Abstract
The randomized phase 3 ENDEAVOR study (N=929) compared carfilzomib and dexamethasone (Kd) with bortezomib and dexamethasone (Vd) in relapsed multiple myeloma (RMM). We performed a subgroup analysis from ENDEAVOR in patients categorized by number of prior lines of therapy or by prior treatment. Median progression-free survival (PFS) for patients with one prior line was 22.2 months for Kd vs 10.1 months for Vd, and median PFS for patients with ⩾2 prior lines was 14.9 months for Kd vs 8.4 months for Vd. For patients with prior bortezomib exposure, the median PFS was 15.6 months for Kd vs 8.1 months for Vd, and for patients with prior lenalidomide exposure the median PFS was 12.9 months for Kd vs 7.3 months for Vd. Overall response rates (Kd vs Vd) were 81.9 vs 65.5% (one prior line), 72.0 vs 59.7% (⩾2 prior lines), 71.2 vs 60.3% (prior bortezomib) and 70.1 vs 59.3% (prior lenalidomide). The safety profile in the prior lines subgroups was qualitatively similar to that in the broader ENDEAVOR population. In RMM, outcomes are improved when receiving treatment with carfilzomib compared with bortezomib, regardless of the number of prior therapy lines or prior exposure to bortezomib or lenalidomide.
Introduction
Several classes of therapy are now available for treating multiple myeloma (MM), including corticosteroids, alkylating agents, anthracyclines and more recently immunomodulatory drugs (IMiDs), proteasome inhibitors, monoclonal antibodies and deacetylase inhibitors. Although a variety of treatment regimens are available in the relapsed/refractory setting, there is no general agreement on the best sequence of salvage therapy.1 The National Comprehensive Cancer Network Guidelines list over 10 preferred regimens that can be used as salvage therapy in MM, and state that patients may be retreated with the same regimen if relapse occurs >6 months after the initial treatment.2 A number of factors should be taken into account when deciding on therapy at relapse, including patient age, pre-existing toxicity, renal function, aggressiveness of disease, type of prior therapies and response to prior therapies.3
Almost all patients with MM will relapse, and patients typically experience lower rates and shorter duration of response (DOR) when receiving successive lines of therapy.4 An International Myeloma Foundation study of MM patients with relapsed disease who received salvage therapy found a progressive decline in overall response rates, from 58% at first relapse to 45% at second relapse and 30% at third relapse.5 Similarly, Kumar et al.6 reported a progressive decline in DOR among relapsed MM (RMM) patients receiving subsequent lines of therapy, from a median of about 7 months for the second therapy line to a median of about 4 months for the fifth therapy line. In a study of the natural history of relapsed/refractory MM disease, the IMWG (International Myeloma Working Group) reported that after patients had become refractory to bortezomib and had also relapsed or become refractory to (or were ineligible to receive) an IMiD, they had a median event-free survival of 5 months and a median overall survival of only 9 months.7 There remains a need to develop more effective treatment approaches for MM patients with relapsed/refractory disease.
Carfilzomib is a second-generation proteasome inhibitor that has demonstrated a high degree of clinical activity in patients with relapsed and/or refractory MM. Unlike bortezomib, which inhibits the proteasome reversibly, carfilzomib is an irreversible inhibitor of the proteasome.8 In the phase 2 PX-171-003-A1 trial, patients with a median of five prior lines of therapy (including bortezomib and an IMiD) had an overall response rate of 23.7% to single agent carfilzomib.9 In the phase 3 ASPIRE trial, carfilzomib, lenalidomide and dexamethasone were compared with lenalidomide and dexamethasone in RMM patients who had received 1–3 prior lines of therapy. The addition of carfilzomib to lenalidomide and dexamethasone led to significant improvements in the overall response rate (87.1 vs 66.7% P<0.001) and in progression-free survival (PFS) (26.3 vs 17.6 months; hazard ratio (HR), 0.69; 95% confidence interval (CI), 0.57–0.83; P=0.0001).10 Recently, results from the phase 3 ENDEAVOR trial were reported, which was a head-to-head comparison between carfilzomib and bortezomib (both in combination with low-dose dexamethasone) in a population of RMM patients who had received 1–3 prior lines of therapy.11 In a preplanned interim analysis of ENDEAVOR, median PFS was significantly higher in the carfilzomib–dexamethasone (Kd) arm than in the bortezomib–dexamethasone (Vd) arm (18.7 vs 9.4 months; HR, 0.53; 95% CI, 0.44–0.65; P<0.0001). The overall response rate was also significantly higher for patients who received Kd vs Vd (77 vs 63% P<0.0001). Whereas patients in ASPIRE received twice-weekly carfilzomib infusions of 27 mg/m2 for 10 min, ENDEAVOR demonstrated that twice-weekly carfilzomib is safe and effective at a higher dose of 56 mg/m2 when infused over the longer length of 30 min.
Because of the challenges associated with treating MM patients in the relapsed setting, we performed a subgroup analysis of the ENDEAVOR trial where patients were categorized based on number of prior treatment lines or by prior therapy. We compared Kd with Vd in a patient subgroup that received just 1 prior therapy line, and in a subgroup that received ⩾2 prior lines. We also compared Kd with Vd in subgroups that had prior exposure to bortezomib or lenalidomide.
