Abstract
Fungi are often inconspicuous in nature and this means it is all too easy to overlook their importance. Often referred to as the “Forgotten Kingdom”, fungi are key components of life on this planet. The phylum Basidiomycota, considered to contain the most complex and evolutionarily advanced members of this Kingdom, includes some of the most iconic fungal species such as the gilled mushrooms, puffballs and bracket fungi. Basidiomycetes inhabit a wide range of ecological niches, carrying out vital ecosystem roles, particularly in carbon cycling and as symbiotic partners with a range of other organisms. Specifically in the context of human use, the basidiomycetes are a highly valuable food source and are increasingly medicinally important. In this review, seven main categories, or ‘roles’, for basidiomycetes have been suggested by the authors: as model species, edible species, toxic species, medicinal basidiomycetes, symbionts, decomposers and pathogens, and two species have been chosen as representatives of each category. Although this is in no way an exhaustive discussion of the importance of basidiomycetes, this review aims to give a broad overview of the importance of these organisms, exploring the various ways they can be exploited to the benefit of human society.
Key words: Agaricomycete, Basidiomycete, Model species, Mushroom
Introduction
The basidiomycetes are a large and incredibly diverse phylum of fungi, which, together with the ascomycetes, make up the subkingdom Dikarya – often referred to as the “higher fungi”. Basidiomycetes are almost exclusively filamentous fungi that have complex lifecycles, reproduce sexually and produce basidiospores on specialised cells called basidia. This review has chosen to focus specifically on the class Agaricomycetes, which make up 98 % of the Agaricomycotina – the largest subphylum of the basidiomycetes. Taxonomically, Agaricomycetes is roughly analogous to the previously accepted Homobasidiomycetes, a name which is no longer used due to the recognition that not all members of this phylogenetic group have homobasidia (undivided basidia). Agaricomycetes includes the most conspicuous basidiomycete species, all of which produce basidiocarps, such as the gilled mushrooms, bracket fungi, puffballs, crust fungi, chanterelles, coral fungi and jelly fungi (Hibbett 2006).
The morphology of agaricomycete fungi is incredibly varied. The cyphelloid fungi, for example, produce small cup or tube-shaped basidiocarps which rarely exceed 2 mm in length and diameter (Bodensteiner et al. 2004). At the other end of the spectrum are species such as Phellinus ellipsoideus, which is responsible for producing the largest fruiting body ever recorded, weighing between 400 and 500 kg (Dai & Cui 2011). Agaricomycetes can also claim to contain some of the largest and oldest organisms on earth. In 1992, a genetically stable individual Armillaria gallica was shown to cover 15 hectares, weigh upwards of 10 000 kg and was aged at 1 500 yr old (Smith et al. 1992). In 2000, an individual colony of Armillaria ostoyae was identified in Oregon which was said to cover an incredible 965 hectares and estimated to be between 1 900 and 8 650 yr old (Ferguson et al. 2003).
In addition to being morphologically varied, agaricomycetes fill a wide range of ecological niches. A very large number of species are wood-decay fungi, which play a vital role in carbon cycling. Other species function as symbiotic partners, including symbionts of insects (Aanen et al., 2002, Mueller et al., 2005), but most notably as mycorrhizal plant-symbionts, which are essential for the survival of many plant species (Kohler et al. 2015). There are also parasitic and pathogenic agaricomycete species, with pathogens of timber and crop species being of particular importance (Brazee and Wick, 2009, Farid et al., 2009). Human pathogenic fungi are generally limited to the ascomycetes, although Schizophyllum commune has been known to cause serious lung infections (Chowdhary et al. 2013). Although less common, agaricomycete species are increasingly being discovered in a variety of freshwater (Frank et al. 2010), marine (Hibbett and Binder, 2001, Binder et al., 2006, Amend et al., 2012) and mangrove environments (Baltazar et al. 2009). Recently a new aquatic species of gilled mushroom belonging to the genus Psathyrella has been identified, which produces completely submerged fruiting bodies (Frank et al. 2010).
The ecological roles of agaricomycetes make them important within human societies due to the roles they play in industries such as forestry and agriculture, but they also impact us more directly, as a valuable source of nutrients and as medicinally relevant species. The vast majority of edible fungi are agaricomycetes, with the exception of the truffles and morels, which are ascomycete species. The most toxic fungi are also agaricomycetes however, such as the death cap, Amanita phalloides, which is responsible for the majority of fatal mushroom poisonings (Litten 1975).
Despite the relatively conspicuous nature of agaricomycete species and their relevance to human societies, they have not always garnered as much attention within the scientific community as other fungi, largely due to the relative difficulty in growing and studying them within laboratory settings. However, with the advent of modern molecular techniques, such as transformation systems and next generation sequencing, this fascinating and indispensable group of organisms are now becoming more and more accessible, and therefore the ways in which they can be exploited for human endeavours is increasing greatly.
This review will attempt to give an overarching perspective of the importance of the Agaricomycetes, by focusing upon selected species which we feel are representative of the main roles these fungi play within natural ecosystems and in the context of serving a particular purpose to human societies: as model species, as edible species, as decomposers, as toxic species, as medicinal mushrooms, as symbionts and as pathogens.
Model agaricomycetes
The in-depth study of model species within all Kingdoms serves to deepen our understanding of the key biological processes shared by all life-forms, including evolution, genetics, cell biology, meiosis and pathogenesis. Fungi play a unique role in understanding such broad biological themes, sitting as they do between plants and animals in the tree of life, and in fact being more closely related to animals than plants, with the Fungi and Animalia Kingdoms being part of a monophyletic clade (Baldauf & Palmer 1993).
Being well understood and genetically tractable, model species not only inform our general understanding of life, but also serve as the foundation for extended research into the biology of other related species. As previously mentioned, one factor that has hampered the study of basidiomycetes has been the relative difficulty in studying these organisms in laboratory conditions. Therefore, detailed and systematic investigations into model species, and the subsequent development of molecular tools specifically adapted for basidiomycetes, have been vital in making the phylum as a whole more accessible.
This review will consider two model agaricomycetes: Coprinopsis cinerea and Schizophyllum commune. Both species have been studied in great detail, with thousands of peer-reviewed papers published elucidating key aspects of basidiomycete biology and describing the development of tools and techniques applicable to such species. This resulting body of knowledge has formed the basis of much of our understanding of this fascinating phylum.
Coprinopsis cinerea
Coprinopsis cinerea (Fig. 1A), commonly known as the “gray shag”, is a model multicellular basidiomycete (Redhead et al. 2001), which has been studied extensively. In nature, C. cinerea is found globally where it employs a saprotrophic lifestyle, favouring habitats containing dung and compost (Kjalke et al., 1992, Kues, 2000). Coprinopsis cinerea is a coprinoid mushroom, collectively known as the “inky caps”, due to the fact that their caps liquefy on maturation to aid the dispersal of basidiospores. As deliquescence occurs from the bottom of the gills upwards, the edges of the cap curl to expose mature spores to wind currents for dispersal (Pukkila 2011). The coprinoid basidiomycetes are an excellent example of convergent evolution, as recent molecular analyses have demonstrated that although they share this common trait, they are not in fact all closely related in evolutionary terms (Redhead et al. 2001).
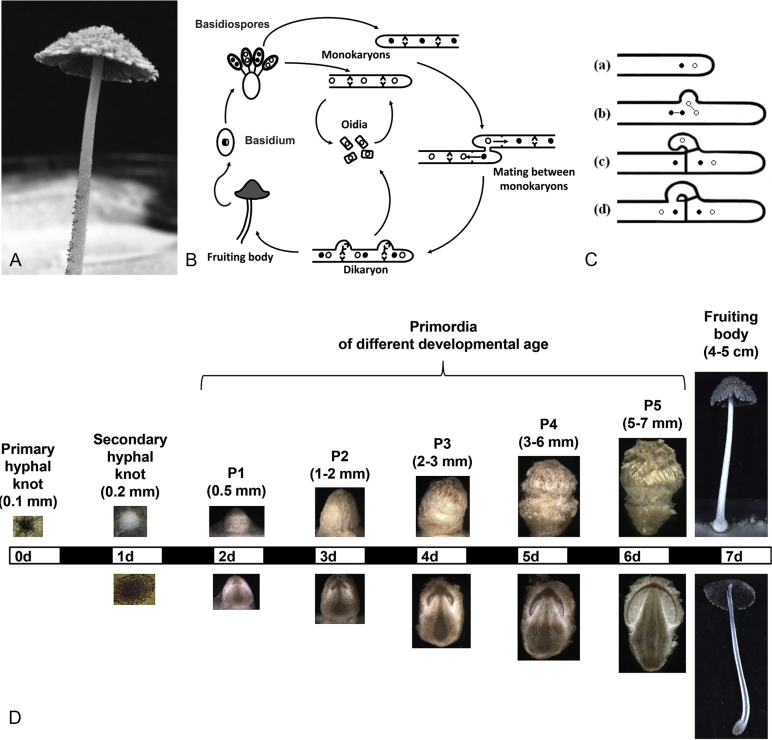
Fig. 1.
A. Under laboratory conditions mature Coprinopsis cinerea fruiting bodies form from dikaryotic mycelia, allowing mushroom development to be studied. Reproduced from Stajich et al. (2010) © National Academy of Sciences. B. The lifecycle of Coprinopsis cinerea, reprinted from Raudaskoski (2015) with permission from Elsevier. C. Clamp connections are a unique feature of dikaryotic basidiomycetes, and are likely to be involved in maintaining the presence of both nuclei types in each cell during mitosis. D. Fruiting body development of C. cinerea strain AmutBmut. The central bar represents the light and dark phases (12 h each) over a seven day period. Structures were harvested at the beginning of each light phase – outer views and longitudinal sections are shown. Fruiting development for C. cinerea is hemiangiocarpic, in that the developing fruiting body is initially enclosed (angiocarpic), opening before full maturity (gymnocarpic). Reprinted from Kües & Navarro-González (2015) with permission from Elsevier.
Much of C. cinerea's value as a model species lies in the fact that it is easy to cultivate on petri dishes in the laboratory, where mating and the full life cycle can be completed in just two weeks. A suite of molecular tools have now been developed for C. cinerea, including an established transformation system (Binninger et al., 1987, Granado et al., 1997, Collins et al., 2010, Dörnte and Kües, 2012), a selection of marker genes (Kilaru et al. 2009b), gene silencing methods (Namekawa et al., 2005, Wälti et al., 2006, Heneghan et al., 2007, Costa et al., 2008) and strains which have been engineered to allow targeted gene disruption, a technique which is generally not feasible for agaricomycetes (Nakazawa et al. 2011). The genome sequence for C. cinerea also became available in 2010, revealing a haploid genome size of 37.5 Mbp (Stajich et al. 2010). This relative ease of working with C. cinerea and the available tools means extensive research has been carried out on many fundamental aspects of the biology of this species.
For example, the sexual reproduction of C. cinerea has been studied in great detail. As is now known to be typical for basidiomycetes, C. cinerea has two distinct stages to its lifecycle: as a primary monokaryote (from the Greek, mono = one; karyos = kernel or nucleus), which contains only one haploid nucleus per cell, and as a fertile dikaryote which contains two nuclei per cell (Fig. 1B). Dikaryotic mycelium forms when two sexually compatible monokaryotic hyphae fuse in a process called plasmogamy (Kues, 2000, Raudaskoski and Kothe, 2010). Unlike in the lifecycle of many plants and animals, karyogamy (nuclear fusion to produce a diploid cell) does not occur immediately for basidiomycetes and instead the dikaryotic state, with two distinct haploid nuclei per cell, is stably maintained indefinitely. Dikaryotic tissues can generally be recognised due to their vigorous growth and the presence of clamp connections at the hyphal septa, which are specialised structures that elegantly maintain the presence of two distinct nuclei in each cell (Fig. 1C).
When the necessary genetic and environmental factors coincide, fruiting bodies will then form from the dikaryotic mycelia, differentiating into three main tissue types: gill, stalk and cap. This differentiation process is incredibly complex and occurs via a well-defined developmental process that requires light/dark cycles and takes approximately one week (Fig. 1D). It is on the external surfaces of the gills in the basidia that nuclear fusion then occurs, followed by meiosis, and finally, haploid basidiospore formation (Kues 2000). After release and dispersal, these basidiospores germinate to produce a monokaryotic mycelium, thus completing the sexual lifecycle. Like 90 % of basidiomycetes, C. cinerea is heterothallic, meaning that two different and compatible monokaryons are required for mating to produce fertile dikaryons that are capable of fruiting and producing sexual spores. The remaining 10 % of basidiomycetes are homothallic, meaning that their spores germinate to produce fertile mycelia capable of fruiting (Raper 1966). Agaricus bisporus, one of the species discussed later in this review, is an interesting example of a basidiomycete where different varieties employ different lifestyles, including homothallic and heterothallic.
Investigations into the lifecycle of C. cinerea have been instrumental in uncovering the genetic regulation of sexual compatibility in basidiomycete fungi. In C. cinerea, compatibility is controlled at two genetic loci: the A (MAT-A) and B (MAT-B) mating type loci (Raudaskoski and Kothe, 2010, Plaza et al., 2014). The MAT-A locus of C. cinerea (now also known as the HD locus) contains genes encoding two classes of homeodomain transcription factors. These need to heterodimerize to become an active transcription factor, which in turn activates the A-regulated developmental pathway, controlling nuclear division and the formation of clamp connections in the dikaryotic tissue (Kues 2015). This active dimer can only form in non-self combinations, meaning that the nuclei from the two original monokaryotic strains must have different alleles at this locus to be compatible. The MAT-B locus of C. cinerea (now also known as the PR locus) encodes small pheromone peptides and G protein-coupled receptors. A successful interaction between pheromones and receptors can also only take place if they are from different B loci (non-self), and the binding of these pheromones to the receptors induces a signal transduction pathway that regulates nuclear migration and the fusion of clamp connections with the adjacent cell. This need for compatibility at two separate loci is known as a tetrapolar system because the progeny of a dikaryon each display one of four mating types (e.g. AB, Ab, aB and ab). The need to differ at both loci means that a fertile cross between sibling progeny can only occur 25 % of the time, but the existence of a high number of different alleles at both loci in the general population means that the chances of being compatible with a non-related individual is very high. The total population of C. cinerea has been estimated to contain 164 A alleles and 79 B alleles (Day 1963), meaning that there are over 12 000 different mating types.
In addition to being a model species, C. cinerea has been shown to produce a wide profile of enzymes and secondary metabolites including lectins, laccases, peroxidases, and terpenoids (Bouws et al., 2008, You et al., 2014). One major group of secreted compounds produced by C. cinerea are the laccases, which play a role in the degradation of lignin in plant fibres, vital for C. cinerea’s role as a decomposer (Kilaru et al., 2006a, Kilaru et al., 2006b, Courty et al., 2009) and of potential use in various industries such as fibre board production and waste treatments. A novel antimicrobial peptide, copsin, has also been discovered from this species, which was previously thought not to produce any antimicrobial compounds (Essig et al. 2014). This recent discovery suggests that despite the thorough analysis of this model organism to date, there is likely to be a wealth of capabilities yet to be discovered.
Schizophyllum commune
Schizophyllum commune, or the “split-gill” mushroom (Fig. 2), is a generalist, saprotrophic species that is found worldwide and has been utilised as a model system for studying basidiomycete mating and mushroom development since the early 20th century (Kniep, 1920, Essig, 1922, Linder, 1933, Whitehouse, 1949, Raper and Miles, 1958). Schizophyllum commune is classed as a white rot fungus (see ‘Decomposing basidiomycetes’ section), which has been responsible for extensive post-harvest losses of hay and silage and has been reported as an occasional pathogen of woody species (Brady et al. 2005). Schizophyllum commune is also an emerging pathogen of mammals and humans, with reports of bone and soft tissue infection in dogs (Kano et al., 2002, Tanaka et al., 2008), and sinusitis and infections of soft tissue, lung and nails in humans, although largely in immunocompromised patients (Chowdhary et al., 2013, Tsukatani et al., 2015). Most recently, S. commune has been isolated from an infected harbour seal (Hanafusa et al. 2016).
Fig. 2.
Shizophyllum commune, the split gill fungus. A. Mature S. commune fruiting bodies growing on a felled beech trunk. B–E. Dorsal (B and C) and ventral (D and E) views of a wild-collected S. commune fruiting body, under dry conditions where the fruiting body is enrolled (B and D), and moist conditions where the fruiting body is unfolded (C and E). F–H. Cross sections through a mature fruiting body, demonstrating the morphological differences under dry (F and G) and moist conditions (H). I–J. Show the outer edges of the lamellae in dry and moist conditions respectively. It is the ‘splitting’ of the pseudolamellae (gills) under dry conditions, as seen in I, which earns this species its common name. K–L. Show the outer edges of the fruiting body under dry and moist conditions respectively. Under dry conditions, the outer edge with abhymenial hairs is enrolled over the youngest hymenia (fertile surfaces). Under moist conditions, the hymenia are exposed. Reprinted from Kües & Navarro-González (2015) with permission from Elsevier.
This incredibly flexible lifestyle means that S. commune can be found in a very wide range of habitats, and is considered to be the most wide-spread and abundant basidiomycete, with the single species existing on every continent except Antarctica (Watling et al. 1991). One unusual trait, which helps facilitate such wide geographic distribution, is its adaptations to climatic variability. The fruiting bodies of S. commune can dehydrate and rehydrate many times over the course of a growing season, maintaining the ability to sporulate and removing the need to produce costly fruiting bodies multiple times. These fruiting bodies can also function as exceptional longer-term survival structures: basidiocarps desiccated and vacuum-stored for 35 yr have been shown to retain the ability to sporulate following rehydration (Essig, 1922, Bisby, 1945).
