Abstract
In the brain, myelin is important in regulating nerve conduction and neurotransmitter release by providing insulation at axons. Myelin is a specialized yet continuous sheet structure of differentiated oligodendrocytes (OLs) that is enriched in lipids, specifically galactosylceramides (GalCer) originated at the endoplasmic reticulum (ER). GalCer are known to affect OL differentiation. However, the mechanism whereby GalCer affect OL differentiation is not well understood. Sigma-1 receptors (Sig-1Rs), shown by us to exist in detergent-insoluble lipid microdomains at lipid-enriched loci of ER in NG108 cells, are important in the compartmentalization/transport of ER-synthesized lipids and in cellular differentiation. In this study, we used rat primary hippocampal cultures and found that Sig-1Rs form GalCer-enriched lipid rafts at ER lipid droplet-like structures in the entire myelin sheet of mature OLs. In rat OL progenitors (CG-4 cells), levels of lipid raft-residing Sig-1Rs and GalCer increase as cells differentiate. Sig-1Rs also increase in OLs and myelin of developing rat brains. Sig-1R, GalCer, and cholesterol are colocalized and are resistant to the Triton X-100 solubilization. Treating cells with a Sig-1R agonist or targeting Sig-1Rs at lipid rafts by overexpression of Sig-1Rs in CG-4 cells enhances differentiation, whereas reducing Sig-1Rs at lipid rafts by transfection of functionally dominant-negative Sig-1Rs attenuates differentiation. Furthermore, Sig-1R siRNA inhibits differentiation. Our findings indicate that, in the brain, Sig-1Rs targeting GalCer-containing lipid microdomains are important for OL differentiation and that Sig-1Rs may play an important role in the pathogenesis of certain demyelinating diseases.
Oligodendrocytes (OLs) are the myelin-forming cells of the CNS. OL progenitors originate from subventricular zones of the brain and migrate along axonal tracts to various regions, where they differentiate into immature and mature OLs (1-5). Differentiated OLs synthesize large amounts of myelin that insulate the axons and regulate nerve conduction (2, 3). A noteworthy characteristic of myelin is its enrichment in lipids. Myelin contains ≈70% of total brain cholesterol (4). Myelin also contains unique glycosphingolipids, such as galactosylceramides (GalCer) and sulfatides, which together comprise 27% of total myelin lipids (4, 5). GalCer synthesized on the endoplasmic reticulum (ER) are transported to the outer leaflet of the OL plasma membrane at a critical point of differentiation when OL progenitors stop proliferation and commence terminal differentiation (3, 6). GalCer, at least in part, are assembled in lipid microdomains in the Golgi apparatus and regulate sorting of myelin specific proteins (7). Recent studies using GalCer antibodies suggest that GalCer function as negative regulators of OL differentiation (6). However, it is unclear at present how myelin-specific lipids are transported to the plasma membrane and how they regulate OL differentiation.
Sigma-1 receptors (Sig-1Rs) are brain-enriched ER proteins that bind neurosteroids and cocaine and are implicated in certain psychiatric disorders (8-11). Sig-1Rs modulate ion channel activities (e.g., K+ channel and inositol 1,3,4-trisphosphate receptors) (12, 13) and neurotransmitter release (14). Sig-1Rs are also involved in learning and memory and the neuroadaptation to drug-induced reward processes (15, 16). Sig-1Rs have no homology to any mammalian protein (17). Although Sig-1Rs have a 30% identity to the fungal sterol C8-C7 isomerase, they lack the sterol isomerase activity (17, 18).
Sig-1Rs dynamically change their distribution pattern on the ER when stimulated (13, 19). Thus, a portion of Sig-1Rs is observed in neurites with cytoskeletal adaptor protein ankyrin (13) and can move anterogradely toward the tip in NG108 cells (19). Sig-1R agonists and up-regulation of this protein potentiate nerve growth factor- or epidermal growth factor-induced neurite sprouting in PC12 cells by changing the lipid constituents of plasma membrane lipid microdomains (lipid rafts) (20, 21). In NG108 cells, Sig-1Rs specifically target intracellular lipid microdomains on ER-associated lipid droplets that contain cholesterol and neutral lipids (22, 23). Also, in NG108 cells, dysfunction of Sig-1Rs causes retention and a diffused distribution of neutral lipids and cholesterol within the entire ER and a decrease of cholesterol in the Golgi apparatus and the plasma membrane (22). We hypothesized, therefore, that Sig-1Rs on the ER might regulate cellular differentiation by controlling compartmentalization and transport of ER-synthesized lipids that eventually are used for formation of plasma membrane lipid microdomains (21). To extend our findings from cell lines on unique properties of Sig-1Rs and to test the relevance of our hypothesis in the brain, we examined cellular distribution of Sig-1Rs and lipids in primary cultures from rat hippocampus. Here, we found that OLs possess GalCer/cholesterol-containing lipid microdomains on the ER where Sig-1Rs specifically target. We also found that Sig-1Rs residing in these GalCer-containing lipid microdomains play an important role in OL differentiation.
Materials and Methods
Cell Culture. Rat hippocampus from 3-day-old Wistar rats were prepared as described in ref. 24. Mixed glial culture was maintained in DMEM containing 5% FCS, 5% horse serum, 2 mM glutamine, and 110 mg/liter pyruvate. OL-type 2 astrocyte progenitor cells isolated from the rat brain (GC-4 cells) were cultured as described in ref. 25. The cells were grown in DMEM-N1, 10 ng/ml biotin, and 30% conditioned medium from rat neuroblastoma B104 cells (proliferation medium). For differentiation of CG-4 cells to OLs, cells were cultured in DMEM-N1 and 15 μg/ml insulin (differentiation medium). Differentiation was assessed by counting numbers of processes longer than a cell-body length and by numbers of cells forming myelin-like structures (>4 processes per cell with extensive network formation).
