Abstract
This paper provides a look at how modulated broad-band noises modulate the thalamic response evoked by brief probe sounds in the awake animal. We demonstrate that noise not only attenuates the response to probe sounds (masking) but also changes the temporal response pattern (scrambling). Two brief probe sounds, a Gaussian noise burst and a brief sinusoidal tone, were presented in silence and in three ongoing noises. The three noises were targeted at activating the auditory system in qualitatively distinct ways. Dynamic ripple noise, containing many random tone-like elements, is targeted at those parts of the auditory system that respond well to tones. International Collegium of Rehabilitative Audiology noise, comprised of the sum of several simultaneous streams of Schroeder-phase speech, is targeted at those parts of the auditory system that respond well to modulated sounds but lack a well defined response to tones. Gaussian noise is targeted at those parts of the auditory system that respond to acoustic energy regardless of modulation. All noises both attenuated and decreased the precise temporal repeatability of the onset response to probe sounds. In addition, the modulated noises induced context-specific changes in the temporal pattern of the response to probe sounds. Scrambling of the temporal response pattern may be a direct neural correlate of the unfortunate experience of being able to hear, but not understand, speech sounds in noisy environments.
This paper examines how neurons in the auditory thalamus [medial geniculate body (MGB)] of awake rats respond to transient probe sounds in silence and in the presence of three ongoing noises. We demonstrate that noise changes not only probe response magnitude but also changes the temporal pattern of the probe-evoked response.
The unwanted acoustic interference in natural listening situations is more closely modeled by modulated noise than by Gaussian, pink noise. This situation forces audiologists to employ complex noises, such as multispeaker “babble,” to predict the ability of hearing-aid users to comprehend speech in noisy environments. This study selected two complementary noises from the vast space of possible modulated noises. One noise was derived from a standardized babble noise developed by the International Collegium of Rehabilitative Audiology (ICRA) (1). The noise contains speech-like temporal modulations but without the narrow-band, tone-like elements of speech. Complementing the ICRA-derived noise is dynamic-ripple noise (DRN) (2), which was originally conceived as an aid to study responses of auditory neurons by the white-noise system identification approach (3). DRN presents many combinations of tone-like elements in a random order. Together, these noises should randomly stimulate parts of the auditory system that respond quite selectively to tones within a relatively narrow frequency range and parts of the auditory system that respond better to broad-band sounds.
This study focused on the MGB because, in anesthetized animals, thalamic neurons respond differentially to spectrotemporally shaped noises. In the ventral division (MGBv), neurons respond well to narrow-band stimuli (4) and to ongoing dynamic ripples similar to those used in this study (5) but not to unmodulated Gaussian noise. In contrast, within the dorsal division (MGBd), neuronal response magnitude is greater to broad-band sounds than to sinusoidal tones (6). Our preliminary findings in anesthetized rats suggest that both MGBv and MGBd respond better to ICRA noise than to either DRN or Gaussian noise.§ Such differential response is of great interest, because it potentially changes the balance between parallel ascending pathways into the primary auditory cortex. Whereas MGBv projects almost exclusively to the middle cortical layers, MGBd also projects to dorsal and ventral layers (7).
This paper provides a look at how modulated broad-band noises modulate the thalamic response evoked by brief probe sounds in the awake animal. It extends existing knowledge (i) by examining the effects of both Gaussian, pink noise and of spectrally and temporally modulated noises and (ii) by reporting masking-related responses in the auditory thalamus of awake animals, avoiding the possible effects of anesthesia.
Materials and Methods
Preparation and Recording. Studies were conducted in albino rats (350-400 g) according to approved protocols at the University of Florida. National, state, and institutional animal welfare guidelines were followed. A surgical plane of anesthesia was induced by a halothane/oxygen mixture. The airway was stabilized by orotracheal intubation. After i.v. access was established in the tail vein, anesthesia was maintained with sodium pentobarbital. The rat was mechanically ventilated. Heart rate and reflexes to deep pressure and glabella tap were monitored. Pentobarbital was titrated to eliminate reflexes without causing undue cardiorespiratory suppression. Electrode arrays were placed in the auditory thalamus stereotaxically (8), and the location was confirmed by acutely recording evoked neuronal responses. In separate acute recording experiments where electrode location was verified histologically, this procedure leads to a bias toward recording from ventral MGB. Rats were recovered and housed singly.
