Abstract
Background:
Patients with head and neck squamous cell carcinoma (HNSCC) undergoing radical chemo-radiation (CRT) frequently receive transfusion with packed red cells (PRCT) during radiotherapy on the basis that PRCT increases tumour oxygenation and overcomes hypoxia-induced radio-resistance. This is likely to be a significant oversimplification given the fact that tumour hypoxia is the result of several intrinsic and extrinsic factors, including many that are not directly related to serum haemoglobin (Hb). Therefore, we have studied the effect of PRCT on tumour oxygenation in a prospective cohort of patients who developed low Hb during radical CRT for HNSCC.
Methods:
This was a prospective study of 20 patients with HNSCC receiving radical CRT undergoing PRCT for Hb<11.5 g dl−1. Patients underwent pretransfusion and posttransfusion intrinsic susceptibility-weighted (SWI) MRI and dynamic contrast-enhanced (DCE) MRI. Blood samples were obtained at the time of MRI scanning and two further time points for measuring Hb and a panel of serum cytokine markers of tumour hypoxia. 3D T2* and Ktrans maps were calculated from the MRI data for primary tumours and cervical lymph node metastases.
Results:
PRCT produced no change (11 patients) or reduced (1 patient) T2* (tumour oxygenation) in 12 of the 16 (75%) evaluable primary tumours. Three of the four patients with improved tumour oxygenation progressed or had partial response following treatment completion. There were variable changes in Ktrans (tumour perfusion or vessel permeability) following PRCT that were of small magnitude for most tumours. Pre- and Post-PRCT levels of measured cytokines were not significantly different.
Conclusions:
This study suggests that PRCT during radical CRT for HNSCC does not improve tumour oxygenation. Therefore, oncologists should consider changing practice according to NICE and American Association of Blood Banks guidelines on PRCT for anaemia.
Keywords: head and neck cancer, anaemia, MRI, blood transfusion, radiotherapy
The presence of tumour hypoxia is associated with an adverse prognosis in patients with head and neck squamous cell carcinomas (HNSCC) (Nordsmark et al, 2005). Similarly, low pretreatment haemoglobin (Hb) is associated with poor outcome for patients with HNSCC (Lee et al, 1998; Prosnitz et al, 2005). Low Hb has been shown to correlate with poor tumour oxygenation (Becker et al, 2000) and many clinicians take the view that anaemia and tumour hypoxia are causally linked. Therefore, packed red cell transfusions (PRCT) are frequently used to correct anaemia (Hb<11.5 g dl−1) during radical chemo-radiation (CRT) for HNSCC and other squamous cell carcinomas (Becker et al, 2000). However, in a retrospective analysis of 169 patients treated with CRT at our institution, PRCT was found to be a negative prognostic factor for relapse-free and overall survival (Bhide et al, 2009). Detrimental effects of PRCT have also been observed in patients having radical radiotherapy or surgery for non-HNSCC tumours (Busch et al, 1993; Varlotto and Stevenson, 2005; Lim et al, 2008).
Attempting to minimise tumour hypoxia during CRT by giving PRCT assumes that low Hb accurately predicts tumour hypoxia and that increasing Hb will reliably reduce its presence. This is likely to be a significant oversimplification given the fact that tumour hypoxia is the result of several intrinsic and extrinsic factors, including many that are not directly related to Hb (Koukourakis et al, 2004). Therefore, we have studied the effect of PRCT on tumour oxygenation in a prospective cohort of patients who developed low Hb during radical CRT for HNSCC.
Intrinsic susceptibility-weighted (SWI) MRI characterised the oxygenation status within the tumour before and after PRCT. T2* measured by SWI MRI is proportional to the total amount of deoxy-Hb in an imaging voxel and, by extension, the tissue partial pressure of oxygen. This parameter provides information on the oxygenation status of tumour tissue and previous research has shown that, in head and neck tumours, a change in T2* can enable calculation of the corresponding change in pO2. (Panek et al, 2016).
It is also possible that PCRT may deliver more than just Hb to the patient. For example, a unit of packed red cells may also contain cytokines and chemokines that may mediate effects on tumour and normal tissue vasculature that may influence tumour oxygenation. Therefore, we also measured circulating levels of biologically relevant cytokines/chemokines before and after PRCT.
Radiotherapy fractions delivered between pre- and post-PRCT MRI scans and cytokines present in PRCT may potentially have affected the tumour perfusion and vascular permeability. This, in turn, may have influenced tumour oxygenation and this was assessed using dynamic contrast-enhanced (DCE) immediately after SWI MRI.
Materials and methods
A prospective longitudinal cohort study was performed in patients with stages III–IV (Edge and Compton, 2010) HNSCC undergoing radical CRT. All patients required PRCT during treatment owing to a fall in Hb to <11.5 g dl−1. All patients had MRI-assessable disease of >2 cm in diameter. Twelve healthy volunteers provided control serum samples. The study was approved by the institutional board and National Research Ethics Committee (no.12/LO/0631).
Treatment
All but one patient received induction chemotherapy (IC) followed by radical CRT (cisplatin 100 mg m−2 days 1 and 29) according to institutional protocols (described in Supplementary Section). All patients were planned using simultaneous integrated boost intensity-modulated RT (SIB-IMRT) techniques (Miah et al, 2015) delivering 65 Gy in 30 fractions over 6 weeks to the primary tumour and involved lymph nodes, and 54 Gy in 30 fractions to prophylactically treated nodal regions.
MRI data acquisition
Patients were imaged on either a 3-T Philips Achieva or a 3-T Siemens Skyra MRI scanner using an eight-channel phased-array head coil. Anatomical coronal and axial T2W images were acquired first to assess the extent of the disease. SWI MRI was obtained using 2D GRE (Gradient Echo) (matrix 240, FOV: 240 × 240, 24 × 2.5 mm slices, FA=60, TR=1000 ms, TE: 4.6–39.1, delta 6.9 ms). DCE MRI was obtained using a 3D Spoiled GRE (TE/TR: 4.5/2.3 ms, matrix 160, FOV: 240 × 240, 24 × 2.5 mm slices, SENSE/GRAPPA parallel imaging: Philips (Amsterdam, Netherlands)/Siemens (Munich, Germany)). A series of 10 proton density-weighted volumes (flip angle, FA=3°) was initially acquired, followed by 100 T1W acquisitions (FA=16°) obtained sequentially with 3.5 s temporal resolution. Gadolinium-based contrast was injected intravenously at the start of the tenth dynamic scan as a bolus through a peripherally placed cannula using an automatic injector (0.2 ml per kg body mass, 2 ml s−1 injection rate, Dotarem, Guerbet, France) and followed by a saline flush (20 ml at 2 ml s−1).
