Abstract
Background:
We investigated the prognostic value of the pretreatment-derived neutrophil–lymphocyte ratio (dNLR) and original NLR in relation to the commonly used inflammation marker C-reactive protein (CRP) in a large cohort of patients with clear cell renal cell carcinoma (RCC).
Methods:
Clinicopathological data from 587 consecutive non-metastatic clear cell RCC patients, operated between 2000 and 2010 at a single tertiary academic center, were evaluated retrospectively. Patients were categorised according to a cutoff value derived from receiver operating curve analysis. Overall (OS), cancer-specific (CSS) as well as metastasis-free survival (MFS) were assessed using the Kaplan–Meier method and multivariate Cox proportional models were applied. Spearman's rank correlation coefficient tested the association between dNLR and other markers of the systemic inflammatory response.
Results:
The significant correlation between pretreatment NLR and dNLR was strong (ρ=0.84), whereas between dNLR and CRP it was weak (ρ=0.18). In multivariate analyses, dNLR achieved independent predictor status regarding CSS (P=0.037) and MFS (P=0.041), whereas CRP was confirmed as independent predictor of OS (P=0.010), CSS (P=0.039) and MFS (P=0.005), respectively. The NLR failed to reach independent predictor status regarding OS, CSS and MFS when CRP was included into the multivariate model.
Conclusions:
In the cohort studied, an elevated (⩾10.0) pretreatment CRP level and elevated dNLR (>2) were robust independent predictors of CSS and MFS. Our data suggest that CRP might be superior to both NLR and dNLR.
Keywords: CRP, derived neutrophil–lymphocyte ratio, prognosis, renal cell carcinoma, survival
Currently representing 2–3% of all cancers, renal cell carcinoma (RCC) accounts for the third most common malignancy of the urinary tract (Ljungberg et al, 2015). In 2012, there were ∼84 400 new cases of RCC diagnosed and 34 700 kidney cancer-related deaths observed within the European Union (Ferlay et al, 2013). The worldwide incidence rates of RCC show a slight (∼2%) increase within the past two decades (Ferlay et al, 2013), whereby due to the more widespread use of radiological imaging techniques, a migration towards small and organ-confined tumours has been observed (Sun et al, 2011; Pichler et al, 2012a). Different histopathological RCC subtypes, linked to distinct genetic characteristics, are described, whereby clear cell RCC represents the majority of cases, accounting for up to 80% (Sun et al, 2011; Pichler et al, 2012a; Ferlay et al, 2013; Srigley et al, 2013; Ljungberg et al, 2015). In all RCC subtypes, prognosis worsens with pathologic T-stage and histopathological tumour grade (Pichler et al, 2012b; Ferlay et al, 2013; Ljungberg et al, 2015). The 5-year overall survival (OS) for all types of RCC is an estimated 49%, which has further improved since 2006 probably due to an increase in incidentally detected tumours, as well as by the introduction of systemic therapies in the metastatic setting (mainly tyrosine kinase inhibitors and immune checkpoint inhibitors; Sun et al, 2011; Albiges et al, 2015; Ljungberg et al, 2015). A complete surgical resection (partial/radical nephrectomy) remains the mainstay of therapy for clinically localised disease, albeit up to 20% of RCC patients subsequently develop metastases after curative surgery (Ferlay et al, 2013; Ljungberg et al, 2015). The prognosis of metastatic RCC remains poor, despite the advances in new targeted therapies to prolong progression-free and cancer-specific survival (CSS) rates (Albiges et al, 2015; Ljungberg et al, 2015).
There is increasing evidence to support the role of the systemic inflammatory response as an important factor in human cancer development and progression. Several markers of the immune response have been proposed as potential prognosticators in RCC, such as plasma fibrinogen (Pichler et al, 2013a; Erdem et al, 2014), interleukin 6 (IL-6) and C-reactive protein (CRP; Karakiewicz et al, 2007; Hrab et al, 2013; Hu et al, 2014) as well as the pretreatment neutrophil–lymphocyte ratio (NLR; de Martino et al, 2013; Pichler et al, 2013b; Ohno et al, 2014; Viers et al, 2014), the lymphocyte–monocyte ratio (LMR; Hutterer et al, 2014) or the granulocyte-to-dendritic cell ratio (Riemann et al, 2014). In particular, the NLR has been demonstrated to influence clinical outcomes in various types of cancer, including cervical (Lee et al, 2012), colorectal (Ding et al, 2010), lung cancer (Sarraf et al, 2009) as well as upper tract urothelial carcinoma (Dalpiaz et al, 2014a). Proctor et al (2012), recently implemented a combined index, named derived neutrophil–lymphocyte ratio (dNLR), which is composed of the neutrophil count to (white cell count minus neutrophil count). The authors proposed that this simplified blood index might be easier to use in clinical trial data, where commonly only leukocyte and neutrophil counts are documented. They evaluated the potential prognostic value of the dNLR in cancer patients including kidney cancer, and were able to demonstrate that the dNLR had similar prognostic value as the classical NLR. In a recent study, our own group was able to confirm the pretreatment dNLR as an independent prognostic factor in patients with upper tract urothelial carcinoma (Dalpiaz et al, 2014b).
