Abstract
The KoreaN Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD) was developed to investigate various clinical courses and risk factors for progression of Korean chronic kidney disease (CKD). The KNOW-CKD study consists of nine clinical centers in Korea, and patients aged between 20 and 75 years with CKD from stage 1 to 5 (predialysis) were recruited. At baseline, blood and urine samples were obtained and demographic data including comorbidities, drugs, quality of life, and health behaviors were collected. Estimated glomerular filtration rate (eGFR) was calculated by 4-variable Modification of Diet in Renal Disease (MDRD) equation using isotope dilution mass spectrometry (IDMS)-calibrated serum creatinine measured at a central laboratory.
As a dynamic cohort, a total of 2,341 patients were enrolled during the enrollment period from 2011 until 2015, among whom 2,238 subjects were finally analyzed for baseline profiles. The mean age of the cohort was 53.7 ± 12.2 year and 61.2% were men. Mean eGFR was 50.5 ± 30.3 mL/min/1.73 m2. The participants with lower eGFR had a tendency to be older, with more comorbidities, to have higher systolic blood pressure (BP) and pulse pressure, with lower income level and education attainment. The patients categorized as glomerulonephritis (GN) were 36.2% followed by diabetic nephropathy (DN, 23.2%), hypertensive nephropathy (HTN, 18.3%), polycystic kidney disease (PKD, 16.3%), and other unclassified disease (6.1%). The KNOW-CKD participants will be longitudinally followed for 10 years. The study will provide better understanding for physicians regarding clinical outcomes, especially renal and cardiovascular outcomes in CKD patients.
Keywords: Chronic Kidney Disease, Cohort, Diabetes, Hypertension, Polycystic Kidney Disease, Glomerulonephritis, Epidemiology
Graphical Abstract
INTRODUCTION
As aging populations are growing worldwide, the number of patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD) is increasing. CKD is a major threat to the all-cause and cardiovascular mortality (1,2). Besides, medical expenses for management of this disease entity are increasing, as well. Because CKD is a progressive disease and is associated with many complications including cardiovascular disease (CVD), mineral-bone disease (MBD), and anemia, early intervention for the prevention of CKD progression is crucial. Especially, it is well known that early referral to a nephrologist is associated with improved clinical outcomes in CKD patients (3,4,5).
To advance our knowledge regarding the risk factors for CKD and CVD and epidemiology of CKD, a few cohort studies have been established recently. The Chronic Renal Insufficiency Cohort (CRIC) study of the United States started in 2001, Canadian Study of Prediction of Death, Dialysis and Interim Cardiovascular Events (CanPREDDICT) (6) of Canada was established in 2009. It is expected that the results from the large-scale longitudinal cohort studies may provide an insight into the various clinical courses and risk factors of CKD. However, the above CKD cohort studies include very few Asian CKD patients.
The risks of CKD progression to ESRD or the risk of cardiovascular (CV) events or mortality differ significantly depending on the socio-demographic background of the population (7,8). Both the racial differences and socioeconomic disparities may play a significant role in the clinical course and complications of CKD (7,9,10). Therefore, with the purpose to elucidate the progression and clinical courses of the Korean CKD, which may or may not be different from the Caucasian CKD population, the KoreaN Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD) was launched in 2011, funded by the Korea Centers for Disease Control and Prevention (KCDC). Furthermore, since the KNOW-CKD cohort includes, unlike other CKD cohort studies (11,12,13,14), the patients with earlier stages of CKD (stage 1 and 2), as well as advanced stage, the study is also aimed to explore the clinical courses of mild CKD subpopulation.
The objectives of the KNOW-CKD cohort study are to explore the clinical courses of Korean CKD, to identify the factors related to CKD progression or adverse clinical outcomes, and to identify high-risk subgroups of CKD progression and CVD development. We enrolled the subjects from 2011 until January, 2016.Here, in the present article, we report the baseline characteristics of total enrolled patients of KNOW-CKD according to CKD stages and specific causes of CKD.
MATERIALS AND METHODS
Study organization
Details of the rationale and design of the KNOW-CKD study have been described elsewhere previously (15). Briefly, 9 university-affiliated tertiary-care hospitals throughout Korea participated in the patient enrollment. Nephrologists, epidemiologists, specialists in laboratory medicine, and biostatisticians have been participating in this study. The study is funded and supervised by the KCDC.
Study design and study populations
The KNOW-CKD was designed as a prospective cohort study and participants who provide written informed consent were enrolled at nine participating centers in Korea—i.e., Seoul National University Hospital, Seoul National University Bundang Hospital, Yonsei University Severance Hospital, Kangbuk Samsung Medical Center, Seoul St. Mary's Hospital, Gil Hospital, Eulji General Hospital, Chonnam National University Hospital, and Pusan Paik Hospital.
The participants were all ethnic Koreans, aged between 20 and 75 years with CKD stages from 1 to 5 (pre-dialysis). CKD stages were defined on the basis of the estimated glomerular filtration rate (eGFR) calculated using the 4-variable Modification of Diet in Renal Disease (MDRD) equation as follows (16):
| eGFR (mL/min/1.73 m2) = 175 × (serum Cr [mg/dL])−1.154 × (age)−0.203 × (0.742 if female) × (1.212 if black) |
The serum creatinine was measured at a central laboratory, using an assay traceable to the international reference material (16,17). We excluded those who 1) were unable or unwilling to give informed consent, 2) had previously received chronic dialysis or organ transplantation, 3) had heart failure (New York Heart Association [NYHA] class 3 or 4) or liver cirrhosis (Child-Pugh class 2 or 3), 4) had a past or current history of malignancy, 5) were currently pregnant, or 6) had a single kidney due to trauma or kidney donation.
All enrolled patients were categorized as the following subgroups; glomerulonephritis (GN), diabetic nephropathy (DN), hypertensive nephropathy (HTN), and polycystic kidney disease (PKD) and unclassified, based on the etiologic disease. The definition of the subgroup categorization was decided by the pathologic diagnosis when the biopsy result is available. Otherwise, the subgroup classification depends on the clinical diagnosis made by nephrologists. The clinical diagnosis of GN is based on the glomerular hematuria or albuminuria with or without an underlying systemic disease causing GN. The patients who had albuminuria and diabetic retinopathy with type 2 diabetes mellitus were categorized into DN. Subjects with hypertension and CKD in the absence of other illness that may cause kidney disease was classified into HTN. Diagnosis of PKD was based on the unified criteria (18). The rest of the patients were categorized as ‘unclassified.’ Baseline comorbidity was scored by using age-adjusted modified Charlson comorbidity index (CCI) (19).
Enrollment, follow-up, and study data collection
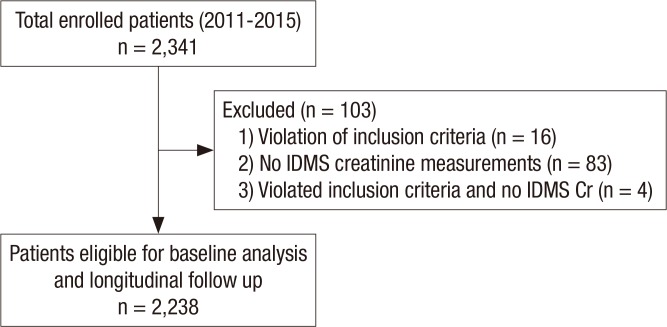
A total 2,341 CKD patients consented to the study enrollment. Among these patients, sixteen subjects violated the inclusion criteria, eighty-three failed to test for the IDMS-calibrated creatinine level, and 4 subjects violated the inclusion criteria and failed to test for IDMS-calibrated creatinine simultaneously. After exclusion (n = 103), 2,238 subjects were finally selected for the analysis of baseline profiles and future follow-up (Fig. 1). The sequence and schedule of KNOW-CKD cohort clinic visits and procedures was showed in Table 1.
