tumor originated from cerebral hemisphere in 19 patients, midline structures including basal ganglia in 9, and cerebellum in 2. Gross total resection or near total resection (> 90% resection) was possible in 11 patients and subtotal resection (50%–90% resection) or biopsy (< 50% resection) was performed in the remaining 19 patients. Four patients had leptomeningeal seeding at diagnosis.
Table 1. Patient characteristics.
| Characteristics | No. (%) |
|---|---|
| Sex | |
| Male | 21 (66.7) |
| Female | 9 (33.3) |
| Age at diagnosis, yr | |
| < 3 | 3 (10.0) |
| > 3 | 27 (90.0) |
| Histology | |
| Glioblastoma | 16 (53.3) |
| Anaplastic astrocytoma | 7 (23.3) |
| Gliomatosis cerebri | 2 (6.7) |
| High-grade astroblastoma | 3 (10.0) |
| Anaplastic pleomorphic astrocytoma | 1 (3.3) |
| Anaplastic glioneuronal tumor | 1 (3.3) |
| Location | |
| Cerebral hemisphere | 19 (63.3) |
| Midline structures | 9 (30.0) |
| Basal ganglia | 8 |
| Midbrain | 1 |
| Cerebellum | 2 (6.7) |
| Leptomeningeal seeding | |
| No | 26 (86.7) |
| Yes | 4 (13.3) |
| Results of surgery | |
| GTR/NTR | 11 (36.7) |
| STR | 6 (20.0) |
| Biopsy | 13 (43.3) |
| Front-line treatment after surgery | |
| RT | 14 (46.7) |
| Chemotherapy | 16 (53.3) |
| Results of induction treatment | |
| CR | 5 (16.7) |
| PR | 4 (13.3) |
| SD | 1 (3.3) |
| Transfer to other hospital in SD | 1 (3.3) |
| TRM (ICH) in SD | 1 (3.3) |
| PD | 15 (50.0) |
| PR2 after PD | 3 (10.0) |
| Tumor status at first HDCT/auto-SCT (n = 13) | |
| CR | 4 (30.8) |
| PR | 4 (30.8) |
| PR2 after PD | 3 (23.1) |
| PD | 2 (15.4) |
GTR = gross total resection, NTR = near total resection, STR = subtotal resection, RT = radiotherapy, CR = complete response, PR = partial response, SD = stable disease, PR2 = second PR, PD = progressive disease, TRM = treatment-related mortality, ICH = intracranial hemorrhage, HDCT = high dose chemotherapy, SCT = stem cell transplantation.
Induction treatment
Chemotherapy was the front-line treatment after surgery in 16 patients including 3 patients younger than 3 years. Among them, concomitant (n = 8) or subsequent (n = 4) RT was given later except 4 patients who refused further treatment after progression (n = 1) or who were younger than 3 years (n = 3). RT was the front-line treatment after surgery in the remaining 14 patients and subsequent chemotherapy was given except 2 patients who was transferred to other hospital or refused further treatment after RT. Cisplatin + etoposide + cyclophosphamide + vincristine (CECV) and vincristine + ifosfamide + carboplatin + etoposide (VICE) regimens were used as front-line chemotherapy in 24 patients (Table 2). Both regimens were used in alternation (17,18). Other front-line regimens were prednisolone + CCNU + vincristine (PCV) in 2, carboplatin + etoposide (CE) in one, and TMZ in one. Local RT dose to the primary site was in the range of 54.0–60.0 Gy and craniospinal RT (23.4–44.0 Gy) was given in 4 patients with leptomeningeal seeding.
Table 2. Chemotherapy regimens.
| Regimens | Drugs | Doses, mg/m2/day | Schedules | Total doses, mg/m2 |
|---|---|---|---|---|
| Induction regimens | ||||
| CECV* | Cisplatin | 90 | Day 0 | 90 |
| Etoposide | 75 | Days 0–2 | 225 | |
| Cyclophosphamide | 1,500 | Days 1 and 2 | 3,000 | |
| Vincristine | 1.5 | Days 0 and 7 | 3 | |
| VICE* | Carboplatin | 300 | Days 0 and 1 | 600 |
| Etoposide | 75 | Days 0–4 | 375 | |
| Ifosfamide | 1,500 | Days 0–4 | 7,500 | |
| Vincristine | 1.5 | Days 0 and 7 | 3 | |
| First HDCT regimen | ||||
| CTE | Carboplatin | 500 | Days –8, –7, –6 | 1,500 |
| Thiotepa | 300 | Days –5, –4, –3 | 900 | |
| Etoposide | 250 | Days –5, –4, –3 | 750 | |
| Second HDCT regimen | ||||
| CyM | Cyclophosphamide | 1,500 | Days –8, –7, –6, –5 | 6,000 |
| Melphalan | 60 | Days –4, –3, –2 | 180 |
CECV = cisplatin + etoposide + cyclophosphamide + vincristine, VICE = vincristine + ifosfamide + carboplatin + etoposide, CTE = carboplatin + thiotepa + etoposide, CyM = cyclophosphamide + melphalan, HDCT = high-dose chemotherapy.