To the best of our knowledge, the potential influence of the pretreatment dNLR has never been explored in large RCC patient cohorts yet, and there is no study until now addressing the question whether this blood-based cellular ratio might contain any prognostic significance, when including the well-established pretreatment CRP level into a prognostic model. Therefore, we decided to evaluate the correlation and the potential prognostic significance of preoperatively assessed dNLR and CRP in a large European cohort of patients with non-metastatic clear cell RCC.
Materials and methods
This retrospective analysis included data from 587 non-metastatic clear cell RCC patients who underwent curative radical or partial nephrectomy at the Department of Urology at the Medical University of Graz between January 2000 and December 2010. Overall, 1 190 RCC patients underwent radical or partial nephrectomy at the Department of Urology at the Medical University of Graz during this period. Nine hundred sixty-six out of 1 190 (81.2%) patients had clear cell RCC, whereby 587 out of 966 (60.8%) patients had complete medical records regarding all parameters for inclusion into this study. Thus, 224 out of 1 190 (18.8%) patients with non-clear cell histologic subtypes were excluded from analyses. All clinicopathological as well as laboratory data were retrieved from medical records from the Department of Urology, as well as from pathology reports from the Institute of Pathology at the same institution. Pathological T-stage was uniformly adjusted according to the seventh edition of the TNM 2009 classification system (Sobin et al, 2009). Other clinicopathological parameters included clear cell histological subtype, tumour grade, presence or absence (not quantitatively assessed) of histological tumour necrosis as well as patients' age and gender. All laboratory data were obtained within 1 week before surgical intervention. All patients in this study cohort were selected for surgery based on preoperative in-hospital anaesthesiological evaluation of their clinical as well as laboratory conditions. All patients with acute severe infection or leukaemia were excluded from surgery and consequently from the analysis. The pretreatment dNLR was calculated as the absolute neutrophil count divided by the absolute count of leukocytes minus the absolute count of neutrophils. Patients' post-operative surveillance included routine clinical and laboratory examination as well as imaging methods, whereby X-rays of the chest and abdominal ultrasound were predominantly used, especially in patients with a low relapse risk (pT-1, G1–2), whereas computed tomography or magnetic resonance imaging was performed in all other patients as previously reported (Pichler et al, 2011). Follow-up evaluations were performed every 6 months for the first 5 years and annually thereafter for locally advanced tumours. In organ-confined cancers, imaging was performed twice in the first year after surgery and annually thereafter. Dates of death were obtained from the central registry of the Austrian Bureau of Statistics. This study was approved by the ethical committee of the Medical University of Graz (28–189 ex 15/16). No neoadjuvant or adjuvant treatment was administered.
Statistical analyses
The primary end points of the study were OS, CSS as well as metastasis-free survival (MFS). OS was defined as the time from the date of surgery to individuals' death of any cause. CSS was defined as the time from the date of surgery to a cancer-related death. MFS was defined as the time from the date of surgery to the recurrence of radiologically or histologically confirmed distant metastases. The median time of follow-up was calculated using the time to patients' last follow-up or death. Patients' date of death was obtained from the central registry of the Austrian Bureau of Statistics.
The potential correlations between the pretreatment laboratory parameters NLR, dNLR and CRP were evaluated with Spearman's rank correlation coefficient as nonparametric test, where ρ=1 implies the strongest agreement among ranks and ρ=0 implies no agreement at all.
The cutoff value (2.0) for the pretreatment dNLR was selected according to Proctor et al (2012) for the purpose to validate their findings. Patients' OS, CSS and MFS were calculated using the Kaplan–Meier method and compared by the log-rank test. Multivariate Cox proportion analysis was performed. Hazard ratios (HR's) estimated from the Cox analysis were reported as relative risks with corresponding 95% confidence intervals (CI's). All statistical analyses were performed using the Statistical Package for Social Sciences version 18.0 (SPSS Inc., Chicago, IL, USA) or the MedCalc software package version 16.8.4. A two-sided P<0.05 was considered statistically significant.
