Abstract
The present study investigated the temporal pattern and cellular localization of nestin in the adult mouse retina with pharmaceutically induced retinal degeneration using N-methyl-N-nitrosourea (MNU). After a single intraperitoneal injection of MNU in 8-week-old C57BL/6 mice, the animals were sacrificed at 1, 3, 5, 7, and 21 days (n = 6, in each stage). The eyes were examined by means of immunohistochemical tests using nestin, ionized calcium-binding adaptor molecule (Iba-1), CD11b, F4/80, and glial fibrillary acidic protein (GFAP). Western blot analysis and manual cell counting were performed for quantification. Nestin expression was increased after MNU administration. Nestin+/Iba-1+ cells were migrated into outer nuclear layer (ONL) and peaked at day 3 post injection (PI). Nestin+/CD11b+ cells were also mainly identified in ONL at day 3 PI and peaked at day 5. Nestin+/F4/80+ cells were shown in the subretinal space and peaked at day 3 PI. Nestin+/GFAP+ cells were distinctly increased at day 1 PI and peaked at day 5 PI. The up-regulation of nestin expression after MNU administration in adult mouse retinal microglia, and monocyte/macrophage suggests that when retinal degeneration progresses, these cells may revert to a more developmentally immature state. Müller cells also showed reactive gliosis and differentiational changes.
Keywords: Nestin, N-methyl-N-nitrosourea (MNU), Retinal Degeneration, Mouse
Graphical Abstract
INTRODUCTION
Nestin is a class VI intermediate filament (IF) protein. Nestin was first described in the developing central nervous system (CNS) as a gene whose expression distinguishes stem cells from the more differentiated cells in the neural tube (1). It is now known as a reliable marker of neural stem cells and progenitor cells (2). However, recent studies have shown that nestin can be expressed in other cells including proliferative endothelial cells (3), pericytes in adult CNS capillary (4), or reactive astrocytes after CNS injury (5,6).
With regards to the retina, as a part of the CNS, nestin is expressed in fetal retinal cells (7). Additional studies have revealed that Müller cells express nestin after an acute injury such as experimentally induced glaucoma (8), optic nerve transection (9), laser photocoagulation (10), and pharmaceutically induced retinal degeneration (11). One study reported expression of nestin in reactive astrocytes in experimental retinal detachment rat model (12). Another study evidenced nestin expression in retinal microglia after optic nerve transection in adult rats (13).
In this study, we investigated the temporal pattern and cellular localization of nestin in the adult mouse retina with pharmaceutically induced retinal degeneration using N-methyl-N-nitrosourea (MNU). Although, previous studies have demonstrated nestin expression in inherited retinal degeneration in a genetic rat model (14), and in pharmaceutically induced retinal degeneration adult rat model (11), these studies localized nestin expression using Müller cell maker, that is glutamine synthetase (GS) or glial fibrillary acidic protein (GFAP). In the present study, we examined the co-localization of nestin and microglia/monocyte/macrophage cell markers using ionized calcium-binding adaptor molecule (Iba-1), CD11b, and F4/80 as well as the Müller cell marker GFAP. Expression of nestin after MNU injection was quantified by means of Western blot analysis.
MATERIALS AND METHODS
Animals
C57BL/6 female mouse of 8-week-old obtained from Charles River Laboratories (Bundang, Korea). All the animals were housed in standard cages under a 12-hour light-dark cycle, with food and drinking water available ad libitum. All animals and experiment procedures were kept in accordance with the Association of Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. The study protocol was reviewed and approved by the institutional animal care and use committee of the Soonchunhyang University Bucheon Hospital (No. SCHBC-animal-2013-12).
Induction of retinal degeneration
The MNU (Sigma-Aldrich, St. Louis, MO, USA) was kept at −20°C in the dark. Prior to its use, MNU powder was dissolved in physiologic saline, and a single intraperitoneal injection of MNU (60 mg/kg of bodyweight) was given to induce retinal degeneration in each of 15 mice to be used for immunohistochemical testing (n = 3 in each of the five stages [at days 1, 3, 5, 7, and 21]) and in each of the 15 mice to be used for western blot analysis (n = 3 in each of the five stages). The animals were sacrificed at 1, 3, 5, 7, and 21 days after the injection. Six additional age-matched, untreated control animals (3 mice for the immunohistochemical testing and 3 mice for the western blot analysis) were kept under the same conditions and were examined using the same methods and procedures that were used for the MNU-treated animals.
Tissue preparation
For cryosectioning, the eyes were enucleated and the anterior segments including the cornea, iris, and lens were removed. The posterior eye cups were fixed in 4% paraformaldehyde (PFA) for 1 hour at 4°C and then immersed in 30% sucrose overnight at 4°C, and embedded and frozen in frozen section compound (Leica Biosystems, Richmond, IL, USA). Completely frozen molds were stored at −70°C until used. Sagittally oriented, 8 µm sections were prepared and only the central sections, which included the optic nerve head were used for measurement purposes. All slides were air-dried for 1 day.
