Abstract
Background:
The effectiveness of faecal immunochemical test (FIT)-based screening programs is highly dependent on consistent participation over multiple rounds. We evaluated adherence to FIT screening over four rounds and aimed to identify determinants of participation behaviour.
Methods:
A total of 23 339 randomly selected asymptomatic persons aged 50–74 years were invited for biennial FIT-based colorectal cancer screening between 2006 and 2014. All were invited for every consecutive round, except for those who had moved out of the area, passed the upper age limit, or had tested positive in a previous screening round. A reminder letter was sent to non-responders. We calculated participation rates per round, response rates to a reminder letter, and differences in participation between subgroups defined by age, sex, and socioeconomic status (SES).
Results:
Over the four rounds, participation rates increased significantly, from 60% (95% CI 60–61), 60% (95% CI 59–60), 62% (95% CI 61–63) to 63% (95% CI 62–64; P for trend<0.001) with significantly higher participation rates in women in all rounds (P<0.001). Of the 17 312 invitees eligible for at least two rounds of FIT screening, 12 455 (72%) participated at least once, whereas 4857 (28%) never participated; 8271 (48%) attended all rounds when eligible. Consistent participation was associated with older age, female sex, and higher SES. Offering a reminder letter after the initial invite in the first round increased uptake with 12% in subsequent screening rounds this resulted in an additional uptake of up to 10%.
Conclusions:
In four rounds of a pilot biennial FIT-screening program, we observed a consistently high and increasing participation rate, whereas sending reminders remain effective. The substantial proportion of inconsistent participants suggests the existence of incidental barriers to participation, which, if possible, should be identified and removed.
Keywords: CRC screening, faecal immunochemical test, participation, adherence, rescreening, uptake, colorectal cancer screening
Colorectal cancer (CRC) is a major cause of cancer-related death (Jemal et al, 2011) and its prognosis is largely dependent on stage at diagnosis. Population-based CRC screening aims to detect CRC in an early stage, and to detect and remove precursor lesions, thereby reducing CRC morbidity and mortality (Shaukat et al, 2013). Faecal occult blood test (FOBT)-based screening using guaiac FOBT (gFOBT) has been shown to result in a reduction in CRC-related mortality in a number of randomised controlled trials (Mandel et al, 1993; Hardcastle et al, 1996; Kronborg et al, 1996), with a 15% reduction in CRC-related mortality in a meta-analysis (Hewitson et al, 2008).
In the last decade, several studies have shown that the performance of the faecal immunochemical test (FIT) is superior to that of gFOBT (Allison et al, 2007; Hewitson et al, 2007; van Rossum et al, 2008). Although FIT-based randomised controlled trials with long-term follow-up are lacking, a recent observational study demonstrated a 22% reduction in CRC mortality in areas, where FIT-screening programs were implemented compared with areas without screening (Zorzi et al, 2015). However, FIT has a relatively low sensitivity for CRC and its precursors, and one round of FIT-screening results in a cancer miss rate of 12–25% depending on the cutoff used (de Wijkerslooth et al, 2012). Screening invitations are therefore usually repeated every 2 years, and the effectiveness of a FIT-screening program is highly dependent on participation in multiple rounds. Ideally, eligible invitees accept the invitation to be screened in every screening round (consistent participation; Gellad et al, 2011; Steele et al, 2013).
A high rate of consistent participation increases the program sensitivity of FIT screening (Winawer et al, 1993; Launoy et al, 1998; Zauber et al, 2012; Nishihara et al, 2013). On the other hand, the succes of a biennial FIT-based screening program might be overestimated if the willingness to participate in multiple rounds is low. Knowing possible determinants of inconsistent participation could help in targeting the information to specific groups. Previous studies showed, for example, that especially socioeconomically deprived persons are less likely to accept CRC-screening invitations (Wee et al, 2005; Pornet et al, 2010, 2014; Moss et al, 2012; Leuraud et al, 2013; Lo et al, 2015b).
Several studies on FOBT screening are available, usually reporting on participation rates in a single round. We aimed to examine patterns in participation in an invitational program of biennial FIT-based screening over four screening rounds and to identify possible predictors for consistent and inconsistent screening behaviour.
