Abstract
The aim of this study was to identify the risk factors for presence and severity of diabetic ketoacidosis (DKA) at the onset of type 1 diabetes mellitus (T1DM) in Korean children and adolescents. A retrospective chart review of children and adolescents newly diagnosed with T1DM was conducted in seven secondary and tertiary centers in Korea. Eligible subjects were < 20 years of age and had records on the presence or absence of DKA at the time of T1DM diagnosis. DKA severity was categorized as mild, moderate, or severe. Data were collected on age, height, body weight, pubertal status, family history of diabetes, delayed diagnosis, preceding infections, health insurance status, and parental education level. A total of 361 patients (male 46.3%) with T1DM were included. Overall, 177 (49.0%) patients presented with DKA at T1DM diagnosis. Risk factors predicting DKA at T1DM diagnosis were age ≥ 12 years, lower serum C-peptide levels, presence of a preceding infection, and delayed diagnosis. Low parental education level and preceding infection increased the severity of DKA. These results suggest that alertness of the physician and public awareness of diabetes symptoms are needed to decrease the incidence and severity of DKA at T1DM diagnosis.
Keywords: Type 1 Diabetes Mellitus, Diabetic Ketoacidosis, Risk Factors
Graphical Abstract
INTRODUCTION
The incidence of type 1 diabetes mellitus (T1DM) is increasing worldwide (1,2). In particular, regions with a low incidence have experienced a more rapid increase in newly diagnosed T1DM cases (1). The T1DM population is increasing continuously in Korea, the nation with very low prevalence of T1DM in the world (3,4,5). Diabetic ketoacidosis (DKA), which is caused by absolute insulin deficiency, is the most serious life-threatening acute complication of T1DM. It is related to 0.15%–0.31% of mortalities among patients with T1DM (6). Cerebral edema is responsible for 0.46%–4.60% of mortalities and is the leading cause of mortality related to DKA (6). In addition, DKA is related to impaired cognitive functions, such as short-term memory and long-term intelligence (7,8). The incidence of DKA at the time of T1DM diagnosis ranges from 15% to 67% (2,9,10) depending on the geographic region. Although the incidence of T1DM has increased worldwide, the incidence of DKA at diagnosis has decreased according to several studies (11,12), or has not changed according to other studies (2,13).
In previous studies, young age, particularly < 2 years, low accessibility to medical care, and absence of a family history of T1DM were identified as risk factors for DKA at T1DM diagnosis (14). Particular geographic regions (10) and ethnicities (15) with a low background incidence of T1DM are associated with a higher risk of DKA at T1DM diagnosis. The higher incidence of DKA associated with a lack of T1DM family history or with a low incidence of T1DM in the population suggests that diabetes awareness reduces the incidence of DKA. No study has reported the incidence of or risk factors for DKA in Asian children who have a very low incidence of T1DM.
The objective of this multicenter study was to evaluate the incidence of DKA at T1DM diagnosis in Korean children and adolescents and to determine the risk factors for developing DKA at T1DM diagnosis. We also evaluated the factors affecting DKA severity. If these risk factors are controllable, it would be possible to reduce the rate and severity of DKA, which is a life-threatening complication of T1DM.
MATERIALS AND METHODS
Subjects
The subjects were recruited from seven secondary and tertiary centers in Korea and were diagnosed with T1DM from January 2000 to May 2015. The participating centers are located throughout the country (Seoul National University Children's Hospital and Seoul Metropolitan Government-Seoul National University (SMG-SNU) Boramae Medical Center in Seoul; Dong-A University Hospital in Busan; Chosun University Hospital in Gwangju; Dankook University Hospital in Cheonan; Seoul National University Bundang Hospital in Seongnam; and Inje University Ilsan Paik Hospital in Goyang).
T1DM was defined as insulin dependency with one of the following conditions: 1) serum C-peptide level < 0.6 ng/mL; 2) 24-hour urine C-peptide level < 30 µg/24 hr; 3) DKA at diagnosis; or 4) positivity for one or more diabetes-associated autoantibodies. DKA was defined as hyperglycemia with venous blood pH < 7.3 or a bicarbonate level < 15 mEq/dL. DKA severity was classified by venous blood pH: severe < 7.1; moderate ≥ 7.1 and < 7.2; mild ≥ 7.2 and < 7.3.