Antibody characterization and immunofluorescence staining
To identify the cellular responses in the retina after MNU-induced retinal degeneration, we obtained primary antibodies against nestin (mouse monoclonal, 1:500; Millipore, Billerica, MA, USA), Iba-1 (a marker for microglia; rabbit polyclonal, 1:1,000; Wako Pure Chemicals, Osaka, Japan) (15), CD11b (a marker for monocyte/macrophage; rat monoclonal, 1:1,000; AbD Serotec, Oxford, UK) (16), F4/80 (a marker for monocyte/macrophage; rat monoclonal, 1:2,000; AbD Serotec) (17), and GFAP (a marker for Müller cells; rabbit polyclonal, 1:500; Millipore) (18). Secondary antibodies included Alexa Fluor-488 conjugated goat anti-mouse for nestin; Alexa Fluor-568 anti-rat for CD11b and F4/80; and Alexa Fluor-568 anti-rabbit for GFAP and Iba-1(1:2,000; Molecular Probes, Eugene, OR, USA). For immunofluorescence staining, the cryosections were permeabilized with 0.1% Triton-X100 (Sigma-Aldrich) in 5% goat serum diluted in phosphate buffered saline (PBS; pH 7.4) for 1 hour, followed by overnight incubation with primary antibodies in 5% goat serum at 4°C. The slides were then washed three times for 5 minutes in PBS, and were further incubated with the appropriate secondary antibodies diluted to 1:2,000 in 5% goat serum. Finally, nuclei were counterstained with 4-6-diamino-2-phenylindole (DAPI) and the sections were mounted using fluorescent mounting medium (Dako, Glostrup, Denmark). Additionally, the cellular markers Iba-1, CD11b, and F4/80 have a cross reaction of staining monocytes, microglia, and macrophages, these cells likely represent overlapping spectra of cells of the same lineage. Therefore, we defined microglia as ramified or amoeboid cells in the neural retina and along the retinal vessels, monocytes as round cells in the vessels or still attached to the vessel walls, and macrophages as ramified or amoeboid cells in the nonneural tissue (17).
Western blot analysis
After the careful dissection of the retinas from the eye cups, the retinal tissue was flash frozen in −80°C liquid nitrogen until samples at all time points were collected. A 100 µL ice-cold extraction buffer containing 10 mM of Tris-HCl (pH = 7.5), 5 mM of ethylenediaminetetraacetic acid (EDTA), 200 mM of NaCl, 1 mM of phenylmethylsulfonyl fluoride, 1 µg/mL of leupeptin, and 28 µg/mL of aprotinin was added to both retinal tissues from one mouse. The retinal samples were sonicated and centrifuged at 12,000 rpm for 30 minutes at 4°C. The supernatant was collected, and protein concentrations were measured according to the manufacturer's instructions (BCA Protein Assay Kit; Thermo Scientific, Rockford, IL, USA). Proteins (30 µg of each sample) were fractionated on 8% polyacrylamide precast gels (Bio-Rad, Cambridge, MA, USA), and transferred to a polyvinylidene difluoride (PVDF) membrane using the Trans-Blot Turbo Transfer System (Bio-Rad). The membranes were blocked for 2 hours at room temperature in Tris-buffered saline (TBS) containing 5% skim milk and were then incubated with nestin (mouse monoclonal, 1:5,000; Millipore) or GFAP antibody (rabbit polyclonal, 1:1,000; Sigma-Aldrich) overnight at 4°C. The membranes were finally incubated with peroxidase-conjugated anti-mouse or anti-rabbit IgG (Sigma-Aldrich) for 2 hours at room temperature. Enhanced chemoluminescence (ECL) detection of nestin or GFAP was performed according to the manufacturer's instructions (Amersham Biosciences, Piscataway, NJ, USA). The expression of those markers was used to quantify and compare them with each other using ImageJ software, version 1.47c (Image Processing and Analysis in Java; National Institutes of Health, Hamilton, MO, USA).
Cell counts
Cells positive for Iba-1, CD11b, F4/80, and nestin were counted in the MNU-treated retinal sections. Cell counts were performed for different time periods. The total number of immunofluorescent-labeled cells in whole retinal sections that contained the optic nerve head was counted manually, and two experienced counters were given the unlabeled sample slides to prevent bias.
Statistical analysis
The Mann-Whitney U test was used to assess differences in cell counts at the five designated time points after MNU administration. Statistical analysis was conducted using SPSS Statistics Version 21 (IBM, Somers, NY, USA). All tests were two-tailed, and differences having P values of less than 0.05 were considered statistically significant.
RESULTS
Degenerative retinal changes after MNU treatment
Intraperitoneal MNU injection was used to induce retinal degenerative changes. At 3 days after MNU injection, retinal thickness had obviously decreased owing to the selective loss of photoreceptor cells, and the arrangement of retinal neurons in the outer nuclear layer (ONL) was distorted (Fig. 1). Progressive loss of photoreceptor cells led to a nearly complete loss of ONL by day 7 PI (Fig. 1).