Like C. cinerea, S. commune arose as an ideal agaricomycete for study due to its ease of manipulation in the laboratory: it fruits readily in vitro and can complete its lifecycle within 10 d (Kües & Navarro-González 2015). Also like C. cinerea, it was used in pioneering research into basidiomycete sexual reproduction; elucidating the tetrapolar system and uncovering the genetic basis of compatibility control (Giasson et al., 1989, Stankis et al., 1992, Specht, 1995, Kothe, 1999). Schizophyllum commune has a very similar tetrapolar system to that of C. cinerea, with two unlinked genetic complexes, one encoding homeodomain transcription factors and the other encoding pheromones and receptors. However, it is worth noting that there are differences between the two species, both in the number of gene at each locus and the genetic organisation (Raudaskoski 2015). In the case of S. commune, there are estimated to be 288 different alleles at the MAT-A locus and 81 alleles at the MAT-B locus (Raper 1966), meaning that this species is estimated to have over 23 000 different mating types – an incredibly efficient way of maintaining out-breeding whilst optimising the chances of being compatible with any non-related isolates. The simultaneous uncovering of the tetrapolar mating systems of C. cinerea and S. commune has since facilitated the discovery of similar mating loci in various species, including Laccaria bicolor (Niculita-Hirzel et al. 2008), Flammulina velutipes (van Peer et al. 2011), Lentinula edodes (Wu et al. 2013b) and Pleurotus eryngii (Kim et al. 2014).
Although both model species discussed here employ a tetrapolar heterothallic lifestyle, it must be noted that 30–40 % of basidiomycetes are bipolar, with compatibility being controlled by only one locus (Kues 2000). This can occur when the two mating loci become genetically linked, effectively acting like a single locus (Lee et al., 1999, Lengeler et al., 2002), but there are also examples where one locus has lost its self/non-self discrimination (James et al., 2006, James et al., 2011).
Schizophyllum commune was utilised in many early studies of fruiting, demonstrating that environmental conditions including light, temperature, CO2 and nutrient availability regulate fruiting (Essig, 1922, Niederpruem and Wessels, 1969, Perkins and Gordon, 1969). In dikaryotic S. commune, light induces asymmetrical growth at colony margins which initiates the formation of the hyphal knots that develop into primordia and then mature into the split-gill basidiocarps bearing the haploid basidiospores (Kües & Navarro-González 2015).
Moving into the modern molecular era, S. commune has continued to be at the forefront of agaricomycete molecular genetic research. It was the first basidiomycete for which a transformation system was established (Munoz-Rivas et al. 1986) and some of the initial regulatory sequences used for heterologous gene expression in agaricomycetes were obtained from S. commune (Harmsen et al. 1992). It was also the first agaricomycete in which GFP, now a ubiquitous molecular tool, was expressed. This highlighted the importance of introns for efficient expression in many basidiomycetes, a requirement not seen in other fungal phyla such as the Ascomycetes (Lugones et al., 1999, Scholtmeijer et al., 2001). Such discoveries concerning the fundamental nature of basidiomycete genetics have paved the way for similar work in other agaricomycete species (Ma et al., 2001, Burns et al., 2005, Kilaru et al., 2009a).
Correspondingly, numerous molecular tools are now available for S. commune. The genome was sequenced in 2010 (Ohm et al. 2010b), there are several selectable markers, both auxotrophic and antibiotic (Munoz-Rivas et al., 1986, Schuren and Wessels, 1994, Scholtmeijer et al., 2001), a range of strong and inducible promoters (Munoz-Rivas et al., 1986, Harmsen et al., 1992) and various tools for functional gene analysis, including RNAi-mediated gene silencing (de Jong et al. 2006) and unusually for agaricomycete fungi, an efficient method of gene knockout by homologous recombination (de Jong et al., 2010, Ohm et al., 2010a). The availability of these tools has facilitated analysis of gene function in S. commune, for example identifying genes that are essential in mushroom development (Ohm et al. 2011) and identifying homologues of the blue light receptors in Neurospora crassa, which are important for light recognition in S. commune (Ohm et al. 2013).
Schizophyllum commune has also been vital in the discovery and understanding of hydrophobins, and was the first species from which a hydrophobin was isolated (Wessels et al. 1991). Hydrophobins are a very important class of proteins produced exclusively by fungi that are capable of spontaneously self-assembling at hydrophilic–hydrophobic interfaces, creating amphipathic monolayers. This layer is typically found on the surface of aerial hyphae and spores. In the former, it is thought to reduce the surface tension of the medium in which the fungus is growing, allowing the hyphae to breach the air–water interface, and also stops water logging from occurring (Lo et al. 2014). By making the surface of spores hydrophobic, the hydrophobin layer is thought to be essential for dispersal. Hydrophobins are also thought to play a protective role during fruiting body formation (De Groot et al. 1996), and there is evidence for various roles in pathogenic fungi, including masking the immunogenicity of airborne fungal spores, effectively by acting as an invisibility cloak (Aimanianda et al. 2009). The various unique properties of hydrophobins mean they have potential applications in many industries, including nanotechnologies such as coating implanted medical devices, as biosensors and as cell growth surfaces (Lo et al. 2014). One hydrophobin from S. commune, SC3, demonstrates immunomodulatory activity (Akanbi et al. 2013) and thus has potential as a therapeutic agent in cancer treatment.
As a prolific producer of plant cell wall degrading enzymes including cellulases, hemicellulases and pectinases, S. commune also has potential use in bioremediation (Irshad and Asgher, 2011, Saini et al., 2013) – a research area discussed in more depth in the “Decomposing basidiomycetes” section of this review.
Edible basidiomycetes
Perhaps the most obvious value of basidiomycetes to human cultures is as a food source. Mushrooms are an excellent source of nutrients, being low in calories and fats and free of cholesterol whilst providing proteins, vitamins, fibre and minerals such as potassium, iron and phosphorus. The consumption of mushrooms goes back to ancient times, with Ancient Romans referring to them as a “divine food”, believing that they were the result of lightning thrown down by Jupiter. The Egyptians believed that they were a gift from the god Osiris (Manzi et al. 1999).
There are considered to be thousands of species of edible mushrooms worldwide and the foraging of such mushrooms is conducted globally. Many species, such as the Chanterelle, are considered delicacies. However, only 25 or so species have been cultivated and only 10 commercialised. The consumption of mushrooms increased steadily during the second half of the 20th century as commercial production became widespread, with consumption recorded as 495 127 tonnes in 1961, increasing to 4 210 714 tonnes in 2000 (FAOSTAT). An even more dramatic increase has been seen so far in the 21st century, with the latest FAO statistic from 2013 being 9 926 966 tonnes.
As basidiomycetes are cultivated using lignocellulosic substrates and other organic waste matter, this industry can be considered as the only known industry that simultaneously addresses two of the major challenges faced by today’s growing population: providing food with high nutritional value whilst reducing waste and environmental pollution.
In this section, we will discuss the two most cultivated species worldwide, Agaricus bisporus and Pleurotus ostreatus.
Agaricus bisporus
Agaricus bisporus is probably the most famous of the edible mushrooms. It is known by various common names, depending on its colour and maturity, including the button mushroom, chestnut mushroom, portobello mushroom and champignon mushroom. Agaricus bisporus is a litter-decomposing saprotroph which is found globally in habitats such as forests and grasslands (Kerrigan, 1995, Hildén et al., 2013). The wild varieties typically have a pale grey-brown cap, which is 5–10 cm in diameter when mature (Fig. 3A). It is the most widely cultivated species in the world, produced on the scale of millions of tons every year, with a global market of several billion US dollars (Kerrigan 2013).
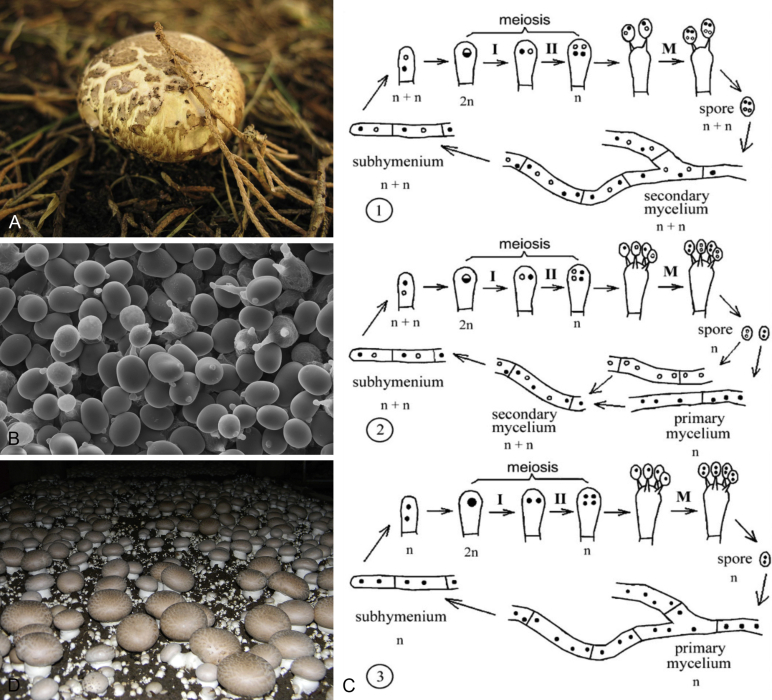
Fig. 3.
A.Agaricus bisporus, commonly known as the button mushroom, growing in the wild (Copyright © Nathan Wilson). B. An electron micrograph of A. bisporus var. bisporus showing the release of spores from bisporic basidia, courtesy of Louisa Howard, Dartmouth College. C. A representation of the three life cycles of Agaricus bisporus. 1: In A. bisporus var. bisporus pseudohomothallism is predominant, where multinucleate spores germinate to produce dikaryotic mycelia. 2: In A. bisporus var. burnettii heterothallism is prevalent. Spores from tetrasporic basidia, containing only one nucleus each, germinate to produce non-fertile homokaryotic mycelia that needs to mate to complete the lifecycle. 3: In A. bisporus var. eurotetrasporus homothallism is predominant. Homokaryotic spores germinate to produce mycelia which, although homokaryotic, is fertile and capable of fruiting (Kamzolkina et al. 2006). This image is reprinted with permission from Mycologia. (Copyright © The Mycological Society of America). D.A. bisporus being cultivated in mushroom beds (Copyright © Tim Sage).
Agaricus bisporus owes its Latin name to the unusual spore production of A. bisporus var. bisporus, the most abundant variety in wild populations and the variety from which all of the traditional cultivars are derived. In most basidiomycetes, four spores form on each basidium (tetrasporic), each containing one of the four products of meiosis. Each spore then germinates into monokaryotic mycelia, which needs to mate with another compatible monokaryotic strain to produce the fertile dikaryotic mycelium. Agaricus bisporus var. bisporus has predominantly bisporic basidia (Fig. 3B), meaning that it produces two spores per basidium. Each of the two spores contain two non-sister post-meiotic nuclei, resulting in the germination of a fertile dikaryotic strain (Kerrigan et al. 1993). This life-cycle is known as secondary homothallism, or pseudohomothallism. A second variety of the button mushroom, called A. bisporus var. burnettii, was discovered in California in the early 1990s, and was shown instead to have a predominantly heterothallic life cycle, with tetrasporic basidia being formed in approximately 90 % of the cases (Kerrigan et al. 1994). Each spore contains one type of nucleus and thus germinates to produce non-fertile monokaryotic mycelia, which need to mate to complete the lifecycle. As A. bisporus is a bipolar species, with mating compatibility being controlled by one locus, the offspring of an individual will be compatible with one another 50 % of the time. Lastly, a rare third variety was discovered by Callac et al. (2003), and named A. bisporus var. eurotetrasporus. The specimens ascribed to this variety, collected in France and Greece, have tetrasporic basidia like A. bisporus var. burnettii but with much longer spores, and this variety employs a predominantly homothallic life cycle (Kamzolkina et al. 2006), meaning that monokaryotic mycelia which germinate from an individual spore are capable of fruiting. See Fig. 3C for a schematic representation of the life cycles of the three varieties.
Cultivation of A. bisporus has been refined over the years, but is believed to have begun around 1650 in France (Chang & Hayes 1978). Commercial farming takes place in mushroom houses under controlled conditions of temperature, humidity and aeration. A few crucial steps can be identified in this process (Hudson, 1986, Carlile et al., 2001), such as preparation of the substrate, or “compost”, which is typically made of horse manure and wheat straw. Several pathogens and competitors can hamper cultivation, so pasteurisation of the substrate is carried out prior to inoculation. Inoculum of pure culture (also known as “spawn”) is prepared by growing the fungus on a sterile substrate made of wheat or rye. This spawn is mixed with the pasteurised compost and incubated at 25 °C to allow for mycelial growth. Fruiting is then induced by performing the “casing” which involves covering the mycelium with peat and chalk and placing the trays at 16–18 °C. Overall harvesting of mushrooms usually occurs 9–11 wk after inoculation (Fig. 3D).
The intensive cultivation and study of A. bisporus has served to highlight the types of microbes that infect mushrooms. There are several ascomycete fungi that parasitise the fruiting bodies or vegetative mycelia of basidiomycetes, such as Lecanicillium fungicola, the causative agent of “dry bubble” (Amey et al., 2003, Bailey et al., 2013b) and Mycogone perniciosa, the causative agent of ‘wet bubble’ (Gea et al. 2010). Symptoms of infection include masses of undifferentiated mycelia rather than fruiting bodies, or developmental aberrations and distortions such as comb-like protrusions of gill tissue on the upper surfaces of the cap (called rosecomb), as seen in M. perniciosa infections (Umar & Van Griensven 1999). Bacterial pathogens, including a number of Pseudomonas species, cause necrotic lesions on fruiting bodies (Abou-Zeid 2012). Various mycoviruses cause disease in A. bisporus, such as brown cap mushroom disease (Eastwood et al. 2015) and La France disease (Borodynko et al. 2010), which impact both the yield and quality. And finally, insect pests have a significant impact of the commercial cultivation of A. bisporus, with the two major classes of pests being the sciarids and phorids (Jess & Bingham 2004).
In addition to being a well-known culinary species, A. bisporus is also known for its ecological role as a lignocellulose decomposer (Wood & Leatham 1983). This was investigated at the molecular level by Morin et al. (2012), who sequenced the genome of the fungus and carried out transcriptomic analyses to compare gene expression at different developmental stages and culturing conditions. They found that when the fungus is grown on compost, there is considerable upregulation of genes involved in the degradation of lignocellulose, including those encoding carbohydrate-active enzymes (CAZy) and oxidoreductases, as well as protein degradation and peptide transporters. Rather than being attributed to either of the two most popular classes of wood degrading fungi (white rots and brown rots), A. bisporus is considered to be a humicolous species that has found its ecological niche in humic-rich environment, where partially decomposed organic matter prevails (Morin et al. 2012). A protease called serine proteinase 1 (SPR1) is expressed by A. bisporus in response to environmental cues such as nitrogen availability (Heneghan et al. 2009), and has recently been shown to play a key role in nutrient acquisition for A. bisporus, allowing colonisation of compost (Heneghan et al. 2016).
Pleurotus ostreatus
Pleurotus ostreatus is the second most cultivated mushroom after Agaricus bisporus (Rühl et al. 2008). Pleurotus ostreatus was first scientifically named Agaricus ostreatus in 1774, later being moved to the newly recognised genus Pleurotus in 1871 by the German mycologist Paul Kummer (Naraian et al. 2016). As with many fungal species, the name is descriptive. The Latin Pleurotus (sideways) refers to the sideways growth of the stem with respect to the cap seen in fungi of this genus. Ostreatus (Latin: oyster) refers to the shape of the cap, which resembles, and is considered by some to taste similar to, the bivalve oyster (Fig. 4). This is also the origin of the common name for this species: the “oyster mushroom”, although it is additionally known as the tree oyster mushroom or grey oyster mushroom to differentiate it from other Pleurotus species. In Japan it is known as Hiratake, which means “flat mushroom”. As well as the offset position of the stipe and fan-shaped cap, P. ostreatus can be recognised by various morphological features. The cap ranges in diameter from 5–25 cm, is smooth and is usually white to light brown but can also be grey or even dark brown, with the flesh being white. The gills are decurrent and the spore print is white to lilac grey (Glaeser & Smith 2010).
Fig. 4.
A. The basidiocarps of Pleurotus ostreatus growing on the trunk of a fallen Red Oak (Quercus Rubra) tree in Washington State Park, New Jersey. (Copyright © I. G. Safonov). B. On a Coast Live Oak (Quercus agrifolia) in Oakland, California. (Copyright © Alan Rockefeller). C. And in a cultivated setting, where a polyethylene bag filled with a suitable lignocellulosic substrate has been inoculated with spawn (Copyright © Alan Rockefeller). D. A paralysed nematode worm being penetrated by P. ostreatus hyphae, which obtain valuable nitrogen from the worm (image courtesy of Prof. George Barron, University of Guelph). E.Pleurotus ostreatus spores (Copyright © Alan Rockefeller).
Pleurotus ostreatus is a saprotrophic species, being a primary decomposer of wood, and of beech trees in particular. It is typically found growing out from trees in shelf-like clusters. This species is sometimes confused with Lentinellus ursinus, a species that occupies the same niche, is widely distributed in North America and has a strong acrid taste.
In nature, P. ostreatus is found throughout the North Temperate Zone, including most of Asia where it is particularly popular as a culinary mushroom, Britain and Ireland, and most of mainland Europe. It is also present in North America, with the exception of the Pacific Northwest where other closely related species can be found. Taxonomically, P. ostreatus is part of a complex of very closely related and morphologically similar species, such as P. pulmonarius and P. populinus (also known as oyster mushrooms) and as such distribution data can be unclear.
Unlike A. bisporus, the history of P. ostreatus cultivation is very recent. The first attempts to grow P. ostreatus as a food source were during the Second World War in Germany as a subsistence measure. A study on the breeding potential of P. ostreatus, published in 1976, described P. ostreatus as a “New Cultivated Mushroom”, proposing its potential as a “future crop” (Eger et al. 1976). Since that time, the cultivation of P. ostreatus has rapidly become a worldwide phenomenon. Although a variety of cultivation techniques are employed, the general process of cultivation is the same as for other edible mushrooms, with a pure culture (spawn) being used to inoculate a suitable substrate. In the case of P. ostreatus, a very wide range of lignocellulosic substrates can be used, including – but not limited to – coffee pulp, sawdust, weed plants, cotton from the textile industry, molasses from the sugar industry, peanut and coconut shells and cassava peels. A mixture of cottonseed hulls and wheat straw, which has a high water holding capacity, is a commonly used substrate. The substrate is generally milled, pasteurised, spawned and then used to fill polyethylene bags (Fig. 4C). Various free-standing or suspended systems are then used, which produce mushrooms for harvesting 3–4 wk after spawning.
