Abstract
Single-molecule spectroscopy can provide insight into the fundamental photophysics of large macromolecules containing tens of thousands of carbon atoms by circumventing disorder broadening. We apply this technique to comparatively ordered ladder-type poly(para-phenylene) and highly disordered poly(phenylenevinylene) (PPV), both of which are materials of substantial technological interest. Identical spectroscopic features are observed on the single-chromophore level, independent of the chemical structure or the chain morphology. Both materials exhibit narrow fluorescence lines down to 0.5 nm wide, which we attribute to the single-chromophore zero-phonon line, accompanied by a discrete vibronic progression providing a signature of the chemical structure. The chromophore units display spectral diffusion, giving rise to dynamic disorder on the scale of the linewidth. Although the energetic range of spectral diffusion is small, it can influence intramolecular excitation energy transfer and thus the overall molecular emission. The spectral diffusion dynamics of single chromophores are identical in both material systems and follow a universal Gaussian distribution. In the case of emission from multiple chromophores situated on the molecule, which we observe for PPV, spectral diffusion follows Lorentzian-like statistics. The fundamental difference between the two materials is the possibility of coherent interchromophoric coupling in PPV, resulting in strong spectral broadening caused by aggregation or superradiance. Such behavior is absent in the ladder-type polymers, where the linewidth of the emissive species is identical for all molecules. Our results demonstrate that structure-property correlations in conjugated polymers derive mainly from chain morphology rather than chromophoric properties and should be considered extrinsic in nature.
The diversity of synthetic organic chemistry has opened up effectively endless options for the creation of organic semiconducting materials, revealing a rich spectrum of correlations between chemical structure and physical properties (1). A fundamental understanding of these correlations is vital to the design of novel materials for optoelectronic applications such as light-emitting diodes, solar cells, and photodiodes. Structure-property relationships can be particularly complex in conjugated polymers (CPs), because the chemical structure not only controls the electronic properties of the π-electron systems, but also strongly influences the chain morphology (2, 3). As in proteins, secondary and tertiary structure such as chain folding can be equally if not even more important than the primary chemical structure of the polymer. The chain conformation can have dramatic consequences on the overall electronic and photophysical properties of the molecule as a whole (4, 5). CPs are generally thought of as long chains of more or less isolated conjugated segments, which are referred to as chromophores (6–8). There has been much debate in the literature about the effective conjugation length of CPs, i.e., the spatial distance over which electronic delocalization may occur (6–13). The picture at present is that the degree of delocalization of the excited state may be considerably smaller than the overall conjugation length because of phonon-assisted self-trapping. All of the evidence to date for the chromophore model of CPs has been based on ensemble measurements, most notably site-selective fluorescence spectroscopy (6, 8). Single-molecule (SM) spectroscopy should be the ideal tool for identifying quasi-molecular subunits on CP chains by allowing a direct differentiation between homogeneous and inhomogeneous broadening (14). Most of the SM studies on CPs thus far have been carried out at room temperature (4, 5, 15–18). Although revealing a surprising richness of photoluminescence (PL) properties such as fluorescence intermittency and uncovering important correlations between chain morphology and photophysical properties, these investigations have not succeeded in actually identifying individual chromophore units. The question of what the fundamental underlying photophysical properties of the individual emissive sites of CPs actually are thus remains unanswered. Developing a picture of the nature and interaction pathways of chromophores on a microscopic level is extremely important to material design for optoelectronic devices (19, 20), but also bears fundamental relevance to understanding the physics of other multichromophoric systems such as natural light-harvesting complexes (21).
We recently were able to identify single chromophores in a highly ordered CP based on a methyl-substituted ladder-type poly(para-phenylene) (MeLPPP) and demonstrated that the efficiency of intramolecular excitation energy transfer (EET) is dominated by the temperature-dependent interchromophoric spectral overlap (22). Because this material is characterized by an exceptionally low level of inhomogeneous disorder broadening (≈30 meV full width at half maximum) and contains only a comparatively small number of repeat units (≈70), it lends itself well to studies of the single-chromophore emission. Surprisingly, we now find that the highly disordered CP poly(2-methoxy-5-(2′-ethyl-hexyloxy)-1,4-phenylene-vinylene) (MEH-PPV) exhibits very similar photophysical properties on the single-chromophore level, although the SM properties of the two CPs are very different (5, 16, 22, 23). Both materials are characterized by virtually identical single-chromophore PL lines of ≈2.5 meV in width. We show that the single chromophore exhibits spectral diffusion, which gives rise to dynamic disorder on the meV scale. Single-chromophore spectroscopy allows us to pinpoint the nature and strength of intramolecular vibrational coupling, which clearly demonstrates the expected correlation with chemical structure. The most important difference between both materials arises from the strength of interactions between chromophores. Whereas chromophore coupling in rigid MeLPPP molecules is principally incoherent in nature, so that all single chains exhibit virtually identical spectroscopic properties, MEH-PPV displays evidence for both free chromophore and aggregated chromophore emission, leading to strong temporal fluctuations of the SM fluorescence linewidth.
