We studied motor unit firing rates (MUFRs) at various voluntary contraction intensities in the hamstrings, one of the only major lower limb muscles to have MUFRs affected by muscle length changes. Within the hamstrings muscle-specific differences have greater impact on MUFRs than length changes, with the biceps femoris having reduced neural drive compared with the semimembranosus-semimembranosus. Comparing our results to other lower limb muscles, flexors have inherently higher firing rate compared with extensors.
Keywords: EMG, muscle length, maximal isometric voluntary contraction, neural drive, strength
Abstract
Neuromuscular properties of the lower limb in health, aging, and disease are well described for major lower limb muscles comprising the quadriceps, triceps surae, and dorsiflexors, with the notable exception of the posterior thigh (hamstrings). The purpose of this study was to further characterize major muscles of the lower limb by comprehensively exploring contractile properties in relation to spinal motor neuron output expressed as motor unit firing rates (MUFRs) in the hamstrings of 11 (26.5 ± 3.8) young men. Maximal isometric voluntary contraction (MVC), voluntary activation, stimulated contractile properties including a force-frequency relationship, and MUFRs from submaximal to maximal voluntary contractile intensities were assessed in the hamstrings. Strength and MUFRs were assessed at two presumably different muscle lengths by varying the knee joint angles (90° and 160°). Knee flexion MVCs were 60–70% greater in the extended position (160°). The frequency required to elicit 50% of maximum tetanic torque was 16–17 Hz. Mean MUFRs at 25–50% MVC were 9–31% less in the biceps femoris compared with the semimembranosus-semitendinosus group. Knee joint angle (muscle length) influenced MUFRs such that mean MUFRs were greater in the shortened (90°) position at 50% and 100% MVC. Compared with previous reports, mean maximal MUFRs in the hamstrings are greater than those in the quadriceps and triceps surae and somewhat less than those in the tibialis anterior. Mean maximal MUFRs in the hamstrings are influenced by changes in knee joint angle, with lower firing rates in the biceps femoris compared with the semimembranosus-semitendinosus muscle group.
NEW & NOTEWORTHY We studied motor unit firing rates (MUFRs) at various voluntary contraction intensities in the hamstrings, one of the only major lower limb muscles to have MUFRs affected by muscle length changes. Within the hamstrings muscle-specific differences have greater impact on MUFRs than length changes, with the biceps femoris having reduced neural drive compared with the semimembranosus-semimembranosus. Comparing our results to other lower limb muscles, flexors have inherently higher firing rate compared with extensors.
neuromuscular properties of human lower limb muscle groups have been studied extensively (Connelly et al. 1999; Dalton et al. 2009; Graham et al. 2016; Kamen and Knight 2004; Kennedy and Cresswell 2001; Kirk et al. 2016; Oya et al. 2009; Roos et al. 1999), with the notable exception of the knee flexors of the posterior thigh. Three of the four muscles of this group (semimembranosus, semitendinosus, and long head of the biceps femoris) are two-joint muscles acting to flex the knee and extend the hip, while the short head of the biceps femoris acts only as a knee flexor (Moses et al. 2005). In relation to other lower limb muscles, muscles of the posterior thigh have been largely overlooked. This is likely because of the experimental challenges of the anatomic location of this extensive two-joint muscle group for recording voluntary activation, stimulated contractile responses, and electromyography (EMG). However, the hamstrings are no less functionally important than other lower limb muscles in bipedal humans. In addition, the posterior thigh muscles provide unique associations with tissue harvesting for reconstructive surgery (Inacio et al. 2012; Taylor et al. 2009) and injury from exercise (Fyfe et al. 2013; Horst et al. 2014). Because of unique limb development in humans, the posterior thigh group are considered flexor muscles and of similarity to the dorsiflexor group of the leg, whereas the quadriceps and triceps surae developmentally are extensors (Sadler and Langman 2012).
Contractile and motor unit (MU) properties are well described for many lower limb muscles such as the vastus medialis, vastus lateralis, tibialis anterior (TA), gastrocnemii, and soleus in health, aging, exercise, and disease (Doherty 2001; Heckman and Enoka 2012; Hepple and Rice 2016; Hunter 2015; Piasecki et al. 2016; Roos et al. 1997). In the lower limb, motor unit firing rates (MUFRs) have shown a great range, from 16 to 22 Hz in the triceps surae (Dalton et al. 2009; Graham et al. 2016; Kirk et al. 2016) and ∼25 Hz in the anterior thigh (Kamen and Knight 2004; Roos et al. 1999) to ∼42 Hz in the dorsiflexors (Connelly et al. 1999). Although voluntary strength (Sanfilippo et al. 2013), electromechanical delay (Blackburn et al. 2009), surface EMG (sEMG) (Gretchen and Christopher 2009; Henriksen et al. 2011; Herda et al. 2008; Jacobson et al. 1995; Lunnen et al. 1981; Marateb et al. 2011; McAllister et al. 2014; McHugh et al. 2001; Rutherford et al. 2011; Watanabe et al. 2016), and intramuscular EMG (Altenburg et al. 2009b; Onishi et al. 2002) have been reported for the hamstrings group under variable situations, no studies have explored MUFRs during a range of contraction intensities.
Hip and knee joint angles have a large influence on overall contractile capacity with muscle length changes (Lunnen et al. 1981; Onishi et al. 2002; Sanfilippo et al. 2013). Studies report that strength and EMG activation in the hamstrings group are likely influenced by muscle length when altered by hip and knee joint positions. When the muscle is shortened, contractile capacity is decreased (Lunnen et al. 1981; Onishi et al. 2002; Sanfilippo et al. 2013); however, there are conflicting reports on the activity in the long head of the biceps. Lunnen et al. (1981) reported that sEMG activity decreases as the muscle is lengthened, whereas Onishi et al. (2002) found intramuscular EMG activity increases.
Our aim was to characterize the contractile and MU properties related to muscle activity and MUFRs in the biceps femoris (BF) and semimembranosus-semitendinosus (SS). These were determined during electrical stimulation and brief voluntary isometric knee flexion contractions [from 25% to 100% maximal isometric voluntary contraction (MVC)] at two knee joint angles (90° and 160° extension). We hypothesized that, as a flexor, hamstring MUFRs at maximal levels of activation would be greater than those found in the antagonist quadriceps group (Kamen and Knight 2004; Roos et al. 1999) and, secondarily, that MUFRs in the BF would be higher than those of the SS because of greater intramuscular EMG activity in the BF at knee joint extended positions (Onishi et al. 2002), where the greatest amount of torque can be produced (Sanfilippo et al. 2013).
METHODS
Subjects.