DCE data were analysed using the software package MRIW (Institute of Cancer Research, London, UK) (d'Arcy et al, 2006) with the extended Kety model (Tofts et al, 1999) and a population-based arterial input function (Orton et al, 2008). The volume transfer constant between blood plasma and extracellular extravascular space (Ktrans) was calculated for each voxel and displayed as a functional map. Signal changes on the multiple gradient echo images were used to calculate 3D T2* relaxivity maps. SWI data processing was performed using the in-house MATLAB software (MathWorks, Natick, MA, USA).
Anatomical posttransfusion MRI images were manually co-registered to pretransfusion images using rigid body manipulation within the Pinnacle (Philips Radiation Oncology Systems, Fitchburg, MA, USA) Radiotherapy Treatment Planning System (RTPS). The resulting transformation matrices were then applied to the posttransfusion MRI parametric maps. Gross tumour volumes (GTV) for primary tumours and cervical lymph node metastases within the SWI and DCE MRI FOV were delineated on co-registered anatomical MR images by a head and neck oncologist (LW) and a radiologist (AR) using Pinnacle (Supplementary Figure S1). 3D voxel-wise data from within the primary and nodal GTVs of the co-registered pretransfusion and posttransfusion Ktrans and T2* parametric maps were exported from Pinnacle for quantitative analysis using the in-house software written in R (R Core Team, 2013) and MATLAB.
Serum markers of tumour hypoxia
Peripheral blood samples were collected before (at the time of pretransfusion MRI) and 24 (at the time of posttransfusion MRI), 48 and 72 h after PRCT and on two separate days during the same week from healthy volunteers. Three to five milliliters of transfusate that remained in the bag after transfusion was collected for analysis. Blood samples were processed according to established standard operating procedures (Supplementary Material). A panel of 20 cytokines derived from a meta-gene signature established by (Winter et al, 2007; Byers et al, 2010) was measured in serum and transfusate by magnetic multiplex bead assay. The panel comprised: eotaxin, osteopontin (OPN), vascular endothelial growth factor (VEGF), interleukin (IL) IL-1β, IL-4, IL-8, IL-10, IL-12, IL-18, interferon (IFN-α), Gro-α, stromal cell-derived factor 1α (SDF1α), basic fibroblast growth factor (FGFb), tumour necrosis factor (TNF-α), transforming growth factor beta (TGF-β), platelet-derived growth factor (PDGF), granulocyte colony-stimulating factor (GCSF), hepatocyte growth factor (HGF), macrophage migration-inhibitory factor (MIF-1), and leptin. Multiplex magnetic bead assay kits were provided by Bio-Rad Laboratories (Bio-Rad, Hercules, CA, USA). Assays were performed in 96-well format according to the manufacturer's instructions. Cytokine concentrations were calculated based on a standard curve derived by performing eight serial dilutions of a protein standard in assay diluents. Serum samples were tested in duplicate, each sample was diluted 1 : 4 and 50 μl was added to each well, and the mean values were calculated.
Statistics
Voxelwise MRI parameter data were analysed using R. Histograms of T2* and Ktrans were plotted for each of the primary and lymph node tumours before and after infusion, and summary statistics were calculated. Bland–Altman limits of agreement (LOA) for T2* (2.9 ms) and log10 Ktrans (0.14 min−1) were determined based on separate reproducibility studies by (Panek et al, 2016) for T2* and unpublished work by Panek et al for Ktrans (see Supplementary Figure S2). We also plotted changes of T2* together with changes of volume transfer constant between blood plasma and extracellular extravascular space, to investigate whether MR relaxation time changes might be linked to vascular alterations induced by vasoactive cytokines present in PRC. Only changes of magnitude greater than the respective LOA were deemed to be statistically significant. Unpaired Wilcoxon tests were used to compare median serum protein concentrations between groups and paired Wilcoxon tests to compare differences in median serum cytokine concentrations.
Results
Twenty patients were recruited to the study (Table 1). The primary tumour was located in the oropharynx in 15 patients (75%), of which 10 (66%) were human papillomavirus infection (HPV) positive. Seventeen patients (85%) had Hb within the gender-specific laboratory normal reference ranges (men: 13.0–17.0 g dl−1; women: 12.0–15.0 g dl−1) at pretreatment. At the time of PRCT, all patients had measureable disease. Seventeen (85%) patients had measurable primary tumours, and 13 (65%) patients had 19 measurable involved cervical LN metastases (range 1–3, Supplementary Table S1).
Table 1. Summary of patient and tumour characteristics.
| Number of patients | 20 |
| Age (years), median (range) | 62 (53–70) |
| Sex (%) | |
| Female | 6 (30) |
| Male | 14 (70) |
| Primary site (%) | |
| Oropharynx | 15 (75) |
| Hypopharynx/larynx | 4 (20) |
| Sinonasal | 1 (5) |
| HPV status for oropharynx (%) | |
| Positive | 10 (66) |
| Negative | 5 (34) |
| T-stage (%) | |
| 1 | 1 (5) |
| 2 | 7 (35) |
| 3 | 7 (35) |
| 4 | 5 (25) |
| N-Stage (%) | |
| 0 | 3 (15) |
| 1 | 2 (10) |
| 2 | 15 (75) |
| Induction chemotherapy (%) | |
| Cisplatin/5-FU | 8 (40) |
| Carboplatin/5-FU | 5 (25) |
| TPF | 6 (30) |
| None | 1 (5) |
| Concomitant chemotherapy (%) | |
| Cisplatin | 9 (45) |
| Carboplatin | 11 (55) |
| Hb at first presentation (%) | |
| <11.5 g dl−1 | 3 (15) |
| >11.5 g dl−1 | 17 (85) |
Abbreviations: Hb=haemoglobin; HPV=human papillomavirus; TPF=docetaxel, cisplatin, 5-fluorouracil; 5-FU=5-fluorouracil.