For awake recording, rats were placed in a test cage, within a double-wall, sound-attenuating chamber. Rats were free to move about the test cage, tethered to the second-stage amplifier by a flexible cable. They were observed on a video monitor and were aroused if they appeared to fall asleep or attended if they showed interest in the recording apparatus. Signals were amplified by a head-mounted high-impedance head stage amplifier. Acoustic stimulation, signal discrimination, and recording were controlled by a computer running brainware (Tucker-Davis Technologies, Alachua, FL). In later experiments, analog waveforms were recorded and then filtered offline with matlab (MathWorks, Natick, MA), and common mode potentials were removed with an array-processing algorithm (9). Spikes were identified by using a variant of Lewicki's Baysian algorithm (10). A total of 21 recordings were obtained from 20 electrodes in six rats. One unit recording appeared to be a clearly separable, single-unit recording, whereas the other recordings were conservatively classified as multiunit.
Acoustic Stimulation. Three ongoing noises were tested: Gaussian noise, modified ICRA noise, and DRN. Two types of transient probe sounds were tested: Gaussian noise bursts and sinusoidal tones. Sequences of five probe sounds were presented during the noise. The sounds were precomputed and digitally stored so that the identical, frozen sound was presented on each of 30 repeated trials. All three noises had the same equal energy-per-octave long-term average power spectrum. The noises were split into two bands. The low bands (0.2-20 kHz) were presented by a powered monitor speaker (model LSR25P, JBL Professional, Northridge, CA), whereas the high bands (20-36 kHz) were presented through a ES1 tweeter (Tucker-Davis Technologies) mounted atop the JBL speaker. Noise duration was 5,500 msec, gated with 5 msec cosine on- and off-ramps. The level of all noises was set to a 60-dB sound-pressure level.
Gaussian noise was synthesized by drawing random samples with matlab function rand and filtering it so that the spectral roll-off was 6 ± 1.5 dB per octave. The Gaussian burst probe had the same spectrum.
The ICRA noise was derived from the standard ICRA noise, track 7 of the version 0.3 compact disc. The noise on this track is the sum of six streams of three-band Schroeder-phase speech (1, 11). So that the ICRA noise covered a wider range of frequencies, a time-shifted version of the 5- to 20-kHz band was copied to the 20- to 35-kHz band by means of the Hilbert transform and added to the original noise. This wide-band ICRA noise was filtered so that its spectrum matched the spectra of the other noises.
DRN was synthesized as the sum of 16 independent dynamic ripples (2). Each dynamic ripple was the sum of 324 logarithmically spaced sinusoidal carriers, with phase uniformly distributed between 0 and 2π and instantaneous amplitude set by a randomly changing spectral envelope. For each of the independent ripples, the spectral envelope was sinusoidal, with uniformly distributed ripple density (0-4 cycles per octave), uniformly distributed ripple-phase (0-2π), and 30-dB modulation depth. The ripple density rate of change was limited to <3 Hz, and the ripple-phase rate of change was limited to <1.5 Hz. The carrier amplitude was set so that the spectrum rolled off at 6 dB per octave.
A sequence of five probe sounds was presented during the ongoing noise. Both Gaussian noise bursts and sinusoidal tones were tested. Tone frequency was near the recording's characteristic frequency, estimated by manually adjusting the frequency of test tones. Probe duration was 200 msec, gated with 5-msec cosine on- and off-ramps. The level of all probes was set to a 60-dB sound pressure level, for an overall probe-to-noise intensity ratio of 0 dB. If rat critical bandwidths are assumed to be ≈1/3 octave, the within channel probe-to-noise ratio was 13.5 dB. If rat critical bandwidths are assumed to be ≈1 octave, as in the mouse (12), the within channel probe-to-noise ratio was 5.5 dB. Probe tones began at 1.2, 2.3, 3.0, 4.3, and 5.1 sec after ongoing noise onset. Gaussian burst probes began at the same times, except that the third noise probe began at 3.3 sec after ongoing noise onset. Statistics were derived from the four windows for which tone onset and burst onset were identical.
