Abstract
Parkinson's disease is a debilitating neurodegenerative disorder that is pathologically characterized by intracellular inclusions comprised primarily of alpha-synuclein (αSyn) that can also be transmitted from neuron to neuron. Several lines of evidence suggest that these inclusions cause neurodegeneration. Thus exploring strategies to improve neuronal survival in neurons with αSyn aggregates is critical. Previously, exposure to αSyn pre-formed fibrils (PFFs) has been shown to induce aggregation of endogenous αSyn resulting in cell death that is exacerbated by either starvation or inhibition of mTOR by rapamycin, both of which are able to induce autophagy, an intracellular protein degradation pathway. Since mTOR inhibition may also inhibit protein synthesis and starvation itself can be detrimental to neuronal survival, we investigated the effects of autophagy induction on neurons with αSyn inclusions by a starvation and mTOR-independent autophagy induction mechanism. We exposed mouse primary cortical neurons to PFFs to induce inclusion formation in the presence and absence of the disaccharide trehalose, which has been proposed to induce autophagy and stimulate lysosomal biogenesis. As expected, we observed that on exposure to PFFs, there was increased abundance of pS129-αSyn aggregates and cell death. Trehalose alone increased LC3-II levels, consistent with increased autophagosome levels that remained elevated with PFF exposure. Interestingly, trehalose alone increased cell viability over a 14-d time course. Trehalose was also able to restore cell viability to control levels, but PFFs still exhibited toxic effects on the cells. These data provide essential information regarding effects of trehalose on αSyn accumulation and neuronal survival on exposure to PFF.
Abbreviations: AraC, cytosine arabinoside; αSyn, α-synuclein; BSA, bovine serum albumin; Con, control; CQ, chloroquine; DIV, days in vitro; DMSO, dimethyl sulfoxide; E. coli, Escherichia coli; FBS, fetal bovine serum; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GFAP, glial fibrillary acidic protein; HMW, high molecular weight; HSC70, heat shock cognate 70; HSP104, heat shock protein 104; ICC, immunocytochemistry; KO, knockout; LAMP1, lysosomal-associated membrane protein 1; LAMP2A, lysosome-associated membrane protein 2A; LC3, microtubule-associated proteins 1 light chain 3; MAP2, microtubule-associated protein 2; mTOR, mammalian target of rapamycin; MTT, , 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide); p-αSyn, phosphorylated-α-synuclein; PBS, phosphate buffered saline; PD, Parkinson's disease; Penn/Strep, Penicillin/Streptomycin; PFFs, pre-formed fibrils; PLL, poly-l-lysine; Pon S, ponceau S; p-S129, phosphorylated serine 129; PVDF, polyvinylidene difluoride; RIPA, radioimmunoprecipitation assay buffer; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; TFEB, transcription factor EB; Tre, trehalose; TX-100, triton X-100; US FDA, United States Food and Drug Administration; WT, wildtype
Keywords: Parkinson's disease, Alpha-synuclein fibrils, P-alpha-synuclein trehalose, Autophagy, LC3-II
1. Introduction
Parkinson's disease (PD) is a neurodegenerative condition that clinically manifests with abnormalities in movement, olfactory senses, gastrointestinal function and an overall decline in cognitive abilities as the disease progresses. These symptoms are associated with the progressive loss of dopaminergic neurons in the substantia nigra [1]. Sharing with other neuropathologies, including dementia with Lewy bodies and multiple systems atrophy, the majority of PD patient postmortem brains exhibit accumulation of α-synuclein (αSyn) protein in highly phosphorylated, ubiquitinated and insoluble aggregates observed as Lewy bodies and Lewy neurites [2], [3], [4]. Recent studies indicate that αSyn may be released from neurons and seed aggregations in adjacent neurons thus propagating disease progression [5].
Recently studies have started to investigate how αSyn intracellular inclusion formation impacts recipient neuronal survival using a model in which αSyn pre-formed fibrils (PFFs) can enter the cell and recruit endogenous αSyn in the neuron to form insoluble, ubiquitinated and phosphorylated high molecular weight αSyn, characteristic of those in human Lewy bodies and Lewy neurites [5], [6], [7]. Since one of the strategies to help maintain intracellular protein homeostasis is through macroautophagy [8], determining the impact of macroautophagy regulators on accumulation of αSyn aggregates and neuronal survival in response to PFF exposure is thus essential to understanding PD.
Autophagy can be activated by the inhibition of the mammalian target of rapamycin (mTOR) by rapamycin. Many in vitro and in vivo studies have reported positive results using rapamycin to mitigate the effects of αSyn toxicity [9]. However, clinical use of rapamycin carries with it disadvantages that make its prolonged use in humans undesirable [10]. In addition, previous studies have demonstrated that autophagy induction by starvation or rapamycin post PFF transduction did not decrease PFF-induced intracellular αSyn aggregations and further exacerbated cell death [6]. Given that targeting of mTOR has limited therapeutic applications in humans in the realm of neurodegenerative treatments, compounds that activate autophagy independently of mTOR have been tested. One compound that has been found to be effective in activating autophagy in cell lines is the disaccharide trehalose [11], which appears to mediate its effects through both initiation of autophagy and activation of TFEB, turning on genes for increased lysosomal biogenesis [12], [13], [14]. Important for our study, neurons lack the enzyme trehalase that breaks down trehalose, thus the level of trehalose should be persistent over long periods of time in culture [15]. In humans, a single bolus of 50 g or less has been deemed safe, and in 2000 the US FDA gave notice that trehalose is generally regarded as safe for human consumption [16]. Although its administration has not been studied in the context of humans and PD, trehalose decreases α-synuclein in PC12 cells [11], decreases Tau accumulation in mice [17], and reduces neurodegeneration in amyotrophic lateral sclerosis [18], Huntington's [19], and Alzheimer's [20] disease models. In this study we specifically test the effects of trehalose on decreasing αSyn aggregation and cell death upon exposure to PFFs, thus helping to evaluate the therapeutic potential of trehalose in PD.