Many different factors affect the productivity of cultivation, including the strain, the substrate, the type of spawn, the moisture level and various physicochemical conditions, and much work has been done investigating such variables for Pleurotus ostreatus (Sánchez 2010). In terms of the strain used, natural variation can be utilised, but strain improvement, particularly genetic manipulation, is increasingly applied to develop desirable characteristics. Long-term exposure to P. ostreatus spores has been known to trigger various respiratory allergies and as such, one aspect of strain improvement is focusing on the development of sporeless mutants (Sharma & Sharma 2014).
Beyond its role as a culinary basidiomycete, being a white rot fungus P. ostreatus plays an important role in decomposition and carbon cycling and has garnered significant attention for its potential use as a bioremediation agent (Eggen and Majcherczyk, 1998, Hirano et al., 2000, Byss et al., 2008, Miele et al., 2010, Purnomo et al., 2010). Genome sequencing has recently revealed the wood-degrading machinery of P. ostreatus to be typical of white rot fungi (Riley et al. 2014). Pleurotus ostreatus has also been shown to produce β-glucans, which have marked anticarcinogenic and cardioprotective properties, and as such could fall into the medicinal category. Finally, P. ostreatus is one of the basidiomycete species known to be capable of carnivory, killing and digesting nematodes as a nitrogen source (Fig. 4D) (Thorn and Barron, 1984, Thorn et al., 2000). There has been some interest in such carnivorous basidiomycetes as biocontrol agents to circumvent the environmental concerns over the use of conventional nematicides (Morton et al., 2004, Balaes and Tanase, 2016).
Decomposing basidiomycetes
One of the most vital ecological roles played by fungi is that of the recyclers. By decomposing substrates such as lignocellulose in wood, which cannot be degraded by any other organisms, they release vital nutrients back into the environment. As such, it can be said that all life relies on the presence of fungi to recycle the basic building blocks needed for growth and survival. Different types of basidiomycetes have evolved to degrade specific types of organic compounds and wood decaying species and genera are traditionally divided into two main categories; brown rot and white rot. Brown rot fungi primarily decompose the white-coloured cellulose present in wood via a Fenton reaction-based mechanism, leaving the brown-coloured lignin largely intact. White rot fungi in comparison, are unique in their ability to completely degrade the brown-coloured lignin, before enzymatically degrading the cellulose layer (Riley et al. 2014). This initial degradation of the brown-coloured lignin polymers gives the decomposing wood a bleached appearance, hence the name ‘white rot’.
This section will discuss two species which are representative of the two wood-degrading categories: the brown rot fungus Serpula lacrymans, and the model white rot fungus Phanerochaete chrysosporium. S. lacrymans is the causative agent of the dry rot of timber, causing hundreds of millions of pounds worth of damage each year globally (Palfreyman 2001). P. chrysosporium on the other hand is a species with the potential to be highly beneficial to human societies, by harnessing its metabolic capabilities for use in a wide range of industries.
Serpula lacrymans
Brown rot fungi are exclusively basidiomycete species and account for 6 % of wood-decomposing fungi (Gilbertson 1980). The most destructive brown rot species is considered to be Serpula lacrymans (Fig. 5), a crust fungus which causes a condition known as dry rot, due to the ability of this species to grow on surfaces containing very little or no apparent moisture (Jennings & Bravery 1991).
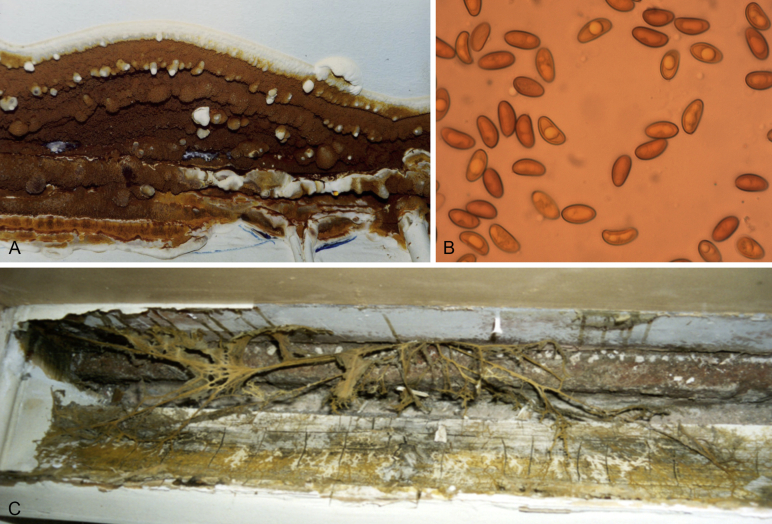
Fig. 5.
A. The fruiting body of Serpula lacrymans, the causative agent of dry rot of timber (Copyright © Malcolm Storey/www.discoverlife.org). B.S. lacrymans spores are released from the fruiting body for air dispersal (photograph taken by Andrzej Majcherczyk from the University of Göttingen, courtesy of Ursula Kües). C. When nutrients become scarce, S. lacrymans produces rhizormorphs. Also known as mycelial cords, rhizomorphs can travel considerable distances, even penetrating brickwork, plaster and masonry to reach another food source.
The history of dry rot by S. lacrymans dates back several centuries, with the first reliable reports coming from wooden vessels in the 17th century (Ramsbottom, 1937, Ramsbottom, 1953). Regarding its general biology, this species is found in nature exclusively on conifers, including species of the genera Pinus and Abies, tending to colonise large trunks with a diameter of more than 1 m at the base (Kauserud et al. 2012), and its temperature range spans between 3 and 26 °C, with an optimum of 21 °C (Jennings & Bravery 1991). Serpula lacrymans is believed to have a typical basidiomycete life cycle, with a predominant dikaryotic stage and dispersal of monokaryotic basidiospores. Flat brown fruiting bodies form primarily at the base of the trunk of the host plant, and have incredibly varying dimensions, from a few centimetres to more than one metre (Kauserud et al. 2012).
Phylogenetic studies have been conducted with the aim of understanding the evolutionary history of S. lacrymans. High genetic variation seen for specimens collected in north-east Asia suggests that this species may have originated in this region (Kauserud et al. 2007), and it is believed that S. lacrymans originally existed on mountains in temperate regions, typically at altitudes of more than 2 000 m. (Kauserud et al. 2012). From this primary environment, S. lacrymans is believed to have spread to the other presently colonised areas, which include Japan, Oceania, Europe and North and South America. Phylogenetic studies also indicate that current isolates of S. lacrymans could be ascribed to two different lineages: var. shastensis (found growing on conifer forests) and var. lacrymans (the cause of dry rot of timber) (Kauserud et al. 2007).
This species has become publically notorious for its extraordinary ability to grow on and decompose wooden building elements, particularly softwoods, and it has been estimated that the damage caused in the US is comparable to that of termites (Alexopoulos et al. 1996). It is commonly claimed that fungi cannot grow on wood that has a water content of less than 20 %, which is more than normally found in buildings. However, conditions such as the proximity of dripping pipes and general dampness can increase the water content of timber sufficiently to promote proliferation of S. lacrymans.
A feature that enables S. lacrymans to colonise dead wood and survive in spatially intermittent conditions of moisture and nutrients is the ability to produce rhizomorphs, like the honey fungus Armillaria mellea discussed later in this review. In S. lacrymans the rhizomorphs, which are linear aggregates of somatic hyphae, arise from a colonised wooden structure when residual nutrients are scarce (Kauserud et al. 2012). This organ-like cluster of hyphae grows five times faster than normal hyphae, and can extend a considerable distance away from the substrate, thus enabling the fungus to reach another host to colonise through the formation of a new mycelium (Fig. 5C). A unique system of vessels inside the hyphae of the rhizomorph allows for translocation of water and nutrients (Isaac 1991). These structures can also grow through plaster, brickwork and masonry, allowing the fungus to spread through buildings (Singh 1994).
Decomposition of wood by S. lacrymans, as with other brown rot fungi, is initiated by the biological disruption of wood polymers by hydroxyl radicals. The hydroxyl radicals are produced extracellularly via a Fenton reaction, mediated by reductants such as 2,5-dimethoxy-1,4-benzoquinone (2,5-DMBQ), which are produced by the fungus (Shimokawa et al., 2004, Korripally et al., 2013). The small size of the reductants needed to drive this reaction means that they can penetrate the intact cell wall, without the need for initial enzymatic degradation of lignin. Once the lignocellulose matrix has been disrupted by the hydroxy radicals, cellulose-degrading enzymes can then gain access. This non-enzymatic way of achieving initial degradation of the lignocellulose differs from the enzymatic approach seen in the ancestral white rot fungi (see next section). The recent sequencing of the S. lacrymans genome, together with a comparative genomic and transcriptomic analysis assessing the evolution of the brown rot lifestyle, demonstrated that brown rot species have indeed lost the enzymatic apparatus of ligninolysis (Eastwood et al. 2011).
Serpula lacrymans represents a clear example of how evolution can act both at the metabolic level – through generation of a particular degradation apparatus – and at the morphological level – via the production of specialised organ-like structures – to enable the fungus to grow in unfavourable conditions and establish its own ecological niche, making it become the most aggressive rot fungus known to the construction industry.
Phanerochaete chrysosporium
Phanerochaete chrysosporium is another resupinate, or “crust” fungus, meaning that rather than producing the fruiting bodies known as mushrooms or toadstools, which are typical of agaricomycete fungi, the fruiting body lies flat on the substrate with the hymenium at the periphery or over the whole surface of the colony, appearing as no more than a crust. It is adapted to moderate to high temperatures and as such has a fairly wide geographical distribution, covering North America, Europe and extending across to Iran.
In terms of lifestyle, P. chrysosporium is the model white rot fungus. White rot fungi are the only species capable of lignin degradation and it is this initial selective degradation of the brown-coloured lignin in the wood, leaving primarily the white-coloured crystalline cellulose, which gives them their name (Fig. 6A). The ability to degrade lignin lies, at least partly, in the ability to produce extracellular lignocellulolytic enzymes, the main examples being lignin peroxidase and manganese peroxidase. Lignin peroxidase, first isolated from P. chrysosporium in 1983 (Tien & Kirk 1983), degrades lignin via an oxidative process, the mechanism of which is thought to involve free radical pathways, utilising hydrogen peroxide as an oxygen source. Manganese peroxidase was isolated from P. chrysosporium in 1985 by two research groups simultaneously (Glenn and Gold, 1985, Paszczyński et al., 1985). This enzyme preferentially oxidises manganese(II) ions and the resulting Mn(III) ions then oxidise the phenolic compounds in lignin directly, thus attacking the numerous and varied bonds in the lignin complex (Hofrichter 2002). In nature, P. chrysosporium therefore plays an essential ecological role recycling recalcitrant lignocellulose plant debris, especially when you consider that lignin is the most abundant aromatic carbon in the biosphere (Hofrichter 2002). Scarce nutrients such as nitrogen, phosphorus and sulphur, which are embedded in lignin tissues, are also released and made available. The degradation products of lignin can then be internalised by P. chrysosporium, where diverse intracellular enzymes such as cytochrome P450-dependant oxidases further metabolise them. An incredible repertoire of P450s (more than 150) has been identified within the genome of P. chrysosporium (Doddapaneni et al. 2005). The free radical based mechanisms of lignases, giving them broad specificity, combined with the incredible enzymatic capabilities of P. chrysosporium combine to make this species capable of degrading a very wide range of compounds, including aromatic, alicyclic, and aliphatic compounds.
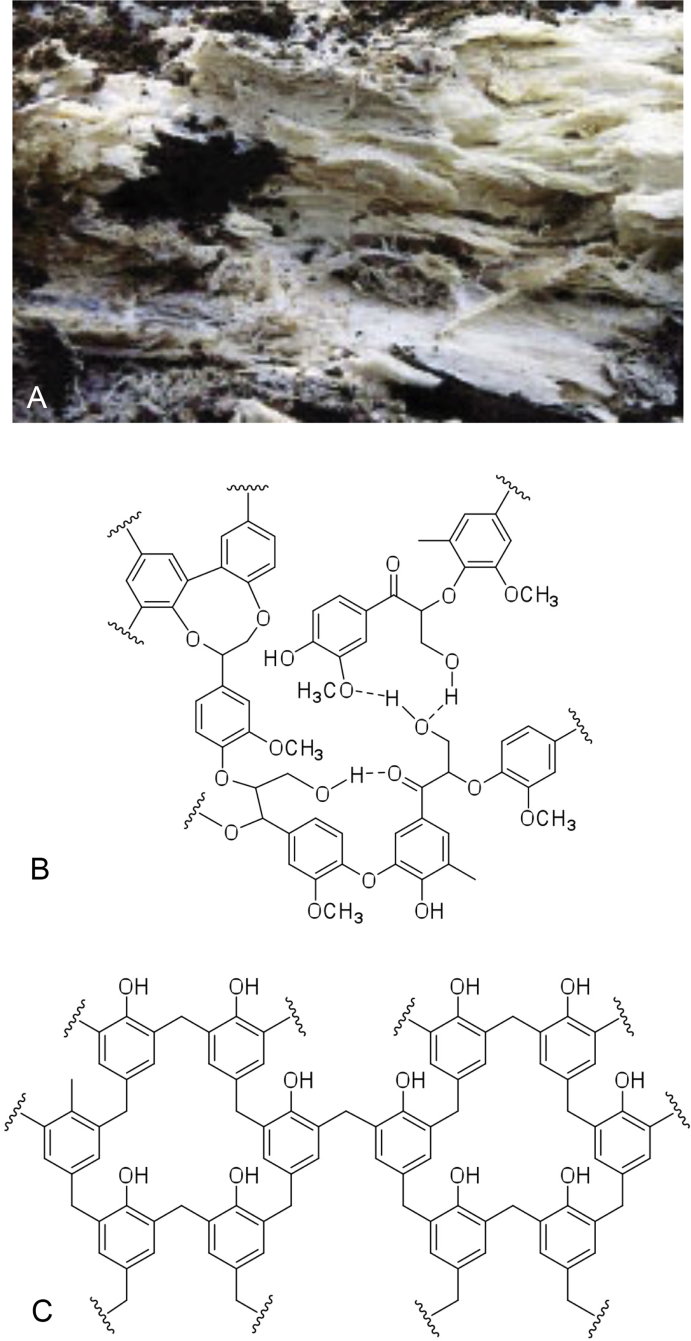
Fig. 6.
A.Phanerocheate chrysosporium, a white rot fungus, so-called due to the bleached appearance of wood degraded by such species. B–C. Show the structures typical of lignin and phenol-formaldehyde resins respectively. The structural similarities mean that the natural metabolic capabilities of fungi such as P. chrysosporium can be used to degrade – and therefore recycle – persistent man-made materials such the phenol-formaldehyde resins.
It is these metabolic capabilities of P. chrysosporium, which gives this species huge potential in various applications. Firstly, within the biofuel industry, where lignocellulosic biomasses from either agricultural or forestry wastes are abundant, but to be used in biofuel production they require hydrolysis into simple sugars. Harnessing the lignin-degrading repertoire of enzymes produced by white rot fungi such as P. chrysosporium is one approach being used to release the cellulosic material from its lignin component (Chaturvedi & Verma 2013).
The high specificity with which P. chrysosporium degrades lignin, leaving cellulose largely intact, combined with its fast growth rate and thermostability, means it could also be employed by the paper pulping industry as a method of biobleaching. The dark colour of pulp and paper mill effluent makes it a significant environmental polluter. By limiting photosynthesis and increasing water temperature, the effluent leads to a decrease in dissolved oxygen concentrations in aquatic ecosystems (Kringstad & Lindstroem 1984), and traditional bleaching agents are also environmental pollutants, making biobleaching an attractive alternative.
Phanerochaete chrysosporium could also be used to recycle or degrade materials previously thought to be entirely non-biodegradable. For example, in 2006, P. chrysosporium became the first organism known to be capable of degrading phenolic resins (PRs) (Gusse et al. 2006), which have structural similarities with lignin (Fig. 6B–C). PRs are complex synthetic polymers made from phenol and formaldehyde, which are thermoset and are incredibly stable and resistant to degradation. It is this durability that makes them so popular, particularly in the construction industry, but it also makes them extremely difficult to degrade or recycle, so most PR products become permanent fixtures in landfills after their initial use. Although methods for recycling the synthetic polymer have been developed, they typically involve intensive heat and the use of solvents, which introduce additional waste management problems and make them too costly to be adopted on a large scale. With the global PR consumption recorded as 3 200 000 metric tons in 2004, and demand still growing (Pilato 2010), it is important that a method is developed to recycle and reuse these materials.
In addition to recycling PRs, another application of this species to the phenolic residue industry is to detoxify the phenol containing effluents. Such phenolic industrial effluents are severely toxic to aquatic life, and are harmful to human health, even in low concentrations, so represent a severe environmental pollutant, which requires addressing. A recent publication has demonstrated the applicability of specialised P. chrysosporium pellets made up of cells called chlamydospore-like cells (CLCs) for treating industrial wastewater containing phenolic compounds (Hailei et al. 2016). In CLC cells the ligninolytic enzymes are not excreted and as such they act as naturally immobilised enzymes, providing a low cost biomaterial for phenol wastewater treatment. This would also apply to phenol containing effluents from refineries, petrochemical, iron and steel industries.
The degradation of phenolic compounds is just one example of the bioremediation potential of white rot fungi such a P. chrysosporium. Other toxic compounds degraded by P. chrysosporium include DDT, TNT, PCBs and PAHs. PAHs are of particular concern due to their carcinogenic and mutagenic effects, such as the highly carcinogenic benzo(a)pyrene found in coal tar, which has been shown to be metabolised by P. chrysosporium (Bhattacharya et al. 2012). One potential application of such fungal degradation capabilities would be to decontaminate soil in industrial sites to allow safe redevelopment.
The final application of P. chrysosporium that will be mentioned here is as a biosorption agent. Biosorption (the binding and concentration) of heavy metals has been demonstrated by various fungal species. P. chrysosporium has been shown to be efficient at absorbing cadmium(II), lead(II) and copper(II), so there is great potential to use this species to safely remove metals from wastewater and contaminated environments (Yetis et al. 1998). A technique has recently been developed to use modified P. chrysosporium to remove the highly toxic chromium(VI) (Chen et al. 2011).