Immunocytochemistry and Confocal Microscopy. Immunocytochemistry was performed as described in refs. 19 and 22. Dilution ratios and sources of antibodies/fluorescent dyes are given in the legend to Fig. 6, which is published as supporting information on the PNAS web site. Cells were examined with a laser scanning confocal system (Zeiss) (22).
In Situ Solubilization of Cells with Triton X-100 and Semiquantification of Proteins in Lipid Rafts. OLs grown on glass coverslips were kept at 4°C for 5 min, followed by a treatment with HBS (5.4 mM KCl, 0.4 mM KH2PO4, 137 mM NaCl, 3 mM NaHCO3 and 0.3 mM Na2HPO4)-containing Triton X-100 (0.1-1.0%) for 20 min at 4°C. Triton X-100-treated cells were triple-labeled with GalCer antibody, filipin, and an antibody against the protein of interest. Mean intensities of each staining at OL sheets were measured as described in the legend to Fig. 6.
Preparation of Triton X-100-Insoluble Microdomains and Western Blotting. CG-4 cell lysates were prepared in Triton X-100-containing TNE buffer as described in ref. 22. After centrifugation at 12,000 × g for 10 min, supernatants were subjected to ultracentrifugation (82,000 × g for 1 h) to yield soluble fraction and raft fractions. Proteins were analyzed by Western blotting with an enhanced chemiluminescence kit (ECL, Amersham Pharmacia). Results were quantified by using nih image software.
Extraction of Lipids and TLC. Total lipids were extracted and subjected to the silicic acid chromatography according to Dasgupta and Hogan (26) to yield cholesterol and monoglycosyl ceramide fractions. Lipids were resolved by high-performance TLC, and respective lipids were visualized as follows: neutral lipids, hexane/ether/acetic acid (80:20:1), FeCl3/H2SO4 spray; GalCer: chloroform/methanol/water (85:15:0.5), dephenylamine-aniline-phosphoric acid spray.
Construction and Transfection of Vectors Encoding Enhanced Yellow Fluorescent Protein (EYFP)-Tagged Sig-1Rs and Sig-1R siRNA. Constructions of vectors for EYFP-tagged Sig-1Rs were described in refs. 19 and 22. For Sig-1R siRNA, two PAGE-purified complementary oligonucleotides were synthesized: 5′-GATCCACACGTGGATGGTGGAGTATTCAAGAGATACTCCACCATCCACGTGTTTTTTTGCTAGCG-3′ and 5′-AATTCGCTAGCAAAAAAACACGTGGATGGTGGAGTATCTCTTGAATACTCCACCATCCACGTGTG-3′, where bold letters stand for rat Sig-1R gene 516-534, italics stand for either BamHI or EcoRI overhangs, and bold italics stand for hairpin loop sequences. These two oligonucleotides were annealed and ligated in pSIREN vector (Clontech). For transfection of vectors into CG-4 cells, a vector (2 μg per well)/Lipofectamine-2000 (2.5 μl per well) (GIBCO) mixture was applied to CG-4 cells in proliferation medium in 12-well plates. After incubation for 6-8 h, the medium was replaced with differentiation medium.
Results
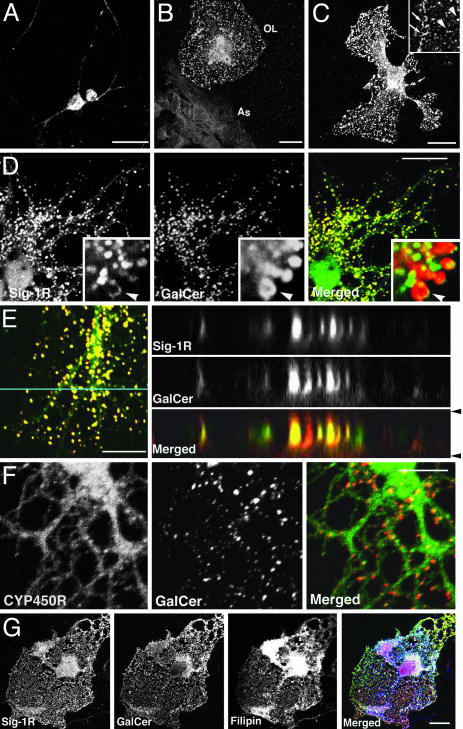
Cellular Distribution of Sig-1Rs in Rat Hippocampal Primary Cells. Sig-1R immunocytochemistry showed that Sig-1Rs are expressed in hippocampal neurons. A majority of Sig-1Rs was present in the cell body of neurons (Fig. 1A). The expression level of Sig-1Rs was low in hippocampal astrocytes but high in terminally differentiated OLs (Fig. 1B). Levels of Sig-1Rs in OLs were significantly higher when they were cocultured with astrocytes (data not shown). Therefore, mixed glial culture was used in this study. Sig-1Rs target globular structures that distribute over the wide flat myelin-like sheets of OLs (Fig. 1C). Those structures were negative to markers for Golgi apparatus, mitochondria, endosome, and lysosome (Fig. 7, which is published as supporting information on the PNAS web site) and were unlikely to be cytosolic vesicles because their sizes and shapes varied and some of them showed polarized or tubular shapes at higher magnification (arrows in Fig. 1C).