Identical Spectroscopic Signatures: Single-Chromophore Emission
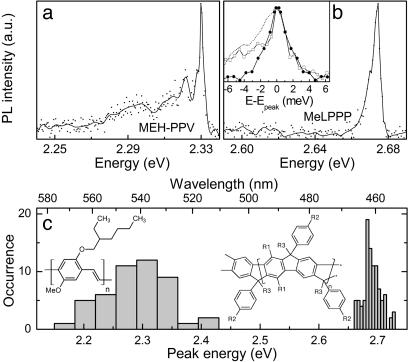
We carried out low-temperature SM fluorescence measurements by dissolving the CPs in toluene at typical concentrations of 10–6 mg/ml for MeLPPP and 10–8 mg/ml for MEH-PPV and dispersing them in a polystyrene matrix. The solutions were spin-coated on top of SiO2-covered silicon wafers, yielding films ≈20 nm thick, and the substrates were mounted in a helium cold finger microscope cryostat under a vacuum of <10–6 millibar. Fluorescence was detected through a long working distance microscope objective (numerical aperture 0.55) in a wide-field imaging configuration, dispersed by using a monochromator with a spectral resolution of 0.12 nm (0.55 meV at 520 nm), and imaged onto a cooled front-illuminated charge-coupled device (Sensicam QE, PCO AG, Kelheim, Germany). Fig. 1a shows a PL spectrum of an MEH-PPV molecule (American Dye Source, Quebec, Mn 200,000, corresponding to ≈800 repeat units) at 5 K, excited by using the 488-nm line of an Ar+ ion laser at an intensity of 500 W/cm2. A narrow feature of 2.6-meV width is seen at 2.335 eV, followed by a progression of peaks offset by 8.6 and 31 meV. For clarity we present the raw data as points and the spectrum with the high-frequency noise removed by using a fast Fourier transform low pass filter as the solid line. Fig. 1b shows the PL of a single MeLPPP molecule (Mn 25,000, corresponding to ≈31 repeat units), excited at 426 nm with 70 W/cm2 by using 100-fs-long pulses (i.e., 8 nm in width) from a frequency-doubled mode-locked Ti:sapphire laser. In this case we observe only one narrow peak at 2.674 eV, which exhibits a slight asymmetry and is broadened to the red side. The peak width of 4.6 meV is, however, comparable to that of MEH-PPV. We note that neither SM nor site-selective fluorescence spectroscopy have previously yielded signatures narrower than 50 meV for MEH-PPV (5, 15, 16, 19, 23). Narrow zero-phonon lines (ZPLs) have been observed in site-selective fluorescence measurements on PPV oligomers, but have thus far remained elusive for polymers (7, 8).
Fig. 1.
SM PL spectra of MEH-PPV and MeLPPP dispersed in a polystyrene matrix at 5 K. (a) SM PL spectra of MEH-PPV, exhibiting a narrow zero-phonon feature of 2.6 meV width. The data points correspond to raw data, and the lines were obtained by using a weak fast Fourier transform low-frequency pass filter. (b) SM spectrum of a MeLPPP molecule with a spectral width of 4.6 meV. (Inset) The overlaid ZPLs of the two CPs (MEH-PPV, □; MeLPPP, •). To compensate for the low-energy phonon wing of the MeLPPP line (dashed line), the mirror image of the high-energy tail is plotted. (c) The histogram shows the distribution of peak positions measured for 97 MeLPPP and 47 MEH-PPV molecules, demonstrating the significantly larger disorder in the case of MEH-PPV. The chemical structure of MEH-PPV and MeLPPP is also given (R1, n-hexyl; R2, n-decyl; R3, methyl).
We attribute the narrow MeLPPP spectrum in Fig. 1b to emission from a single chromophore unit. The PL is linearly polarized in emission and absorption (22, 24, 25). We recently reported multiple line emission from single MeLPPP molecules and suggested that this corresponds to emission from a number of chromophore units on the chain (22). For the present study we have extended both our measurement sensitivity and statistics and have also studied four different molecular weights of MeLPPP. We found that the number of fluorescence lines measured in a SM increases with chain length, whereas the shape of the individual lines is constant. For the present study it is sufficient to note that we can physically count individual chromophores on MeLPPP and find a monotonic increase of the number of narrow lines observed from SMs with increasing molecular weight Mn following the approximate relationship NChromophores ≈(4 × 10–5 ± 1.5 × 10–5) Mn. This estimate is somewhat below previous estimates of the effective length of chromophores in MeLPPP of six to seven repeat units (11) with oligomers, suggesting that the conjugated segment is on average two to three times longer than the size of the excitation (F.S., J.M.L., J.F., U.S., J. Josemon, A. C. Grimsdale, and K. Müllen, unpublished work). The similarity between the narrow spectral features in both polymers leads us to the proposition that the narrow spectrum in Fig. 1a also corresponds to a single-chromophore ZPL convoluted with a slight phonon sidewing to the red and followed by a discrete vibronic progression.