Eligible subjects were children and adolescents diagnosed with T1DM before 20 years of age who also had medical records regarding the presence or absence of DKA at T1DM diagnosis. Patients diagnosed before 1 year of age were excluded.
Methods
Medical records were reviewed retrospectively. Data were collected on age, height, weight, pubertal status, season (spring, March–May; summer, June–August; autumn, September–November; winter, December–February), family history of diabetes, delayed diagnosis, presence of preceding infections, health insurance status, and parents' education level at diagnosis. The date of diagnosis was defined as the first day of insulin administration. Laboratory results at diagnosis, such as serum glucose, C-peptide, glycated hemoglobin (HbA1c), venous pH and bicarbonate levels, were also investigated.
Weight loss was estimated as the difference between weight after recovery from acute diabetes and weight at diagnosis. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). The BMI standard deviation score (SDS) was calculated for each subject based on 2007 Korean National Growth Charts (16). Delayed diagnosis was defined as a diagnosis of a disease other than diabetes at the first medical consultation. A high parental education level was defined if both parents graduated college or university. A low parental education level was defined when at least one parent had an education of high school or less.
All Koreans are covered by one of the following three healthcare programs depending on their household income: 1) the national health insurance program; 2) copayment subsidies for the project; and 3) the medical aid program (healthcare for the poor). Thus, health insurance status in Korea is considered an indicator of socioeconomic status.
Statistical analysis
Statistical analyses were performed using SPSS ver. 21.0 for Windows software (SPSS Inc., Chicago, IL, USA). Variables were assessed for a normal distribution. All continuous variables are described as means ± standard deviation. Student's t-test and the χ2 test were used to evaluate differences between patients with and without DKA at T1DM diagnosis. Analysis of variance and the χ2 test for trend analysis were used to compare variables between DKA severity groups. Multivariate logistic regression analysis was used to evaluate risk factors for 1) the presence of DKA at T1DM diagnosis and 2) DKA severity at T1DM diagnosis. A P value < 0.05 was considered statistically significant.
Ethics statement
The present study protocol was reviewed and approved by the Institutional Review Board of Seoul National University Hospital (IRB No. 1507-025-686), Ilsan Paik Hospital (IRB No. 2015-06-024), Seoul National University Bundang Hospital (IRB No. B-1607-356-401), Dong-A University Hospital (IRB No. 15-127), Chosun University Hospital (IRB No. 2015-07-016-001), Dankook University Hospital (IRB No. DKUH 2016-02-019), SMG-SNU Boramae Medical Center (IRB No.16-2015-104). Informed consent was waived by each IRB.
RESULTS
Subject characteristics
A total of 361 patients with T1DM were included in this study. Overall, 49.0% (177/361) of the patients with T1DM presented with DKA at T1DM diagnosis. Male patients comprised 46.3% of the study population, and their mean age at T1DM diagnosis was 8.9 ± 4.0 years. In total, 65.1% of the study population was prepubertal. The mean height SDS was 0.48 ± 1.18, mean weight SDS at diagnosis was −0.46 ± 1.16, mean weight SDS after stabilization was 0.20 ± 0.96, and change in the weight SDS was 0.69 ± 0.58. The mean BMI SDS at diagnosis was −0.88 ± 1.44, mean BMI SDS after stabilization was 0.08 ± 1.10, and change in the BMI SDS was 1.00 ± 0.82. Of the total patients, 30% were diagnosed in spring, 26.6% in summer, 22.2% in autumn, and 21.1% in winter. Approximately 40% had a family history of diabetes. Of the total study population, 46.6% were not diagnosed with diabetes mellitus (DM) at their first hospital visit (delayed diagnosis), and 60.9% had parents with a high education level. Most patients were covered by the national health insurance program, 3.1% were receiving copayment subsidies for the near poor project, and 2.8% were covered by the medical aid program. The proportion who were receiving copayment subsidies was higher among patients with T1DM than among the total population (0.6%), and the proportion receiving medical aid was the same as that in the total population (2.8%) (17).
Comparison between patients with and without DKA
The comparison between patients with and without DKA at diagnosis is shown in Table 1. No differences in sex ratio or mean age at diagnosis were detected between the two groups, but more children were aged < 3 years and ≥ 12 years in the DKA group than in the non-DKA group (14.1% and 29.4% in DKA group vs. 6.5% and 21.2%, in non-DKA group, respectively; P = 0.004).