*Dose was determined based on body weight in children under 3 years of age.
Response to induction treatment
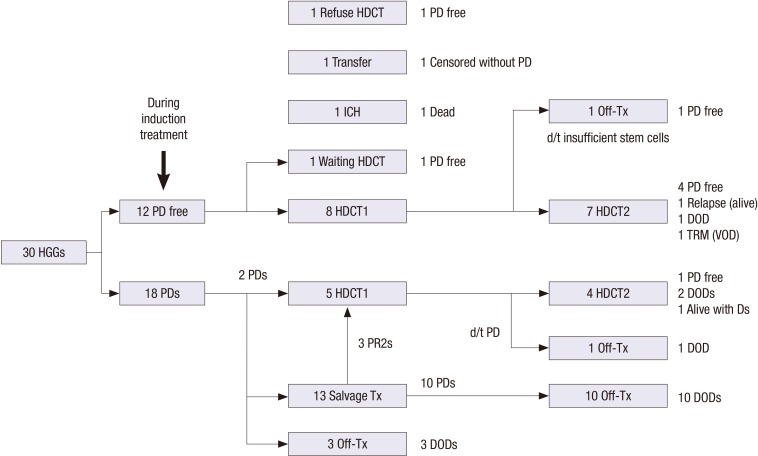
Fig. 1 summarizes the flow of patients. A total of 12 patients remained progression free during induction treatment. Among them, 8 patients (4 CRs and 4 PRs) proceeded to the first HDCT/auto-SCT and one is waiting HDCT/auto-SCT. However, the remaining 3 patients could not proceed to the HDCT/auto-SCT due to death from intracranial hemorrhage during induction treatment in one, transfer to other hospital in one, and refusal of further treatment in one. The remaining 18 patients experienced progression during induction treatment. Among them, 13 received conventional salvage treatment including second-look surgery, RT, or chemotherapy, and 3 of them achieved second PR (PR2) and proceeded to the first HDCT/auto-SCT. Another 2 patients proceeded to the first HDCT/auto-SCT as salvage treatment without preceding conventional salvage treatment. The other 3 patients gave up further treatment after initial progression.
Fig. 1.
Flow of patients. Treatment flow and outcome of all patients are illustrated.
HGGs = high-grade gliomas, PD = progressive disease, HDCT = high-dose chemotherapy, ICH = intracranial hemorrhage, HDCT1 = first high-dose chemotherapy, Tx = Treatment, HDCT2 = second high-dose chemotherapy, Ds = disease, DOD = died of disease, TRM = treatment-related mortality, VOD = veno-occlusive disease.
Tumor progression during induction treatment was more frequent in subtotal or less resection group than in gross or near total resection group (78.9% vs. 27.3%, P = 0.009). Glioblastoma group as compared to other HGG group and chemotherapy as compared to RT as front-line treatment after surgery were not associated with a higher progression rate during induction treatment. Peripheral blood stem cells (PBSCs) were collected during the recovery phase of chemotherapy cycle and the aim was to collect a minimum of 2 × 106 CD34+ cells/kg, with an optimal collection of greater than 5 × 106/kg to be used for bone marrow rescue during tandem HDCT/auto-SCT. The median number of CD34+ cells collected was 40.0 × 106 cells/kg (range 2.4–127.1).
Tandem HDCT/auto-SCT
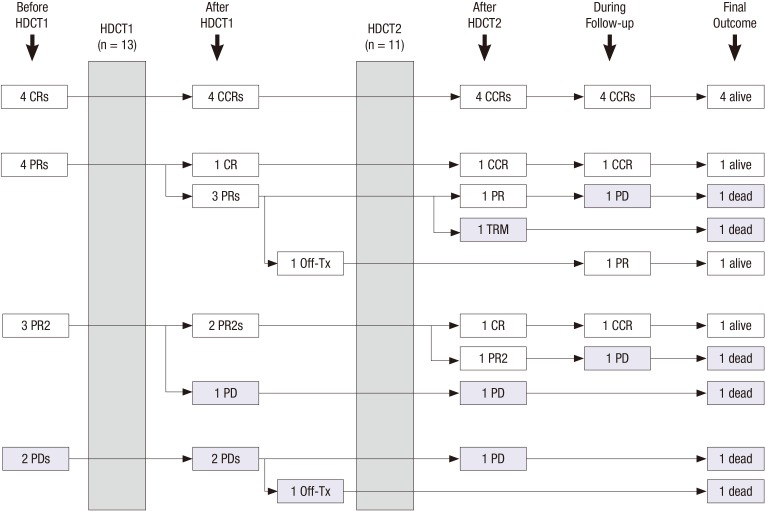
Fig. 2 summarizes the flow of patients during and after tandem HDCT/auto-SCT. A total of 13 patients (CR in 4, PR in 4, PR2 in 3, and PD in 2) underwent the first HDCT/auto-SCT and 11 of them proceeded to the second HDCT/auto-SCT. The remaining 2 patients could not proceed to the second HDCT/auto-SCT due to insufficient stem cells in one and tumor progression after the first HDCT/auto-SCT in 1. Tumor status at the second HDCT/auto-SCT was CR in 5, PR in 2, PR2 in 2, and PD in 2. Carboplatin + thiotepa + etoposide (CTE) was used for the first HDCT/auto-SCT and cyclophosphamide + melphalan (CyM) regimen was used for the second HDCT/auto-SCT (Table 2). The median interval from the first PBSC infusion to initiation of the second HDCT/auto-SCT was 84 days (range 79–102). Table 3 compares the frequency of grade 3 and 4 toxicities that developed during tandem HDCT/auto-SCT. A median of 20.8 × 106 CD34+ cells/kg (range 1.1–56.7), and 15.2 × 106 CD34+ cells/kg (range 2.2–44.6) were infused for the first and second HDCT/auto-SCT, respectively. Neutrophil and platelet counts recovered rapidly during the first and second HDCT/auto-SCT. The number of days with fever was higher in the first HDCT/auto-SCT than in the second (P < 0.001); however, we found no difference in the number of positive blood cultures. Grade 3 and 4 stomatitis and elevation of liver enzymes were more frequent in the first HDCT/auto-SCT than in the second. In the second HDCT/auto-SCT, the frequency and severity of mucositis-related toxicity was lower; however, the frequency of hepatic veno-occlusive disease (VOD) was higher with borderline significance (P = 0.082) and one patient died from hepatic VOD during the second HDCT/auto-SCT.