The fact that the narrow laser line should excite only one single chromophore in the multichromophoric assembly of MEH-PPV is rather surprising, because similar experiments at room temperature, but also at 20 K, have previously yielded spectra comparable in width to the bulk (5, 15, 16, 23). Very recently, single-chromophore emission was asserted from MEH-PPV at 20 K, which was still >10 times broader than the present feature (26). However, as the excitation occurs nonresonantly into the vibronic manifold of the molecule, which narrows dramatically with decreasing temperature (27), it may be possible to pick out just one single conjugated segment selectively. Alternatively, single-chromophore emission in MEH-PPV may become possible because of highly efficient intramolecular EET to a low-energy single-chromophore emissive trap state. Then again, emission in only one narrow line could also result from selective quenching of all but one of the chromophores excited, by either on-chain chemical defects, radicals, or nonemissive interchromophoric aggregate states leading to polaron-pair formation (6). Because we can only speculate about the single-chromophore absorption linewidth at present, the question of how a single chromophore can be addressed in an assembly containing potentially hundreds of chromophores must be left for future studies.
Fig. 1b Inset shows a direct comparison of the PL lines of the two polymers. The MeLPPP line is broadened slightly to the red, which is most likely caused by inelastic scattering of the excitation with low-frequency acoustic phonons. Although this process also occurs for MEH-PPV, it appears to be less substantial, possibly because of the much smaller physical dimensions of the optically active segments of this molecule. The Stokes phonon wing in MeLPPP can be removed by taking the mirror image of the high-energy side of the line, which yields a line (Fig. 1b Inset, •) absolutely identical to that of MEH-PPV (Fig. 1b Inset, □). It is gratifying to note that resonant Rayleigh scattering measurements on PPV at 5 K yielded a dephasing time of ≈0.5 ps (28), corresponding to our measured linewidth of 2.6 meV and suggesting that this is actually the homogeneous linewidth. Similar linewidths were also recently measured in hole-burning experiments on MeLPPP (29).
Fig. 1c shows the distribution of peak energies measured for 47 MEH-PPV and 97 MeLPPP molecules. Whereas the peak positions scatter over a range of 250 meV for MEH-PPV, they cover only 60 meV for MeLPPP. The inhomogeneity and disorder of MEH-PPV is much larger as expected from the differences in chemical structure. Whereas the backbone of MeLPPP is rigid and cannot undergo folding (11), numerous conformational degrees of freedom exist for the MEH-PPV chain (4). Apparently, very different CP material systems with large differences in the level of disorder can exhibit universal fluorescence signatures on the single-chromophore level. Assuming that the measured linewidth provides an upper bound for the homogeneous linewidth in CPs, this idea implies that homogeneous broadening in MEH-PPV is 2 orders of magnitude smaller than inhomogeneous broadening.
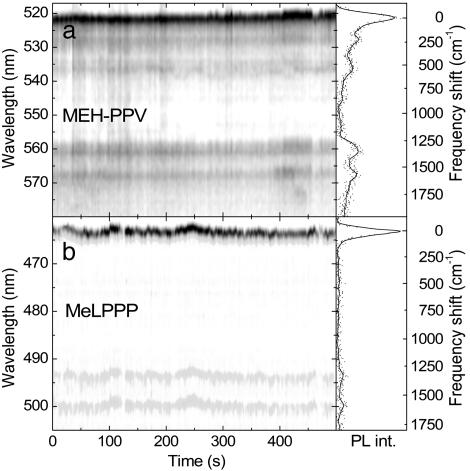
Our microscope allows us to record time sequences of PL spectra with up to 1-s resolution. Fig. 2 shows two examples of PL spectra measured over 500 s. We make two important observations. First, both spectra exhibit a narrow peak followed by equally narrow emission from discrete vibrational levels. The time-integrated spectra are shown in Fig. 2 Right on a frequency axis, shifted by their peak position. The main vibrational emission displayed by both molecules is at 1,318 and 1,578 cm–1, corresponding to the standard Raman interring and intraring C C stretching modes (7). Whereas no substantial low-frequency modes are detected in MeLPPP (Fig. 2b), two lower-energy modes at 250 and 555 cm–1 are clearly visible in MEH-PPV (Fig. 2a). A comparison with the spectrum in Fig. 1a suggests that a third very low energy mode at 70 cm–1 is actually merged with the ZPL, in this particular case because of the exceptionally clear and strong vibrational coupling exhibited by this molecule. The second observation relates to the temporal fluctuation in emission. Occasional fluorescence intermittency is observed; however, more importantly the peak maximum drifts with time. This finding is particularly evident in the MeLPPP spectra at 250 s. Identical spectral drifts are observed simultaneously in the vibronic replica. Spectral drifts and jumps are well known for SMs at both high (30) and low temperatures (14, 27, 31), and a few reports on room-temperature single-polymer spectroscopy also suggest spectral fluctuations with time (16, 18). This effect is, however, mainly caused by photo-oxidation and the inhibition of EET to lower energy segments, resulting in irreversible spectral bluing (5). In the present case we consider the fluorescence from one single chromophore only and find that this drifts with time to both higher and lower energies. This observation immediately puts under question the common notion of a chromophore being a static entity defined by the conjugation length (6, 8, 11). We find that a single chromophore of MeLPPP can drift by up to 15 meV over time at 5 K, covering a quarter of the inhomogeneous distribution. Shifts spanning the entire range of inhomogeneous broadening can be achieved by heating the molecule up briefly. This process results in the average spectral position of SMs remaining temperature independent (22), whereas substantial thermochromism is typically observed in the bulk. These observations suggest that chromophores in CPs are not actually static entities defined by an effective conjugation length.