Eleven young men [age 26.5 (3.8) yr, body mass 83.2 (9.4) kg, and height 181.8 (4.0) cm] volunteered for this study. Participants were recreationally active and not highly sedentary or trained systematically. Exclusion criteria included known neuromuscular or orthopedic pathologies of the lower limb, diabetes, alcoholism, caffeine consumption prior to participation, and recreational drug use. This study was reviewed and approved by the local University's research ethics board for human experimentation and conforms to the latest revision of the Declaration of Helsinki.
For optimal response of electrically stimulated contractile properties, stimulations were performed in the seated upright position because of the limitations of the dynamometer and weight of the leg acting on the lower limb. Intramuscular EMG and sEMG were recorded in a prone position at various voluntary contractile intensities. At each position the hamstrings were tested at two different knee joint angles.
Muscle contractile properties and stimulation.
For the first session, subjects (n = 11) were seated upright on a dynamometer (Cybex HUMAC NORM; CSMi Medical Solutions) with the left (nondominant) leg fixed to an adaptor arm on the anterior tibia just proximal to the malleoli. The hip was extended to 100°, the ankle was positioned at 90°, and the lateral femoral condyle was aligned to the axis of rotation of the dynamometer. Measurements were taken at two knee joint angles, which affected the length of the hamstrings. One knee joint position was flexed to 90° (shortened length) and the other extended to 160°. The subject was firmly positioned in the dynamometer chair by seatbelts at the shoulder and hip, and a Velcro strap secured the distal thigh just above the knee. Calibrated torque was recorded from the dynamometer and sampled at 500 Hz (Cambridge Electronic Design, Cambridge, UK). Torque production was displayed on a computer screen for visual feedback.
For electrical stimulation, large stimulation electrodes (5 × 15 cm, aluminum foil) covered in electro-stimulation gel, were wrapped in paper towel and saturated in saline water. The first electrodes were taped transversely over the proximal posterior thigh (inferior to the gluteal fold and slightly medial to avoid activation of the vastus lateralis) and the second placed distally over the posterior thigh muscles (∼5 cm superior to the popliteal fossa). Stimulation (model DS7AH; Digitimer, Welwyn Garden City, UK) was applied through the electrodes at 400 V with a pulse duration of 50 μs for the tetanic stimulation protocol and 200 μs for the modified twitch stimulation protocol and with current intensity ranging between 250 and 450 mA. For all stimulation events the current intensity was incremented to a level that would activate as much of the muscle as possible (as measured by torque output) without activation of muscles in the anterior or medial thigh compartments assessed by maximal torque output, visual inspection, and palpation.
Two or three 5- to 7-s MVCs were performed without stimulation for knee extension and knee flexion MVCs at the two knee joint angles to assess maximal voluntary torque production. Electrically stimulated twitch properties were recorded at rest and during and after knee flexion MVCs in the extended knee joint position. This was found to be the optimal position to record stimulated contractile responses in this muscle group. A modified twitch interpolation technique (Hales and Gandevia 1988) was used to test the ability of the subjects to maximally activate the hamstrings during MVC. For this test, electrical impulses (2 pulses at 100 Hz) were applied to the muscle at rest ∼1 s before, during the peak plateau of the 5- to 7-s MVC, and ∼1 s after the MVC when at rest. Visual feedback and strong verbal encouragement were given during MVC. Two or three separate trials were performed with 5-min rest to control for fatigue, with the highest value being recorded as maximum.
The final test was the force-frequency protocol, in which the muscle was stimulated at 2, 5, 8, 10, 12, 15, 20, 30, 40, 50, 80, and 100 Hz (Roos et al., 1999) for 1-s durations with the muscle at rest with a 30-s interval between each stimulation. The stimulus intensity was determined by the maximum tolerable intensity at the 100-Hz stimulation, which ranged from 16% to 35% of MVC, remaining at this intensity for all stimulation frequencies. The order of stimulation frequencies was randomized for each subject.
Electromyography.
On a separate day, subjects (n = 10) were positioned prone on the same dynamometer with the left (nondominant) leg fixed to the adaptor arm as described above. The hip was extended to 170° with the ankle fixed at 90°. The lateral femoral condyle was aligned to the axis of rotation, and the knee was positioned at two angles, one flexed to 90° and one extended to 160° (modified from Onishi et al. 2002). For this setup the subject was firmly fastened by large Velcro straps on the posterior lower back and posterior distal thigh to prevent extraneous movements. Intramuscular EMG was only possible in this position because of accessibility of the posterior thigh for needle insertions and manipulations.
To assess neuromuscular activation, sEMG in a bipolar configuration for the medial and lateral hamstring muscle groups was used; for the antagonist (quadriceps) an overall EMG assessment was employed by a monopolar configuration. For the flexors, an interelectrode distance of 2 cm center to center was used with a common ground on the patella; electrode pairs were placed over the lateral midthigh on BF and the medial midthigh over SS. For the quadriceps, a single electrode was placed on the anterior midthigh, and the ground electrode was positioned on the lateral femoral condyle. sEMG signals were recorded with self-adhering cloth Ag-AgCl electrodes (H59P monitoring electrodes; Kendall, Mansfield, MA). The sEMG electrode placement sites were swabbed vigorously with 70% ethanol before placement. All sEMG signals were preamplified (×1,000), wide-band filtered between 10 Hz and 10 kHz, further appropriately low- and high-pass filtered (Neurolog, NL844; Digitimer), and sampled at 2 kHz (Power 1401; Cambridge Electronic Design).
For sampling of MUFRs during voluntary contractions, two intramuscular tungsten electrodes (Bellemare et al. 1983; Fuglevand et al. 1993) were inserted into the hamstring muscles, one into the lateral musculature (BF) and one into the medial (SS) musculature half the distance from the head of the fibula to the greater trochanter. Before insertion, each insertion site was cleansed with 70% ethanol on the skin surface over the muscle bellies. The two sterile electrodes were connected to separate channels, and each was manipulated independently by an operator. The electrodes were custom-made insulated tungsten wire needles (123 μm in diameter and 45 mm in length; FHC, Bowdoin, ME). The advantage of this technique is to manipulate the electrode during contractions, allowing the operator to sample from many discrete MUs from each subject, building an overall MU profile range at each contraction intensity. The EMG signals were preamplified (×100), wide-band filtered between 10 Hz and 10 kHz, further appropriately low- and high-pass filtered (Neurolog, NL844; Digitimer), and sampled at 20 kHz per channel (Power 1401; Cambridge Electronic Design). Reference electrodes for the intramuscular electrodes were positioned over the patella of the left lower limb. Audio and visual feedback were provided to each operator independently.