Fig. 2.
Response to tandem HDCT/auto-SCT. Responses before and after HDCT/auto-SCT are illustrated. A total of 13 patients (CR in 4, PR in 4, PR2 in 3, and PD in 2) underwent the first HDCT/auto-SCT and 11 of them proceeded to the second HDCT/auto-SCT. Overall, 7 patients are alive after HDCT/auto-SCT.
HDCT/auto-SCT = high-dose chemotherapy and autologous stem cell transplantation, CR = complete response, PR = partial response, PR2 = second PR, PD = progressive disease, CCR = continuous, HDCT1 = first high-dose chemotherapy, HDCT2 = second high-dose chemotherapy, TRM = treatment-related mortality.
Table 3. Characteristics of tandem HDCT/auto-SCT.
| Parameters | CTE regimen (n = 13) | CyM regimen (n = 11) | P value |
|---|---|---|---|
| Hematologic toxicity | |||
| CD34+ cells (× 106/kg) | 20.8 × 106 (1.1–56.7)* | 34.2 (2.2–44.6)* | 0.649 |
| Time (days) to reach an ANC 500/µL† | 8 (8–13) | 9 (8–12) | 0.424 |
| Time (days) to reach a PLT count 20,000/µL‡ | 20 (15–195) | 26 (15–77) | 0.235 |
| Days of BT ≥ 38.0℃ | 5 (3–13) | 0 (0–4) | < 0.001 |
| Positive blood culture | 1 (7.7) | 3 (27.3) | 0.300 |
| Non-hematologic toxicity | |||
| Stomatitis | 7 (63.6) | 0 (0) | 0.004 |
| Vomiting | 3 (23.1) | 2 (18.2) | 0.585 |
| Diarrhea | 10 (76.9) | 5 (45.5) | 0.206 |
| Elevation of liver enzymes | 6 (46.2) | 0 (0) | 0.013 |
| Renal insufficiency§ | 0 (0) | 0 (0) | 1.000 |
| Hypokalemia | 3 (23.1) | 2 (18.2) | 0.585 |
| Hyponatremia | 2 (15.4) | 1 (9.1) | 0.565 |
| Hepatic VOD∥ | 0 (0) | 3 (27.3) | 0.082 |
| Myocarditis | 0 (0) | 0 (0) | 1.000 |
| Seizure | 1 (7.7) | 0 (0) | 0.542 |
| Treatment-related mortality | 0 (0) | 1 (9.1) | 0.471 |
Values are presented as number (%).
CTE = carboplatin + thiotepa + etoposide, CyM = cyclophosphamide + melphalan, HDCT = high-dose chemotherapy, ANC = absolute neutrophil count, PLT = platelet, BT = body temperature, VOD = veno-occlusive disease.
*Median (range); †The first day when ANC exceeded 500/μL for 3 consecutive days; ‡The first day when PLT count exceeded 20,000/μL without transfusion for 7 days; §Elevation of serum creatinine more than threefold of baseline; ∥At least 2 of the following 3 events within 20 days of transplantation: bilirubin level > 2 mg/dL, hepatomegaly or right upper quadrant pain of liver origin, or > 2% weight gain secondary to fluid accumulation.