Table 1. Characteristics of patients according to the presence of DKA at diagnosis.
| Characteristics | With DKA (n = 177) | Without DKA (n = 184) | P value |
|---|---|---|---|
| Male | 78 (44.1) | 89 (48.4) | 0.460 |
| Age, yr | 8.80 ± 4.30 | 8.80 ± 3.70 | 0.911 |
| < 3 | 25 (14.1) | 12 (6.5) | |
| 3–12 | 100 (56.5) | 133 (72.3) | |
| ≥ 12 | 52 (29.4) | 39 (21.2) | |
| Puberty | 62 (37.1) | 57 (32.8) | 0.398 |
| Height SDS | 0.56 ± 1.27 | 0.41 ± 1.10 | 0.229 |
| Weight SDS | |||
| At diagnosis | −0.49 ± 1.21 | −0.44 ± 1.12 | 0.140 |
| After stabilization | 0.28 ± 1.03 | 0.12 ± 0.88 | 0.697 |
| Change | −0.77 ± 0.66 | −0.61 ± 0.48 | 0.015 |
| BMI SDS | |||
| At diagnosis | −0.98 ± 1.52 | −0.78 ± 1.36 | 0.212 |
| After stabilization | 0.11 ± 1.07 | 0.05 ± 1.07 | 0.586 |
| Change | 0.93 ± 0.08 | 0.68 ± 0.06 | 0.014 |
| Delayed diagnosis | 77 (44.5) | 51 (28.5) | 0.002 |
| Family history of diabetes | 69 (41.6) | 68 (37.8) | 0.510 |
| Health insurance/co-payment/medical aid | 142/4/7 (92.8/2.6/4.6) | 164/6/2 (95.3/3.5/1.2) | 0.353 |
| High parental education | 64 (58.2) | 73 (63.5) | 0.495 |
| Preceding infection | 34 (19.8) | 13 (7.2) | 0.001 |
| Serum C-peptide, ng/mL | 0.44 ± 0.40 | 0.73 ± 0.70 | < 0.001 |
| Glucose, mg/dL | 537.20 ± 249.80 | 453.30 ± 176.30 | < 0.001 |
| HbA1c, % | 12.60 ± 1.90 | 12.10 ± 2.40 | 0.071 |
Data are expressed as the mean ± standard deviation or number (%).
DKA = diabetic ketoacidosis, SDS = standard deviation score, BMI = body mass index, HbA1c = glycated hemoglobin.
Pubertal status, height, and weight at diagnosis did not differ between the two groups. Patients with DKA at diagnosis lost more weight than did patients without DKA. Delayed diagnosis (44.5% vs. 28.5%) and preceding infections (19.8% vs. 7.2%) were more prevalent in the DKA than non-DKA group at diagnosis. However, a family history of diabetes, health insurance status, and parental education level did not differ between the two groups. The serum C-peptide level was lower and initial serum glucose level higher in the DKA than the non-DKA group. No difference was found in HbA1c levels at diagnosis.
Risk factors for DKA at T1DM diagnosis
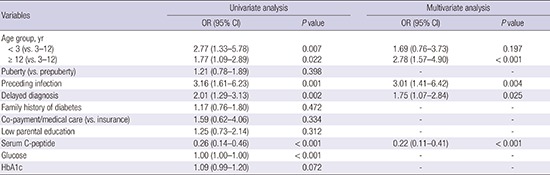
Univariate analysis revealed that age < 3 years or ≥ 12 years increased the risks for DKA (odds ratio [OR], 2.77; 95% confidence interval [CI], 1.33–5.78; P = 0.007 and OR, 1.77; 95% CI, 1.09–2.89; P = 0.022, respectively), compared with the 3–12-year age group (Table 2). A preceding infection (OR, 3.16; 95% CI, 1.61–6.23; P = 0.001) and a delayed diagnosis (OR, 2.01; 95% CI, 1.29–3.13; P = 0.002) also increased the risk for DKA. Serum C-peptide levels were lower in patients with DKA than in those without DKA (OR, 0.26; 95% CI, 0.14–0.46; P < 0.001). Multivariate analysis revealed that age ≥ 12 years (OR, 2.78; 95% CI, 1.57–4.90; P < 0.001), a preceding infection (OR, 3.01; 95% CI, 1.41–6.42; P = 0.004), delayed diagnosis (OR, 1.75; 95% CI, 1.07–2.84; P = 0.025), and a low serum C-peptide level (OR, 0.22; 95% CI, 0.11–0.41; P < 0.001) increased the risk for DKA at T1DM diagnosis (Table 2).