Fig. 1.
Participants' recruitment and follow-up flow diagram.
IDMS = isotope dilution mass spectrometry, Cr = creatinine.
Table 1. Sequence and schedule of KNOW-CKD Study clinic visits and procedures.
| Parameters | Screen | Baseline | 6 mon | 1 yr | 2 yr | 3 yr | 4 yr | 5 yr | 6 yr | 7 yr | 8 yr | 9 yr | 10 yr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Informed consent | ● | ||||||||||||
| Demographic information | ● | ||||||||||||
| Medical history | ● | ||||||||||||
| Eligibility confirmation | ● | ||||||||||||
| Recent events | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||
| Medications | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |
| Health related questionnaire | ● | ● | |||||||||||
| HRQOL questionnaire | ● | ● | |||||||||||
| BP | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |
| Anthropometric measures | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |
| CBC, chemistry | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |
| IDMS-traceable Cr, eGFR | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |
| Cystatin C | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |
| HbA1c (diabetic subjects) | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |
| Lipid panel, CRP, iron panel | ● | ● | ● | ● | ● | ● | |||||||
| Intact PTH, 25(OH)D, 1,25(OH)D, troponin T | ● | ● | ● | ● | ● | ● | ● | ||||||
| UA with microscopy | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |
| 24HU – Ca/P/Cr/Urea/Uric acid/protein | ● | ● | ● | ● | ● | ||||||||
| ECG, chest X-ray | ● | ● | ● | ● | ● | ● | |||||||
| Spot urine e', albumin, protein, osmolality, Cr | ● | ● | ● | ● | ● | ● | |||||||
| EchoCG, PWV, ABI, Coronary CT, LS spine lateral X-ray, DEXA BMD | ● | ● | ● | ||||||||||
| Biosamples for DNA | ● | ||||||||||||
| Plasma/urine biosample | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |
| Fundus exam (DNsubgroup) | ● | ||||||||||||
| Abdomen CT (PKD subgroup) | ● | ● | ● | ● | ● | ● | |||||||
| Family screen (PKD subgroup) | ● |
KNOW-CKD = KoreaN Cohort Study for Outcomes in Patients With Chronic Kidney Disease, HRQOL = health-related quality of life, BP = blood pressure, CBC = complete blood count, Cr = creatinine, eGFR = estimated glomerular filtration rate, HbA1c = hemoglobin A1c, CRP = C-reactive protein, UA = urinalysis, 24HU = 24-hour urine, ECG = electrocardiogram, PTH = parathyroid hormone, 25(OH)D = 25-hydroxyvitamin D, 1,25(OH)D = 1,25-dihydroxyvitamin D, EchoCG = echocardiography, PWV = pulse wave velocity, ABI = ankle-brachial index, CT = computerized tomography, LS = lumbosacral spine, DEXA BMD = dual energy x-ray absorptiometry bone mineral density, DN = diabetic nephropathy, PKD = polycystic kidney disease.
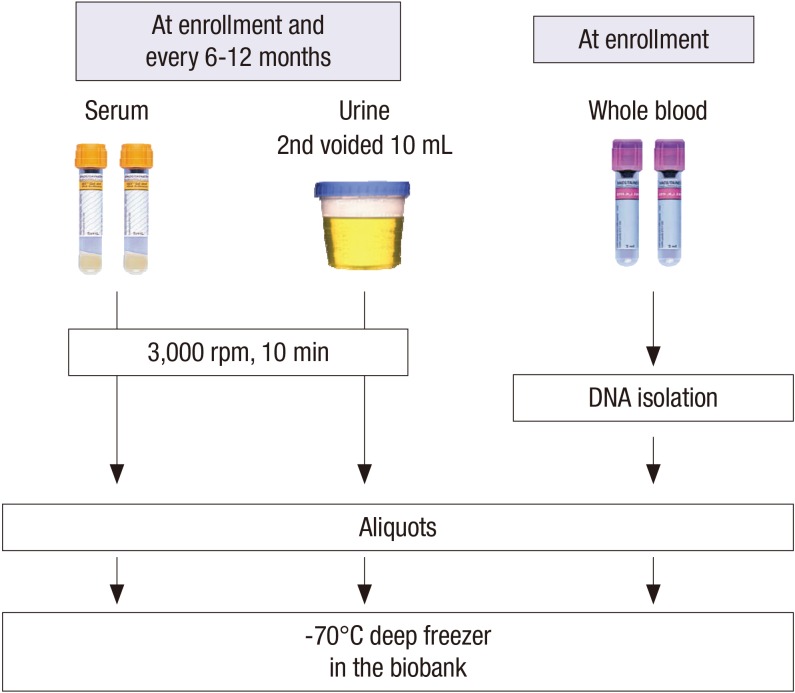
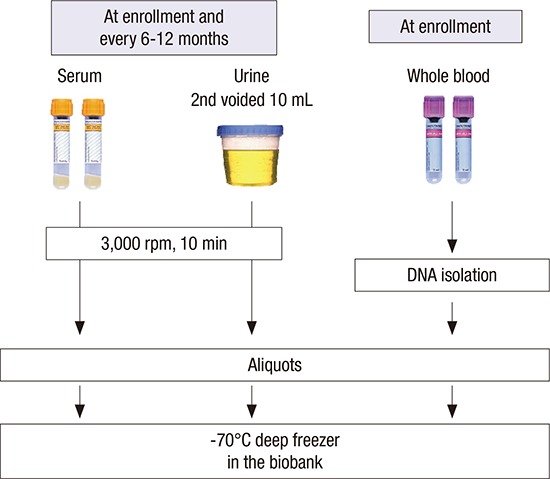
At the baseline visits, socio-demographic characteristics, smoking, alcohol consumption, medication, and personal and family medical histories were collected. The anthropometric measurements such as height, weight, and waist/hip ratios were collected. The resting blood pressure (BP) and pulse pressure in the clinic were measured by an electronic sphygmomanometer. Evaluation of CV parameters is assessed via measurements of ankle-brachial index (ABI), pulse wave velocity (PWV), and echocardiography. Each center assessed coronary calcium score (CCS) through Agatston score which was calculated based on the extent of coronary artery calcification detected by an electron-beam computed tomography (CT) scanner or a multi-detector CT. The subjects filled out questionnaires regarding quality of life using Korean version of Kidney Disease Quality Of Life Short Form (KDQOL-SF) instrument (20), socioeconomic status (SES), educational attainment, physical activity, health behaviors, and health care facility utilization. Hypertension was defined as either BP measured at the clinic ≥ 140/90 mmHg or taking anti-hypertensive medication. Serum and urine samples were collected for initial study measures and rest of samples were stored for further studies (Fig. 2). Serum concentrations of creatinine, intact parathyroid hormone (iPTH), 25-hydroxyvitamin D3 (25[OH]D3), 1,25-dihydroxyvitamin D3 (1,25[OH]2D3), cystatin C, troponin T were measured at the central laboratory. Urine concentrations of albumin, protein, creatinine, calcium, phosphorus, urea, uric acid, sodium, potassium, chloride, osmolality were measured at a random urine at the central laboratory.