Results
Overall, a total of 587 patients with non-metastatic clear cell RCC were included into this study. Descriptive clinicopathological parameters of the study cohort are shown in Table 1. The median pretreatment NLR was 2.95 (IQR=2.24–4.00) and the median pretreatment dNLR 1.96 (IQR=1.48–2.61). Preoperatively assessed CRP levels were available in 516 out of 587 (87.9%) patients with a median of 2.80 mg dl−1 (IQR=1.40–7.38). Spearman's rank correlation coefficient between the pretreatment NLR and dNLR was 0.84 (P<0.001, indicating a strong correlation), whereas between dNLR and CRP it was weak (ρ=0.18, P<0.001), and between NLR and CRP it was 0.31 (P<0.001).
Table 1. Descriptive clinicopathological parameters of the study cohort comprising of patients with non-metastatic clear cell renal cell carcinoma (n=587).
| Parameter | No (%) |
|---|---|
|
Age at operation (years) | |
| Mean±SD | 64.0±11.7 |
| Median | 65.0 |
| Range | 20.0–88.0 |
|
Gender | |
| Male | 349 (59.5) |
| Female | 238 (40.5) |
|
Pathologic T-stage (TNM 2010) | |
| pT1a | 300 (51.1) |
| pT1b | 96 (16.3) |
| pT2a | 27 (4.6) |
| pT2b | 5 (0.8) |
| pT3a | 143 (24.4) |
| pT3b | 14 (2.4) |
| pT3c | 1 (0.2) |
| pT4 | 1 (0.2) |
|
Tumour grade | |
| G1 | 156 (26.6) |
| G2 | 345 (58.8) |
| G3 | 82 (13.9) |
| G4 | 4 (0.7) |
|
Presence of histologic tumour necrosis | |
| No | 443 (75.5) |
| Yes | 144 (24.5) |
|
Preoperative CRP (mg dl−1) | |
| Mean±SD | 14.18±35.20 |
| Median | 2.80 |
| Range | 0–325 |
|
NLR | |
| Mean±SD | 3.50±2.50 |
| Median | 2.95 |
| Range | 0.77–31.20 |
|
dNLR | |
| Mean±SD | 2.27±2.13 |
| Median | 1.96 |
| <2.25 | 374 (63.7) |
| ⩾2.25 | 213 (36.3) |
Abbreviations: CRP=C-reactive protein; dNLR=derived neutrophil–lymphocyte ratio; NLR=neutrophil–lymphocyte ratio; SD=standard deviation.
Comparing the area under the curve (AUC) after receiver operating curve (ROC) analysis to discriminate between events in all three end points is shown in Supplementary Figures 1-3. The ROC curves using CSS as an end point for pretreatment of CRP, NLR and dNLR are shown in Supplementary Figure 1. The AUC for CRP, NLR and dNLR were 0.787 (95% CI=0.75–0.82), 0.711 (95% CI=0.67–0.75) and 0.686 (95% CI=0.64–0.73), respectively, (no statistically significant difference between AUCs was observed). The ROC curves using OS as an end point for pretreatment of CRP, NLR and dNLR are shown in Supplementary Figure 2. The AUCs for CRP, NLR and dNLR were 0.685 (95% CI=0.64–0.73, P=0.045 compared with AUC dNLR), 0.660 (95% CI=0.62–0.70, P=0.007 compared with AUC dNLR) and 0.580 (95% CI=0.54–0.62), respectively. The ROC curves using MFS as an end point for pretreatment of CRP, NLR and dNLR are shown in Supplementary Figure 3. The AUCs for CRP, NLR and dNLR were 0.757 (95% CI=0.72–0.79), 0.672 (95% CI=0.63–0.71) and 0.667 (95% CI=0.63–0.71), respectively, (no statistically significant difference between AUCs was observed).
