Abstract
The prognostic role of aberrant serum miRNA expression for predicting response to sorafenib treatment in advanced hepatocellular carcinoma (HCC) patients has not been well characterized. We aimed to identify specific serum miRNAs that are associated with positive radiologic responses or improved survival in sorafenib-treated HCC patients. miR-18a, miR-21, miR-139-5p, miR-221, miR-224, and miR-10b-3p, were selected for analysis. Serum samples from 24 patients with advanced stage HCC and 25 patients with liver cirrhosis (LC) were analyzed. All of the miRNAs except miR-21 were found to be upregulated in serum samples from HCC patients. None of the miRNAs assayed differed significantly in terms of expression between the responder and non-responder groups among HCC patients. However, miR-10b-3p levels were significantly higher in the subgroup of HCC patients with worse overall survival (fold change = 5.8, P = 0.008). Serum miRNA-10b-3p was upregulated in the presence of macrovascular invasion (MVI), and those with higher serum miRNA-10b-3p had significantly shorter survival during treatment (P = 0.042). Although no single serum miRNA was predictive of response to sorafenib treatment, analysis of serum miR-10b-3p levels may be valuable for diagnosis of HCC and prediction of survival of sorafenib-treated patients.
Keywords: Serum MicroRNA, Hepatocellular Carcinoma, Sorafenib, Survival, Macrovascular Invasion
Graphical Abstract
INTRODUCTION
Sorafenib is the only therapy that has been proven to result in a survival benefit in hepatocellular carcinoma (HCC) patients with advanced stage (1). However, the efficacy of sorafenib is variable, and prognostic biomarkers of positive response to sorafenib are needed to facilitate patient-tailored therapy. To date, no reliable biomarkers specific for response to sorafenib have been identified outside of well-known clinical parameters, such as alpha-fetoprotein (AFP) expression, the presence of macrovascular invasion (MVI), or extra-hepatic spread (2).
miRNAs are small non-coding RNA molecules that control various cellular processes by binding to target mRNAs and repressing transcription or translation. Analysis of miRNA dysregulation can be used in the detection of cancer, the discrimination of tumor characteristics, and the prediction of prognosis (3). Numerous key miRNAs have been reported to be dysregulated in HCC. For example, miR-18a, miR-221, and miR-224 have been shown to be upregulated in HCC compared with non-cancerous liver tissue (4). miR-21 has been confirmed to be upregulated in human HCC samples in many studies (5,6,7,8). The tumor suppressor phosphatase and tensin homologue (PTEN) has been shown to be a target of miR-21 (6). miR-221 has an oncogenic function in hepatocarcinogenesis in vitro and was found to be upregulated in HCC samples compared to cirrhosis samples (9). miR-10b-3p was found to be upregulated in resected HCC tissue specimens compared with non-tumorous liver tissue (10). In addition to the oncogenic miRNAs, miR-139-5p downregulation contributes to metastasis of HCC in vitro (11). In addition, overexpression of miR-139-5p reduced the incidence of metastatic HCC and correlates with good prognosis in vivo (12). Therefore, a single miRNA or multiple combinations of these miRNAs may have a potential role in the prediction of response to sorafenib treatment of HCC.
Screening for miRNAs in HCC is complicated by the fact that obtaining liver tissue samples requires an invasive procedure that can be accompanied by rare, but serious, complications, such as episodes of uncontrolled internal bleeding. Furthermore, monitoring miRNA expression in these tissues over the course of treatment further complicates the situation. Therefore, a better approach would be to analyze serum miRNAs, which avoid the limitations of tissue-based analysis. Thus, our goal was to identify serum miRNAs that are associated with good radiologic response or longer survival in patients with advanced HCC who were treated with sorafenib.
MATERIALS AND METHODS
Patients and samples
We retrospectively analyzed the medical records of patients with advanced stage disease who were treated with sorafenib for measurable HCC lesions in the imaging study. Sorafenib was administered to patients with advanced stage HCC according to the Barcelona clinic liver cancer staging system, whose HCC lesions accompanied any of the findings of portal vein invasion, lymph node involvement, or distant metastases was the criteria for defining advanced stage HCC. This study was conducted in the Department of Hepatology at Guro Hospital, Korea University College of Medicine between 2009 and 2011. Among 24 HCC patients, 12 patients were diagnosed with HCC by pathologic confirmation. Others were diagnosed with HCC by identification of typical nodular lesions in the liver (i.e., hypervascularity in the arterial phase and washout in the portal venous or delayed phase) via a 4-phase multi-detector CT scan or dynamic contrast enhanced MRI (1). Serum samples from 24 HCC patients were collected just prior to the administration of sorafenib. Serum samples from 25 liver cirrhosis (LC) patients who were on several antiviral agents were also obtained as controls. Liver function and severity of LC were matched between LC controls and patients with HCC by Child-Pugh classification. The presence of cirrhosis was identified through combinations of multiple clinical and imaging findings. Sera were frozen at −70°C until use.