Materials and methods
The ENDEAVOR trial has previously been described by Dimopoulos et al.11 Briefly, this was a randomized, phase 3, open-label study (NCT01568866). Patients enrolled in ENDEAVOR were at least 18 years of age, had an Eastern Cooperative Oncology Group performance status of 0–2 and had detectable MM that was relapsing or progressing at study entry. Patients needed to have received 1–3 prior lines of therapy, and to have achieved a partial response or better to at least 1 prior therapy line. Prior bortezomib or carfilzomib was allowed as long as a patient achieved a partial response or better to prior bortezomib or carfilzomib, did not discontinue prior bortezomib or carfilzomib due to toxicity, and had an interval of at least 6 months between stopping prior bortezomib or carfilzomib and starting study treatment. Within 21 days before randomization, patients were required to have adequate hepatic function (bilirubin <1.5 times and alanine aminotransferase <3 times the upper limit of normal), absolute neutrophil count ⩾1000/mm3, hemoglobin ⩾8.0 g/dl, platelet count ⩾50 000/mm3 (or ⩾30 000/mm3 if bone marrow involvement is >50%) and creatinine clearance of ⩾15 ml/min. Patients also needed to have left ventricular ejection fraction ⩾40%, and patients were excluded if they had myocardial infarction within 4 months before randomization; active congestive heart failure (New York Heart Association Class III to IV); symptomatic ischemia; or conduction abnormalities uncontrolled by conventional intervention. Patients with significant neuropathy (grade 2 with pain, or grade 3–4) within 14 days before randomization were also excluded. Written consent was obtained from all patients, and the study protocol received institutional review board or ethics committee approval by all participating institutions.
Patients were randomized 1:1 to the Kd group (carfilzomib and dexamethasone) or the Vd group (bortezomib and dexamethasone). Stratification factors used for randomization were prior proteasome inhibitor therapy, prior lines of treatment, International Staging System stage and planned route of bortezomib administration. Carfilzomib was given on days 1, 2, 8, 9, 15 and 16 of 28-day cycles until disease progression or unacceptable toxicity. Patients received carfilzomib as a 30-min infusion at a dose of 56 mg/m2 (20 mg/m2 on days 1 and 2 of cycle 1 only). Bortezomib was given at a dose of 1.3 mg/m2 (intravenous bolus or subcutaneous injection) on days 1, 4, 8 and 11 of 21-day cycles until disease progression or unacceptable toxicity. In the Kd group, patients received dexamethasone 20 mg on days 1, 2, 8, 9, 15, 16, 22 and 23, and in the Vd group, patients received dexamethasone 20 mg on days 1, 2, 4, 5, 8, 9, 11 and 12.
In this secondary analysis, patients enrolled in ENDEAVOR were assigned to a subgroup according to lines of prior therapy: 1 prior line or ⩾2 prior lines. The intent-to-treat population was used for efficacy analyses, and all patients who received at least one dose of study treatment were used for safety analyses. The IMWG Uniform Response Criteria were used to evaluate response and disease progression.12 Overall response included a best response of partial response, very good partial response, complete response or stringent complete response. The Kaplan–Meier method was used to assess PFS and DOR. Disease responses were evaluated by an independent review committee that was blinded to treatment arm. PFS was compared between treatment groups using a log-rank test, and a Cox regression model was used to estimate the corresponding HR. The overall response rate (ORR) was compared between treatment groups using a Mantel-Haenszel test. No adjustments were made for multiple comparisons of the subgroup analysis, and the P-values reported here for the subgroup analysis are descriptive in nature.
Results
The cutoff date for the preplanned interim analysis of ENDEAVOR was November 10, 2014. Nine hundred twenty-nine patients were enrolled in the intent-to-treat population; 464 and 465 patients were randomized to Kd and Vd, respectively. In the 1 prior line subgroup, 232 patients (50%) received Kd and 232 (50%) received Vd, and in the ⩾2 prior lines subgroup, 232 patients (50%) received Kd and 233 (50%) received Vd. In general, patient demographic and baseline characteristics were well balanced between treatment arms in both subgroups, including age, presence of high-risk cytogenetics, and prior exposure to lenalidomide or bortezomib (Table 1).
Table 1. Patient demographics and baseline disease characteristics.
|
1 Prior line |
⩾2 Prior lines |
|||
|---|---|---|---|---|
| Kd (n=232) | Vd (n=232) | Kd (n=232) | Vd (n=233) | |
| Age, median years (range) | 66.0 (36.0–89.0) | 63.5 (39.0–88.0) | 64.0 (35.0–89.0) | 66.0 (30.0–86.0) |
| ECOG PS, n (%) | ||||
| 0 | 110 (47.4) | 131 (56.5) | 111 (47.8) | 101 (43.3) |
| 1 | 104 (44.8) | 92 (39.7) | 107 (46.1) | 111 (47.6) |
| 2 | 18 (7.8) | 9 (3.9) | 14 (6.0) | 21 (9.0) |
| Cytogenetic risk by FISH at study entry, n (%) | ||||
| High risk | 44 (19.0) | 53 (22.8) | 53 (22.8) | 60 (25.8) |
| Standard risk | 149 (64.2) | 144 (62.1) | 135 (58.2) | 147 (63.1) |
| Unknown/missing | 39 (16.8) | 35 (15.1) | 44 (19.0) | 26 (11.1) |
| Creatinine clearance, n (%) | ||||
| <30 ml/min | 14 (6.0) | 17 (7.3) | 14 (6.0) | 11 (4.7) |
| 30 to <50 ml/min | 26 (11.2) | 27 (11.6) | 31 (13.4) | 44 (18.9) |
| 50 to <80 ml/min | 97 (41.8) | 85 (36.6) | 89 (38.4) | 92 (39.5) |
| ⩾80 ml/min | 95 (40.9) | 103 (44.4) | 98 (42.2) | 86 (36.9) |
| ISS stage, n (%) | ||||
| Stage 1 | 109 (47.0) | 115 (49.6) | 103 (44.4) | 90 (38.6) |
| Stage 2 | 68 (29.3) | 62 (26.7) | 70 (30.2) | 89 (38.2) |
| Stage 3 | 55 (23.7) | 55 (23.7) | 59 (25.4) | 54 (23.2) |
| Prior therapy, n (%) | ||||
| Bortezomib | 96 (41.4) | 101 (43.5) | 154 (66.4) | 151 (64.8) |
| Lenalidomide | 51 (22.0) | 47 (20.3) | 126 (54.3) | 130 (55.8) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridization; ISS, International Staging System; Kd, carfilzomib and dexamethasone; Vd, bortezomib and dexamethasone.