Materials and methods
Study population/study design
This study was performed in our ongoing pilot program of population-based CRC screening. Details about the design of our program have been described previously (van Rossum et al, 2008; Denters et al, 2013; Kapidiz et al, 2014; Stegeman et al, 2015). In short, demographic data of persons between 50 and 74 years living in the southwest and northwest of The Netherlands were obtained from municipal population registers. Selection of the regions in 2006 was based on a known average uptake of invitees for the national breast cancer screening program. For the southwest region, random samples were taken from the target population by a computer-generated algorithm (Tenalea, Amsterdam, The Netherlands). In the northwest region random samples of selected postal code areas were taken.
The study was conducted in a dynamic cohort. Persons in the target age range that had moved into the targeted postal code area at any time during the recruitment period were included, as well as those that reached the lower age limit of 50 years.
No national screening program had been implemented at the start of this pilot program, and thus the target population was screening naive when first contacted. In the Netherlands, a national FIT-based CRC screening program was gradually initiated from January 2014 onwards. Invitees for our cohort were not invited for the national program.
The selected persons were invited for each consecutive round, except for those who had moved out of the area, those that had passed the upper age limit, institutionalised people, invitees unable to give informed consent, and those who had tested positive in a previous screening round. In our information leaflet and in our informed consent form, persons with a history of inflammatory bowel disease, proctocolectomy, or CRC were advised not to participate CRC screening, but report this reason for non-participation back to our screening organisation via the informed consent form. Participants reporting a colonoscopy in the past 2 years during intake after a positive FIT result were excluded from further participation, as well as those with an estimated life expectancy of <5 years.
Recruitment took place between June 2006 and December 2014 (first round from June 2006 to February 2007; second round from August 2008 to June 2009; third round from February 2011 to February 2012; and fourth round from March 2014 to December 2014). During the first round, invitees from the northwest region were randomly allocated to receive either a gFOBT or a FIT as screening test. Invitees who received a gFOBT in this first round were excluded from our analyses.
Date of birth, sex, and postal codes of all invitees were collected using the municipal population register. Socioeconomic status (SES) was based on social status scores provided by the Netherlands Institute of Social Research (www.scp.nl). The social status score of a postal code area is based on the unemployment rate, education level, average income, and position on the labour market. Social status scores are available for almost all postal codes in the Netherlands. The average status score of 0 and the s.d. in the Netherlands in 2006 was used to divide persons into three categories into high (status scores >0.96), average (status scores between −0.96 and 0.96), and low SES (status scores <−0.96). The first available postal code of the invitee was used to categorise invitees.
FIT screening
Every 2 years, all invitees received a pre-announcement letter about the screening program by mail, followed 2 weeks later by an invitation kit containing an invitation letter, information leaflet, and a single FIT device with testing instructions. In the first, second, and third round, all invitees received an OC-Sensor (Eiken Chemical Co, Tokyo, Japan) as a FIT device. In the fourth round, invitees were randomised to receive either an OC-Sensor (Eiken Chemical Co, Tokyo, Japan) or a FOB Gold (Sentinel Diagnostics SpA, Milan, Italy). As no differences in participation behaviour were seen between the two tests, we included both arms in our analysis (Grobbee et al, 2016). The FIT devices were returned to one of our two selected specialised laboratories and dates of return were registered. A test positivity threshold of ⩾10 μg Hb g−1 faeces was used. People with a positive FIT result were referred for colonoscopy.
All non-responders received a reminder letter by mail after 2–6 weeks. Date of dispatch was registered. A positive response after the reminder letter was defined as a FIT device arriving at the laboratory 3 or more days after sending out the reminder letter. This interval of 3 days was based on the mail system delivery times, which maximally take 3 days between sending and delivering. Date of dispatch of the reminder and date of return of the FIT device at the laboratories were recorded for calculating return time.
Statistical analysis
The participation rate was calculated as the number of participants relative to all eligible invitees. For each screening round, we calculated participation rates per sex. For our analyses of adherence to FIT screening, we only included invitees who were eligible in at least two rounds to be able to observe the three different screening patterns (see below).
Differences in screening behaviour were used to assign participants to one of three groups: consistent participation (i.e., attending all rounds when eligible), inconsistent participation (i.e., attending at least once but less than the total times eligible), and non-participants (not participating in any round of FIT screening).
The percentage of consistent participants was defined as the number of invitees attending all rounds, for which they were eligible relative to the total number of invitees. The percentage of inconsistent participation was defined, as the number of invitees attending inconsistently relative to the total number of invitees. Similarly, the percentage of non-participants was defined as the number of invitees, who never responded to any of the screening invitations.