Fig. 1.
Cellular distribution of Sig-1Rs in primary cells of rat hippocampus. (A) Sig-1R expression in hippocampal neurons. (B) Sig-1Rs in glial cells. As, astrocyte. (C) Sig-1Rs in a mature OL. Arrowheads show Sig-1R-containing globules; arrows show tubular structures containing Sig-1R. (D) Colocalization of Sig-1R (green) with GalCer (red) in globular structures of OLs. Arrowheads in insets show GalCer and Sig-1R on the surface of a globule at high magnification. (E) Orthogonal distributions of Sig-1R (green) and GalCer (red) in a myelin sheet. Z-dimensional images were captured along the blue line. Arrowheads show positions of plasma membranes. (F) GalCer-containing globules (red) connecting to ER network (CYP450R in green) in an OL sheet. (G) Colocalization of Sig-1R (green), GalCer (red), and free cholesterol (filipin staining in blue) in a mature OL. [Bar, 20 μm (A-C and G) and 10 μm (D-F).]
Colocalization of Sig-1R with GalCer and Cholesterol on the Globular Structures Associated with the ER. To characterize the Sig-1R-targeting globules in OL sheets, we tested specific markers for differentiated OLs. The confocal microscopic observations demonstrated the apparent colocalization between GalCer and Sig-1R on the globular structures (Fig. 1D). Orthogonal sectioning (Fig. 1E) and a 3D y-axis rotation analysis (Fig. 8, which is published as supporting information on the PNAS web site) of myelin sheets confirmed that GalCer and Sig-1Rs coexist in the globular structures (as ovals in Z-dimension) that localize between upper and lower plasma membranes. Sig-1Rs and GalCer are present on the surface of globular structures (arrowheads in insets of Fig. 1D), indicating that these two molecules are membrane components of the unique intracellular globules. Myelin basic protein (MBP) and proteolipid protein were highly expressed in mature hippocampal OLs but did not colocalize with Sig-1Rs (Fig. 7).
We found recently that Sig-1Rs target similar ring-like structures associated with ER (e. g., ER lipid droplets) in NG108 cells that contain neutral lipids and cholesterol (19, 22). Thus, spatial distributions of the globular structures and ER membranes in myelin-like sheets were examined. When stained with NADPH cytochrome P450 reductase (CYP450R) antibodies, smooth ER showed reticular network structures throughout the sheets of OLs (Fig. 1F). Although GalCer and CYP450R were not colocalized, the merged image revealed that GalCer-containing globular structures were connected to the ER reticular network (Fig. 1F). This finding indicates that the globular structures are subcompartments of the ER. Immunoglobulin heavy chain binding protein (BiP) immunostaining showed a restricted distribution of rough ER in cell bodies (Fig. 7). Thus, GalCer- and Sig-1R-containing globules in OLs share similar structural and spatial characteristics to those of ER lipid droplets seen in NG108 cells (19, 22). Because the ER lipid droplet contains moderate amounts of neutral lipids and free cholesterol in NG108 cells, we examined next the lipid contents of ER globules in OLs. Nile red staining for neutral lipids was unexpectedly low in mature OLs, in contrast to its enrichment in immature OLs and NG108 cells (Fig. 8). On the other hand, filipin staining revealed an abundance of free cholesterol in Sig-1R/GalCer-containing globular structures (Fig. 1G). Filipin staining also labeled membrane domains that are negative in both Sig-1R and GalCer, suggesting the existence of different classes of cholesterol-rich membrane domains in the sheet of OLs. Taken together, these findings indicate that OL sheets possess unique GalCer- and cholesterol-enriched ER subcompartments where Sig-1Rs specifically target. Thus, although lipid contents in those ER globules are different in part from those in ER lipid droplets in NG108 cells, we conclude that Sig-1R-containing globules seen in primary OLs are structurally equivalent to ER lipid droplets in NG108 cells (19, 22).