Table 2. Risk factors for DKA at T1DM diagnosis.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age group, yr | ||||
| < 3 (vs. 3–12) | 2.77 (1.33–5.78) | 0.007 | 1.69 (0.76–3.73) | 0.197 |
| ≥ 12 (vs. 3–12) | 1.77 (1.09–2.89) | 0.022 | 2.78 (1.57–4.90) | < 0.001 |
| Puberty (vs. prepuberty) | 1.21 (0.78–1.89) | 0.398 | - | - |
| Preceding infection | 3.16 (1.61–6.23) | 0.001 | 3.01 (1.41–6.42) | 0.004 |
| Delayed diagnosis | 2.01 (1.29–3.13) | 0.002 | 1.75 (1.07–2.84) | 0.025 |
| Family history of diabetes | 1.17 (0.76–1.80) | 0.472 | - | - |
| Co-payment/medical care (vs. insurance) | 1.59 (0.62–4.06) | 0.334 | - | - |
| Low parental education | 1.25 (0.73–2.14) | 0.312 | - | - |
| Serum C-peptide | 0.26 (0.14–0.46) | < 0.001 | 0.22 (0.11–0.41) | < 0.001 |
| Glucose | 1.00 (1.00–1.00) | < 0.001 | - | - |
| HbA1c | 1.09 (0.99–1.20) | 0.072 | - | - |
DKA = diabetic ketoacidosis, T1DM = type 1 diabetes mellitus, OR = odds ratio, CI = confidence interval, HbA1c = glycated hemoglobin.
Comparisons between groups according to DKA severity
We analyzed 163 of 177 patients with DKA at diagnosis, excluding 14 patients whose DKA severity could not be determined due to incomplete records. A lower socioeconomic status assumed by insurance status, lower parental education level, preceding infection, and lower serum C-peptide level were related to severe DKA (Table 3). The proportion of patients with health insurance (vs. copayment subsidies and the medical aid program) was 98.4% in the mild DKA group, 93.0% in the moderate DKA group, and 84.8% in the severe DKA group (P for trend = 0.037). Approximately 68% of parents had a high education levels in the mild DKA group, 59.4% in the moderate DKA group, and 37.9% in the severe DKA group (P for trend = 0.014). As the DKA severity increased, the rate of preceding infections increased (P for trend = 0.010), and the mean serum C-peptide level decreased (P for trend = 0.024). No differences in mean age, sex, puberty, delayed diagnosis, or family history of DM were observed among the mild, moderate, and severe DKA groups.
Table 3. Characteristics of the patients with DKA according to severity of DKA.