Sample preparation and RNA extraction
Total RNA was isolated from serum samples using TRIzol® reagent (Invitrogen, Carlsbad, CA, USA). In general, 600–3,750 ng/µL total RNA was obtained from 700 µL of serum. A NanoDrop ND-1000 spectrophotometer® (NanoDrop Technologies, Wilmington, DE, USA) was used to assess the quality of the isolated RNA.
Real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) analysis of miRNA expression
Reverse transcription and real-time RT-PCR were performed using a TaqMan® MicroRNA Reverse Transcription kit and TaqMan® MicroRNA Assays (Applied Biosystems, Carlsbad, CA, USA). miR-18a, miR-21, miR-139-5p, miR-221, miR-224, and miR-10b-3p, which have previously been reported to be aberrantly expressed in HCC or to play important roles in HCC tumorigenesis, were evaluated (5,6,7,8,10,13,14,15,16,17). Expression of the target miRNAs was normalized to expression of miR-16 (18). The comparative CT method (2-ΔΔCT) was used for analysis of relative miRNA expression (19).
Study design and definitions
Patients were treated with 400 mg sorafenib twice daily. To assess treatment response, patients who underwent radiologic evaluation after one month of treatment were included. The median duration of sorafenib treatment was 2.18 months (95% confidence interval [CI], 2.13–6.96; range, 0.43–27.70). All but one patient was administered sorafenib for more than 5 weeks. Therapy was discontinued after 12 days of treatment in one patient who had definite aggravation of lung metastases on chest X-ray while taking sorafenib. This patient was included in our analysis. Treatment response was evaluated for viable target lesions according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) (20). Responses to treatment were characterized as either complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). Responders were defined as patients with non-PD after sorafenib treatment, including CR, PR, and SD. Non-responders were defined as patients with PD despite sorafenib treatment. Based on the median overall survival reported from the Asian-Pacific sorafenib trial, the better survival group was defined as patients who lived longer than 6.5 months after the initiation of sorafenib treatment (21). The worse survival group included patients who survived less than 6.5 months after the initiation of therapy.
Outcomes
The primary endpoint was to determine the most significant miRNA predictive of the response to sorafenib, based on non-PD according to mRECIST criteria. Secondary endpoints included identification of miRNAs that are differentially expressed in serum samples of patients with HCC compared to patients with LC. Additionally, we also sought to identify serum miRNAs that could be used to differentiate better survival from worse survival and serum miRNAs that are significantly dysregulated in patients with invasive tumor characteristics.
Statistical analysis
Categorical and continuous variables were analyzed by the χ2 test and the Mann-Whitney U-test, respectively. Overall survival was calculated by the Kaplan-Meier method, and differences between groups were compared using the log-rank test. Logistic regression analysis was used to identify factors significantly affecting survival. P values less than 0.05 were considered statistically significant. Statistical analyses of experimental and clinical data were performed using GraphPad® Prism 4 software (GraphPad, San Diego, CA, USA) and SPSS 17.0 (SPSS Inc., Chicago, IL, USA), respectively.
Ethics statement
This study was approved from Institutional Review Board of Korea University College of Medicine, Guro Hospital (KUGH12111). Written informed consent was obtained from all patients whose samples were stored at our center.
RESULTS
Baseline characteristics of LC and HCC patients
Baseline characteristics were similar between the LC and HCC patients. Chronic hepatitis B was the most common etiology in both the LC and HCC groups. Severity of cirrhosis was similar between the groups (Table 1).
Table 1. Comparison of the baseline characteristics of patients with LC and HCC.
| Characteristics | LC (n = 25) | HCC (n = 24) | P value |
|---|---|---|---|
| Sex (male) | 15 (60.0) | 21 (87.5) | 0.051 |
| Age, yr | 51.8 ± 7.7 | 57.3 ± 10.2 | 0.065 |
| Etiology | |||
| Alcoholism | 0 (0.0) | 4 (16.7) | 0.120 |
| CHB | 24 (96.0) | 18 (75.0) | |
| CHC | 0 (0.0) | 1 (4.2) | |
| Combined | 1 (4.0) | 1 (4.2) | |
| Cirrhosis | 25 (100.0) | 20 (83.3) | 0.051 |
| Child-Pugh class | |||
| A | 17 (68.0) | 19 (79.2) | 0.520 |
| B | 8 (32.0) | 5 (20.8) | |
| Albumin, g/dL | 3.8 ± 0.5 | 3.7 ± 0.3 | 0.277 |
| Total bilirubin, mg/dL | 1.4 ± 0.9 | 1.3 ± 0.8 | 0.516 |
| Prothrombin time, INR | 1.2 ± 0.2 | 1.1 ± 0.2 | 0.873 |
Values are expressed as number (percentage) or median ± standard deviation where specified.
LC = liver cirrhosis, HCC = hepatocellular carcinoma, CHB = chronic hepatitis B, CHC = chronic hepatitis C, INR = international normalized ratio.