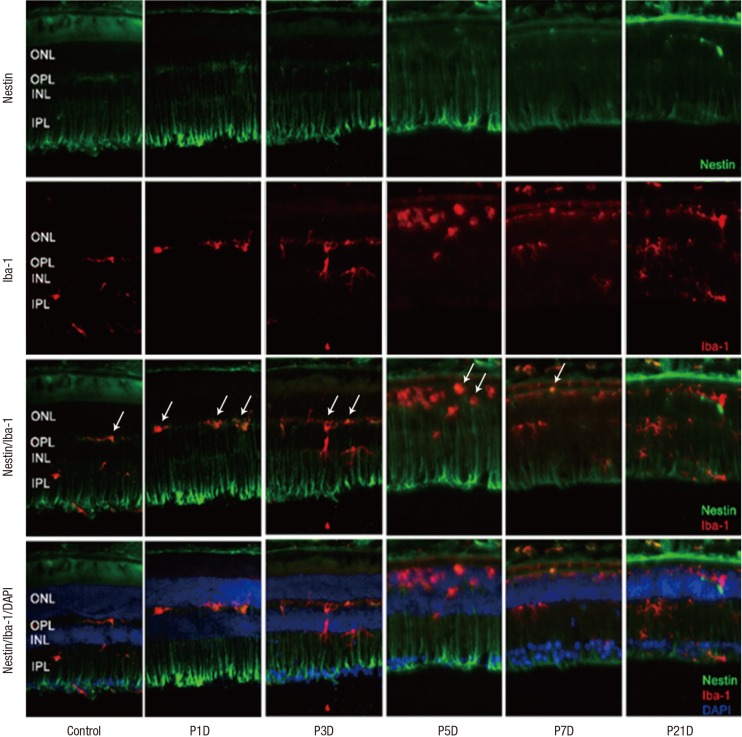
Fig. 1.
Nestin and Iba-1 expression in control and degenerated adult mouse retina.
Immunofluorescent labeling with nestin (green), Iba-1(red), and DAPI (blue) is shown. DAPI was used for nuclei staining to visualize the retinal layers. The decrease in thickness of the ONL due to selective loss of photoreceptor cells was becoming obvious by day 3 after MNU injection. Progressive loss of photoreceptor cells ultimately led to a complete loss of the ONL at day 7 PI. Immunoreactivity of nestin was increased at day 1 PI, peaked at day 3, and declined gradually until day 21. MNU treatment resulted in migrating nestin+/Iba-1+ cells into the ONL. The number of nestin+/Iba-1+ cells peaked at day 3 PI. After that, the number of nestin+/Iba-1+ cells diminished progressively, particularly at day 7, and the cells were sparsely distributed by day 21. Arrows indicate nestin/Iba-1 co-immunolabeling.
Iba-1 = ionized calcium-binding adaptor molecule, DAPI = 4-6-diamino-2-phenylindole, ONL = outer nuclear layer, MNU = N-methyl-N-nitrosourea, PI = post injection, OPL = outer plexiform layer, INL = inner nuclear layer, IPL = inner plexiform layer.
Nestin expression in resting retina
In the normal adult mouse retina, nestin was rarely expressed except for some vascular profiles in the outer plexiform layer (OPL) (Fig. 1).
Up-regulation of nestin expression after MNU treatment
Nestin expressing Iba-1+ microglia with amoeboid shape were identified mainly in outer plexiform layer at 1 day after retinal degeneration induced by MNU injection (Fig. 1). Nestin expressing microglia, previously located mainly in the OPL, had migrated into ONL. The number of microglia showing nestin expression was markedly increased at day 3 PI (Fig. 1). At day 5 PI, cell dimensions of nestin+ microglia were more extended (Fig. 1). At day 7 PI, nestin expressing microglia were significantly decreased compared with day 5 PI, and the immunoreactivity of nestin in the whole retina was also significantly diminished (Fig. 1). By 21 days after MNU, most of the retinal neurons nuclei were degraded and nestin expression had decreased to the baseline levels.