C stretching modes (7). Whereas no substantial low-frequency modes are detected in MeLPPP (Fig. 2b), two lower-energy modes at 250 and 555 cm–1 are clearly visible in MEH-PPV (Fig. 2a). A comparison with the spectrum in Fig. 1a suggests that a third very low energy mode at 70 cm–1 is actually merged with the ZPL, in this particular case because of the exceptionally clear and strong vibrational coupling exhibited by this molecule. The second observation relates to the temporal fluctuation in emission. Occasional fluorescence intermittency is observed; however, more importantly the peak maximum drifts with time. This finding is particularly evident in the MeLPPP spectra at 250 s. Identical spectral drifts are observed simultaneously in the vibronic replica. Spectral drifts and jumps are well known for SMs at both high (30) and low temperatures (14, 27, 31), and a few reports on room-temperature single-polymer spectroscopy also suggest spectral fluctuations with time (16, 18). This effect is, however, mainly caused by photo-oxidation and the inhibition of EET to lower energy segments, resulting in irreversible spectral bluing (5). In the present case we consider the fluorescence from one single chromophore only and find that this drifts with time to both higher and lower energies. This observation immediately puts under question the common notion of a chromophore being a static entity defined by the conjugation length (6, 8, 11). We find that a single chromophore of MeLPPP can drift by up to 15 meV over time at 5 K, covering a quarter of the inhomogeneous distribution. Shifts spanning the entire range of inhomogeneous broadening can be achieved by heating the molecule up briefly. This process results in the average spectral position of SMs remaining temperature independent (22), whereas substantial thermochromism is typically observed in the bulk. These observations suggest that chromophores in CPs are not actually static entities defined by an effective conjugation length.
Fig. 2.
Time-dependent PL spectra for a single MEH-PPV (a) and MeLPPP (b) molecule at 5 K. The temporal resolution is 3 s in a and 2 s in b. The grayscale corresponds to the PL intensity. The integrated PL spectra are shown (Right) and are shifted in frequency by their peak position to allow a direct comparison of the vibrational modes.
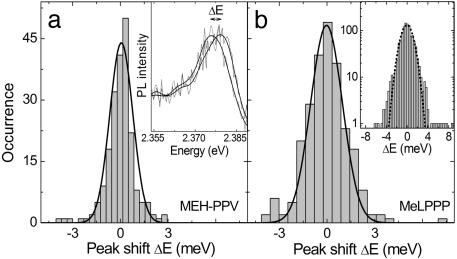
Although the MEH-PPV spectra in Fig. 2 are slightly broader because of the merging of the low-frequency vibrational mode with the ZPL, comparable jumps in the SM spectra are observed. The Fig. 3a Inset illustrates two consecutive spectra taken from Fig. 2a at 405 and 408 s. The histogram shows the distribution of jumps in energy ΔE between consecutive spectra, which is well described by a Gaussian of width 1.5 meV. Fig. 3b shows the ΔE distribution extracted for MeLPPP from Fig. 2b. It is virtually identical to that for MEH-PPV with a width of 2.0 meV, demonstrating that the spectral jitter of single chromophores follows a universal pattern in both molecules. Fig. 3b Inset gives the sum of five single-chromophore histograms on a logarithmic scale. As the jitter of different molecules is comparable, so are the ΔE histograms, and the sum provides unambiguous evidence for Gaussian statistics underlying the spectral jitter of the single chromophore. The similarity in spectral jitter between both types of molecules suggests that the spectral diffusion may be caused by interactions with the matrix. We therefore also investigated single MeLPPP molecules deposited directly on the SiO2 surface. Although the fluorescence intermittency was found to be enhanced substantially in the absence of the matrix, we found no change in the ΔE distribution. This finding is not too surprising, because the chromophores on the CP chain are in effect embedded in a polymer matrix made up by the remainder of the chain. The presence of an inert matrix around the entire CP molecule therefore only has a limited effect.
Fig. 3.
Frequency of jumps of energy ΔE between the peak position of subsequent spectra for the data in Fig. 2 for MEH-PPV (a) and MeLPPP (b) together with Gaussian fits of width 1.5 and 2.0 meV, respectively. (a Inset) Shown is the peak of two subsequent spectra recorded at 405 and 408 s, separated by an energy ΔE. (b Inset) The sum of five histograms for MeLPPP is given on a logarithmic scale together with a Gaussian fit of width 2.2 meV.