Voluntary contractions were held for 5–10 s at each of three (25%, 50%, and 100% of MVC) contraction levels with rest periods between contractions of ∼5 min to minimize fatigue. The order was pseudorandomized, with MU trains being sampled during the steady-state plateau portion of the contractions (Fig. 1). Visual feedback of force was provided to the participant for each contraction. To sample from as many discrete MUs as possible, the microelectrode was manipulated and advanced slowly through the muscle (∼0.5 cm per contraction). Over a series of contractions, electrodes were repositioned or reinserted into different portions of the muscle in a similar region of the thigh to achieve a representative sample from each muscle. Several (∼3–6) contractions were made at each contraction intensity until the MVC level was ∼5% lower than the baseline (initial). To acquire a large number of MU trains without the influence of fatigue, some subjects returned to the lab to repeat these procedures on an additional day.
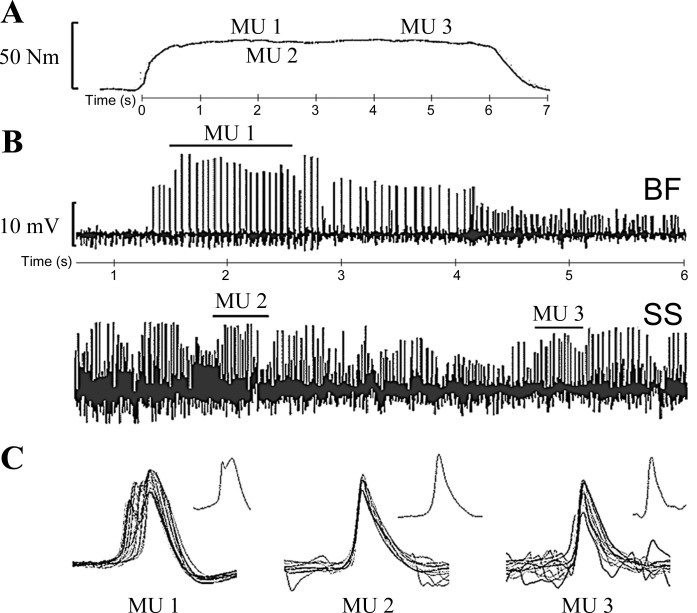
Fig. 1.
Example of a typical motor unit (MU) action potential train recorded at 50% MVC with the knee joint angle at 90°. A: voluntary isometric torque. B: expanded view of unprocessed electromyographic signals recorded through tungsten intramuscular electrodes individually inserted into the biceps femoris (BF) and semimembranosus-semitendinosus (SS). C: example of an action potential shape and overlay of all action potentials from 3 identified MU trains: MU1 is an overlay of 23 MU action potentials with a coefficient of variation of 9.7 discharging at 13.8 Hz. MU2 is an overlay of 8 MU action potentials with a coefficient of variation of 15.9, discharging at 30.7 Hz. MU3 is an overlay of 16 MU action potentials with a coefficient of variation of 19.7, discharging at 19.3 Hz.
Data acquisition and analyses.
The contractile property and MU analyses were performed off-line with Spike2 (Cambridge Electronic Design) as described previously (Connelly et al. 1999; Roos et al. 1999). For contractile properties of the modified evoked twitch the following measurements were made: peak twitch amplitude, twitch time to peak tension, one-half relaxation time, contraction duration, and potentiated peak twitch amplitude (Roos et al. 1999; Vandervoort and McComas 1983). Voluntary activation was calculated from the interpolated twitch technique as previously described (Todd et al. 2004). The force-frequency curve was normalized to the 100-Hz stimulation. To measure sEMG, for a 1-s epoch about the maximal torque during the knee flexor MVC, root-mean-squared (RMS) amplitude was calculated and used to normalize the sEMG for all other contraction intensities. For coactivation, knee extension RMS of a 1-s epoch about the peak torque during MVC was calculated. Quadriceps femoris sEMG recorded during knee flexor contractions was normalized to the maximal knee extension sEMG.
For the MU train analysis a template shape algorithm facilitated the process, but visual inspection by an experienced operator was required to confirm spike allocation to each specific MU train. For inclusion, in addition to shape overlay of MU potentials, a MU train required a minimum of five contiguous action potentials (4 interspike intervals). Doublets (>100 Hz) were excluded from analysis, and a coefficient of variation <30% for the interspike interval duration of each MU train was required for inclusion (Fuglevand et al. 1993). An example of three discrete MU action potential trains extracted during 50% MVC from a participant is shown in Fig. 1. For statistical comparisons, the MU trains were grouped into three bins based on torque level: a 100% bin contained torque levels between 100% and 87.5% of MVC; a 50% bin was 62.5-37.5% MVC; and a 25% bin was 37.5-12.5% MVC.
Statistics.
The R software environment (R version 3.2.3) was used for statistical analysis. To assess differences in strength between flexors and extensors at different joint angles, unpaired t-tests were used. To assess differences in sEMG, a 1 × 3 analysis of variance (ANOVA) was used (sEMG | muscle × torque × angle). To assess differences in MUFRs, a 1 × 3 ANOVA was also used (MUFR | muscle × torque × angle). When statistical significance was found (P < 0.05) the Tukey honestly significant difference post hoc test was used to determine differences within the interaction. Regression analyses were used to determine relationships between torque and MUFRs for each muscle and joint angle. To determine dependence of two variables (MUFR × %MVC) the Pearson's correlation (r) was used. To explore whether expected joint angle-related differences in MUFRs were greater in one muscle than the other, effect sizes (Cohen's d) were calculated. Numerical outcomes are reported as means (±SD).
RESULTS
Contractile properties and surface electromyography.
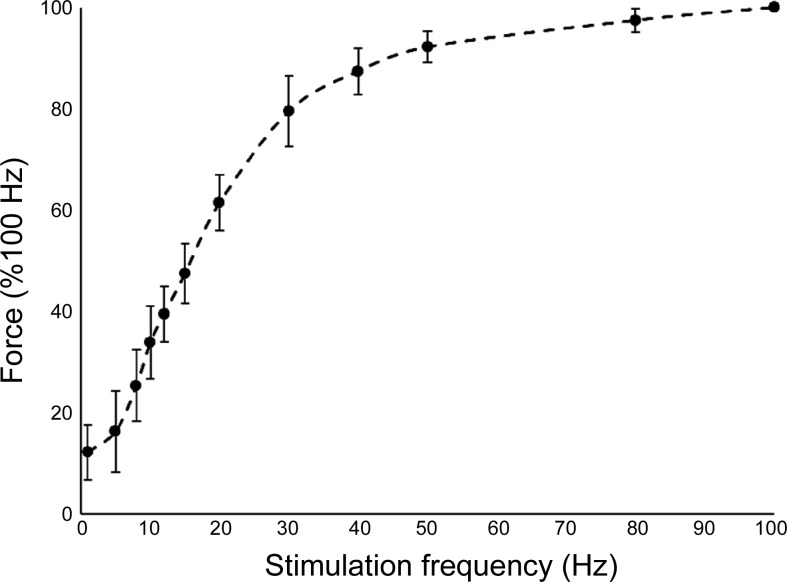
MVC during knee flexion and knee extension, voluntary activation, and contractile properties are presented in Table 1. Torque produced during knee flexion MVCs was 60–70% greater with the knee extended compared with the knee flexed in both the prone and seated positions (Table 1). In the prone position, knee flexion torque was ∼50% less than in the seated position (Table 1). In the seated position, voluntary activation (98.7%) and contractile properties from electrically stimulated twitch characteristics are presented in which the maximum twitch tension equaled 14% of MVC (Table 1). The normalized stimulated force-frequency relationship is shown in Fig. 2, and the maximum stimulated fused tetanic force generated was between 16% and 35% MVC. A frequency of 16–17 Hz was required to achieve 50% of the maximum normalized force.