| Variables | Mild (n = 67, 41.1) | Moderate (n = 52, 31.9) | Severe (n = 44, 27.0) | P value |
|---|---|---|---|---|
| Male | 27 (40.3) | 28 (53.8) | 18 (40.9) | 0.281 |
| Age, yr | 8.81 ± 4.24 | 8.72 ± 4.37 | 9.24 ± 4.17 | 0.827 |
| < 3 | 8 (11.9) | 6 (11.5) | 5 (11.4) | |
| 3–12 | 40 (59.7) | 31 (59.6) | 24 (54.5) | |
| ≥ 12 | 19 (28.4) | 15 (28.8) | 15 (34.1) | |
| Height SDS | 0.54 ± 1.06 | 0.59 ± 1.52 | 0.62 ± 1.36 | 0.948 |
| Weight SDS | ||||
| At diagnosis | −0.46 ± 1.26 | −0.52 ± 1.25 | −0.57 ± 1.16 | 0.914 |
| After stabilization | 0.29 ± 1.01 | 0.38 ± 1.18 | 0.17 ± 0.84 | 0.632 |
| Change | 0.74 ± 0.46 | 0.90 ± 0.81 | 0.71 ± 0.75 | 0.358 |
| BMI SDS | ||||
| At diagnosis | −0.94 ± 1.51 | 0.19 ± 0.99 | 1.10 ± 0.72 | 0.293 |
| After stabilization | −1.05 ± 1.68 | 1.13 ± 0.17 | 1.27 ± 1.10 | 0.852 |
| Change | −1.11 ± 1.44 | 1.04 ± 0.18 | 1.01 ± 1.05 | 0.448 |
| Puberty | 25 (38.5) | 19 (37.3) | 15 (38.5) | 0.989 |
| Delayed diagnosis | 31 (47.0) | 24 (47.1) | 19 (44.2) | 0.951 |
| Family history of diabetes | 28 (43.1) | 19 (39.6) | 18 (43.9) | 0.903 |
| Co-payment/medical aid (vs. insurance) | 1 (1.6) | 3 (7.0) | 5 (15.2) | 0.037 |
| High parental education | 28 (68.3) | 19 (59.4) | 11 (37.9) | 0.014 |
| Preceding infection | 8 (12.1) | 9 (17.6) | 14 (32.6) | 0.010 |
| Serum C-peptide, ng/mL | 0.54 ± 0.53 | 0.38 ± 0.26 | 0.35 ± 0.23 | 0.024 |
| Glucose, mg/dL | 464.80 ± 186.30 | 583.60 ± 338.40 | 591.10 ± 200.30 | 0.010 |
| HbA1c, % | 12.70 ± 1.70 | 12.50 ± 2.00 | 12.60 ± 1.90 | 0.923 |
Data are expressed as the mean ± standard deviation or number (%).
DKA = diabetic ketoacidosis, SDS = standard deviation score, BMI = body mass index, HbA1c = glycated hemoglobin.
Risk factors for severe DKA at T1DM diagnosis
Univariate analysis revealed that preceding infections (OR, 2.84; 95% CI, 1.25–6.44; P = 0.013), low parental education level (OR, 2.96; 95% CI, 1.22–7.20; P = 0.017), and copayment subsidies or medical aid (OR, 4.55; 95% CI, 1.15–18.09; P = 0.031) increased the risk for severe DKA at T1DM diagnosis (Table 4). Multivariate logistic regression analysis of the factors determined to be statistically significant in the univariate analysis showed that a preceding infection and low parental education level increased the risk for severe DKA (OR, 3.94; 95% CI, 1.32–11.77; P = 0.013 and OR, 2.80; 95% CI, 0.98–8.01; P = 0.047, respectively).
Table 4. Risk factors for severe DKA (vs. mild to moderate DKA).
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age group, yr | ||||
| < 3 (vs. 3–12) | 1.06 (0.34–3.24) | 0.944 | - | - |
| ≥ 12 (vs. 3–12) | 1.31 (0.61–2.80) | 0.496 | - | - |
| Puberty (vs. prepuberty) | 1.02 (0.49–2.16) | 0.953 | - | - |
| Preceding infection | 2.84 (1.25–6.44) | 0.013 | 3.94 (1.32–11.77) | 0.013 |
| Delayed diagnosis | 0.89 (0.44–1.80) | 0.751 | - | - |
| Family history of diabetes | 1.09 (0.53–2.26) | 0.798 | - | - |
| Co-payment/medical aid (vs. insurance) | 4.55 (1.15–18.09) | 0.031 | 1.63 (0.14–19.72) | 0.701 |
| Low parental education | 2.96 (1.22–7.20) | 0.017 | 2.80 (0.98–8.01) | 0.047 |
| Serum C-peptide | 0.34 (0.10–1.16) | 0.085 | - | - |
| Glucose | 1.00 (1.00–1.00) | 0.119 | - | - |
| HbA1c | 0.99 (0.83–1.19) | 0.940 | - | - |
DKA = diabetic ketoacidosis, OR = odds ratio, CI = confidence interval, HbA1c = glycated hemoglobin.
DISCUSSION
In the present study, 49% of patients newly diagnosed with T1DM had DKA. Age ≥ 12, lower serum C-peptide levels, presence of a preceding infection, and delayed diagnosis significantly increased the risk for DKA at T1DM diagnosis. Low parental education level and preceding infection increased the risk of severe DKA.