Spectroscopic Differences: Interchromophoric Interactions
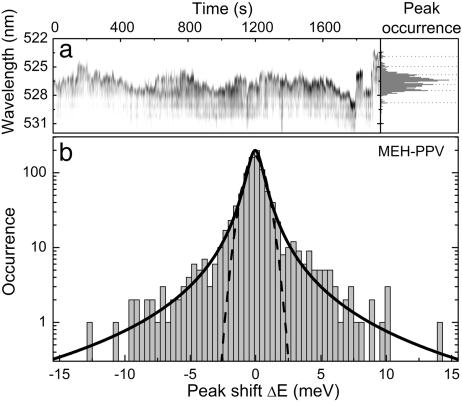
All of the single-line MeLPPP traces examined exhibited ΔE distributions comparable to that in Fig. 3b. In contrast, a MEH-PPV molecule should contain many more chromophore units than a MeLPPP chain, so that multiple-chromophore excitation should also occur under the narrow-band excitation conditions used. Fig. 4 demonstrates the case of multichromophoric emission of narrow, discrete lines over a detection time of 30 min. The spectral jitter covers a considerably broader range than in Fig. 2, although the PL linewidth is typically of order 0.5 nm (2.5 meV). The histogram of the peak positions shown in Fig. 4a Right reveals the presence of seven distinct peaks (marked by dashed lines), suggesting that the molecule switches between different emitting states. The ΔE histogram, displayed on a logarithmic scale in Fig. 4b, exhibits a clear deviation from the Gaussian in Fig. 3 (shown by the dashed line), with the tail of the distribution being more Lorentzian-like, as indicated by the solid line. We propose that whereas the emission from a single chromophore obeys Gaussian spectral diffusion, similar to the emission from single colloidal nanocrystals (32, 33), multichromophoric emission and jumping between discrete emitting states results in a more Lorentzian-like distribution. In our case the temporally integrated line shape (Fig. 4a Right) is quite definitely not Lorentzian in origin, whereas the jump statistics evidently are. In contrast, Lorentzian line shapes are frequently used to describe the origin of SM lines in low-temperature PL excitation spectra (34), which are broadened because of spectral diffusion. Typically, however, the energy range over which this kind of spectral diffusion occurs, which is often discussed in the framework of Levy-type statistics, is >3 orders of magnitude smaller than in the present case (34).
Fig. 4.
Spectral diffusion in the emission of a multichromophoric molecule. (a) Fluorescence trace of a single MEH-PPV molecule consisting of 960 spectra (2-s resolution) exhibiting multichromophoric emission together with a histogram of the peak positions displaying a number of discrete maxima (marked by dashed lines) caused by the presence of different chromophore units. (b) Corresponding jump frequency histogram. The solid line shows a Lorentzian fit (width 1.3 meV) to the data. A Gaussian (width 1.5 meV) is also plotted for comparison.
The distribution of small ΔE drifts is similar to that in Fig. 3 and exhibits an identical width of 1.5 meV, so that the peak of the multichromphoric ΔE distribution in Fig. 4b exhibits the same width as the single-chromophore distribution in Fig. 3a. The small ΔE values are thus mainly controlled by the jitter of the single chromophores, which can be equally well described by the Gaussian statistics of Fig. 3. In contrast, the large ΔEs arise from spectral jumping between chromophores, which are considerably rarer events, leading to the Lorentzian tails in the distribution when compared with the single-chromophore case. Studying the distribution of spectral jumps ΔE can thus allow the discrete identification of single chromophores in multichromophoric CPs. The universal Gaussian distribution, which is virtually identical for the two classes of CPs studied, also demonstrates that spectral diffusion in CPs is photochemical rather than thermal in nature, because the jumps observed are much larger than kT (≈0.5 meV) and the distribution is fully symmetrical. The precise origin of the spectral jitter is presently not clear, but might be linked to the presence of charges (30–33) on and around the polymer chain, which modify the electronic properties of the effective chromophore unit. Alternatively, slight structural rearrangements of the CP side groups may also play a role. The chromophore is therefore not defined intrinsically by the conjugation length of the polymer, but by external perturbations of the π-electron system.
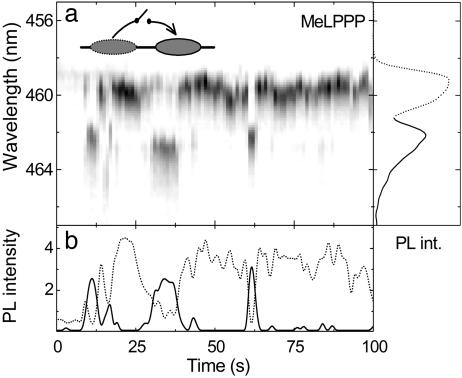
From the complicated fluorescence trace in Fig. 4 it is hard to tell whether correlated emission of individual chromophores occurs or whether the emission from the different narrow lines is predominantly random in nature. We recently demonstrated that polarization anisotropy measurements on single MeLPPP chains can provide detailed insight into intramolecular EET processes at low temperatures and yield conformational information on the rigid rod-like molecules (24). Intramolecular EET can, however, also be manifested by correlated emission between two discrete emitting units on the chain. Fig. 5 shows an example of two emitting states, which emit in dependence of one another. Two units are identified, at 459.5 and 462 nm. We studied the spectral diffusion of >70 single MeLPPP molecules and never observed jumps >3–4 meV. The two peaks in the present case, however, are separated by 15 meV, so we conclude that the two emitting species are caused by two chromophores present on the chain. Because the integrated intensities of the two lines are very different, we plot the envelopes of the two peaks to the right of the fluorescence trace rather than the integrated PL spectrum. Fig. 5b shows the peak intensities of the two emitting states marked by dashed (459.5 nm) and solid (462 nm) lines. Emission from the higher-energy chromophore dominates most of the time, but a decrease in emission from the higher lying state is always correlated with an increase in emission from the band at 462 nm. The observed switching between the two units is not discrete, but gradual, as is particularly apparent at time 35 s. A possible interpretation of the correlated behavior of the two chromophore units is that only one of the units may absorb, a phenomenon we have previously been able to observe using polarization-sensitive SM spectroscopy (24). Under certain conditions EET can occur between the two chromophore units, so that all excitation energy is transferred to the lower energy segment. At present we can only speculate about why this effect may depend on time. One conceivable reason is that the spectral diffusion of both chromophores will control the spectral overlap between the two lines and therefore influence the efficiency of EET. Slight conformational rearrangements or the presence of reversibly formed chemical defects such as charge-transfer states (14, 16, 18) between the two units may be additional reasons for the appearance of this switchable intramolecular EET.