Comparison of serum miRNA expression between LC and HCC patients
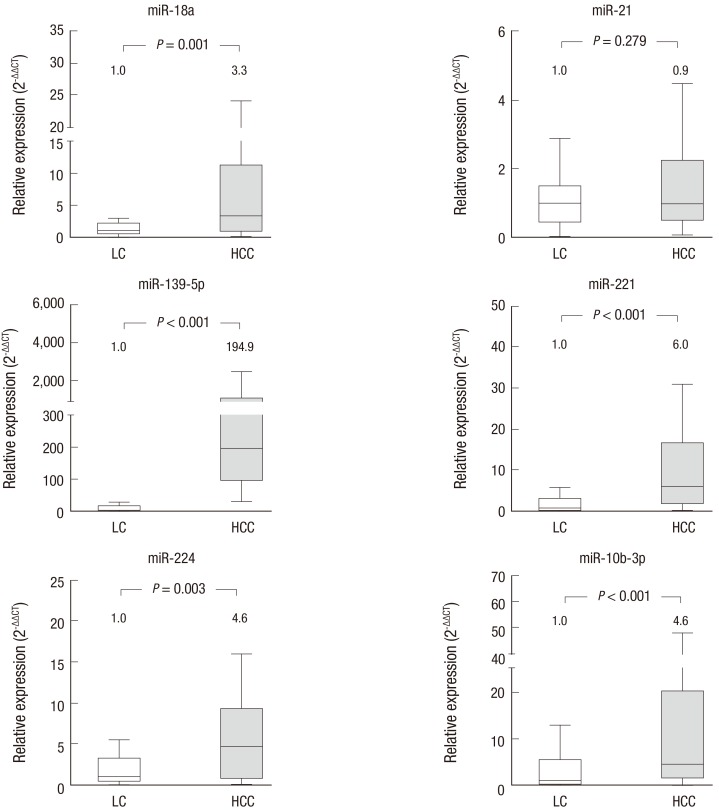
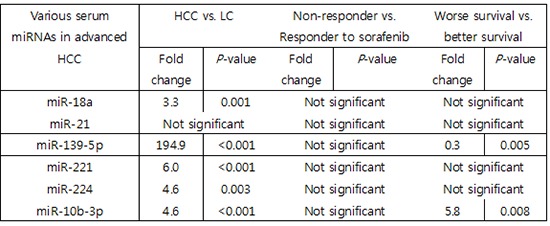
Six miRNAs were selected for analysis based on previous results reporting their relevance to HCC. Expression of each miRNA was measured by real-time RT-PCR in 24 HCC patients and 25 cirrhotic patients and subsequently compared between the 2 patient groups. miR-18a, miR-221, miR-224, and miR-10b-3p were significantly upregulated in the HCC group by 3- to 6-fold compared to the LC group. Moreover, expression of miR-139-5p was over 190 times higher in the HCC group than in the LC group (P < 0.001). All of the selected miRNAs except miR-21 were able to differentiate the HCC group from the LC group (Fig. 1).
Fig. 1.
Expression of serum miRNAs in LC and HCC patients.
We selected 6 candidate miRNAs reported to be aberrantly expressed in HCC tissue specimens compared with non-cancerous liver tissues. With the exception of miR-21, all of the chosen miRNAs are significantly overexpressed in serum samples from HCC patients compared with those from LC patients. The numbers shown above each box represent the median fold changes compared to the median value of the samples obtained from LC patients. The box plots show the median (horizontal bar) and the interquartile ranges, and the bars show the minimum and maximum values. LC (n = 25); HCC (n = 24).
LC = liver cirrhosis, HCC = hepatocellular carcinoma.
Expression of serum miRNAs and radiologic responses to sorafenib treatment
To identify miRNAs that could be useful for prediction of sorafenib responders, we compared the expression of miR-18a, miR-21, miR-139-5p, miR-221, miR-224, and miR-10b-3p between the responder and non-responder groups. The baseline characteristics were similar between the 2 groups. None of the candidate miRNAs were expressed at significantly different levels between the groups (P > 0.050).
Expression of serum miRNAs and overall survival
We compared the expression of the 6 miRNAs between the better survival and the worse survival groups to identify serum miRNAs that were associated with longer survival. Although most baseline characteristics were similar between the 2 survival groups, the presence of MVI was significantly less frequent in the better survival group (P = 0.001; Table 2).
Table 2. Comparison of the baseline characteristics between the better and worse survival groups.
| Characteristics | HCC patients with better survival (n = 14) | HCC patients with worse survival (n = 10) | P value |
|---|---|---|---|
| Sex (male) | 13 (92.9) | 8 (80.0) | 0.348 |
| Age, yr | 56.2 ± 11.8 | 58.9 ± 7.9 | 0.472 |
| Etiology | 0.811 | ||
| Alcoholism | 2 (14.3) | 1 (10.0) | |
| CHB | 9 (64.3) | 6 (60.0) | |
| CHC | 2 (14.3) | 1 (10.0) | |
| Combined | 1 (7.1) | 2 (20.0) | |
| Cirrhosis | 11 (78.6) | 9 (90.0) | 0.459 |
| Child-Pugh class | 0.051 | ||
| A | 13 (92.9) | 6 (60.0) | |
| B | 1 (6.7) | 4 (40.0) | |
| Albumin, g/dL | 3.8 ± 0.3 | 3.7 ± 0.4 | 0.438 |
| Total bilirubin, mg/dL | 1.0 ± 0.7 | 1.6 ± 0.8 | 0.056 |
| Prothrombin time, INR | 1.1 ± 0.2 | 1.2 ± 0.1 | 0.625 |
| Baseline high AFP (> 200 ng/mL) | 6 (42.9) | 7 (70.0) | 0.188 |
| Baseline high PIVKA-II (> 125 mAU/mL) | 8 (61.5) | 9 (90.0) | 0.123 |
| MVI | 3 (21.4) | 9 (90.0) | 0.001 |
| Metastasis | 11 (78.6) | 5 (50.0) | 0.143 |
| AJCC staging | 0.143 | ||
| 3 | 3 (21.4) | 5 (50.0) | |
| 4 | 11 (78.6) | 5 (50.0) |
Values are expressed as number (percentage) or median ± standard deviation where specified.