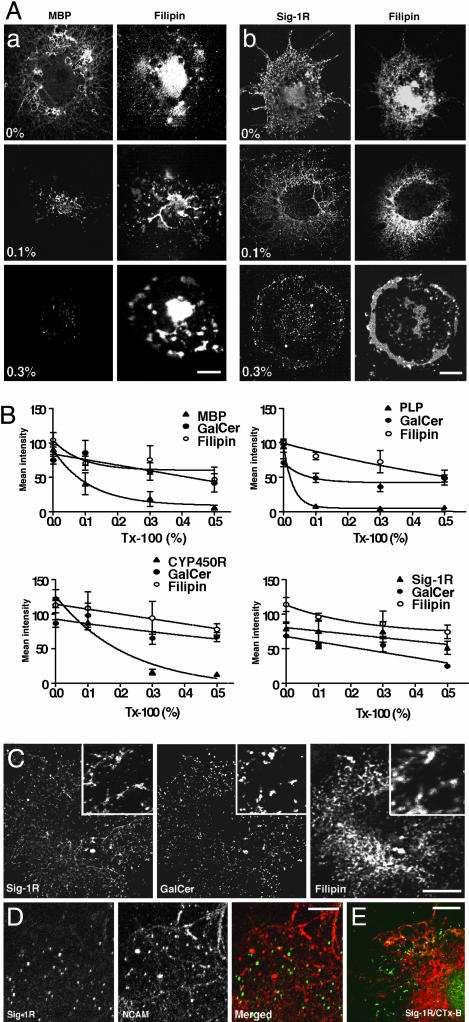
GalCer Form a Distinct Class of Lipid Rafts on the ER of OLs. It has been recently established that cholesterol and glycosphingolipids, particularly gangliosides, form detergent-resistant lipid microdomains (lipid rafts) that play important roles in cellular functions (27, 28). However, less is known about the involvement, if any, of GalCer in lipid raft formation. Inasmuch as Sig-1Rs can exist in Triton X-100-resistant microdomains in NG108 cells (22) and Sig-1Rs and GalCer colocalize in OL sheets (Fig. 1), OL globular structures might thus contain ER lipid rafts composed of cholesterol and GalCer instead of gangliosides. Accordingly, we tested the detergent resistance of ER membrane domains by using an in situ solubilization with Triton X-100 at 4°C. Here, we chose not to employ biochemical approaches using cell homogenates for the lipid raft examination because our cultures contain not only mature OLs but also immature OLs and astrocytes. Because Triton X-100 caused morphological changes at concentrations of >1%, we set the highest concentration of Triton X-100 at 0.5%. As shown in Fig. 2A, although general intensities of filipin stainings were reduced by increasing concentrations of Triton X-100, cholesterol-rich membrane domains remained detergent-resistant even at the highest concentration of Triton X-100. This result suggests the presence of lipid rafts on OL sheets. On the contrary, MBP-containing membranes were highly susceptible to Triton X-100 solubilization. Sig-1R-containing membrane domains, which are positive for both filipin and GalCer, were highly resistant to Triton X-100 (Fig. 2 A). To semiquantify the resistance of OL proteins against the Triton X-100 solubilization, cells were triple-labeled with filipin, GalCer antibody, and an antibody against the protein of interest. Fluorescence intensities derived from each staining were compared (see Materials and Methods and Fig. 6). As shown in Fig. 2B, MBP and proteolipid protein were susceptible to Triton X-100 solubilization with proteolipid protein disappearing even at 0.1% Triton X-100, whereas accompanying stainings for cholesterol and GalCer were resistant to the solubilization. CYP450R on the ER membrane of OL sheets (Fig. 1F) was susceptible to Triton X-100 solubilization. Apparently different from the other three proteins tested here, Sig-1Rs remained at the ER domains after Triton X-100 solubilization, showing a resistance to Triton X-100 similar to that shown by GalCer or cholesterol (Fig. 2B). Importantly, the colocalization of Sig-1R, GalCer, and cholesterol remained intact even after the Triton X-100 solubilization (Fig. 2C). These results indicate that GalCer can form lipid rafts on the OL ER globules where Sig-1Rs also target.
Fig. 2.
GalCer-containing lipid rafts in OLs. (A) In situ solubilization for detecting lipid rafts in myelin sheets. OLs were incubated at 4°C in HBS containing Triton X-100 (0-0.3%) (see Materials and Methods). Cells were double-stained with an antibody (MBP or Sig-1R) and filipin. (B) Semiquantifying the resistance of myelin proteins to Triton X-100 (Tx-100) solubilization. After in situ solubilization, cells were triple-labeled with filipin, GalCer antibody, and a specific antibody for either MBP, proteolipid protein (PLP), CYP450R, or Sig-1R. Mean intensities of respective stainings in OL sheets were densitometrically measured. Data represent mean ± SE from three to five individual coverslips (total of 48-104 cells). (C) Colocalization of Sig-1R, GalCer, and cholesterol after solubilization with 0.5% Triton X-100 in an OL. Insets are at higher magnification. (D) Differential distribution between membrane domains containing Sig-1R-(green) or neuronal cell adhesion molecule (NCAM; red) in a myelin sheet. (E) No colocalization between Sig-1R (green) and cholera toxin subunit B (CTx-B)-labeled gangliosides (red) in a myelin sheet. [Bar, 20 μm(A and C) and 10 μm(D and E).]
Sig-1R-positive GalCer rafts were next compared with plasma membrane rafts regarding the distribution pattern and the lipid components therein. Neuronal cell adhesion molecule, a glycosylphosphatidylinositol-anchored protein specific for plasma membrane rafts, was detected in membrane domains on OL sheets (Fig. 2D), which were stained by filipin and cholera toxin subunit B, a marker for gangliosides (data not shown). However, none of these membrane domains colocalized with the ER globules containing Sig-1Rs (Fig. 2D). Furthermore, Sig-1R-targeted membrane domains were negative to cholera toxin subunit B labeling (Fig. 2E). Taken together, we conclude that mature OLs possess a unique subclass of Sig-1R-containing cholesterol- and GalCer-enriched lipid rafts on the ER globular structures.
Developmental Expression of GalCer-Enriched ER Globules and Sig-1Rs in OLs. Because we found that Sig-1R-containing ER globules exist in terminally differentiated OLs, it was of interest to determine whether Sig-1Rs on GalCer-enriched ER globules have any functional significance on OL differentiation. Accordingly, we observed the expression levels of Sig-1Rs and GalCer in different stages of OL differentiation in vitro. Sig-1R immunoreactivity in OL precursors or immature OLs was low and diffuse across cytoplasm (Fig. 3 A and B). A substantial number of cholesterol-rich membrane domains, however, already existed in immature OLs (Fig. 3 A and B). In mature OLs, Sig-1Rs clustered and targeted ER globules, apparently in perfect alignment with the GalCer expression and accumulation, suggesting that GalCer might affect the distribution of Sig-1Rs on the ER.