Toxic basidiomycetes
The production of mycotoxins by basidiomycetes, and the resulting risk associated with ingesting certain species, is well known. Most mushroom poisoning, also known as mycetism or mycetismus, occurs following misidentification of a toxic species as an edible one and this accounts for hundreds of deaths globally each year (Graeme 2014). There are also, however, reports of poisonous mushrooms being used in assassinations. One such story is that of the Roman Emperor Claudius, said to have been deliberately poisoned by his wife Agrippina who laced a meal of Caeser's mushrooms (Amanita caesarea) with the death cap mushroom (Amanita phalloides) (Wasson 1972). Certain mycotoxin producing basidiomycetes are also intentionally ingested due to their psychedelic effects (Tyls et al. 2014).
In this section we will discuss Psilocybe semilanceata, the best known of the ‘magic mushrooms’, and Amanita muscaria, the quintessential toadstool and type species of the Amanita genus. Although reports of fatalities from A. muscaria poisoning are extremely rare, it is closely related to some of the most poisonous species known, including the destroying angels (A. virosa and A. bisporigera) and the death cap (A. phalloides) (Allen et al. 2012).
Psilocybe semilanceata
Psilocybe semilanceata is one of the best-known of the psychedelic mushrooms. This species is commonly known as the liberty cap due to its resemblance to the Phrygian cap; a cap which originated in the ancient country of Phrygia in Asia Minor but became a symbol of liberty during the French revolution, gaining the name of the ‘liberty cap’. The Psilocybe genus is distributed worldwide, existing in most biomes, with the greatest species diversity being found in the neotropic ecozone, and particularly Mexico (Guzmán et al., 1998, Ramírez-Cruz et al., 2013). The genus is now restricted to the hallucinogenic species with P. semilanceata as the conserved type species (Redhead et al., 2007, Ramírez-Cruz et al., 2013). This species is most commonly found in the cool temperate regions of the northern hemisphere with maritime influence, particularly bicoastal Northern America and Europe (Guzmán, 1983, Redhead, 1989). It has been recorded in some warmer or more southern climates, such as India (Barthakur et al. 2000) and Chile (Guzmán 2009), but in these regions related species are more prevalent and the reports are now suspect.
The preferred habitats of P. semilanceata are grassy meadows and fields, particularly north facing fields that have been fertilised by the faeces of ruminants such as sheep and cattle. Unlike the related psychedelic species P. cubensis, P. semilanceata does not grow directly on dung but is saprobic and feeds off decaying grass roots nearby (Keay & Brown 1990). It typically has fruiting bodies with a small cap (5–25 mm diameter and approximately twice as tall), which are hygrophanous, meaning the colour and appearance depends on the state of hydration. When dry it is pale in colour, a light yellow brown, but when wet it ranges from light to dark chestnut brown and has radial striations that correspond to the gills underneath (Fig. 7A). The gills are only very slightly attached to the stalk (adnexed), and are pale when young, becoming a darker purple-black as the spores mature (Musshoff et al. 2000).
Fig. 7.
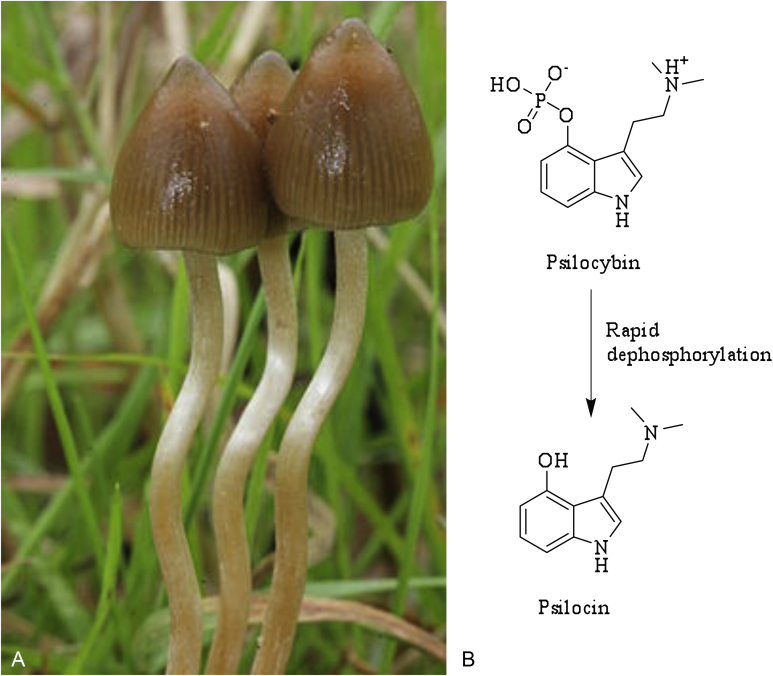
A.Psilocybe semilanceata (Copyright © 2012 Alan Rockefeller), commonly known as the liberty cap, is one of many basidiomycetes which produce the metabolite psilocybin. B. In the body psilocybin is quickly dephosphorylated into the active compound Psilocin.
Plants and fungi have been exploited for their psychotropic effects since prehistoric times (Guzmán 2009), and although some such ritualistic traditions – particularly by the Aztecs – are well documented, there is a relatively sparse record for P. semilanceata use. The first reliable record of P. semilanceata intoxication, which was accidental rather than intentional, was published in 1799 (Brande 1799). A British family had collected mushrooms from Green Park in London to use in a meal. The family's father and four children were reported to experience symptoms now known to be typical of psilocybin ingestion, including spontaneous laughter, delirium and pupil dilation. Evidence for the ritual use of a Psilocybe species in prehistoric Europe has recently been uncovered for the first time, in the form of post-Paleolithic rock art in Spain, which is thought to be 6 000 yr old (Akers et al. 2011). However, this is thought to depict the use of Psilocybe hispanica, a closely related mushroom endemic to a neighbouring region of Spain. The oldest suspected depiction of psilocybin use (approx. 7 000–9 000 yr old) is a painted mural from southeast Algeria, portraying mushrooms that have tentatively been identified as P. mairei (Akers et al. 2011).
The psychotropic effects of Psilocybe mushrooms are due to the presence of the compounds psilocybin and psilocin. These compounds were first isolated, identified and named by the chemist Albert Hoffmann in 1959, from samples of P. mexicana sent to him by the French mycologist Roger Heim (Hofmann et al. 1959). Psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine) is a prodrug which is quickly hydrolysed in the body to give psilocin (4-hydroxy-N,N-dimethyltryptamine), the pharmacologically active compound (Fig. 7B). This compound acts on serotonin receptors in the brain by acting as a partial agonist at the serotonergic receptors 5-HT1A and 5-HT2A (Passie et al. 2002). The commonly reported physical effects include pupil dilation, changes in heart rate, nausea, tremors, changes in blood pressure and dysmetria. The psychological effects are highly variable and influenced by the mindset and environment of the user, but can include giddiness, euphoria and joy but also depression, anxiety and paranoia. Hallucinations and distortions in the perception of time are also common, so although the mind-altering effects of psilocin typically last from two to six hours, it is reported that time can seem significantly longer for the user.
Although most cases of ingesting the liberty cap occur without incident or hospitalisation, and death from psilocybin alone has never been recorded, some isolated cases have had severe consequences. In 1998, an 18-yr-old man suffered from Wolff–Parkinson–White syndrome, arrhythmia and a myocardial infarction. In 2009, a teenager was reported to suffer cardiac abnormalities usually seen in Tako-Tsubo cardiomyopathy (Nef et al. 2009). Inaddition to the direct effects of P. semilanceata ingestion, there have been cases of novice collectors confusing P. semilanceata for a toxic basidiomycete called Cortinarius rubellus. In at least one case this resulted in end-stage renal failure, requiring a kidney transplant (Franz et al. 1996).
As well as being produced by P. semilanceata, psilocybin is produced by upwards of 200 fungal species. The majority (over 150 species) belong to the Psilocybe genus, with multiple other species belonging to the genera Gymnopilus, Panaeolus and Copelandia, amongst others (Guzmán, 2005, Guzmán, 2009). Possession of any psilocybin containing “magic mushroom”, including P. semilanceata, is now illegal in most countries, although for a time after its discovery, pure psilocybin was marketed and sold to physicians and clinicians by Sandox (now Novartis), for use in psychedelic psychotherapy (Livingstone 2015). Recently there has been a resurgence in interest in the potential medicinal benefits of psilocybin, particularly as a treatment for OCD (Wilcox 2014), and cluster headaches (Sewell et al. 2006).
Amanita muscaria
Amanita is one of the best-known fungal genera, containing some of the most poisonous species of basidiomycetes known. Since this genus contains a broad range of equally interesting and conspicuous species, we have decided to describe the Amanita taxon in general terms, with emphasis on the type-species, Amanita muscaria. Amanita muscaria, the fly agaric, has long been recognised for its acknowledged toxicity, its use for recreational purposes and its insecticidal properties.
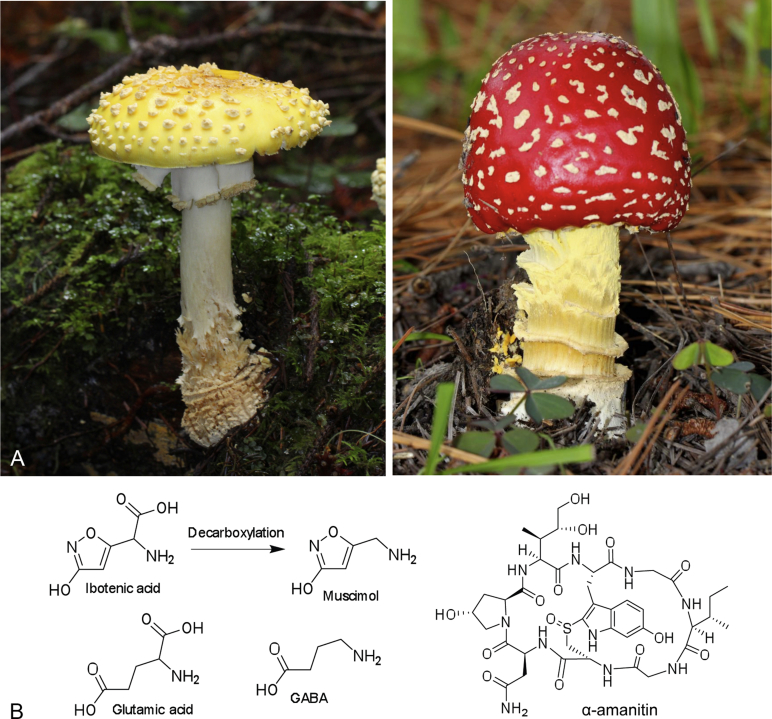
Amanita are mycorrhizal in nature, having symbiotic relationships with a wide range of deciduous and coniferous trees, while related genera (Limacella, Saproamanita) in the family Amanitaceae are likely saprotrophic (Redhead et al. 2016). Indeed, a recent project that sequenced the genome of A. muscaria found that much of the wood-degrading machinery has been lost from this species, likely due to its adaptation to a mycorrhizal life-style (Wolfe et al., 2012, Kohler et al., 2015). Individual species can often establish interactions with multiple host plants belonging to different taxa (Geml et al. 2008); A. muscaria for example has been reported to colonise the root environment of birch (Betula spp.), as well as that of larch, pine and the Norway spruce, Picea abies (Hudson 1986). The basidiocarps formed by fungi of this genus are generally characterised by the presence of annulus, volva, free gills and white spore print, and can be a diverse range of colours (Alexopoulos et al. 1996). The most famous representatives of this taxon include Amanita caesarea (Caesar's mushroom), which produces an orange-yellow edible mushroom, the poisonous Amanita virosa (the European destroying angel), with a white basidiocarp, as well as the deadly Amanita phalloides (the death cap), which produces an olive-green toadstool, and is estimated to cause a striking 90–95 % of all deaths from fungal poisoning in Europe (Litten 1975). The basidiocarps of A. muscaria can reach up to 50 cm diameter (Michelot & Melendez-Howell 2003), and can be bright red, orange or yellow, scattered with white scales or patches (Fig. 8A), and can sometimes be mistaken for that of the edible A. caesarea. A. muscaria owes both its scientific and common names to its documented insecticidal properties against the house fly (musca in Latin). The toxicity of the fly agaric, as well as that of the related species A. pantherina, is believed to be based upon the activity of two specific toxic compounds: ibotenic acid and muscimol (Takemoto et al., 1964, Chilton and Ott, 1976). Ibotenic acid is an amino acid analogue of glutamic acid, and once ingested, the low pH of the gastrointestinal tract causes it to be decarboxylated into muscimol (Fig. 8B). Muscimol acts as an analogue of gamma-aminobutyric acid (GABA). Both ibotenic acid and muscimol can cause neuronal degeneration by targeting glutamic acid and GABA receptors, respectively (Schwarcz et al., 1979, Taira et al., 1993).
Fig. 8.
A.Amanita muscaria toadstools demonstrating the range of colours and fruiting body shapes observed for this species. The scales seen on the caps of this species are remnants of the universal veil that surrounds the immature fruiting body (Copyright © Alan Rockefeller). B. Toxic compounds produced by Amanita fungi. Ibotenic acid and muscimol act as analogues of the amino acid glutamic acid, and the neurotransmitter GABA, respectively. Ibotenic acid is decarboxylated to muscimol in the body. α-amanitin is considered one of the most deadly of the amatoxins, all of which inhibit RNA polymerase.
Looking at the whole taxon, fungi of the genus Amanita can produce a wide range of mycotoxins that can be classified into three main groups of cyclic peptides: amatoxins, phallotoxins and virotoxins (Alexopoulos et al. 1996). Amatoxins are inhibitors of DNA-dependent RNA polymerase II, leading to impaired gene transcription, a resulting reduction in protein synthesis, and ultimately apoptosis of the cell or necrosis (Buku et al. 1971). The structure of α-amanitin, considered to be one of the most deadly of all the amatoxins, is shown in Fig. 8B. Phallotoxins and virotoxins induce polymerisation of G-actin, promoting an anomalous stabilisation of F-actin filaments (Wieland 1977). Beyond their importance as toxins, these natural products from Amanita species are significant as the first ribosomally synthesised and post-translationally modified peptides (RiPPs) identified from fungi (Hallen et al., 2007, Walton et al., 2010). RiPPs are a recently recognised class of secondary metabolites that have been gaining significant attention in recent years (Arnison et al. 2013).
Poisoning by toxic Amanita species typically proceeds through four stages post ingestion (Alexopoulos et al. 1996). The first stage is a latent phase that can last between 6 and 12 h, where, due to the slow action of the mycotoxins, no symptoms are reported. In the second phase there is appearance of vomiting, diarrhoea, and abdominal pain, which all together can also lead to severe dehydration. The third stage is characterised by a temporary and apparent improvement in the condition of the patient. However, the toxins have by now started inhibiting cellular metabolism. In the fourth stage liver and kidney failure usually occur, and may be accompanied by internal bleeding due to an altered coagulation capability of the blood. This can lead to death as fast as 6 to 8 d after consumption of the poisonous mushrooms. The outcome of the poisoning depends however on several factors, such as the amount of mushrooms consumed, the health and weight of the consumer, as well as the timing of the medical treatment offered. It should also be noted that each of the Amanita species contain its own pool of toxic substances, which may be more or less dangerous for humans. In the specific case of A. muscaria poisoning, death is not the usual outcome (Michelot & Melendez-Howell 2003).
The fly agaric has long been known for its psychotropic and hallucinogenic effects, which are believed to be due to the two toxic amino acids mentioned above, ibotenic acid and muscimol (Scotti de Carolis et al. 1969). Amanita muscaria usually contains only trace amounts of muscarine, which makes this species an attractive choice for drug consumers. In low concentrations, muscarine can cause unpleasant side effects by mimicking acetylcholine, which include sweating, salivation and pupillary contraction (Bhatnagar et al. 1971). However, in high doses, muscarine can lead to death. It is believed that the recreational use of A. muscaria dates back many centuries and spreads across different civilisations, largely being consumed as an entheogen during ceremonies by Siberian tribes (Rolfe & Rolfe 1925), as well as being the basis of a fertility cult in Christianity (Allegro 1970). Wasson hypothesised that soma, a ritual drink consumed in honour of the homonymous Hindu God of the moon, was made using A. muscaria extracts (Wasson 1968).
Medicinal basidiomycetes
The medicinal properties of mushrooms have been known about for millennia and were exploited for the benefit of humans by many ancient civilisations, including those in Ancient China, Eastern Europe, Mesoamerica and Africa (Wasser 2011). This tradition has continued into the present day, particularly in China, Korea and Japan, with modern clinical practices involving the use of mushroom preparations. Polysaccharide Kureha (PSK) – a natural product from Trametes versicolor – is approved for use in cancer treatment in Japan. Isolated in the 1960s, by 1987 over 25 % of the Japanese expenditure on anticancer agents was spent on PSK (Sullivan et al. 2006), showing the value placed on medicinal mushroom products in certain cultures.
The health promoting activities attributed to mushrooms are many, including antibacterial, antiviral, antiparasitic, anticancer, cardioprotective and cholesterol lowering. For instance, over 200 species of mushroom have been reported to markedly inhibit the growth of various tumours (Wasser & Weis 1999). Although traditionally whole mushroom extracts have been used, some fruiting bodies are scare and with a greater understanding of natural product science, individual compounds with beneficial pharmacokinetic properties can now be identified and purified.
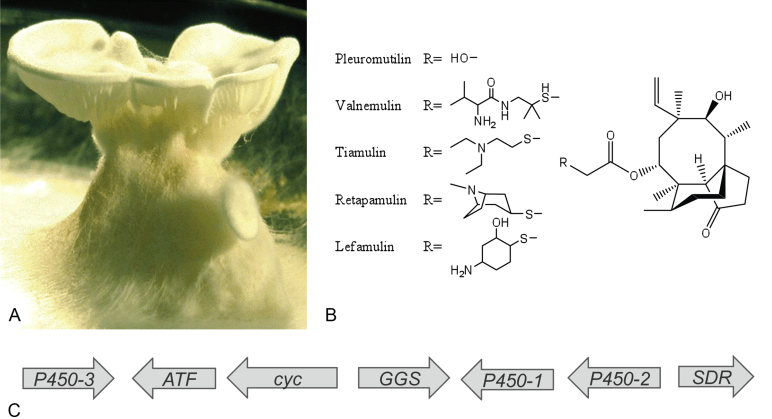
In this review, we will discuss two basidiomycetes that have contributed to medicine in very different ways: Ganoderma lucidum, a fungus which has been used in traditional medicine for thousands of years, and Clitopilus passeckerianus, which produces pleuromutilin, the lead compound for the pleuromutilin class of antibiotics.