2. Methods
2.1. Cell culture
Primary wildtype (C57BL/6 strain bred in house from mice ordered from Charles River and WT mice bred from a cathepsin D knockout colony) [21], [22], [23], or αSyn knockout (The Jackson Labs C57BL/6N-Sncatm1Mjff/J) [24] cortical mouse neurons were derived from p0 pups. All mouse experiments were done in compliance with the University of Alabama at Birmingham Institutional Animal Care and Use Committee guidelines (IACUC09019 and IACUC20354). Briefly, cells were seeded on plates coated with Poly-L-Lysine solution containing 0.1 mg/mL Poly-L-Lysine, 50 mM Boric acid, and 10 mM Borax for a minimum of 1 h. Cells were grown and maintained in Neural Basal A medium supplemented with Glutamax, Penn/Strep, and B27 neuronal supplement. Treatments were performed by removing half the media and adding back the same volume of media with the treatment media. Tissue culture medium and reagents were obtained from Life Technologies. Trehalose (T0167–25G), chloroquine (C6628–25G) sucrose (S-5016) and AraC (C1768-500MG) were obtained from Sigma.
2.2. Generation of αSyn PFFs
A human wildtype αSyn gene was cloned into pRK172 and expressed in E. coli as previously reported [25]. Bacteria grown under antibiotic selection were harvested, homogenized and dialyzed before purification through size exclusion and ionic exchange columns. Five mg/mL of protein was incubated at 37 °C for 1 week to produce fibrils. Before applying to the cells, fibrils were sonicated 60 times over 40 s [6]. Using the Pierce LAL chromogenic endotoxin quantification kit, we have determined that ≤0.004 ng/mL of endotoxin was present in the PFF samples.
2.3. Cell viability
Cell viability was measured in two ways. First, viability was measured utilizing the trypan blue exclusion method. Cells were trypsinized and then trypan blue was added to the cells. Cells excluding the dye were counted. Second, viability was measured using the MTT cell death/proliferation assay. Briefly, a media and tetrazolium salt mixture was added to the cells where it is reduced to insoluble formazan crystals. These crystals were dissolved in DMSO and measured at 550 nm using a plate reader. Cells were plated on 96-well plates at 80,000 cells per well for both assays.
2.4. Immunocytochemistry
Cells were seeded at 240,000 cells per well on autoclaved glass coverslips that were placed in 24-well plates. After treatment, cells were fixed with a mixture of 4% paraformaldehyde and 4% sucrose. The addition of 1% Triton X-100 to the fixative was used to determine the soluble from insoluble protein in the cell. After fixing, cells were permeabilized with 0.1% Triton X-100 and then blocked with 3% BSA in PBS. Cells were probed with antibodies for p-Ser-129 using either Affinity Bioreagents (PA1-4686 1:2000) for Fig. 1G or Covance-81A (MMS-5091 1:5000) for the remaining figures. For non-αSyn staining, cells were plated and fixed as above but were blocked with 10% horse serum and 5% FBS in PBS. Cells were probed with either LC3 (L8918 Sigma 1:500), MAP2 (Sigma M4403 1:1000), or GFAP (Dako Z0334 1:500). Alexa Fluor 488 (Invitrogen A11001, A11008 1:500) or 568 (Invitrogen A11004 1:500) secondary antibodies were subsequently added to the wells. Cells were then counter stained with nuclear dye Hoechst 33342 (Sigma 861405) and mounted with Fluoromount-G (Southern Biotechnology). All images were acquired using a Leica TCS SP5 V confocal laser scanning microscope.
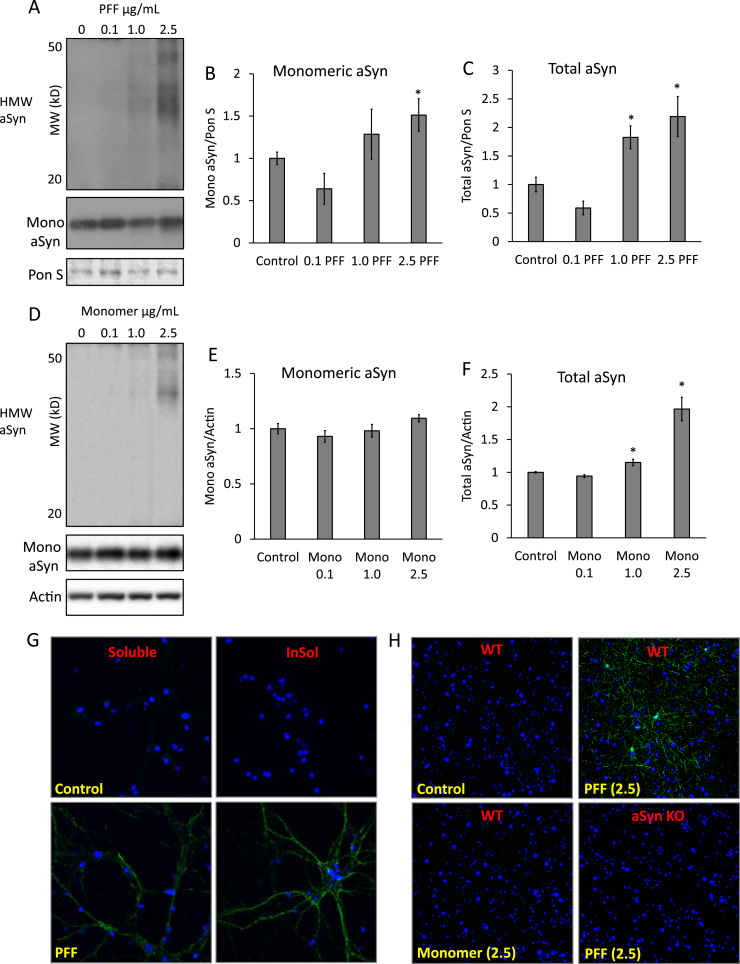
Fig. 1.