Fig. 3.
Developmental expression of Sig-1R in OLs. (A-C) In vitro OLs at different stages of differentiation. Cells were triple-labeled with Sig-1R antibody, GalCer antibody, and filipin. (A) Early progenitor. (B) Immature OL. (C) Mature OL. (D) Sig-1R in corpus callosum of developing rat brains. Arrowheads show Sig-1R in interfascicular OLs. P, postnatal day. (E) Sig-1R (green) and A2B5-positive OL progenitors (red) in rat subventricular zone on postnatal day 1. LV, lateral ventricule. (F) Sig-1R (green) and MBP (red) in rat hippocampal CA2 on postnatal day 35. Arrowheads show myelin sheath identified as double outlines. *, A pyramidal neuron. (Bar, 20 μm.)
In rat brain, Sig-1R increased significantly in corpus callosum during development (Fig. 3D). Sig-1Rs were seen in interfascicular OLs in corpus callosum on postnatal day 35 but not in A2B5-positive OL progenitors in subventricular zone on postnatal day 1 (Fig. 3 D and E), consistent with the results from in vitro studies. Although Sig-1Rs exist in pyramidal neurons on postnatal day 35 in hippocampal CA2 region, denser Sig-1R-immunoreactivities were observed in MBP-positive myelin sheaths surrounding pyramidal neurons (Figs. 3F and 8). These results confirm the physiological relevance of our findings in vitro.
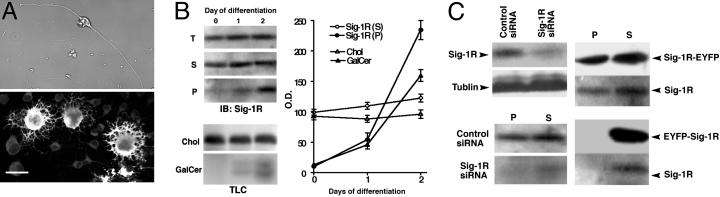
Regulation of OL-Type 2 Astrocyte Progenitor Differentiation by Sig-1R. To examine the functional significance of Sig-1Rs in OL differentiation, we used OL-type 2 astrocyte progenitor cells purified from rat brain (CG-4 cells) (25). By using these cells, we were able to obtain homogeneous cell populations and to induce cell differentiation at a specific time point by withdrawing the proliferation medium. When CG-4 cells were cultured on poly-d-ornithine-coated coverslips, 98% of cells showed a bipolar lineage (Fig. 4A). In differentiation medium, all cells became multipolar and ≈30% of cells became MBP-positive on day 2 (Fig. 4A). Western blotting showed that Sig-1R protein levels increased with the differentiation of CG-4 cells. Importantly, Sig-1R in lipid rafts (P in Fig. 4B) increased much greater than those in the soluble fraction. TLC analysis showed that GalCer appeared after differentiation, but free cholesterol levels remained constant (Fig. 4B), consistent with the result in Fig. 3. Notably, Sig-1R levels in lipid rafts paralleled the expression levels of GalCer (Fig. 4B Right). These results suggest that GalCer might be involved in the recruitment of Sig-1Rs to lipid rafts on the ER.
Fig. 4.
Up-regulation and lipid raft targeting of Sig-1R during differentiation of CG-4 cells. (A) CG-4 cells in proliferation medium (Upper) and differentiation medium (Lower) in MBP immunostaining. (Bar, 20 μm.) (B) Levels of Sig-1R proteins, cholesterol (Chol), and GalCer in CG-4 cells during differentiation. T, total protein lysate; S, soluble fraction; P, raft fraction; IB, immunoblotting. The graph shows the densitometrical analyses of Sig-1R and lipids (n = 4). See Materials and Methods for detailed analyses of lipids. (C) Transfection of Sig-1R siRNA (Left) or EYFP-tagged Sig-1Rs (Right) in CG-4 cells. Note the disappearance of endogenous Sig-1Rs in rafts in EYFP-Sig-1R-transfected cells.