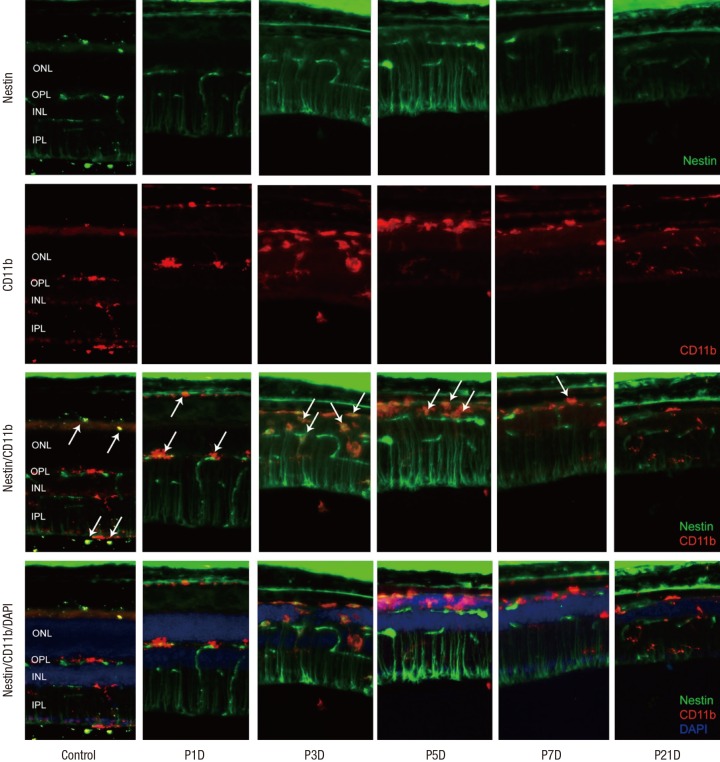
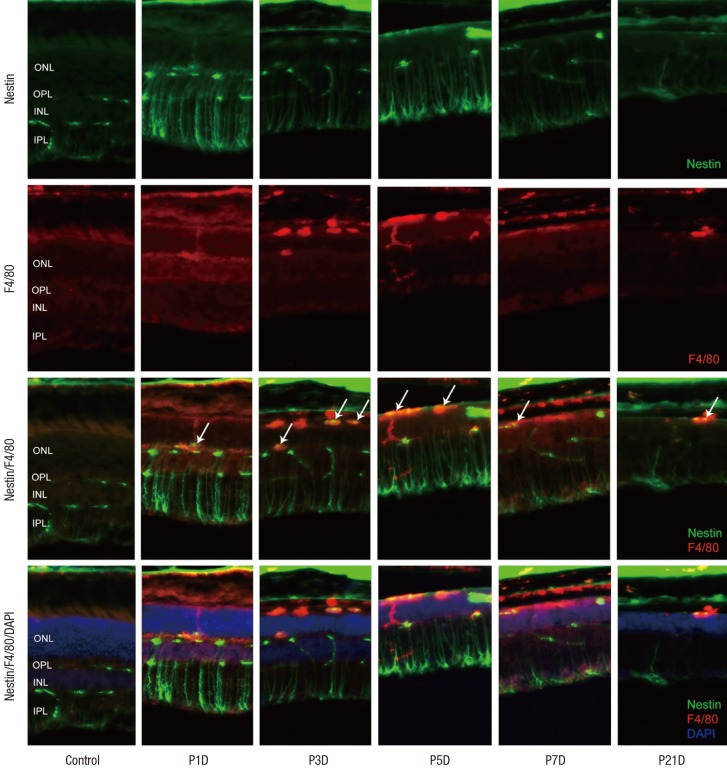
Expression of CD11b and F4/80 were rarely identified in the physiologic state prior to the MNU injection except for their expression in the endothelium (Figs. 2 and 3). At day 1 after MNU injection, nestin+/CD11b+ cells were evident in the OPL. Nestin+/CD11b+ cells migrated into the ONL at day 3PI (Fig. 2), peaked at day 5 (Fig. 2), and decreased thereafter (Fig. 2). F4/80+ cells observed in the subretinal space revealed oval or spindle shaped cytoplasm without ramified processes at day 1 PI (Fig. 3). The number of amoeboid nestin+/F4/80+ cells increased markedly from day 3 to day 5 PI and these cells were heavily confined within the subretinal space and OPL (Fig. 3). Although the number of F4/80+ cells decreased thereafter, it was relatively preserved at 7 days after MNU injection (Fig. 3).
Fig. 2.
Nestin and CD11bexpression in control and degenerated adult mouse retina.
Immunofluorescent labeling with nestin (green), CD11b (red), and DAPI (blue) is shown. Arrows indicate nestin/CD11b co-immunolabeling. After a single injection of MNU, nestin+/CD11b+ cells migrated into the ONL at day 3 and showed a peak at day 5. The number of nestin+/CD11b+ cells diminished progressively, particularly after 7 days, with sparse distribution by day 21.
DAPI = 4-6-diamino-2-phenylindole, ONL = outer nuclear layer, MNU = N-methyl-N-nitrosourea, OPL = outer plexiform layer, INL = inner nuclear layer, IPL = inner plexiform layer.
Fig. 3.
Nestin and F4/80 expression in control and degenerated adult mouse retina.
Immunofluorescent labeling with nestin (green), F4/80 (red), and DAPI (blue) is shown. Arrows indicate nestin and F4/80 co-immunolabeling. The number of amoeboid nestin+/F4/80+ cells increased markedly from day 3 to day 5 post injection and decreased thereafter.