Fig. 2.
Collection of clinical specimens in KNOW-CKD cohort.
KNOW-CKD = KoreaN Cohort Study for Outcomes in Patients With Chronic Kidney Disease, rpm = revolution per minute.
All of the data, including demographics, laboratory test and clinical outcomes were recorded in a web-based, electronic case-reporting form (eCRF, http://www.phactax.org) developed by the Division of Data Management in Seoul National University Medical Research Collaborating Center.
Outcome variables
Composite renal events were defined as > 50% decrease in eGFR from the baseline eGFR, doubling of serum creatinine, or development of ESRD. CV events are defined as myocardial infarction, unstable angina, coronary revascularization, stroke and new development or aggravation of congestive heart failure. Data on the time and specific causes of mortality will also be collected. The mortality and cause of death will also be obtained from KOrean Statistical Information Service (KOSIS). Other events such as all-cause hospitalization, surgery, and fracture will also be collected during the follow-up period.
Statistical analysis
The baseline characteristics of the study participants are described using means with standard deviation (SD) or medians and interquartile ranges for continuous variables, and frequency is described using percentages for categorical variables. We used t-test and one-way analysis of variance (ANOVA) for comparisons of continuous variables and the χ2 test for categorical variables as appropriate. A statistically significant difference was defined as a 2-sided P < 0.05.
Ethics statement
The study protocol was approved by the Institutional Review Board at each participating clinical center—i.e., Seoul National University Hospital (1104-089-359), Seoul National University Bundang Hospital (B-1106/129-008), Yonsei University Severance Hospital (4-2011-0163), Kangbuk Samsung Medical Center (2011-01-076), Seoul St. Mary's Hospital (KC11OIMI0441), Gil Hospital (GIRBA2553), Eulji General Hospital (201105-01), Chonnam National University Hospital (CNUH-2011-092), and Pusan Paik Hospital (11-091) in 2011. This study was conducted in accordance with the principles of the Declaration of Helsinki. The protocol summary is also registered at the ClinicalTrials.gov with accession number NCT01630486. Written informed consent was obtained from all the subjects when they were enrolled.
RESULTS
Baseline demographic and clinical characteristics of enrolled participants
Two thousand two hundred thirty-eight subjects with CKD were analyzed for baseline demographic and clinical characteristics (Table 2). Mean age was 53.7 ± 12.2 years, and 61.2% were men. 40.8% of the total subjects had completed the college-level education. Current smokers comprised 15.7% (n = 349) and former smokers 30.4% (n = 677). Coronary artery disease was prevalent in 5.3% of the total subjects. Peripheral artery disease and cerebrovascular disease were prevalent in 3.5% and 6.0%, respectively. Seven hundred fifty-five subjects (33.7%) were diabetic (either type 1 or type 2 diabetes). Mean systolic and diastolic BPs were 127.8 ± 16.2 mmHg and 77.0 ± 11.1 mmHg, respectively, 96.1% of the patients either had a history of hypertension or was taking antihypertensive drugs. Mean body mass index (BMI) was 24.6 ± 3.4 kg/m2 with 68% of total subjects being in the categories of overweight or obesity.
Table 2. Baseline demographic and clinical characteristics of KNOW-CKD participants according to specific cause of CKD.
| Causes of CKD | Total (n = 2,238) | GN (n = 810) | DN (n = 519) | HTN (n = 409) | PKD (n = 364) | P | |
|---|---|---|---|---|---|---|---|
| Age, yr | 53.7 ± 12.2 | 49.8 ± 12.1 | 59.3 ± 9.4 | 59.6 ± 10.8 | 47.0 ± 10.6 | < 0.001* | |
| Age (category), yr | < 0.001† | ||||||
| 20 to 29 | 77 (3.4) | 48 (5.9) | 0 (0.0) | 3 (0.7) | 20 (5.5) | ||
| 30 to 39 | 248 (11.1) | 110 (13.6) | 18 (3.5) | 25 (6.1) | 79 (21.7) | ||
| 40 to 49 | 449 (20.1) | 223 (27.5) | 58 (11.2) | 46 (11.2) | 110 (30.2) | ||
| 50 to 59 | 671 (30.0) | 250 (30.9) | 168 (32.4) | 99 (24.2) | 110 (30.2) | ||
| 60 to 69 | 578 (25.8) | 146 (18.0) | 191 (36.8) | 158 (38.6) | 45 (12.4) | ||
| 70 to 75 | 215 (9.6) | 33 (4.1) | 84 (16.2) | 78 (19.1) | 0 (0.0) | ||
| Gender | < 0.001† | ||||||
| Male | 1,369 (61.2) | 451 (55.7) | 357 (68.8) | 295 (72.1) | 184 (50.5) | ||
| Female | 869 (38.8) | 359 (44.3) | 162 (31.2) | 114 (27.9) | 180 (49.5) | ||
| Monthly income, KRW | <0.001† | ||||||
| < 1,500,000 | 367 (22.7) | 103 (18.9) | 112 (30.9) | 72 (21.7) | 45 (17.3) | ||
| 1,500,000 to 4,500,000 | 861 (53.1) | 297 (54.5) | 190 (52.3) | 179 (53.9) | 127 (48.8) | ||
| > 4,500,000 | 392 (24.2) | 145 (26.6) | 61 (16.8) | 81 (24.4) | 88 (33.8) | ||
| Educational attainment | < 0.001† | ||||||
| Illiteracy | 15 (0.8) | 6 (0.9) | 1 (0.2) | 5 (1.4) | 2 (0.6) | ||
| Graduated elementary school | 239 (10.8) | 56 (8.0) | 71 (16.0) | 53 (14.7) | 19 (5.6) | ||
| Graduated middle school | 259 (11.7) | 62 (8.9) | 76 (17.2) | 41 (11.4) | 24 (7.0) | ||
| Graduated high school | 785 (35.3) | 268 (38.3) | 145 (32.7) | 110 (30.6) | 98 (28.7) | ||
| Graduated college or more | 906 (40.8) | 308 (43.9) | 150 (33.8) | 151 (42.0) | 198 (58.1) | ||
| Smoking | < 0.001† | ||||||
| Never smoker | 1,202 (53.9) | 478 (59.1) | 240 (46.8) | 183 (44.9) | 228 (62.6) | ||
| Current smoker | 349 (15.7) | 115 (14.2) | 84 (16.4) | 71 (17.4) | 55 (15.1) | ||
| Former smoker | 677 (30.4) | 216 (26.7) | 189 (36.8) | 154 (37.7) | 81 (22.3) | ||