Differences in proportions between groups were evaluated for statistical significance using the χ2-test statistic. We evaluated participation over rounds with the χ2-test statistic for trend. Differences in medians between groups were tested using the Kruskal–Wallis test statistic. P<0.05 were considered to correspond to statistically significant differences. Data analysis was performed using SPSS22 for Windows (SPSS Inc., Chicago, Ill, USA).
Ethics approval
The Dutch National Health Council approved the study. All included screenees gave written informed consent to participate in the study.
Results
Population
Our dynamic cohort consisted of 23 339 invitees, of whom 323 had to be excluded because they did not meet the inclusion criteria; 49 invitees had moved out, 12 invitees had died, and 262 invitees met one or more of the exclusion criteria (see Materials and Methods section) leaving 23 016 eligible invitees.
Baseline characteristics of the eligible invitees are summarised in Table 1.
Table 1. Basic characterics of eligible invitees of the dynamic FIT-based screening cohort.
| Round 1 | Round 2 | Round 3 | Round 4 | P-value | |
|---|---|---|---|---|---|
| Inviteesa (n) | 14.651 | 14.059 | 16.042 | 16.495 | <0.001 |
| Age (median; IQR) | 59 (54–65) | 60 (55–65) | 59 (54–65) | 61 (56–67) | <0.001 |
| Sex (male; n (%)) | 7264 (50) | 6880 (49) | 7841 (49) | 7955 (48) | 0.127 |
| SES (n (%)) | <0.001 | ||||
| Low | 1328 (9) | 1412 (10) | 1637 (10) | 2281 (14) | |
| Average | 10 602 (72) | 10 004 (71) | 11 296 (70) | 11 117 (67) | |
| High | 2721 (19) | 2643 (19) | 3094 (19) | 3088 (19) | |
| Missing | 0 | 0 | 15 (0.1) | 9 (0.1) |
Abbreviations: FIT=faecal immunochemical test; IQR=interquartile range; SES=socioeconomic status.
Eligible invitees.
Participation
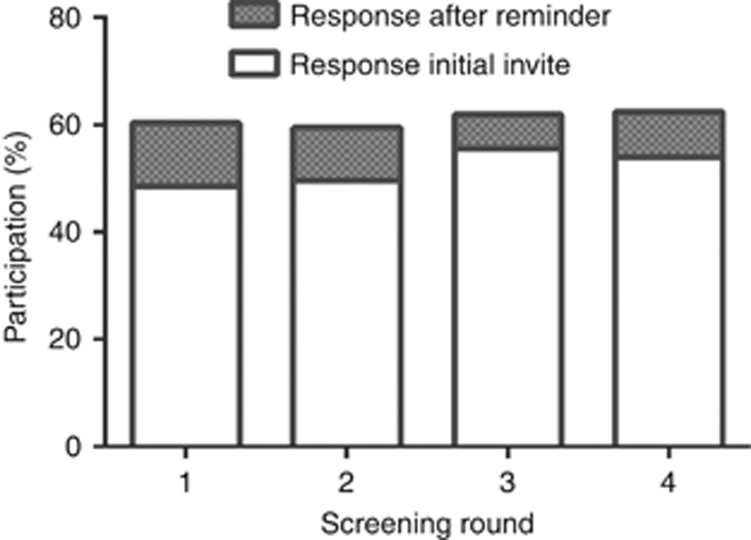
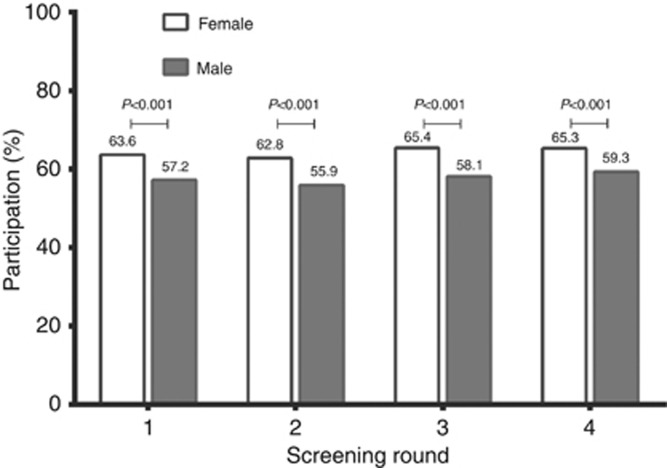
Over the four rounds, participation rates increased significantly, from 60% (95% CI 60–61), 60% (95% CI 59–60), 62% (95% CI 61–63) to 63% (95% CI 62–64), respectively (Figure 1; P for trend <0.001). Differences between men and women over four rounds of FIT screening are shown in Figure 2, with significantly higher participation rates for women in all four rounds (P<0.001).