Survival
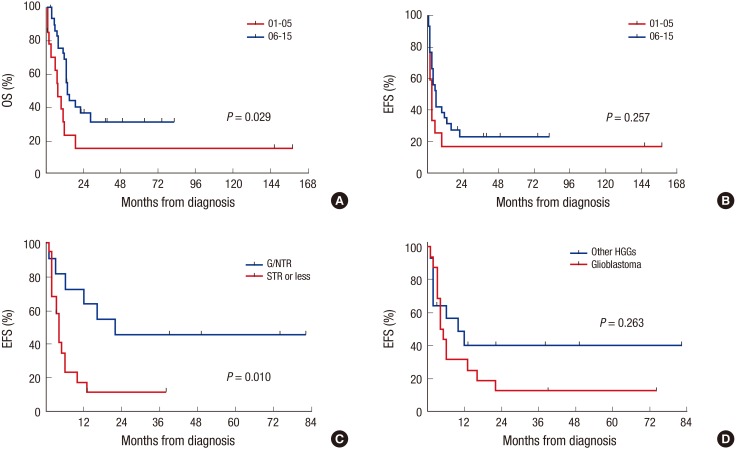
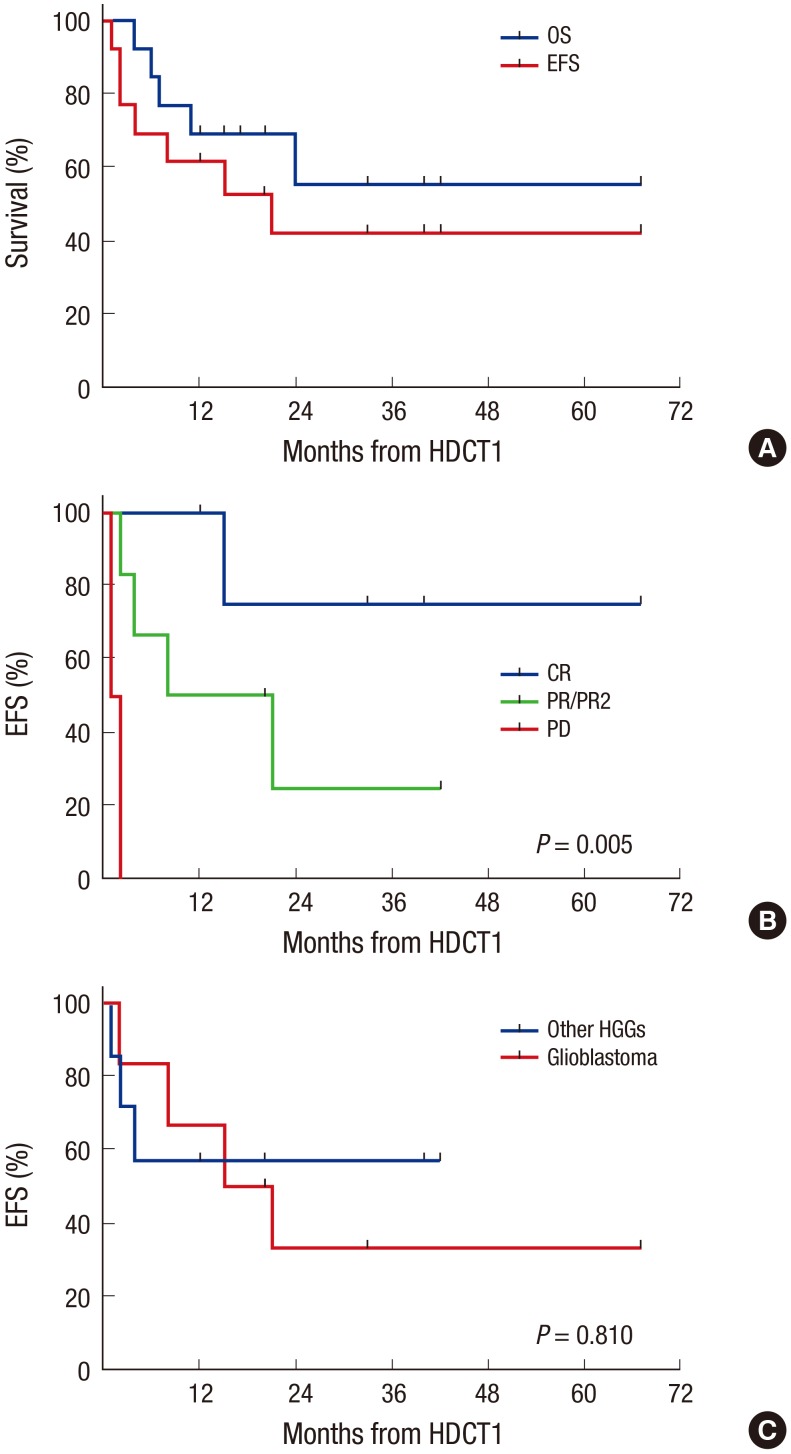
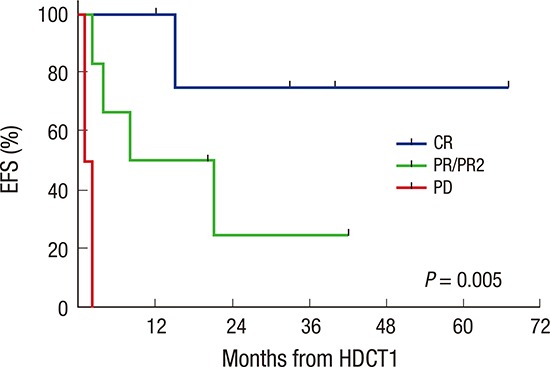
Overall, 9 patients remain progression free, 19 patients experienced progression at least once, and 2 patients died from toxicities (intracranial hemorrhage and hepatic VOD). Two of 19 patients who experienced progression remain alive after salvage treatment (one in CR and one in PD). Therefore, a total of 11 patients remain alive for a median of 38 months (range 3–82) after diagnosis. The probabilities of 3-year OS and EFS after diagnosis were 31.5% ± 9.2% and 23.7% ± 8.1%, respectively. The probability of 3-year OS is higher than that in 14 patients during 5 years (2001–2005) prior to the present study period, most of whom were treated with conventional modalities alone (Fig. 3A), although the EFS rate was not different (Fig. 3B). Subtotal or less resection of the primary tumor was associated with inferior EFS (Fig. 3C). However, glioblastoma (Fig. 3D), age under 3 years, chemotherapy compared to RT as front-line treatment after surgery were not associated with inferior OS or EFS. In the multivariate analysis for EFS, subtotal or less resection was the only significant unfavorable factor (hazard ratio [HR] 3.95, 95% confidence interval [CI] 1.19–13.18, P = 0.025, Table 4). In analysis confined only to 13 patients who underwent the first HDCT/auto-SCT, 8 patients remain alive for a median of 27 months (range 12–67) after the first HDCT/auto-SCT. Seven of them remain progression free for a median of 33 months (range 12–67) after the first HDCT/auto-SCT, the other one patient remains alive with disease after progression. The remaining 5 patients died due to progression in 4 and toxicity in 1. The probabilities of 3-year OS and EFS after the first HDCT/auto-SCT were 55.4% ± 16.1% and 43.1% ± 14.8%, respectively (Fig. 4A). In analysis confined only to 11 patients who were in CR, PR, or PR2 at the first HDCT/auto-SCT, the probabilities of 3-year OS and EFS after the first HDCT/auto-SCT were 58.2% ± 16.9% and 49.9% ± 16.4%, respectively. Tumor status less than PR at the first HDCT/auto-SCT was associated with inferior EFS (Fig. 4B); however, there was no difference in survival between glioblastoma and other HGGs (Fig. 4C).
Fig. 3.