| Comorbid disease | |||||||
| Coronary artery disease | 118 (5.3) | 14 (1.7) | 60 (11.6) | 32 (7.8) | 2 (0.5) | < 0.001† | |
| Peripheral vascular disease | 78 (3.5) | 13 (1.6) | 32 (6.2) | 20 (4.9) | 1 (0.3) | < 0.001† | |
| Cerebrovascular disease | 135 (6.0) | 19 (2.3) | 51 (9.8) | 38 (9.3) | 21 (5.8) | < 0.001† | |
| Diabetes | 755 (33.7) | 70 (8.6) | 519 (100.0) | 72 (17.6) | 12 (3.3) | < 0.001† | |
| Hypertension | 2,150 (96.1) | 785 (96.9) | 513 (98.8) | 407 (99.5) | 314 (86.3) | < 0.001† | |
| Congestive heart failure | 34 (1.5) | 7 (0.9) | 12 (2.3) | 10 (2.4) | 2 (0.5) | 0.025‡ | |
| Arrhythmia | 56 (2.5) | 18 (2.2) | 16 (3.1) | 18 (4.4) | 2 (0.5) | 0.006† | |
| Age-adjusted modified CCI | 2.3 ± 1.6 | 1.5 ± 1.2 | 4.2 ± 0.9 | 2.4 ± 1.2 | 1.1 ± 1.2 | < 0.001* | |
| Age-adjusted modified CCI | < 0.001† | ||||||
| Low (≤ 3) | 1,174 (52.5) | 627 (77.4) | 24 (4.6) | 153 (37.4) | 317 (87.1) | ||
| Moderate (4–5) | 652 (29.1) | 155 (19.1) | 204 (39.3) | 193 (47.2) | 45 (12.4) | ||
| High (6–7) | 347 (15.5) | 25 (3.1) | 242 (46.6) | 56 (13.7) | 2 (0.5) | ||
| Very high (8–9) | 65 (2.9) | 3 (0.4) | 49 (9.4) | 7 (1.7) | 0 (0.0) | ||
| BMI, kg/m2 | 24.6 ± 3.4 | 24.2 ± 3.3 | 25.2 ± 3.2 | 25.1 ± 3.5 | 23.5 ± 3.0 | < 0.001* | |
| BMI category, kg/m2 | < 0.001† | ||||||
| < 18.5 (underweight) | 54 (2.4) | 27 (3.4) | 5 (1.0) | 9 (2.2) | 8 (2.2) | ||
| 18.5 to 22.9 (normal) | 654 (29.6) | 250 (31.4) | 118 (23.0) | 95 (23.5) | 163 (44.8) | ||
| 23.0 to < 24.9 (overweight) | 585 (26.4) | 215 (27.0) | 153 (29.8) | 91 (22.5) | 86 (23.6) | ||
| 25.0 to 30.0 (obese I) | 778 (35.2) | 272 (34.2) | 189 (36.8) | 175 (43.2) | 96 (26.4) | ||
| ≥ 30.0 (obese II) | 142 (6.4) | 32 (4.0) | 49 (9.5) | 35 (8.6) | 11 (3.0) | ||
| BP variables, mmHg | |||||||
| Systolic BP | 127.8 ± 16.2 | 123.4 ± 14.2 | 134.3 ± 18.5 | 127.8 ± 15.9 | 128.3 ± 13.3 | < 0.001* | |
| Diastolic BP | 77.0 ± 11.1 | 75.6 ± 10.1 | 75.7 ± 11.7 | 77.7 ± 11.5 | 81.0 ± 10.4 | < 0.001* | |
| Pulse pressure | 50.8 ± 12.2 | 47.8 ± 10.6 | 58.5 ± 13.4 | 50.1 ± 11.3 | 47.3 ± 9.8 | < 0.001* | |
| Creatinine, mg/dL | 1.8 ± 1.1 | 1.6 ± 1.0 | 2.4 ± 1.3 | 2.0 ± 1.2 | 1.3 ± 0.9 | < 0.001* | |
| Cystatin C, mg/L | 1.76 ± 0.92 | 1.57 ± 0.85 | 2.27 ± 0.97 | 1.92 ± 0.85 | 1.30 ± 0.74 | < 0.001* | |
| eGFR, mL/min/1.73 m2 | |||||||
| MDRD study equation | 50.5 ± 30.3 | 56.9 ± 31.8 | 35.2 ± 20.6 | 40.8 ± 21.2 | 68.1 ± 33.3 | < 0.001* | |
| CKD-EPI creatinine equation | 53.1 ± 30.7 | 60.1 ± 31.4 | 36.6 ± 21.9 | 42.3 ± 21.7 | 72.9 ± 32.9 | < 0.001* | |
| CKD-EPI cystatin equation | 52.6 ± 32.4 | 60.1 ± 33.0 | 34.5 ± 21.9 | 42.1 ± 23.1 | 74.0 ± 35.5 | < 0.001* | |
| Urine protein/24 hr, mg/day | 1,353 ± 2,139 | 1,292 ± 1,524 | 2,664 ± 3,168 | 766 ± 1,133 | 178 ± 256 | < 0.001* | |
| ACEI or ARB therapy | 1,907 (85.4) | 726 (89.7) | 448 (86.8) | 333 (81.6) | 285 (78.3) | < 0.001† | |
| CKD stage | < 0.001† | ||||||
| Stage 1 | 265 (11.8) | 131 (16.2) | 13 (2.5) | 11 (2.7) | 94 (25.8) | ||
| Stage 2 | 419 (18.7) | 180 (22.2) | 44 (8.5) | 48 (11.7) | 118 (32.4) | ||
| Stage 3a | 403 (18.0) | 163 (20.1) | 72 (13.9) | 95 (23.2) | 52 (14.3) | ||
| Stage 3b | 484 (21.6) | 158 (19.5) | 131 (25.2) | 112 (27.4) | 45 (12.4) | ||
| Stage 4 | 522 (23.3) | 138 (17.0) | 198 (38.2) | 118 (28.9) | 42 (11.5) | ||
| Stage 5 | 145 (6.5) | 40 (4.9) | 61 (11.8) | 25 (6.1) | 13 (3.6) | ||
Values are mean ± SD or No. (%).
KNOW-CKD = KoreaN Cohort Study for Outcomes in Patients With Chronic Kidney Disease, SD = standard deviation, GN = glomerulonephritis, DN = diabetic nephropathy, HTN = hypertensive nephropathy, PKD = polycystic kidney, KRW = South Korean Won rates, CCI = Charlson comorbidity index, BMI = body mass index, BP = blood pressure, eGFR = estimated glomerular filtration rate, MDRD = Modification of Diet in Renal Disease, CKD-EPI = chronic kidney disease epidemiology collaboration, ACEI = angiotensin converting enzyme inhibitor, ARB = angiotensin receptor blocker, CKD = chronic kidney disease.
*P value evaluated by Kruskal-Wallis test; †P value evaluated by χ2 test; ‡P value evaluated by Fisher's exact test.
Mean eGFR calculated by MDRD, chronic kidney disease epidemiology collaboration (CKD-EPI) creatinine equation, and CKD-EPI cystatin equation for the cohort were 50.5 ± 30.3 mL/min/1.73 m2, 53.1 ± 30.7 mL/min/1.73 m2, and 52.6 ± 32.4 mL/min/1.73 m2, respectively. Mean cystatin C level was 1.76 ± 0.92 mg/L. Mean proteinuria was 1,353 ± 2,139 mg per 24 hours.
Baseline characteristics by specific cause of CKD
The baseline characteristics according to specific cause of CKD were reported in Table 2. The patients were categorized into GN (n = 810, 36.2%), DN (n = 519, 23.2%), HTN (n = 409, 18.3%), PKD (n = 364, 16.3%), and unclassified cause (n = 136, 6.1%). The proportion of men was higher in GN, DN, and HTN subgroups (GN, 55.7%; DN, 68.8%; HTN, 72.1%). Mean age was highest in HTN (59.6 ± 10.8 years), and followed by DN (59.3 ± 9.4 years), GN (49.8 ± 12.1 years) and PKD (47.0 ± 10.6 years). The modified CCI was the highest in the patients with DN and lowest in the GN and PKD subgroups. In particular, the prevalence of cardiovascular comorbidities such as coronary artery diseases, PVD, cerebrovascular disease, and congestive heart failure were the highest in DN subgroup, respectively. The percentage of advanced CKD (stage 4 and 5) was the highest in DN group and the lowest in PKD. The level of albuminuria and proteinuria were the highest in DN groups.