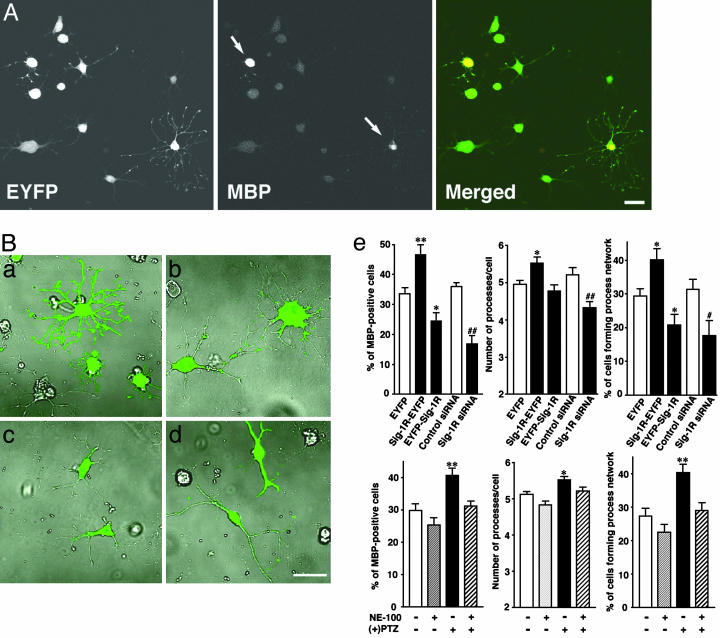
To investigate a causal role of Sig-1R in CG-4 differentiation, vectors containing Sig-1R cDNA or Sig-1R siRNA were transfected to CG-4 cells and cells were then allowed to differentiate for 2 days in differentiation medium. Transfection of Sig-1R siRNA knocked down Sig-1Rs to a level <20% of that seen in vector-transfected cells, suppressing Sig-1Rs in both raft and nonraft fractions (Fig. 4C Left). C-terminally EYFP-tagged Sig-1Rs (Sig-1R-EYFP), which have the same properties as those of endogenous Sig-1Rs in NG108 cells (19, 22), appeared in the detergent-insoluble fraction, forming rafts that were the same as endogenous Sig-1Rs in CG-4 cells (Fig. 4C Right). We also tested the transfection of N-terminally EYFP-tagged Sig-1Rs (EYFP-Sig-1Rs) that not only failed to target ER lipid droplets but also prevented endogenous Sig-1Rs to target ER lipid rafts in NG108 cells (22). Transfection of EYFP-Sig-1Rs did not affect total protein levels of endogenous Sig-1Rs but caused a reduction of endogenous Sig-1Rs in lipid rafts (Fig. 4C Right). These vector transfection tools were used in the following studies to provide a causal role of Sig-1Rs in OL differentiation. For identification of Sig-1R siRNA-transfected cells, cells were cotransfected with one-fifth the amount of EYFP vectors. In addition to morphological analysis, “percent of differentiation” was calculated by dividing the number of cells expressing both EYFP and MBP by the total of EYFP-expressing cells (see Fig. 5A). Thus, only cells expressing yellow fluorescence were subjected to assessments of cell differentiation.
Fig. 5.
Effects of Sig-1R levels and ligands on OL differentiation. (A) After transfection with EYFP vectors, CG-4 cells were allowed to differentiate for 2 days. Only cells expressing yellow fluorescence were used for assessments of differentiation. Arrows indicate MBP-positive OLs. (B) Effect of vector transfection on CG-4 differentiation. EYFP- (a), Sig-1R-EYFP- (b), EYFP-Sig-1R- (c), and Sig-1R siRNA- (d) transfected CG-4 cells on differentiation day 2. siRNA vectors were cotransfected with one-fifth amounts of EYFP-vectors. (e) Graphs showing percentage of differentiated cells in vector-transfected or Sig-1R ligand-treated cells. Values are mean ± SE of 20-60 fields (10 fields were captured from one coverslip). (+)pentazocine [(+)PTZ] and/or NE-100 (both at 300 nM) were applied to cells for 2 days. (Bar, 20 μm.)
Expression of EYFP per se did not affect differentiation (nontransfected cells: 31.9 ± 2.1%, 753 cells from five coverslips; EYFP-transfected cells: 33.7 ± 2.5%, 562 cells from four coverslips). In contrast to the diffuse cytoplasmic distribution of EYFP (Fig. 5B a and b), Sig-1R-EYFP formed clusters and targeted ER subdomains in differentiated CG-4 cells. EYFPSig-1Rs localized on the entire ER reticular network and not on the ER lipid droplets (Fig. 5Bc). Expression of Sig-1R-EYFP significantly potentiated the OL differentiation. The EYFP-Sig-1R expression partially but significantly inhibited the OL differentiation (Fig. 5B). Transfection of Sig-1R siRNA also decreased the OL differentiation (Fig. 5B d and e). Although bipolar lineage disappeared on CG-4 cells transfected with EYFP, Sig-1R-EYFP, or EYFP-Sig-1R in differentiation medium on day 2, 23% of Sig-1R siRNA-transfected cells still exhibited bipolar lineage (Figs. 5Bd and 8). Some Sig-1R siRNA-transfected cells extended a few asymmetrical and twirled processes varying in lengths (Fig 8F), whereas EYFP- or Sig-1R-EYFP-transfected cells showed numerous long symmetrical processes in a radial pattern branching into a wide network (Fig. 5B a and b). These results indicate that Sig-1Rs are important in oligodendrocyte differentiation. The result of EYFP-Sig-1R transfection suggests that existing in ER lipid rafts would be crucial for Sig-1Rs to exert their function in regulating OL differentiation. Finally, to test the pharmacological relevance of Sig-1Rs in the OL differentiation, we tested the effects of Sig-1R ligands on CG-4 cells. As shown in Fig. 5Be, (+)pentazocine, a Sig-1R agonist, potentiated the differentiation of CG-4 cells. The potentiation caused by (+)pentazocine was blocked by Sig-1R antagonist NE-100. Thus, Sig-1R ligands may affect myelination in the brain.