Data Analysis. The main assertions of this paper, that ongoing noise both masks the response to probe sounds and scrambles the temporal response pattern, were assessed by examining the spike trains in time windows locked to probe-sound onset. Evoked responses were quantified by the windowed spike counts and by a coefficient of reliability. Estimates of spontaneous rate, response driven by noise alone, and reliability of activity evoked by noise alone were based on a window at 0.5-5.5 sec after noise onset. In Figs. 1 and 2, bar graphs show the mean and the 95% confidence limits based on a univariate t test. Probe-evoked spike counts were combined across the four analyzed probe times, and the reported reliability was the average reliability for the four tested probe times. Activity was evaluated in three time intervals: one interval 0-50 msec after probe onset (”onset”), a second interval 50-200 msec after probe onset (”steady”), and a third interval 40-115 msec after the probe ended (”offset”). Because responses in the onset intervals were so different, they were analyzed separately from responses in the steady and offset intervals.
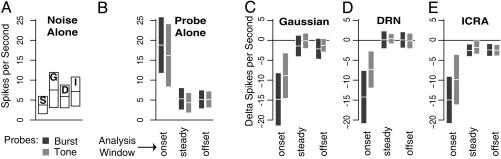
Fig. 1.
Response magnitude: noise, probes, and masking. Bars express response to test sounds, ± 95% confidence limits, in spikes per second. (A) Response to noise alone. S, silence (no noise or probe sound); G, Gaussian noise; D, DRN; I, modified ICRA noise. (B) Response to probes alone. Bars show the response to transient probe sounds in time intervals relative to tone onset. Dark bars represent the response to a Gaussian noise burst; light bars represent the response to a brief tone. (C-E) Excess masking. Bars show the difference between the observed spike rate and the spike rate predicted by the linear combination of the responses to the probe sound and ongoing Gaussian noise (C), ongoing DRN (D), or ongoing modified ICRA noise (E).
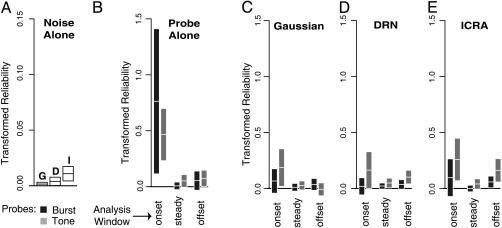
Fig. 2.
Temporal reliability: noise, probes, and scrambling. Bars express the Fisher-transformed temporal reliability of the response to test sounds, ± 95% confidence limits, in spikes per second. (A) Response to noise alone. Bars show the temporal reliability of the response to noise. G, Gaussian noise; D, DRN; I, modified ICRA noise. Note that the vertical range for A is 10 times less than the range for B-E. (B) Response to probes alone. Bars show the reliability of the response to transient probe sounds in time intervals relative to tone onset. Dark bars represent the response to a Gaussian noise burst; light bars represent the response to a brief tone. (C-E) Probe and noise. Bars show the temporal reliability of the response to transient probe sounds presented simultaneously with ongoing Gaussian noise (C), ongoing DRN (D), or ongoing modified ICRA noise (E).
An ANOVA evaluated the possibility of parametric effects of the types of noise, probe, or analysis window. If the ANOVA is conducted recognizing that the responses are repeated measurements of the same recordings in different conditions, the reported P values decrease, but only in one instance did an additional term become significant. Specific pairwise comparisons were made with a t test, and specific multiway comparisons were made by using Tukey's method for testing significance of multiple comparisons.