Baseline characteristics across CKD stages
Table 3 summarizes the baseline characteristics of the total participants across CKD stages. CKD stages were categorized on the basis of eGFR calculated by MDRD formula using IDMS-calibrated creatinine level. The largest number of the participants were categorized in CKD stage 4 (n = 522, 23.3%). The patients with advanced stages of CKD had a tendency to be older, or to have a lower income level and lower education attainment in comparison with those with early CKD. The relationships between advanced CKD and a higher prevalence of comorbidities including hypertension, diabetes, cerebrovascular disease, peripheral vascular disease and congestive heart failure were also remarkable. Age-adjusted modified CCI tended to be higher in advanced stages of CKD. Individuals with advanced CKD tended to have higher systolic BP and pulse pressure. Among the CKD stage 1, 2, 3a, and 3b, GN was the most common subgroup, while DN was the most common among individuals with stage 4 and 5 CKD. There was increased amount of albuminuria and proteinuria, with advanced stage of CKD.
Table 3. Baseline demographic and clinical characteristics of KNOW-CKD participants according to CKD stages.
| Characteristics | CKD stage | P | P for trend | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Stage 1 (n = 265) | Stage 2 (n = 419) | Stage 3a (n = 403) | Stage 3b (n = 484) | Stage 4 (n = 522) | stage 5 (n = 145) | ||||
| Age, yr | 43.2 ± 11.7 | 50.5 ± 11.7 | 54.9 ± 11.7 | 56.7 ± 11 | 57.2 ± 11.1 | 55.7 ± 11.4 | < 0.001* | < 0.001 | |
| Age (category), yr | 20 to 29 | 37 (14.0) | 16 (3.8) | 8 (2.0) | 5 (1.0) | 9 (1.7) | 2 (1.4) | < 0.001† | < 0.001 |
| 30 to 39 | 69 (26.0) | 63 (15.0) | 43 (10.7) | 29 (6.0) | 32 (6.1) | 12 (8.3) | |||
| 40 to 49 | 74 (27.9) | 106 (25.3) | 70 (17.4) | 96 (19.8) | 79 (15.1) | 24 (16.6) | |||
| 50 to 59 | 59 (22.3) | 136 (32.5) | 126 (31.3) | 138 (28.5) | 167 (32.0) | 45 (31.0) | |||
| 60 to 69 | 24 (9.1) | 80 (19.1) | 115 (28.5) | 150 (31.0) | 160 (30.7) | 49 (33.8) | |||
| 70 to 75 | 2 (0.8) | 18 (4.3) | 41 (10.2) | 66 (13.6) | 75 (14.4) | 13 (9.0) | |||
| Gender | < 0.001† | 0.719 | |||||||
| Male | 127 (47.9) | 277 (66.1) | 276 (68.5) | 307 (63.4) | 314 (60.2) | 68 (46.9) | |||
| Female | 138 (52.1) | 142 (33.9) | 127 (31.5) | 177 (36.6) | 208 (39.8) | 77 (53.1) | |||
| Monthly income, KRW | < 0.001† | ||||||||
| < 1,500,000 | 70 (27.9) | 108 (26.7) | 98 (25.0) | 101 (21.5) | 96 (18.9) | 18 (12.8) | |||
| 1,500,000 to 4,500,000 | 146 (58.2) | 230 (56.9) | 222 (56.6) | 256 (54.5) | 245 (48.1) | 76 (53.9) | |||
| > 4,500,000 | 35 (13.9) | 66 (16.3) | 72 (18.4) | 113 (24.0) | 168 (33.0) | 47 (33.3) | |||
| Educational attainment | < 0.001† | ||||||||
| Illiteracy | 1 (0.4) | 2 (0.5) | 3 (0.7) | 4 (0.8) | 4 (0.8) | 1 (0.7) | |||
| Graduated elementary school | 9 (3.8) | 26 (7.2) | 33 (9.4) | 56 (13.1) | 67 (14.7) | 22 (17.6) | |||
| Graduated middle school | 19 (8.1) | 31 (8.6) | 30 (8.5) | 61 (14.3) | 64 (14.0) | 17 (13.6) | |||
| Graduated high school | 78 (33.1) | 103 (28.5) | 113 (32.1) | 150 (35.1) | 160 (35.1) | 49 (39.2) | |||
| Graduated college or more | 129 (48.7) | 199 (47.5) | 173 (42.9) | 156 (32.2) | 161 (30.8) | 36 (24.8) | |||
| Smoking | < 0.001† | 0.009 | |||||||
| Never smoker | 173 (65.3) | 226 (54.1) | 194 (48.4) | 253 (52.5) | 271 (52.2) | 85 (59.4) | |||
| Current smoker | 42 (15.8) | 69 (16.5) | 78 (19.5) | 66 (13.7) | 76 (14.6) | 18 (12.6) | |||
| Former smoker | 50 (18.9) | 123 (29.4) | 129 (32.2) | 163 (33.8) | 172 (33.1) | 40 (28.0) | |||
| Comorbid disease | |||||||||
| Coronary artery disease | 2 (0.8) | 10 (2.4) | 17 (4.2) | 33 (6.8) | 48 (9.2) | 8 (5.5) | < 0.001† | < 0.001 | |
| Peripheral vascular disease | 3 (1.1) | 7 (1.7) | 18 (4.5) | 16 (3.3) | 29 (5.6) | 5 (3.4) | 0.006† | 0.002 | |
| Cerebrovascular disease | 6 (2.3) | 15 (3.6) | 28 (6.9) | 32 (6.6) | 45 (8.6) | 9 (6.2) | 0.003† | < 0.001 | |
| Diabetes | 40 (15.1) | 87 (20.8) | 119 (29.5) | 192 (39.7) | 249 (47.7) | 68 (46.9) | < 0.001† | < 0.001 | |
| Hypertension | 225 (84.9) | 399 (95.2) | 394 (97.8) | 475 (98.1) | 515 (98.7) | 142 (97.9) | < 0.001† | < 0.001 | |
| Congestive heart failure | 1 (0.4) | 2 (0.5) | 1 (0.2) | 7 (1.4) | 19 (3.6) | 4 (2.8) | 0.001‡ | < 0.001 | |
