Abstract
Adverse changes in nutrition are prevalent and are strong indicators of adverse outcomes in patients with chronic kidney disease (CKD). The International Society of Renal Nutrition and Metabolism (ISRNM) proposed a common nomenclature and diagnostic criteria to identify protein-energy wasting (PEW) in CKD patients. We examined the nutritional status in 1,834 adults with predialysis CKD enrolled in the KoreaN cohort study for Outcome in patients With Chronic Kidney Disease (KNOW-CKD) study. As there was a need for further understanding of nutritional status and associated factors in CKD, we evaluated the prevalence and associated factors of PEW in adults with predialysis CKD. The prevalence of PEW was about 9.0% according to ISRNM criteria and tended to increase with advanced stage in predialysis CKD. Those who concurrently had PEW, inflammation, and CVD were a small proportion (0.4%). In multivariate logistic regression model, PEW was independently associated with estimated glomerular filtration rate (eGFR) (odds ratio [OR], 0.98; 95% confidence interval [CI], 0.96–0.99), total CO2 (OR, 0.93; 95% CI, 0.87–0.99), physical activity (OR, 0.43; 95% CI, 0.26–0.69), comorbid diabetes (OR, 1.68; 95% CI, 1.09–2.59), and high sensitivity C-reactive protein (hs-CRP) (OR, 1.03; 95% CI, 1.01–1.06). Our study suggests that PEW increases with advanced CKD stage. PEW is independently associated with renal function, low total CO2, low physical activity, comorbid diabetes, and increased hs-CRP in adults with predialysis CKD.
Keywords: Nutrition, Protein-Energy Wasting, Inflammation, Chronic Kidney Disease
INTRODUCTION
Chronic kidney disease (CKD) is recognized as a global health problem (1). The health and economic burdens of CKD are high. Patients with CKD are at increased risk for cardiovascular disease (CVD) and end-stage renal failure. Adverse changes in nutrition are prevalent with decreasing renal function and are a strong indicator of adverse outcomes in patients with CKD (2).
Multiple mechanisms are involved in adverse nutritional changes in CKD patients. Poor food intake caused by uremia-induced anorexia alone does not explain adverse nutritional changes. There are additional contributing causes, such as persistent inflammation, acidosis, multiple hormonal disturbance, comorbidities, and physical inactivity (3). Many studies about nutritional status of CKD patients revealed that wasting and protein-energy abnormalities might be induced by inflammatory processes (2). Simple malnutrition refers to nutritional abnormalities induced by inadequate nutritional intake, whereas wasting caused by CKD refers to nutritional abnormalities that may be associated with inflammation and cannot be corrected solely by increasing the diet. The International Society of Renal Nutrition and Metabolism (ISRNM) (4) proposed a common nomenclature and diagnostic criteria for this adverse nutritional status in the context of CKD. Protein-energy wasting (PEW) was proposed to denote concurrent losses in protein and energy stores in CKD patients.
PEW is a strong indicator of adverse outcome and is associated with CVD. Persistent inflammatory processes in CKD could act as links between inflammation, PEW, and CVD. Several studies (5,6,7,8) have reported that strong interactions exist among inflammatory parameters, nutritional parameters, and CVD in CKD patients.
In this study, we examined the nutritional status of predialysis CKD in adults enrolled in the KoreaN cohort study for Outcome in patients with Chronic Kidney Disease (KNOW-CKD) study. As there was a need for further understanding of nutritional status and associated factors in CKD, we described nutritional status, inflammatory markers, CVD, and other clinical characteristics according to estimated glomerular filtration rate (eGFR) and PEW parameters. We also explored factors associated with PEW in adults with predialysis CKD.