Masking was quantified by comparing the magnitude of the response evoked by a probe and noise together with the response predicted from the responses to probe and noise presented separately. Masking was mathematically defined as the response of (probe in noise) - (probe alone + noise alone) + spontaneous activity. Spontaneous activity was added because it was presumed to be subtracted twice, once with the probe-alone data and once with the noise-alone data.
Scrambling was evaluated by comparing the reliability of the response evoked by a probe and noise together, across repeated trials, with the reliability of the response to the probe alone. The coefficient of reliability¶ is a quantitative measure of the variance of spike time firing to repeated identical stimuli, adapted from multiple crosscorrelation analysis (13). Each single trial recording, considered as a point process (14), was convolved with a Gaussian smoothing function (standard deviation of 10 msec) truncated with a total width of three standard deviations. The reliability coefficient is the mean crosscorrelation of each smoothed single-trial spike train with every other smoothed single-trial spike train in a block of trials. If either spike train in a pair had no activity, the crosscorrelation of the pair was taken to be zero. Compared with other reliability measures (15-18), this coefficient more robustly quantifies the presence of reliable events among random phase responses. Because it is based on the crosscorrelation of responses across repeated trials, the reliability coefficient shares the limitations of crosscorrelation as a measure of the relationship between non-Poisson point processes (19, 20). The reliability coefficients reported here have been Fisher-transformed [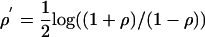 ] so that the distribution of coefficient values approximates a Gaussian and parametric statistics can be applied.
] so that the distribution of coefficient values approximates a Gaussian and parametric statistics can be applied.
Results
Ongoing noise slightly increased the ongoing activity over spontaneous activity (P < 0.05), but there was no significant effect of noise type on the increase (Fig. 1A). Temporal reliability of the activity evoked by repeated presentations of the same, frozen noise was small and significantly dependent on noise type (Fig. 2A). Simultaneous comparisons showed that the reliability in ICRA noise was greater than in DRN (P < 0.05) or in Gaussian noise (P < 0.01).
In silence, the magnitude of the onset response to tone and noise probes was similar and well above the spontaneous rate (Fig. 1B), but the magnitude of the response in the steady and offset intervals did not significantly differ from the spontaneous rate. The temporal reliability of the onset response to probe sounds was large (Fig. 2B). The temporal reliability of the onset response to the burst probe varied widely across recordings and was significantly larger for the burst probe than for the tone probe (t test, P < 0.01) but did not significantly differ from zero in the steady and offset intervals.
All noises masked probe onset responses, but, in the steady and offset intervals, excess masking was only seen with ongoing ICRA noise (Fig. 1 C-E). A noise·probe ANOVA of the probe onset response shows a significant effect of probe type (P < 0.05) but not noise type on onset response magnitude. A t test confirmed that excess masking was greater for the burst probe than for the tone probe (P < 0.05). In the steady and offset intervals, a noise·probe·interval ANOVA shows a significant effect of only noise type (P < 0.01). Simultaneous comparisons confirm that excess masking is greater in ICRA noise compared with DRN (P < 0.001). Before correcting for multiple comparisons, excess masking was greater in Gaussian noise than in DRN (P < 0.05 corrected to P = 0.064). Excess masking in ICRA noise was not significantly greater than in Gaussian noise.
In noise, probe onset responses had less temporal reliability than probe onset response in silence, especially for the burst probe. The reliability of the response to the tone probe during the offset interval was greater than expected with ongoing modulated noises (Fig. 2 C-E). A noise·probe ANOVA of the onset reliability showed a significant effect of only probe type on reliability (P < 0.01). A t test confirmed that the reliability of the tone onset response in noise was more reliable than the burst onset response (P < 0.01). In the steady and offset intervals, a noise·probe·interval ANOVA showed significant effects of noise (P < 0.05), probe (P < 0.05), interval (P < 0.01), and an interaction between noise type and phase (P < 0.05). Simultaneous comparison of all combinations of noise type, interval, and probe (66 comparisons) confirms the temporal reliability of the response to the tone probe with ICRA noise was significantly more reliable than all of the other probe responses in the steady and offset intervals, with the exception of the response to the tone probe during the steady interval with ongoing ICRA noise, for which it was still somewhat greater (P = 0.06). Before correction for multiple comparisons, the temporal reliability of the response to the tone probe during the offset interval with ongoing DRN was also greater than the reliability of many of the other responses during the steady and offset intervals.