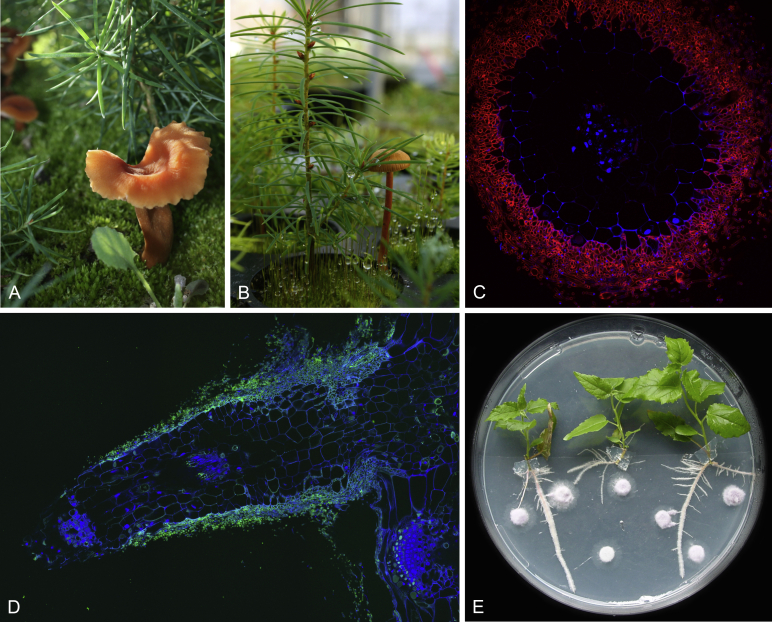
Ganoderma lingzhi and the G. lucidum species complex
Ganoderma lingzhi is a highly revered medicinal mushroom, which has been attributed with a plethora of health benefits and has been used in traditional medicine for thousands of years (Bishop et al. 2015). Belonging to the G. lucidum species complex (Zhou et al. 2015), the taxonomy of G. lingzhi and related species has only recently begun to be elucidated. Differentiating between the members of the species complex using traditional morphological techniques is problematic, with all species being morphologically similar, and all producing conspicuous fruiting bodies with red, shiny, kidney-shaped caps, the flesh of which are soft and corky (Fig. 9A–B). The advent of sequencing is now allowing a better understanding of the species complex to emerge (Hseu et al., 1996, Zheng et al., 2009), and there are currently considered to be 13 member species found globally (Zhou et al. 2015). There is a growing consensus that the Ganoderma species traditionally used in Asian medicine is G. lingzhi (Cao et al., 2012, Kwon et al., 2016), and as such a large proportion of the research conducted on the medicinal benefits of Ganoderma species will have been conducted using G. lingzhi, not the type-species G. lucidum. The authors of this review will thus refer to the Ganoderma lucidum species complex when referencing previous work, and advise caution when assuming species identity.
Fig. 9.
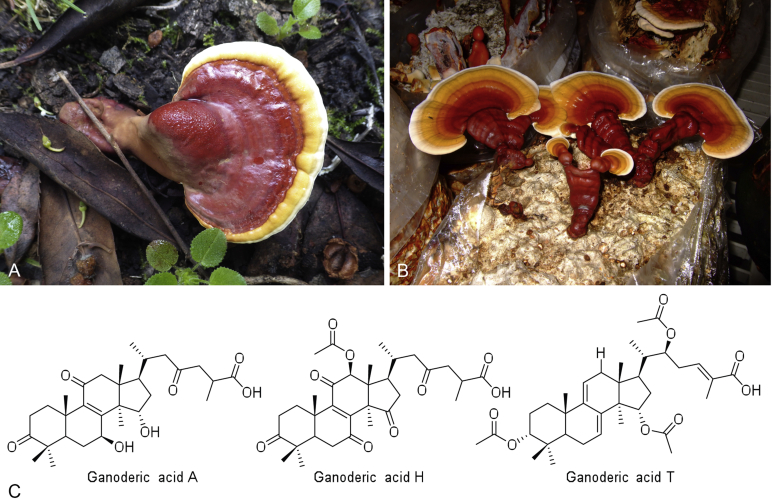
A–B. The brightly coloured kidney shaped basidiocarp of Ganoderma lucidum species, in the wild (A: Copyright © zaca) and in cultivation (B). C.G. lingzhi produces a wealth of active secondary metabolites including hundreds of ganodermic acids. Ganoderic acids A and H have been shown to suppress the growth and spread of breast cancer (Jiang et al. 2008). Ganoderic acid T has been shown to induce mitochondria-mediated apoptosis in lung cancer cells (Tang et al. 2006).
The genus Ganoderma, which means shining skin, was named by the Finnish mycologist Petter Adolf Karsten in 1881 (Karston 1881). Ganoderma belongs in the Polyporaceae family of Agaricomycetes, so called due to the presence of many small pores on the underside of the fruiting bodies, which contain the reproductive spores. The generic type-species, Ganoderma lucidum, was initially reported from the UK and has since been considered to be distributed globally, although the actual distribution of individual member species is unclear due to the historical taxonomic confusion surrounding this species complex. See Zhou et al. (2015) for a recent study of the global phylogeny of this group of fungi.
Ganoderma lucidum species are known as reishi or mannamtake (10 000-yr mushroom) in Japan and ling zhi (mushroom of immortality) in China, where they are seen depicted in ancient art such as ceramics, paintings and carpets. Ganoderma fruiting bodies can even be seen on the facades of the Emperors Palace of the Forbidden City in Beijing (Jones 1998). The use of these mushrooms is not, however, restricted to ancient times. Various products have been developed in the form of powders, teas and dietary supplements and worldwide sales of such reishi extracts have been estimated to exceed $2.5 billion annually (Li et al. 2013), with sales increasing at 18 % per year (Sudheer et al. 2016).
The medicinal effects attributed to members of the G. lucidum species complex are said to include anti-cancer, cardioprotective, immunomodulatory, anticonvulsant, neuroprotective, hypoglycaemic, hepatoprotective, cholesterol lowering, anti-inflammatory, fungicidal, antibacterial and anti-viral, including anti-HIV. Although many of these claims are based on anecdotal evidence, an increasing number are now being demonstrated using more rigorous scientific approaches. Studies using mouse models, for example, have demonstrated the hypoglycaemic (Xiao et al. 2012) and LDL-cholesterol lowering (Oluba et al. 2012) effects of Ganoderma extracts. One study using rats found that Ganoderma extracts enhanced the activity of heart mitochondrial enzymes and respiratory chain complexes, partially ameliorating the age-related decline in cellular energy production (Sudheesh et al. 2009). The most widely studied aspects of G. lucidum species, however, are the immunomodulatory and anti-cancer properties.
The effects of Ganoderma species on a wide range of cancers have been investigated, including leukaemia, bladder cancer, breast cancer, cervical cancer, colon cancer, hepatoma and gastric carcinoma. A meta-analysis concerning the overall efficacy of extracts found that when used in conjunction with chemotherapy, the outlook for cancer patients was significantly improved and immune stimulation was observed (Jin et al. 2012). However, it also concluded that Ganoderma extracts are not suitable as a first-line treatment for cancer, as when used alone no significant effect on tumour size could be demonstrated.
The mechanisms behind the observed anti-cancer effects are complex and multiple. Different studies have identified different mechanisms, and the contribution of individual mechanisms is likely to vary depending on the chemical composition of the extract used and the type of cancer. A recent review, which analysed over 270 patents and peer-reviewed articles concluded that the anti-cancer effects of Ganoderma were due to a combination of at least five different mechanisms: (1) activation/modulation of the immune response of the host, (2) direct cytotoxicity to cancer cells, (3) inhibition of tumour-induced angiogenesis, (4) inhibition of cancer cell proliferation and metastasis and (5) deactivation of carcinogens (Boh 2013).
Significant work has been done towards identifying the specific bioactive compounds of Ganoderma lucidum species. To date over 300 bioactive compounds have been detected from fruiting bodies, including fatty acids, nucleotides, polysaccharides, glycoproteins, sterols, steroids, proteins, peptides and triterpenoids (Xia et al. 2014). Peptidoglycans, polysaccharides and triterpenoids have attracted particular attention as the major physiologically active constituents. For example, G. lucidum proteoglycan (GLPG) has been shown to exhibit antiviral activity against type 1 and type 2 herpes simplex virus (Li et al. 2005). Specific polysaccharides have demonstrated antitumour (Miyazaki & Nishijima 1981), hypoglycaemic (Hikino et al., 1985, Tomoda et al., 1986) and immunological (Bao et al. 2001) activities. The anticancer effects of G. lucidum triterpenoids (GLTs), of which over 140 have been isolated and characterised (Wu et al. 2013a), have been repeatedly demonstrated using both in vitro and in vivo studies. For example, ganoderic acid T has been shown to induce mitochondria-mediated apoptosis in lung cancer cells, and has therapeutic potential as an agent against high metastatic lung carcinoma (Tang et al., 2006, Xu et al., 2010). Ganoderic acids A and H have been shown to suppress both the growth, and invasive behaviour of human breast cancer cells (Jiang et al. 2008) (Fig. 9C).
In nature, Ganoderma lucidum species grow as saprotrophs at the base, or on stumps, of deciduous trees, particularly maples. Ganoderma lucidum species are widely, though not abundantly, distributed, being found in tropical and subtropical climates on various continents, including Europe, North and South America and Asia (Zhou et al. 2015). Due to the natural scarcity of Ganoderma fruiting bodies, and the increased demand for Ganoderma extracts, various cultivation methods have been developed. The specific variables favoured for cultivation depend on the species or isolate used and also the desired product. For example, classical basidiocarp cultivation techniques have been applied, using substrates such as grain, sawdust and wood logs (Erkel, 2009, Cilerdzic et al., 2014). However, this process is very time-consuming, so an alternative approach uses submerged fungal cultures, which produce only the vegetative fungal mycelia. This has been demonstrated as a promising approach for the production of various compounds including polysaccharides (Fang and Zhong, 2002, Wagner et al., 2003, Habijanic et al., 2015). Genetically engineering strains to increase the production of ganodermic acids in submerged cultures has also enjoyed some success (Zhou et al., 2014, Xu et al., 2015, Li et al., 2016). As well as being a faster approach, the use of submerged cultures allows improved control over various fermentation conditions such as nutrient availability, pH and oxygen, which allows optimisation and greater reproducibility. Reproducibility, both in terms of fungal yields, but also the chemical composition of the resulting extracts, will become increasingly important if Ganoderma extracts and purified compounds are to become recognised and regulated medicinal products. The development of such specific bioactive compounds as targeted therapeutic agents would be a great step forward for Ganoderma research.
In 2012, two research groups simultaneously published draft genomes for G. lucidum of 39.9 Mb (Liu et al. 2012) and 43.3 Mb (Chen et al. 2012). A rich array of genes linked to secondary metabolite biosynthesis were revealed, including P450s, regulatory genes and transporters (Chen et al. 2012) and a putative ganodermic acid pathway has been proposed (Liu et al. 2012). Both groups also recognised the presence of a diverse set of wood degrading enzymes, as is fitting with the white rot lifestyle of Ganoderma lucidum species.
Clitopilus passeckerianus
Clitopilus passeckerianus may not be as publically well known as most of the species described in this review. However, it was included here for being the only basidiomycete to date to produce an antibiotic that has led to the generation of commercial derivatives, which are successfully used both in human and veterinary medicine.
Clitopilus passeckerianus is an Agaricale of the taxon Entolomataceae, a family that contains more than 1 500, predominately saprotrophic, species (Co-David et al. 2009). As with other species of the Clitopilus genus, C. passeckerianus is endemic to temperate northern regions, and produces characteristic pink angular spores with longitudinal ribs (Co-David et al. 2009). Relatively little is known about the general biology of this fungus, although it has been reported as an occasional contaminant of commercial mushrooms beds. What is presently understood is that the isolate of C. passeckerianus currently used for the industrial production of pleuromutilin is dikaryotic, as inferred from the production of hyphal knots and mature fruiting bodies (Fig. 10A) (Hartley et al. 2009), and the sequencing of multiple alleles for certain genes (Bailey et al. 2016).
Fig. 10.
A. A fruiting body of Clitopilus passeckerianus, the pleuromutilin producing basidiomycete (Photograph taken by Tim Colborn). B. The chemical structure of pleuromutilin, the natural product, and the various pleuromutilin derivatives. C. The pleuromutilin gene cluster, recently identified by Bailey et al. (2016), contains the seven genes required to biosynthesise the antibiotic pleuromutilin: A geranylgeranyl disphosphate synthase (GGS), a cyclase, (cyc), three cytochrome P450s, an acetyl transferase (ATF) and a short-chain dehydrogenase/reductase (SDR).
The first report of antibiotic production in C. passeckerianus came in 1951 from Kavanagh et al. (Kavanagh et al. 1951), who first discovered that two species then classified in the genus Pleurotus (now known as Clitopilus species), Pleurotus passeckerianus and Pleurotus mutilus, were producing an antibacterial substance active on Gram-positive bacteria, and named it pleuromutilin. Despite several reports of production of pleuromutilin from other Agaricales, such as Drosophila subatrata (now known as Parasola conopilus) (Kavanagh et al. 1952), controversy on the taxonomy of pleuromutilin-producing fungi was clarified only recently when eleven pleuromutilin-producing isolates were found to fall within a distinct clade of the genus Clitopilus (Hartley et al. 2009).
Ten years after its discovery, Arigoni (Arigoni 1962) and Birch (Birch et al. 1966) independently unveiled the chemical formula of pleuromutilin (Fig. 10B), also proving its diterpene nature. Although pleuromutilin itself has modest antibacterial activity and has not been used as a commercial antibiotic, extensive structure–activity relationship studies have been done on more than 1 200 semi-synthetic derivatives with the aim of enhancing MIC and improving pharmacokinetic properties (Novak 2011). This led first to the commercialisation of two pleuromutilin-derived antibiotics employed in veterinary field: tiamulin (Sandoz) (Drews et al. 1975) and valnemulin (Novartis) (Aitken et al. 1999), both used on swine and poultry as a treatment and prophylaxis agent for dysentery caused by Gram-positive bacteria and Mycoplasma spp. infections. In 2007 the first pleuromutilin antibiotic was approved for the use in human medicine, retapamulin (GlaxoSmithKline) (Rittenhouse et al. 2006), and has since been used as a topical treatment for impetigo skin infections, typically caused by Gram-positive bacteria like Staphylococcus aureus and Streptococcus pyogenes. Research is still ongoing to develop novel pleuromutilin derivatives that could be employed as systemic antibiotics in human medicine (Ling et al., 2014, Zhang et al., 2015). One very promising compound is lefamulin, a pleuromutilin derivative developed by Nabriva Therapeutics that is soon to be entering phase III clinical trials for the treatment of moderate to severe CAPB (community-acquired bacterial pneumonia) (Prince et al., 2013, Rubino et al., 2015, Waites et al., 2016). Fig. 10B shows the structures of pleuromutilin and the various derivatives.
The success of pleuromutilin antibiotics, as the first class of antimicrobial agents produced from a basidiomycete fungus to be commercialised, is partly due to their low potential to induce resistance in targeted bacteria, as seen with retapamulin and the pathogens S. pyogenes and S. aureus (Kosowska-Shick et al., 2006, Rittenhouse et al., 2006). Moreover, pleuromutilin antibiotics have a specific mode of action that relies on binding to the 50S ribosomal subunit in bacteria at the level of the peptidyl transferase centre (PTC) (Hodgin & Högenauer 1974), which shows low cross-resistance with antibiotics having a similar mode of action (Jones et al., 2006, Williams et al., 2007).
The rising need for new antibiotics and the full exploitation of those already known, means increasing attention has been paid to pleuromutilin and its producing organism in recent years. Firstly, studies were conducted with the aim of enhancing antibiotic titres from C. passeckerianus by the traditional approach of random mutagenesis (Papa et al. 2006). The biosynthetic pathway to the antibiotic was proposed by Tsukagoshi et al. (2007) based on the results of feeding experiments with isotopically labelled predicted intermediates. Total synthesis has been achieved, so far adopting three different strategies, as reviewed by Fazakerley and Procter (Fazakerley & Procter 2014), however so far these have always resulted in low production yields that could not be translated into an industrial route of production. A protocol for the transformation of C. passeckerianus has been established (Kilaru et al. 2009a), and subsequent attempts to increase pleuromutilin production through genetic engineering of the native host have met with some success (Bailey et al. 2013a). Finally, the most recent publication has reported the discovery of the pleuromutilin biosynthetic gene cluster of C. passeckerianus (Fig. 10C) and exploited it in a heterologous system to establish increased production titres (Bailey et al. 2016).
It is worth noting that the various bioactivities demonstrated by fungal natural products, such as antibacterial, antiviral, and antifungal can be harnessed not only for medical purposes, but also to protect crop species from disease and therefore play an important role in food security. The strobilurins for example, are a class of antifungal compounds first isolated from the basidiomycete species Strobilurus tenacellus. First launched in 1996, the strobilurins quickly became one of the most important classes of agricultural fungicides (Bartlett et al. 2002).
Symbiotic basidiomycetes
Symbiotic relationships are found across all Kingdoms of life and are vital to the healthy functioning of ecosystems. Mycorrhizal fungi, which form close symbiotic associations with plants, are a perfect example of this. They colonise their host’s roots, either intracellularly as arbuscular mycorrhizal fungi, or extracellularly as ectomycorrhizal fungi. In these relationships the plant provides the fungus with sugars produced by photosynthesis and in return the fungus provides the plant with various nutrients such as nitrogen and phosphorus. The fungi also play a vital role in protecting the plants from pollutants, for example by retaining heavy metals. As a result of this interaction, mycorrhizal plants have an increased growth rate and are less susceptible to various stress factors (Feng et al., 2002, Schützendübel and Polle, 2002, Woodward et al., 2012). This plant/fungal partnership is thought to have been essential for the early colonisation of land by plants. The surprising ability of endophytic fungi to colonise genetically divergent crop species forms the basis of symbiogenic technology, where seeds or seedlings are inoculated with fungi that then confer fitness benefits to the crops. This technology is increasingly being developed and applied as a way of sustaining agricultural productivity in the face of 21st century climate change (Woodward et al. 2012).