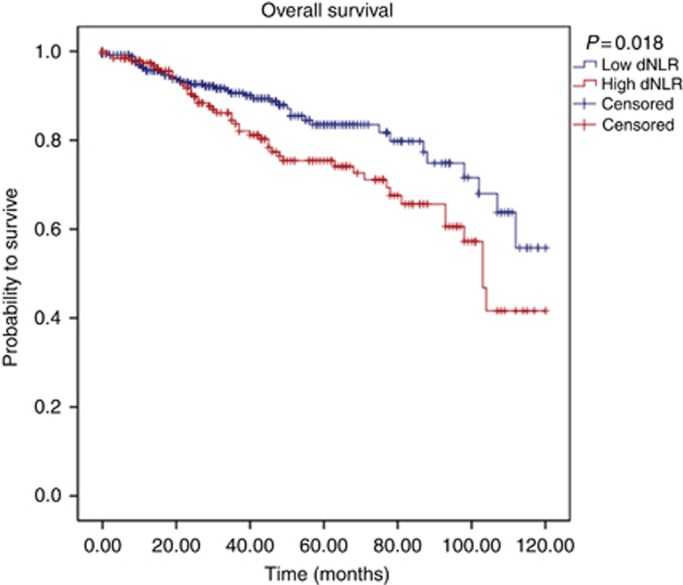
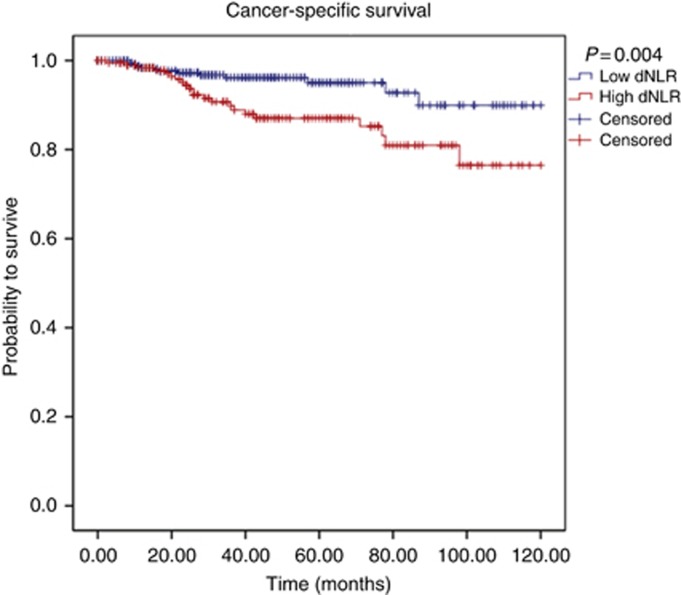
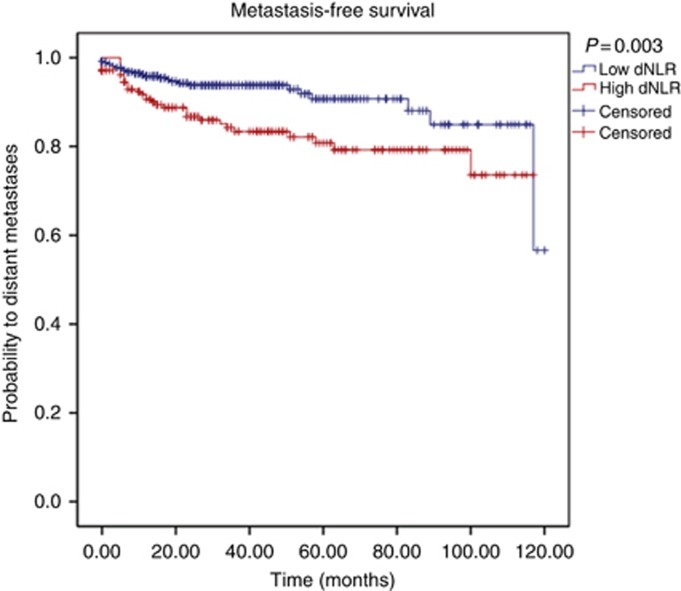
According to our own ROC analysis, we identified a cutoff value of 2.25 to be optimal to discriminate between groups with regard to CSS. We identified 374 (63.7%) patients with a low (<2.25) dNLR and 213 patients (36.3%) with an elevated (⩾2.25) dNLR. Figures 1, 2, 3 show the Kaplan–Meier curves for OS, CSS and MFS. The figures demonstrate that an elevated pretreatment dNLR represents a robust and consistent factor for decreased survival at 10 years after curative partial/radical nephrectomy, as evidenced by a log-rank P=0.018, P=0.004 and P=0.003, for OS, CSS and MFS, respectively.
Figure 1.
Kaplan–Meier curves predicting OS, groups categorised by the pretreatment dNLR. A full colour version of this figure is available at the British Journal of Cancer online.
Figure 2.
Kaplan–Meier curves predicting CSS, groups categorised by the pretreatment dNLR. A full colour version of this figure is available at the British Journal of Cancer online.
Figure 3.
Kaplan–Meier curves predicting MFS, groups categorised by the pretreatment dNLR. A full colour version of this figure is available at the British Journal of Cancer online.
In addition to implementing our own cutoff value, our study's aim was to validate the previously published cutoff value of 2.0 for dNLR (Proctor et al, 2012).