DAPI = 4-6-diamino-2-phenylindole, ONL = outer nuclear layer, OPL = outer plexiform layer, INL = inner nuclear layer, IPL = inner plexiform layer.
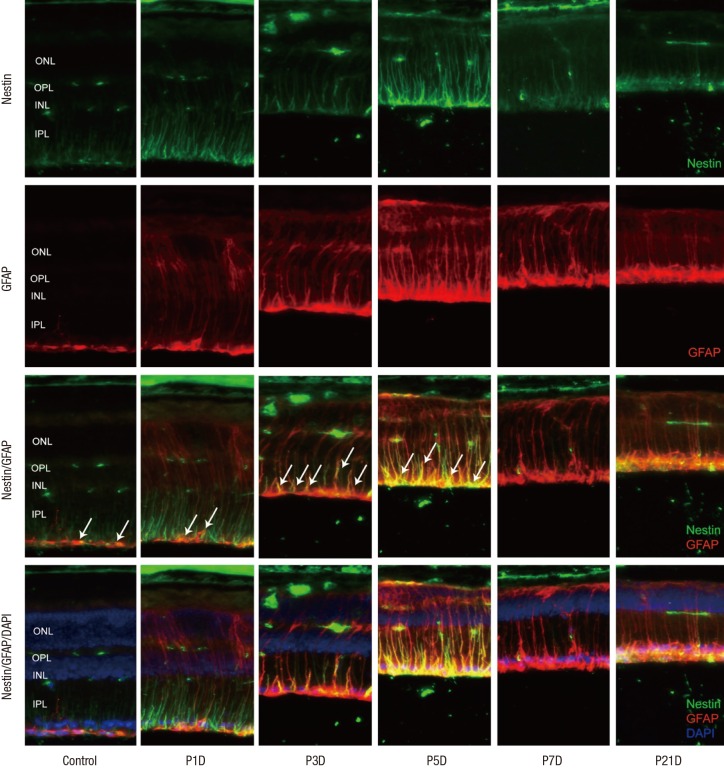
GFAP labeled Müller cells were distinctly increased at 1 day after MNU (Fig. 4). Nestin immunoreactivity of GFAP+ astrocytes in the ganglion cell layer (GCL) and Müller cells was progressively increased at day 3 PI (Fig. 4), and revealed peak activity at day 5 (Fig. 4). After that, the number of nestin+/GFAP+ Müller cells was markedly reduced. However, GFAP expression remained in an activated state in the retinal tissue at day 7 (Fig. 4) and day 21 after MNU despite the diminution in retinal thickness (Fig. 4).
Fig. 4.
Nestin and GFAP expression in control and degenerated adult mouse retina.
Immunofluorescent labeling with nestin (green), GFAP (red), and DAPI (blue) is shown. Arrows indicate nestin/GFAP co-immunolabeling. The number of nestin+/GFAP+ Müller cells increased progressively at day 3 after MNU treatment, peaked at day 5, and decreased thereafter. GFAP-positive cells remained in an activated state at day 7 and day 21 post injection, despite the diminution in retinal thickness.
GFAP = glial fibrillary acidic protein, DAPI = 4-6-diamino-2-phenylindole, ONL = outer nuclear layer, OPL = outer plexiform layer, INL = inner nuclear layer, IPL = inner plexiform layer.
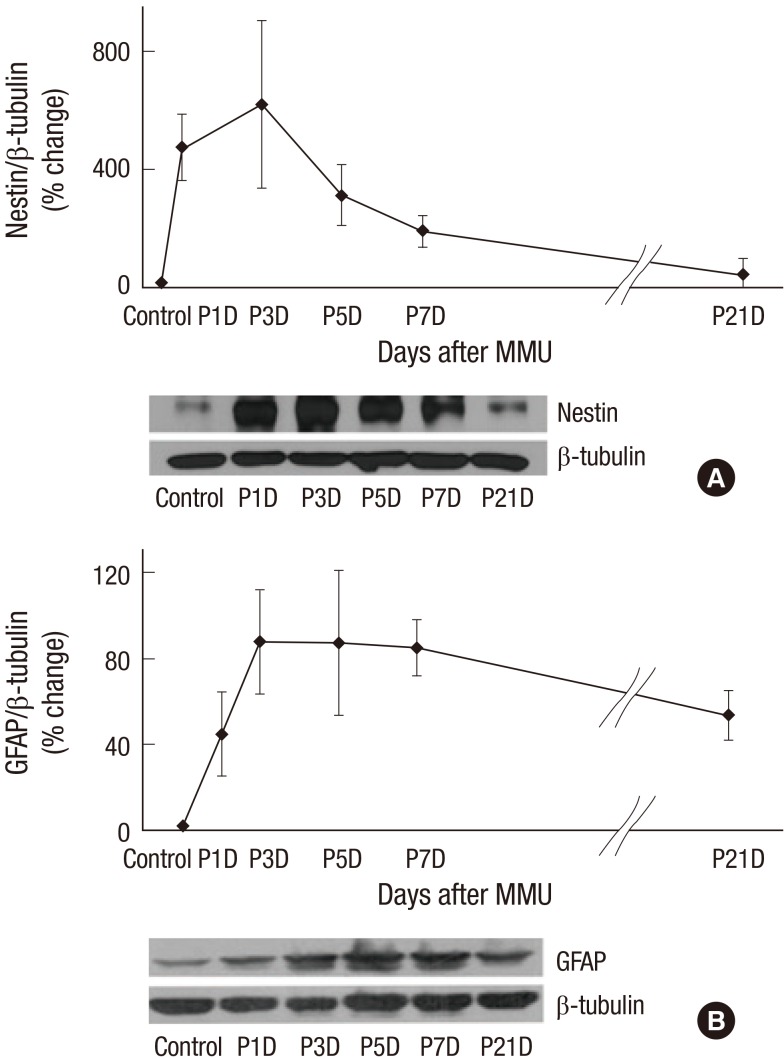
Western blot analysis
We used western blot analysis to quantify the expression of nestin and GFAP. Nestin showed increased immunoreactivation at day 1 PI. The expression of nestin peaked at day 3 PI and declined at day 7 PI. At day 21 PI, the level declined to baseline when compared with the control retinas (Fig. 5). GFAP expression initially showed a gradual increase for the first 3 days after the MNU injection, and this level was maintained from day 3 to day 7 PI. GFAP expression decreased at day 21 PI; however, unlike nestin expression, GFAP expression was higher than that in the control retina (Fig. 5).
Fig. 5.
Western blot analyses of nestin and GFAP expression.
(A) Significantly increased immune-reactivity of nestin at day 1 and significantly decreased to mostly the level of control group at 21 days after MNU injection. (B) Immune-reactivity of GFAP showed gradually increasing for 3 days after MNU injection, and declined at 21 days. Nevertheless, GFAP remained in activated state compared to control group even after 21 days after MNU injection.
GFAP = glial fibrillary acidic protein, MNU = N-methyl-N-nitrosourea.
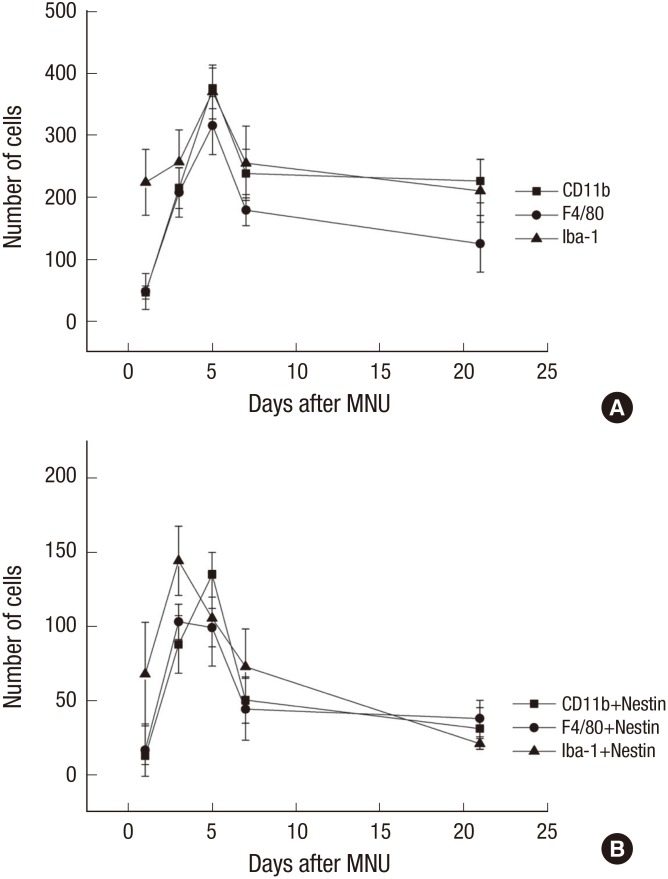
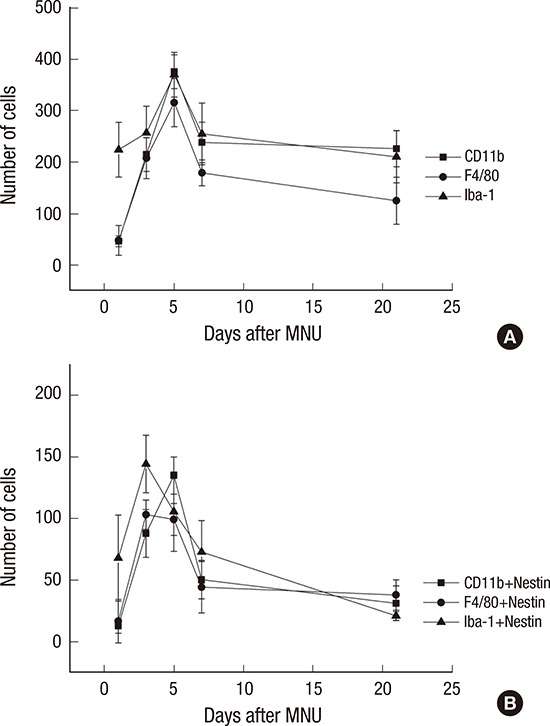
Cell count
The number of nestin-expressing cells labeled with CD11b, Iba-1, and F4/80 was calculated in the retinal sections. After the MNU injection, the number of microglia/monocyte/macrophage marker-positive cells was increased at day 1 after MNU, peaked at day 5 (P < 0.001), and then decreased significantly at day 7 PI (P < 0.001) (Fig. 6). The number of nestin co-expressing microglia/ monocytes/macrophages was also increased at day 1 PI. The number of nestin+/Iba-1+ and nestin+/F4/80+ cells peaked at day 3 PI (P < 0.001). The number of nestin+/Iba-1+ cells decreased significantly at day 5 (P < 0.001), and the number of nestin+/F4/80+ cells was significantly reduced at day 7 (P < 0.001). The number of nestin+/CD11b+ cells peaked at day 5 PI (P < 0.001) and then decreased at day 7 (P < 0.001) (Fig. 6B).