Figure 1.
Overall participation per screening round with percentage distribution of type of response to participation (initial response vs response after reminder letter).
Figure 2.
Participation rates per round of FIT-based screening subdivided by sex.
Adherence to screening and determinants of adherence
A total of 17 312 invitees were eligible for two or more rounds of FIT screening (Table 2). In this group, 8271 invitees (48%) were consistent participants and 4184 (24%) were inconsistent participants. Overall, 12 455 (72%) invitees participated at least once, whereas 4857 (28%) never participated in the FIT-screening program. Of the 8795 invitees that were eligible for all four rounds, 4345 (49%) participated in four rounds, 2370 (27%) in one or more rounds, and 2080 (24%) participated in none.
Table 2. Adherence to FIT-based CRC screening over multiple rounds.
|
Times participated (n, %) |
||||||
|---|---|---|---|---|---|---|
| Times eligible | 0 | 1 | 2 | 3 | 4 | Total |
| 2 | 1766 (34%) | 905 (17%) | 2561 (49%) | — | — | 5232 |
| 3 | 1011 (31%) | 427 (13%) | 482 (15%) | 1365 (42%) | — | 3285 |
| 4 | 2080 (24%) | 656 (8%) | 686 (8%) | 1028 (12%) | 4345 (49%) | 8795 |
| Total | 4.857 | 1.988 | 3.729 | 2.393 | 4.345 | 17312 |
Abbreviations: CRC=colorectal cancer; FIT=faecal immunochemical test.
Analysis restricted to invitees who were eligible at least two screening rounds. Highlighted light purple blocks represent consistent participation.
Table 3 lists the differences between consistent, inconsistent, and non-participants. Consistent participants were significantly older, more often female, and more likely to have a high SES.
Table 3. Determinants of FIT-screening participation behaviour.
| Consistent (n=8271, 48%) | Inconsistent (n=4184, 24%) | Never (n=4857, 28%) | P-value | |
|---|---|---|---|---|
| Median agea (IQR) | 57 (52–63) | 55 (51–61) | 55 (51–62) | <0.001 |
| Males | 45% | 50% | 54% | <0.001 |
| SESa (%) | <0.001 | |||
| Low | 7% | 11% | 14% | |
| Average | 71% | 72% | 70% | |
| High | 22% | 17% | 16% |
Abbreviations: FIT=faecal immunochemical test; IQR=interquartile range; SES=socioeconomic status.
Age when first eligible.
Reminder letter
In the first screening round, 49% (95% CI 48–49) of the invitees responded within the first 2–6 weeks after receiving the initial invitation kit, and 12% (95% CI 11–12) participated after having been sent a reminder letter (Figure 1). The percentage of participants responding to the initial invitation increased after the first round, with participation rates of 50%, 56%, and 54% for the second, third, and fourth round, respectively. An additional uptake of up to 10% was observed after sending a reminder letter (Figure 1) within each round. On average, the FIT devices were returned within 15 days after sending a reminder letter (first round after 12 days (IQR 7–21); second round after 13 days (IQR 7–32); third round after 15 days (IQR 8–27); and fourth round after 14 days (IQR 7–28)).
Discussion
In four rounds of a pilot biennial FIT-screening program, we observed consistently high and increasing participation rates of 60–63% in each round. Sending a reminder letter after an initial non-response resulted in an increased participation rate, adding 10–12% in each screening round. Almost half of the invitees that were eligible for two or more screening rounds were consistent participants, while almost a quarter never participated. Consistent participants were typically older, more often female, and more likely to have a high SES.