PFFs induce high molecular weight (HMW) and insoluble aggregates. (A) Western blot analysis of total monomeric and HMW αSyn from primary cortical neurons treated for 14 days with PFFs. (B, C) Quantification of western blots from A. (D) Western blot analysis of total and monomeric αSyn of primary cortical neurons exposed to αSyn monomer for 14 days. (E, F) Quantification of western blots D. Data represent mean±SEM (n=3). *p<0.05 compared to control; the Student t-test. (G) Cortical neurons exposed to 2.5 µg/mL PFF for 14 days and then probed for p-αSyn (green) in the presence (InSol) or absence (Soluble) of 1% Triton X-100 and counter stained with Hoechst (blue). Images were taken at 63× magnification. (H) Wildtype or αSyn knockout neurons exposed to vehicle, PFF, or αSyn Monomer at 2.5 µg/mL for 14 days and then probed for 1% Triton X-100 insoluble p-αSyn (green) and counterstained with nuclear dye Hoechst (blue), 40× magnification. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.5. Western blot analysis
For immunoblot analysis, cells were plated at 240,000 cells per well on 48 well plates or 480,000 per well on 24 well plates and then washed with ice cold PBS and then lysed with RIPA buffer (50 mM Tris pH 7.8, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100%, and 0.1% SDS) in the presence of protease (Roche) and phosphatase inhibitors (Sigma) after treatments. After 20 min on ice, cells were scraped from the wells and placed in 1.5 mL Eppendorf tubes for centrifugation at 16,800×g for 20 min at 4 °C. Protein content in supernatant was determined by DC Protein assay (Bio-Rad). Equal amounts of protein for each sample were loaded and separated by SDS-PAGE using 12% or 15% gels. Protein was wet-box transferred to PVDF membranes and probed with the following antibodies: LC3 (Sigma L8918 1:2000), p62 (Abnova H00008878-M01 1:2000), α-synuclein (Santa Cruz sc-7011-R 1:2500), and β-actin (Sigma A5441 1:2500). Blots were visualized by film or Amersham™ Imager 600 (GE Healthcare Biosciences; Pittsburg PA).
2.6. Statistical analysis
All data are reported as the mean±SEM, p values of less than 0.05 were deemed statistically significant after being analyzed by Student's t-test or ANOVA.
3. Results
3.1. αSyn Pre-formed fibrils (PFF) induce intracellular αSyn protein aggregation
To measure the dose-dependent effects of extracellular exposure to αSyn pre-formed fibrils, we cultured primary cortical neurons from mouse pups, and at DIV7 exposed them to human wildtype αSyn PFFs for 14 days at concentrations 0.1–2.5 µg/mL for western blot analysis of total αSyn and immunocytochemistry for insoluble S129 p-αSyn. Western blot analysis revealed a small but significant increase in the monomeric αSyn at 2.5 µg/mL of PFFs. An approximately 2-fold increase of total αSyn (monomeric and high molecular weight αSyn) was found in response to PFF at concentrations of 1.0 and 2.5 µg/mL (Fig. 1A-C). Whether accumulation of αSyn is more severe in response to PFF, compared to un-aggregated monomeric αSyn, was determined by exposing cells to similar concentrations of monomeric αSyn. In the latter case, we observed no changes in the αSyn monomer, whereas a modest increase of total αSyn was observed with 1 µg/mL and 2-fold increase with exposure to 2.5 µg/mL monomeric αSyn (Fig. 1D-F).
To determine if these αSyn aggregates shared common features with both classical Lewy bodies and previously characterized PFF-induced aggregates [5], [6], [7], we performed immunocytochemistry analyses after exposing fixed cells to 1% Triton X-100 [6]. As shown in Fig. 1G, S129 phosphorylated αSyn that is insoluble in 1% Triton X-100 was significantly increased in response to 2.5 µg/mL PFF. Under the same condition, exposure of cells to 2.5 µg/mL of αSyn monomer resulted in no p-αSyn accumulation as demonstrated by immunocytochemistry analyses. αSyn knockout cells exposed to 2.5 µg/mL PFFs also resulted in no insoluble p-αSyn under the same condition indicating endogenous αSyn is required for accumulation of insoluble p-αSyn (Fig. 1H).
3.2. Effects of PFFs on autophagy markers
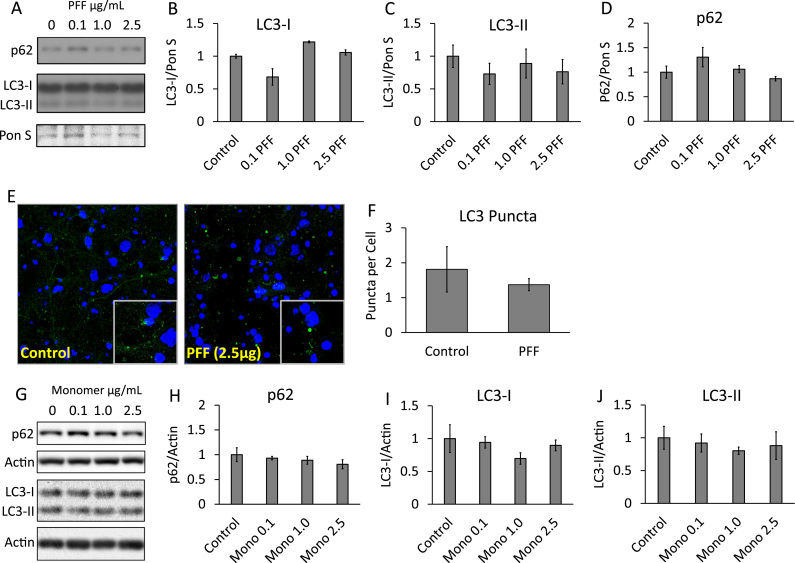
Previous studies have demonstrated that mouse primary hippocampal neurons exposed to 5 µg PFFs for 14 days exhibit increased LC3-II and levels of Triton X-100 insoluble p62 [26]. To determine whether this occurs at a lower concentration with primary neurons from a different brain region, we performed western blot analyses of LC3 and p62 after exposing primary cortical neurons to PFFs for 14 days (Fig. 2). We found that increasing concentrations of PFFs up to 2.5 µg did not significantly change LC3-II or total levels of soluble p62 (Fig. 2A-D). The levels of LC3-II were confirmed by immunocytochemistry, where LC3-II puncta per cell were counted and no significant difference was observed between control and cells exposed to PFFs (Fig. 2E, F). Nor did we observe changes in either p62 or LC3-II in cells exposed to non-aggregated αSyn monomer for 14 days (Fig. 2G-J).