Fig. 6.
Cell counts of Iba-1+, CD11b+, F4/80+, and nestin+ cells.
(A) Iba-1+, CD11b+, F4/80+ cells were peaked at 5 days after injection. (B) Nestin/Iba-1 co-immunolabelling cells and Nestin/F4/80 co-immunolabelling cells were peaked at 3 days after injection. Nestin/CD11b co-immunolabelling cells were peaked at 5 days after injection.
Iba-1 = ionized calcium-binding adaptor molecule, MNU = N-methyl-N-nitrosourea.
DISCUSSION
Recently, nestin expression in the retina after acute injury has been widely studied (8,9,10,11,12,13). Previous studies have shown nestin expression in a degenerative retinal disease model. Wan et al. (11) investigated the possibility of photoreceptor regeneration from Müller glia after MNU induced retinal degeneration in the adult rat retina. In that study, Müller glia underwent reactive gliosis with the up-regulation of nestin and GFAP after MNU administration, and some Müller glia derived cells were induced to express rhodopsin exclusively. Valamanesh et al. (14) examined the temporal pattern and cellular localization of nestin in the newborn rat retina in a neurodegenerative inherited disease model using genetically mutated rats. The study demonstrated that the level of nestin expression remained low after postnatal day 20 up to 1 year in normal animals but was up-regulated from postnatal day 30 in the dystrophic animals, and they concluded that nestin might be involved in mechanisms of migration, generation of new neurons or glial cells and/or retinal modeling (14).
In the present study, we assessed nestin expression in the adult mouse retina with MNU induced retinal degeneration model, and showed the up-regulation of nestin expression in microglia, monocytes/macrophages, Müller glia cells and astrocytes after MNU treatment. To the best of our knowledge, this is the first report of localized nestin expressing cells using microglia and monocyte/macrophage markers as well as a Müller cell marker in the adult mouse degenerative retinal disease model.
Microglia and monocytes/macrophages showed differentiational changes in the adult mouse retina after MNU administration. Nestin up-regulation in the Iba-1 positive and CD11b/F4/80 positive cells suggests that these cells may revert to a more developmentally immature state, since nestin serves as a cell cycle reentry marker (12). In the resting retina, microglia marker positive cells were identified in the inner plexiform layer (IPL), the OPL, and the GCL. After MNU administration, these cells migrated toward the ONL and simultaneously showed increased nestin expression. Monocytes/macrophages marker positive cells were rarely recognized in the normal retina, but after MNU treatment, monocyte/macrophages were observed and also showed nestin co-expression.
The Müller cells also showed dedifferentiational changes after MNU treatment. Previous studies have shown that nestin expression in differentiated Müller cell can be induced following retinal injury (7,10,12). Consistent with the results of previous studies, the present study demonstrated reactive gliosis and nestin up-regulation of Müller cell after MNU injection. Moreover, it is thought that, similar to GFAP, the nestin expression represent not only progenitor cells but also reactive gliosis in the pathologic retina (10,19,20). From this perspective, nestin might be used as an early stage marker of acute retinal injury when compared with the GFAP.
In conclusion, retinal microglia, macrophage, and Müller cells showed up-regulation of nestin expression in the adult mouse with pharmaceutically induced retinal degeneration model using MNU, and the results suggest that these cells may revert to a more developmentally immature state. In addition, nestin expression levels were sharply increased immediately after MNU administration and then returned to baseline levels. This finding implies that nestin might be used as an early-stage marker of acute retinal injury.
Footnotes
Funding: This work was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF) as funded by the Ministry of Education, Science, and Technology (Grant number 2016R1A2B4008376), and partially by the Soonchunhyang University Research Fund.