| Arrhythmia | 5 (1.9) | 9 (2.1) | 11 (2.7) | 9 (1.9) | 19 (3.6) | 3 (2.1) | 0.498† | 0.157 | |
| Age-adjusted modified CCI | < 0.001† | < 0.001 | |||||||
| Low (≤ 3) | 257 (97.0) | 352 (84.0) | 186 (46.2) | 176 (36.4) | 155 (29.7) | 48 (33.1) | |||
| Moderate (4–5) | 7 (2.6) | 54 (12.9) | 141 (35.0) | 185 (38.2) | 207 (39.7) | 58 (40.0) | |||
| High (6–7) | 1 (0.4) | 11 (2.6) | 63 (15.6) | 109 (22.5) | 131 (25.1) | 32 (22.1) | |||
| Very high (8–9) | 0 (0.0) | 2 (0.5) | 13 (3.2) | 14 (2.9) | 29 (5.6) | 7 (4.8) | |||
| Etiology of CKD | < 0.001† | 0.011 | |||||||
| GN | 131 (49.4) | 180 (43.0) | 163 (40.4) | 158 (32.6) | 138 (26.4) | 40 (27.6) | |||
| DN | 13 (4.9) | 44 (10.5) | 72 (17.9) | 131 (27.1) | 198 (37.9) | 61 (42.1) | |||
| HTN | 11 (4.2) | 48 (11.5) | 95 (23.6) | 112 (23.1) | 118 (22.6) | 25 (17.2) | |||
| PKD | 94 (35.5) | 118 (28.2) | 52 (12.9) | 45 (9.3) | 42 (8.0) | 13 (9.0) | |||
| Unclassified | 16 (6.0) | 29 (6.9) | 21 (5.2) | 38 (7.9) | 26 (5.0) | 6 (4.1) | |||
| BMI, kg/m2 | 24.2 ± 3.8 | 24.6 ± 3.4 | 24.7 ± 3.3 | 24.8 ± 3.2 | 24.4 ± 3.4 | 24.2 ± 3.4 | 0.068* | 0.838 | |
| BMI category, kg/m2 | 0.084† | 0.949 | |||||||
| < 18.5 (underweight) | 8 (3.0) | 8 (1.9) | 9 (2.3) | 8 (1.7) | 16 (3.1) | 5 (3.5) | |||
| 18.5 to 22.9 (normal) | 97 (36.6) | 123 (29.6) | 103 (26.1) | 120 (25.2) | 164 (31.6) | 47 (32.9) | |||
| 23.0 to < 24.9 (overweight) | 60 (22.6) | 97 (23.4) | 116 (29.4) | 153 (32.1) | 123 (23.7) | 36 (25.2) | |||
| 25.0 to 30.0 (obese I) | 82 (30.9) | 159 (38.3) | 141 (35.8) | 169 (35.4) | 180 (34.7) | 47 (32.9) | |||
| ≥ 30.0 (obese II) | 18 (6.8) | 28 (6.7) | 25 (6.3) | 27 (5.7) | 36 (6.9) | 8 (5.6) | |||
| BP variables, mmHg | |||||||||
| Systolic BP | 126.6 ± 14.3 | 126.4 ± 14.7 | 126.5 ± 15.7 | 126.8 ± 15.5 | 129.7 ± 17.6 | 135.1 ± 20.0 | < 0.001* | < 0.001 | |
| Diastolic BP | 78.6 ± 10.5 | 78.3 ± 11.3 | 76.6 ± 10.3 | 76.0 ± 10.5 | 76.4 ± 12.1 | 77.4 ± 11.8 | 0.050* | 0.003 | |
| Pulse pressure | 48.0 ± 10.4 | 48.1 ± 9.8 | 49.9 ± 12.3 | 50.8 ± 12.2 | 53.4 ± 12.8 | 57.7 ± 14.8 | < 0.001* | < 0.001 | |
| Creatinine, mg/dL | 0.7 ± 0.1 | 1.0 ± 0.2 | 1.3 ± 0.2 | 1.7 ± 0.3 | 2.7 ± 0.6 | 4.8 ± 1.6 | < 0.001* | < 0.001 | |
| Cystatin C, mg/L | 0.75 ± 0.14 | 1.02 ± 0.20 | 1.33 ± 0.22 | 1.77 ± 0.33 | 2.61 ± 0.52 | 3.86 ± 0.71 | < 0.001* | < 0.001 | |
| eGFR, mL/min/1.73 m2 | |||||||||
| MDRD study equation | 110.9 ± 20.6 | 73.3 ± 8.6 | 52.2 ± 4.3 | 37.3 ± 4.2 | 23.1 ± 4.4 | 11.8 ± 2.4 | < 0.001* | < 0.001 | |
| CKD-EPI creatinine equation | 110.9 ± 10.4 | 80.9 ± 10.7 | 56 ± 5.2 | 39.2 ± 4.8 | 23.7 ± 4.8 | 11.8 ± 2.5 | < 0.001* | < 0.001 | |
| CKD-EPI cystatin equation | 109.5 ± 16.2 | 80.7 ± 19.2 | 55.7 ± 12.5 | 38.1 ± 10.2 | 22.9 ± 7.6 | 13.3 ± 3.2 | < 0.001* | < 0.001 | |
| Urine protein/24 hr, mg/day | 621 ± 1,084 | 753 ± 1,481 | 1,088 ± 1,814 | 1,338 ± 1,927 | 2,062 ± 2,645 | 2,717 ± 3,171 | < 0.001* | < 0.001 | |
| ACEI or ARB therapy | 205 (77.4) | 369 (88.3) | 354 (88.3) | 421 (87.2) | 437 (83.9) | 121 (84.0) | 0.006† | ||
| Lipid profile, mg/dL | |||||||||
| Total cholesterol | 185.3 ± 38.1 | 179.5 ± 35.7 | 173.3 ± 35.9 | 170.6 ± 39.3 | 169.6 ± 42.3 | 168.9 ± 42.8 | < 0.001* | < 0.001 | |
| LDL cholesterol | 107.3 ± 33.1 | 102.3 ± 30.5 | 96.5 ± 30.4 | 92.3 ± 30.2 | 92.7 ± 32.4 | 93.7 ± 34.4 | < 0.001* | < 0.001 | |
| HDL cholesterol | 56.9 ± 15.6 | 52.6 ± 15.6 | 49.6 ± 14.1 | 47.2 ± 14.3 | 45.3 ± 14.7 | 45.3 ± 16.6 | < 0.001* | < 0.001 | |
| Triglycerides | 136.7 ± 86.6 | 148.5 ± 84.3 | 158.1 ± 100.2 | 166.4 ± 106.9 | 168.1 ± 107.7 | 150.7 ± 77.5 | < 0.001* | < 0.001 | |
| Hemoglobin, g/dL | 14.0 ± 1.5 | 14.1 ± 1.7 | 13.5 ± 1.9 | 12.7 ± 1.8 | 11.5 ± 1.5 | 10.5 ± 1.2 | < 0.001* | < 0.001 | |
| Serum calcium, mg/dL | 9.2 ± 0.4 | 9.3 ± 0.4 | 9.2 ± 0.4 | 9.2 ± 0.5 | 9.0 ± 0.5 | 8.5 ± 0.8 | < 0.001* | < 0.001 | |
| Serum phosphorus, mg/dL | 3.5 ± 0.5 | 3.5 ± 0.6 | 3.5 ± 0.5 | 3.6 ± 0.6 | 3.9 ± 0.7 | 4.7 ± 0.8 | < 0.001* | < 0.001 | |
| iPTH, pg/mL | 40.2 ± 17.4 | 43.0 ± 21.1 | 51.9 ± 29.7 | 61.6 ± 31.2 | 104.7 ± 70.8 | 240.8 ± 214.1 | < 0.001* | < 0.001 | |
| 25(OH)D, ng/mL | 16.3 ± 6.4 | 18.5 ± 7.4 | 18.8 ± 8.0 | 18.0 ± 7.9 | 17.6 ± 8.6 | 16.3 ± 8.3 | < 0.001* | 0.066 | |
Values are mean ± SD or No. (%).