Fig. 2.
PFFs do not significantly alter the levels of autophagosomal protein LC3-II or autophagy adaptor/substrate protein p62. (A) Western blot analysis of LC3-I, LC3-II, and p62 from primary cortical neurons exposed to PFFs for 14 days. (B-D) Quantification of western blots from A. (E) Immunocytochemistry of cells exposed for 14 days to 2.5 µg/mL PFFs and probed for LC3 (green) and counterstained with nuclear dye Hoechst (blue). Images were taken at 63×. (F) Quantification of images from E. (G) Western blot analysis of LC3-I, LC3-II, and p62 from primary cortical neurons exposed to αSyn monomer for 14 days. (H-J) Quantification of western blots from G. Data represent mean±SEM (n=3). *p<0.05 compared to control; the Student t-test. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Effects of trehalose on neuronal autophagy
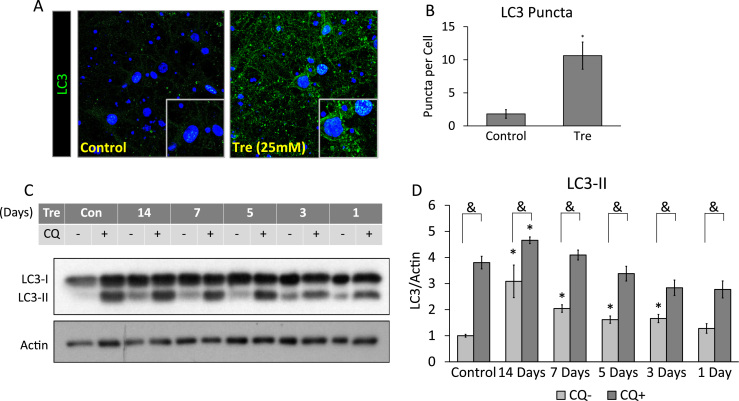
Previous studies have demonstrated that autophagy induction by starvation or rapamycin post PFF transduction did not decrease PFF-induced intracellular αSyn aggregations and only exacerbated cell death [26]. Here we investigated whether induction of autophagy using trehalose attenuates PFF-induced αSyn accumulation thus enhancing neuronal survival. We exposed neurons to 25 mM trehalose for 14 days and found significant increases in LC3 puncta as assessed by immunocytochemistry analyses (Fig. 3A, B).
Fig. 3.
Trehalose increases LC3-II and autophagic flux. (A) Immunocytochemistry of cells exposed for 14 days to 2.5 µg/mL PFFs and probed for LC3 (green) and counterstained with nuclear dye Hoechst (blue). Images were taken at 63×. (B) Quantification of images from A. (C) Autophagic flux analysis of neurons exposed for up to 14 days to PFF with and without trehalose and then to 40 µM chloroquine (CQ) 5 h before protein collection. Control (Con) was 14 days without Trehalose. (D) Quantification of western blot C. Data represent mean±SEM (n=3). *p<0.05 compared to control; the Student t-test. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To determine if autophagic flux through the autophagy pathway was increased, we exposed cells from 1 to 14 days with 25 mM trehalose, and then with 40 µM Chloroquine (CQ) for 5 h which prevents lysosomal degradation of autophagosomes. We found that trehalose alone significantly increased LC3-II beginning at 3 days that continued to increase until the conclusion of the experiment at 14 days. CQ induced significant increases in LC3-II over non-CQ treated neurons for all time points. However, only at 14 days did the trehalose and CQ treated cells exhibit significantly more LC3-II than the CQ only control cells, indicating increased autophagic flux at 14 days (Fig. 3C, D).