MATERIALS AND METHODS
Study participants
The KNOW-CKD study is an ongoing multicenter prospective, observational study of adults with CKD in Korea. The study design was published previously (9), and is summarized here. Each participating center enrolled a baseline of 2,238 adults with CKD from 2011 to 2015. All enrolled participants were ethnic Korean patients between 20 and 75 years old with predialysis CKD stage 1 to 5, which was staged based on eGFR. Those who had a history of malignancy, liver cirrhosis, advanced heart failure, or who had a single kidney were excluded according to the KNOW-CKD protocol. We included a total of 1,834 participants in the analysis who underwent complete 24-hour urine chemistry, serum high sensitivity C-reactive protein (hs-CRP) concentration measurement, and baseline laboratory tests. All included patients also completed a health questionnaire
PEW parameters and definition
Using data from the initial KNOW-CKD study, indicators for PEW were created based on the ISRNM diagnostic criteria (4). An ISRNM expert panel recommended four main categories for diagnosing PEW: biochemical parameter, low body mass, decreased muscle mass, and low protein intake. PEW parameters and diagnostic criteria in this study included the following: 1) Biochemical parameter: serum albumin < 3.8 g/dL; 2) Body mass: body mass index (BMI) < 23.0 kg/m2; 3) Muscle mass: 24-hour urine creatinine excretion (UCE) < the sex-specific lower quartile; and 4) Dietary intake: dietary protein intake (DPI) < 0.6 g/kg/day. Three or more of these indicators must be present for a diagnosis of PEW. Twenty-four-hour urine collection began after the bladder was emptied in the morning and continued for 24 hours, including the last urination the following morning. We excluded patients from whom less than 500 mL/day of urine was collected, because of concerns regarding urine collection errors. UCE and urine urea excretion were measured. UCE was evaluated according to sex. DPI was calculated using the following formula (10,11):
6.25 × ([24-hour urine urea nitrogen excreted in grams] + [weight in kilograms × 0.031 g nitrogen/kg/day]) + 24-hour urine proteinuria (g/day, if urinary protein loss > 5 g/day)
Other variables
Data regarding demographics, medical history, causes of CKD and comorbid conditions were collected at baseline by a well-trained study coordinator using a standardized case report form and protocol. Serum samples were collected at baseline according to our standardized protocol and sent to a central laboratory for measurement. Serum creatinine concentration was measured using an isotope-dilution mass spectrometry-traceable method. eGFR was calculated using the 4-variable Modification of Diet in Renal Disease (MDRD) formula as follows (12):
eGFR mL/min/1.73 m2 = 175 × serum creatinine (mg/dL)−1.154 × age−0.203 × 0.742 (if female)
Inflammatory marker was investigated by measuring hs-CRP. Inflammation was defined as hs-CRP > 3 mg/L (13). A patient was defined as an active smoker if a person currently smoked. Alcohol drinking was defined as having more than 2 times of standard drinking (about one bottle of beer, 14 grams of alcohol)/week. Physical activity for health was defined as more than 150 min/week moderate activity, or 75 min/week vigorous activity, or an equivalent combination according to global recommendations on physical activity for health of World Health Organization (14). Cardiovascular disease was defined as having medical history of myocardial infarction, heart failure, peripheral vascular disease, or stroke.
Statistical analysis
Data were expressed as a percentage for categorical variables and as the mean ± standard deviation (SD) for continuous variables. We used χ2 test for categorical variables and the analysis of variance (ANOVA) for continuous variables to show differences in baseline characteristics according to CKD stage and PEW score. The covariates for PEW were selected according to our baseline data and other PEW studies of CKD. In logistic analyses of PEW, the associated factors were expressed as odds ratios (ORs) and 95% confidence intervals (CIs).
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) PASW statistics version 18.0 (IBM, Armonk, NY, USA).
Ethics statement
The study protocol was approved by the relevant ethics committees, and all participants were informed of their inclusion in this registry. The KNOW-CKD study was supervised by the Korea Centers for Disease Control and Prevention. The design of the present study was approved by Institutional Review Board of the Kangbuk Samsung Hospital (2011-01-076-011).
RESULTS
Nutritional and inflammatory status
A total of 1,834 adults with predialysis CKD were included in the study. The mean age was 53.9 ± 12.2 years old, and 60.4% were male. In the study population, 34.5% had diabetes and 96.1% had hypertension.
Characteristics of the patients are shown in Tables 1 and 2. Those with an advanced CKD stage tended to have more CVD, lower Total CO2, and greater weight loss. Inflammation prevalence and neutrophil percent increased with CKD stage. PEW prevalence was 2.2%, 4.4%, 8.3%, 6.2%, 15.6%, and 24.6% in CKD stage 1, 2, 3a, 3b, 4, and 5, respectively. Patients with an advanced eGFR stages had lower serum albumin, lower UCE, and lower DPI (Table 1). Those with more PEW parameters tended to have advanced CKD, diabetes, and CVD. They also had lower TCO2, low physical activity, and higher weight loss. The hs-CRP were 1.84 ± 4.61, 1.79 ± 5.01, 2.32 ± 5.32, and 2.82 ± 7.65 mg/L in patients with 0, 1, 2, ≥ 3 PEW parameters, respectively (Table 2).