Using multivariate analyses, the pretreatment dNLR represented an independent prognostic factor regarding CSS (HR=2.67, 95% CI=1.06–6.71, P=0.037) as well as MFS (HR=2.02, 95% CI=1.03–3.94, P=0.041). However, the pretreatment NLR (using a cutoff value of 3.3 as previously published by our own group, Pichler et al, 2013b) failed to achieve independent predictor status regarding all three end points when CRP was included into the multivariate Cox model, regarding OS (NLR <3.3 vs ≥3.3, HR=1.38, 95% CI=0.82–2.32, P=0.221), CSS (HR=1.90, 95% CI=0.76–4.73, P=0.171) and MFS (HR=1.69, 95% CI=0.86–3.33, P=0.129). Overall, the preoperative CRP level represented a superior prognosticator in multivariate analyses compared with both, dNLR (OS/CRP <10.0 vs >10.0, HR=2.00, 95% CI=1.18–3.39, P=0.010), MFS (HR=2.65, 95% CI=1.34–5.23, P=0.005) as well as to NLR (OS/CRP <10.0 vs>10.0, HR=1.90, 95% CI=1.11–3.25, P=0.019), CSS (HR=2.24, 95% CI=0.95–5.29, P=0.065), MFS (HR=2.54, 95% CI=1.27–5.10, P=0.009) regarding these end points (Table 2 and Supplementary Table 1).
Table 2. Multivariate analysis of clinicopathological parameters for the prediction of OS, CSS, as well as MFS at 10 years in patients with non-metastatic clear cell renal cell carcinoma (n=587).
|
OS |
CSS |
MFS |
||||
|---|---|---|---|---|---|---|
| Parameter | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|
Gender | ||||||
| Male | 1 (reference) | 0.510 | 1 (reference) | 0.552 | 1 (reference) | 0.782 |
| Female | 0.849 (0.521–1.382) | 1.252 (0.598–2.623) | 1.086 (0.605–1.950) | |||
|
Age at operation (years) | ||||||
| <65 | 1 (reference) | 0.006 | 1 (reference) | 0.416 | 1 (reference) | 0.951 |
| ⩾65 | 2.078 (1.230–3.510) | 1.373 (0.640–2.945) | 1.019 (0.565–1.838) | |||
|
Tumour grade | ||||||
| G1+G2 | 1 (reference) | 0.126 | 1 (reference) | 0.090 | 1 (reference) | 0.036 |
| G3+G4 | 1.583 (0.878–2.854) | 2.041 (0.895–4.654) | 2.059 (1.049–4.041) | |||
|
Pathologic T-stage | ||||||
| pT1 | 1 (reference) | 0.007 | 1 (reference) | <0.001 | 1 (reference) | <0.001 |
| pT2–4 | 1.993 (1.209–3.286) | 7.805 (2.888–21.095) | 4.273 (2.161–8.451) | |||
|
Presence of tumour necrosis | ||||||
| No | 1 (reference) | 0.729 | 1 (reference) | 0.069 | 1 (reference) | 0.086 |
| Yes | 1.098 (0.646–1.868) | 2.035 (0.946–4.378) | 1.724 (0.925–3.214) | |||
|
dNLR | ||||||
| <2.0 | 1 (reference) | 0.568 | 1 (reference) | 0.037 | 1 (reference) | 0.041 |
| ⩾2.0 | 1.154 (0.706–1.885) | 2.669 (1.062–6.707) | 2.015 (1.031–3.939) | |||
|
Preoperative CRP (mg dl−1) | ||||||
| <10.0 | 1 (reference) | 0.010 | 1 (reference) | 0.039 | 1 (reference) | 0.005 |
| ⩾10.0 | 1.999 (1.180–3.386) | 2.408 (1.045–5.549) | 2.651 (1.344–5.230) | |||
Abbreviations: 95% CI=95% confidence interval; CRP=Creactive protein; CSS=cancer-specific survival; dNLR=derived neutrophil–lymphocyte ratio; HR=hazard ratio; MFS=metastasis-free survival; OS=overall survival.
Discussion
The results of the present study show that in multivariate analyses, categorised dNLR achieved independent predictor status regarding CSS (P=0.037) and MFS (P=0.041), whereas categorised CRP was confirmed as independent predictor of OS (P=0.010), CSS (P=0.039) and MFS (P=0.005), respectively.