Anaemia and PRCT
Seventeen patients (85%) developed anaemia during treatment and three remaining patients were anaemic at presentation (Table 2). Patients were transfused at a median of 11 fractions of chemo-radiation (range 2–23 fractions). One patient (number 3; Table 3) developed symptomatic anaemia following two cycles of IC and, therefore, underwent PRCT immediately prior to starting chemo-radiation. Median Hb at the time of pretransfusion MRI scanning was 10.0 g dl−1 (range 9.4–11.4 g dl−1). A median of 2 units (range 1–3 units) of PRCs were transfused, resulting in an increment in Hb to a median of 12.3 g dl−1 (range 11.3–14.4 g dl−1) at the time of the posttransfusion MRI scan (Table 2).
Table 2. Summary of outcome data 3 months after RT with median K trans and T2* changes after blood transfusion.
|
Primary |
Lymph nodes |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient no. | Subsite/staging | HPV status | Hb before transfusion (g dl−1) | Hb after transfusion (g dl−1) | Significant median T2* change | Significant median Ktrans change | Significant median T2* change | Significant median Ktrans change | Outcome 3 months after RT |
| 1 | T3N1 OPC | −ve | 9.4 | 10.6 | ↑ | No | No | NA | CR |
| 2 | T2N1 OPC | +ve | 10.5 | 13.7 | No | No | No, ↓ | ↑, No | CR |
| 3 | T2N2b OPC | +ve | 9.7 | 13.2 | No | No | ↓ | No | CR |
| 4 | T2N2a OPC | +ve | 9.9 | 14.1 | No | ↑ | ↑ | No | CR |
| 5 | T4N0 HypoPC | Unk | 11.3 | 12.6 | No | No | — | — | PD |
| 6 | T4N2c Larynx | Unk | 9.8 | 11.3 | No | No | No,↑, ↑ | No,↑, ↑ | PD |
| 7 | T3N2c Larynx | Unk | 10.2 | 11.7 | No | NA | ↓ | NA | CR |
| 8 | T3N0 OPC | −ve | 9.5 | 11.5 | No | No | — | — | CR |
| 9 | T3N2b OPC | +ve | 10.3 | 12.6 | — | — | ↓ | No | CR |
| 10 | T4N2c OPC | +ve | 10.2 | 11.3 | No | No | — | — | PD |
| 11 | T1N2c OPC | −ve | 9.5 | 14.4 | — | — | No, ↓ | No,↑ | PD |
| 12 | T3N2b OPC | −ve | 10.6 | 11.7 | ↑ | No | ↓ | ↑ | PD |
| 13 | T4N0 Ethmoid | Unk | 9.7 | 12.6 | No | No | — | — | PD |
| 14 | T2N2c OPC | +ve | 9.5 | 12.1 | No | ↑ | ↓ | No | CR |
| 15 | T3N2c OPC | +ve | 11.4 | 12.3 | ↑ | ↑ | — | — | PD |
| 16 | T2N2a OPC | +ve | 10.4 | 12.3 | NA | NA | ↓ | ↓ | CR |
| 17 | T2N2b Larynx | Unk | 11.4 | 12.5 | ↓ | ↓ | NA | NA | PD |
| 18 | T4N2c OPC | +ve | 9.7 | 11.4 | No | No | No | ↓ | CR |
| 19 | T2N2b OPC | −ve | 10.1 | 11.8 | ↑ | ↑ | No, No | No,↓ | PD |
| 20 | T3N2b OPC | +ve | 9.7 | 11.5 | No | No | No | ↑ | CR |
Abbreviations: CR=complete remission; Hb=haemoglobin; HPV=human papillomavirus; NA=insufficient or poor quality data for analysis; PD=persistent or progressive disease; RT=radiation therapy.
Table 3. Summary of median serum concentration (pg ml−1) of the panel of hypoxia-associated cytokines for healthy volunteers and patients and the median concentration (pg ml−1) of the same panel of cytokines in the PRC transfusate.
|
Patients |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Volunteers |
Transfusate |
MRI 1 (pretransfusion) |
MRI 2 (posttransfusion) |
|||||||||
| Cytokine | Median | Min. | Max. | Median | Min. | Max. | Median | Min. | Max. | Median | Min. | Max. |
| Eotaxin | 27.0 | 4.4 | 78.5 | 10.8 | 3.3 | 83.4 | 36.6 | 4.7 | 9965.4 | 24.9 | 15.7 | 10 114.6 |
| OPN | 5178.2 | 2466.9 | 11 483.2 | 2095.6 | 486.5 | 24 578.2 | 13 258.0 | 1853.7 | 18 647.8 | 12 632.9 | 2024.0 | 21 178.8 |
| HGF | 47.9 | 24.1 | 124.4 | 14.0 | 4.28 | 4426.8 | 65.6 | 18.7 | 9714.8 | 60.3 | 14.4 | 7418.6 |
| MIF | 26.9 | 11.8 | 77.5 | 799.5 | 20.6 | 5461.3 | 38.0 | 10.9 | 761.9 | 47.5 | 15.7 | 612.6 |
| GROα | 21.3 | 12.6 | 54.0 | 14.6 | 1.54 | 24.9 | 18.6 | 0.0 | 102.8 | 14.9 | 0.0 | 55.1 |
| PDGF | 958.9 | 280.7 | 1469.2 | 5.8 | 0.8 | 470.6 | 472.9 | 12.5 | 1161.4 | 387.0 | 21.2 | 1467.6 |
| VEGF | 11.3 | 3.5 | 47.6 | 7.6 | 1.3 | 33.9 | 23.6 | 1.9 | 264.3 | 28.2 | 7.4 | 274.0 |
| SDF1α | 182.1 | 98.8 | 241.1 | 13.7 | 4.62 | 161.5 | 126.7 | 41.6 | 405.4 | 112.0 | 69.0 | 495.2 |
| FGFb | 5.4 | 0.0 | 11.3 | 5.6 | 0.6 | 34.0 | 2.7 | 0.8 | 9.3 | 4.1 | 0.5 | 11.4 |
| Leptin | 646.8 | 71.8 | 6886.2 | 218.5 | 17.9 | 3237.4 | 1965.5 | 55.7 | 14 519.1 | 856.8 | 84.0 | 7469.6 |
| IL-18 | 11.2 | 6.7 | 33.3 | 7.1 | 2.0 | 23.3 | 13.9 | 5.6 | 603.8 | 14.0 | 5.7 | 660.5 |
| IL-10 | 0.0 | 0.0 | 19.8 | 0.8 | 0.0 | 4.2 | 0.8 | 0.0 | 2177.35 | 0.3 | 0.0 | 1436.6 |
Abbreviations: FGFb=basic fibroblast growth factor; GROα=growth regulated alpha protein; HGF=hepatocyte growth factor; IL=interleukin; MIF=migration-inhibitory factor; MRI=magnetic resonance imaging; OPN=osteopontin; PDGF=platelet-derived growth factor; PRC=packed red cell; SDF1α=stromal cell-derived factor 1α; VEGF=vascular endothelial growth factor.