Table 1. Characteristics of 1,834 adults with predialysis CKD based on eGFR stage.
| Characteristics | All (n = 1,834) | eGFR stage, mL/min/1.73 m2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Stage 1 (³ 90) (n = 231) |
Stage 2 (60–89) (n = 339) |
Stage 3a (45–59) (n = 327) |
Stage 3b (30–44) (n = 405) |
Stage 4 (15–29) (n = 418) |
Stage 5 (< 15) (n = 114) |
P value | ||
| Age, yr | 53.9 ± 12.2 | 43.7 ± 11.9 | 50.9 ± 11.6 | 54.8 ± 11.7 | 57.2 ± 10.7 | 57.3 ± 11.0 | 55.9 ± 11.5 | < 0.001 |
| Male sex | 60.4 | 46.8 | 65.8 | 67.6 | 62.5 | 60.5 | 43.0 | 0.872 |
| eGFR, mL/min/1.73 m2 | 51 ± 30 | 110 ± 20 | 73 ± 9 | 52 ± 4 | 37 ± 4 | 23 ± 4 | 12 ± 2 | < 0.001 |
| 24-hr UP, g/day | 1,340 ± 2,137 | 615 ± 1,103 | 723 ± 1,399 | 1,089 ± 1,829 | 1,307 ± 1,897 | 2,092 ± 2,682 | 2,734 ± 3,199 | < 0.001 |
| Diabetes | 34.5 | 15.7 | 21.4 | 30.9 | 40.7 | 48.1 | 50.0 | < 0.001 |
| Hypertension | 96.1 | 85.7 | 95.3 | 98.2 | 98.0 | 98.6 | 97.4 | < 0.001 |
| CVD | 11.1 | 3.9 | 7.1 | 11.6 | 13.8 | 16.0 | 8.8 | < 0.001 |
| Total CO2, meq/L | 25.7 ± 3.6 | 27.8 ± 2.9 | 27.7 ± 3.0 | 26.9 ± 3.0 | 25.3 ± 3.1 | 23.6 ± 3.2 | 21.7 ± 3.3 | < 0.001 |
| Active smoker | 15.5 | 16.0 | 17.1 | 18.0 | 12.8 | 15.6 | 11.4 | 0.163 |
| Alcohol consumption ³ 2 times/wk | 13.0 | 16.9 | 17.4 | 13.8 | 12.1 | 10.3 | 2.6 | < 0.001 |
| Physical activity | 38.4 | 40.8 | 48.0 | 49.2 | 40.2 | 35.5 | 38.4 | 0.015 |
| Weight loss > 3 kg/yr | 16.7 | 12.6 | 14.2 | 15.6 | 14.6 | 22.2 | 23.7 | < 0.001 |
| WBC, cell/mL | 6,579 ± 1,905 | 6,248 ± 1,730 | 6,377 ± 1,854 | 6,520 ± 1,889 | 6,635 ± 1,889 | 6,972 ± 1,994 | 6,372 ± 1,952 | 0.031 |
| Neutrophil | 58.2 ± 9.0 | 55.1 ± 9.6 | 57.0 ± 8.6 | 56.7 ± 8.6 | 58.9 ± 8.7 | 60.4 ± 8.6 | 62.2 ± 9.6 | < 0.001 |
| hs-CRP, mg/L | 2.00 ± 5.23 | 1.36 ± 4.80 | 1.59 ± 3.44 | 1.98 ± 5.57 | 1.87 ± 3.99 | 2.92 ± 7.33 | 1.71 ± 3.47 | 0.081 |
| hs-CRP > 3 mg/L | 13.6 | 10.0 | 9.7 | 11.3 | 15.8 | 18.7 | 13.2 | < 0.001 |
| PEW parameters | ||||||||
| Serum albumin, g/dL | 4.18 ± 0.42 | 4.13 ± 0.40 | 4.31 ± 0.34 | 4.20 ± 0.40 | 4.19 ± 0.37 | 4.02 ± 0.46 | 3.97 ± 0.44 | < 0.001 |
| Serum albumin < 3.8 g/dL | 16.0 | 9.5 | 5.0 | 14.4 | 14.1 | 27.3 | 31.6 | < 0.001 |
| BMI, kg/m2 | 24.58 ± 3.39 | 24.04 ± 3.75 | 24.76 ± 3.39 | 24.73 ± 3.28 | 24.74 ± 3.17 | 24.50 ± 3.40 | 24.38 ± 3.51 | 0.355 |
| BMI < 23.0 kg/m2 | 33.2 | 42.4 | 32.2 | 29.7 | 28.6 | 34.9 | 37.7 | 0.321 |
| UCE, g/day | 1,179 ± 416 | 1,202 ± 401 | 1,308 ± 453 | 1,243 ± 425 | 1,163 ± 402 | 1,088 ± 368 | 948 ± 341 | < 0.001 |
| Low quartile of UCE (sex-specific) | 25.0 | 13.9 | 19.2 | 24.4 | 25.4 | 34.4 | 43.0 | < 0.001 |
| DPI, g/kg/day | 0.80 ± 0.35 | 0.87 ± 0.28 | 0.86 ± 0.33 | 0.83 ± 0.36 | 0.81 ± 0.39 | 0.74 ± 0.36 | 0.65 ± 0.28 | < 0.001 |
| DPI < 0.6 g/kg/day | 26.5 | 16.5 | 20.4 | 25.7 | 23.7 | 34.2 | 49.1 | < 0.001 |
| PEW | 9.0 | 2.2 | 4.4 | 8.3 | 6.2 | 15.6 | 24.6 | < 0.001 |
Date are represented as mean ± standard deviation (SD) or percentage (%).
CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate, 24-hr UP = 24-hour urine protein, CVD = cardiovascular disease, WBC = white blood cell, hs-CRP = high sensitivity C-reactive protein, PEW = protein-energy wasting, BMI = body mass index, UCE = urine creatinine excretion, DPI = dietary protein intake.
Table 2. Characteristics of 1,834 adults with predialysis CKD according to numbers of PEW parameters.
| Characteristics | No. of PEW Parameters | ||||
|---|---|---|---|---|---|
| 0 (n = 694) | 1 (n = 611) | 2 (n = 364) | ≥ 3 (n = 165) | P for trend | |
| Age, yr | 54.3 ± 11.9 | 51.9 ± 12.1 | 55.4 ± 12.6 | 55.8 ± 11.7 | 0.069 |
| Male sex | 69.0 | 51.7 | 58.1 | 60.0 | 0.001 |
| eGFR, mL/min/1.73 m2 | 54 ± 28 | 56 ± 34 | 45 ± 29 | 34 ± 23 | < 0.001 |
| 24-hr UP, g/day | 975 ± 1,223 | 1,201 ± 2,065 | 1,712 ± 2,781 | 2,579 ± 3,056 | < 0.001 |
| Diabetes | 31.4 | 30.5 | 41.0 | 48.5 | < 0.001 |
| Hypertension | 97.6 | 93.5 | 97.0 | 97.6 | 0.799 |
| CVD | 8.4 | 10.3 | 15.2 | 17.0 | < 0.001 |
| Total CO2, meq/L | 26.1 ± 3.4 | 26.1 ± 3.5 | 25.2 ± 3.8 | 23.9 ± 3.6 | < 0.001 |
| Physical exercise | 49.7 | 41.5 | 37.1 | 25.4 | < 0.001 |
| Active smoking | 16.9 | 13.9 | 13.5 | 19.4 | 0.842 |
| Alcohol consumption ≥ 2 times/wk | 16.3 | 11.3 | 11.3 | 9.1 | 0.003 |
| Weight loss > 3 kg/yr | 13.5 | 17.5 | 18.7 | 23.0 | 0.001 |
| WBC, cell/mL | 6,628 ± 1,795 | 6,395 ± 1,930 | 6,663 ± 2,008 | 6,870 ± 1,994 | 0.246 |
| Neutrophil, % | 56.8 ± 9.1 | 58.5 ± 8.6 | 59.2 ± 9.3 | 61.1 ± 8.6 | < 0.001 |
| hs-CRP, mg/L | 1.84 ± 4.61 | 1.79 ± 5.01 | 2.32 ± 5.32 | 2.82 ± 7.65 | 0.021 |
| hs-CRP > 3 mg/L | 12.7 | 12.1 | 17.1 | 15.8 | 0.067 |
| PEW parameters | |||||
| Serum albumin, g/dL | 4.32 ± 0.28 | 4.19 ± 0.37 | 4.07 ± 0.47 | 3.74 ± 0.56 | < 0.001 |
| Serum albumin < 3.8 g/dL | 0 | 14.7 | 27.3 | 63.0 | < 0.001 |
| BMI, kg/m2 | 26.22 ± 2.47 | 23.76 ± 3.37 | 23.77 ± 3.80 | 22.46 ± 2.95 | < 0.001 |
| BMI < 23.0 kg/m2 | 0 | 49.1 | 53.2 | 70.3 | < 0.001 |
| UCE, g/day | 1,438 ± 389 | 1,159 ± 321 | 908 ± 313 | 756 ± 257 | < 0.001 |
| Low quartile of UCE (sex-specific) | 0 | 12.6 | 65.8 | 93.9 | < 0.001 |
| DPI, g/kg/day | 0.95 ± 0.36 | 0.83 ± 0.31 | 0.63 ± 0.28 | 0.48 ± 0.18 | < 0.001 |
| DPI < 0.6 g/kg/day | 0 | 23.6 | 53.7 | 88.5 | < 0.001 |
Date are represented as mean ± standard deviation (SD) or percentage (%).