Strengths of our study include that our large cohort consists of an average risk population, comprising all the age ranges that are usually invited for CRC-screening programs worldwide. This population was screen naive when first approached, without the presence of any other CRC-screening initiatives in the population. Moreover, it covers four FIT-based screening rounds, although the majority of long-term studies so far were based on gFOBT-based screening. However, some study limitations have to be acknowledged. SES could only be assigned by postal code, as a proxy for individual-level SES. Regrettably, no data were available on the ethnicity of all invitees, nor their marital status, both factors that could also be associated with participation (El-Haddad et al, 2015). Our pilot program started in 2006, at a time when general awareness of CRC and CRC screening in The Netherlands was limited. That awareness has likely increased over time, especially after 2014, when a national Dutch CRC-screening program was launched. This might have positively affected participation rates in the third and fourth screening round in our pilot program. Moreover, in 2006, we selected our cohort based on uptake data from our national breast cancer screening program that has been implemented in 1990. We selected postal code areas within our regions with a known average uptake in breast cancer screening. This could have resulted in the relatively low proportion of low SES in our cohort.
Similar participation rates, ranging between 56 and 63%, have been reported for a pilot study over four rounds of biennial FIT screening in Italy (Crotta et al, 2012). Our percentage of consistent participants are in line with these data. Studies reporting on adherence to FIT screening over a longer time interval are scarce (Crotta et al, 2012; Kapidzic et al, 2014; Jensen et al, 2016). Most reports are based on studies using gFOBT, reporting consistent adherence rates over multiple rounds, ranging from 39–44% (Steele et al, 2013; Pornet et al, 2014; Lo et al, 2015a).
As in several studies, women were more likely to participate in our FIT-screening program than men. A study from the United Kingdom also described sex differences in participation within a gFOBT-screening pilot consisting of three rounds (Moss et al, 2012). Denis et al (2015) reported an overall 6% higher participation rate for women in a first screening round within a gFOBT-screening program that consisted of four rounds, with a gradually decreasing difference over time. In contrast to these studies, the sex difference in our study remained comparable and significantly different, though this difference was small. A possible explanation could be that women are generally more familiar with the concept of screening. In The Netherlands, women are invited for cervical cancer screening every five years, since 1996 (invitations between the age of 30–60 years), and for breast cancer screening every 2 years, since 1990 (invitations between the age of 50–75 years). So far, no other national screening programs have targeted men. Yet the fact that the difference between participation in men and women did not decrease over four rounds, in contrast to what Denis et al reported, suggests that there may be other factors involved. Possibly, men are less likely to respond the mailed invitations as compared to women and would, for instance, endorsement of the test by their general practitioner encourage them to participate.
A higher SES and older age were also significantly associated with consistent participation. These determinants for adherence to FIT screening are comparable with those in the previously reported one-time FOBT-screening studies and gFOBT-based CRC-screening studies (Wee et al, 2005; Pornet et al, 2010, 2014; Moss et al, 2012; Leuraud et al, 2013; Hurtado et al, 2015; Lo et al, 2015b). Pornet et al compared occasional participants with compliant participants in a gFOBT-screening program and also reported that occasional adherence was positively associated with living in socioeconomically deprived areas.
Response times for participation varied over screening rounds, with prompter participation in later screening rounds. A potential explanation could be that most invitees grew familiar with the program and the FIT as a screening test over successive rounds, thereby lowering the barrier to participate and to perform the test. An alternative, additional factor could be the increased awareness of CRC and CRC screening over time.
Response rates further increased after sending reminder letters to non-participants, and this effect was seen in each of the four rounds. Previous one-time screening studies with varying intervals for sending reminder letters also showed a positive effect on uptake from sending a reminder letter (Baron et al, 2008). Santare et al (2015) reported a very high proportion of 29% FIT devices returned (OC-Sensor) after sending a reminder letter after 21 days, but this was studied in Latvia, which has an opportunistic screening program with very low uptake (7.6%). Tinmouth et al (2015) reported a 9.7% increase in participation after sending a reminder letter in a gFOBT-based CRC screening after 6 months. Participation rates doubled after sending a new gFOBT kit. Our results indicate that sending a reminder letter to all non-responders after 6 weeks, in every screening round, consistently results in a positive contribution to overall participation and that reminders remain effective over multiple rounds.
About one in four invitees eligible for more than one round participated once or more often, but not in all screening rounds for which they had been invited. This indicates that the decision to participate in screening is not always the outcome of a one-time assessment. It is possible that eligible citizens change their behaviour in time, and one must acknowledge that also practical issues, such as work-related responsibilities, could prevent consistent participation.