HCC = hepatocellular carcinoma, CHB = chronic hepatitis B, CHC = chronic hepatitis C, AFP = alpha-fetoprotein, INR = international normalized ratio, PIVKA-II = protein induced by vitamin K absence/antagonist-II, MVI = macrovascular invasion, AJCC = American Joint Committee on Cancer.
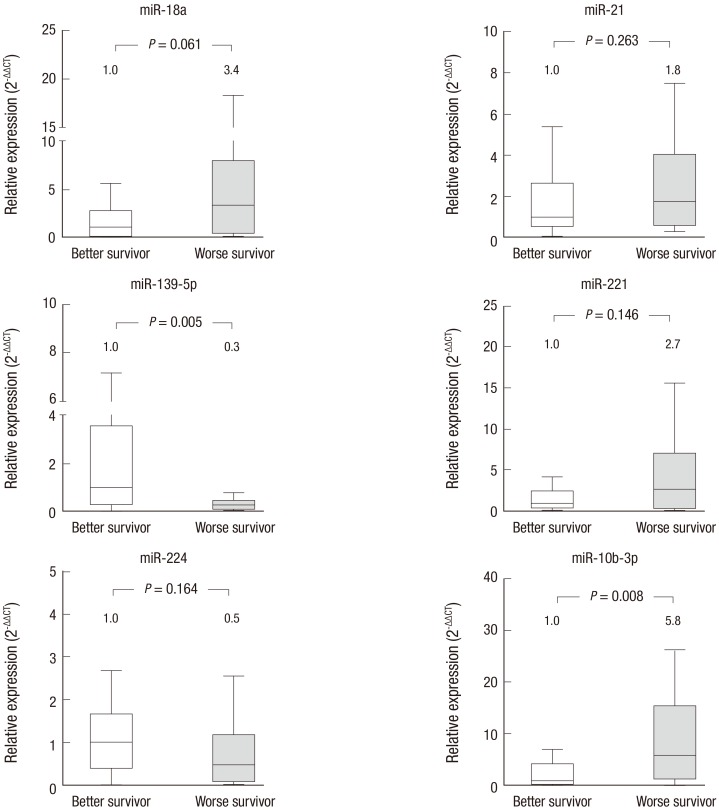
miR-18a and miR-10b-3p were expressed 3.4 times and 5.8 times higher, respectively, in the worse survival group (P = 0.061 and P = 0.008, respectively). miR-139-5p was downregulated in the worse survival group compared with the better survival group (P = 0.005). Among these differences, only miR-10b-3p and miR-139-5p were significant (Fig. 2).
Fig. 2.
Expression of serum miRNAs and overall survival.
The expression of 6 candidate miRNAs was compared between the better and the worse survival groups of HCC patients. miR-139-5p is significantly downregulated in serum samples from the worse survival group (P = 0.005), while miR-10b-3p was significantly upregulated in samples from the worse survival group (P = 0.008). The numbers shown above each box represent median fold changes compared to the median value of samples for the better survival group. Better survival (n = 14); worse survival (n = 10).
HCC = hepatocellular carcinoma.
Significant predictors of longer survival in patients with advanced HCC treated with sorafenib
We identified baseline factors predictive of longer survival in sorafenib-treated patients with advanced HCC by logistic regression analysis. To determine the prognostic values of serum miR-10b-3p and miR-139-5p, their levels of expression in serum were also included in the analysis. Univariate analysis revealed that the presence of MVI had a significant negative association with longer survival (P = 0.007). Higher baseline serum miR-10b-3p expression based on a median fold change of 2.2 exhibited a tendency toward a negative relationship with longer survival but there was no statistical significance (odds ratio [OR], 0.167; 95% CI, 0.024–1.164; P = 0.071). Expression of miR-139-5p was not significantly associated with longer survival on univariate analysis (P = 0.244). When the presence of MVI and serum miR-10b-3p expression were included in the multivariate analysis, the presence of MVI was the only factor that was significantly associated with survival (OR, 0.038; 95% CI, 0.003–0.482; P = 0.012). Higher baseline serum miR-10b-3p levels were not statistically significant in the multivariate analysis (OR, 0.522; 95% CI, 0.045–6.032; P = 0.603; Table 3).