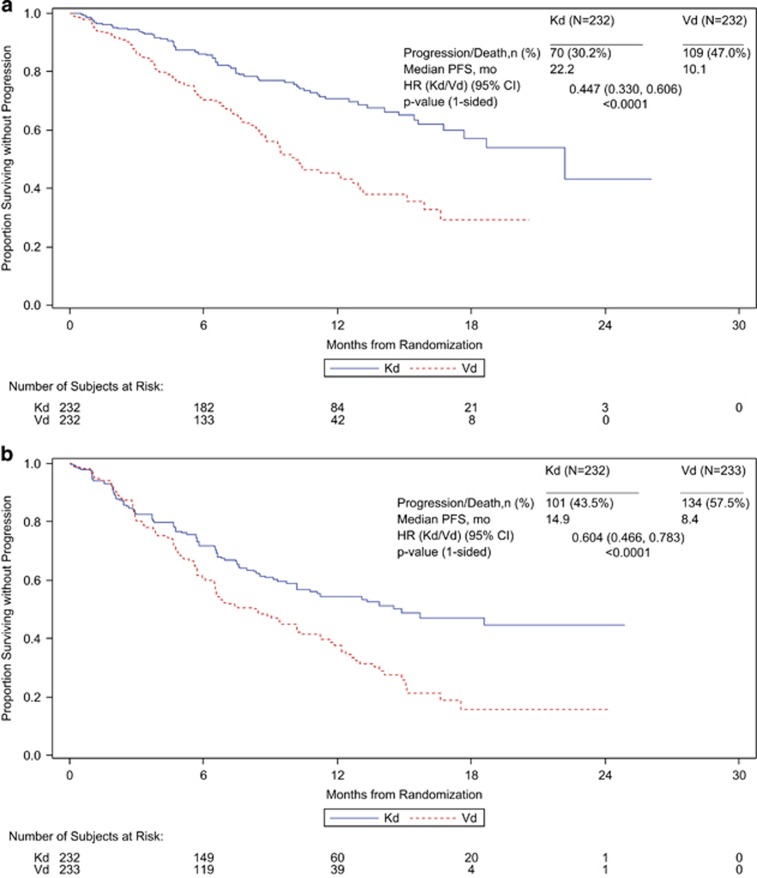
PFS was longer for the Kd arm than the Vd arm in both prior line subgroups. In the 1 prior line subgroup the median PFS was 22.2 months for Kd and 10.1 months for Vd (HR, 0.45; 95% CI, 0.33–0.61; P<0.0001) (Figure 1). In the ⩾2 prior lines subgroup the median PFS was 14.9 months for Kd and 8.4 months for Vd (HR, 0.60; 95% CI, 0.47–0.78; P<0.0001) (Figure 1). The ORR was also higher for Kd than Vd in both prior line subgroups. In the 1 prior line subgroup the ORR was 81.9% (95% CI, 76.3–86.6) for Kd and 65.5% (95% CI, 59.0–71.6) for Vd (P<0.0001) (Table 2). In the ⩾2 prior lines subgroup the ORR was 72.0% (95% CI, 65.7–77.7) for Kd and 59.7% (95% CI, 53.1–66.0) for Vd (P=0.0026) (Table 2). A higher proportion of patients in the Kd arm than in the Vd arm also achieved complete response or better in both the 1 prior line subgroup and the ⩾2 prior lines subgroup (Table 2). The median DOR (Kd vs Vd) was 21.3 vs 14.1 months in the 1 prior line subgroup and not estimable (NE) vs 10.3 months in the ⩾2 prior lines subgroup (Table 2).
Figure 1.
Kaplan–Meier PFS curves by prior therapy lines (a) 1 prior line (b) ⩾2 prior lines.
Table 2. Responses by prior lines of therapy.
|
1 Prior line |
⩾2 Prior lines |
|||
|---|---|---|---|---|
| Kd (n=232) | Vd (n=232) | Kd (n=232) | Vd (n=233) | |
| Best overall response, n (%) | ||||
| Stringent complete response | 6 (2.6) | 6 (2.6) | 2 (0.9) | 3 (1.3) |
| Complete response | 21 (9.1) | 12 (5.2) | 29 (12.5) | 8 (3.4) |
| Very good partial response | 117 (50.4) | 53 (22.8) | 77 (33.2) | 51 (21.9) |
| Partial response | 46 (19.8) | 80 (34.5) | 58 (25.0) | 77 (33.0) |
| Overall response rate, % (95% CI) | 81.9 (76.3–86.6) | 65.5 (59.0–71.6) | 72.0 (65.7–77.7) | 59.7 (53.1–66.0) |
| Median duration of response, months (95% CI) | 21.3 (17.6–NE) | 14.1 (8.6–NE) | NE (13.9–NE) | 10.3 (9.0–12.2) |
Abbreviations: CI, confidence interval; Kd, carfilzomib and dexamethasone; NE, not estimable; Vd, bortezomib and dexamethasone.