Fig. 5.
Switchable interchromophoric energy transfer in a SM. (a) PL trace of a single MeLPPP molecule (1-s resolution) showing correlated emission of two chromophore units. The envelopes of the two peaks are also given for clarity. (b) Temporal evolution of the peak intensities of the two discrete emitting units.
This demonstration of interchromophoric EET on the SM level serves to illustrate that the underlying photophysical signatures we observe from MeLPPP molecules are virtually always identical, with linewidths typically in the range of 0.5 to 1 nm (3–6 meV). Although naturally every SM behaves slightly differently, many molecules exhibit such a correlated single-chromophore emission, which complements our previous polarization anisotropy measurements of intramolecular EET (24). We conclude that the emitting state is always the same and corresponds to a principally isolated unit on the chain. This finding is not further surprising, because aggregation effects are not overly important in the photophysics of even bulk films of MeLPPP (35). The case is very different for MEH-PPV and indeed highlights the principal dissimilarity between these two CP materials. Some molecules exhibit PL line-widths in excess of 50 meV, and indeed previous reports of low-temperature MEH-PPV SM PL have only documented features over an order of magnitude broader than the single-chromophore line (23, 26). The reason for this dramatic spread in linewidths may be the simultaneous emission of multiple chromophores, aggregation, very rapid electronic dephasing, or exceptionally strong vibrational coupling.
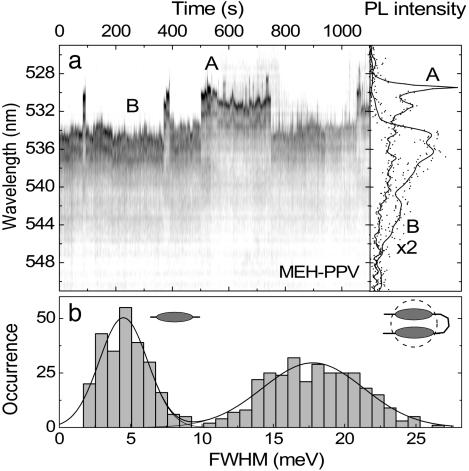
Below, we demonstrate that one and the same molecule can exhibit both narrow and broad emission features. Fig. 6a shows a fluorescence trace consisting of 500 spectra of a single MEH-PPV molecule displaying a strong switching behavior between two rather different emissive species, labeled A and B. Whereas A is dominated by a narrow (≈0.5 nm, 2.5 meV) ZPL followed by the vibronic replicas discussed above, B consists of a broad featureless spectrum. The distribution of linewidths observed is plotted in Fig. 6b and reveals two distinct emitting species, one narrow (≈4 meV) and the other broad (≈18 meV). The clear minimum in the distribution at 10 meV full width at half maximum documents the presence of two distinct emitting species in the CP. Aggregation effects are known to occur in MEH-PPV and are most apparent in solvent-mediated photophysics (2, 4–6). The presence of directional EET (16) and even controlled single-photon emission (36) from single CP chains has provided further evidence for aggregate formation at room temperature. Broad, red-shifted spectral features in bulk films or concentrated solutions are often discussed in terms of aggregation or even excimer formation (6). Interestingly, this transition observed in the SM is very reminiscent of the recently reported switching between isolated and superradiant states in bichromophoric molecules, which also reveal reversible transitions from states exhibiting narrow ZPLs to broad, red-shifted emission features (37, 38). Future measurements of the SM PL lifetime will clarify whether the broadening arises because of superradiance (resulting in an increased decay rate) or excimer formation, leading to a slower decay. In any case, the dramatic spectral broadening is indicative of strong, coherent interchromophoric coupling. The fundamental insight provided by this observation of linewidth switching is that emission from one and the same molecule may be both molecular and band-like (delocalized) in nature, depending on the strength of interchromophore coupling.
Fig. 6.
Switching between aggregated and free chromophore emission in MEH-PPV. (a) PL trace of a single MEH-PPV molecule (2-s resolution) revealing discrete jumps between narrow, single-chromophore emission (A) and broad, aggregated emission (B). (b) Histogram showing the occurrence of a particular spectral width for 500 spectra.