Table 3. Significant factors predictive of longer survival in patients treated with sorafenib.
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | |
| Sex (male) | 1.300 (0.155–10.899) | 0.809 | - | - |
| Age, yr | 0.984 (0.914–1.059) | 0.665 | - | - |
| Cirrhosis | 0.364 (0.033–4.035) | 0.410 | - | - |
| Child-Pugh class | - | - | ||
| A | 1 | 0.053 | - | - |
| B | 0.100 (0.010–1.029) | - | - | |
| High baseline AFP (> 200 ng/mL) | 0.438 (0.091–2.106) | 0.303 | - | - |
| High baseline PIVKA-II (> 125 mAU/mL) | 0.360 (0.055–2.338) | 0.284 | - | - |
| High baseline serum miR-10b-3p (Fold change > 2.2) | 0.167 (0.024–1.164) | 0.071 | 0.522 (0.045–6.032) | 0.603 |
| High baseline Serum miR-139-5p (Fold change > 0.5) | 2.700 (0.057–14.372) | 0.244 | - | - |
| MVI | 0.073 (0.011–0.487) | 0.007 | 0.038 (0.003–0.482) | 0.012 |
| Metastasis | 4.000 (0.730–21.838) | 0.109 | - | - |
RR = relative risk, CI = confidence interval, AFP = alpha-fetoprotein, PIVKA-II = protein induced by vitamin K absence/antagonist-II, MVI = macrovascular invasion.
The relationship between miR-10b-3p expression and the presence of MVI
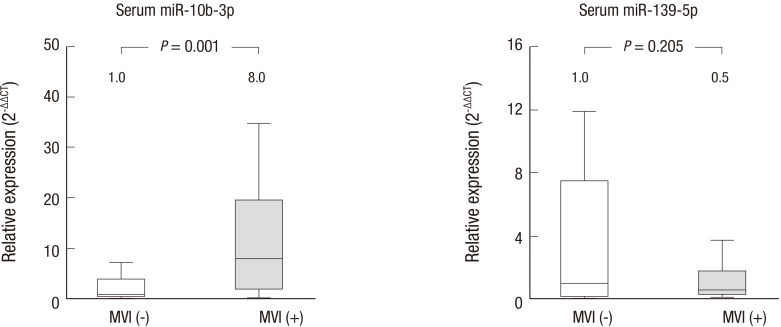
Expression of miR-10b-3p was compared between subgroups of patients with the presence or absence of MVI. In the 12 patients with MVI, serum miR-10b-3p was significantly upregulated compared to the 12 patients without MVI, with a fold change of 8.0 (P = 0.001). For reference, expression of miR-139-5p in the serum was compared between patients with or without MVI, and no difference in expression was observed (Fig. 3).
Fig. 3.
Expression of miR-10b-3p and miR-139-5p and the invasiveness of HCC.
Levels of serum miR-10b-3p were significantly higher in patients with MVI (P = 0.001). Expression of miR-139-5p in serum samples did not differ significantly between patients with and without MVI. The numbers shown above each box represent the median fold changes compared to the median value of samples obtained from HCC patients without MVI. MVI (−), without MVI (n = 12); MVI (+), with MVI (n = 12).
HCC = hepatocellular carcinoma, MVI = macrovascular invasion.
Relationship between baseline serum miR-10b-3p levels and median overall survival in advanced HCC patients
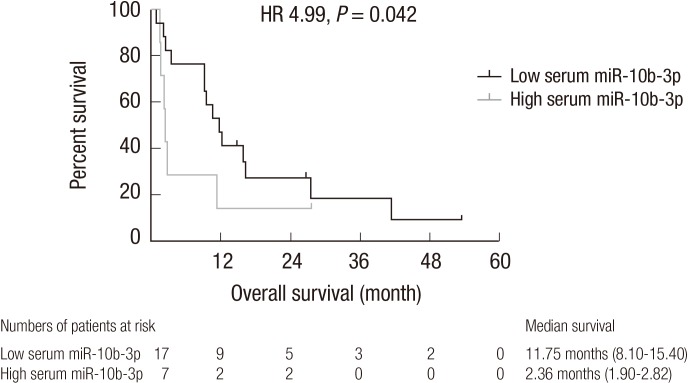
We compared the median overall survival of the low and high serum miR-10b-3p groups based on a median fold change of 2.2. The median overall survival times in the low and high serum miR-10b-3p groups were 11.75 months (95% CI, 8.10–15.40) and 2.36 months (95% CI, 1.90–2.82), respectively. Median overall survival was significantly longer in the low serum miR-10b-3p group (hazard ratio [HR], 4.99; 95% CI, 4.60–5.57; P = 0.042; Fig. 4). The median duration of sorafenib treatment in the low and high serum miR-10b-3p groups were 3.21 months (95% CI, 1.28–5.15) and 2.07 months (95% CI, 1.37–2.77), respectively, and were not significantly different (P = 0.055).
Fig. 4.
Comparison of median overall survival between the low and high serum miR-10b-3p groups.