KNOW-CKD = KoreaN Cohort Study for Outcomes in Patients With Chronic Kidney Disease, SD = standard deviation, GN = glomerulonephritis, DN = diabetic nephropathy, HTN = hypertensive nephropathy, PKD = polycystic kidney, KRW = South Korean Won rates, BMI = body mass index, CCI = Charlson comorbidity index, BP = blood pressure, eGFR = estimated glomerular filtration rate, MDRD = Modification of Diet in Renal Disease, CKD-EPI = chronic kidney disease epidemiology collaboration, ACEI = angiotensin converting enzyme inhibitor, ARB = angiotensin receptor blocker, CKD = chronic kidney disease, LDL = low-density lipoprotein, HDL = high-density lipoprotein, iPTH = intact parathyroid hormone, 25(OH)D = 25-hydroxyvitamin D.
*P value evaluated by Kruskal-Wallis test; †P value evaluated by χ2 test; ‡P value evaluated by Fisher's exact test.
DISCUSSION
As the first and largest-scale long-term CKD cohort study in Korea, the KNOW-CKD has been established with 2,238 Korean CKD patients at stages 1 to 5 (predialysis). The KNOW-CKD study will provide the key clinical information regarding the baseline comorbidity, quality of life issues of CKD, socioeconomic disparities related to CKD, progression of CKD, diverse complications including mineral bone disorder, anemia, and cardiovascular complications, and adverse clinical outcomes of Asian CKD population. Various biomarkers from the serum, and urine samples will also be sought for, as well as genomic and epigenetic markers in order to identify the risk factors of adverse outcomes.
Particularly, the KNOW-CKD categorized the subjects into four etiologic disease subgroups—GN, DN, HTN, and PKD, based on the 4 most common etiologies of CKD in Korea. Other causes of CKD or unknown etiologic diseases were categorized as “unclassified.” One of the aims of the KNOW-CKD study is to compare the clinical courses and progression patterns of CKD between the four etiologic subgroups, the study oversampled HTN and PKD in order to establish a cohort in which all the 4 etiologic disease subgroups are similar in size. While other major CKD cohort studies dichotomized the total CKD patients into diabetic and nondiabetic subgroups, (6,12,14), the KNOW-CKD study subdivided the total CKD into four major etiologic causes.
Unlike recently established CKD cohorts elsewhere (12,13), the KNOW-CKD also comprises mild CKD patients at stages 1 and 2 (11.8% and 18.7%, respectively). Hence, unlike other cohort studies, it is relevant for the KNOW-CKD study to clarify natural course and progression of the early stage CKD. We hope that the KNOW-CKD will also elucidate how the mild CKD is clinically different from the moderate-to-severe CKD population.
Approximately, among the individuals of the KNOW-CKD cohort, 33.7% were diagnosed with diabetes (either type 1 or type 2), and 96.1% were with hypertension. The prevalence of diabetes and hypertension among the general population was 12.8% and 32.4% in men, 9.1% and 22.2% in women, respectively, according to Korean National Health and Nutrition Examination Survey (https://knhanes.cdc.go.kr/knhanes/index.do) in 2013. As expected, the prevalence of diabetes and hypertension in patients with CKD was higher than the general Korean population.
The baseline characteristics in KNOW-CKD are similar to other CKD cohort studies, but several important differences were observed. One of the major differences in baseline characteristics between KNOW-CKD and other western cohort studies is the ethnicity. CRIC populations consist of 45% white, 46% black, and 5% Hispanic (14), while the KNOW-CKD comprises only ethnic Koreans. In a previous study conducted in the United Kingdom, stage 3 CKD was less prevalent in South Asian groups compared to whites and black groups, however, stage 4 and 5 CKD was higher than the whites (21). There is a lack of studies regarding ethnicity and CKD prevalence. Therefore, the efforts for investigating the relationships between ethnicity and clinical course of CKD are warranted. The KNOW-CKD will provide reliable information about Asian CKD populations.
Compared to CRIC study, male patients were more common in Asian cohort studies (61.2% were male in KNOW-CKD, 62.1% in Chronic Kidney Disease Japan Cohort [CKD-JAC], 54% in CRIC) (12,14). The proportion of diabetes within the KNOW-CKD and CRIC were 33.7% and 46.6%, respectively. It is well known that obesity is 1 of the independent risk factors for CKD progression (22,23,24). In the CRIC, the mean BMI of total subjects (32.1 ± 7.9 kg/m2 vs. 24.6 ± 3.4 kg/m2) and the proportion of individuals with BMI > 25 kg/m2 was higher than KNOW-CKD participant (83.0% vs. 41.6%). The subjects in the CRIC study are more obese and more likely to have diabetes at enrollment than those in the KNOW-CKD.
When we compared the baseline characteristics with CKD-JAC (12) which was conducted in Japan, the KNOW-CKD subjects are younger (53.7 ± 12.2 vs. 60.8 ± 11.6 years) and the serum creatinine level is lower (1.8 ± 1.1 vs. 2.15 ± 1.06 mg/dL). eGFR was higher in Korean patients because of enrollment of CKD stages 1 and 2 in KNOW-CKD cohort, compared with CKD-JAC study which included only CKD stages from 3 to 5.
The age distribution was different among the 4 etiologic subgroups of CKD. Subjects in the GN or PKD subgroups were younger than DN and HTN subgroups. These two subgroups exhibited lower comorbidity score such as coronary artery disease, peripheral vascular disease, cerebrovascular disease, hypertension, diabetes, and congestive heart failure. The proportion of current smokers was higher in DN and HTN group.
Since the KNOW-CKD includes only ethnic Korean CKD population, it cannot analyze the differences between various ethnic groups. As a hospital-based cohort, it included only patients referred to nephrologists, which may be different from community-based CKD population.
Planned long-term follow-up will be conducted and the accurate information on the exposures and events will be captured prospectively. International collaborations with other cohort study investigators such as CRIC, and CKD-JAC will be undertaken in order to expand the size of the CKD population under investigation, to facilitate scientific discovery and to generalize the scientific findings by validating them in another ethnic groups.
To conclude, the KNOW-CKD cohort study will provide new insights regarding the natural course and clinical outcomes of CKD in Asian populations. Additionally, the knowledge discovered from KNOW-CKD cohort study will help to find modifiable factors for prevention of CKD progression and adverse outcomes.
ACKNOWLEDGMENT
We also thank the clinical research coordinators of each participating institution and the Medical Research Collaborating Center, Seoul National University Hospital for the data management and data quality control. This is a group study by multi-center collaboration by theKNOW-CKD Investigator Group.