This incidence of DKA at T1DM diagnosis in this study is relatively high compared with that in Western countries where there is a higher prevalence of T1DM (11,14,18,19). This might be explained by ascertainment bias, as this study was conducted at secondary and tertiary centers, which tend to admit patients with more severe symptoms or delayed visits to the hospital after the initial presentation. As most patients with T1DM are referred to secondary and tertiary centers, the incidence of DKA in this study may not be exaggerated and may indicate the overall incidence of DKA at diagnosis in Korea.
Previous studies have reported that younger age at T1DM diagnosis is a risk factor for DKA (13,14). In this study, children aged < 3 years or ≥ 12 years had a higher risk for DKA at T1DM diagnosis. However, after adjusting for serum C-peptide levels, age < 3 years was not a significant risk factor for DKA, suggesting that the higher risk of DKA at a younger age may be due to more decreased β-cell function. In fact, aggressive diabetes and delayed detection of diabetes symptoms are more frequent in young children. Participating in a prospective cohort that informed the parents about diabetes symptoms decreased the risk of DKA at T1DM diagnosis in young children (19). On the other hand, children and adolescents ≥ 12 years continued to have a significantly higher risk of DKA at T1DM diagnosis after adjusting for C-peptide levels, puberty, and delayed diagnosis. Children and adolescents at this age have likely escaped parental control; thus, detection or reporting of symptoms may be delayed. A study in New Zealand reported an increased risk for DKA at age around 11 years (13). Because earlier detection of symptoms in young children is possible through involvement in toileting and provision of water by parents, the authors suggested that greater self-awareness and earlier reporting of symptoms in children older than 11 years are needed to prevent DKA.
C-peptide is a surrogate marker of endogenous insulin secretion. A low C-peptide level implies decreased β-cell function. Thus, patients with DKA due to a significant β-cell failure are likely to have lower C-peptide and higher glucose levels. As expected, our study showed that C-peptide levels of DKA group were lower than those of non-DKA group.
Infection is related to T1DM and an increased risk for DKA. Infection can accelerate destruction of β-cells through an autoimmune process (20). Therefore, destruction of β-cells may increase the risk for DKA and its severity. Infection also results in inflammation, release of pro-inflammatory cytokines, and a counter regulatory response related to insulin resistance and metabolic decompensation (21). On the other hand, symptoms of infection may mask the symptoms of T1DM, delay diagnosis, and increase the risk for DKA.
A delayed diagnosis in this study referred to a missed T1DM diagnosis at the first medical counseling visit for symptoms such as polyuria, polydipsia, and weight loss. As mentioned earlier, some patients were misdiagnosed with gastroenteritis or a respiratory infection and diagnosed with T1DM later, after DKA occurred. After adjusting for preceding infections, a delayed diagnosis still increased the risk for DKA. Thus, initial suspicion and monitoring blood sugar levels could prevent a delayed diagnosis and reduce development of DKA.
Public education about diabetes symptoms alone can reduce the frequency of DKA through preventing a delayed diagnosis. An Italian study reported that a decrease in the frequency of DKA at T1DM diagnosis from 78% to 12.5% 8 years after the education program for the public and primary physicians (22). Other studies from Sweden (23) and Finland (11) also reported that education reduces the frequency of DKA at T1DM diagnosis.
We found that DKA severity was influenced by socioeconomic factors as well as the presence of preceding infection. An education level higher than college in both parents reduced the risk for severe DKA in this study. Furthermore, if the education level of one or both parents was high school or lower, the risk for severe DKA at T1DM diagnosis was 2.8-fold higher compared with parents with more education. This result is consistent with previous studies. A lower education level in the mother was related to a higher risk of DKA at onset in Lithuania (24). A lower parental education level was related to a higher prevalence of DKA according to the US Search for Diabetes in Youth study (18).
Those receiving copayment subsidies and medical aid with low income probably have less time and capacity to take care of their children and may not detect diabetic symptoms early. However, the relationship between DKA severity and insurance status was not significant after adjusting for other factors, which may be due to the small sample size of the copayment subsidies and medical aid groups.