Table 1.
Contractile properties during knee contractions
| Knee Joint Angle |
||||
|---|---|---|---|---|
| Muscle Property | Setup | Muscle Action | 90° | 160° |
| MVC, Nm | Seated | Extension | 320.1 (20.1) | 106.3 (14.9)* |
| Flexion | 101.3 (39.1) | 172.3 (89.6)* | ||
| Prone | Extension | 285.6 (91.5) | 115.5 (32.5)* | |
| Flexion | 53.8 (14.91) | 86.3 (17.4)* | ||
| VA, % | Seated | Flexion | 98.7 (1.3) | |
| Pt, Nm | 24.7 (8.8) | |||
| TPT, ms | 95.8 (43.6) | |||
| HRT, ms | 85.6 (53.4) | |||
| CD, ms | 181.5 (67.8) | |||
| PTP, Nm | 28.1 (7.0) | |||
Values are means (SD). Isometric torque was measured in the prone position for knee extension (n = 6) and knee flexion (n = 11) and in the seated position for knee extension (n = 11) and knee flexion (n = 11). Electrically stimulated contractile properties of knee flexion in the seated position were measured with a modified twitch technique (n = 11). MVC, maximal voluntary isometric contraction; VA, voluntary activation; Pt, evoked peak twitch; TPT, time to peak tension; HRT, half-relaxation time; CD, contraction duration; PTP, evoked potentiated twitch.
Significance in a knee joint angle interaction (P < 0.05).
Fig. 2.
Stimulated force-frequency relationship of the posterior thigh in the seated position with the knee joint angle at 160°. Peak tetanic tension of was normalized to the maximal peak tension of each subject recorded at 100 Hz. Mean maximal peak tension achieved was 30.0 (5.2)% MVC from the 11 participants. Error bars represent SD; n = 10.
In the prone position, intramuscular EMG and sEMG were sampled simultaneously (Table 2). For sEMG a three-way interaction (torque × muscle × angle) was nonsignificant (P = 0.75). The significant main effect of torque (P < 0.01) indicated that sEMG in both muscle groups were greater with successive torque levels. At 25% MVC in the 90° flexed position there was a significant difference in sEMG between muscles (P < 0.01), with 33% more sEMG activity in the BF compared with the SS group (Table 2). The main effect of knee joint angle (P = 0.06) presented a strong trend, and at 50% MVC there was 19% and 44% more sEMG activity at the 90° flexed position in the BF and SS, respectively (Table 2).
Table 2.
Surface electromyography during knee flexion contraction
| % RMS |
|||||
|---|---|---|---|---|---|
| Muscle | Setup | Muscle Action | % MVC | Knee joint angle 90° | Knee joint angle 160° |
| BF | Prone | Flexion | 50 | 54.7 (13.2) | 45.6 (14.0) |
| 25 | 26.9 (9.6)* | 24.9 (11.4) | |||
| SS | 50 | 58.6 (30.7) | 40.8 (10.9) | ||
| 25 | 20.2 (8.6)* | 21.7 (5.9) | |||
| QF | 100 | 27.3 (15.2) | 29.4 (13.3) | ||
| 50 | 13.0 (9.9) | 11.8 (4.9) | |||
| 25 | 5.2 (11.4) | 6.2 (4.7) | |||
Values are mean (SD) surface electromyography properties of the knee extensors and flexors of the thigh in the prone position. All muscles had root mean square (RMS) amplitude values normalized to 100% MVC at each knee joint angle position: biceps femoris muscle (BF; n = 10); semimembranosus-semitendinosus muscles (SS: n = 10); and for coactivation quadriceps femoris (QF; n = 6). There was significant increase in % RMS in each muscle and each angle as % MVC increased (P < 0.05).
Significance in a muscle-to-muscle interaction (P < 0.05).
Intramuscular electromyography.
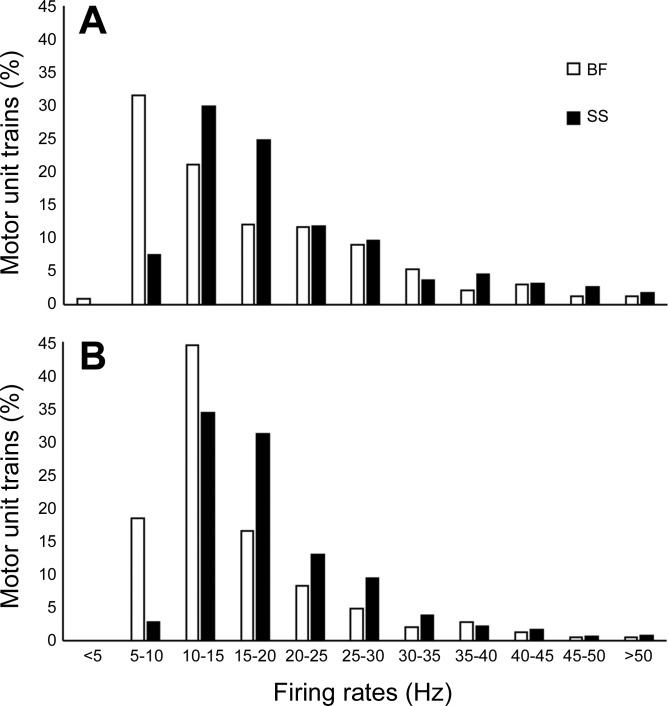
A total of 1,720 MU spike trains were identified from the BF and SS. Of these, 755 MU trains were identified in the BF, with 29% being recorded at 90° knee flexion, and 965 MU trains were identified in the SS, with 38% being recorded at 90° knee flexion. For each muscle and knee joint angle the greatest numbers of MU trains were identified at 25% MVC (∼800) while the fewest were identified at 100% MVC (∼190). In the BF at 25%, 50%, and 100% MVC the numbers of trains were 301, 293, and 111, respectively. In the SS at 25%, 50%, and 100% MVC the numbers of trains were 493, 338, and 82, respectively. For all MU trains the range of interspike intervals defining a train was 4–88, with a mean of 9.4 (7.4). The range of the coefficient of variation for interspike intervals of all MU trains was 0.8–29.8, with a mean of 14.2 (5.8). The range of firing rates for all MU trains was 4.5–76.2 Hz. The contribution by each subject to the sum of total collected MUFRs was 10.0 (2.9)%, and the normalized distributions of all collected MU trains for each muscle and knee joint angle are presented in Fig. 3.