Survival from diagnosis. The probability of 3-year OS (A) is higher than that in 14 patients during 5 years (2001–2005) prior to the present study period, most of whom were treated with conventional modalities alone, although the EFS rate is not different (B). Subtotal or less resection of the primary tumor is associated with inferior EFS (C). There is no difference in survival between glioblastoma and other HGGs (D).
HGGs = high-grade gliomas, NTR = near total resection, STR = subtotal resection, OS = overall survival, EFS = event-free survival.
Table 4. Multivariate analysis for factors affecting survival from diagnosis.
| Risk factors | EFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age < 3 yr | 0.58 | 0.10–3.21 | 0.529 | 0.37 | 0.06–2.24 | 0.277 |
| Glioblastoma | 2.10 | 0.80–5.50 | 0.133 | 1.92 | 0.66–5.54 | 0.229 |
| Subtotal or less resection | 3.95 | 1.19–13.18 | 0.025 | 3.09 | 0.84–11.37 | 0.089 |
| Chemotherapy as front-line treatment | 1.38 | 0.51–3.76 | 0.531 | 1.15 | 0.38–3.48 | 0.803 |
EFS = Event-free survival, OS = overall survival, HR = hazard ratio, CI = confidence interval.
Fig. 4.
Survival after first HDCT/auto-SCT. Survival analysis was confined only to 13 patients who underwent the first HDCT/auto-SCT. The probabilities of 3-year OS and EFS after the first HDCT/auto-SCT are 55.4% ± 16.1% and 43.1% ± 14.8%, respectively (A). Tumor status less than PR at the first HDCT/auto-SCT is associated with inferior EFS (B). There is no difference in survival between glioblastoma and other HGGs (C).
HDCT/auto-SCT = high-dose chemotherapy and autologous stem cell transplantation, OS = overall survival, EFS = event-free survival, CR = complete response, PR = partial response, PR2 = second PR, PD = progressive disease, HGGs = high-grade gliomas.
DISCUSSION
Although previous studies for children and adolescent with HGGs showed the effectiveness of adjuvant chemotherapy, current conventional therapies are not yet sufficient with the far majority of patients succumbing to their disease (3,4,5). For this reason, we intended to give tandem HDCT/auto-SCT during the study period in order to improve the outcome by increasing the intensity of chemotherapy although it was not performed in a prospective trial. As results, toxicities during HDCT were generally acceptable and OS was higher as compared to the 5 years (2001–2005) prior to the present study period, most of whom were treated with conventional modalities alone. In particular, survival after HDCT/auto-SCT was encouraging in patients who could remain progression free until the first HDCT/auto-SCT. Results of our study suggest the possible effectiveness of tandem HDCT/auto-SCT.