Exposure to study treatment was longer for the Kd arm than for the Vd arm in both the 1 prior line subgroup (mean duration of 41.6 vs 31.0 weeks) and the ⩾2 prior lines subgroup (mean duration of 38.0 vs 29.0 weeks). The cycle time for Kd was also longer than the cycle time for Vd (28 vs 21 days). In the 1 prior line subgroup, the rates of adverse events (Kd vs Vd) were 97.4 vs 98.7% for any grade adverse event, 69.8 vs 63.9% for grade ⩾3 adverse events and 44.0 vs 31.3% for serious adverse events (Table 3). Treatment discontinuations due to adverse events occurred in 17.2% of patients receiving Kd and 18.5% of patients receiving Vd, and deaths due to adverse events occurred in 4.3% of Kd patients and 3.1% of Vd patients (Table 3). In the ⩾2 prior lines subgroup, the rates of adverse events (Kd vs Vd) were 99.1 vs 97.4% for any grade adverse event, 76.6 vs 69.9% for grade ⩾3 adverse events and 52.8 vs 39.7% for serious adverse events (Table 3). Treatment discontinuations due to adverse events occurred in 22.5% of patients receiving Kd and 23.1% of patients receiving Vd, and deaths due to adverse events occurred in 6.5% of Kd patients and 6.1% of Vd patients (Table 3). Grade ⩾3 diarrhea and peripheral neuropathy occurred more frequently (by at least 5%) in the Vd arm than in the Kd arm in the ⩾2 prior lines subgroup (Table 3). Grade ⩾3 hypertension occurred more frequently (⩾5%) in the Kd arm than in the Vd arm in both the 1 prior line subgroup and the ⩾2 prior lines subgroup (Table 3). Grade ⩾3 anemia, decreased lymphocyte count, dyspnea, cardiac failure and renal failure also occurred slightly more frequently (<5%) in the Kd arm than in the Vd arm in both prior line subgroups (Table 3). Even though the incidence of adverse events was generally higher in the ⩾2 prior lines subgroup for both treatment arms, the safety profile of Kd compared with Vd was consistent between the two groups.
Table 3. Adverse events, treatment discontinuations and deaths.
|
1 Prior line |
⩾2 Prior lines |
|||
|---|---|---|---|---|
| Kd (n=232) | Vd (n=227) | Kd (n=231) | Vd (n=229) | |
| Any grade adverse event (n, %) | 226 (97.4) | 224 (98.7) | 229 (99.1) | 223 (97.4) |
| Grade ⩾3 adverse event (n, %) | 162 (69.8) | 145 (63.9) | 177 (76.6) | 160 (69.9) |
| Serious adverse event (n, %) | 102 (44.0) | 71 (31.3) | 122 (52.8) | 91 (39.7) |
| Adverse event leading to treatment discontinuation (n, %) | 40 (17.2) | 42 (18.5) | 52 (22.5) | 53 (23.1) |
| Adverse event leading to death (n, %) | 10 (4.3) | 7 (3.1) | 15 (6.5) | 14 (6.1) |
| Grade ⩾3 adverse events reported in ⩾5% of patients in any subgroup (n, %) | ||||
| Anemia | 30 (12.9) | 19 (8.4) | 37 (16.0) | 26 (11.4) |
| Diarrhea | 5 (2.2) | 11 (4.8) | 11 (4.8) | 23 (10.0) |
| Dyspnea | 12 (5.2) | 5 (2.2) | 13 (5.6) | 5 (2.2) |
| Fatigue | 14 (6.0) | 18 (7.9) | 11 (4.8) | 14 (6.1) |
| Hypertension | 24 (10.3) | 8 (3.5) | 17 (7.4) | 4 (1.7) |
| Lymphocyte count decreased | 13 (5.6) | 3 (1.3) | 13 (5.6) | 5 (2.2) |
| Peripheral neuropathy | 5 (2.2) | 10 (4.4) | 1 (0.4) | 14 (6.1) |
| Platelet count decreased | 8 (3.4) | 8 (3.5) | 9 (3.9) | 16 (7.0) |
| Pneumonia | 15 (6.5) | 14 (6.2) | 17 (7.4) | 22 (9.6) |
| Thrombocytopenia | 15 (6.5) | 18 (7.9) | 24 (10.4) | 25 (10.9) |
| Other select grade ⩾3 adverse events of interest (n, %) | ||||
| Cardiac failure | 5 (2.2) | 2 (0.9) | 5 (2.2) | 1 (0.4) |
| Lymphopenia | 10 (4.3) | 6 (2.6) | 10 (4.3) | 6 (2.6) |
| Neutropenia | 2 (0.9) | 4 (1.8) | 8 (3.5) | 6 (2.6) |
| Renal failure | 6 (2.6) | 2 (0.9) | 1 (0.4) | 0 |
Abbreviations: Kd, carfilzomib and dexamethasone; Vd, bortezomib and dexamethasone.