This study had some limitations, including the retrospective medical record review. Some records contained incomplete data regarding parental education level and previous visits to other hospitals before the T1DM diagnosis. In addition, data from one secondary and six tertiary centers, where more critical patients tend to visit, may have caused selection bias. Further prospective studies inclusive of more complete data are needed. Despite these limitations, this study is the first nationwide multicenter study to evaluate the risk factors for DKA, including socioeconomic factors, at T1DM diagnosis, performed in East Asia, where the incidence of T1DM is very low. In the future, more studies on T1DM and DKA incidence time trends will be helpful to understand the risk factors for DKA.
In conclusion, the incidence of DKA at T1DM diagnosis in Korean children and adolescents in this study was 49%. The risk factors for DKA at T1DM diagnosis were age ≥ 12 years, low C-peptide levels, delayed diagnosis, and a preceding infection. A low parental education level and preceding infection were more likely to cause severe DKA. Thus, public education and the physician's alertness are important to reduce the incidence and severity of DKA, a life-threatening complication due to T1DM.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Lee HJ, Jung HW, Lee YA, Shin CH, Lee SY. Data curation: Yu HW, Kim JH, Chung HR, Yoo J, Kim E, Yu J, Lee SY. Investigation: Lee HJ, Jung HW, Lee YA, Kim JH, Chung HR, Yoo J, Kim E, Yu J, Shin CH, Yang SW, Lee SY. Writing - original draft: Lee HJ. Writing - review & editing:Kim JH, Lee YA, Shin CH, Yang SW, Lee SY.
References
- 1.DIAMOND Project Group. Incidence and trends of childhood type 1 diabetes worldwide 1990-1999. Diabet Med. 2006;23:857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 2.Wojcik M, Sudacka M, Wasyl B, Ciechanowska M, Nazim J, Stelmach M, Starzyk JB. Incidence of type 1 diabetes mellitus during 26 years of observation and prevalence of diabetic ketoacidosis in the later years. Eur J Pediatr. 2015;174:1319–1324. doi: 10.1007/s00431-015-2537-1. [DOI] [PubMed] [Google Scholar]
- 3.Ko KW, Yang SW, Cho NH. The incidence of IDDM in Seoul from 1985 to 1988. Diabetes Care. 1994;17:1473–1475. doi: 10.2337/diacare.17.12.1473. [DOI] [PubMed] [Google Scholar]
- 4.Shin CH. Epidemiologic characteristics of type 1 diabetes in children aged 14 years or under in Korea, 1985-2000. Korean J Pediatr. 2008;51:569–575. [Google Scholar]
- 5.Kim JH, Lee CG, Lee YA, Yang SW, Shin CH. Increasing incidence of type 1 diabetes among Korean children and adolescents: analysis of data from a nationwide registry in Korea. Pediatr Diabetes. 2016;17:519–524. doi: 10.1111/pedi.12324. [DOI] [PubMed] [Google Scholar]
- 6.Edge JA, Ford-Adams ME, Dunger DB. Causes of death in children with insulin dependent diabetes 1990-96. Arch Dis Child. 1999;81:318–323. doi: 10.1136/adc.81.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron FJ, Scratch SE, Nadebaum C, Northam EA, Koves I, Jennings J, Finney K, Neil JJ, Wellard RM, Mackay M, et al. Neurological consequences of diabetic ketoacidosis at initial presentation of type 1 diabetes in a prospective cohort study of children. Diabetes Care. 2014;37:1554–1562. doi: 10.2337/dc13-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghetti S, Lee JK, Sims CE, Demaster DM, Glaser NS. Diabetic ketoacidosis and memory dysfunction in children with type 1 diabetes. J Pediatr. 2010;156:109–114. doi: 10.1016/j.jpeds.2009.07.054. [DOI] [PubMed] [Google Scholar]
- 9.Dunger DB, Sperling MA, Acerini CL, Bohn DJ, Daneman D, Danne TP, Glaser NS, Hanas R, Hintz RL, Levitsky LL, et al. ESPE/LWPES consensus statement on diabetic ketoacidosis in children and adolescents. Arch Dis Child. 2004;89:188–194. doi: 10.1136/adc.2003.044875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lévy-Marchal C, Patterson CC, Green A, EURODIAB ACE Study Group Europe and Diabetes. Geographical variation of presentation at diagnosis of type I diabetes in children: the EURODIAB study. European and Dibetes. Diabetologia. 2001;44(Suppl 3):B75–B80. doi: 10.1007/pl00002958. [DOI] [PubMed] [Google Scholar]