The median survival of the low serum miR-10b-3p group was significantly longer than that of the high serum miR-10b-3p group (HR, 4.99; 95% CI, 4.60–5.57; P = 0.042). The numbers shown below the graph represent patients who survived until the time on the x-axis after the initiation of sorafenib treatment.
HR = hazard ratio, CI = confidence interval.
DISCUSSION
This study is the first to analyze dysregulated serum miRNA profiles in advanced stage HCC patients treated with sorafenib. Aberrant expression of tissue miRNA has been widely evaluated as promising biomarkers for HCC (22). In contrast to studies on tissue miRNAs, the significance of serum miRNAs in HCC has not been well characterized to date. Moreover, few previous studies evaluating serum miRNAs were more focused on differentiating HCC from control groups consisting of LC or chronic hepatitis patients. Our study is unique in that miRNA expression was evaluated with a goal of identifying potential biomarkers of radiologic response to sorafenib. We chose 6 candidate miRNAs that were previously reported to be significantly dysregulated in HCC tissue samples compared with non-tumoral liver tissues. We sought to determine whether those miRNAs were aberrantly expressed in serum samples from HCC patients compared with those from LC patients. In agreement with our hypothesis, miR-18a, miR-139-5p, miR-221, miR-224, and miR-10b-3p were significantly overexpressed in serum samples from HCC patients. miR-21 did not exhibit aberrant expression in HCC patients, as has been reported in other studies. Previous studies on miR-21 were primarily focused on the identification of early HCC patients who could be treated with curative resection (14,23). Thus, miR-21 might play an important role in the early development of HCC, but not in advanced stage HCC. In addition, liver injury in advanced stage HCC patients might have much progressed to such a degree that miR-21 may not have been able to differentiate non-responders and those with worse survival from responders and those with better survival in the present study.
There were no differences in expression of the 6 candidate serum miRNAs between the responders and non-responders. When comparing the responder and non-responder groups, miR-139-5p tended to be downregulated in the non-responder group (P = 0.067). The significance of miR-139-5p as a predictor of response may have been obscured due to the limited number of patients. We also compared the expression of these miRNAs between the CR + PR group and SD + PD group. However, the miRNAs were not differently expressed between the 2 groups (data not shown). To date, considerable effort has been directed toward identifying a serum marker predictive of good radiologic response to sorafenib (24,25). Llovet et al. (24) analyzed ten plasma cytokines, but failed to prove that any of these plasma cytokines were predictive of a good response to sorafenib (23).
In addition to miR-10b-3p, expression of miR-139-5p was downregulated in sera samples from the worse survival group, and the difference between the groups was significant. Previous studies reported a relationship between miR-139-5p overexpression and a lower incidence of metastasis or a good prognosis (11,12). However, in our study, overexpression of serum miR-139-5p was not an independent predictor of longer survival. Additionally, comparison of miR-139-5p expression based on the presence or absence of MVI exhibited no significant difference. Similar results were found for comparison of expression between patients with and without distant metastases (data not shown). However, it still may be worthwhile to validate a potential association between serum miR-139-5p and survival in a large study population in the future, given the wide distribution of miR-139-5p expression in the radiologic responder group, better survival group, and patients without MVI.
The role of miR-10b-3p has not been studied in any malignancies, including HCC, to date. It has not been determined whether miR-10b-3p is similar to miR-10b-5p in terms of target mRNAs or functions. miR-10b-5p was first shown to be highly expressed in metastatic breast tumors (26,27). Upregulation of miR-10b-5p has been detected in several highly metastatic cancers, such as breast cancer, esophageal cancer, malignant glioma, and pancreatic cancer, and has been shown to promote cell migration and invasion (26,27,28,29,30,31). However, few studies have analyzed the effects of miR-10b-5p overexpression in HCC, although it is assumed that miR-10b-5p may play a role in the HCC migration and invasion (32). After cleavage of the hairpin loop structure during miRNA gene processing, 2 arms of the hairpin form a miRNA-5p:miRNA-3p duplex in the cytoplasm (33). Thus, it can be inferred from our results that miR-10b-3p may have an oncogenic effect similar to miR-10b-5p in HCC. However, we failed to identify an association between miR-10b-3p expression and radiologic response to sorafenib treatment. Nevertheless, it had a marginally significant association with longer survival during sorafenib treatment on logistic regression analysis. It is not evident that the differences in survival observed in comparison of the low and high serum miR-10b-3p groups are related to the effects of sorafenib treatment, but rather miR-10b-3p expression appeared to be associated with aggressive tumor characteristics, such as the presence of MVI. Loss of function and gain of function analyses with transfection of miR-10b-3p mimics or inhibitors should be performed to differentiate between the effects of this miRNA on tumor characteristics and sorafenib treatment. Expression of miR-10b-3p did not differ between patients with and without distant metastases in this study (data not shown) (32).
Analysis of serum miRNAs has several advantages over analysis of tissue-based miRNAs for HCC. First, serum miRNAs have the potential to be ideal biomarkers, as serum can be easily obtained in a minimally invasive and safe method, unlike tissue-based miRNAs, especially in advanced HCC patients. Second, serum miRNAs can be repetitively monitored before, during, and after treatment to enable more accurate prediction of prognosis.