Serum cytokine concentrations
Median serum cytokine concentrations for volunteers and patients are shown in Table 3. There were no significant differences in the concentrations measured for volunteers on separate days. Measured serum concentrations for eight of the cytokines (IL-1β, IL-4, IL-8, IL-12, IFN-α, TNF-α, TGF-β and GCSF) were below the level of detection for both volunteers and patients, resulting in usable serum cytokine concentration data for only 12 of the 20 measured cytokines (Eotaxin, FGFb, GRO-α, HGF, IL-10, IL-18, Leptin, MIF, OPN, PDGF, SDF1α, VEGF). There were no statistically significant changes in median serum concentrations for any of the cytokines when measured at the time of pretransfusion and posttransfusion MRI scans (Table 3). Each of the 12 serum cytokines measurable in both patients and volunteers were also measurable in PRC transfusate (Table 3). The level of MIF in the transfusate was significantly higher than in volunteer serum (P<0.0001). The concentrations of OPN (P<0.001) and MIF (P=0.02) in the patients' sera were significantly higher than in volunteers both before and after the transfusion.
MRI parameters of tumour hypoxia
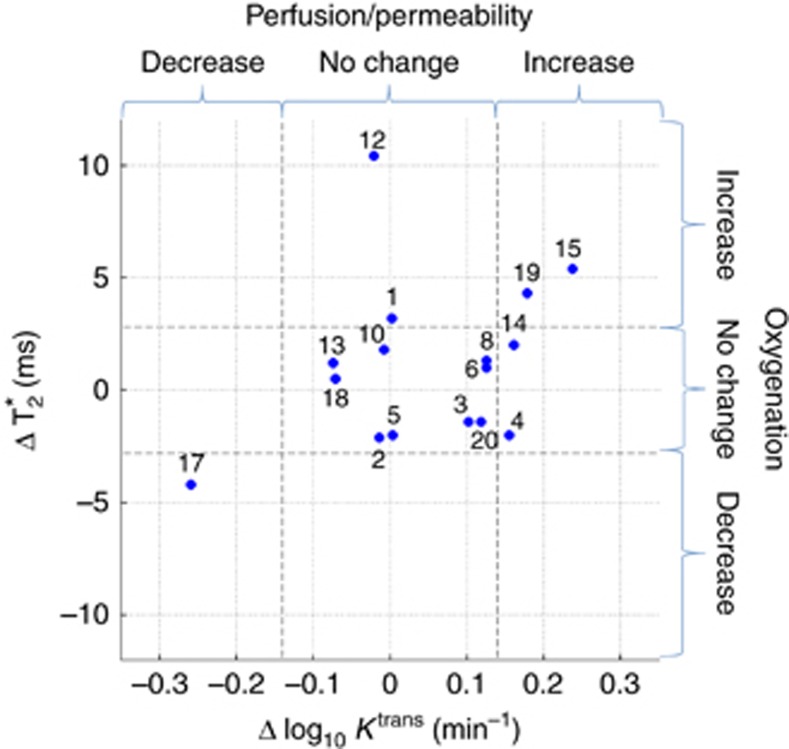
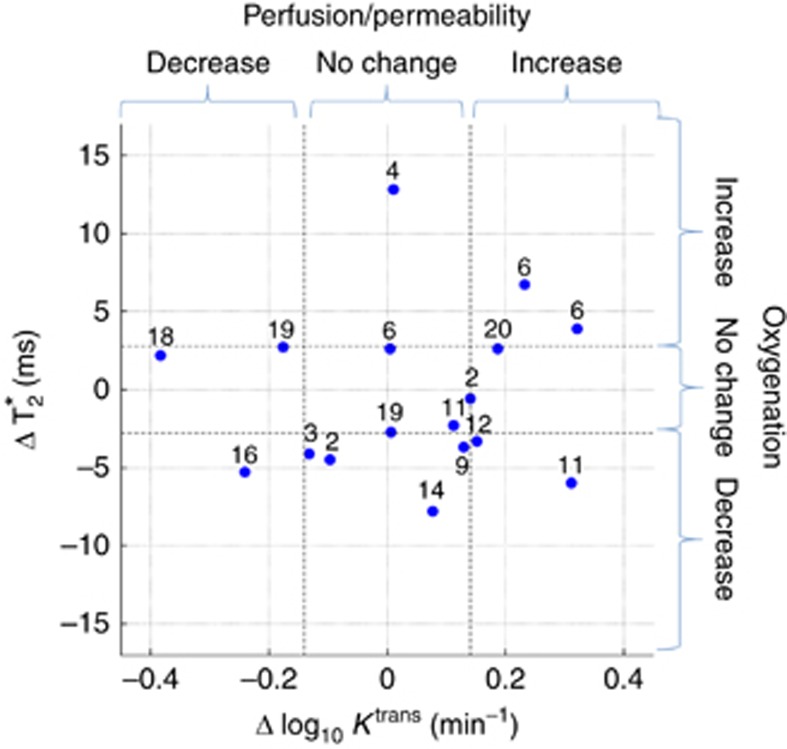
Pre- and post-PRCT T2* and Ktrans were evaluable for primary tumours in 16 patients and for LN metastases in 13 patients. These are summarised in Table 2 (statistically significant net changes) and Supplementary Table S1 (median voxel-wise changes). Scatterplots showing change in median T2* versus change in log10 median Ktrans for primary tumours and lymph nodes are detailed in Figures 1 and 2, respectively. Only changes of magnitude greater than the respective LOA are statistically significant. As seen in Figure 1, improved tumour oxygenation in primary tumours was observed in four patients (nos. 1, 12, 15 and 19). Figure 2 shows that similar changes in tumour oxygenation were not observed in the lymph nodes in these four patients. Similarly, no change in primary tumour oxygenation was observed in two patients (nos. 4 and 6) for whom involved lymph nodes showed improved oxygenation, demonstrating a differential response in primary tumours and lymph nodes to PCRT. No change in primary tumour oxygenation was observed in 11 patients (no. 2–6, 8, 10, 13, 14, 18 and 20). Reduced primary tumour oxygenation and perfusion was observed in one patient (no. 17).