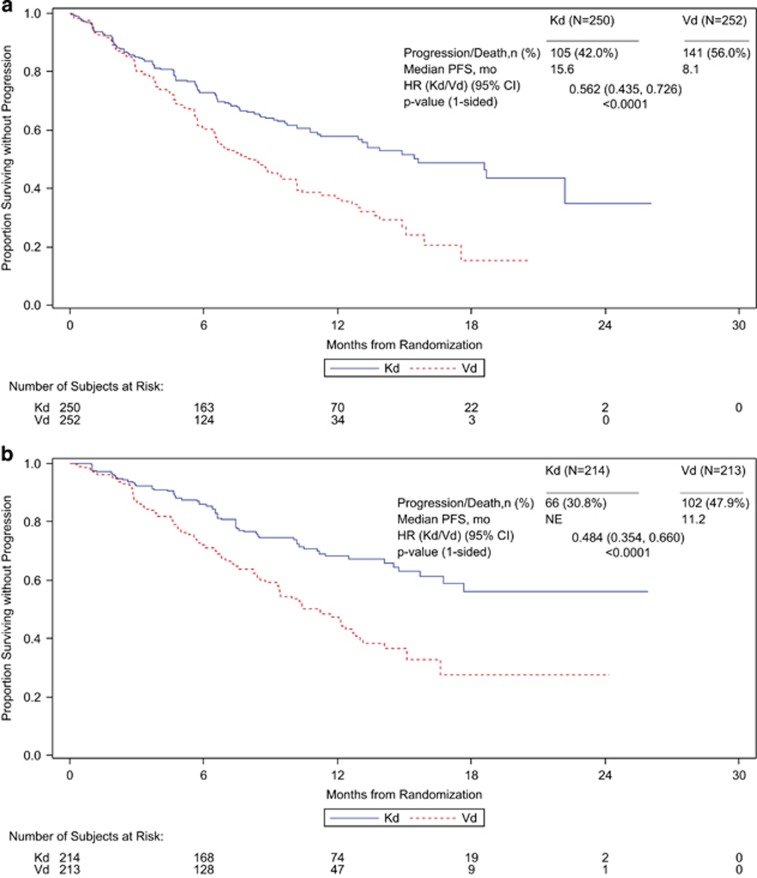
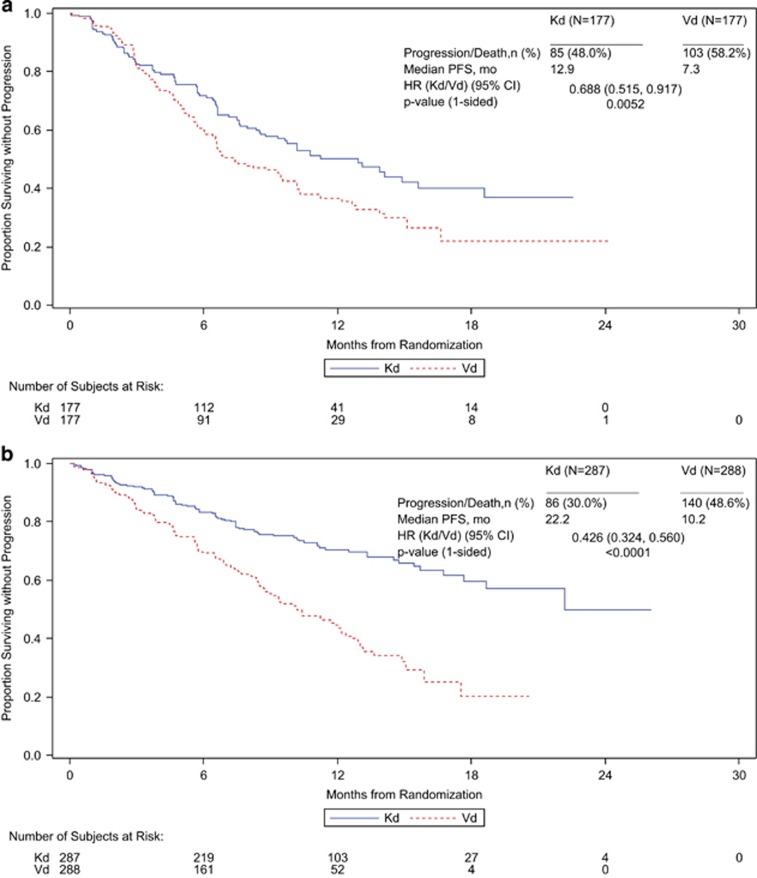
Median PFS was higher for patients receiving Kd than for patients receiving Vd regardless of prior exposure to bortezomib or lenalidomide. In the subgroup of patients with prior bortezomib exposure, the median PFS (Kd vs Vd) was 15.6 vs 8.1 months (HR, 0.56; 95% CI, 0.44–0.73; P<0.0001), and in the subgroup of patients without prior bortezomib exposure, the median PFS was NE vs 11.2 months (HR, 0.48; 95% CI, 0.35–0.66; P<0.0001) (Table 4; Figure 2). In the subgroup of patients with prior lenalidomide exposure, the median PFS (Kd vs Vd) was 12.9 vs 7.3 months (HR, 0.69; 95% CI, 0.52–0.92; P=0.0052), and in the subgroup of patients without prior lenalidomide exposure, the median PFS was 22.2 vs 10.2 months (HR, 0.43; 95% CI, 0.32–0.56; P< 0.0001) (Table 4; Figure 3). The interaction between study treatment and prior lenalidomide exposure status had a two-sided P-value of 0.0257. The ORRs (Kd vs Vd) for the prior treatment subgroups were 71.2 vs 60.3% for patients with prior bortezomib exposure, 83.6 vs 65.3% for patients without prior bortezomib exposure, 70.1 vs 59.3% for patients with prior lenalidomide exposure and 81.2 vs 64.6% for patients without prior lenalidomide exposure (Table 4). Similar trends in PFS and ORR were observed for Kd vs Vd when patients were stratified both by number of prior lines of therapy and by prior treatment with bortezomib or lenalidomide (Table 4; Supplementary Figures 1 and 2).