Many of the species discussed in this review form mycorrhizal relationships with plant species, including many of the Amanita mushrooms, but in this section we will specifically discuss Laccaria bicolor. L. bicolor has been intensively studied and being the first ectomycorrhizal fungus to be fully sequenced, this species has become the basis for much of our understanding of these fascinating symbiotic fungi.
For our second species, we have chosen Leucoagaricus gongylophorus, an intriguing species that has an obligate mutualistic relationship with many different species of leaf-cutter ants. This is one of a number of fascinating symbiotic relationships basidiomycetes have with insect species. Others include Amylostereum areolatum, a white rot basidiomycete that provides digestive enzymes to wood wasps (Kukor & Martin 1983) and Septobasidium retiforme, which infects scale insects, obtaining nutrients that the insects get from plant sap and in return forming protective chambers in which the insects are housed (Paracer & Ahmadjian 2000).
Laccaria bicolor
Laccaria bicolor is a mycorrhizal symbiont that forms ectomycorrhizal associations with a range of tree species including varieties of birch, pine and spruce (Podila et al. 2002). L. bicolor forms small tan-coloured basidiocarps with lilac gills, which dull on ageing and can be found near the base of symbiotic tree species (Fig. 11). This tendency for the colours to fade has led to the common name of “deceiver” as the faded hues lead to difficulties in identification. The preferred habitat of the fungus is temperate, boreal forest, primarily in Northern Europe and North America (Mueller and Gardes, 1991, Mueller, 1992, Baar et al., 1994, Plett et al., 2012).
Fig. 11.
A–B.Laccaria bicolor growing as a mycorrhizal association with Douglas fir, showing fruiting bodies in nursery bed (A) and greenhouse (B) conditions. C–D. The fungus grows as an extensive mycelial network (called the mantle shealt) surrounding the root, with occasional mycelial ingrowths between cells of the root epidermis: the so-called Harting net. A transverse section (C) and longitudinal section (D) of colonised Poplar, stained with DAPI and WGA633. E. Colonisation of the root can be observed under laboratory conditions (colonisation of Poplar seedling roots on agar plates) where typically the roots show reduced extension and extraradical hyphae can be seen growing from the mantle out into the surrounding media. Images courtesy of Francis Martin, Feng Zhang, Aurélie Deveau and Anne Jambois, INRA.
Typically for basidiomycetes, the life cycle of L. bicolor is comprised of a monokaryotic and a dikaryotic stage and it is the dikaryotic stage which dominates in vegetative growth and generally forms the symbiotic relationship (Kropp 1997). Both ploidy states are capable of indefinite growth, which facilitates maintaining cultures for scientific study. L. bicolor is also capable of a dual saprotrophic and biotrophic lifestyle, capable of growing both freely in the soil and in living roots. This dual lifestyle means that unusually for an ectomycorrhizal fungus, L. bicolor can be maintained in a pure culture before co-cultivation with roots of mycorrhizal partner trees in order to study the interactions (Iwase, 1997, Siri-in et al., 2014), making it an ideal model for studying mycorrhizal fungi. An Agrobacterium-mediated transformation system has also been established for L. bicolor allowing further exploration and understanding of this ecologically important species (Kemppainen et al. 2005).
Laccaria bicolor was the first mycorrhizal mutualistic symbiont to have its entire genome sequenced, which was completed in 2008 and revealed a genome size of 65 Mbp (Martin et al., 2008, Martin and Selosse, 2008), at the time the largest fungal genome to be sequenced. The large size is thought to be partly due to an expansion in gene families allowing for the adaptation to multiple complex lifestyles. An analysis of the genome sequence, along with transcriptomic data, revealed various insights into mycorrhizal symbiosis. A large array of small-secreted proteins (SSPs) were identified, several of which were expressed exclusively in symbiotic tissues (Martin et al. 2008). The most highly expressed SSP, mycorrhiza-induced SSP 7 (MiSSP7), has now been well characterised and shown to be necessary for the establishment of the symbiotic association. MiSSP7 is secreted by L. bicolor and is targeted to the plant nucleus where it alters the transcription of the plant cell (Plett et al., 2011, Plett et al., 2012). Interestingly, it does not do this directly, but by interacting with a plant protein, JAZ6, which is a negative regulator of jasmonic acid (JA)-induced gene regulation. In this way, L. bicolor inhibits the plants JA-mediated defence signalling, allowing colonisation to occur (Plett et al. 2014).
Genome analysis of L. bicolor also identified an increased potential for nitrogen uptake when compared to other basidiomycetes, with a relatively large number of secreted proteases and a higher than average number of transporters, including ammonia transporters. This is consistent with the role of the fungus in supplying the plant host with nitrogen (Lucic et al. 2008).
Slightly surprisingly, like A. muscaria discussed earlier, genome sequencing has uncovered a reduced enzymatic capability in L. bicolor, which lacks the degradation enzymes required for the breakdown of carbohydrates in plant cell walls (Martin et al. 2008). However, unlike in the case of A. muscaria, this has not led to an obligatory symbiotic life-style. L. bicolor does appear to have the enzymatic capacity to metabolise non-plant (bacterial and animal) cell wall polysaccharides (Martin et al. 2008) and the ability to use nitrogen of animal origins, which probably contribute to its ability to live as a saprotroph as well as a biotrophic symbiont. This is compatible with reports that L. bicolor is capable of carnivory, and has been found to target springtails. The fungus is thought to produce a toxin that paralyses the insects, immobilising them to allow hyphae to penetrate the exoskeleton of the insect to utilise the nitrogen in the springtail bodies (Klironomos & Hart 2001).
Laccaria bicolor's symbiotic tendencies have long been exploited in both agriculture and horticulture where the fungus is used as a soil inoculant (Selosse et al., 1998, Müller et al., 2013), and it has also been used to aid reforestation projects by increasing the survival rates of saplings. Such intentional partnering of fungi and plants, a form of symbiogenic technology, is an exciting and rapidly growing field of research.
Leucoagaricus gongylophorus
Leucoagaricus gongylophorus, known as Attamyces bromatificus when in its asexual anamorphic state (Kreisel 1972), is a basidiomycete that has an obligate mutualistic relationship with approximately 40 species of leaf-cutter ants found throughout warm-temperate Central and South America and southern North America (Weber, 1966, Kooij et al., 2014a). The higher attine ants, of the genera Atta and Acromyrmex, cultivate L. gongylophorus by cutting sections of leaves and flowers (Fig. 12A) from various plants to create a subterranean nest: a “fungal garden”, consisting of plant material and L. gongylophorus and housing the ant queen and her brood (Fig. 12B). In this mutualism, the ants provide the fungus with nutrition and protection from competitors and pathogens and the fungus produces specialised swollen hyphal tips known as gongylidia (Fig. 12C) to feed the worker ants and the growing larvae (Weber, 1966, Fisher et al., 1994a, Aylward et al., 2012).
Fig. 12.
A.Atta sexdens workers carrying leafs back to their colony in Gamboa, Panama. B. One year old Atta colombica colony with queen on top in Gamboa, Panama. C. Gongylidia of Leucoagaricus gongylophorus – specialised swollen hyphal tips, which are the principal food source for ant larvae. Bar: 40 μm. Reprinted from Fisher et al. (1994b), with permission from Elsevier. D. Colony of Atta cephalotes collected in Gamboa, Panama, with L. gongylophorus fruiting body. (Photographs A, B and D were kindly provided by Pepijn Kooij of the Royal Botanic Gardens, Kew).
Fungus–ant mutualisms were first described in the late 1800s (Belt, 1874, Möller, 1893) and are estimated to be at least 50 million years old (Mueller et al., 2005, Schultz and Brady, 2008), with the specific relationship between L. gongylophorus and the leaf-cutter ants evolving around 2–8 million years ago (Mikheyev et al. 2010). Leucoagaricus gongylophorus and its associated ant species play an important ecological role in nutrient recycling, but with millions of worker ants in one colony, they can also be serious agricultural pests (Fowler et al., 1989, Zanetti et al., 2014).
Leucoagaricus gongylophorus fungal gardens are actively maintained by the ants, which establish vertical strata in their gardens (Huang et al. 2014). In the middle section of the garden, L. gongylophorus produces the gongylidia that contain laccases, proteases, pectinases, hemicelluloses, cellulases and xylanases for digestion of plant tissues and are ingested by the ants and their larvae. The top of the garden contains limited fungal biomass and it is here that the ants add new plant material, masticating it into smaller fragments and depositing faecal fluid containing the fungal-derived digestive enzymes from the ingested gongylidia to facilitate degradation (Grell et al., 2013, Huang et al., 2014). The ants also inoculate new plant material with fragments of mycelium from the older, lower part of the garden to expedite digestion. In the lower strata of the garden, ants remove the spent plant material and there is complete turnover of substrate over a 4–6 wk period (Aylward et al., 2013, Grell et al., 2013).
The fungus is unable to suppress microbial competitors and pathogens itself: excised fragments of fungal gardens and detached fruiting bodies are quickly overcome by other microorganisms (Fisher et al., 1994b, Fisher et al., 1996). Instead, the ants attempt to maintain the nest axenicity by licking the plant material to sterilise it prior to integration into the fungal garden, “grooming” the fungus garden to remove spores of fungal pathogens including Trichoderma and Escovopsis to prevent contamination and “weeding” the fungal garden when pathogens are established by removing sections of the nest containing hyphae from species other than L. gongylophorus (Currie and Stuart, 2001, dos Santos Reis et al., 2015). Acromyrmex ant colonies go even further to preserve their monoculture and will strive to maintain a specific strain of L. gongylophorus: the queen will carry fragments of the old nest containing L. gongylophorus when she is establishing a new colony and cultivate a new fungal garden consisting of the same specific strain. Leucoagaricus gongylophorus can reproduce sexually, but the ants endeavour to maintain its asexuality through suppressing basidiocarp formation by biting emerging primordia, and any basidiocarps that do form are hollowed out from the inside by the ants, preventing maturation and ensuring no basidiospores are formed, facilitating retention of their preferred fungal isolate (Fisher et al., 1994b, Kooij et al., 2015a). Mature basidiocarps can be observed within fungal gardens farmed by Atta species, suggesting that these ant species are not as meticulous about strain maintenance (Fig. 12D).
In recent years, genomic (Aylward et al., 2013, De Fine Licht et al., 2013), transcriptomic (Grell et al. 2013) and proteomic data (Kooij et al., 2014b, Aylward et al., 2015) has greatly advanced our understanding of the association between L. gongylophorus and the leaf-cutter ants. The genome of L. gongylophorus was recently sequenced from a strain isolated from an Atta cephalotes leaf-cutter ant colony in Gamboa, Panama (Aylward et al. 2013). 101 Mbp of total sequence was generated as a draft genome, suggesting that L. gongylophorus has a relatively large genome for a basidiomycete. Combining the sequence data with metaproteomic data for fungal gardens of Atta cephalotes and Acromyrmex echinatior colonies produced a detailed picture of not only the enzymatic capabilities of L. gongylophorus, but also how these are applied to biomass degradation within the fungal gardens (Aylward et al. 2013). 145 lignocelluloses were identified, in addition to many pectinases, xylanases, amylases and cellulases. Distinct enzymatic cocktails were identified in the different strata of the fungal garden, for example with certain cellulases being increasingly present in the lower strata, suggesting that these enzymes come into play once the more yielding polymers have been consumed. This analysis also provided further evidence for the role of fungal enzymes in detoxifying plant secondary metabolites, as had been suggested previously (De Fine Licht et al. 2013).
Research is ongoing to resolve the phylogeny of the Leucoagaricus genus, which appears to be polyphyletic (Johnson, 1999, Vellinga et al., 2003, Kooij et al., 2014b, Pereira et al., 2015), and to decipher the specific relationships of the leaf-cutter ant genera with their fungal symbiont, as there are some indications that Atta and Acromyrmex are cultivating different clades of L. gongylophorus (Mikheyev et al., 2010, Kooij et al., 2015a, Kooij et al., 2015b).
Pathogenic basidiomycetes
As well as containing many species that play vital roles as symbionts, the basidiomycete fungi include many pathogenic species, infecting a wide range of hosts and often having devastating effects on hosts in terms of mortality and loss of crop yields. Basidiomycete pathogens primarily infect plants, both monocotyledonous and dicotyledonous, infecting species of both agricultural and horticultural importance.
The first pathogenic species to be discussed in this review is the major plant pathogen Armillaria mellea, which has a broad host range, spanning from broadleaf trees to grapevines, and is found widely distributed across the Northern hemisphere, causing significant damage to wild, crop and ornamental species. We also consider Moniliophthora perniciosa, the causal agent of Witches Broom Disease, a pathogen of the cocoa tree, which is currently limited to Central and South America, but which has the potential to threaten cacao production worldwide.
Armillaria mellea
Armillaria mellea (Physalacriaceae) (Fig. 13A), commonly known as the honey fungus, is a necrotrophic pathogen that causes Armillaria root disease (Baumgartner et al. 2011). With taxonomic descriptions dating back to the 1700s (Vahl 1790), A. mellea was believed to be a highly polymorphic pathogen until the species complex began to be unravelled in the late 1970s, revealing approximately 40 described species with lifestyles that range from saprotrophic to pathogenic (Korhonen, 1978, Anderson and Ullrich, 1979, Rishbeth, 1982, Watling et al., 1991, Volk and Burdsall, 1995). Fungi in this genus produce a range of unique secondary metabolites and are unusual within the basidiomycetes in that their persistent vegetative state is diploid rather than dikaryotic (Korhonen & Hintikka 1974, Shim et al. 2006, Misiek et al., 2011, Mihail, 2015). Most, if not all, Armillaria species are bioluminescent, with Armillaria being one of three fungal lineages responsible for the ‘fox fire’ phenomenon that has been described for millennia. Light in the range of 520–530 nm is emitted from the mycelium growing in decaying wood, which can cause the entire forest floor to appear aglow. Bioluminescence is only apparent in hyphae and rhizomorphs and has not been observed in fruiting bodies (Murrill, 1915, Ramsbottom, 1953, Desjardin et al., 2008, Mihail, 2015). Armillaria mushrooms are edible, although it is advisable to cook them thoroughly beforehand to avoid stomach upsets, and some work has been done toward realising their commercial cultivation (Shim et al. 2006).
Fig. 13.
Signs and symptoms of Armillaria root disease. A.Armillaria mellea fruiting bodies. B. Mycelial fans underneath bark. C. Rhizomorphs growing from a decaying log. D. An A. mellea disease centre in an infected vineyard in California. (Photos courtesy of K. Baumgartner, USDA).
Armillaria mellea sensu stricto is a virulent pathogen found throughout forest, agricultural and urban environments in Europe, North America and in parts of Asia and Africa. It has an extremely large host range and is an economically important pathogen on many fruit and nut crops in Europe and North America, such as walnut (Juglans spp.), stone fruits (Prunus spp.), apple (Malus spp.) and highbush blueberry (Vaccinium corymbosum) (Baumgartner and Rizzo, 2001, Prodorutti et al., 2009, Thomidis and Exadaktylou, 2012, Baumgartner et al., 2013, Elías-Román et al., 2013). Losses can be substantial: 10 % in infected pear (Pyrus spp.) orchards and up to 40 % in infected vineyards in California (Rizzo et al., 1998, Baumgartner, 2004). Armillaria mellea is also a significant pathogen of ornamental species and has been reported from public gardens in South Africa infecting trees such as oak (Quercus spp.) and horse chestnut (Aesculus spp.) (Coetzee et al. 2001) and herbaceous species including Chrysanthemum spp., Cynara spp. and Cyclamen spp. in Europe (Guillaumin et al. 1993). Furthermore, a role for A. mellea in other tree diseases including chronic oak decline (Denman et al. 2010) and ash dieback (Gross et al. 2014) has also been suggested, where it is implicated in weakening trees and predisposing them to infection by other pathogens.
Armillaria root disease is spread by foraging reddish-black rhizomorphs, hyphae and through contact with infected roots. Basidiospores are unimportant in infection and no asexual reproductive stage is known (Mwenje et al., 1998, Rizzo et al., 1998, Hood et al., 2008). To penetrate plant roots, rhizomorphs grow appressed to the root surface and produce lateral branches that secrete an assortment of cell wall degrading enzymes such as laccases, pectin lyases, peroxidases, polygalacturonases and suberinases (Mwenje and Ride, 1999, Baumgartner et al., 2011, Ross-Davis et al., 2013). After penetrating the root, hyphae subsequently spread through the phloem and secondary xylem in parallel to the cambium and colonise the surrounding tissues, growing as a mycelial fan through the roots and causing necrotic lesions (Fig. 13B). Infected plants display symptoms that allude to a reduced root system including wilting, early senescence, leaf abscission, dieback and rapid onset of death, with healthy mature trees often succumbing to infection within a few years. The presence of fruiting bodies at the base of diseased trees, internal mycelial fans, epiphytic rhizomorphs (Fig. 13C) and disease centres characterised by dead trees in the centre (Fig. 13D) are indicative of severe Armillaria root disease (Baumgartner and Rizzo, 2002, Brazee and Wick, 2009). After the death of the host plant, A. mellea remains viable in residual roots and stumps in soil for decades, feeding saprotrophically until the area is replanted, at which time new infections are initiated. It is this saprotrophic ability, together with a broad host range, that makes control of Armillaria root disease especially challenging (Baumgartner and Rizzo, 2002, Guillaumin and Legrand, 2013), particularly if planting orchards or vineyards on land recently cleared of forest trees.