Fig. 3.
Histograms of the distribution of motor unit firing rates across all torque levels of the biceps femoris (BF) and semimembranosus-semitendinosus (SS) with the knee joint flexed at 90° (A) and extended at 160° (B). Percentages of total motor unit trains are sorted into 5-Hz bins; n = 10.
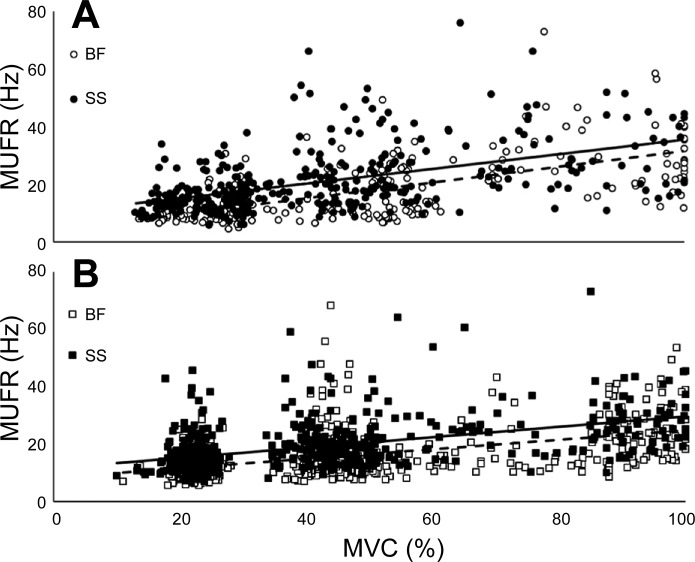
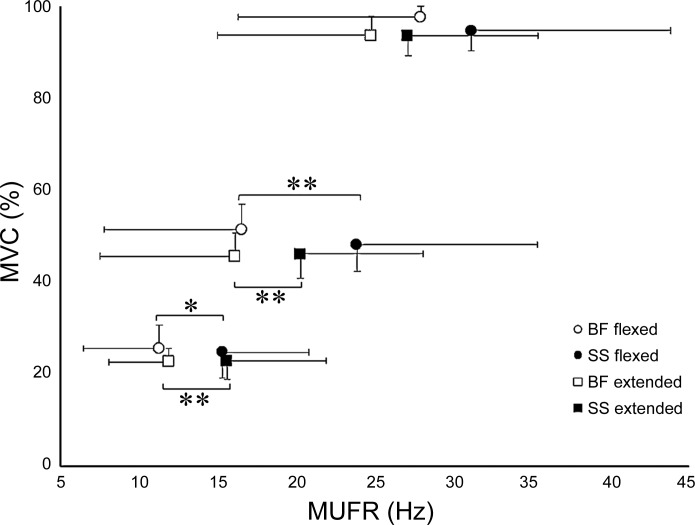
To compare the relationship between MUFRs and normalized torque, the data sets for each muscle group and knee joint angle were modeled by linear regression (Fig. 4). Additionally, to compare the dependence of MUFRs and %MVC against each muscle and joint angle, Pearson's correlations were performed (Fig. 4). To investigate the mean MUFR relationship to torque, muscle, and knee joint angle, MUs were grouped into three target levels normalized to the MVC (see methods). The significant main effect of torque (P < 0.001) indicated that in both muscle groups MUFRs were greater at each successive torque level from 25% to 100% MVC (Fig. 4). Mean MUFRs were 9–31% less in the BF than in the SS, achieving statistical significance at 25% and 50% MVC (Fig. 5). There was a trend in the muscle × knee joint angle interaction (P = 0.07) and a main effect of torque × knee joint angle interaction (P < 0.05) such that mean MUFRs were greater in the knee joint flexed-muscle shortened position in the BF and SS at 50% and 100% MVC (Fig. 5). To explore whether knee joint angle-related differences in MUFRs were larger for the BF than the SS, effect sizes (Cohen's d) were calculated; MUFRs were combined from all torque levels for each muscle, and the effect size, although small, was greater in the BF (16.8 Hz, Cohen's d 0.20) than the SS (19.8 Hz, Cohen's d 0.16).
Fig. 4.
Relationship between torque and motor unit firing rates (MUFRs). A: scatterplot of all MUFRs in the biceps femoris (BF) and semimembranosus-semitendinosus (SS) to their normalized torque levels with the knee joint angle at 90°. Linear regression equations are frequency(Hz) BF = 0.27 × torque(%MVC) + 4.08, R2 = 0.39 (dashed line); frequency(Hz) SS = 0.25 × torque(%MVC) + 10.09, R2 = 0.25 (solid line). Pearson's correlation coefficient for the BF (r = 0.62) was greater than for the SS (r = 0.50) with the knee joint angle at 90°. B: scatterplot of all MUFRs in the BF and SS to their normalized torque levels with the knee joint angle at 160°. Linear regression equations are frequency(Hz) BF = 0.16 × torque(%MVC) + 8.33, R2 = 0.24 (dashed line); frequency(Hz) SS = 0.18 × torque(%MVC) + 11.60, R2 = 0.24 (solid line). Pearson's correlation coefficient for the BF (r = 0.49) was similar to that for the SS (r = 0.49) with the knee joint angle at 160°; n = 10.
Fig. 5.
Mean motor unit firing rates (MUFRs) of the biceps femoris (BF) and semimembranosus-semitendinosus (SS) at knee joint angles of 90° and 160°. Axes are reversed from Fig. 4 to be consistent with typical methods of plotting stimulated force-frequency curves (as seen in Fig. 2). The MUFRs progressively increased with contraction intensity in both muscle groups and both knee joint angles (P < 0.001); n = 10. Values are means, with error bars representing SDs. *P < 0.01, **P < 0.001.
DISCUSSION
We report a comprehensive evaluation of neuromuscular properties in the medial and lateral compartments of the posterior thigh in young men. In comparing voluntary isometric measures at two knee joint angles, there were differential muscle and muscle length responses of neural drive. MUFRs increased with voluntary torque output, and overall rates were higher (∼25% more at MVC) than those previously reported for the quadriceps (Kalmar and Cafarelli 1999; Kamen and Knight 2004; Roos et al. 1999), with the SS group having greater MUFRs than the BF at all contraction intensities. The hamstrings were weaker in the shortened position when the knee joint angle was at 90°, being further impaired in the prone position when the hip joint was extended (Table 1). sEMG indicated more activity in the hamstrings in a shortened position, with muscle-specific differences between the BF and SS at low (<50% MVC) contraction levels.