The demonstration of masking is amplified by more detailed consideration of a specific example (Fig. 3). On average, there is an unanticipated decrease in activity in an offset time window, specific to the combination of the burst probe and ICRA noise. Other changes in temporal response pattern that vary according to the sequential position of the probe are also illustrated in Fig. 3.
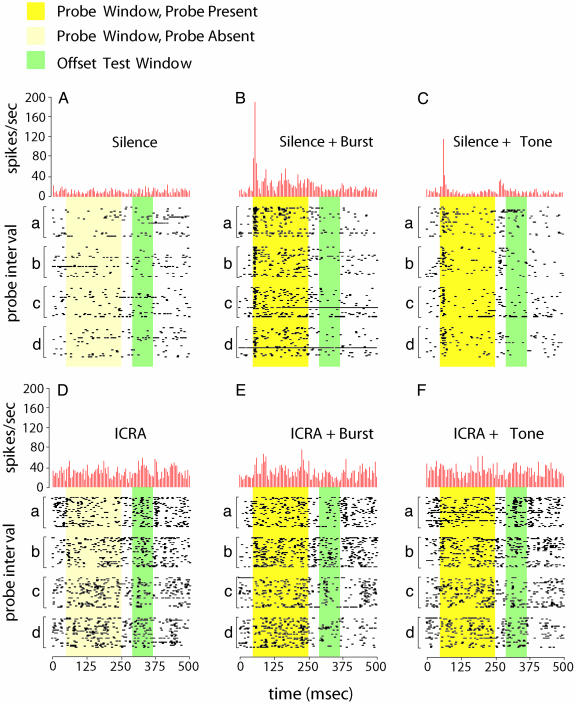
Fig. 3.
Individual recording example. Shown are the response, 500-msec time windows around the time of probe presentation. Raster subplots (a-d) show the response to 30 stimulus repetitions and are organized sequentially so that section a is drawn from an early segment of the recording, with sections b, c, and d drawn from successively later sections of the same recording. Yellow background denotes the probe presentation window, with darker yellow in panels that show when the probe was actually presented. Green background denotes an offset response analysis window. Histograms express the average firing rate across all four segments, in 5-msec bins. Details of the response patterns are described in the text. Probes: no probe (A and D); gated burst of Gaussian noise (B and E); and gated sinusoidal tone (C and F). Noise: silence (A-C) and ICRA noise (D-F).
The example of Fig. 3 demonstrates stimulus-specific masking and scrambling and that the scrambled response has aspects related to the average probe/ongoing-noise combination and to the particular placement of the probe sound within the noise. In silence, the magnitude of the onset response to both probes was large, and there was an offset response immediately after the tone probe. For neither probe did the spike count in the offset test interval differ from spontaneous activity. In ICRA noise, the response to the tone was effectively masked, and, in particular, the spike count in the offset test interval did not differ from that in the comparable interval for ICRA noise with no probe (P > 0.01). The count in the offset test interval for the combined ongoing ICRA noise and Gaussian probe was significantly less than the count in the corresponding interval for both the ICRA alone and the ICRA plus tone conditions. This recording had offset attenuation only during the ICRA noise, not during the Gaussian or ripple noises. Closer examination reveals reliable unexpected distinctions between the ICRA plus burst and ICRA alone conditions on a probe-by-probe basis. Only for probe b was there a reliable offset response just after the probe offset. Only for probe c did the offset suppression extend to a more reliable response near the end of the plotted interval. Only for probe d did the offset response suppression extend through the entire plotted interval. During the interval when the Gaussian probe is present, response features resemble features in the corresponding probe plus silence and ICRA alone conditions, but the highly reliable onset response is conspicuously absent.