Table 4. Efficacy outcomes by prior bortezomib or lenalidomide treatment and prior therapy line.
|
Prior bortezomib exposure |
No prior bortezomib exposure |
Prior lenalidomide exposure |
No prior lenalidomide exposure |
|||||
|---|---|---|---|---|---|---|---|---|
| Kd (n=250) | Vd (n=252) | Kd (n=214) | Vd (n=213) | Kd (n=177) | Vd (n=177) | Kd (n=287) | Vd (n=288) | |
| 1–3 prior lines, n | 250 | 252 | 214 | 213 | 177 | 177 | 287 | 288 |
| Median PFS, months | 15.6 | 8.1 | NE | 11.2 | 12.9 | 7.3 | 22.2 | 10.2 |
| HR for progression, Kd vs Vd (95% CI) | 0.56 (0.44–0.73) | 0.48 (0.35–0.66) | 0.69 (0.52–0.92) | 0.43 (0.32–0.56) | ||||
| ORR, % | 71.2 | 60.3 | 83.6 | 65.3 | 70.1 | 59.3 | 81.2 | 64.6 |
| 1 prior line, n | 97 | 98 | 134 | 131 | 51 | 45 | 180 | 184 |
| Median PFS, months | 18.7 | 8.7 | NE | 11.2 | 15.6 | 10.3 | 22.2 | 10.1 |
| HR for progression, Kd vs Vd (95% CI) | 0.48 (0.31–0.76) | 0.43 (0.28–0.66) | 0.62 (0.33–1.17) | 0.41 (0.29–0.58) | ||||
| ORR, % | 78.4 | 64.3 | 83.6 | 65.6 | 82.4 | 64.4 | 81.1 | 65.2 |
| 2–3 prior lines, n | 153 | 154 | 80 | 82 | 126 | 132 | 107 | 104 |
| Median PFS, months | 13.1 | 7.4 | 15.7 | 9.4 | 9.7 | 6.6 | NE | 10.4 |
| HR for progression, Kd vs Vd (95% CI) | 0.62 (0.45–0.85) | 0.56 (0.36–0.89) | 0.73 (0.53–1.01) | 0.45 (0.29–0.70) | ||||
| ORR, % | 66.7 | 57.8 | 83.8 | 64.6 | 65.1 | 57.6 | 81.3 | 63.5 |
Abbreviations: CI, confidence interval; HR, hazard ratio; Kd, carfilzomib and dexamethasone; NE, not estimable; ORR, overall response rate; PFS, progression-free survival; Vd, bortezomib and dexamethasone.
Figure 2.
Kaplan–Meier PFS curves by prior bortezomib exposure (a) received prior bortezomib (b) no prior bortezomib.
Figure 3.
Kaplan–Meier PFS curves by prior lenalidomide exposure (a) received prior lenalidomide (b) no prior lenalidomide.
In the Kd arm, patients without prior lenalidomide exposure had better median PFS than patients with prior lenalidomide exposure (22.2 vs 12.9 months; P<0.0001) (Table 4). A consistent trend across lenalidomide exposure groups was observed in subgroups of Kd patients with 1 prior line of therapy (22.2 vs 15.6 months; P=0.1147) and ⩾2 prior lines of therapy (NE vs 9.7 months; P=0.0007) (Table 4). To investigate this phenomenon, we categorized patients with prior lenalidomide exposure according to length of prior lenalidomide exposure or whether they were refractory to prior lenalidomide. Kd patients who were refractory to prior lenalidomide (n=113) had a median PFS of 8.6 months, whereas Kd patients who were not refractory to prior lenalidomide (n=64) had a median PFS that was NE (P<0.0001). Kd patients who had a median exposure to prior lenalidomide of <18 months (n=124) had a median PFS of 10.8 months, and Kd patients who had a median exposure to prior lenalidomide of ⩾18 months (n=53) had a median PFS of 15.6 months (P=0.7836). The relationship between prior lenalidomide status and median PFS was not as pronounced for patients in the Vd arm (no prior lenalidomide, 10.2 months; prior lenalidomide, 7.3 months; P=0.0506). However, similar to the results observed in the Kd arm, patients in the Vd arm who were refractory to prior lenalidomide had a shorter median PFS than those who were not refractory (n=122, PFS=6.6 months; n=55, PFS=11.2 months; P=0.0076), and patients who had an exposure to prior lenalidomide of <18 months had shorter PFS than patients whose median exposure to prior lenalidomide was ⩾18 months (n=131, PFS=6.7 months; n=46, PFS=10.2 months; P=0.1039).
Discussion
In the phase 3 ENDEAVOR trial, a head-to-head comparison was performed between Kd and Vd, and Kd was found to significantly improve PFS compared with Vd in RMM patients with 1–3 prior lines of therapy.11 In this secondary analysis of ENDEAVOR, we compared Kd with Vd in subgroups of patients that had 1 prior line of therapy and ⩾2 prior lines of therapy. We found that regardless of number of prior lines of therapy, treatment with Kd was associated with greater clinical benefit than treatment with Vd. In the 1 prior line subgroup, the median PFS was 22.2 months for patients receiving Kd and 10.1 months for patients receiving Vd, and in the ⩾2 prior lines subgroup the median PFS was 14.9 months for Kd and 8.4 months for Vd. The rates of ORR and the proportion of patients achieving complete response or better were also higher with Kd than with Vd in both prior line subgroups. The efficacy findings reported in this subgroup analysis are consistent with the results reported in the primary analysis of ENDEAVOR.11
MM patients in the relapsed and/or refractory setting represent a challenging-to-treat population, as benefit from drugs typically diminishes with subsequent lines of therapy in patients with MM.4 The results from this study demonstrate a clinically meaningful improvement in PFS for patients treated with Kd compared with Vd, including for a subgroup of more heavily pretreated patients who had received multiple prior therapy lines, and establish the efficacy of a doublet regimen containing carfilzomib. These results should be interpreted in the context of the structural and functional differences between bortezomib and carfilzomib: bortezomib is a dipeptide boronic acid analog that is a reversible inhibitor of the proteasome, while carfilzomib is an epoxyketone-based irreversible proteasome inhibitor.13, 14
In the safety analysis, we found that rates of treatment discontinuation due to adverse events and rates of death due to adverse events were similar (<2% difference) between the Kd arm and the Vd arm in both the 1 prior line subgroup and the ⩾2 prior lines subgroup. Rates of grade ⩾3 hypertension were higher (⩾5% difference between treatment arms) in patients receiving Kd than in patients receiving Vd in both prior lines subgroups. Hypertension is a recognized but manageable complication of carfilzomib. Grade ⩾3 cardiac failure, renal failure, anemia and dyspnea also occurred more frequently in the Kd arm than in the Vd arm in both prior line subgroups, although the difference was not as pronounced (<5% between arms). Patients receiving Vd had higher rates of grade ⩾3 peripheral neuropathy than patients receiving Kd in the ⩾2 prior lines subgroup (difference ⩾5%), and also in the 1 prior line subgroup (<5% between arms). These trends are generally similar to what was observed in the safety analysis of the primary ENDEAVOR population. Also, we observed here that the incidence of adverse events in the ⩾2 prior lines subgroup was generally higher than the incidence of adverse events in the 1 prior line subgroup for both treatment arms.