Figure 1.
Scatterplot showing change in median T2* versus change in median Ktrans (log10transformed) for primary tumours. Dashed lines show Bland–Altman limits of agreement.
Figure 2.
Scatterplot showing change in median T2* versus change in median Ktrans (log10transformed) for cervical lymph node tumours. Dashed lines show Bland–Altman limits of agreement.
No statistically significant associations were found between median T2* or median Ktrans and the median serum concentrations of any of the measured cytokines for either primary tumours or LNs. Therefore, neither the radiation delivered between pre- and post-PRCT scans nor the cytokines present in the transfusate altered perfusion or vascular permeability in the tumour to a degree sufficient to affect tissue oxygenation.
Treatment outcomes
Treatment outcomes at 3 months post-CRT are summarised in Table 2. Complete clinical and radiological responses (CR) to CRT at this time were achieved for 11 out of 20 (55%) patients, but the remaining 9 out of 20 (45%) patients had evidence of persistent or progressive disease (PD). Three of the four patients (nos. 12, 15 and 19) who demonstrated improved primary tumour oxygenation (Figure 1) had PD (Table 2). Therefore, the observed magnitude of improved tumour oxygenation following PRCT did not translate into improved treatment outcomes in these patients.
Correlations between MRI parameters and blood Hb
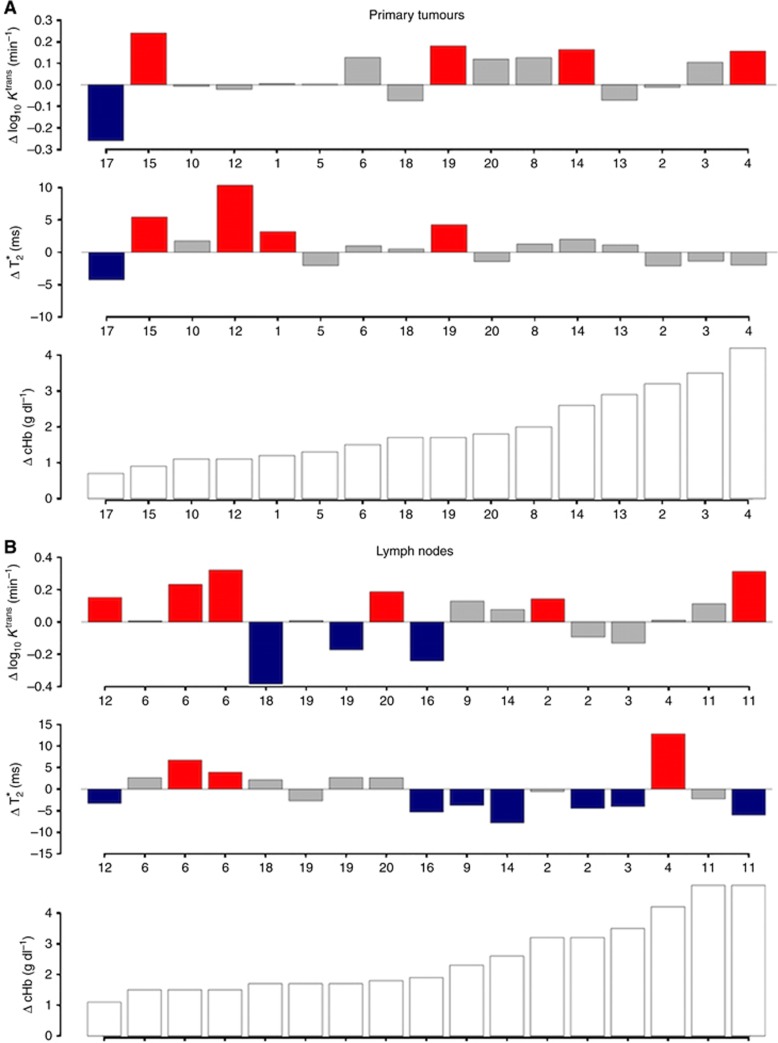
The relationships between change in blood Hb and change in median T2* and median Ktrans for primary tumours and cervical LN metastases are summarised in Figures 3A and B. There was no significant correlation between change in Hb and change in primary tumour median T2* following PRCT (Kendall's tau −0.286, P=0.13).
Figure 3.
Barplots summarising the relationship between changes in median T2*, median Ktrans, and blood cHb before and after PRC transfusion for primary tumours (A) and LNs (B).