Discussion
Here, we found that mature OLs possess cholesterol- and GalCer-stored compartments in myelin sheets. The structures are characteristic of (i) globular membrane structures associated with ER tubular elements, (ii) expressing highly clustered Sig-1R, and (iii) forming GalCer-containing lipid microdomains on the surface. Because these structures share the same characteristics with ER lipid droplets identified in NG108 cells (19, 22), we conclude that the ER globular structures in myelin sheets are equivalents of ER lipid droplets in NG108 cells. A difference in lipid components, i.e., an abundance of GalCer and few neutral lipids in OL ER globules, could be due to differences between OLs and NG108 cells in lipid contents and metabolism. Certain lipids synthesized on the ER are first accumulated between the leaflets of the ER membranes (e.g., ER lipid droplets). After reaching a critical size, the ER lipid droplets can bud off to form cytosolic lipid droplets (23). Because in mature OLs unesterified cholesterol and GalCer are actively synthesized, OLs might thus use ER globular structures as loci for compartmentalization and transport of GalCer and cholesterol. A number of GalCer-enriched ER globules are present in the periphery of myelin sheets (Fig. 1) but are apart from the Golgi apparatus (Fig. 7). GalCer, if transported to the Golgi apparatus, are converted to sulfatides by enzymes residing in the Golgi apparatus (29). Thus, there should exist an ER-plasma membrane transport pathway for GalCer inside OLs that bypasses the Golgi apparatus just like that known for transport of cholesterol. How GalCer and cholesterol are concentrated in the ER globules and how they are transported to the myelin membrane in OLs warrant investigation.
We found that Sig-1Rs are expressed in mature OLs. This finding is consistent with previous studies (30, 31). A subcellular fractionation study demonstrated that (+)[3H]SKF-10,047-binding Sig-1R sites in the rat brain are enriched in the P2A myelin fraction as well as in the microsome P3 fraction (30). A recent immunohistochemical study showed that Sig-1Rs are present in OLs in the adult animal brain (31). In the present study, we demonstrate that Sig-1Rs in OLs play a role in OL differentiation (Fig. 5). Accordingly, an increase in MBP-positive OLs by Sig-1R overexpression and a decrease of them by Sig-1R knockdown indicate that Sig-1Rs might regulate terminal differentiation of OLs and/or maintenance of mature OLs. Furthermore, the stalling of OL progenitors at bipolar lineage stage by Sig-1R siRNA (Fig. 5Bd) suggests the possibility that Sig-1Rs regulate the initiation of differentiation that includes multiple cellular polarization or proper process extension. Further experiments are required to clarify which stage(s) of differentiation are regulated by Sig-1Rs. Sig-1Rs are known to bind neurosteroids (8, 15). Progesterone, possessing the highest affinity among neurosteroids for Sig-1Rs (8), promotes sciatic nerve regeneration and remyelination in male mice (32). A Sig-1R agonist eliprodil was shown to promote myelination in neuron-OL cocultures (33). Thus, although the exact molecular mechanism remains to be elucidated, Sig-1Rs are apparently important in the regulation of myelination in the brain. Sig-1R ligands may be of therapeutic importance in treating demyelinating diseases, such as multiple sclerosis in humans.
Lipid rafts on the plasma membrane regulate a variety of signal transduction pathways, for example, by recruiting tropic factor receptors or kinases to the microdomains (27, 28). However, roles of lipid rafts residing at intracellular organelle membranes (e.g., ER) are unknown at present. Although ER membranes lack gangliosides in particular, they contain moderate amounts of cholesterol and certain sphingolipids (29), ceramides in most cells, and GalCer in OLs. Transition temperatures of GalCer are typically in the range 60-80°C, much higher than those for glycerophospholipids (≈0o) (7). Therefore, GalCer tend to separate out as solid domains of gel-phase lipid in phospholipid membranes and, together with cholesterol, form liquid-ordered microdomains. Indeed, by using fluorophore-labeled GalCer, it has been shown that GalCer partition into cholesterol-enriched microdomains in a cholesterol-dependent manner (34). Because GalCer are synthesized in the lumen of the ER reticular network by UDP-galactose/ceramide galactosyltransferase (29), nascent GalCer may move laterally on the ER network to corroborate with cholesterol to form ER lipid rafts in OLs. The ceramide rafts on the ER have been shown to recruit and segregate specific ER-residing proteins in the yeast (35). Our result with the EYFP-Sig-1R overexpression (Fig. 5) also suggests, indirectly, that the ceramide lipid rafts may invoke functioning of Sig-1Rs by recruiting them to the rafts on the ER.
Based on previous (19-22) and present results, we conclude that Sig-1R-containing, GalCer-enriched ER globules might represent machinery whereby OLs regulate GalCer and cholesterol transport from the ER to myelin membranes. Sig-1Rs and their ligands might modulate myelination by affecting lipid constituents of the myelin sheet by means of this transport pathway.
Supplementary Material
Acknowledgments
We thank Dr. V. Ganapathy (Medical College of Georgia, Augusta) for providing pSPORT-1 Sig-1R cDNA, Dr. F. Cambi (Thomas Jefferson University, Philadelphia) for a generous gift of GC-4 cells and valuable suggestions, and Dr. Rao Rapaka (National Institute on Drug Abuse) for (+)pentazocine. We also thank Dr. K. Anderson for technical advice on dephenylamine-aniline-phosphoric acid spray. This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, U.S. Department of Health and Human Services.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Sig-1R, sigma-1 receptor; OL, oligodendrocyte; GalCer, galactosylceramides; ER, endoplasmic reticulum; MBP, myelin basic protein; CYP450R, NADPH cytochrome P450 reductase; EYFP, enhanced yellow fluorescent protein.