Based on our results, miR-18a, miR-139-5p, miR-221, miR-224, and miR-10b-3p can be used to differentiate HCC patients from LC patients. Our findings can serve as the basis of further, larger studies, especially considering the high median fold changes that were observed despite the small number of patients included in this study.
There are several limitations in our study. Firstly, as this study was based on biomaterials, the study population was small. Differences in the absolute values of fold changes between the responders and the non-responders might have retained statistical significance if the analysis was performed with a larger patient sample. These results should be prospectively validated in a well-matched cohort of patients to ensure their generalizability. Secondly, additive analyses of other serum miRNAs should be performed in order to potentially identify prognostic factors of good radiologic response to sorafenib. Thirdly, there has been no unified miRNAs for normalization as control in the analysis of fold change of serum miRNA expression. Therefore, the role as control of miR-16 in normalization should be evaluated further. Fourthly, direct comparison of tissues among the healthy liver, cirrhotic liver, and HCC was not possible due to difficulty in collecting healthy liver. Additionally, it was not possible to compare the expression of miRNA in tissue and serum at the same time. As pathologic confirmation is not prerequisite in the diagnosis of HCC, especially in the setting of advanced stage of HCC, it was not seemed to be ethical to perform liver biopsy in patients who are waiting for the prompt treatment.
In conclusions, treatment with sorafenib is limited in that some patients do not show satisfactory results in terms of radiologic response. We analyzed the expression of serum miRNAs and attempted to identify a predictive marker of good response to sorafenib treatment. There was no single serum miRNA that was prognostic of a good radiologic response to sorafenib in advanced HCC patients, nor had association with longer survival of HCC patients after sorafenib treatment. However, the presence of MVI was significantly associated with survival in sorafenib-treated advanced HCC on multivariate analysis. Serum miR-10b-3p was overexpressed in patients with MVI when compared to patients without MVI. The expression of serum miR-10b-3p may prove valuable in the diagnosis of HCC and in the prediction of survival in patients treated with sorafenib by the association with MVI.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Yoon EL, Yeon JE. Resources: Ko E. Formal analysis: Yoon EL. Writing - original draft: Yoon EL. Writing - review & editing: Yeon JE.
References
- 1.European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Shao YY, Hsu CH, Cheng AL. Predictive biomarkers of sorafenib efficacy in advanced hepatocellular carcinoma: are we getting there? World J Gastroenterol. 2015;21:10336–10347. doi: 10.3748/wjg.v21.i36.10336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borel F, Konstantinova P, Jansen PL. Diagnostic and therapeutic potential of miRNA signatures in patients with hepatocellular carcinoma. J Hepatol. 2012;56:1371–1383. doi: 10.1016/j.jhep.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Li W, Xie L, He X, Li J, Tu K, Wei L, Wu J, Guo Y, Ma X, Zhang P, et al. Diagnostic and prognostic implications of microRNAs in human hepatocellular carcinoma. Int J Cancer. 2008;123:1616–1622. doi: 10.1002/ijc.23693. [DOI] [PubMed] [Google Scholar]
- 5.Connolly E, Melegari M, Landgraf P, Tchaikovskaya T, Tennant BC, Slagle BL, Rogler LE, Zavolan M, Tuschl T, Rogler CE. Elevated expression of the miR-17-92 polycistron and miR-21 in hepadnavirus-associated hepatocellular carcinoma contributes to the malignant phenotype. Am J Pathol. 2008;173:856–864. doi: 10.2353/ajpath.2008.080096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang J, Gusev Y, Aderca I, Mettler TA, Nagorney DM, Brackett DJ, Roberts LR, Schmittgen TD. Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res. 2008;14:419–427. doi: 10.1158/1078-0432.CCR-07-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Lee AT, Ma JZ, Wang J, Ren J, Yang Y, Tantoso E, Li KB, Ooi LL, Tan P, et al. Profiling microRNA expression in hepatocellular carcinoma reveals microRNA-224 up-regulation and apoptosis inhibitor-5 as a microRNA-224-specific target. J Biol Chem. 2008;283:13205–13215. doi: 10.1074/jbc.M707629200. [DOI] [PubMed] [Google Scholar]
- 9.Fornari F, Gramantieri L, Ferracin M, Veronese A, Sabbioni S, Calin GA, Grazi GL, Giovannini C, Croce CM, Bolondi L, et al. MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene. 2008;27:5651–5661. doi: 10.1038/onc.2008.178. [DOI] [PubMed] [Google Scholar]