Discussion
In this study, we have used SWI MRI to characterise the change in oxygenation status within tumours before and after PRCT. As T2* is proportional to the total amount of deoxy-Hb in an imaging voxel (Ogawa et al, 1990) and, by extension, the tissue partial pressure of oxygen, this parameter provides information on the oxygenation status of tumour tissue (Panek et al, 2016). An absolute measurement of tumour oxygen partial pressure using SWI MRI poses a serious challenge as it requires knowledge on additional confounding factors, such as blood volume and tissue hematocrit (Zhang et al, 2014). In addition, local magnetic field inhomogeneity induced by air–tissue boundaries or the presence of metallic implants can also affect T2* susceptibility measurements. In this study, changes in T2* rather than absolute values were used. By measuring T2* before and after PRCT, the effect of factors other than change in Hb should be controlled, leaving change in tissue deoxy-Hb as the main factor driving change in T2*. The variation of T2* due to different patient positioning and MR system optimisation (i.e., iterative local field shimming) were taken into account adopting 3T repeatability thresholds calculated in a cohort of HNSCC patients (Panek et al, 2016). It would be interesting to extend such analysis to hypoxia-specific PET markers (Lopci et al, 2014), which could help to identify severely hypoxic regions of HNSCC tumours. Similarly, MR imaging could be used to identify avascular parts of tumour, such as necrotic lymph nodes, with impaired delivery of a PET tracer.
This study demonstrates that tumour oxygenation remained stable (11 out of 16) or was significantly reduced (1 out of 16) following PRCT in 12 out 16 (75%) evaluable patients (Figure 1). In addition, for three of the four patients with improved tumour oxygenation following PRCT, this did not translate into improved outcome (nos. 12, 15, and 19, Table 3). For the four primary tumours with a significant increase in median T2* after PRC transfusion, the median increase was 4.3 ms (range 3.3–8.4) corresponding to an approximate increase in median tumour tissue oxygen tension of the order of 4 mm Hg (Panek et al, 2016). The overall magnitudes of these observed increases in median T2* are relatively small and correspond to changes in tissue pO2 that are unlikely to be of radiobiological significance and would not therefore be expected to influence treatment outcome.
In addition, it is possible that anaemia and hypoxia act independently and that tumours with a more aggressive adverse phenotype cause anaemia via activation of catabolic pathways, increased secretion of inflammatory cytokines, extracorpuscular haemolysis and reduction in the level of haematopoetic precursors (Hoff, 2012). The CR rate of 55% in patients who developed anaemia was inferior to CR rates of ∼75% that we have previously reported in an unselected group of patients (Bhide et al, 2008; Miah et al, 2015). This supports the assertion that patients who develop anaemia may have more aggressive tumours with poor prognosis. The transitory reversal of anaemia using PRCT is unlikely to reverse the biology underlying the anaemia and improve treatment outcomes.
Previous studies have demonstrated that pro-inflammatory cytokines are present in leucocyte-depleted and non-depleted blood and that their concentration increases with storage time (Benson et al, 2012). We hypothesised that PRC transfusate might contain physiologically significant concentrations of vasoactive and pro-inflammatory cytokines that could influence tumour hypoxia, vasculature and growth, independent of changes in blood Hb. Our data demonstrate that vasoactive and pro-inflammatory cytokines are present in PRC, in agreement with previous studies. MIF was the only cytokine that was present in PRC in significantly greater concentration than in serum from healthy volunteers (Table 3). However, there was no statistically significant change in the concentration of any of the measured serum cytokines following PRCT, including MIF.
We specifically investigated the possibilities that cytokines present in PRCT, or radiotherapy fractions delivered between pre- and post-PRCT MRI scans, might affect tumour perfusion and/or vascular permeability, by calculating tumour Ktrans from DCE MRI. Only small changes in median Ktrans across PRCT, for both primary tumours and LNs, were observed (Table 2; Figures 1 and 2). Cytokines present in the PRCT, or radiotherapy fractions delivered between pre- and post-PRCT MRI scans, are therefore unlikely to have resulted in changes in tumour vasculature and/or vessel permeability. Therefore, we can conclude that it is unlikely that PRCT results in administration of biologically significant quantities of cytokines that might enhance tumour growth or alter tumour vasculature and vessel permeability. The median Ktrans values were in agreement with a range of values reported for stage IV HNSCC in the literature (Chawla et al, 2011; Shukla-Dave et al, 2012; Bernstein et al, 2014).
Our study included small patient numbers. However, it included a spectrum of HNSCC tumour profiles encountered in clinical practice for radical non-surgical treatment, such as oropharyngeal (HPV positive and negative), laryngeal and hypopharyngeal cancers. In addition, we have attempted to minimise bias by using LOA set by previous studies on MRI reproducibility (Panek et al, 2016) to determine the clinical relevance of observed changes in MRI parameters and by using adequate controls when analysing the cytokine data. In this study, we have only studied the effect of PRCT on changes in tumour oxygenation and have not modelled the other effects of PRCT on the tumour and the patient.
Many institutions follow a policy of using PRCT to maintain Hb levels >12 g dl−1 during radical treatment for HNSCC and other tumour types under the assumption that this mitigates hypoxia-induced radio-resistance. This is based on older studies that have correlated low Hb with reduced tumour oxygenation and adverse outcomes (Tarnawski et al, 1997; Stadler et al, 1999; Becker et al, 2000). Our data obtained using modern imaging and molecular techniques do not support the hypothesis that PRCT improves oxygenation and tumour outcomes. Given the existing clinical data demonstrating lack of benefit for PRCT in HNSCC patients (Hoff et al, 2011; Hoff, 2012), our data add significant weight to concerns that current clinical practice of routine PRCT during radical treatment for HNSCC to enhance tumour oxygenation may be futile. Importantly, however, our study has demonstrated that PRCT does not contain biologically significant quantities of cytokines that could promote tumour growth. PRCT, therefore, may be administered for other clinical indications and should be carried out as per relevant expert guidelines, such as those of NICE and American Association of Blood Banks guidelines on PRCT for anaemia (Murphy et al, 2001; Carson et al, 2012). These guidelines do not recommend routine PRCT for Hb⩾10 g dl−1. The only definite indication for PRCT is Hb<7 g dl−1 or Hb<8 g dl−1 in patients with reduced tolerance for anaemia (aged >65 years and with preexisting cardiovascular and/or respiratory disorders). In addition, change in clinical practice would help mitigate the impact of projected future shortages of blood products (as predicted by NHS Blood and Transplant) as well as preventing unnecessary exposure of patients to the risks associated with PRCT.