3.4. Effects of trehalose on αSyn protein aggregation and autophagy
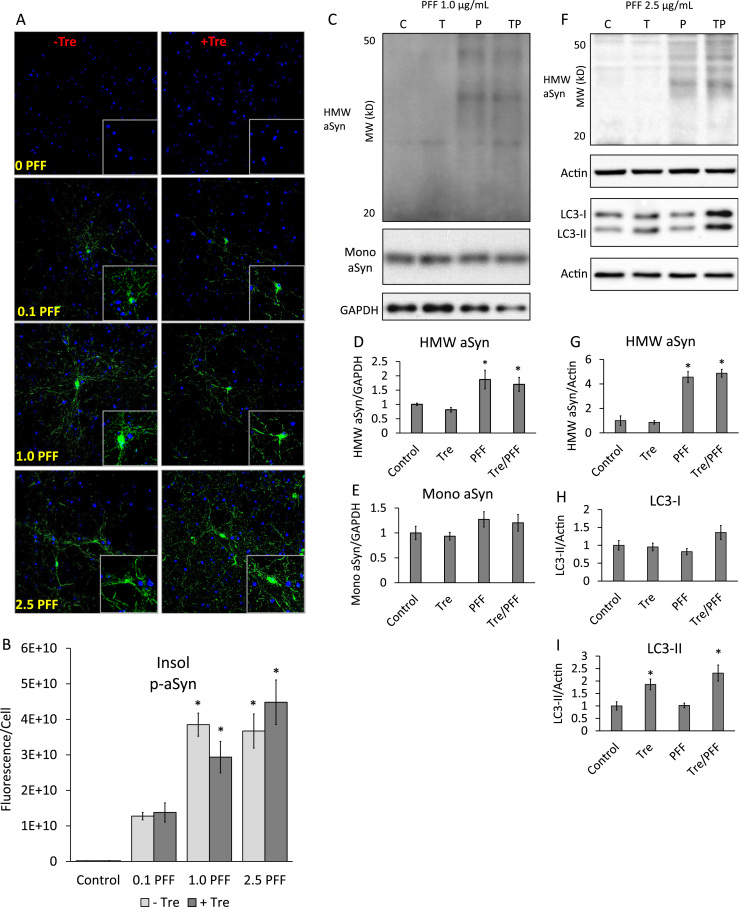
To determine if trehalose impacts PFF-induced αSyn aggregation, we added 25 mM trehalose to cells at the time of PFF exposure and performed immunocytochemistry of 1% Triton X-100 insoluble p-αSyn over a range of PFF concentrations with and without trehalose for 14 days. Without trehalose, there was a strong trend toward increased p-αSyn at 0.1 µg/mL that became significant at 1.0 µg/mL and remained at similar levels at 2.5 µg/mL. In the presence of trehalose, similar trends of p-αSyn were observed with significant increases seen at 1.0 µg/mL and continuing to 2.5 µg/mL similar to cells without trehalose. No statistical differences were observed for cells exposed to PFFs with or without trehalose over a range of PFF concentrations, indicating that trehalose failed to remove p-αSyn aggregates (Fig. 4A, B). To further confirm these findings, we measured total αSyn in the presence or absence of both trehalose and PFFs by western blot. Trehalose exposure alone for 14 days did not change endogenous αSyn levels, while at 1.0 µg/mL (Fig. 4C-E) or 2.5 µg/mL (Fig. 4F, G) PFFs significantly increased total high molecular weight αSyn, even in the presence of trehalose, again suggesting trehalose fails to remove PFF induced aggregations.
Fig. 4.
Trehalose fails to remove PFF-induced aggregations. (A) Immunocytochemistry of primary cortical mouse neurons exposed to PFFs (0, 0.1, 1.0, 2.5 µg/mL)±25 mM trehalose for 14 days and probed for 1% Triton X-100 insoluble phosphorylated αSyn (green) and counterstained with nuclear dye Hoechst (blue), 40× magnification. (B) Quantification of total fluorescence from PFF exposed cells normalized to nuclei per field. Data represent mean±SEM (n=3). *p<0.05 to control; 2-way ANOVA. (C) Western blot analysis of cortical neurons exposed for 14 days to 1 µg/mL PFFs±25 mM trehalose. (D, E) Quantification of western blots C. (F) Western blot analysis of cortical neurons exposed for 14 days to 2.5 µg/mL PFFs±25 mM trehalose. (G-I) Quantification of western blots F. Data represent mean±SEM (n=3–4). *p<0.05 compared to control; the Student t-test. C=Control, T=Trehalose, P=PFF, TP=Both. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Noting that trehalose alone increases LC3-II (Fig. 3), we measured whether this is still the case in the presence of PFF by western blot analyses lysates from cells exposed for 14 days to 25 mM trehalose, 2.5 µg/mL PFFs, or both. No significant changes were noted in LC3-I. However, we again observed increases in LC3-II with trehalose alone but not PFF alone. LC3-II remained elevated in the presence of both trehalose and PFFs at similar levels as trehalose alone (Fig. 4F, H, I).
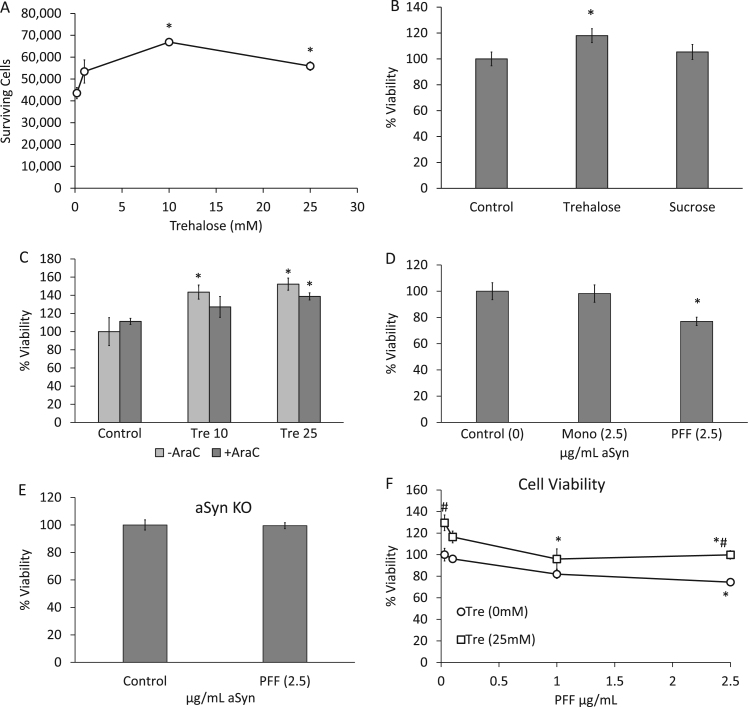
3.5. Trehalose impacts cell viability
Although trehalose does not appear to remove αSyn aggregations, it may help remove toxic species of αSyn that are below detection sensitivity. Alternatively, it may physically interact with the αSyn aggregates reducing their toxicity or assisting in clearing cellular organelles damaged by αSyn, and thus attenuate cell death. To test whether trehalose alone alters the basal viability of neurons in culture, we exposed primary neurons to 3 different concentrations of trehalose over a 15-d period. We saw no change in cell viability at 1 mM but beginning at 10 mM and continuing to 25 mM we saw significant increases in cell viability with trehalose exposure alone (Fig. 5A). Next we determined if the enhancement of viability was specific to trehalose alone or if another disaccharide could have similar effect. We thus compared cell viability after exposing cells to either trehalose or a similar disaccharide, sucrose, for 14 days both at 25 mM concentrations. Sucrose had no impact on cell viability while 25 mM trehalose increased the number of surviving cells (Fig. 5B).