- 11.Hekkala A, Knip M, Veijola R. Ketoacidosis at diagnosis of type 1 diabetes in children in northern Finland: temporal changes over 20 years. Diabetes Care. 2007;30:861–866. doi: 10.2337/dc06-2281. [DOI] [PubMed] [Google Scholar]
- 12.de Vries L, Oren L, Lebenthal Y, Shalitin S, Lazar L, Phillip M. Decrease in frequency of ketoacidosis at diabetes onset over the past two decades - perspectives of a paediatric tertiary care centre. Diabet Med. 2012;29:e170–5. doi: 10.1111/j.1464-5491.2012.03679.x. [DOI] [PubMed] [Google Scholar]
- 13.Jefferies C, Cutfield SW, Derraik JG, Bhagvandas J, Albert BB, Hofman PL, Gunn AJ, Cutfield WS. 15-year incidence of diabetic ketoacidosis at onset of type 1 diabetes in children from a regional setting (Auckland, New Zealand) Sci Rep. 2015;5:10358. doi: 10.1038/srep10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Usher-Smith JA, Thompson MJ, Sharp SJ, Walter FM. Factors associated with the presence of diabetic ketoacidosis at diagnosis of diabetes in children and young adults: a systematic review. BMJ. 2011;343:d4092. doi: 10.1136/bmj.d4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvi NS, Davies P, Kirk JM, Shaw NJ. Diabetic ketoacidosis in Asian children. Arch Dis Child. 2001;85:60–61. doi: 10.1136/adc.85.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, Oh K, Jang MJ, Hwang SS, Yoo MH, et al. 2007 Korean national growth charts: review of developmental process and an outlook. Korean J Pediatr. 2008;51:1–25. [Google Scholar]
- 17.National Health Insurance Service (KR) Major health insurance statistics: 2015 [Internet] [accessed on 27 June 2016]. Available at http://www.nhis.or.kr.
- 18.Rewers A, Klingensmith G, Davis C, Petitti DB, Pihoker C, Rodriguez B, Schwartz ID, Imperatore G, Williams D, Dolan LM, et al. Presence of diabetic ketoacidosis at diagnosis of diabetes mellitus in youth: the search for diabetes in youth study. Pediatrics. 2008;121:e1258–66. doi: 10.1542/peds.2007-1105. [DOI] [PubMed] [Google Scholar]
- 19.Elding Larsson H, Vehik K, Bell R, Dabelea D, Dolan L, Pihoker C, Knip M, Veijola R, Lindblad B, Samuelsson U, et al. Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care. 2011;34:2347–2352. doi: 10.2337/dc11-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Z, Zhou F, Dorman J, Wang H, Zu X, Mazumdar S, LaPorte RE. Association between infectious diseases and type 1 diabetes: a case-crossover study. Pediatr Diabetes. 2006;7:146–152. doi: 10.1111/j.1399-543X.2006.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rayfield EJ, Ault MJ, Keusch GT, Brothers MJ, Nechemias C, Smith H. Infection and diabetes: the case for glucose control. Am J Med. 1982;72:439–450. doi: 10.1016/0002-9343(82)90511-3. [DOI] [PubMed] [Google Scholar]
- 22.Vanelli M, Chiari G, Ghizzoni L, Costi G, Giacalone T, Chiarelli F. Effectiveness of a prevention program for diabetic ketoacidosis in children. An 8-year study in schools and private practices. Diabetes Care. 1999;22:7–9. doi: 10.2337/diacare.22.1.7. [DOI] [PubMed] [Google Scholar]
- 23.Samuelsson U, Stenhammar L. Clinical characteristics at onset of type 1 diabetes in children diagnosed between 1977 and 2001 in the south-east region of Sweden. Diabetes Res Clin Pract. 2005;68:49–55. doi: 10.1016/j.diabres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Sadauskaite-Kuehne V, Samuelsson U, Jasinskiene E, Padaiga Z, Urbonaite B, Edenvall H, Ludvigsson J, DEBS Study Group Severity at onset of childhood type 1 diabetes in countries with high and low incidence of the condition. Diabetes Res Clin Pract. 2002;55:247–254. doi: 10.1016/s0168-8227(01)00328-x. [DOI] [PubMed] [Google Scholar]