Although screening uptake was high and increased over rounds, and about half of the FIT invitees were consistent participants, almost a quarter of the invitees never participated in any of the rounds of FIT screening. It would be relevant to investigate whether these invitees made an informed decision not to participate, or whether participation was hampered by barriers, such as limited health literacy, distrust of government initiated health initiatives, cost considerations, or other issues. Health literacy is an individual's capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions. Limited health literacy has been shown to be associated with a restricted use of preventive health services, such as cancer screening (Kobayashi et al, 2014). A questionnaire study performed in the second round of our pilot program of FIT screening showed that one of the more frequently reported reasons for non-participation in FOBT screening was lack of abdominal complaints, which suggests limitations in CRC knowledge in this group (Denters et al, 2015). Adequate CRC knowledge was found to be a strong predictor for participation in successive rounds (van Dam et al, 2013). It is conceivable that we need to diversify our invitation and information strategy, taking into account differences between groups, to achieve equity, enabling men and women, in all age groups, and socioeconomic layers, in making well-informed decisions about participation in CRC screening.
Acknowledgments
We thank The Netherlands Organization for Health Research and Development of the Dutch Ministry of Health (ZonMW) for funding (project numbers 120710007, 63000004, 12010095420, and 200340001). Also, we acknowledge Karin de Groot, research nurse, for her professional work. The authors especially thank Mieke Janssen and Kirsten Izelaar, and all involved co-workers of the Regional Organization for Population Screening Mid-West and South-West (Bevolkingsonderzoek MiddenWest, Bevolkingsonderzoek ZuidWest) for their important contributions to the study. The Netherlands Organisation for Health Research and Development of the Dutch Ministry of Health was not involved in the analysis and interpretation of the data nor in the writing of the manuscript or decision to submit the paper.
Author contributions
M van der Vlugt: study concept and design, acquisition of data; analysis and interpretation of data; drafting of the manuscript; and statistical analysis. EJ Grobbee: study concept and design; and analysis. Critical revision of the manuscript for important intellectual content. PMM Bossuyt: study concept and design; and statistical analysis. Critical revision of the manuscript for important intellectual content. E Bongers: made substantial contributions to the acquisition of data. Critical revision of the manuscript for important intellectual content. W Spijker: made substantial contributions to the acquisition of data. Critical revision of the manuscript for important intellectual content. EJ Kuipers: study concept and design. Critical revision of the manuscript for important intellectual content. I Lansdorp-Vogelaar: study concept and design. Critical revision of the manuscript for important intellectual content. M-L Essink-Bot: study concept and design. Critical revision of the manuscript for important intellectual content. MCW Spaander: study concept and design. Contributed to the acquisition of data. Critical revision of the manuscript for important intellectual content. E Dekker: study concept, and design and interpretation of data. Contributed to the acquisition of data. Critical revision of the manuscript for important intellectual content.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
References
- Allison JE, Sakoda LC, Levin TR, Tucker JP, Tekawa IS, Cuff T, Pauly MP, Shlager L, Palitz AM, Zhao WK, Schwartz JS, Ransohoff DF, Selby JV (2007) Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst 99: 1462–1470. [DOI] [PubMed] [Google Scholar]
- Baron RC, Rimer BK, Breslow RA, Coates RJ, Kerner J, Melillo S, Habarta N, Kalra GP, Chattopadhyay S, Wilson KM, Lee NC, Mullen PD, Coughlin SS, Briss PA Task Force on Community Preventive Services (2008) Client-Directed Interventions to Increase Community Demand for Breast, Cervical, and Colorectal Cancer Screening. Am J Prev Med 35: S34–S55.18541187 [Google Scholar]