Conventional control methods for Armillaria root disease include the use of fungicides and soil fumigants, but these chemicals are being phased out globally, are expensive and often not completely effective (West and Fox, 2002, Baumgartner et al., 2011, Percival et al., 2011). Cultural management practices consist of root collar excavation (Baumgartner, 2004, Percival et al., 2011) and removal of residual roots prior to replanting in infected areas. The latter of these is particularly effective but laborious and expensive (Cleary et al. 2013). Biological control treatments including the use of bacterial soil inoculants and antagonistic species such as Trichoderma have been trialled (Baumgartner and Warnock, 2006, Percival et al., 2011), but are yet to progress from the laboratory to use in the field.
Recent advances have provided a variety of genetic and molecular tools for A. mellea, including a genome sequence (Collins et al. 2013) which has revealed a 58.35 Mb genome, proteomic and transcriptomic data (Collins et al., 2013, Ross-Davis et al., 2013), an Agrobacterium tumefaciens-based method for transformation of basidiospores (Baumgartner et al. 2010) together with a system of producing basidiospores in vitro (Ford et al. 2015) and expression of fluorescent proteins GFP and mRFP (Ford et al. 2016a). A new infection assay for A. mellea using herbaceous plants under controlled conditions has also been recently reported (Ford et al. 2016b). These developments should lead to a more comprehensive understanding of A. mellea virulence factors and infection strategies to enable the establishment of robust management practices for this pathogen.
Moniliophthora perniciosa
Moniliophthora perniciosa, previously classified as Crinipellis perniciosa, is a pathogen of the cocoa tree (Theobroma cacao) and is the causal agent of “Witches' Broom Disease” (WBD) (Aime & Phillips-Mora 2005). Different M. perniciosa biotypes have been shown to infect a range of plant taxa, including bignoniaceous lianas, solanaceous hosts, and the shrub Heteropterys acutifolia (Griffith et al. 2003), but it is best known for the huge losses to cocoa crops it causes, with up to 90 % yield losses recorded (Paim et al. 2006). When M. perniciosa infection spread in the Bahia region of Brazil in 1989 (Periera et al. 1990), Brazil went from being the third largest exporter of chocolate to an importer (Meinhardt et al. 2008). M. perniciosa was first reported in the Brazilian Amazon in 1785 and is considered to have evolved in this area. Subsequent recordings of the disease were made in Surinam, Guyana, Ecuador, Trinidad, Colombia and Grenada over the following 200 years, revealing the spread of the pathogen through Central and South America. M. perniciosa is currently thought to be restricted to the Americas and to date there have not been any reported cases of the disease in T. cacao crops grown in West Africa or South East Asia (Meinhardt et al. 2008).
Moniliophthora perniciosa is unusual for a plant pathogen in that it has a hemibiotrophic nature and requires young, living, green shoots to maintain pathogenicity. It is also able to directly infect aerial parts of plants – a trait that is rarely seen in agaricomycete pathogens (Mondego et al. 2008). Infection is established when basidiospores infect the active meristematic tissues of the cacao plant. Proposed entry mechanisms have included stomatal entry or entry via wounds or sheared trichomes (Frias et al., 1991, Silva and Matsuoka, 1999), but it has also been suggested that germ tubes can penetrate leaf surface cuticles and may be capable of penetrating directly into the meristems (Sreenivasan & Dabydeen 1989). A recent study (Sena et al. 2014) has shown that M. perniciosa does indeed display multiple modes of penetration, including through the base of trichomes, via natural openings in the cuticular surface, through stomata, and directly through intact cuticle, with the latter occurring via both mechanical and enzymatic means (Sena et al. 2014).
Infection begins with the chaotic proliferation of new shoots due to hypertrophy and hyperplasia of host cells, which produces clusters of shoots known as “brooms” (Chaves & Gianfagna 2006). The brooms are short lived and after 1–2 mo they change from a bright green to brown and remain in the canopy, which lead to the characteristic “witches broom” appearance (Frias et al. 1991). Infection of the flower cushions induces ectopic shoots instead of flowers, and can cause small, hardened parthenocarpic (seedless) fruits (Silva et al. 2014). Infection that spreads to more mature pods can often be more cryptic, and in some cases, is only discovered on harvesting (Fig. 14A) (Griffith et al. 2003). Basidiocarps (Fig. 14B) form during the wet season under highly moist conditions, and small pink mushrooms can be seen fruiting from the dead brooms (Fig. 14C). The resulting basidiospores are then readily dispersed in the humid canopy conditions of cacao plantations. High humidity is essential for the dispersal of basidiospores, which is usually only over short distances, with dispersal over larger distance believed to result from human actions spreading basidiospores on asymptomatic infected pods (Griffith et al. 2003). Typically there is only one disease cycle per rainy season (Maddison et al. 1996).
Fig. 14.
A–C. The basidiocarps of Moniliophthora perniciosa (A: Copyright © Scott Bauer, USDA, Bugwood.org), which form during the wet season, can often be seen emerging from the dead tissues of infected plants (B: Copyright © James A. Saunders, Bugwood.org). The valuable beans of cacao pods are destroyed when the pods are infected with M. perniciosa (C: Copyright © Scott Bauer, USDA, Bugwood.org).
Relatively little is known about the causes of the specific symptoms. It is not fully understood which effects are the result of fungal toxin production within the host tissues and which symptoms are the result of host defence mechanisms. Certain symptoms point towards an imbalance of plant growth regulators, which may be responsible for the loss of apical growth during the primary stages of infection (Mondego et al. 2008). However, the switch from the biotrophic, broom producing stage of infection is thought to be triggered by senescence signalling within the host plant (Teixeira et al. 2014).
Existing prevention methods include the efficient removal of infected material, with a focus on pruning the brooms. An additional control method is the application of a spray of mineral oil over the ground, which inhibits the sporulation of pruned material on the ground. Phytosanitisation by the frequent removal of sources of inoculum has proved to be the most effective method for disease control (Andebrhan & Furtek 1994). Moniliophthora perniciosa is tolerant to many widely used fungicides, even in higher doses, and as a result there are currently no viable chemical control methods for the treatment of WBD (Farquharson 2014). The application of chemical fungicides is also problematic as the disease is located in the aerial canopy. This means that fungicides require spraying over entire canopies. Alternatively chemicals could be applied to the soil and taken up by the cocoa tree root system but this raises concerns over chemical residues in the mature pods and may be environmentally damaging (Rudgard et al. 2012).
An alternative approach to tackling the problem of WBD is the cultivation of disease resistant varieties of cocoa tree. Basidiospores exposed to the extracts of more resistant varieties of cocoa exhibited abnormal growth and stunted germination (Evans & Bastos 1980). Field studies have also evaluated the resistance to WBD in flower cushions of T. cacao, to identify isolates with disease resistant phenotypes (Silva et al. 2014). It has also been shown that the biological control agent Gliocladium catenulatum was effective as an endophytic symbiont, and reduced the incidence of WBD by 70 % in cacao seedlings (Rubini et al. 2005).
Taxonomically, M. perniciosa belongs to the Marasmiaceae family of basidiomycetes, and has now been shown to be a sister species to M. roreri (Evans et al., 2002, Evans et al., 2003, Aime and Phillips-Mora, 2005). M. roreri, the causative agent of another devastating disease of T. cacao crops known as Frosty Pod Rot, is an unusual basidiomycete pathogen which was originally described as a conidia-producing anamorphic ascomycete due to an absence of fruiting bodies (Ciferri & Parodi 1934). Work has now shown that M. roreri is in fact a teleomorph basidiomycete that produces abundant meiospores, which are thought to be highly modified probasidia (Evans et al. 2003). Moniliophthora roreri and M. perniciosa represent an interesting model for the evolution of pathogenicity in fungi, sitting as they do within a taxonomic clade which is dominated by saprotrophic species (Teixeira et al. 2015). Tiburcio et al. (2010) recently demonstrated that M. perniciosa and M. roreri probably acquired the required genes for pathogenicity by horizontal gene transfer, including a family of necrosis-inducing proteins from Phytophthora oomycetes. Moniliophthora rorei is a highly invasive species that has been spreading in recent years, and once established, leads to yield reductions of over 80 % (Phillips-Mora et al., 2006, Krauss et al., 2010), further increasing the importance of understanding this unusual taxonomic group.
The genome of M. perniciosa was sequenced and published in 2008 and is estimated to have a genome size of 39 Mbp (Mondego et al. 2008). A transformation system had been developed using the PEG-mediated transformation of protoplasts which, along with other molecular tools, opens up the potential for a greater understanding of the pathogenicity mechanisms for this species (Lima et al., 2003, Leal et al., 2010, Santana et al., 2012).
Conclusions
We hope that this review has highlighted just how crucial the Agaricomycetes are. They are an astoundingly diverse group of organisms, which are vital in nature, recycling nutrients and thus allowing ecosystems to function, as well as directly enabling the survival of plant and animal species through symbiotic relationships. Harnessing these various unique traits, for example in bioremediation, environmentally beneficial waste disposal and the production of biofuels, is something that has been expanding as a fascinating and exciting field of research. More directly, basidiomycetes supply us with food and medicine. Even the apparently negative traits of the basidiomycetes, as pathogens and toxic species, have potential uses to human societies, for example as producers of bioactive compounds or biocontrol agents. Understanding such species, particularly the pathogens of important crop plants, is also important for reducing their negative impacts, particularly at a time when global food-security is so important.
Although our understanding of this fascinating phylum has grown hugely in recent decades, we have only just begun to exploit their true potential. As our understanding of the basidiomycete fungi grows, through the study of model organisms, the development of various tools and techniques and the continuing discovery of novel species, our ability to exploit their unique abilities and characteristics will also grow. Basidiomycetes have the potential to provide us with the next generation of antibiotics, to reduce environmental pollution, and even produce our fuel. They currently represent one of the great untapped resources of nature.
Acknowledgements
Authors would like to thank the following for help with the wonderful images within this review: Dr. Kendra Baumgartner (USDA) for images of A. mellea; Prof George Barron (University of Guelph) for an image of P. ostreatus colonising a nematode; Prof Francis Martin, Dr Feng Zhang, Dr Aurélie Deveau and Dr Anne Jambois (INRA) for images of L. bicolor; Dr Pepijn Kooij (Kew Gardens) for images of L. gongylophorus; Prof. Ursula Kues and Prof Andrzej Majcherczyk (Göttingen University) for an image of S. lacrymans spores.; Prof Louisa Howard (Dartmouth College) for an electron micrograph of A. bisporus var. bisporus spores. The authors were supported by grants from Royal Horticultural Society, U.S. Department of Agriculture, University of Bristol, and Biotechnology and Biological Sciences Research Council BB/K002341/1.
Footnotes
Peer review under responsibility of CBS-KNAW Fungal Biodiversity Centre.
References
- Aanen D.K., Eggleton P., Rouland-Lefevre C. The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14887–14892. doi: 10.1073/pnas.222313099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Zeid M.A. Pathogenic variation in isolates of Pseudomonas causing the brown blotch of cultivated mushroom, Agaricus bisporus. Brazilian Journal of Microbiology. 2012;43:1137–1146. doi: 10.1590/S1517-838220120003000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimanianda V., Bayry J., Bozza S. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460:1117–1121. doi: 10.1038/nature08264. [DOI] [PubMed] [Google Scholar]
- Aime M.C., Phillips-Mora W. The causal agents of witches' broom and frosty pod rot of cacao (chocolate, Theobroma cacao) form a new lineage of Marasmiaceae. Mycologia. 2005;97:1012–1022. doi: 10.3852/mycologia.97.5.1012. [DOI] [PubMed] [Google Scholar]
- Aitken I.A., Morgan J.H., Dalziel R. Comparative in vitro activity of valnemulin against porcine bacterial pathogens. Veterinary Record. 1999;144:128. doi: 10.1136/vr.144.5.128. [DOI] [PubMed] [Google Scholar]
- Akanbi M.H.J., Post E., van Putten S.M. The antitumor activity of hydrophobin SC3, a fungal protein. Applied Microbiology and Biotechnology. 2013;97:4385–4392. doi: 10.1007/s00253-012-4311-x. [DOI] [PubMed] [Google Scholar]
- Akers B., Ruiz J., Piper A. A prehistoric mural in Spain depicting neurotropic psilocybe mushrooms. Economic Botany. 2011;65:121–128. [Google Scholar]
- Alexopoulos C.J., Mims C.W., Blackwell M. 4th edn. John Wiley & Sons Ltd; New York: 1996. Introductory mycology. [Google Scholar]
- Allegro J.M. Doubleday; New York: 1970. The sacred mushroom and the cross. [Google Scholar]
- Allen B., Desai B., Lisenbee N. Amatoxin: a review. ISRN Emergency Medicine. 2012;2012:1–4. [Google Scholar]
- Amend A.S., Barshis D.J., Oliver T.A. Coral-associated marine fungi form novel lineages and heterogeneous assemblages. The ISME Journal. 2012;6:1291–1301. doi: 10.1038/ismej.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amey R.C., Mills P.R., Bailey A. Investigating the role of a Verticillium fungicola beta-1,6-glucanase during infection of Agaricus bisporus using targeted gene disruption. Fungal Genetics and Biology. 2003;39:264–275. doi: 10.1016/s1087-1845(03)00061-6. [DOI] [PubMed] [Google Scholar]
- Andebrhan T., Furtek D.B. Random amplified polymorphic DNA (RAPD) analysis of Crinipellis perniciosa isolates from different hosts. Plant Pathology. 1994;43:1020–1027. [Google Scholar]
- Anderson J.B., Ullrich R.C. Biological species of Armillaria mellea in North America. Mycologia. 1979;71:402–414. [Google Scholar]
- Arigoni D. La struttura di un terpene di nuovo genere. Gazzetta Chimica Italiana. 1962;92:884–901. [Google Scholar]
- Arnison P.G., Bibb M.J., Bierbaum G. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Natural Product Reports. 2013;30:108–160. doi: 10.1039/c2np20085f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward F.O., Burnum-Johnson K.E., Tringe S.G. Leucoagaricus gongylophorus produces diverse enzymes for the degradation of recalcitrant plant polymers in leaf-cutter ant fungus gardens. Applied and Environmental Microbiology. 2013;79:3770–3778. doi: 10.1128/AEM.03833-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward F.O., Currie C.R., Suen G. The evolutionary innovation of nutritional symbioses in leaf-cutter ants. Insects. 2012;3:41–61. doi: 10.3390/insects3010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward F.O., Khadempour L., Tremmel D.M. Enrichment and broad representation of plant biomass-degrading enzymes in the specialized hyphal swellings of Leucoagaricus gongylophorus, the fungal symbiont of leaf-cutter ants. PLoS ONE. 2015;10:e0134752. doi: 10.1371/journal.pone.0134752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar J., Ozinga W.A., Kuyper T.W. Spatial distribution of Laccaria bicolor genets reflected by sporocarps after removal of litter and humus layers in a Pinus sylvestris forest. Mycological Research. 1994;98:726–728. [Google Scholar]
- Bailey A.M., Alberti F., Kilaru S. Identification and manipulation of the pleuromutilin gene cluster from Clitopilus passeckerianus for increased rapid antibiotic production. Scientific Reports. 2016;6:25202. doi: 10.1038/srep25202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AM, Collins C, Crawford A, et al. (2013a). Inventors; Glaxo Wellcome House (Greenford, Middlesex, GB), assignee. Methods of increasing yields of pleuromutilin. United States patent EP2494028 A2.