Knee flexion torque produced at MVC was 60–70% greater with the knee extended in both the prone and seated positions, in agreement with previous reports (Lunnen et al. 1981; Onishi et al. 2002; Sanfilippo et al. 2013). Assessment of voluntary activation with the twitch interpolation technique has been performed in many muscles of the lower limb (Connelly et al. 1999; Dalton et al. 2009; Kirk et al. 2016; Roos et al. 1999), and here we report for the hamstrings that young healthy men are able to achieve near-complete activation (∼99%) with this technique (Table 1), which is similar to the other limb muscles tested. Compared with electrically evoked contractile properties of the knee extensors in young healthy men (Roos et al. 1999), twitch torque in the hamstrings reached 14% MVC compared with 10% MVC in the knee extensors (Roos et al. 1999). The stimulated force-frequency curve of the hamstrings required 16–17 Hz to achieve 50% of the maximum normalized force (Fig. 2), which is greater than the 11–12 Hz in the quadriceps of young men who were of similar age and activity status (Roos et al. 1999), indicating that the force-frequency relationship is shifted to the right compared with the quadriceps. Fiber type distributions in the hamstrings have been reported to be similar to those for the quadriceps. The long head of the BF has ∼45–67% type I (Garrett et al. 1984; Johnson et al. 1973), the semitendinosus has ∼45% type I, and the semimembranosus has ∼50% type I (Garrett et al. 1984), whereas in the quadriceps the vastus lateralis, vastus intermedius, vastus medialis, and rectus femoris are composed of ∼45%, 55%, 50%, and 40% type I, respectively (Garrett et al. 1984; Johnson et al. 1973). From these two reports utilizing cadavers, it appears that muscles of the posterior thigh may have a slightly greater type I fiber composition compared with the quadriceps but within the posterior thigh constitutive muscles are similar. These small compositional differences between the quadriceps and hamstrings are likely not functionally meaningful, and it is unlikely that fiber types could explain the observed differences in contractile properties and MUFRs.
In the posterior thigh, at lower contraction levels more sEMG activity was elicited in the BF than in the SS group (Table 2), and at more extended knee joint angles (longer muscle length) there was less relative sEMG activity in the BF and SS groups at 50% MVC. These results provide support to previous reports (Lunnen et al. 1981; Onishi et al. 2002) that muscle length differentially affects the activation of the BF and SS. Thus, in contrast to Onishi et al. (2002) but in support of Lunnen et al. (1981), we found activity in the BF to be decreased when the muscle was in a lengthened state (when the knee joint angle was at 160°). Muscle length had an effect on sEMG in both muscles, such that when shortened there was a statistical trend (P = 0.06) of increased sEMG activity, with more sEMG activity in the BF at 25% MVC (Table 2). Knee joint angle influenced sEMG; however, muscle-independent differences between the BF and SS had greater determination on neural drive at the various isometric contraction intensities than changes in length.
From the 1,720 individual MU spike trains identified in the BF and SS at two knee joint angles (Fig. 4), the distribution of MUFRs in the BF had >50% occurring at or below 15 Hz (Fig. 3). When the knee joint was extended (lengthening the muscle), the distribution of firing rates in the BF shifted (Fig. 3). In contrast, changes of the firing rate distribution in the SS seemed to be less affected by knee joint angle (Fig. 3). At submaximal contraction intensities (25% and 50% MVC) the SS had statistically significant greater MUFRs than the BF at both knee joint angles (Fig. 5), and at MVC the same directional difference was evident, although not achieving statistical significance.
The present investigation adds to the limited literature on MUFRs by quantifying these properties at moderate to maximal contractile intensities in the hamstrings and completes the description for major lower limb muscles in young humans. In the BF, mean MUFRs ranged from 25 to 28 Hz at MVC in the extended and flexed knee positions, respectively (Fig. 5). In the SS, mean MUFRs ranged from 27 to 31 Hz at MVC in the extended and flexed knee positions, respectively (Fig. 5). In the vastus lateralis, with a different intramuscular EMG technique, mean MUFRs were observed to be ∼25 Hz (Kamen and Knight 2004), and in the vastus medialis, with the same collection and analysis methodology as our investigation (Roos et al. 1999), mean MUFRs were ∼26 Hz at MVC. At the knee joint angle of 90°, mean MUFRs at MVC are ∼25% greater in the BF and SS than in quadriceps (extensors). In muscles of the leg, with the same collection and analysis methodologies as in this study, the TA had greater mean MUFRs of ∼42 Hz at MVC (Connelly et al. 1999) compared with the gastrocnemii of ∼22 Hz at MVC (Graham et al. 2016; Kirk et al. 2016) and ∼16.5 Hz at MVC in the soleus (Dalton et al. 2009). In the extensor hallucis longus (a flexor), a relatively small number of units (13) were tracked over 10–30 s during a fatiguing MVC with firing rates between 11 and 30 Hz (Macefield et al. 2000). In general, when major thigh and leg muscles are compared, muscles with embryological origin as a flexor (hamstrings and dorsiflexors) seem to have a greater range of MUFRs compared with extensors (quadriceps and triceps surae) (Sadler and Langman 2012). In the leg, flexors and extensors are reported to have unique innervation characteristics, such that flexors have a greater percentage of efferent monosynaptic corticospinal projections and a greater percentage of afferent polysynaptic circuits, whereas in the extensors a greater percentage of efferent synapses are polysynaptic and a greater percentage of afferent feedback is monosynaptic (Brouwer and Ashby 1991; Cowan et al. 1986). These innervation characteristics perhaps related to functional development may explain some of the differences of inherent neural drive, at least as expressed through rate coding between flexors and extensors of the leg, which likely are also relevant in the thigh. These differences in the lower limb may help explain why assessment of neural output by MUFRs finds higher rates in muscles that have embryological origin as flexors.
Muscle length has been shown to affect MUFRs (Andrew 1985; Kennedy and Cresswell 2001; Lunnen et al. 1981; Soderberg et al. 1991; Vander Linden et al. 1991), but not all report the same relationship (Altenburg et al. 2009a; Bigland-Ritchie et al. 1992; Cresswell et al. 1995; Del Valle and Thomas 2004). The majority of reports exploring MUFRs have tested muscles simply at two lengths (comparing short and long) based on relevant joint positions, and those that report a difference found higher MUFRs with the muscle in a more shortened state. In the present investigation, mean MUFRs for both the BF and SS were 85–97% lower in the lengthened muscle position compared with shortened muscle at moderate to maximal contraction intensities (Fig. 5). In agreement with some of the aforementioned reports, muscle length seemed to affect neural drive (MUFRs) in the hamstrings, but individual muscle differences had a greater effect, with the SS having higher firing rates regardless of knee joint position. There is a paucity of reports that have systematically explored muscle length effects on neural drive in human lower limb muscles. The hamstrings, which is primarily a large two-joint muscle group, undergo substantial length changes during locomotion and therefore could be a good candidate for length-dependent studies.