Discussion
This paper demonstrates that in the auditory thalamus, noise not only attenuates the probe-evoked neuronal response but also changes its temporal response pattern. Attenuating the neuronal response, either by decreasing the total number of evoked spikes or because of decreasing response modulation relative to ongoing activity, makes the evoked response less obvious and is a likely correlate of the complex neurophysiological processes that take place during psychophysical masking (21). Changing the temporal response pattern may change how downstream circuits interpret the neuronal activity. Such circuits still receive an obvious message, but the message is scrambled. This model may be a correlate of the ability to hear, but not interpret, complex sound sequences in noisy environments.
As expected, noise adds spikes to the recording's spike train and makes the response to probe sounds less obvious. Noise masks the response to the probe sound not only by placing the probe response on a higher background but by decreasing the incremental number of spikes evoked at probe onset. For ICRA noise, this decrease extends, on average, through the steady and offset periods.
Interestingly, modulated noise also scrambles the probe-evoked response pattern, changing both response magnitude and response timing. On average, the temporal reliability of the onset response is decreased by all three noises. During the offset interval, the reliability of the response to the tone probe is on average significantly reliable in the modulated noises, especially in ICRA noise. In the detailed example, the temporal pattern was stimulus specific, changing only for one of the two probe stimulus classes. An entirely new type of response component appeared, which was different in both timing and sign, and only appeared in one of three noise conditions. Repeatable, distinct temporal response patterns were observed for identical probe sounds in different contexts. Modulated neuronal activity signals that a sound is present in silence and in the presence of noise, but the neurons communicate different messages in different specific contexts.
The consistent feature in the detailed example is a suppression of the response to the ongoing noise after the offset of a Gaussian probe, in a time window corresponding to a temporal frequency of slightly less than 10 Hz. Thalamocortical dynamics on this time scale have been posited to play an important role in temporal integration, and the extraction of sequence information from a continuous sensory stream, as in the parsing of speech sounds (22). Disruption of such dynamics is thought to underlie certain language learning impairments (23). Some authors have argued that such dynamics are characteristic only of the sleeping and anesthetized states, rather than the awake state (24); a counterexample is provided here.
It is interesting for human ecology that the most extreme effects were seen with the modified ICRA noise (1, 11). This noise, an International Organization for Standardization draft standard, was originally developed by audiologists to improve their assessment of hearing aid patients' ability to understand speech in noise and allow fair comparisons of performance regardless of the patient's native language. ICRA noise, on average, induced both more temporally extended masking and the most clear change in the reliability of the response to a probe tone in the offset window. Given the design of this experiment, it is not clear what features of the ICRA noise make its effects extreme. One way to extend this finding would be to test sets of designed noises. One set might have speech-like temporal modulation, varying local spectral contrast, and another might have speech-like spectral contrast, varying temporal modulation. It would be interesting to compare the spectrotemporal modulation of the best masker for an individual neuron with its acoustic spectrotemporal receptive field parameters. We note, however, that on average there was no significant effect of ongoing noise type on masking in the probe onset interval.
A common complaint in noisy environments is that a listener can hear, but not understand or interpret, complex sounds, such as speech. These observations demonstrate that modulated background noise can scramble the temporal response pattern to simple probe sounds, which may be the direct neural correlate of this unfortunate experience.
Acknowledgments
We thank Mark Kvale (University of California, San Francisco) for the spike-sorting program. This work was supported by the Whitaker Foundation and the Evelyn F. and William L. McKnight Brain Institute.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ICRA, International Collegium of Rehabilitative Audiology; DRN, dynamicripple noise; MGB, medial geniculate body.
Footnotes
Martin, E. M. & Bedenbaugh, P. H., 34th Annual Meeting of the Society for Neuroscience, Oct. 23-27, 2004, San Diego, Session 305.11 (abstr.).
Martin, E. M., West, M. F. & Bedenbaugh, P. H., 31st Annual Meeting of the Society for Neuroscience, Nov. 10-15, 2001, San Diego, Session 823.3 (abstr.).