A higher proportion of patients remained progression free or could achieve CR or PR in gross or near total resection group than in subtotal or less resection group, and the extent of surgery was an independent prognostic factor for EFS. Previous reports also suggest that the amount of surgical resection is one of the most important clinical prognostic factors identified to date in children with supratentorial HGGs (6,7). Therefore, every attempt should be made to maximize the extent of surgical resection whenever it is safe and feasible. However, complete resection was not possible in many cases, especially in patients with HGGs originating from or invading critical structures.
Although there is no accepted standard of care for HGGs, RT to the tumor bed following maximal surgical resection with or without concomitant chemotherapy plus additional post-RT chemotherapy has been the most commonly used treatment approach for children older than 3 years. To the contrary, in our experience, chemotherapy was the front-line treatment in many patients including young patients less than 3 years of age. However, chemotherapy as front-line treatment after surgery was not associated with inferior OS or EFS as compared to immediate RT after surgery. Pre-RT chemotherapy and/or concomitant chemotherapy might enhance the efficacy of RT by reducing the bulk of tumor.
Although many prospective studies demonstrated the benefit of adjuvant chemotherapy with various regimens, there is no gold standard chemotherapy regimen for HGGs (4,6,7). Since concomitant TMZ and RT for adult with glioblastoma led to prolonged survival and is now considered the standard of care (8), several studies have tested the efficacy of TMZ in pediatric patients. However, the results were disappointing and failed to demonstrate the benefit of TMZ in children (4,9,10). We used CECV and VICE regimens in alternation as front-line chemotherapy in most patients. Although one patient died from tumor bleeding during concomitant chemotherapy and RT, toxicities of both regimens were generally acceptable except neutropenic fever. Non-hematologic toxicity was not common during induction chemotherapy (19).
Although HDCT/auto-SCT is still considered controversial and is not universally accepted, previous studies suggest that there may be a role for children with recurrent HGGs (20,21). However, studies for newly diagnosed HGGs are limited to date (22,23,24). In the treatment of recurrent or high-risk brain tumors, several recent studies have suggested tandem HDCT/auto-SCT might further improve outcomes and/or reduce RT dose without jeopardizing survival rates (16,17,18,19). For this reason, we used tandem HDCT/auto-SCT for HGGs in order to improve the survival rates. As results, survival after tandem HDCT/auto-SCT was encouraging. All 4 patients in CR and 3 of 7 patients in PR or PR2 at the first HDCT/auto-SCT maintained CR, achieved CR, or remained progression free after tandem HDCT/auto-SCT. These findings suggest that tandem HDCT/auto-SCT might improve the survival if the patient remains progression free before HDCT/auto-SCT or could achieve second response (PR2 or more) after initial progression. However, our results also showed that tandem HDCT/auto-SCT could not change the fate of patients with progressed tumors at HDCT/auto-SCT.
We used different drug combinations during the first and second HDCT to curtail or prevent drug resistance and overlapping drug toxicity. As we reported previously (25), acute toxicities during tandem HDCT/auto-SCT were generally acceptable except that one patient died from hepatic VOD. However, dose-escalation during tandem HDCT/auto-SCT might be associated with more significant late adverse effects. Therefore, longer follow-up is needed to assess whether the possible survival benefits of tandem HDCT/auto-SCT ultimately outweigh the adverse effects associated with dose-intense tandem HDCT/auto-SCT.
Our results showed that survival of patients who could achieve CR or PR before HDCT/auto-SCT was encouraging; however, less than one-third of patients could remain progression free before HDCT/auto-SCT. In addition, only 3 patients could achieve second response with salvage treatment after initial progression. These findings suggest that conventional front-line or salvage treatment modalities are not effective in achieving or maintaining responses in patients with HGGs. Therefore, more effective therapeutic approach is needed to improve the outcome of patients with HGGs. In this context, strategies using new drugs such as anti-angiogenic agent, receptor tyrosine kinase inhibitors, or histone deacetylase inhibitors have been evaluated (26,27,28). Novel treatment approach using target therapy based on genomic profiles of the tumor might also be another option to improve patient survival with minimal treatment toxicities (29). Effective tumor control before HDCT/auto-SCT with strategies mentioned above might improve the overall outcome of HGGs.
In conclusion, results of our study suggest that the outcome of HGGs in children and adolescents after HDCT/auto-SCT might be encouraging if the patient could remain progression free before HDCT/auto-SCT. However, this study is a retrospective review and the size of cohorts is small. Therefore, prospective studies with larger cohorts of patients are needed to answer the controversy and some debate about the efficacy of HDCT/auto-SCT for HGGs in children.
Footnotes
Funding: This study was supported by a Samsung Medical Center Grant (#PHO3110265).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Sung KW, Shin HJ. Data curation: Lee JW, Lim DH, Sung KW, Lee HJ, Yi ES. Investigation: Lee JW, Lim DH, Sung KW, Yoo KH, Koo HH, Suh YL. Writing - original draft: Lee JW, Lim DH, Sung KW.