- Crotta S, Segnan N, Paganin S, Dagnes B, Rosset R, Senore C (2012) High rate of advanced adenoma detection in 4 rounds of colorectal cancer screening with the fecal immunochemical test. Clin Gastroenterol Hepatol 10: 633–638. [DOI] [PubMed] [Google Scholar]
- Denis B, Gendre I, Perrin P (2015) Participation in four rounds of a French colorectal cancer screening programme with guaiac faecal occult blood test: a population-based open cohort study. J Med Screen 22: 76–82. [DOI] [PubMed] [Google Scholar]
- Denters MJ, Bossuyt PM, Deutekom M, Fockens P, Dekker E (2015) Most participate in faecal immunochemical test-based colorectal cancer screening out of curiosity about their chances of developing cancer. Eur J Cancer Prev 24: 176–179. [DOI] [PubMed] [Google Scholar]
- Denters MJ, Deutekom M, Bossuyt PM, van Rijn AF, Fockens P, Dekker E (2013) Involvement of previous non-participants cannot fully compensate for lower participation in a second round of FIT-screening. Cancer Epidemiol 37: 330–335. [DOI] [PubMed] [Google Scholar]
- de Wijkerslooth TR, Stoop EM, Bossuyt PM, Meijer GA, van Ballegooijen M, van Roon AH, Stegeman I, Kraaijenhagen RA, Fockens P, van Leerdam ME, Dekker E, Kuipers EJ (2012) Immunochemical fecal occult blood testing is equally sensitive for proximal and distal advanced neoplasia. Am J Gastroenterol 107: 1570–1578. [DOI] [PubMed] [Google Scholar]
- El-Haddad B, Dong F, Kallail KJ, Hines RB, Ablah E (2015) Association of marital status and colorectal cancer screening participation in the USA. Colorectal Dis 17: 108–114. [DOI] [PubMed] [Google Scholar]
- Gellad ZF, Stechuchak KM, Fisher DA, Olsen MK, McDuffie JR, Ostbye T, Yancy Jr WS (2011) Longitudinal adherence to fecal occult blood testing impacts colorectal cancer screening quality. Am J Gastroenterol 106: 1125–1134. [DOI] [PubMed] [Google Scholar]
- Grobbee EJ, van der Vlugt M, van Vuuren AJ, Stroobants AK, Mundt MW, Spijker WJ, Bongers EJ, Kuipers EJ, Lansdorp-Vogelaar I, Bossuyt PM, Dekker E, Spaander MC (2016) Comparison of OC-Sensor and FOB-gold in population-based colorectal cancer screening based on FIT. Gut e-pub ahead of print 9 August 2016 doi:10.1136/gutjnl-2016-311819. [DOI] [PubMed]
- Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM (1996) Randomised controlled trial of faecal- occult blood screening for colorectal cancer. Lancet 348: 1472–1477. [DOI] [PubMed] [Google Scholar]
- Hewitson P, Glasziou P, Irwig L, Towler B, Watson E (2007) Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst Rev 1: CD001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitson P, Glasziou P, Watson E, Towler B, Irwig L (2008) Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol 103: 1541–1549. [DOI] [PubMed] [Google Scholar]
- Hurtado JL, Bacigalupe A, Calvo M, Esnaola S, Mendizabal N, Portillo I, Idigoras I, Millán E, Arana-Arri E (2015) Social inequalities in a population based colorectal cancer screening programme in the Basque Country. BMC Public Health 15: 1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. [DOI] [PubMed] [Google Scholar]
- Jensen CD, Corley DA, Quinn VP, Doubeni CA, Zauber AG, Lee JK, Zhao WK, Marks AR, Schottinger JE, Ghai NR, Lee AT, Contreras R, Klabunde CN, Quesenberry CP, Levin TR, Mysliwiec PA (2016) Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med 164: 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapidzic A, Grobbee EJ, Hol L, van Roon AH, van Vuuren AJ, Spijker W, Izelaar K, van Ballegooijen M, Kuipers EJ, van Leerdam ME (2014) Attendance and yield over three rounds of population-based fecal immunochemical test screening. Am J Gastroenterol 109: 1257–1264. [DOI] [PubMed] [Google Scholar]
- Kobayashi LC, Wardle J, von Wagner C (2014) Limited health literacy is a barrier to colorectal cancer screening in England: evidence from the English longitudinal study of ageing. Prev Med 61: 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O (1996) Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 348: 1467–1471. [DOI] [PubMed] [Google Scholar]
- Launoy G, Duffy SW, Prevost TC, Bouvier V (1998) Detection of cancer, sensitivity of the test and sensitivity of the screening program. Rev Epidemiol Sante Publique 46: 420–426. [PubMed] [Google Scholar]
- Leuraud K, Jezewski-Serra D, Viguier J, Salines E (2013) Colorectal cancer screening by guaiac faecal occult blood test in France: evaluation of the programme two years after launching. Cancer Epidemiol 37: 959–967. [DOI] [PubMed] [Google Scholar]