References
- 1.Dreschler, W. A., Verschuure, H., Ludvigsen, C. & Westermann, S. (2001) Audiology 40, 148-157. [PubMed] [Google Scholar]
- 2.Escabi, M. A. (2000) Dissertation (Univ. of California, San Francisco).
- 3.Marmarelis, P. Z. & Marmarelis, V. Z. (1978) Analysis of Physiological Systems: The White Noise Approach (Plenum, New York).
- 4.Calford, M. B. & Webster, W. R. (1981) J. Neurophysiol. 45, 1013-1028. [DOI] [PubMed] [Google Scholar]
- 5.Miller, L. M., Escabi, M. A., Read, H. L. & Schreiner, C. E. (2002) J. Neurophysiol. 87, 516-527. [DOI] [PubMed] [Google Scholar]
- 6.Calford, M. B. (1983) J. Neurosci. 3, 2350-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linden, J. F. & Schreiner, C. E. (2003) Cereb. Cortex 13, 83-89. [DOI] [PubMed] [Google Scholar]
- 8.Nicolelis, M. A. L., Ghazanfar, A. A., Faggin, B. M., Votaw, S. & Oliveira, L. M. O. (1997) Neuron 18, 529-537. [DOI] [PubMed] [Google Scholar]
- 9.Musial, P. G., Baker, S. N., Gerstein, G. L., King, E. A. & Keating, J. G. (2002) J. Neurosci. Methods 115, 29-43. [DOI] [PubMed] [Google Scholar]
- 10.Lewicki, M. S. (1994) Neural Comput. 6, 1005-1030. [Google Scholar]
- 11.Westermann, S. (1997) ICRA Noise Signals [audio compact disc] (Widex, Vaerloese, Denmark), Version 0.3.
- 12.Fay, R. R. (1988) Hearing in Vertebrates: A Psychophysics Databook (Hill-Fay, Chicago).
- 13.Lees, J. M. (1998) Bull. Seismol. Soc. Am. 88, 1127-1143. [Google Scholar]
- 14.Perkel, D. H., Gerstein, G. L. & Moore, G. P. (1967) Biophys. J. 7, 391-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mainen, Z. F. & Sejnowski, T. J. (1995) Science 268, 1503-1506. [DOI] [PubMed] [Google Scholar]
- 16.Victor, J. D. & Purpura, K. P. (1996) J. Neurophysiol. 76, 1310-1326. [DOI] [PubMed] [Google Scholar]
- 17.Hunter, J. D., Milton, J. G., Thomas, P. J. & Cowan, J. D. (1998) J. Neurophysiol. 80, 1427-1438. [DOI] [PubMed] [Google Scholar]
- 18.Tiesinga, P. H. E., Fellous, J. M. & Sejnowski, T. J. (2002) Neural Comput. 14, 1629-1650. [DOI] [PubMed] [Google Scholar]
- 19.Bedenbaugh, P. H. & Gerstein, G. L. (1994) Biol. Cybern. 70, 219-225. [DOI] [PubMed] [Google Scholar]
- 20.Bedenbaugh, P. H. & Gerstein, G. L. (1997) Neural Comput. 9, 1265-1275. [DOI] [PubMed] [Google Scholar]
- 21.Delgutte, B. (1996) in Physiological Models for Basic Auditory Percepts, eds. Hawkins, H. L., McMullen, T. A., Popper, A. N. & Fay, R. R. (Springer, New York), pp. 157-220.
- 22.Merzenich, M. M., Schreiner, C. E., Jenkins, W. M. & Wang, X. (1993) Ann. N.Y. Acad. Sci. 682, 1-22. [DOI] [PubMed] [Google Scholar]
- 23.Merzenich, M. M., Jenkins, W. M., Johnston, P., Schreiner, C., Miller, S. L. & Tallal, P. (1996) Science 271, 77-81. [DOI] [PubMed] [Google Scholar]
- 24.Steriade, M. (2001) Nat. Neurosci. 4, 671 (lett.). [DOI] [PubMed] [Google Scholar]