Fig. 5.
Effects of trehalose on cell viability. (A) Viability of primary cortical neurons exposed to 1, 10, or 25 mM trehalose for 15 days was determined by trypan blue exclusion assays and plotted. *p<0.05 (n≥3). (B) Viability of primary cortical neurons exposed to either 25 mM trehalose or 25 mM sucrose for 14 days was determined by trypan blue exclusion assays. Data represent mean±SEM (n≥3). *p<0.05 compared to control. (C) Viability of primary neurons exposed to trehalose in the presence or absence of 2.5 µM cytosine arabinoside (AraC) was determined by trypan blue exclusion. Data represent mean±SEM (n≥3). *p<0.05 compared to control. (D) Viability of primary cortical neurons treated with 2.5 µg/mL αSyn monomer or PFF for 14 days was determined by MTT. Data represent mean±SEM (n=5). *p<0.05 compared to control; 1-way ANOVA. (E) Viability of αSyn KO neurons exposed to control or 2.5 µg/mL PFF for 14 days was determined by MTT. Data represent mean±SEM (n=6). Student t-test. (F) Viability of primary neurons exposed to PFFs plus or minus 25 mM trehalose for 14 days was determined by MTT. Data represent mean±SEM (n=5). *p<0.05 compared to respective control (no PFFs). #p<0.05 between + and - trehalose treated cells; 2-way ANOVA.
It is currently unknown whether trehalose will increase glial cell proliferation in neuronal culture, even while using Neurobasal medium without serum. To rule out the possible effects of trehalose inducing glial proliferation, we used a glial cell proliferation inhibitor, cytosine arabinoside (AraC). As before, trehalose increased cell viability at 10 and 25 mM. At 10 mM trehalose, AraC slightly blunted cell viability but still trended upwards. At 25 mM trehalose, AraC did not blunt cell viability, indicating that glial proliferation is not responsible for the enhanced viability in response to trehalose (Fig. 5C). Furthermore, we confirmed this finding by comparing the number of GFAP positive cells to the total number of nuclei and determined that only approximately 5% of cultures stained positive for GFAP and that this number did not change with Trehalose or PFF exposure (Supplemental Fig. 1).
To determine if PFFs induce cell death and are more toxic than monomeric αSyn, we compared cells exposed to both 2.5 µg/mL αSyn monomer and PFFs for 14 days. We found that PFFs induced significant cell loss, while treatment with αSyn monomer had no effect on cell viability, indicating that αSyn protein in its monomeric state is not sufficient to decrease viability (Fig. 5D). Additionally, cells from αSyn KO mice exposed to PFFs did not show any decrease in cell viability, further demonstrating that endogenous αSyn is required to potentiate cell toxicity in response to PFFs (Fig. 5E). To determine if trehalose would enhance cell survival in the presence of PFFs, we exposed cells for a period of 14 days to 3 concentrations of PFFs with or without 25 mM trehalose. Using an MTT based cell death assay, we measured the effect of PFFs on cell viability. We observed that at 2.5 µg/mL PFFs decreased cell viability. In cells exposed to trehalose, there was an increase in basal cell viability after 14 days as observed above, and an increase in cell viability after 2.5 µg/mL PFF+trehalose compared to the same concentration of PFF -trehalose (Fig. 5F). Cell survival is comparable in +trehalose+PFF compared to –PFF-trehalose. However, in the presence of trehalose, PFFs still induced cell death compared to +trehalose+PFF indicating that PFF toxicity still persists.
4. Discussion
In this study we have shown that exogenously applied αSyn pre-formed fibrils (PFFs) induce significant protein aggregation in primary neurons (Fig. 1A-C, G) consistent with prior reports [6], [7]. The addition of αSyn monomer does not induce S129 phosphorylated insoluble inclusions in primary neurons (Fig. 1D-F, H). Furthermore, endogenous αSyn is required for the propagation of PFFs as αSyn KO neurons exposed to 2.5 µg/mL PFFs do not accumulate insoluble S129 phosphorylated αSyn inclusions (Fig. 1H).
In our study, PFFs did not change levels of p62 and LC3-II as previous studies have shown (Fig. 2A-D). This is likely due to a lower amount of PFFs added, and that in our system we used cortical neurons where Lewy body pathology occurs later in Parkinson's disease [27]. Additionally, our cultures were isolated from postnatal mice opposed to embryonic cultures, thus it is possible that a higher threshold is required for PFFs to attenuate autophagy. By these measures our model used a less severe exposure to PFFs and concurrently we observed a less severe phenotype.