CKD = chronic kidney disease, PEW = protein-energy wasting, eGFR = estimated glomerular filtration rate, 24-hr UP = 24-hour urine protein, CVD = cardiovascular disease, WBC = white blood cell, hs-CRP = high sensitivity C-reactive protein, BMI = body mass index, UCE = urine creatinine excretion, DPI = dietary protein intake.
Inflammation, CVD, and PEW
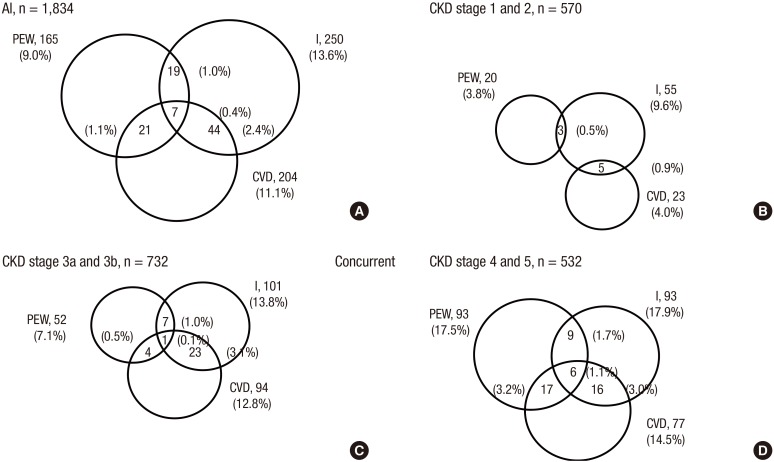
In the study population, 11.1% had CVD, 13.6% had inflammation, and 9.0% had PEW. The prevalence of PEW, inflammation, and CVD according CKD stage was shown in Fig. 1. Among patients with CKD 1 and 2 stages, prevalence was 3.8% for PEW, 9.6% for inflammation, and 4.0% for CVD. Among patient with advanced CKD 4 and 5 stages, prevalence was 17.5% for PEW, 17.9% for inflammation, and 14.5% for CVD. Those who had concurrently PEW, inflammation, and CVD were a small proportion (0.4%), but their proportion increased with advanced CKD stage; 0% (CKD 1 and 2 stages), 0.1% (CKD 3a and 3b stages), and 1.1% (CKD 4 and 5 stages).
Fig. 1.
Concurrent prevalence of PEW, I, and CVD in 1,834 adults with predialysis CKD according to eGFR stage. Inflammation is defined as hs-CRP > 3 mg/L.
PEW = protein-energy wasting, I = inflammation, CVD = cardiovascular disease, CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate, hs-CRP = high sensitivity C-reactive protein.
The associated factors of PEW
We investigated which factors were predictive of PEW among 1,834 adults with predialysis CKD. In multivariate logistic regression models for PEW, male sex, diabetes, higher hs-CRP levels, lower eGFR, lower total CO2, and no physical activity were associated with PEW. PEW was independently associated with the inflammatory marker, hs-CRP in univariate (OR, 1.02; 95% CI, 1.01–1.05) and multivariate analyses (OR, 1.03; 95% CI, 1.01–1.06; Table 3).
Table 3. Logistic regression analysis model for PEW in 1,834 adults with predialysis CKD.
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age, yr | 1.02 (1.01–1.03) | 0.033 | 1.00 (0.98–1.02) | 0.997 |
| Male | 0.98 (0.71–1.36) | 0.921 | 1.46 (0.93–2.29) | 0.099 |
| eGFR, mL/min/1.73 m2 | 0.97 (0.96–0.98) | < 0.001 | 0.98 (0.96–0.99) | < 0.001 |
| Total CO2, meq/L | 0.86 (0.82–0.91) | < 0.001 | 0.93 (0.87–0.99) | 0.043 |
| Physical exercise | 0.43 (0.29–0.66) | < 0.001 | 0.43 (0.26–0.69) | < 0.001 |
| Diabetes | 1.90 (1.38–2.62) | < 0.001 | 1.68 (1.09–2.59) | 0.02 |
| Hypertension | 0.59 (0.21–1.62) | 0.303 | 1.30 (0.37–4.66) | 0.684 |
| CVD | 1.73 (1.12–2.68) | 0.013 | 1.09 (0.61–1.95) | 0.783 |
| hs-CRP, mg/L | 1.02 (1.01–1.05) | 0.043 | 1.03 (1.01–1.06) | 0.044 |
PEW = protein-energy wasting, CKD = chronic kidney disease, OR = odds raito, CI = confidence interval, eGFR = estimated glomerular filtration rate, CVD = cardiovascular disease, hs-CRP = high sensitivity C-reactive protein.