In the thigh, we report that MUFRs of flexors are greater than those of extensors (Kalmar and Cafarelli 1999; Kamen and Knight 2004; Roos et al. 1999). The same relationship is also found in the leg, where MUFRs of the flexors are greater than those of the extensors (Bellemare et al. 1983; Connelly et al. 1999; Dalton et al. 2009; Graham et al. 2016; Kirk et al. 2016). Curiously, in the upper limb this relationship is reversed, such that MUFRs of the biceps brachii (flexors) are less than those of the triceps (extensors) (Bellemare et al. 1983; Dalton et al. 2010). Within the posterior thigh, we report that the BF has lower firing rates than the SS and that muscle length likely affects neural drive; however, others have shown that muscle length has a nonsignificant influence on MUFRs during isometric contractions in the quadriceps (Altenburg et al. 2009a), TA (Bigland-Ritchie et al. 1992), and triceps (Del Valle and Thomas 2004). In the lower limb, flexors have greater MUFRs at submaximal and maximal voluntary isometric contractions independent of muscle length, possibly related to the unique aspects of embryological development (flexor vs. extensor). These differences should be considered when comparing different muscle groups in paradigms of exercise, disease, and aging.
GRANTS
The Natural Sciences and Engineering Research Council (NSERC) of Canada supported this work.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.A.K. and C.L.R. performed experiments; E.A.K. and C.L.R. analyzed data; E.A.K. and C.L.R. interpreted results of experiments; E.A.K. and C.L.R. prepared figures; E.A.K. and C.L.R. drafted manuscript; E.A.K. and C.L.R. edited and revised manuscript; E.A.K. and C.L.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank all participants and acknowledge David B. Copithorne and Christopher W. Stolworthy for their help with MUFR collection.
REFERENCES
- Altenburg TM, de Haan A, Verdijk PW, van Mechelen W, de Ruiter CJ. Vastus lateralis single motor unit EMG at the same absolute torque production at different knee angles. J Appl Physiol 107: 80–89, 2009a. [DOI] [PubMed] [Google Scholar]
- Altenburg TM, de Ruiter CJ, Verdijk PW, van Mechelen W, de Haan A. Vastus lateralis surface and single motor unit electromyography during shortening, lengthening and isometric contractions corrected for mode-dependent differences in force-generating capacity. Acta Physiol (Oxf) 196: 315–328, 2009b. [DOI] [PubMed] [Google Scholar]
- Andrew PD. Motor unit activity under low tensions as muscle changes length. Am J Phys Med 64: 235–254, 1985. [PubMed] [Google Scholar]
- Bellemare F, Woods JJ, Johansson R, Bigland-Ritchie B. Motor-unit discharge rates in maximal voluntary contractions of three human muscles. J Neurophysiol 50: 1380–1392, 1983. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie BR, Furbush FH, Gandevia SC, Thomas CK. Voluntary discharge frequencies of human motoneurons at different muscle lengths. Muscle Nerve 15: 130–137, 1992. [DOI] [PubMed] [Google Scholar]
- Blackburn TJ, Bell DR, Norcross MF, Hudson JD, Engstrom LA. Comparison of hamstring neuromechanical properties between healthy males and females and the influence of musculotendinous stiffness. J Electromyogr Kinesiol 19: e362–e369, 2009. [DOI] [PubMed] [Google Scholar]
- Brouwer B, Ashby P. Altered corticospinal projections to lower limb motoneurons in subjects with cerebral palsy. Exp Brain Res 83: 649–654, 1991. [Google Scholar]
- Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol 87: 843–852, 1999. [DOI] [PubMed] [Google Scholar]
- Cowan JM, Day BL, Marsden C, Rothwell JC. The effect of percutaneous motor cortex stimulation on H reflexes in muscles of the arm and leg in intact man. J Physiol 377: 333–347, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell AG, Löscher WN, Thorstensson A. Influence of gastrocnemius muscle length on triceps surae torque development and electromyographic activity in man. Exp Brain Res 105: 283–290, 1995. [DOI] [PubMed] [Google Scholar]
- Dalton BH, Harwood B, Davidson AW, Rice CL. Triceps surae contractile properties and firing rates in the soleus of young and old men. J Appl Physiol 107: 1781–1788, 2009. [DOI] [PubMed] [Google Scholar]
- Dalton BH, Jakobi JM, Allman BL, Rice CL. Differential age-related changes in motor unit properties between elbow flexors and extensors. Acta Physiol (Oxf) 200: 45–55, 2010. [DOI] [PubMed] [Google Scholar]
- Del Valle A, Thomas CK. Motor unit firing rates during isometric voluntary contractions performed at different muscle lengths. Can J Physiol Pharmacol 82: 769–776, 2004. [DOI] [PubMed] [Google Scholar]
- Doherty TJ. The influence of aging and sex on skeletal muscle mass and strength. Curr Opin Clin Nutr Metab Care 4: 503–508, 2001. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Winter DA, Patla AE. Models of recruitment and rate coding organization in motor-unit pools. J Neurophysiol 70: 2470–2488, 1993. [DOI] [PubMed] [Google Scholar]
- Fyfe JJ, Opar DA, Williams MD, Shield AJ. The role of neuromuscular inhibition in hamstring strain injury recurrence. J Electromyogr Kinesiol 23: 523–530, 2013. [DOI] [PubMed] [Google Scholar]
- Garrett WE, Califf JC, Bassett FH. Histochemical correlates of hamstring injuries. Am J Sports Med 12: 98–103, 1984. [DOI] [PubMed] [Google Scholar]
- Graham MT, Rice CL, Dalton BH. Motor unit firing rates of the gastrocnemii during maximal brief steady-state contractions in humans. J Electromyogr Kinesiol 26: 82–87, 2016. [DOI] [PubMed] [Google Scholar]
- Gretchen D, Christopher P. Comparison of hamstring and gluteus muscles electromyographic activity while performing the razor curl vs. the traditional prone hamstring curl. J Strength Cond Res 8: 2250–2255, 2009. [DOI] [PubMed] [Google Scholar]
- Hales JP, Gandevia SC. Assessment of maximal voluntary contraction with twitch interpolation: an instrument to measure twitch responses. J Neurosci Methods 25: 97–102, 1988. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Enoka RM. Motor unit. Compr Physiol 2: 2629–2682, 2012. [DOI] [PubMed] [Google Scholar]