As previously stated, the pretreatment dNLR was calculated as the absolute neutrophil count divided by the absolute count of leukocytes minus the absolute count of neutrophils. This combined index was first introduced by Proctor et al (2012), whereby the authors proposed that this simplified blood index might be easier to use in clinical trial data, where commonly only leukocyte and neutrophil counts are documented. The authors tested the potential prognostic benefit of the pretreatment dNLR (in comparison with the classical pretreatment NLR) in >12 000 patients from the Scottish Cancer Registry with different types of cancer. In their retrospective analysis, Proctor et al found a comparable prognostic value of both pretreatment blood-based indices and thus recommended the use of the universally available pretreatment dNLR (as reasonable approximation of the pretreatment NLR) for risk stratification purposes particularly in cancer patients. More recently, other studies confirmed an independent prognostic value of the pretreatment dNLR in different types of cancer; however, the authors proposed different optimal cutoff values (Absenger et al, 2013; Szkandera et al, 2013; Dalpiaz et al, 2014b). Among these reports, our own group tested the potential prognostic role of the pretreatment dNLR in >170 patients with non-metastatic upper tract urothelial carcinoma, whereby multivariate analysis identified pretreatment dNLR as an independent predictor of OS as well as CSS (Dalpiaz et al, 2014b).
Regarding pretreatment NLR, only a few studies evaluated its potential prognostic role in non-metastatic clear cell RCC. Viers et al (2014) evaluated >820 M0 clear cell RCC patients undergoing radical nephrectomy and found pretreatment NLR being independently associated with an increased risk of cancer-specific and all-cause mortality. Pichler et al (2013b) recently demonstrated that an increased pretreatment NLR represented an independent risk factor regarding OS (albeit not CSS, nor MFS), which might reflect a higher risk for severe comorbidities, in >670 patients with non-metastatic clear cell RCC undergoing partial/radical nephrectomy.
A strong correlation between pretreatment NLR and dNLR (Spearman's ρ=0.84, P<0.001) prompted us to perform a multivariate Cox regression analysis including the well-established inflammatory parameter of preoperatively assessed CRP.
Representing an important acute phase protein, CRP is recognised as a stable downstream marker of inflammation, whereby its hepatic secretion appears to be controlled by IL-6 (Karakiewicz et al, 2007; Hrab et al, 2013; Hu et al, 2014; Shrotriya et al, 2015). Additionally, IL-1 as well as tumour necrosis factor were shown to be able to stimulate CRP synthesis (Hu et al, 2014; Shrotriya et al, 2015). During the past decade, the context of chronic inflammation and cancer initiation, as well as cancer progression and metastatic spread, has been re-emphasised by extending the potential clinical use of CRP-level measurements to the prediction of tumour progression and cancer survival. Regarding RCC, increased pretreatment CRP levels have been associated with advanced and progressive tumours (Karakiewicz et al, 2007; Steffens et al, 2012; Hrab et al, 2013; Hu et al, 2014; Shrotriya et al, 2015).
In our recent analysis, we found a very weak correlation between pretreatment dNLR (as well as pretreatment NLR) and CRP (evidenced by Spearman's ρ=0.18 and 0.31, P<0.001, respectively). On the contrary, Spearman's rank correlation coefficient between the pretreatment NLR and dNLR was 0.84, P<0.001 (indicating a strong correlation). When categorically coded, pretreatment CRP was confirmed as independent predictor of OS (P=0.010), CSS (P=0.039) and MFS (P=0.005), respectively.
Regarding the results of the recent analysis, the preoperative CRP level represented a superior prognosticator in multivariate analyses compared with pretreatment NLR regarding OS, CSS as well as MFS. Especially the NLR does not prevail as an independent prognostic factor for all three end points. The dNLR represents an independent prognostic factor for CSS and MFS even after inclusion of CRP. Thus, on the basis of our data, measuring CRP probably is sufficient to substitute the calculation of NLR, whereas dNLR adds some independent value for prognostic purposes. Nevertheless, our results warrant further validation in independent patient cohorts, before this statement can be generalised.
As with all retrospective studies, limitations of our study are inherent to the design, including the retrospective data collection, as well as multiple urologic surgeons involved. Moreover, due to the retrospective design, we were not able to adjust for important comorbidities, such as chronic renal failure, coronary heart disease and diabetes mellitus. Incomplete documentation of the surgical margin status in patients' pathological reports represents another important limitation, since positive surgical margins do heavily influence patients' outcomes (Ljungberg et al, 2015). Nonetheless, even considering these limitations, our data clearly indicate that an elevated pretreatment dNLR represents an independent prognostic factor in non-metastatic clear cell RCC patients.