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Moon CH, Cho H, Park TK. Data curation: Moon CH, Cho H, Kim YK. Funding acquisition: Park TK. Investigation: Moon CH, Cho H, Kim YK, Park TK. Supervision: Cho H, Park TK. Writing - original draft: Moon CH, Cho H, Park TK. Writing - review & editing: Moon CH, Cho H, Park TK.
References
- 1.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki S, Namiki J, Shibata S, Mastuzaki Y, Okano H. The neural stem/progenitor cell marker nestin is expressed in proliferative endothelial cells, but not in mature vasculature. J Histochem Cytochem. 2010;58:721–730. doi: 10.1369/jhc.2010.955609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- 5.Frisén J, Johansson CB, Török C, Risling M, Lendahl U. Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J Cell Biol. 1995;131:453–464. doi: 10.1083/jcb.131.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke SR, Shetty AK, Bradley JL, Turner DA. Reactive astrocytes express the embryonic intermediate neurofilament nestin. Neuroreport. 1994;5:1885–1888. doi: 10.1097/00001756-199410000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Walcott JC, Provis JM. Müller cells express the neuronal progenitor cell marker nestin in both differentiated and undifferentiated human foetal retina. Clin Experiment Ophthalmol. 2003;31:246–249. doi: 10.1046/j.1442-9071.2003.00638.x. [DOI] [PubMed] [Google Scholar]
- 8.Xue LP, Lu J, Cao Q, Hu S, Ding P, Ling EA. Müller glial cells express nestin coupled with glial fibrillary acidic protein in experimentally induced glaucoma in the rat retina. Neuroscience. 2006;139:723–732. doi: 10.1016/j.neuroscience.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 9.Wohl SG, Schmeer CW, Kretz A, Witte OW, Isenmann S. Optic nerve lesion increases cell proliferation and nestin expression in the adult mouse eye in vivo. Exp Neurol. 2009;219:175–186. doi: 10.1016/j.expneurol.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Kohno H, Sakai T, Kitahara K. Induction of nestin, Ki-67, and cyclin D1 expression in Müller cells after laser injury in adult rat retina. Graefes Arch Clin Exp Ophthalmol. 2006;244:90–95. doi: 10.1007/s00417-005-0030-7. [DOI] [PubMed] [Google Scholar]
- 11.Wan J, Zheng H, Chen ZL, Xiao HL, Shen ZJ, Zhou GM. Preferential regeneration of photoreceptor from Müller glia after retinal degeneration in adult rat. Vision Res. 2008;48:223–234. doi: 10.1016/j.visres.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Luna G, Lewis GP, Banna CD, Skalli O, Fisher SK. Expression profiles of nestin and synemin in reactive astrocytes and Müller cells following retinal injury: a comparison with glial fibrillar acidic protein and vimentin. Mol Vis. 2010;16:2511–2523. [PMC free article] [PubMed] [Google Scholar]
- 13.Wohl SG, Schmeer CW, Friese T, Witte OW, Isenmann S. In situ dividing and phagocytosing retinal microglia express nestin, vimentin, and NG2 in vivo. PLoS One. 2011;6:e22408. doi: 10.1371/journal.pone.0022408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valamanesh F, Monnin J, Morand-Villeneuve N, Michel G, Zaher M, Miloudi S, Chemouni D, Jeanny JC, Versaux-Botteri C. Nestin expression in the retina of rats with inherited retinal degeneration. Exp Eye Res. 2013;110:26–34. doi: 10.1016/j.exer.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 16.London A, Itskovich E, Benhar I, Kalchenko V, Mack M, Jung S, Schwartz M. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. J Exp Med. 2011;208:23–39. doi: 10.1084/jem.20101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caicedo A, Espinosa-Heidmann DG, Piña Y, Hernandez EP, Cousins SW. Blood-derived macrophages infiltrate the retina and activate Muller glial cells under experimental choroidal neovascularization. Exp Eye Res. 2005;81:38–47. doi: 10.1016/j.exer.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Erickson PA, Fisher SK, Guérin CJ, Anderson DH, Kaska DD. Glial fibrillary acidic protein increases in Müller cells after retinal detachment. Exp Eye Res. 1987;44:37–48. doi: 10.1016/s0014-4835(87)80023-4. [DOI] [PubMed] [Google Scholar]
- 19.Xue LP, Lu J, Cao Q, Kaur C, Ling EA. Nestin expression in Müller glial cells in postnatal rat retina and its upregulation following optic nerve transection. Neuroscience. 2006;143:117–127. doi: 10.1016/j.neuroscience.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 20.Xue L, Ding P, Xiao L, Hu M, Hu Z. Nestin, a new marker, expressed in Müller cells following retinal injury. Can J Neurol Sci. 2010;37:643–649. doi: 10.1017/s0317167100010830. [DOI] [PubMed] [Google Scholar]