- Henriksen M, Rosager S, Aaboe J, Bliddal H. Adaptations in the gait pattern with experimental hamstring pain. J Electromyogr Kinesiol 21: 746–753, 2011. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Rice CL. Innervation and neuromuscular control in ageing skeletal muscle. J Physiol 594: 1965–1978, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herda TJ, Cramer JT, Ryan EE, McHugh MP, Stout JJ. Acute effects of static versus dynamic stretching on isometric peak torque, electromyography, and mechanomyography of the biceps femoris muscle. J Strength Cond Res 22: 809–817, 2008. [DOI] [PubMed] [Google Scholar]
- Horst K, Dienstknecht T, Sellei RM, Pape HC. Partial rupture of the hamstring muscle complex: a literature review on treatment options. Eur J Orthop Surg Traumatol 24: 285–289, 2014. [DOI] [PubMed] [Google Scholar]
- Hunter SK. Sex differences in human fatigability: mechanisms and insight to physiological responses. Acta Physiol (Oxf) 210: 768–789, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inacio MC, Paxton EW, Maletis GB, Csintalan RP, Granan LP, Fithian DC, Funahashi TT. Patient and surgeon characteristics associated with primary anterior cruciate ligament reconstruction graft selection. Am J Sports Med 40: 339–345, 2012. [DOI] [PubMed] [Google Scholar]
- Jacobson WC, Gabel RH, Brand RA. Surface vs. fine-wire electrode ensemble-averaged signals during gait. J Electromyogr Kinesiol 5: 37–44, 1995. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci 18: 111–129, 1973. [DOI] [PubMed] [Google Scholar]
- Kalmar JM, Cafarelli E. Effects of caffeine on neuromuscular function. J Appl Physiol 87: 801–808, 1999. [DOI] [PubMed] [Google Scholar]
- Kamen G, Knight CA. Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol A Biol Sci Med Sci 59: 1334–1338, 2004. [DOI] [PubMed] [Google Scholar]
- Kennedy PM, Cresswell AG. The effect of muscle length on motor-unit recruitment during isometric plantar flexion in humans. Exp Brain Res 137: 58–64, 2001. [DOI] [PubMed] [Google Scholar]
- Kirk EA, Copithorne DB, Dalton BH, Rice CL. Motor unit firing rates of the gastrocnemii during maximal and sub-maximal isometric contractions in young and old men. Neuroscience 330: 376–385, 2016. [DOI] [PubMed] [Google Scholar]
- Lunnen JD, Yack J, LeVeau BF. Relationship between muscle length, muscle activity, and torque of the hamstring muscles. Phys Ther 61: 190–195, 1981. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Fuglevand AJ, Howell JN, Bigland-Ritchie BR. Discharge behaviour of single motor units during maximal voluntary contractions of a human toe extensor. J Physiol 528: 227–234, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marateb HR, Muceli S, McGill KC, Merletti R, Farina D. Robust decomposition of single-channel intramuscular EMG signals at low force levels. J Neural Eng 8: 066015, 2011. [DOI] [PubMed] [Google Scholar]
- McAllister MJ, Hammond KG, Schilling BK, Ferreria LC, Reed JP, Weiss LW. Muscle activation during various hamstring exercises. J Strength Cond Res 28: 1573–1580, 2014. [DOI] [PubMed] [Google Scholar]
- McHugh MP, Connolly DA, Eston RG, Gartman EJ, Gleim GW. Electromyographic analysis of repeated bouts of eccentric exercise. J Sports Sci 19: 163–170, 2001. [DOI] [PubMed] [Google Scholar]
- Moses KP, Banks JC, Nava PB, Petersen D. Atlas of Clinical Gross Anatomy. Philadelphia, PA: Elsevier, 2005. [Google Scholar]
- Onishi H, Yagi R, Oyama M, Akasaka K, Ihashi K, Handa Y. EMG-angle relationship of the hamstring muscles during maximum knee flexion. J Electromyogr Kinesiol 12: 399–406, 2002. [DOI] [PubMed] [Google Scholar]
- Oya T, Riek S, Cresswell AG. Recruitment and rate coding organisation for soleus motor units across entire range of voluntary isometric plantar flexions. J Physiol 587: 4737–4748, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki M, Ireland A, Jones DA, McPhee JS. Age-dependent motor unit remodelling in human limb muscles. Biogerontology 17: 485–496, 20156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos MR, Rice CL, Connelly DM, Vandervoort AA. Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve 22: 1094–1103, 1999. [DOI] [PubMed] [Google Scholar]
- Roos MR, Rice CL, Vandervoort AA. Age-related changes in motor unit function. Muscle Nerve 20: 679–690, 1997. [DOI] [PubMed] [Google Scholar]
- Rutherford DJ, Hubley-Kozey CL, Stanish WD. Maximal voluntary isometric contraction exercises: a methodological investigation in moderate knee osteoarthritis. J Electromyogr Kinesiol 21: 154–160, 2011. [DOI] [PubMed] [Google Scholar]
- Sadler T, Langman J. Langman's Medical Embryology (12th ed). Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2012. [Google Scholar]
- Sanfilippo J, Silder A, Sharry MA, Tuite MJ, Heiderscheit BC. Hamstring strength and morphology progression after return to sport from injury. Med Sci Sports Exerc 45: 448–454, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg GL, Minor SD, Nelson RM. A comparison of motor unit behaviour in young and aged subjects. Age Ageing 20: 8–15, 1991. [DOI] [PubMed] [Google Scholar]
- Taylor DC, DeBerardino TM, Nelson BJ, Duffey M, Tenuta J, Stoneman PD, Sturdivant RX, Mountcastle S. Patellar tendon versus hamstring tendon autografts for anterior cruciate ligament reconstruction: a randomized controlled trial using similar femoral and tibial fixation methods. Am J Sports Med 37: 1946–1957, 2009. [DOI] [PubMed] [Google Scholar]
- Todd G, Gorman RB, Gandevia SC. Measurement and reproducibility of strength and voluntary activation of lower-limb muscles. Muscle Nerve 29: 834–842, 2004. [DOI] [PubMed] [Google Scholar]
- Vander Linden DW, Kukulka CG, Soderberg GL. The effect of muscle length on motor unit discharge characteristics in human tibialis anterior muscle. Exp Brain Res 84: 210–218, 1991. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA, McComas AJ. A comparison of the contractile properties of the human gastrocnemius and soleus muscles. Eur J Appl Physiol Occup Physiol 51: 435–440, 1983. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kouzaki M, Moritani T. Effect of electrode location on task-dependent electromyographic responses within the human biceps femoris muscle. J Appl Biomech 32: 97–100, 2016. [DOI] [PubMed] [Google Scholar]