Most studies in cell cultures use acute high doses of ≥100 mM trehalose, demonstrating in the short term that trehalose enhances autophagy. In these models, up-regulation of autophagy was implicated as being protective [28], [29]. However, in animal models, chronic trehalose exposure is necessary, and may also be for future patients. To determine the effects of chronic lower concentrations of trehalose in culture, we for the first time show a low concentration of 25 mM trehalose over a 14-d period enhances autophagic flux and increases basal cell viability. And while trehalose in the presence of PFFs was unable to enhance viability to that of cells exposed only to trehalose, it was able to restore viability to the level of untreated controls (Fig. 5F). This protective effect is intriguing especially that the survival of the minor population of astrocytes was not changed by either PFF or trehalose.
It is currently unclear how trehalose enhances cell survival. Trehalose increased LC3-II in the absence and presence of PFF (Fig. 4I-K). Nonetheless, trehalose has been shown to alter the formation of A53T fibrils, tau fibrils, amyloid-beta and polyQ proteins [19], [20], [29], [30], [31], and thus is proposed to act as a molecular chaperone. In yeast, it has been shown that under the protein misfolding and aggregation stress of desiccation, trehalose is essential for maintaining viability by stabilizing cellular proteins, similar in function to HSP104 [32]. It also can interact in synergy with existing chaperones, as is the case with its interaction with p26 to maintain functional citrate synthase protein during heat shock in vitro [33]. Furthermore, unlike rapamycin, trehalose did not exacerbate PFF toxicity. Additionally, trehalose has been shown effective in ameliorating symptoms in an A53T AAV rat model of PD administered via drinking water, suggesting that trehalose may also protect against PFF-induced neurodegeneration in animal models [34]. Nonetheless, this study corroborates previous work that PFFs seem to resist degradation even with enhancement of macroautophagy. Future studies are still urgently needed to identify novel pathways that promote αSyn degradation and enhance neuronal survival in response to exposure to αSyn fibrils.
Conflict of interest
We have no conflict of interest.
Acknowledgments
We would like to thank Dr. Laura Volpicelli-Daley and members of the Zhang and Darley-Usmar laboratories for discussion and technical assistance. This work was supported by NIHR01-NS064090 (to JZ).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2016.12.032.
Appendix A. Supplementary material
Supplementary material Figure 1: Primary cortical neurons exposed to control, 25 mM trehalose, 2.5 µg/mL PFFs, or both for 14 days were probed for MAP2, GFAP and counterstained with nuclear dye Hoechst (blue), 40× magnification. GFAP positive cells were quantified and normalized to total cell number determined by nuclear dye. Data represent mean±SEM (n=3).
.
References
- 1.Damier P., Hirsch E.C., Agid Y., Graybiel A.M. The substantia nigra of the human brain. II. patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain: J. Neurol. 1999;122:1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 2.Spillantini M.G., Schmidt M.L., Lee V.M., Trojanowski J.Q., Jakes R., Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 3.Baba M., Nakajo S., Tu P.H., Tomita T., Nakaya K., Lee V.M., Trojanowski J.Q., Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am. J. Pathol. 1998;152(4):879–884. [PMC free article] [PubMed] [Google Scholar]
- 4.Spillantini M.G., Crowther R.A., Jakes R., Cairns N.J., Lantos P.L., Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci. Lett. 1998;251(3):205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 5.Lee H.J., Cho E.D., Lee K.W., Kim J.H., Cho S.G., Lee S.J. Autophagic failure promotes the exocytosis and intercellular transfer of alpha-synuclein. Exp. Mol. Med. 2013;45:e22. doi: 10.1038/emm.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volpicelli-Daley L.A., Luk K.C., Patel T.P., Tanik S.A., Riddle D.M., Stieber A., Meaney D.F., Trojanowski J.Q., Lee V.M. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luk K.C., Kehm V., Carroll J., Zhang B., O'Brien P., Trojanowski J.Q., Lee V.M. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338(6109):949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuervo A.M., Bergamini E., Brunk U.T., Droge W., Ffrench M., Terman A. Autophagy and aging: the importance of maintaining "clean" cells. Autophagy. 2005;1(3):131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 9.Hochfeld W.E., Lee S., Rubinsztein D.C. Therapeutic induction of autophagy to modulate neurodegenerative disease progression. Acta Pharmacol. Sin. 2013;34(5):600–604. doi: 10.1038/aps.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stallone G., Infante B., Grandaliano G., Gesualdo L. Management of side effects of sirolimus therapy. Transplantation. 2009;87(8 Suppl):S23–S26. doi: 10.1097/TP.0b013e3181a05b7a. [DOI] [PubMed] [Google Scholar]
- 11.Lan D.M., Liu F.T., Zhao J., Chen Y., Wu J.J., Ding Z.T., Yue Z.Y., Ren H.M., Jiang Y.P., Wang J. Effect of trehalose on PC12 cells overexpressing wild-type or A53T mutant alpha-synuclein. Neurochem. Res. 2012;37(9):2025–2032. doi: 10.1007/s11064-012-0823-0. [DOI] [PubMed] [Google Scholar]