Acknowledgments
Writing and drafting of this manuscript has been partly supported by a fund of the Oesterreichische Nationalbank No. 15888 (to GCH) and the Hans and Blanca Moser Foundation for Early Cancer Research (MS).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- Absenger G, Szkandera J, Pichler M, Stotz M, Arminger F, Weissmueller M, Schaberl-Moser R, Samonigg H, Stojakovic T, Gerger A (2013) A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br J Cancer 109(2): 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albiges L, Choueiri T, Escudier B, Galsky M, George D, Hofmann F, Lam T, Motzer R, Mulders P, Porta C, Powles T, Sternberg C, Bex A (2015) A systematic review of sequencing and combinations of systemic therapy in metastatic renal cancer. Eur Urol 67(1): 100–110. [DOI] [PubMed] [Google Scholar]
- Dalpiaz O, Ehrlich GC, Mannweiler S, Hernández JM, Gerger A, Stojakovic T, Pummer K, Zigeuner R, Pichler M, Hutterer GC (2014. a) Validation of pretreatment neutrophil-lymphocyte ratio as a prognostic factor in a European cohort of patients with upper tract urothelial carcinoma. BJU Int 114(3): 334–339. [DOI] [PubMed] [Google Scholar]
- Dalpiaz O, Pichler M, Mannweiler S, Martín Hernández JM, Stojakovic T, Pummer K, Zigeuner R, Hutterer GC (2014. b) Validation of the pretreatment derived neutrophil-lymphocyte ratio as a prognostic factor in a European cohort of patients with upper tract urothelial carcinoma. Br J Cancer 110(10): 2531–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Martino M, Pantuck AJ, Hofbauer S, Waldert M, Shariat SF, Belldegrun AS, Klatte T (2013) Prognostic impact of preoperative neutrophil-to-lymphocyte ratio in localized nonclear cell renal cell carcinoma. J Urol 190(6): 1999–2004. [DOI] [PubMed] [Google Scholar]
- Ding PR, An X, Zhang RX, Fang YJ, Li LR, Chen G, Wu XJ, Lu ZH, Lin JZ, Kong LH, Wan DS, Pan ZZ (2010) Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int J Colorectal Dis 25(12): 1427–1433. [DOI] [PubMed] [Google Scholar]
- Erdem S, Amasyali AS, Aytac O, Onem K, Issever H, Sanli O (2014) Increased preoperative levels of plasma fibrinogen and d dimer in patients with renal cell carcinoma is associated with poor survival and adverse tumour characteristics. Urol Oncol 32(7): 1031–1040. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49(6): 1374–1403. [DOI] [PubMed] [Google Scholar]
- Hrab M, Olek-Hrab K, Antczak A, Kwias Z, Milecki T (2013) Interleukin-6 (IL-6) and C-reactive protein (CRP) concentration prior to total nephrectomy are prognostic factors in localized renal cell carcinoma (RCC). Rep Pract Oncol Radiother 18(5): 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Gou Y, Sun C, Ding W, Xu K, Gu B, Xia G, Ding Q (2014) The prognostic value of C-reactive protein in renal cell carcinoma: a systematic review and meta-analysis. Urol Oncol 32(1): 50.e1–8. [DOI] [PubMed] [Google Scholar]
- Hutterer GC, Stoeckigt C, Stojakovic T, Jesche J, Eberhard K, Pummer K, Zigeuner R, Pichler M (2014) Low preoperative lymphocyte-monocyte ratio (LMR) represents a potentially poor prognostic factor in nonmetastatic clear cell renal cell carcinoma. Urol Oncol 32(7): 1041–1048. [DOI] [PubMed] [Google Scholar]
- Karakiewicz PI, Hutterer GC, Trinh QD, Jeldres C, Perrotte P, Gallina A, Tostain J, Patard JJ (2007) C-reactive protein is an informative predictor of renal cell carcinoma-specific mortality: a European study of 313 patients. Cancer 110(6): 1241–1247. [DOI] [PubMed] [Google Scholar]
- Lee YY, Choi CH, Kim HJ, Kim TJ, Lee JW, Lee JH, Bae DS, Kim BG (2012) Pretreatment neutrophil:lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer Res 32(4): 1555–1561. [PubMed] [Google Scholar]
- Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L, Merseburger AS, Mulders P, Powles T, Staehler M, Volpe A, Bex A (2015) EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 67(5): 913–924. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Nakashima J, Ohori M, Tanaka A, Hashimoto T, Gondo T, Hatano T, Tachibana M (2014) Clinical variables for predicting metastatic renal cell carcinoma patients who might not benefit from cytoreductive nephrectomy: neutrophil-to-lymphocyte ratio and performance status. Int J Clin Oncol 19(1): 139–145. [DOI] [PubMed] [Google Scholar]