References
- 1.Bondy ML, Scheurer ME, Malmer B, Barnholtz-Sloan JS, Davis FG, Il’yasova D, Kruchko C, McCarthy BJ, Rajaraman P, Schwartzbaum JA, et al. Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer. 2008;113:1953–1968. doi: 10.1002/cncr.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broniscer A, Gajjar A. Supratentorial high-grade astrocytoma and diffuse brainstem glioma: two challenges for the pediatric oncologist. Oncologist. 2004;9:197–206. doi: 10.1634/theoncologist.9-2-197. [DOI] [PubMed] [Google Scholar]
- 4.Cohen KJ, Pollack IF, Zhou T, Buxton A, Holmes EJ, Burger PC, Brat DJ, Rosenblum MK, Hamilton RL, Lavey RS, et al. Temozolomide in the treatment of high-grade gliomas in children: a report from the Children’s Oncology Group. Neuro-oncol. 2011;13:317–323. doi: 10.1093/neuonc/noq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finlay JL, Zacharoulis S. The treatment of high grade gliomas and diffuse intrinsic pontine tumors of childhood and adolescence: a historical - and futuristic - perspective. J Neurooncol. 2005;75:253–266. doi: 10.1007/s11060-005-6747-7. [DOI] [PubMed] [Google Scholar]
- 6.Finlay JL, Boyett JM, Yates AJ, Wisoff JH, Milstein JM, Geyer JR, Bertolone SJ, McGuire P, Cherlow JM, Tefft M, et al. Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen. Childrens cancer group. J Clin Oncol. 1995;13:112–123. doi: 10.1200/JCO.1995.13.1.112. [DOI] [PubMed] [Google Scholar]
- 7.Sposto R, Ertel IJ, Jenkin RD, Boesel CP, Venes JL, Ortega JA, Evans AE, Wara W, Hammond D. The effectiveness of chemotherapy for treatment of high grade astrocytoma in children: results of a randomized trial. A report from the Childrens Cancer Study Group. J Neurooncol. 1989;7:165–177. doi: 10.1007/BF00165101. [DOI] [PubMed] [Google Scholar]
- 8.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 9.Lashford LS, Thiesse P, Jouvet A, Jaspan T, Couanet D, Griffiths PD, Doz F, Ironside J, Robson K, Hobson R, et al. Temozolomide in malignant gliomas of childhood: a United Kingdom Children’s Cancer Study Group and French Society for Pediatric Oncology Intergroup Study. J Clin Oncol. 2002;20:4684–4691. doi: 10.1200/JCO.2002.08.141. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson HS, Kretschmar CS, Krailo M, Bernstein M, Kadota R, Fort D, Friedman H, Harris MB, Tedeschi-Blok N, Mazewski C, et al. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: a report from the Children’s Oncology Group. Cancer. 2007;110:1542–1550. doi: 10.1002/cncr.22961. [DOI] [PubMed] [Google Scholar]
- 11.Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, Gerbing RB, London WB, Villablanca JG. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. J Clin Oncol. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philip T, Ladenstein R, Lasset C, Hartmann O, Zucker JM, Pinkerton R, Pearson AD, Klingebiel T, Garaventa A, Kremens B, et al. 1070 myeloablative megatherapy procedures followed by stem cell rescue for neuroblastoma: 17 years of European experience and conclusions. European Group for Blood and Marrow Transplant Registry Solid Tumour Working Party. Eur J Cancer. 1997;33:2130–2135. doi: 10.1016/s0959-8049(97)00324-9. [DOI] [PubMed] [Google Scholar]
- 13.Fangusaro J, Finlay J, Sposto R, Ji L, Saly M, Zacharoulis S, Asgharzadeh S, Abromowitch M, Olshefski R, Halpern S, et al. Intensive chemotherapy followed by consolidative myeloablative chemotherapy with autologous hematopoietic cell rescue (AuHCR) in young children with newly diagnosed supratentorial primitive neuroectodermal tumors (sPNETs): report of the Head Start I and II experience. Pediatr Blood Cancer. 2008;50:312–318. doi: 10.1002/pbc.21307. [DOI] [PubMed] [Google Scholar]
- 14.Mason WP, Grovas A, Halpern S, Dunkel IJ, Garvin J, Heller G, Rosenblum M, Gardner S, Lyden D, Sands S, et al. Intensive chemotherapy and bone marrow rescue for young children with newly diagnosed malignant brain tumors. J Clin Oncol. 1998;16:210–221. doi: 10.1200/JCO.1998.16.1.210. [DOI] [PubMed] [Google Scholar]
- 15.Modak S, Gardner S, Dunkel IJ, Balmaceda C, Rosenblum MK, Miller DC, Halpern S, Finlay JL. Thiotepa-based high-dose chemotherapy with autologous stem-cell rescue in patients with recurrent or progressive CNS germ cell tumors. J Clin Oncol. 2004;22:1934–1943. doi: 10.1200/JCO.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 16.Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, Woo S, Wheeler G, Ahern V, Krasin MJ, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 17.Sung KW, Lim DH, Lee SH, Yoo KH, Koo HH, Kim JH, Suh YL, Joung YS, Shin HJ. Tandem high-dose chemotherapy and auto-SCT for malignant brain tumors in children under 3 years of age. Bone Marrow Transplant. 2013;48:932–938. doi: 10.1038/bmt.2012.263. [DOI] [PubMed] [Google Scholar]