Hypoxia-induced treatment resistance is still a problem in HNSCC management and alternative strategies such as hypoxic sensitisers such as nimorazole or vascular-targeting agents should be explored (Overgaard, 2011; Nyflot et al, 2015) using a personalised targeted strategy based on molecular hypoxic signatures (Betts et al, 2013; Tawk et al, 2015).
Acknowledgments
This work was undertaken in The Royal Marsden NHS Foundation Trust, which received a proportion of its funding from the NHS Executive; the views expressed in this publication are those of the authors and not necessarily those of the NHS Executive. This work was supported by Cancer Research UK Programme Grants C46/A10588 and C7224/A13407, CRUK and EPSRC support to the Cancer Imaging Centre at ICR and RMH in association with the MRC and Department of Health; contract grant numbers:C1060/A10334, C1060/A16464. The authors acknowledge the support of the National Institute for Health Research Royal Marsden and Institute of Cancer Research Biomedical Research Centre and the Clinical Research Facility. The charity Oracle Cancer trust funded a part of the project. MOL is an NIHR Senior Investigator. We thank Alex Dunlop, Dualta McQuaid and Simon Doran (Department of Physics) and Veronica Morgan and Sharon Giles (Department of radiology) for their assistance in running the study.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- Becker A, Stadler P, Lavey RS, Hänsgen G, Kuhnt T, Lautenschläger C, Feldmann HJ, Molls M, Dunst J (2000) Severe anemia is associated with poor tumor oxygenation in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys 46: 459–466. [DOI] [PubMed] [Google Scholar]
- Benson DD, Beck AW, Burdine MS, Brekken R, Silliman CC, Barnett CC Jr. (2012) Accumulation of pro-cancer cytokines in the plasma fraction of stored packed red cells. J Gastrointest Surg 16(3): 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein JM, Homer JJ, West CM (2014) Dynamic contrast-enhanced magnetic resonance imaging biomarkers in head and neck cancer: potential to guide treatment? A systematic review. Oral Oncol 50(10): 963–970. [DOI] [PubMed] [Google Scholar]
- Betts GN, Eustace A, Patiar S, Valentine HR, Irlam J, Ramachandran A, Merve A, Homer JJ, Moller-Levet C, Buffa FM, Hall G, Miller CJ, Harris AL, West CM (2013) Prospective technical validation and assessment of intra-tumour heterogeneity of a low density array hypoxia gene profile in head and neck squamous cell carcinoma. Eur J Cancer 49(1): 156–165. [DOI] [PubMed] [Google Scholar]
- Bhide SA, Ahmed M, Barbachano Y, Newbold K, Harrington KJ, Nutting CM (2008) Sequential induction chemotherapy followed by radical chemo-radiation in the treatment of locoregionally advanced head-and-neck cancer. Br J Cancer 99: 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhide SA, Ahmed M, Rengarajan V, Powell C, Miah A, Newbold K, Nutting CM, Harrington KJ (2009) Anemia during sequential induction chemotherapy and chemoradiation for head and neck cancer: the impact of blood transfusion on treatment outcome. Int J Radiat Oncol Biol Phys 73: 3389–3411. [DOI] [PubMed] [Google Scholar]
- Busch OR, Hop WC, Hoynck van Papendrecht MA, Marquet RL, Jeekel J (1993) Blood transfusions and prognosis in colorectal cancer. N Engl J Med 328(19): 1372–1376. [DOI] [PubMed] [Google Scholar]
- Byers LA, Holsinger FC, Kies MS, William WN, El-Naggar AK, Lee JJ, Hu J, Lopez A, Tran HT, Yan S, Du Z, Ang KK, Glisson BS, Raso MG, Wistuba II, Myers JN, Hong W-K, Papadimitrakopoulou V, Lippman SM, Heymach JV (2010) Serum signature of hypoxia-regulated factors is associated with progression after induction therapy in head and neck squamous cell cancer. Mol Cancer Ther 9: 1755–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, Holcomb JB, Illoh O, Kaplan LJ, Katz LM, Rao SV, Roback JD, Shander A, Tobian AA, Weinstein R, Swinton McLaughlin LG, Djulbegovic B Clinical Transfusion Medicine Committee of the AABB (2012) Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med 157(1): 49–58. [DOI] [PubMed] [Google Scholar]
- Chawla S, Kim S, Loevner LA, Hwang WT, Weinstein G, Chalian A, Quon H, Poptani H (2011) Prediction of disease-free survival in patients with squamous cell carcinomas of the head and neck using dynamic contrast-enhanced MR imaging. AJNR Am J Neuroradiol 32(4): 778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Arcy JA, Collins DJ, Padhani AR, Walker-Samuel S, Suckling J, Leach MO (2006) Informatics in Radiology (infoRAD): Magnetic Resonance Imaging Workbench: analysis and visualization of dynamic contrast-enhanced MR imaging data. Radiographics 26(2): 621–632. [DOI] [PubMed] [Google Scholar]
- Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17: 1471–1474. [DOI] [PubMed] [Google Scholar]
- Hoff CM (2012) Importance of hemoglobin concentration and its modification for the outcome of head and neck cancer patients treated with radiotherapy. Acta Oncol 51(4): 419–432. [DOI] [PubMed] [Google Scholar]
- Hoff CM, Lassen P, Eriksen JG, Hansen HS, Specht L, Overgaard M, Grau C, Johansen J, Bentzen J, Andersen L, Evensen JF, Overgaard J (2011) Does transfusion improve the outcome for HNSCC patients treated with radiotherapy?—results from the randomized DAHANCA 5 and 7 trials. Acta Oncol 50(7): 1006–1014. [DOI] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Sivridis E, Pastorek J, Karapantzos I, Gatter KC, Harris AL Group TaAR (2004) Hypoxia-activated tumor pathways of angiogenesis and pH regulation independent of anemia in head-and-neck cancer. Int J Radiat Oncol Biol Phys 59: 67–71. [DOI] [PubMed] [Google Scholar]