References
- 1.Pfeiffer, S. E., Warrington, A. E. & Bansal, R. (1993) Trends Cell Biol. 3, 191-197. [DOI] [PubMed] [Google Scholar]
- 2.Miller, R. H. (2002) Prog. Neurobiol. 67, 451-467. [DOI] [PubMed] [Google Scholar]
- 3.Barres, B. A. & Raff, M. C. (1999) J. Cell Biol. 47, 1123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietschy, J. M. & Turley, S. D. (2001) Curr. Opin. Lipidol. 12, 105-112. [DOI] [PubMed] [Google Scholar]
- 5.Marcus, J. & Popko, B. (2002) Biochim. Biophys. Acta 1573, 406-413. [DOI] [PubMed] [Google Scholar]
- 6.Bansal, R., Winkler, S. & Bheddah, S. (1999) J. Neurosci. 19, 7913-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee, A. G. (2001) Curr. Biol. 11, R60-R62. [DOI] [PubMed] [Google Scholar]
- 8.Su, T. P., London, E. D. & Jaffe, J. H. (1988) Science 240, 219-221. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto, R. R., Liu, Y., Lerner, M., Howard, E. W. & Brackett, D. J. (2003) Eur. J. Pharmacol. 469, 1-12. [DOI] [PubMed] [Google Scholar]
- 10.Snyder, S. H. & Largent, B. L. (1989) J. Neuropsychiatry Clin. Neurosci. 1, 7-15. [DOI] [PubMed] [Google Scholar]
- 11.Ferris, C. D., Hirsch, D. J., Brooks, B. P. & Snyder, S. H. (1991) J. Neurochem. 57, 729-737. [DOI] [PubMed] [Google Scholar]
- 12.Aydar, E., Palmer, C. P., Klyachko, V. A. & Jackson, M. B. (2002) Neuron 34, 399-410. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi, T. & Su, T. P. (2001) Proc. Natl. Acad. Sci. USA 98, 491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nuwayhid, S. J. & Werling, L. L. (2003) J. Pharmacol. Exp. Ther. 306, 934-940. [DOI] [PubMed] [Google Scholar]
- 15.Maurice, T. & Lockhart, B. P. (1997) Prog. Neuropsychopharmacol. Biol. Psychiatry 21, 69-102. [DOI] [PubMed] [Google Scholar]
- 16.Stefanski, R., Justinova, Z., Hayashi, T., Takebayashi, M., Goldberg, S. R. & Su, T. P. (2004) Psychopharmacology 175, 68-75. [DOI] [PubMed] [Google Scholar]
- 17.Hanner, M., Moebius, F. F., Flandorfer, A., Knaus, H. G., Striessnig, J., Kempner, E. & Glossmann, H. (1996) Proc. Natl. Acad. Sci. USA 93, 8072-8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labit-Le Bouteiller, C., Jamme, M. F., David, M., Silve, S., Lanau, C., Dhers, C., Picard, C., Rahier, A., Taton, M., Loison, G., et al. (1998) Eur. J. Biochem. 256, 342-349. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi, T. & Su, T. P. (2003) J. Pharmacol. Exp. Ther. 306, 726-733. [DOI] [PubMed] [Google Scholar]
- 20.Takebayashi, M., Hayashi, T. & Su, T. P. (2002) J. Pharmacol. Exp. Ther. 303, 1227-1237. [DOI] [PubMed] [Google Scholar]
- 21.Takebayashi, M., Hayashi, T. & Su, T. P. (2004) Synapse 53, 90-103. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi, T. & Su, T. P. (2003) J. Pharmacol. Exp. Ther. 306, 718-725. [DOI] [PubMed] [Google Scholar]
- 23.Murphy, D. J. & Vance, J. (1999) Trends Biochem. Sci. 24, 109-115. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi, T., Kagaya, A., Takebayashi, M., Shimizu, M., Uchitomi, Y., Motohashi, N. & Yamawaki, S. (1995) J. Pharmacol. Exp. Ther. 275, 207-214. [PubMed] [Google Scholar]
- 25.Louis, J. C., Magal, E., Muir, D., Manthorpe, M. & Varon, S. (1992) J. Neurosci. Res. 31, 193-204. [DOI] [PubMed] [Google Scholar]
- 26.Dasgupta, S. & Hogan, E. L. (2001) J. Lipid Res. 42, 301-308. [PubMed] [Google Scholar]
- 27.Anderson, R. G. & Jacobson, K. (2002) Science 296, 1821-1825. [DOI] [PubMed] [Google Scholar]
- 28.Simons, K. & Toomre, D. (2000) Nat. Rev. Mol. Cell. Biol. 1, 31-39. [DOI] [PubMed] [Google Scholar]
- 29.van Meer, G. & Holthuis, J. C. (2000) Biochim. Biophys. Acta 1486, 145-170. [DOI] [PubMed] [Google Scholar]
- 30.McCann, D. J. & Su, T. P. (1990) Eur. J. Pharmacol. 188, 211-218. [DOI] [PubMed] [Google Scholar]
- 31.Palacios, G., Muro, A., Vela, J. M., Molina-Holgado, E., Guitart, X., Ovalle, S. & Zamanillo, D. (2003) Brain Res. 961, 92-99. [DOI] [PubMed] [Google Scholar]
- 32.Baulieu, E. E. (1998) Psychoneuroendocrinology 23, 963-987. [DOI] [PubMed] [Google Scholar]
- 33.Demerens, C., Stankoff, B., Zalc, B. & Lubetzki, C. (1999) Neurology 52, 346-350. [DOI] [PubMed] [Google Scholar]
- 34.Puri, V., Watanabe, R., Dominguez, M., Sun, X., Wheatley, C. L., Marks, D. L. & Pagano, R. E. (1999) Nat. Cell Biol. 1, 386-388. [DOI] [PubMed] [Google Scholar]
- 35.Muniz, M., Morsomme, P. & Riezman, H. (2001) Cell 104, 313-320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.