- Pichler M, Hutterer GC, Chromecki TF, Jesche J, Kampel-Kettner K, Rehak P, Pummer K, Zigeuner R (2011) External validation of the Leibovich prognosis score for nonmetastatic clear cell renal cell carcinoma at a single European center applying routine pathology. J Urol 186(5): 1773–1777. [DOI] [PubMed] [Google Scholar]
- Pichler M, Hutterer GC, Chromecki TF, Jesche J, Kampel-Kettner K, Pummer K, Zigeuner R (2012. a) Renal cell carcinoma stage migration in a single European centre over 25 years: effects on 5- and 10-year metastasis-free survival. Int Urol Nephrol 44(4): 997–1004. [DOI] [PubMed] [Google Scholar]
- Pichler M, Hutterer GC, Chromecki TF, Jesche J, Kampel-Kettner K, Eberhard K, Hoefler G, Pummer K, Zigeuner R (2012. b) Trends of stage, grade, histology and tumour necrosis in renal cell carcinoma in a European centre surgical series from 1984 to 2010. J Clin Pathol 65(8): 721–724. [DOI] [PubMed] [Google Scholar]
- Pichler M, Hutterer GC, Stojakovic T, Mannweiler S, Pummer K, Zigeuner R (2013. a) High plasma fibrinogen level represents an independent negative prognostic factor regarding cancer-specific, metastasis-free, as well as overall survival in a European cohort of non-metastatic renal cell carcinoma patients. Br J Cancer 109(5): 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler M, Hutterer GC, Stoeckigt C, Chromecki TF, Stojakovic T, Golbeck S, Eberhard K, Gerger A, Mannweiler S, Pummer K, Zigeuner R (2013. b) Validation of the pre-treatment neutrophil-lymphocyte ratio as a prognostic factor in a large European cohort of renal cell carcinoma patients. Br J Cancer 108(4): 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ (2012) A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer 107(4): 695–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann D, Hase S, Fischer K, Seliger B (2014) Granulocyte-to-dendritic cell-ratio as marker for the immune monitoring in patients with renal cell carcinoma. Clin Transl Med 3: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E (2009) Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg 137(2): 425–428. [DOI] [PubMed] [Google Scholar]
- Shrotriya S, Walsh D, Bennani-Baiti N, Thomas S, Lorton C (2015) C-reactive protein is an important biomarker for prognosis tumour recurrence and treatment response in adult solid tumors: a systematic review. PLoS One 10(12): e0143080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin LH, Gospodariwicz M, Wittekind C (2009) TNM classification of malignant tumors. UICC International Union Against Cancer 7th edn. Wiley-Blackwell: Chichester, West Sussex, UK, p 255 . Available at http://www.uicc.org/tnm.
- Srigley JR, Delahunt B, Eble JN, Egevad L, Epstein JI, Grignon D, Hes O, Moch H, Montironi R, Tickoo SK, Zhou M, Argani P (2013) The international society of urological pathology (isup) Vancouver classification of renal neoplasia. Am J Surg Pathol 37(10): 1469–1489. [DOI] [PubMed] [Google Scholar]
- Steffens S, Köhler A, Rudolph R, Eggers H, Seidel C, Janssen M, Wegener G, Schrader M, Kuczyk MA, Schrader AJ (2012) Validation of CRP as prognostic marker for renal cell carcinoma in a large series of patients. BMC Cancer 12: 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Thuret R, Abdollah F, Lughezzani G, Schmitges J, Tian Z, Shariat SF, Montorsi F, Patard JJ, Perrotte P, Karakiewicz PI (2011) Age-adjusted incidence, mortality, and survival rates of stage-specific renal cell carcinoma in North America: a trend analysis. Eur Urol 59(1): 135–141. [DOI] [PubMed] [Google Scholar]
- Szkandera J, Stotz M, Eisner F, Absenger G, Stojakovic T, Samonigg H, Kornprat P, Schaberl-Moser R, Alzoughbi W, Ress AL, Seggewies FS, Gerger A, Hoefler G, Pichler M (2013) External validation of the derived neutrophil to lymphocyte ratio as a prognostic marker on a large cohort of pancreatic cancer patients. PLoS One 8(11): e78225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viers BR, Houston Thompson R, Boorjian SA, Lohse CM, Leibovich BC, Tollefson MK (2014) Preoperative neutrophil-lymphocyte ratio predicts death among patients with localized clear cell renal carcinoma undergoing nephrectomy. Urol Oncol 32(8): 1277–1284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.