Patient recruitment. Seoul National University Hospital, Curie Ahn, MD (PI), Kook-Hwan Oh, MD (SubPI), Hajeong Lee, MD, Seungmi Lee, RN, Jiseon Kim, RN, and Aram Lee, RN. Seoul National University Bundang Hospital, Dong Wan Chae, MD (SubPI), Seon Ha Baek MD, and Hyun Jin Cho, RN. Yonsei University, Severance Hospital, Kyu Hun Choi, MD (SubPI), Seung Hyeok Han, MD, Tae Hyun Yoo, MD, and Mi Hyun Yu, RN. Kangbuk Samsung Hospital, Kyu-Beck Lee, MD and Young Youl Hyun, MD. The Catholic University of Korea, Seoul St. Mary's Hospital, Yong-Soo Kim, MD and Min Jung Ahn, RN. Gachon University, Gil Hospital, Wookyung Chung, MD, Ji Yong Jung, MD, Youkyoung Jang, RN, and Ji Hye Park, RN. Eulji Medical Center, Eulji University, Suah Sung, MD, Sung Woo Lee, MD, and Min A Yoo, RN. Chonnam National University Hospital, Soo Wan Kim, MD, Seong Kwon Ma, MD, Eun Hui Bae, MD, Chang Seong Kim, MD, Yong Un Kang, MD, Ha Yeon Kim, MD, and Ji Seon Lee, RN. Inje University, Pusan Paik Hospital, Yeong Hoon Kim, MD, Sun Woo Kang, MD, Tae Hee Kim, MD and A Jin Son, RN.
Polycystic kidney disease research. Seoul National University Boramae Medical Center, Yun Kyu Oh, MD (SubPI). Truewords Dialysis Clinic, Young-Hwan Hwang, MD
Epidemiology and biostatistics. Department of Preventive Medicine, Seoul National University College of Medicine, Byung-Joo Park, MD, Sue K. Park, MD and Ju Yeon Lee.
Data coordinating center. Medical Research Collaborating Center, Seoul National University Hospital and Seoul National University College of Medicine, Joongyub Lee, MD, Dayeon Nam, RN, Soohee Kang, MSc and Heejung Ahn, RN. Central Laboratory. Dong Hee Seo, MD, and Dae Yeon Cho, PhD, LabGenomics, Korea.
Biobank. Korea Biobank, Korea Centers for Disease Control and Prevention.
Korea Center for Disease Control and Prevention. Un Yeong Go, Yeong Taek Kim, Hyejin Lee, Eun Mi Ahn and Seo Heui Jeon.
Footnotes
Funding: This study was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (2011E3300300, 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, and 2016E3300200).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Park SK, Ahn C, Oh KH. Data curation: Han M, Kim H, Lee J, Hyun YY, Kim YS, Chung W, Kim HJ. Investigation: Kang E, Han M, Kim H, Park SK, Lee J, Kim HJ, Oh YK, Oh KH. Writing - original draft: Kang E, Oh KH. Writing - review & editing: Kang E, Han M, Kim H, Park SK, Lee J, Hyun YY, Kim YS, Chung W, Kim HJ, Oh YK, Ahn C, Oh KH.
References
- 1.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Taskapan H, Tam P, Au V, Chow S, Fung J, Nagai G, Roscoe J, Ng P, Sikaneta T, Ting R, et al. Improvement in eGFR in patients with chronic kidney disease attending a nephrology clinic. Int Urol Nephrol. 2008;40:841–848. doi: 10.1007/s11255-008-9360-9. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura S, Nakata H, Yoshihara F, Kamide K, Horio T, Nakahama H, Kawano Y. Effect of early nephrology referral on the initiation of hemodialysis and survival in patients with chronic kidney disease and cardiovascular diseases. Circ J. 2007;71:511–516. doi: 10.1253/circj.71.511. [DOI] [PubMed] [Google Scholar]
- 5.Jones C, Roderick P, Harris S, Rogerson M. Decline in kidney function before and after nephrology referral and the effect on survival in moderate to advanced chronic kidney disease. Nephrol Dial Transplant. 2006;21:2133–2143. doi: 10.1093/ndt/gfl198. [DOI] [PubMed] [Google Scholar]
- 6.Levin A, Rigatto C, Brendan B, Madore F, Muirhead N, Holmes D, Clase CM, Tang M, Djurdjev O. CanPREDDICT investigators. Cohort profile: Canadian study of prediction of death, dialysis and interim cardiovascular events (CanPREDDICT) BMC Nephrol. 2013;14:121. doi: 10.1186/1471-2369-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricardo AC, Lash JP, Fischer MJ, Lora CM, Budoff M, Keane MG, Kusek JW, Martinez M, Nessel L, Stamos T, et al. Cardiovascular disease among hispanics and non-hispanics in the chronic renal insufficiency cohort (CRIC) study. Clin J Am Soc Nephrol. 2011;6:2121–2131. doi: 10.2215/CJN.11341210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehrotra R, Kermah D, Fried L, Adler S, Norris K. Racial differences in mortality among those with CKD. J Am Soc Nephrol. 2008;19:1403–1410. doi: 10.1681/ASN.2007070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez CJ, Sciacca RR, Diez-Roux AV, Boden-Albala B, Sacco RL, Homma S, DiTullio MR. Relation between socioeconomic status, race-ethnicity, and left ventricular mass: the Northern Manhattan study. Hypertension. 2004;43:775–779. doi: 10.1161/01.HYP.0000118055.90533.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutiérrez OM, Anderson C, Isakova T, Scialla J, Negrea L, Anderson AH, Bellovich K, Chen J, Robinson N, Ojo A, et al. Low socioeconomic status associates with higher serum phosphate irrespective of race. J Am Soc Nephrol. 2010;21:1953–1960. doi: 10.1681/ASN.2010020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai E, Matsuo S, Makino H, Watanabe T, Akizawa T, Nitta K, Iimuro S, Ohashi Y, Hishida A, CKD-JAC Study Group Chronic kidney disease Japan cohort (CKD-JAC) study: design and methods. Hypertens Res. 2008;31:1101–1107. doi: 10.1291/hypres.31.1101. [DOI] [PubMed] [Google Scholar]
- 12.Imai E, Matsuo S, Makino H, Watanabe T, Akizawa T, Nitta K, Iimuro S, Ohashi Y, Hishida A. Chronic kidney disease Japan cohort study: baseline characteristics and factors associated with causative diseases and renal function. Clin Exp Nephrol. 2010;14:558–570. doi: 10.1007/s10157-010-0328-6. [DOI] [PubMed] [Google Scholar]
- 13.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, et al. The Chronic renal insufficiency cohort (CRIC) study: design and methods. J Am Soc Nephrol. 2003;14:S148–53. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 14.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, et al. Chronic renal insufficiency cohort (CRIC) study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh KH, Park SK, Park HC, Chin HJ, Chae DW, Choi KH, Han SH, Yoo TH, Lee K, Kim YS, et al. KNOW-CKD (KoreaN cohort study for outcome in patients with chronic kidney disease): design and methods. BMC Nephrol. 2014;15:80. doi: 10.1186/1471-2369-15-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 18.Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, Parfrey P, Cramer B, Coto E, Torra R, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20:205–212. doi: 10.1681/ASN.2008050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Park HJ, Kim S, Yong JS, Han SS, Yang DH, Meguro M, Han CW, Kohzuki M. Reliability and validity of the Korean version of kidney disease quality of life instrument (KDQOL-SF) Tohoku J Exp Med. 2007;211:321–329. doi: 10.1620/tjem.211.321. [DOI] [PubMed] [Google Scholar]
- 21.Hull S, Dreyer G, Badrick E, Chesser A, Yaqoob MM. The relationship of ethnicity to the prevalence and management of hypertension and associated chronic kidney disease. BMC Nephrol. 2011;12:41. doi: 10.1186/1471-2369-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 23.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 24.Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D. Obesity and prevalent and incident CKD: the hypertension detection and follow-up program. Am J Kidney Dis. 2005;46:587–594. doi: 10.1053/j.ajkd.2005.06.007. [DOI] [PubMed] [Google Scholar]