For light-harvesting applications such as those required in organic solar cells, where efficient exciton migration to charge-accepting sites is important, superradiant states are most preferable, in analogy to biological photosynthetic complexes (21). In contrast, molecular photophysical properties characteristic of strongly localized emission are desirable for electroluminescence applications, where a high degree of delocalization and mobility of excitations leads to fluorescence quenching. As for the case of incoherent chromophore coupling demonstrated above for MeLPPP in Fig. 5, the question remains why the chromophore interaction in Fig. 6 suddenly switches on and off. It might again be caused by temporal changes in the energetic separation between adjacent chromophores due to the dynamic disorder of the single emissive species, conformational rearrangement, or one of the units entering a reversible dark state as in the case of well defined bichromophoric systems (38). Because it is unlikely that the broad and narrow emissions arise from the same chromophore, the most probable scenario involves switchable EET from a single chromophore to a lower-energy aggregated trap state. Some molecules, however, also exhibit switching between lower-energy narrow lines and higher-energy broad lines, so that the general situation is evidently somewhat more complex. Future research should be directed toward externally controlling the level of interchromophore coupling manifested by the dramatic spectral transitions. The fundamental message is that one and the same molecule can support both aggregated and nonaggregated (i.e., MeLPPP-like) emission.
Previous photon-echo measurements suggested that the ensemble absorption spectrum of MEH-PPV in toluene is predominantly homogeneously broadened (39). This finding is apparently not generally the case at low temperatures, because disorder is more than a factor 100 greater than the chromophore linewidth. Assuming that the aggregated state of MEH-PPV exhibits a similar broadening with temperature to the single chromophores in MeLPPP (22), the linewidth of the aggregate state may indeed become comparable to the disorder at room temperature. It is important to realize that ref. 39 therefore refers to aggregated states rather than to the intrinsic electronic structure of the π-electron system. This idea is consistent with the fact that MEH-PPV is known to aggregate in toluene (2, 4, 5). Aggregated states such as those observed in Fig. 6 are highly significant for achieving efficient intramolecular EET in MEH-PPV (19). The efficiency of intramolecular EET is controlled by the average spectral overlap between adjacent chromophore units (22). Because the single-chromophore linewidth is 100 times smaller than the disorder in MEH-PPV at 5 K, interchromophoric EET is inefficient (19). Energetically broad, aggregated states, however, can always act as acceptors, thereby enabling highly efficient exciton funneling (16, 19, 36). This process is controlled primarily by the spectral linewidth.
Conclusions
We have demonstrated that very different CP materials exhibit universal fluorescence behavior on the single-chromophore level, providing useful imput to discussions of structure–property relationships. SM spectroscopy allows us to differentiate directly between static and dynamic disorder. Whereas static disorder bears a direct correlation to chemical structure, the chromophoric dynamic disorder appears to be a universal property despite the large differences in intramolecular vibrational coupling. The fundamental difference between rigid rod and flexible CPs is the tendency for intramolecular aggregation, which leads to discrete spectroscopic signatures in the SM emission from flexible chains. Our results provide input into the understanding of the underlying physics of excitonic dipole coupling in molecular systems. Following from the photophysical stability of single-polymer chains observed in the present work, we anticipate that CPs could provide the basis for future generations of SM optoelectronic devices.
Acknowledgments
We thank Josef Müller for the provision of data analysis tools and many helpful discussions and Werner Stadler for technical assistance. This work received financial support from the Deutsche Forschungsgemeinschaft through the Sonderforschungsbereich 486 and a Gottfried Wilhelm Leibniz award.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CP, conjugated polymer; SM, single molecule; MEH-PPV, poly(2-methoxy-5-(2′-ethyl-hexyloxy)-1,4-phenylene-vinylene); MeLPPP, methyl-substituted ladder-type poly(para-phenylene); ZPL, zero-phonon line; PL, photoluminescence; EET, excitation energy transfer.
References
- 1.Kraft, A., Grimsdale, A. C. & Holmes, A. B. (1998) Angew. Chem. Int. Ed. 37, 402–428. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz, B. J. (2003) Annu. Rev. Phys. Chem. 54, 141–172. [DOI] [PubMed] [Google Scholar]
- 3.Scherf, U. & List, E. J. W. (2002) Adv. Mater. 14, 477–487. [Google Scholar]
- 4.Hu, D. H., Yu, J., Wong, K., Bagchi, B., Rossky, P. J. & Barbara, P. F. (2000) Nature 405, 1030–1033. [DOI] [PubMed] [Google Scholar]
- 5.Huser, T., Yan, M. & Rothberg, L. J. (2000) Proc. Natl. Acad. Sci. USA 97, 11187–11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pope, M. & Swenberg, C. E. (1999) Electronic Processes in Organic Crystals and Polymers (Oxford Univ. Press, Oxford), 2nd Ed.