- 18.Sung KW, Lim DH, Son MH, Lee SH, Yoo KH, Koo HH, Kim JH, Suh YL, Joung YS, Shin HJ. Reduced-dose craniospinal radiotherapy followed by tandem high-dose chemotherapy and autologous stem cell transplantation in patients with high-risk medulloblastoma. Neuro-oncol. 2013;15:352–359. doi: 10.1093/neuonc/nos304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sung KW, Lim DH, Yi ES, Choi YB, Lee JW, Yoo KH, Koo HH, Kim JH, Suh YL, Joung YS, et al. Tandem high-dose chemotherapy and autologous stem cell transplantation for atypical teratoid/rhabdoid tumor. Cancer Res Treat. 2016;48:1408–1419. doi: 10.4143/crt.2015.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finlay JL, Dhall G, Boyett JM, Dunkel IJ, Gardner SL, Goldman S, Yates AJ, Rosenblum MK, Stanley P, Zimmerman RA, et al. Myeloablative chemotherapy with autologous bone marrow rescue in children and adolescents with recurrent malignant astrocytoma: outcome compared with conventional chemotherapy: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2008;51:806–811. doi: 10.1002/pbc.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guruangan S, Dunkel IJ, Goldman S, Garvin JH, Rosenblum M, Boyett JM, Gardner S, Merchant TE, Gollamudi S, Finlay JL. Myeloablative chemotherapy with autologous bone marrow rescue in young children with recurrent malignant brain tumors. J Clin Oncol. 1998;16:2486–2493. doi: 10.1200/JCO.1998.16.7.2486. [DOI] [PubMed] [Google Scholar]
- 22.Grovas AC, Boyett JM, Lindsley K, Rosenblum M, Yates AJ, Finlay JL. Regimen-related toxicity of myeloablative chemotherapy with BCNU, thiotepa, and etoposide followed by autologous stem cell rescue for children with newly diagnosed glioblastoma multiforme: report from the Children’s Cancer Group. Med Pediatr Oncol. 1999;33:83–87. doi: 10.1002/(sici)1096-911x(199908)33:2<83::aid-mpo4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 23.Heideman RL, Douglass EC, Krance RA, Fontanesi J, Langston JA, Sanford RA, Kovnar EH, Ochs J, Kuttesch J, Jenkins JJ, et al. High-dose chemotherapy and autologous bone marrow rescue followed by interstitial and external-beam radiotherapy in newly diagnosed pediatric malignant gliomas. J Clin Oncol. 1993;11:1458–1465. doi: 10.1200/JCO.1993.11.8.1458. [DOI] [PubMed] [Google Scholar]
- 24.Massimino M, Gandola L, Luksch R, Spreafico F, Riva D, Solero C, Giangaspero F, Locatelli F, Podda M, Bozzi F, et al. Sequential chemotherapy, high-dose thiotepa, circulating progenitor cell rescue, and radiotherapy for childhood high-grade glioma. Neuro-oncol. 2005;7:41–48. doi: 10.1215/S1152851704000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SH, Son MH, Sung KW, Choi YB, Lee NH, Yoo KH, Koo HH, Lim DH, Shin HJ. Toxicity of tandem high-dose chemotherapy and autologous stem cell transplantation using carboplatin-thiotepa-etoposide and cyclophosphamide-melphalan regimens for malignant brain tumors in children and young adults. J Neurooncol. 2014;120:507–513. doi: 10.1007/s11060-014-1576-1. [DOI] [PubMed] [Google Scholar]
- 26.Lee EQ, Puduvalli VK, Reid JM, Kuhn JG, Lamborn KR, Cloughesy TF, Chang SM, Drappatz J, Yung WK, Gilbert MR, et al. Phase I study of vorinostat in combination with temozolomide in patients with high-grade gliomas: North American Brain Tumor Consortium Study 04-03. Clin Cancer Res. 2012;18:6032–6039. doi: 10.1158/1078-0432.CCR-12-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narayana A, Golfinos JG, Fischer I, Raza S, Kelly P, Parker E, Knopp EA, Medabalmi P, Zagzag D, Eagan P, et al. Feasibility of using bevacizumab with radiation therapy and temozolomide in newly diagnosed high-grade glioma. Int J Radiat Oncol Biol Phys. 2008;72:383–389. doi: 10.1016/j.ijrobp.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 28.Reardon DA, Groves MD, Wen PY, Nabors L, Mikkelsen T, Rosenfeld S, Raizer J, Barriuso J, McLendon RE, Suttle AB, et al. A phase I/II trial of pazopanib in combination with lapatinib in adult patients with relapsed malignant glioma. Clin Cancer Res. 2013;19:900–908. doi: 10.1158/1078-0432.CCR-12-1707. [DOI] [PubMed] [Google Scholar]
- 29.Gajjar A, Bowers DC, Karajannis MA, Leary S, Witt H, Gottardo NG. Pediatric brain tumors: innovative genomic information is transforming the diagnostic and clinical landscape. J Clin Oncol. 2015;33:2986–2998. doi: 10.1200/JCO.2014.59.9217. [DOI] [PMC free article] [PubMed] [Google Scholar]