- Lo SH, Halloran S, Snowball J, Seaman H, Wardle J, von Wagner C (2015. a) Colorectal cancer screening uptake over three biennial invitation rounds in the English bowel cancer screening programme. Gut 64: 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SH, Halloran S, Snowball J, Seaman H, Wardle J, von Wagner C (2015. b) Predictors of repeat participation in the NHS bowel cancer screening programme. Br J Cancer 112: 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel JS, Bond TH, Church TR, Snover DC, Bradley GM, Schuman LM, Ederer F (1993) Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 328: 1365–1371. [DOI] [PubMed] [Google Scholar]
- Moss SM, Campbell C, Melia J, Coleman D, Smith S, Parker R, Ramsell P, Patnick J, Weller DP (2012) Performance measures in three rounds of the English bowel cancer screening pilot. Gut 61: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, Inamura K, Kim SA, Kuchiba A, Yamauchi M, Imamura Y, Willett WC, Rosner BA, Fuchs CS, Giovannucci E, Ogino S, Chan AT (2013) Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 369: 1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pornet C, Dejardin O, Morlais F, Bouvier V, Launoy G (2010) Socioeconomic determinants for compliance to colorectal cancer screening. A multilevel analysis. J Epidemiol Community Health 64: 318–324. [DOI] [PubMed] [Google Scholar]
- Pornet C, Denis B, Perrin P, Gendre I, Launoy G (2014) Predictors of adherence to repeat fecal occult blood test in a population-based colorectal cancer screening program. Br J Cancer 111: 2152–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santare D, Kojalo I, Huttunen T, Rikacovs S, Rucevskis P, Boka V, Leja M (2015) Improving uptake of screening for colorectal cancer: a study on invitation strategies and different test kit use. Eur J Gastroenterol Hepatol 27: 536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, Church TR (2013) Long- term mortality after screening for colorectal cancer. N Engl J Med 369: 1106–1114. [DOI] [PubMed] [Google Scholar]
- Steele RJ, McClements PL, Libby G, Carey FA, Fraser CG (2013) Patterns of uptake in a biennial faecal occult blood test screening programme for colorectal cancer. Colorectal Dis 16: 28–32. [DOI] [PubMed] [Google Scholar]
- Stegeman I, van Doorn SC, Mundt MW, Mallant-Hent RC, Bongers E, Elferink MA, Fockens P, Stroobants AK, Bossuyt PM, Dekker E (2015) Participation, yield, and interval carcinomas in three rounds of biennial FIT-based colorectal cancer screening. Cancer Epidemiol 39: 388–393. [DOI] [PubMed] [Google Scholar]
- Tinmouth J, Patel J, Austin PC, Baxter NN, Brouwers MC, Earle C, Levitt C, Lu Y, Mackinnon M, Paszat L, Rabeneck L (2015) Increasing participation in colorectal cancer screening: results from a cluster randomized trial of directly mailed gFOBT kits to previous non-responders. Int J Cancer 136: E697–E703. [DOI] [PubMed] [Google Scholar]
- van Dam L, Korfage IJ, Kuipers EJ, Hol L, van Roon AH, Reijerink JC, van Ballegooijen M, van Leerdam ME (2013) What influences the decision to participate in colorectalcancer screening with faecal occult blood testing and sigmoidoscopy? Eur J Cancer 49: 2321–2330. [DOI] [PubMed] [Google Scholar]
- van Rossum LG, van Rijn AF, Laheij RJ, van Oijen MG, Fockens P, van Krieken HH, Verbeek AL, Jansen JB, Dekker E (2008) Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 135(1): 82–90. [DOI] [PubMed] [Google Scholar]
- Wee CC, Mc Carthy EP, Philips RS (2005) Factors associated with colon cancer screening: the role of patient factors and physician counseling. Prev Med 41: 23–29. [DOI] [PubMed] [Google Scholar]
- Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF, Ackroyd F, Shike M, Kurtz RC, Hornsby-Lewis L, Gerdes H, Stewart ET *the National Polyp Study Workgroup (1993) Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 329: 1977–1981. [DOI] [PubMed] [Google Scholar]
- Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD (2012) Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 366: 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzi M, Fedeli U, Schievano E, Bovo E, Guzzinati S, Baracco S, Fedato C, Saugo M, Dei Tos AP (2015) Impact on colorectal cancer mortality of screening programmes based on the faecal immunochemical test. Gut 64: 784–790. [DOI] [PubMed] [Google Scholar]