DISCUSSION
As a baseline study of KNOW-CKD, the nutritional status in predialysis CKD adults has been described here. This study demonstrates the prevalence of PEW is increased with advanced CKD stage. The relationships between the nutritional status and inflammatory status and CVD are getting closer in advanced CKD. PEW is associated with metabolic acidosis, low physical activity, comorbid diabetes, and inflammation.
Adverse nutritional changes are serious consequences and a strong indicator of adverse outcomes in CKD. This abnormality is often associated with diminished functional capacity related to uremic metabolic stress (2). Several terms used to describe the adverse nutritional changes in kidney disease include “uremic malnutrition” (15), “malnutrition-inflammation atherosclerosis syndrome” (16), or “malnutrition-inflammation complex syndrome” (17). Ill-defined terminologies and non-uniform definitions may lead to conceptual errors and data misinterpretation. In 2008, ISRNM experts panel recommended the term “PEW” to describe the state of decreased body stores of protein and energy in CKD patients (4). The ISRNM proposed that 4 main categories be recognized for diagnosing PEW: biochemical criteria, low body mass, decreased in muscle mass, and low protein intake. Among biochemical indicators, serum albumin is a strong and consistent indicator for PEW. BMI is the most common measure of body mass, and is often used to predict outcomes in CKD. Muscle mass is the most valid criterion for diagnosing PEW, and is often difficult to diagnose low muscle mass. There are no clinical uniform and reproducible measures of muscle mass especially in patients with catabolic status. However, UCE remains the method for assessing total muscle mass because of its lower cost and easier reproducibility. Recent studies (18,19) showed that UCE effectively predicted clinical outcomes in CKD. In the Chronic Renal Insufficient Cohort (CRIC) study (19), UCE and appendicular muscle mass were modestly correlated. In the NephroTest cohort study (20), lower gender-specific quartile of UCE was associated with higher risk for mortality in early stage CKD. DPI is a reliable marker of nutritional intake and can be estimated by 24-hour urine urea nitrogen in clinically stable CKD patients (10). There have been a few studies of nutritional status in predialysis CKD patients using the ISRNM criteria. In this study, we defined the PEW according to the ISRNM criteria, and we described the nutritional status in CKD patients. The prevalence of PEW was 9.0% and was increased with advanced CKD stage.
PEW, inflammation, and CVD clearly contribute to poor outcomes in CKD patients. Persistent, low-grade inflammation has been recognized as a component of CKD. Inflammation plays a contributing role in CVD as well as contributing to PEW. Stenvinkel et al. (5) reported a strong association between malnutrition, inflammation, and atherosclerosis in 109 patients with terminal chronic renal failure (mean eGFR 7 ± 1 mL/min). The concurrent prevalence of PEW, inflammation, and CVD in their study was 22.0%. They assessed PEW by subjective global assessment (SGA), inflammation by C-reactive protein (CRP) ≥ 10 mg/L, and CVD by the presence of carotid plaque. Another study (6) suggested that inflammation, malnutrition, and CVD appear to be interrelated, each additionally contributing to high mortality in 128 hemodialysis patients; the concurrent prevalence of PEW, inflammation, and CVD was 23.0%. This study assessed CVD according to CVD history. The Netherlands Co-operative Study on the Adequacy of Dialysis (NECOSAD) study (7) reported PEW interacted with inflammation and CVD in 815 patients with dialysis. In the NECOSAD study, the concurrent presence of PEW, inflammation, and CVD was a small proportion of 6%, but was strikingly increased the mortality. In our study, we defined PEW according to ISRNM criteria, inflammation as hs-CRP > 3 mg/L, and CVD as having medical history of CVD. The prevalence of PEW, inflammation, and CVD was 9.0%, 12.7%, and 8.4% respectively, while concurrent presence of PEW, inflammation, and CVD was uncommon (0.4%), but increased with advanced CKD stage. There are possible explanations of the discrepancy between our study and the previous studies. First, non-uniform definitions of PEW, inflammation, and CVD have been assessed in the studies. Second, the study populations of these studies varied in terms of comorbidities and CKD stages.