- Lee WR, Berkey B, Marcial V, Fu KK, Cooper JS, Vikram B, Coia LR, Rotman M, Ortiz H (1998) Anemia is associated with decreased survival and increased locoregional failure in patients with locally advanced head and neck carcinoma: a secondary analysis of RTOG 85-27. Int J Radiat Oncol Biol Phys 42: 1069–1075. [DOI] [PubMed] [Google Scholar]
- Lim MC, Kim J-Y, Kim T-H, Park S, Kong S-Y, Yoon J-H, Kang S, Seo S-S, Park SY (2008) Allogeneic blood transfusion given before radiotherapy is associated with the poor clinical outcome in patients with cervical cancer. Yonsei Med J 49: 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopci E, Grassi I, Chiti A, Nanni C, Cicoria G, Toschi L, Fonti C, Lodi F, Mattioli S, Fanti S (2014) PET radiopharmaceuticals for imaging of tumor hypoxia: a review of the evidence. Am J Nucl Med Mol Imaging 4(4): 365–384. [PMC free article] [PubMed] [Google Scholar]
- Miah AB, Schick U, Bhide SA, Guerrero-Urbano MT, Clark CH, Bidmead AM, Bodla S, Del Rosario L, Thway K, Wilson P, Newbold KL, Harrington KJ, Nutting CM (2015) A phase II trial of induction chemotherapy and chemo-IMRT for head and neck squamous cell cancers at risk of bilateral nodal spread: the application of a bilateral superficial lobe parotid-sparing IMRT technique and treatment outcomes. Br J Cancer 112(1): 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MF, Wallington TB, Kelsey P, Boulton F, Bruce M, Cohen H, Duguid J, Knowles SM, Poole G, Williamson LM British Committee for Standards in Haematology BTTF (2001) Guidelines for the clinical use of red cell transfusions. Br J Haematol 113(1): 24–31. [DOI] [PubMed] [Google Scholar]
- Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, Becker A, Adam M, Molls M, Dunst J, Terris DJ, Overgaard J (2005) Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol 77(1): 18–24. [DOI] [PubMed] [Google Scholar]
- Nyflot MJ, Kruser TJ, Traynor AM, Khuntia D, Yang DT, Hartig GK, McCulloch TM, Wiederholt PA, Gentry LR, Hoang T, Jeraj R, Harari PM (2015) Phase 1 trial of bevacizumab with concurrent chemoradiation therapy for squamous cell carcinoma of the head and neck with exploratory functional imaging of tumor hypoxia, proliferation, and perfusion. Int J Radiat Oncol Biol Phys 91(5): 942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW (1990) Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 87: 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orton MR, d'Arcy JA, Walker-Samuel S, Hawkes DJ, Atkinson D, Collins DJ, Leach MO (2008) Computationally efficient vascular input function models for quantitative kinetic modelling using DCE-MRI. Phys Med Biol 53(5): 1225–1239. [DOI] [PubMed] [Google Scholar]
- Overgaard J (2011) Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck—a systematic review and meta-analysis. Radiother Oncol 100: 22–32. [DOI] [PubMed] [Google Scholar]
- Panek R, Welsh L, Dunlop A, Wong KH, Riddell AM, Koh DM, Schmidt MA, Doran S, McQuaid D, Hopkinson G, Richardson C, Nutting CM, Bhide SA, Harrington KJ, Robinson SP, Newbold KL, Leach MO (2016) Repeatability and sensitivity of T2* measurements in patients with head and neck squamous cell carcinoma at 3T. J Magn Reson Imaging 44(1): 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosnitz RG, Bin Y, Farrell CL, Clough R, Brizel DM (2005) Pretreatment anemia is correlated with the reduced effectiveness of radiation and concurrent chemotherapy in advanced head and neck cancer. Int J Radiat Oncol Biol Phys 61: 1087–1095. [DOI] [PubMed] [Google Scholar]
- R Core Team (2013) A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Veinna, Austria, ISBN 3-900051-07-0. Available at: www.R-project.org/. [Google Scholar]
- Shukla-Dave A, Lee NY, Jansen JF, Thaler HT, Stambuk HE, Fury MG, Patel SG, Moreira AL, Sherman E, Karimi S, Wang Y, Kraus D, Shah JP, Pfister DG, Koutcher JA (2012) Dynamic contrast-enhanced magnetic resonance imaging as a predictor of outcome in head-and-neck squamous cell carcinoma patients with nodal metastases. Int J Radiat Oncol Biol Phys 82(5): 1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler P, Becker A, Feldmann HJ, Hansgen G, Dunst J, Wurschmidt F, Molls M (1999) Influence of the hypoxic subvolume on the survival of patients with head and neck cancer. Int J Radiat Oncol Biol Phys 44(4): 749–754. [DOI] [PubMed] [Google Scholar]
- Tarnawski R, Skladowski K, Maciejewski B (1997) Prognostic value of hemoglobin concentration in radiotherapy for cancer of supraglottic larynx. Int J Radiat Oncol Biol Phys 38(5): 1007–1011. [DOI] [PubMed] [Google Scholar]
- Tawk B, Schwager C, Deffaa O, Dyckhoff G, Warta R, Linge A, Krause M, Weichert W, Baumann M, Herold-Mende C, Debus J, Abdollahi A (2015) Comparative analysis of transcriptomics based hypoxia signatures in head- and neck squamous cell carcinoma. Radiother Oncol 118(2): 350–358. [DOI] [PubMed] [Google Scholar]
- Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ, Port RE, Taylor J, Weisskoff RM (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10(3): 223–232. [DOI] [PubMed] [Google Scholar]
- Varlotto J, Stevenson MA (2005) Anemia, tumor hypoxemia, and the cancer patient. Int J Radiat Oncol Biol Phys 63: 25–36. [DOI] [PubMed] [Google Scholar]
- Winter SC, Buffa FM, Silva P, Miller C, Valentine HR, Turley H, Shah KA, Cox GJ, Corbridge RJ, Homer JJ, Musgrove B, Slevin N, Sloan P, Price P, West CML, Harris AL (2007) Relation of a hypoxia metagene derived from head and neck cancer to prognosis of multiple cancers. Cancer Res 67: 3441–3449. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Hallac RR, Peschke P, Mason RP (2014) A noninvasive tumor oxygenation imaging strategy using magnetic resonance imaging of endogenous blood and tissue water. Magn Reson Med 71(2): 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.