- 12.Dehay B., Bove J., Rodriguez-Muela N., Perier C., Recasens A., Boya P., Vila M. Pathogenic lysosomal depletion in Parkinson's disease. J. Neurosci. 2010;30(37):12535–12544. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Settembre C., Di Malta C., Polito V.A., Garcia Arencibia M., Vetrini F., Erdin S., Erdin S.U., Huynh T., Medina D., Colella P. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332(6036):1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sardiello M., Palmieri M., di Ronza A., Medina D.L., Valenza M., Gennarino V.A., Di Malta C., Donaudy F., Embrione V., Polishchuk R.S. A gene network regulating lysosomal biogenesis and function. Science. 2009;325(5939):473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 15.Sacktor B. Trehalase and the transport of glucose in the mammalian kidney and intestine. Proc. Natl. Acad. Sci. USA. 1968;60(3):1007–1014. doi: 10.1073/pnas.60.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richards A.B., Krakowka S., Dexter L.B., Schmid H., Wolterbeek A.P., Waalkens-Berendsen D.H., Shigoyuki A., Kurimoto M. Trehalose: a review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem. Toxicol.: Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2002;40(7):871–898. doi: 10.1016/s0278-6915(02)00011-x. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Navarro J.A., Rodriguez L., Casarejos M.J., Solano R.M., Gomez A., Perucho J., Cuervo A.M., Garcia de Yebenes J., Mena M.A. Trehalose ameliorates dopaminergic and tau pathology in parkin deleted/tau overexpressing mice through autophagy activation. Neurobiol. Dis. 2010;39(3):423–438. doi: 10.1016/j.nbd.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Castillo K., Nassif M., Valenzuela V., Rojas F., Matus S., Mercado G., Court F.A., van Zundert B., Hetz C. Trehalose delays the progression of amyotrophic lateral sclerosis by enhancing autophagy in motoneurons. Autophagy. 2013;9(9):1308–1320. doi: 10.4161/auto.25188. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka M., Machida Y., Niu S., Ikeda T., Jana N.R., Doi H., Kurosawa M., Nekooki M., Nukina N. Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat. Med. 2004;10(2):148–154. doi: 10.1038/nm985. [DOI] [PubMed] [Google Scholar]
- 20.Liu R., Barkhordarian H., Emadi S., Park C.B., Sierks M.R. Trehalose differentially inhibits aggregation and neurotoxicity of beta-amyloid 40 and 42. Neurobiol. Dis. 2005;20(1):74–81. doi: 10.1016/j.nbd.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Qiao L., Hamamichi S., Caldwell K.A., Caldwell G.A., Yacoubian T.A., Wilson S., Xie Z.L., Speake L.D., Parks R., Crabtree D. Lysosomal enzyme cathepsin D protects against alpha-synuclein aggregation and toxicity. Mol. Brain. 2008;1:17. doi: 10.1186/1756-6606-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shacka J.J., Klocke B.J., Young C., Shibata M., Olney J.W., Uchiyama Y., Saftig P., Roth K.A. Cathepsin D deficiency induces persistent neurodegeneration in the absence of Bax-dependent apoptosis. J. Neurosci. 2007;27(8):2081–2090. doi: 10.1523/JNEUROSCI.5577-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walls K.C., Klocke B.J., Saftig P., Shibata M., Uchiyama Y., Roth K.A., Shacka J.J. Altered regulation of phosphatidylinositol 3-kinase signaling in cathepsin D-deficient brain. Autophagy. 2007;3(3):222–229. doi: 10.4161/auto.3822. [DOI] [PubMed] [Google Scholar]
- 24.Abeliovich A., Schmitz Y., Farinas I., Choi-Lundberg D., Ho W.H., Castillo P.E., Shinsky N., Verdugo J.M., Armanini M., Ryan A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25(1):239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 25.Volpicelli-Daley L.A., Luk K.C., Lee V.M. Addition of exogenous alpha-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous alpha-synuclein to Lewy body and Lewy neurite-like aggregates. Nat. Protoc. 2014;9(9):2135–2146. doi: 10.1038/nprot.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanik S.A., Schultheiss C.E., Volpicelli-Daley L.A., Brunden K.R., Lee V.M. Lewy body-like alpha-synuclein aggregates resist degradation and impair macroautophagy. J. Biol. Chem. 2013;288(21):15194–15210. doi: 10.1074/jbc.M113.457408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braak H., Del Tredici K., Rub U., de Vos R.A., Jansen Steur E.N., Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 28.Sarkar S., Davies J.E., Huang Z., Tunnacliffe A., Rubinsztein D.C. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J. Biol. Chem. 2007;282(8):5641–5652. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- 29.Kruger U., Wang Y., Kumar S., Mandelkow E.M. Autophagic degradation of tau in primary neurons and its enhancement by trehalose. Neurobiol. Aging. 2012;33(10):2291–2305. doi: 10.1016/j.neurobiolaging.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Davies J.E., Sarkar S., Rubinsztein D.C. Trehalose reduces aggregate formation and delays pathology in a transgenic mouse model of oculopharyngeal muscular dystrophy. Hum. Mol. Genet. 2006;15(1):23–31. doi: 10.1093/hmg/ddi422. [DOI] [PubMed] [Google Scholar]
- 31.Yu W.B., Jiang T., Lan D.M., Lu J.H., Yue Z.Y., Wang J., Zhou P. Trehalose inhibits fibrillation of A53T mutant alpha-synuclein and disaggregates existing fibrils. Arch. Biochem. Biophys. 2012;523(2):144–150. doi: 10.1016/j.abb.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 32.Tapia H., Koshland D.E. Trehalose is a versatile and long-lived chaperone for desiccation tolerance. Curr. Biol.: Cb. 2014;24(23):2758–2766. doi: 10.1016/j.cub.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Viner R.I., Clegg J.S. Influence of trehalose on the molecular chaperone activity of p26, a small heat shock/alpha-crystallin protein. Cell Stress Chaperones. 2001;6(2):126–135. doi: 10.1379/1466-1268(2001)006<0126:iototm>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He Q., Koprich J.B., Wang Y., Yu W.B., Xiao B.G., Brotchie J.M., Wang J. Treatment with Trehalose prevents behavioral and neurochemical deficits produced in an AAV alpha-synuclein rat model of Parkinson's disease. Mol. Neurobiol. 2016;53(4):2258–2268. doi: 10.1007/s12035-015-9173-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material Figure 1: Primary cortical neurons exposed to control, 25 mM trehalose, 2.5 µg/mL PFFs, or both for 14 days were probed for MAP2, GFAP and counterstained with nuclear dye Hoechst (blue), 40× magnification. GFAP positive cells were quantified and normalized to total cell number determined by nuclear dye. Data represent mean±SEM (n=3).