The mechanisms involved in PEW are multiple and complicated (3). Although insufficient food intake due to poor appetite and dietary restrictions contribute to PEW, uremia-induced alterations such as increased energy expenditure, persistent inflammation, acidosis, and multiple catabolic endocrine disorders lead to excessive wasting of muscle and fat. We examined the predictors of PEW in 1,834 adults with predialysis CKD. In our multiple adjusted logistic regression models, comorbid diabetes, advanced CKD stage, metabolic acidosis, lack of physical activity, and inflammation were independently associated with PEW. Among the PEW predictors, metabolic acidosis and physical non-exercise are important modifiable factors. Metabolic acidosis induces release of branched-chain amino acids from muscle, also leads to insulin resistance, and induces adrenal glucocorticoid production (21,22). Bicarbonate therapy or low dietary acid load can reduce metabolic acidosis and muscle degradation. Muscle weakness is a reliable marker of poor outcome in CKD and is measured simply by grip strength or gait speed. Exercise-induced benefits include improvement in insulin sensitivity, decrease the muscle degradation, and improvement in cardiovascular functions (23,24). Decreased physical activity likely plays a major role in PEW.
A few considerations are important in the interpretation of our results. First, we sincerely defined the PEW according to ISRNM criteria; however, the cut-off value for each criterion is not validated in our Korean population. Racial differences in nutritional and inflammation status have been observed in CKD patients (25). Second, our study population is all CKD stage of CKD. Adults with CKD stage 1 and 2 (eGFR > 60 mL/min/1.73 m2) made up 31% of the study population. Third, a single hs-CRP measure may not be a standard method for defining the inflammation in CKD patients. The optimal cut-off value of hs-CRP is also uncertain (13,26). Fourth, there are many risk factors associated with PEW; thus may have an unknown potential bias. However, our study also has several distinct strengths. The KNOW-CKD study has well-designed protocol and is a nationally representative prospective cohort study. We used a rigorous protocol to measure demographics, comorbidities, and laboratory data. We are following the adults with various CKD stage and will evaluate the roll of inflammation, metabolic acidosis, and physical activity in PEW.
In summary, nutritional status in predialysis CKD adults indicated that the prevalence of PEW is about 9.0% according to ISRNM criteria and increased with advanced CKD stage. PEW was associated with metabolic acidosis, low physical activity, comorbid diabetes, and inflammation. Future studies are needed to evaluate the complex interaction between PEW, CVD, and inflammation. New strategies to improve metabolic acidosis or physical activity in at-risk adults with CKD are needed to reduce the burden of CKD.
Footnotes
Funding: This research was supported by grants 2011E3300300, 2012E3301100 and 2013E3301600 from Research of Korea Centers for Disease Control & Prevention; by the National Research Foundation of Korea, funded by the Ministry of Science, ICT & Future Planning (2015R1C1A1A01051769); by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Science, ICT and future planning (2016R1A2B4007870); and by a grant of the Korea Health Technology R & D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (HI14C2084).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Oh YK, Chae DW, Ahn C. Data curation: Kim YH, Kim YS, Lee SW. Formal analysis: Hyun YY, Lee KB. Validation: Han SH. Writing - original draft: Hyun YY, Lee KB.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 2.Fouque D, Pelletier S, Mafra D, Chauveau P. Nutrition and chronic kidney disease. Kidney Int. 2011;80:348–357. doi: 10.1038/ki.2011.118. [DOI] [PubMed] [Google Scholar]
- 3.Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar-Zadeh K, Kaysen G, Mitch WE, Price SR, Wanner C, Wang AY, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM) J Ren Nutr. 2013;23:77–90. doi: 10.1053/j.jrn.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 5.Stenvinkel P, Heimbürger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–1911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi AR, Alvestrand A, Divino-Filho JC, Gutierrez A, Heimbürger O, Lindholm B, Bergström J. Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. J Am Soc Nephrol. 2002;13(Suppl 1):S28–36. [PubMed] [Google Scholar]