The decision of which agent(s) to select for salvage therapy after a patient relapses or become refractory to an earlier regimen is an important clinical question. In a subgroup of patients with prior bortezomib exposure, we found that the PFS was improved (median 15.6 vs 8.1 months) and the ORR was higher (71.2 vs 60.3%) in patients treated with Kd compared with patients treated with Vd. Several prior studies have demonstrated that bortezomib retains clinical activity when used to retreat patients who had prior bortezomib exposure. For example, in a population of patients with progressive MM who had previously responded to bortezomib as a single agent or in combination with other drugs, Sood et al.15 reported an ORR (complete plus partial response) of 50% and a median time to progression (from start of retreatment) of 6.6 months when retreating with bortezomib or bortezomib and dexamethasone. In a similar study, Petrucci et al.16 reported an ORR (complete plus partial response) of 40% and a time to progression of 8.4 months among RMM patients who were retreated with bortezomib or bortezomib and dexamethasone. Although prior studies have demonstrated activity of bortezomib in patients with previous exposure, the results from the head-to-head study performed here suggest that patients with prior bortezomib exposure may obtain greater benefit from treatment with carfilzomib rather than retreatment with bortezomib.
We also observed in this subgroup analysis that the Kd doublet retained activity in patients with prior lenalidomide exposure. In the group of patients with prior lenalidomide exposure, the ORR for patients receiving Kd was 70.1% and the median PFS was 12.9 months. In contrast, in the Vd arm the ORR was 59.3% and the median PFS was 7.3 months. The Kd doublet could potentially be considered for patients who received prior lenalidomide, including for patients who progressed on lenalidomide maintenance, as well as for those who were intolerant to lenalidomide. Several studies have demonstrated a benefit for lenalidomide maintenance both in the post-transplant setting and in the transplant-ineligible setting.17, 18, 19, 20 Additionally, the FIRST trial recently demonstrated that in patients with newly diagnosed MM who were ineligible for stem-cell transplantation, lenalidomide and dexamethasone until progression resulted in superior outcomes compared with the combination melphalan–prednisone–thalidomide.21 As front-line lenalidomide and dexamethasone may be increasingly used to treat transplant-ineligible MM patients, Kd could be considered for second-line therapy in this setting.
Although PFS and ORR were higher for Kd than for Vd in patients with prior lenalidomide exposure, we note that PFS and ORR were higher in the Kd arm of the subgroup without prior lenalidomide than in the Kd arm of the subgroup with prior lenalidomide exposure. Although the reason for this is not immediately clear, it is possible that the subgroup with prior lenalidomide exposure was more heavily pretreated and had received a higher number of prior lines of therapy. However, when we further categorized patients with or without previous lenalidomide exposure by number of prior lines of therapy, similar trends were observed within the Kd arm of both the 1 prior lines subgroup and the ⩾2 prior lines subgroup. It is also possible that some patients with prior lenalidomide exposure had disease that was more aggressive and difficult to treat. When we categorized patients with prior lenalidomide exposure based on whether they were refractory to prior lenalidomide, we found that patients who were not refractory to prior lenalidomide had better outcomes on Kd than patients who were refractory to prior lenalidomide. Within the Kd arm, we also found that patients who had a longer exposure to prior lenalidomide had improved outcomes compared with patients who had shorter exposure. These findings suggest that patients who do not respond well to prior lenalidomide may not benefit as much from Kd as patients who respond well to prior lenalidomide (although in both cases, outcomes were better for Kd than for Vd). Therefore, a patient's experience with prior lenalidomide could be taken into account when deciding whether to use Kd as salvage therapy.
Carfilzomib is a second-generation proteasome inhibitor, which, unlike bortezomib, inhibits the proteasome irreversibly. In this exploratory analysis, we found evidence that Kd led to clinically meaningful improvements in PFS, ORR and DOR compared with Vd in several subgroups of patients from the ENDEAVOR trial. These findings are consistent with results from the primary analysis of ENDEAVOR and support use of the Kd doublet for RMM in a variety of clinical scenarios, including in patients with 1 prior line of therapy, patients with ⩾2 prior lines of therapy and patients with prior exposure to bortezomib or lenalidomide.
Acknowledgments
We would like to thank Jesse Potash of Amgen Inc. for medical writing assistance.