- 7.Bässler, H. & Schweitzer, B. (1999) Acc. Chem. Res. 32, 173–182. [Google Scholar]
- 8.Heun, S., Mahrt, R. F., Greiner, A., Lemmer, U., Bässler, H., Halliday, D. A., Bradley, D. D. C., Burn, P. L. & Holmes, A. B. (1993) J. Phys. Condens. Matter 5, 247–260. [Google Scholar]
- 9.Shand, M. L., Chance, R. R., LePostollec, M. & Schott, M. (1982) Phys. Rev. B 25, 4431–4436. [Google Scholar]
- 10.Rossi, G., Chance, R. R. & Silbey, R. (1989) J. Chem. Phys. 90, 7594–7601. [Google Scholar]
- 11.Grimme, J., Kreyenschmidt, M., Uckert, F., Müllen, K. & Scherf, U. (1995) Adv. Mater. 7, 292–295. [Google Scholar]
- 12.Rohlfing, M. & Louie, S. G. (1999) Phys. Rev. Lett. 82, 1959–1962. [Google Scholar]
- 13.Mukamel, S., Tretiak, S., Wagersreiter, T. & Chernyak, V. (1997) Science 277, 781–787. [Google Scholar]
- 14.Moerner, W. E. & Orrit, M. (1999) Science 283, 1670–1676. [DOI] [PubMed] [Google Scholar]
- 15.VandenBout, D. A., Yip, W. T., Hu, D. H., Fu, D. K., Swager, T. M. & Barbara, P. F. (1997) Science 277, 1074–1077. [Google Scholar]
- 16.Yu, J., Hu, D. & Barbara, P. F. (2000) Science 289, 1327–1330. [DOI] [PubMed] [Google Scholar]
- 17.Kumar, P., Mehta, A., Dadmun, M. D., Zheng, J., Peyser, L., Bartko, A. P., Dickson, R. M., Thundat, T., Sumpter, B. G., Noid, D. W. & Barnes, M. D. (2003) J. Phys. Chem. B 107, 6252–6257. [Google Scholar]
- 18.Yip, W.-T., Hu, D., Yu, J., Vanden Bout, D. A. & Barbara, P. F. (1998) J. Phys. Chem. A 102, 7564–7575. [Google Scholar]
- 19.Nguyen, T. Q., Wu, J. J., Doan, V., Schwartz, B. J. & Tolbert, S. H. (2000) Science 288, 652–656. [DOI] [PubMed] [Google Scholar]
- 20.Beljonne, D., Pourtois, G., Silva, C., Hennebicq, E., Herz, L. M., Friend, R. H., Scholes, G. D., Setayesh, S., Müllen, K. & Brédas, J. L. (2002) Proc. Natl. Acad. Sci. USA 99, 10982–10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmann, C., Ketelaars, M., Matsushita, M., Michel, H., Aartsma, T. J. & Köhler, J. (2003) Phys. Rev. Lett. 90, 013004. [DOI] [PubMed] [Google Scholar]
- 22.Müller, J. G., Lemmer, U., Raschke, G., Anni, M., Scherf, U., Lupton, J. M. & Feldmann, J. (2003) Phys. Rev. Lett. 91, 267403. [DOI] [PubMed] [Google Scholar]
- 23.Rønne, C., Trägårdh, J., Hessman, D. & Sundström, V. (2004) Chem. Phys. Lett. 388, 40–45. [Google Scholar]
- 24.Müller, J. G., Lupton, J. M., Feldmann, J., Lemmer, U. & Scherf, U. (2004) Appl. Phys. Lett. 84, 1183–1185. [Google Scholar]
- 25.Müller, J. G., Anni, M., Scherf, U., Lupton, J. M. & Feldmann, J. (2004) Phys. Rev. B 70, 035205. [DOI] [PubMed] [Google Scholar]
- 26.Yu, Z. & Barbara, P. F. (2004) J. Phys. Chem. B 108, 11321–11326. [Google Scholar]
- 27.Kiraz, A., Ehrl, M., Bräuchle, C. & Zumbusch, A. (2003) J. Chem. Phys. 118, 10821–10824. [Google Scholar]
- 28.Kennedy, S. P., Garro, N. & Phillips, R. T. (2001) Phys. Rev. Lett. 86, 4148–4151. [DOI] [PubMed] [Google Scholar]
- 29.Romanovskii, Y. V., Bässler, H. & Scherf, U. (2004) Chem. Phys. Lett. 383, 89–94. [Google Scholar]
- 30.Lu, H. P. & Xie, X. S. (1997) Nature 385, 143–146. [Google Scholar]
- 31.Ambrose, W. P. & Moerner, W. E. (1991) Nature 349, 225–227. [Google Scholar]
- 32.Neuhauser, R. G., Shimizu, K. T., Woo, W. K., Empedocles, S. A. & Bawendi, M. G. (2000) Phys. Rev. Lett. 85, 3301–3304. [DOI] [PubMed] [Google Scholar]
- 33.Müller, J., Lupton, J. M., Rogach, A. L., Feldmann, J., Talapin, D. V. & Weller, H. (2004) Phys. Rev. Lett., in press. [DOI] [PubMed]
- 34.Jung, Y. J., Barkai, E. & Silbey, R. J. (2002) J. Chem. Phys. 117, 10980–10995. [Google Scholar]
- 35.Lupton, J. M. (2002) Chem. Phys. Lett. 365, 366–368. [Google Scholar]
- 36.Hollars, C. W., Lane, S. M. & Huser, T. (2003) Chem. Phys. Lett. 370, 393–398. [Google Scholar]
- 37.Hernando, J., van der Schaaf, M., van Dijk, E., Sauer, M., García-Parajó, M. F. & van Hulst, N. F. (2003) J. Phys. Chem. A 107, 43–52. [Google Scholar]
- 38.Lippitz, M., Hübner, C. G., Christ, T., Eichner, H., Bordat, P., Herrmann, A., Müllen, K. & Basché, T. (2004) Phys. Rev. Lett. 92, 103001. [DOI] [PubMed] [Google Scholar]
- 39.Scholes, G. D., Larsen, D. S., Fleming, G. R., Rumbles, G. & Burn, P. L. (2000) Phys. Rev. B 61, 13670–13678. [Google Scholar]