- 10.Wong CM, Wong CC, Lee JM, Fan DN, Au SL, Ng IO. Sequential alterations of microRNA expression in hepatocellular carcinoma development and venous metastasis. Hepatology. 2012;55:1453–1461. doi: 10.1002/hep.25512. [DOI] [PubMed] [Google Scholar]
- 11.Fan Q, He M, Deng X, Wu WK, Zhao L, Tang J, Wen G, Sun X, Liu Y. Derepression of c-Fos caused by microRNA-139 down-regulation contributes to the metastasis of human hepatocellular carcinoma. Cell Biochem Funct. 2013;31:319–324. doi: 10.1002/cbf.2902. [DOI] [PubMed] [Google Scholar]
- 12.Wong CC, Wong CM, Tung EK, Au SL, Lee JM, Poon RT, Man K, Ng IO. The microRNA miR-139 suppresses metastasis and progression of hepatocellular carcinoma by down-regulating Rho-kinase 2. Gastroenterology. 2011;140:322–331. doi: 10.1053/j.gastro.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Huang YS, Dai Y, Yu XF, Bao SY, Yin YB, Tang M, Hu CX. Microarray analysis of microRNA expression in hepatocellular carcinoma and non-tumorous tissues without viral hepatitis. J Gastroenterol Hepatol. 2008;23:87–94. doi: 10.1111/j.1440-1746.2007.05223.x. [DOI] [PubMed] [Google Scholar]
- 14.Xu J, Wu C, Che X, Wang L, Yu D, Zhang T, Huang L, Li H, Tan W, Wang C, et al. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog. 2011;50:136–142. doi: 10.1002/mc.20712. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Wang Y, Yu W, Chen J, Luo J. Expression of serum miR-221 in human hepatocellular carcinoma and its prognostic significance. Biochem Biophys Res Commun. 2011;406:70–73. doi: 10.1016/j.bbrc.2011.01.111. [DOI] [PubMed] [Google Scholar]
- 16.Su H, Yang JR, Xu T, Huang J, Xu L, Yuan Y, Zhuang SM. MicroRNA-101, down-regulated in hepatocellular carcinoma, promotes apoptosis and suppresses tumorigenicity. Cancer Res. 2009;69:1135–1142. doi: 10.1158/0008-5472.CAN-08-2886. [DOI] [PubMed] [Google Scholar]
- 17.Sato F, Hatano E, Kitamura K, Myomoto A, Fujiwara T, Takizawa S, Tsuchiya S, Tsujimoto G, Uemoto S, Shimizu K. MicroRNA profile predicts recurrence after resection in patients with hepatocellular carcinoma within the Milan Criteria. PLoS One. 2011;6:e16435. doi: 10.1371/journal.pone.0016435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 22.Chauhan R, Lahiri N. Tissue- and serum-associated biomarkers of hepatocellular carcinoma. Biomark Cancer. 2016;8:37–55. doi: 10.4137/BIC.S34413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomimaru Y, Eguchi H, Nagano H, Wada H, Kobayashi S, Marubashi S, Tanemura M, Tomokuni A, Takemasa I, Umeshita K, et al. Circulating microRNA-21 as a novel biomarker for hepatocellular carcinoma. J Hepatol. 2012;56:167–175. doi: 10.1016/j.jhep.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 24.Llovet JM, Peña CE, Lathia CD, Shan M, Meinhardt G, Bruix J, SHARP Investigators Study Group Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012;18:2290–2300. doi: 10.1158/1078-0432.CCR-11-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyahara K, Nouso K, Tomoda T, Kobayashi S, Hagihara H, Kuwaki K, Toshimori J, Onishi H, Ikeda F, Miyake Y, et al. Predicting the treatment effect of sorafenib using serum angiogenesis markers in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:1604–1611. doi: 10.1111/j.1440-1746.2011.06887.x. [DOI] [PubMed] [Google Scholar]
- 26.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 27.Moriarty CH, Pursell B, Mercurio AM. miR-10b targets Tiam1: implications for Rac activation and carcinoma migration. J Biol Chem. 2010;285:20541–20546. doi: 10.1074/jbc.M110.121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, Teruya-Feldstein J, Bell GW, Weinberg RA. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol. 2010;28:341–347. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian Y, Luo A, Cai Y, Su Q, Ding F, Chen H, Liu Z. MicroRNA-10b promotes migration and invasion through KLF4 in human esophageal cancer cell lines. J Biol Chem. 2010;285:7986–7994. doi: 10.1074/jbc.M109.062877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakata K, Ohuchida K, Mizumoto K, Kayashima T, Ikenaga N, Sakai H, Lin C, Fujita H, Otsuka T, Aishima S, et al. MicroRNA-10b is overexpressed in pancreatic cancer, promotes its invasiveness, and correlates with a poor prognosis. Surgery. 2011;150:916–922. doi: 10.1016/j.surg.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Sasayama T, Nishihara M, Kondoh T, Hosoda K, Kohmura E. MicroRNA-10b is overexpressed in malignant glioma and associated with tumor invasive factors, uPAR and RhoC. Int J Cancer. 2009;125:1407–1413. doi: 10.1002/ijc.24522. [DOI] [PubMed] [Google Scholar]
- 32.Li QJ, Zhou L, Yang F, Wang GX, Zheng H, Wang DS, He Y, Dou KF. MicroRNA-10b promotes migration and invasion through CADM1 in human hepatocellular carcinoma cells. Tumour Biol. 2012;33:1455–1465. doi: 10.1007/s13277-012-0396-1. [DOI] [PubMed] [Google Scholar]
- 33.Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]