The ENDEAVOR study was supported by Onyx Pharmaceuticals, Inc., an Amgen subsidiary. PM played a consulting or advisory role in Celgene, Janssen, Millennium, Novartis and Onyx and received honoraria from Celgene, Janssen, Millennium, Novartis and Onyx. DJ participated in a Speakers' bureau for Amgen. W-JC received honoraria from Celgene, Janssen, Novartis and Takeda and received research funding from Celgene, Novartis and Merck. AP played a consulting or advisory role in Amgen, Celgene and Onyx and received honoraria from Amgen, Celgene and Onyx. HG played a consulting or advisory role in Bristol-Myers Squibb, Celgene Corporation, Janssen Pharmaceuticals, Millennium Pharmaceuticals, Novartis and Onyx Pharmaceuticals; received honoraria from Celgene Corporation, Chugai, Janssen Pharmaceuticals, Millennium Pharmaceuticals, Novartis and Onyx Pharmaceuticals and received research funding from Bristol-Myers Squibb, Celgene Corporation, Chugai, Janssen Pharmaceuticals and Novartis. RH received honoraria from Celgene, Janssen, Merck, Onyx and Amgen. HL participated in a Speakers' bureau for Amgen, Bristol-Myers Squibb, Celgene, Novartis and Takeda and received study support from Takeda. RN played a consulting or advisory role in Celgene, Millennium and Onyx and participated in a Speakers' bureau for Celgene, Millennium and Onyx. AO played a consulting or advisory role in Amgen, Celgene and Janssen and participated in a Speakers' bureau for Amgen, Celgene and Janssen. LR received honoraria from Amgen, Celgene and Janssen. GG received honoraria from Amgen and played a consulting or advisory role in Amgen. KW received honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, Novartis, Onyx and Takeda and played a consulting or advisory role in Amgen, Bristol-Myers Squibb, Celgene, Janssen, Novartis, Onyx and Takeda. HHG and NM were formerly employed by Onyx Pharmaceuticals, an Amgen subsidiary. SA and SF are employees of Amgen. MAD received honoraria from Celgene and Onyx and played a consulting or advisory role in Celgene and Onyx. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- van de Donk NW, Lokhorst HM, Dimopoulos M, Cavo M, Morgan G, Einsele H et al. Treatment of relapsed and refractory multiple myeloma in the era of novel agents. Cancer Treat Rev 2011; 37: 266–283. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. NCCN Guidelines Multiple Myeloma (Version 2.2016). [cited October 2015] Available from http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PMC free article] [PubMed]
- Mohty B, El-Cheikh J, Yakoub-Agha I, Avet-Loiseau H, Moreau P, Mohty M. Treatment strategies in relapsed and refractory multiple myeloma: a focus on drug sequencing and 'retreatment' approaches in the era of novel agents. Leukemia 2012; 26: 73–85. [DOI] [PubMed] [Google Scholar]
- Kurtin SE. Relapsed or relapsed/refractory multiple myeloma. J Adv Pract Oncol 2013; 4: 5–14. [PMC free article] [PubMed] [Google Scholar]
- Durie BG, Moreau P, Sonneveld P, Morgan GJ, Lahuerta JJ, Beksac M et al. Regional differences in the treatment approaches for relapsed multiple myeloma: an IMF study. J Clin Oncol 2012; 30: (suppl; abstr 8095). [Google Scholar]
- Kumar SK, Therneau TM, Gertz MA, Lacy MQ, Dispenzieri A, Rajkumar SV et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc 2004; 79: 867–874. [DOI] [PubMed] [Google Scholar]
- Kumar SK, Lee JH, Lahuerta JJ, Morgan G, Richardson PG, Crowley J et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia 2012; 26: 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride A, Klaus JO, Stockerl-Goldstein K. Carfilzomib: a second-generation proteasome inhibitor for the treatment of multiple myeloma. Am J Health Syst Pharm 2015; 72: 353–360. [DOI] [PubMed] [Google Scholar]
- Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood 2012; 120: 2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Spicka I, Oriol A et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med 2015; 372: 142–152. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hajek R et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol 2016; 17: 27–38. [DOI] [PubMed] [Google Scholar]
- Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K et al. International uniform response criteria for multiple myeloma. Leukemia 2006; 20: 1467–1473. [DOI] [PubMed] [Google Scholar]
- Khan ML, Stewart AK. Carfilzomib: a novel second-generation proteasome inhibitor. Fut Oncol (London, England) 2011; 7: 607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood 2007; 110: 3281–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood R, Carloss H, Kerr R, Lopez J, Lee M, Druck M et al. Retreatment with bortezomib alone or in combination for patients with multiple myeloma following an initial response to bortezomib. Am J Hematol 2009; 84: 657–660. [DOI] [PubMed] [Google Scholar]
- Petrucci MT, Giraldo P, Corradini P, Teixeira A, Dimopoulos MA, Blau IW et al. A prospective, international phase 2 study of bortezomib retreatment in patients with relapsed multiple myeloma. Br J Haematol 2013; 160: 649–659. [DOI] [PubMed] [Google Scholar]
- Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med 2012; 366: 1782–1791. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Delforge M, Hajek R, Kropff M, Petrucci MT, Lewis P et al. Lenalidomide, melphalan, and prednisone, followed by lenalidomide maintenance, improves health-related quality of life in newly diagnosed multiple myeloma patients aged 65 years or older: results of a randomized phase III trial. Haematologica 2013; 98: 784–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med 2012; 366: 1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med 2012; 366: 1759–1769. [DOI] [PubMed] [Google Scholar]
- Benboubker L, Dimopoulos MA, Dispenzieri A, Catalano J, Belch AR, Cavo M et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med 2014; 371: 906–917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.