- 7.de Mutsert R, Grootendorst DC, Axelsson J, Boeschoten EW, Krediet RT, Dekker FW, NECOSAD Study Group Excess mortality due to interaction between protein-energy wasting, inflammation and cardiovascular disease in chronic dialysis patients. Nephrol Dial Transplant. 2008;23:2957–2964. doi: 10.1093/ndt/gfn167. [DOI] [PubMed] [Google Scholar]
- 8.Han SS, Shin N, Lee SM, Lee H, Kim DK, Kim YS. Correlation between periodontitis and chronic kidney disease in Korean adults. Kidney Res Clin Pract. 2013;32:164–170. doi: 10.1016/j.krcp.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh KH, Park SK, Park HC, Chin HJ, Chae DW, Choi KH, Han SH, Yoo TH, Lee K, Kim YS, et al. KNOW-CKD (Korean cohort study for outcome in patients with chronic kidney disease): design and methods. BMC Nephrol. 2014;15:80. doi: 10.1186/1471-2369-15-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masud T, Manatunga A, Cotsonis G, Mitch WE. The precision of estimating protein intake of patients with chronic renal failure. Kidney Int. 2002;62:1750–1756. doi: 10.1046/j.1523-1755.2002.00606.x. [DOI] [PubMed] [Google Scholar]
- 11.Kovesdy CP, Kopple JD, Kalantar-Zadeh K. Management of protein-energy wasting in non-dialysis-dependent chronic kidney disease: reconciling low protein intake with nutritional therapy. Am J Clin Nutr. 2013;97:1163–1177. doi: 10.3945/ajcn.112.036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapter 1: definition and classification of CKD. Kidney Int Suppl. 2011;2013:19–62. doi: 10.1038/kisup.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers GL, Christenson RH, Cushman M, Ballantyne CM, Cooper GR, Pfeiffer CM, Grundy SM, Labarthe DR, Levy D, Rifai N, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice guidelines: emerging biomarkers for primary prevention of cardiovascular disease. Clin Chem. 2009;55:378–384. doi: 10.1373/clinchem.2008.115899. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Global Recommendations on Physical Activity for Health. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 15.Pupim LB, Caglar K, Hakim RM, Shyr Y, Ikizler TA. Uremic malnutrition is a predictor of death independent of inflammatory status. Kidney Int. 2004;66:2054–2060. doi: 10.1111/j.1523-1755.2004.00978.x. [DOI] [PubMed] [Google Scholar]
- 16.Pecoits-Filho R, Lindholm B, Stenvinkel P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome -- the heart of the matter. Nephrol Dial Transplant. 2002;17(Suppl 11):28–31. doi: 10.1093/ndt/17.suppl_11.28. [DOI] [PubMed] [Google Scholar]
- 17.Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003;42:864–881. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Di Micco L, Quinn RR, Ronksley PE, Bellizzi V, Lewin AM, Cianciaruso B, Ravani P, Alberta Kidney Disease Network (AKDN) Urine creatinine excretion and clinical outcomes in CKD. Clin J Am Soc Nephrol. 2013;8:1877–1883. doi: 10.2215/CJN.01350213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson FP, Xie D, Anderson AH, Leonard MB, Reese PP, Delafontaine P, Horwitz E, Kallem R, Navaneethan S, Ojo A, et al. Urinary creatinine excretion, bioelectrical impedance analysis, and clinical outcomes in patients with CKD: the CRIC study. Clin J Am Soc Nephrol. 2014;9:2095–2103. doi: 10.2215/CJN.03790414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tynkevich E, Flamant M, Haymann JP, Metzger M, Thervet E, Boffa JJ, Vrtovsnik F, Houillier P, Froissart M, Stengel B. NephroTest Study Group. Urinary creatinine excretion, measured glomerular filtration rate and CKD outcomes. Nephrol Dial Transplant. 2015;30:1386–1394. doi: 10.1093/ndt/gfv047. [DOI] [PubMed] [Google Scholar]
- 21.Franch HA, Raissi S, Wang X, Zheng B, Bailey JL, Price SR. Acidosis impairs insulin receptor substrate-1-associated phosphoinositide 3-kinase signaling in muscle cells: consequences on proteolysis. Am J Physiol Renal Physiol. 2004;287:F700–6. doi: 10.1152/ajprenal.00440.2003. [DOI] [PubMed] [Google Scholar]
- 22.Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol. 2004;15:1537–1545. doi: 10.1097/01.asn.0000127211.86206.e1. [DOI] [PubMed] [Google Scholar]
- 23.Johansen KL, Painter P. Exercise in individuals with CKD. Am J Kidney Dis. 2012;59:126–134. doi: 10.1053/j.ajkd.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeBrasseur NK, Walsh K, Arany Z. Metabolic benefits of resistance training and fast glycolytic skeletal muscle. Am J Physiol Endocrinol Metab. 2011;300:E3–10. doi: 10.1152/ajpendo.00512.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lertdumrongluk P, Kovesdy CP, Norris KC, Kalantar-Zadeh K. Nutritional and inflammatory axis of racial survival disparities. Semin Dial. 2013;26:36–39. doi: 10.1111/sdi.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]