- Bailey A.M., Collopy P.D., Thomas D.J. Transcriptomic analysis of the interactions between Agaricus bisporus and Lecanicillium fungicola. Fungal Genetics and Biology. 2013;55:67–76. doi: 10.1016/j.fgb.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Balaes T., Tanase C. Basidiomycetes as potential piocontrol agents against nematodes. Romanian Biotechnological Letters. 2016;21:11185. [Google Scholar]
- Baldauf S.L., Palmer J.D. Animals and fungi are each other's closest relatives: congruent evidence from multiple proteins. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:11558–11562. doi: 10.1073/pnas.90.24.11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltazar J.M., Trierveiler-Pereira L., Loguercio-Leite C. A checklist of xylophilous basidiomycetes (Basidiomycota) in mangroves. Mycotaxon. 2009;107:221–224. [Google Scholar]
- Bao X.F., Liu C.P., Fang J.N. Structural and immunological studies of a major polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Carbohydrate Research. 2001;332:67–74. doi: 10.1016/s0008-6215(01)00075-1. [DOI] [PubMed] [Google Scholar]
- Barthakur B., Gogoi P., Barua P. Agaricales of Nambar reserve forest, Golaghat, Assam, India. Advances in Plant Sciences. 2000;13:609–613. [Google Scholar]
- Bartlett D.W., Clough J.M., Godwin J.R. The strobilurin fungicides. Pest Management Science. 2002;58:649–662. doi: 10.1002/ps.520. [DOI] [PubMed] [Google Scholar]
- Baumgartner K. Root collar excavation for postinfection control of Armillaria root disease of grapevine. Plant Disease. 2004;88:1235–1240. doi: 10.1094/PDIS.2004.88.11.1235. [DOI] [PubMed] [Google Scholar]
- Baumgartner K., Coetzee M.P.A., Hoffmeister D. Secrets of the subterranean pathosystem of Armillaria. Molecular Plant Pathology. 2011;12:515–534. doi: 10.1111/j.1364-3703.2010.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner K., Fujiyoshi P., Browne G.T. Evaluating paradox walnut rootstocks for resistance to Armillaria root disease. HortScience. 2013;48:68–72. [Google Scholar]
- Baumgartner K., Fujiyoshi P., Foster G.D. Agrobacterium tumefaciens-mediated transformation for investigation of somatic recombination in the fungal pathogen Armillaria mellea. Applied and Environmental Microbiology. 2010;76:7990–7996. doi: 10.1128/AEM.01049-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner K., Rizzo D.M. Distribution of Armillaria species in California. Mycologia. 2001;93:821–830. [Google Scholar]
- Baumgartner K., Rizzo D.M. Spread of Armillaria root disease in a California vineyard. American Journal of Enology and Viticulture. 2002;53:197–203. [Google Scholar]
- Baumgartner K., Warnock A.E. A soil inoculant inhibits Armillaria mellea in vitro and improves productivity of grapevines with root disease. Plant Disease. 2006;90:439–444. doi: 10.1094/PD-90-0439. [DOI] [PubMed] [Google Scholar]
- Belt T. J. Murray; London: 1874. The naturalist in Nicaragua. [Google Scholar]
- Bhatnagar R., Shuttleworth C., Mussell S. Structure and activity of muscarinic stimulants. Nature. 1971;230:439. doi: 10.1038/230439a0. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S., Angayarkanni J., Das A. Mycoremediation of Benzo [a] pyrene by Pleurotus ostreatus isolated from Wayanad district in Kerala, India. International Journal of Pharmacy and Bio Sciences. 2012;2:84–93. [Google Scholar]
- Binder M., Hibbett D.S., Wang Z. Evolutionary relationships of Mycaureola dilseae (Agaricales), a basidiomycete pathogen of a subtidal rhodophyte. American Journal of Botany. 2006;93:547–556. doi: 10.3732/ajb.93.4.547. [DOI] [PubMed] [Google Scholar]
- Binninger D.M., Skrzynia C., Pukkila P.J. DNA-mediated transformation of the basidiomycete Coprinus cinereus. The EMBO Journal. 1987;6:835–840. doi: 10.1002/j.1460-2075.1987.tb04828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch A.J., Holzapfel C.W., Rickards R.W. The structure and some aspects of the biosynthesis of pleuromutilin. Tetrahedron. 1966;22:359–387. [Google Scholar]
- Bisby G.R. Longevity of Schizophyllum commune. Nature. 1945;155:732–733. [Google Scholar]
- Bishop K.S., Kao C.H.J., Xu Y. From 2000 years of Ganoderma lucidum to recent developments in nutraceuticals. Phytochemistry. 2015;114:56–65. doi: 10.1016/j.phytochem.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Bodensteiner P., Binder M., Moncalvo J.-M. Phylogenetic relationships of cyphelloid homobasidiomycetes. Molecular Phylogenetics and Evolution. 2004;33:501–515. doi: 10.1016/j.ympev.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Boh B. Ganoderma lucidum: a potential for biotechnological production of anti-cancer and immunomodulatory drugs. Recent Patents on Anti-Cancer Drug Discovery. 2013;8:255–287. doi: 10.2174/1574891x113089990036. [DOI] [PubMed] [Google Scholar]
- Borodynko N., Hasiów-Jaroszewska B., Rymelska N. La France disease of the cultivated mushroom Agaricus bisporus in Poland. Acta Virologica. 2010;54:217. doi: 10.4149/av_2010_03_217. [DOI] [PubMed] [Google Scholar]
- Bouws H., Wattenberg A., Zorn H. Fungal secretomes – nature's toolbox for white biotechnology. Applied Microbiology and Biotechnology. 2008;80:381–388. doi: 10.1007/s00253-008-1572-5. [DOI] [PubMed] [Google Scholar]
- Brady K., O'Kiely P., Forristal P. Schizophyllum commune on big-bale grass silage in Ireland. Mycologist. 2005;19:30–35. [Google Scholar]
- Brande E. On a poisonous species of Agaric. London Medical and Physical Journal. 1799;11:41–44. [PMC free article] [PubMed] [Google Scholar]
- Brazee N.J., Wick R.L. Armillaria species distribution on symptomatic hosts in northern hardwood and mixed oak forests in western Massachusetts. Forest Ecology and Management. 2009;258:1605–1612. [Google Scholar]
- Buku A., Campadelli-Fiume G., Fiume L. Inhibitory effect of naturally occurring and chemically modified amatoxins on RNA polymerase of rat liver nuclei. FEBS Letters. 1971;14:42–44. doi: 10.1016/0014-5793(71)80270-3. [DOI] [PubMed] [Google Scholar]
- Burns C., Gregory K.E., Kirby M. Efficient GFP expression in the mushrooms Agaricus bisporus and Coprinus cinereus requires introns. Fungal Genetics and Biology. 2005;42:191–199. doi: 10.1016/j.fgb.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Byss M., Elhottová D., Tříska J. Fungal bioremediation of the creosote-contaminated soil: influence of Pleurotus ostreatus and Irpex lacteus on polycyclic aromatic hydrocarbons removal and soil microbial community composition in the laboratory-scale study. Chemosphere. 2008;73:1518–1523. doi: 10.1016/j.chemosphere.2008.07.030. [DOI] [PubMed] [Google Scholar]
- Callac P., Jacobe de Haut I., Imbernon M. A novel homothallic variety of Agaricus bisporus comprises rare tetrasporic isolates from Europe. Mycologia. 2003;95:222–231. [PubMed] [Google Scholar]
- Cao Y., Wu S.-H., Dai Y.-C. Species clarification of the prize medicinal Ganoderma mushroom “Lingzhi”. Fungal Diversity. 2012;56:49–62. [Google Scholar]
- Carlile M.C., Watkinson S.C., Gooday G.W. 2nd edn. Academic Press; San Diego: 2001. The fungi. [Google Scholar]
- Chang S.T., Hayes W.A. Academic Press; New York: 1978. The biology and cultivation of edible mushrooms. [Google Scholar]
- Chaturvedi V., Verma P. An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. 3 Biotech. 2013;3:415–431. doi: 10.1007/s13205-013-0167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves F.C., Gianfagna T.J. Necrotrophic phase of Moniliophthora perniciosa causes salicylic acid accumulation in infected stems of cacao. Physiological and Molecular Plant Pathology. 2006;69:104–108. [Google Scholar]
- Chen G.-Q., Zhang W.-J., Zeng G.-M. Surface-modified Phanerochaete chrysosporium as a biosorbent for Cr(VI)-contaminated wastewater. Journal of Hazardous Materials. 2011;186:2138–2143. doi: 10.1016/j.jhazmat.2010.12.123. [DOI] [PubMed] [Google Scholar]
- Chen S., Xu J., Liu C. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nature Communications. 2012;3:913. doi: 10.1038/ncomms1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton W.S., Ott J. Toxic metabolites of Amanita pantherina, A. cothurnata, A. muscaria and other Amanita species. Lloydia. 1976;39:150–157. [PubMed] [Google Scholar]
- Chowdhary A., Randhawa H.S., Gaur S.N. Schizophyllum commune as an emerging fungal pathogen: a review and report of two cases. Mycoses. 2013;56:1–10. doi: 10.1111/j.1439-0507.2012.02190.x. [DOI] [PubMed] [Google Scholar]
- Ciferri R., Parodi E. Descrizione del fungo che causa la “Moniliasi” del cacao. Phytopathologische Zeitschrift. 1934;5:539–542. [Google Scholar]
- Cilerdzic J., Vukojevc J., Stajic M. Biological activity of Ganoderma lucidum basidiocarps cultivated on alternative and commercial substrate. Journal of Ethnopharmacology. 2014;155:312–319. doi: 10.1016/j.jep.2014.05.036. [DOI] [PubMed] [Google Scholar]
- Cleary M.R., Arhipova N., Morrison D.J. Stump removal to control root disease in Canada and Scandinavia: a synthesis of results from long-term trials. Forest Ecology and Management. 2013;290:5–14. [Google Scholar]
- Co-David D., Langeveld D., Noordeloos M.E. Molecular phylogeny and spore evolution of Entolomataceae. Persoonia. 2009;23:147–176. doi: 10.3767/003158509X480944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee M.P.A., Wingfield B.D., Harrington T.C. The root rot fungus Armillaria mellea introduced into South Africa by early Dutch settlers. Molecular Ecology. 2001;10:387–396. doi: 10.1046/j.1365-294x.2001.01187.x. [DOI] [PubMed] [Google Scholar]
- Collins C., Keane T.M., Turner D.J. Genomic and proteomic dissection of the ubiquitous plant pathogen, Armillaria mellea: toward a new infection model system. Journal of Proteome Research. 2013;12:2552–2570. doi: 10.1021/pr301131t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C.M., Heneghan M.N., Kilaru S. Improvement of the Coprinopsis cinerea molecular toolkit using new construct design and additional marker genes. Journal of Microbiological Methods. 2010;82:156–162. doi: 10.1016/j.mimet.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Costa A.M.S.B., Mills P.R., Bailey A.M. Oligonucleotide sequences forming short self-complimentary hairpins can expedite the down-regulation of Coprinopsis cinerea genes. Journal of Microbiological Methods. 2008;75:205–208. doi: 10.1016/j.mimet.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Courty P.E., Hoegger P.J., Kilaru S. Phylogenetic analysis, genomic organization, and expression analysis of multi-copper oxidases in the ectomycorrhizal basidiomycete Laccaria bicolor. New Phytologist. 2009;182:736–750. doi: 10.1111/j.1469-8137.2009.02774.x. [DOI] [PubMed] [Google Scholar]
- Currie C.R., Stuart A.E. Weeding and grooming of pathogens in agriculture by ants. Proceedings of the Royal Society B: Biological Sciences. 2001;268:1033–1039. doi: 10.1098/rspb.2001.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y.C., Cui B.K. Fomitiporia ellipsoidea has the largest fruiting body among the fungi. Fungal Biology. 2011;115:813–814. doi: 10.1016/j.funbio.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Day P. The structure of the A mating type factor in Coprinus lagopus: wild alleles. Genetical Research. 1963;4:323–325. [Google Scholar]
- De Fine Licht H.H., Schiott M., Rogowska-Wrzesinska A. Laccase detoxification mediates the nutritional alliance between leaf-cutting ants and fungus-garden symbionts. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:583–587. doi: 10.1073/pnas.1212709110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot P.W., Schaap P.J., Sonnenberg A.S. The Agaricus bisporus hypA gene encodes a hydrophobin and specifically accumulates in peel tissue of mushroom caps during fruit body development. Journal of Molecular Biology. 1996;257:1008–1018. doi: 10.1006/jmbi.1996.0219. [DOI] [PubMed] [Google Scholar]
- de Jong J.F., Deelstra H.J., Wösten H.A.B. RNA-mediated gene silencing in monokaryons and dikaryons of Schizophyllum commune. Applied and Environmental Microbiology. 2006;72:1267–1269. doi: 10.1128/AEM.72.2.1267-1269.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong J.F., Ohm R.A., De Bekker C. Inactivation of ku80 in the mushroom-forming fungus Schizophyllum commune increases the relative incidence of homologous recombination. FEMS Microbiology Letters. 2010;310:91–95. doi: 10.1111/j.1574-6968.2010.02052.x. [DOI] [PubMed] [Google Scholar]
- Denman S., Kirk S., Webber J. Forestry Commission; Edinburgh: 2010. Managing acute oak decline. [Google Scholar]
- Desjardin D.E., Oliveira A.G., Stevani C.V. Fungi bioluminescence revisited. Photochemical & Photobiological Sciences. 2008;7:170–182. doi: 10.1039/b713328f. [DOI] [PubMed] [Google Scholar]
- Doddapaneni H., Chakraborty R., Yadav J.S. Genome-wide structural and evolutionary analysis of the P450 monooxygenase genes (P450ome) in the white rot fungus Phanerochaete chrysosporium: evidence for gene duplications and extensive gene clustering. BMC Genomics. 2005;6:92. doi: 10.1186/1471-2164-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörnte B., Kües U. Reliability in transformation of the basidiomycete Coprinopsis cinerea. Current Trends in Biotechnology and Pharmacy. 2012;6:340–355. [Google Scholar]
- dos Santos Reis B.M., Silva A., Del Valle Alvarez M.R. Fungal communities in gardens of the leafcutter ant Atta cephalotes in forest and cabruca agrosystems of southern Bahia State (Brazil) Fungal Biology. 2015;119:1170–1178. doi: 10.1016/j.funbio.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Drews J., Georgopoulos A., Laber G. Antimicrobial activities of 81.723 hfu, a new pleuromutilin derivative. Antimicrobial Agents and Chemotherapy. 1975;7:507–516. doi: 10.1128/aac.7.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood D., Green J., Grogan H. Viral agents causing brown cap mushroom disease of Agaricus bisporus. Applied and Environmental Microbiology. 2015;81:7125–7134. doi: 10.1128/AEM.01093-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood D.C., Floudas D., Binder M. The plant cell wall – decomposing machinery underlies the functional diversity of forest fungi. Science. 2011;333:762–765. doi: 10.1126/science.1205411. [DOI] [PubMed] [Google Scholar]
- Eger G., Eden G., Wissig E. Pleurotus ostreatus – breeding potential of a new cultivated mushroom. Theoretical and Applied Genetics. 1976;47:155–163. doi: 10.1007/BF00278373. [DOI] [PubMed] [Google Scholar]
- Eggen T., Majcherczyk A. Removal of polycyclic aromatic hydrocarbons (PAH) in contaminated soil by white rot fungus Pleurotus ostreatus. International Biodeterioration & Biodegradation. 1998;41:111–117. [Google Scholar]
- Elías-Román R.D., Guzmán-Plazola R.A., Klopfenstein N.B. Incidence and phylogenetic analyses of Armillaria spp. associated with root disease in peach orchards in the State of Mexico, Mexico. Forest Pathology. 2013;43:390–401. [Google Scholar]
- Erkel E.I. The effect of different substrate mediums on yield of Ganoderma lucidum (Fr.) Karst. Journal of Food Agriculture & Environment. 2009;7:841–844. [Google Scholar]
- Essig A., Hofmann D., Münch D. Copsin, a novel peptide-based fungal antibiotic interfering with the peptidoglycan synthesis. The Journal of Biological Chemistry. 2014;289:34953–34964. doi: 10.1074/jbc.M114.599878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essig F.M. The morphology, development, and economic aspects of Schizophyllum commune Fries. University of California Publications in Botany. 1922;7:447–498. [Google Scholar]
- Evans H., Holmes K., Reid A. Phylogeny of the frosty pod rot pathogen of cocoa. Plant Pathology. 2003;52:476–485. [Google Scholar]
- Evans H.C., Bastos C.N. Basidiospore germination as a means of assessing resistance to Crinipellis perniciosa (witches' broom disease) in cocoa cultivars. Transactions of the British Mycological Society. 1980;74:525–536. [Google Scholar]
- Evans H.C., Holmes K.A., Phillips W. What's in a name: Crinipellis, the final resting place for the frosty pod rot pathogen of cocoa? Mycologist. 2002;16:148–152. [Google Scholar]
- Fang Q.-H., Zhong J.-J. Submerged fermentation of higher fungus Ganoderma lucidum for production of valuable bioactive metabolites—ganoderic acid and polysaccharide. Biochemical Engineering Journal. 2002;10:61–65. [Google Scholar]
- Farid A.M., Lee S., Maziah Z. Pathogenicity of Rigidoporus microporus and Phellinus noxius against four major plantation tree species in Peninsular Malaysia. Journal of Tropical Forest Science. 2009;21:289–298. [Google Scholar]
- Farquharson K.L. The fungus, the witches' broom, and the chocolate tree: deciphering the molecular interplay between Moniliophthora perniciosa and Theobroma cacao. The Plant Cell. 2014;26:4231. doi: 10.1105/tpc.114.133462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazakerley N.J., Procter D.J. Synthesis and synthetic chemistry of pleuromutilin. Tetrahedron. 2014;70:6911–6930. [Google Scholar]
- Feng G., Zhang F., Li X. Uptake of nitrogen from indigenous soil pool by cotton plant inoculated with arbuscular mycorrhizal fungi. Communications in Soil Science and Plant Analysis. 2002;33:3825–3836. [Google Scholar]
- Ferguson B., Dreisbach T., Parks C. Coarse-scale population structure of pathogenic Armillaria species in a mixed-conifer forest in the Blue Mountains of northeast Oregon. Canadian Journal of Forest Research. 2003;33:612–623. [Google Scholar]
- Fisher P.J., Stradling D.J., Pegler D.N. Leaf cutting ants, their fungus gardens and the formation of basidiomata of Leucoagaricus gongylophorus. Mycologist. 1994;8:128–131. [Google Scholar]
- Fisher P.J., Stradling D.J., Pegler D.N. Leucoagaricus basidiomata from a live nest of the leaf-cutting ant Atta cephalotes. Mycological Research. 1994;98:884–888. [Google Scholar]
- Fisher P.J., Stradling D.J., Sutton B.C. Microfungi in the fungus gardens of the leaf-cutting ant Atta cephalotes: a preliminary study. Mycological Research. 1996;100:541–546. [Google Scholar]
- Ford K.L., Baumgartner K., Henricot B. A reliable in vitro fruiting system for Armillaria mellea for evaluation of Agrobacterium tumefaciens transformation vectors. Fungal Biology. 2015;119:859–869. doi: 10.1016/j.funbio.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Ford K.L., Baumgartner K., Henricot B. A native promoter and inclusion of an intron is necessary for efficient expression of GFP or mRFP in Armillaria mellea. Scientific Reports. 2016;6:29226. doi: 10.1038/srep29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford K.L., Henricot B., Baumgartner K. A faster inoculation assay for Armillaria using herbaceous plants. The Journal of Horticultural Science and Biotechnology. 2016;2016 [Google Scholar]
- Fowler H.G., Pagani M.I., da Silva O.A. A pest is a pest is a pest? The dilemma of neotropical leaf-cutting ants: keystone taxa of natural ecosystems. Environmental Management. 1989;13:671–675. [Google Scholar]
- Frank J.L., Coffan R.A., Southworth D. Aquatic gilled mushrooms: Psathyrella fruiting in the Rogue River in southern Oregon. Mycologia. 2010;102:93–107. doi: 10.3852/07-190. [DOI] [PubMed] [Google Scholar]
- Franz M., Regele H., Kirchmair M. Magic mushrooms: hope for a ‘cheap high’ resulting in end-stage renal failure. Nephrology Dialysis Transplantation. 1996;11:2324–2327. doi: 10.1093/oxfordjournals.ndt.a027160. [DOI] [PubMed] [Google Scholar]
- Frias G., Purdy L., Schmidt R. Infection biology of Crinipellis pernicosa on vegetative flushes of Cacao. Plant Disease. 1991;75:552–556. [Google Scholar]
- Gea F.J., Tello J.C., Navarro M.-J. Efficacy and effects on yield of different fungicides for control of wet bubble disease of mushroom caused by the mycoparasite Mycogone perniciosa. Crop Protection. 2010;29:1021–1025. [Google Scholar]
- Geml J., Tulloss R.E., Laursen G.A. Evidence for strong inter-and intracontinental phylogeographic structure in Amanita muscaria, a wind-dispersed ectomycorrhizal basidiomycete. Molecular Phylogenetics and Evolution. 2008;48:694–701. doi: 10.1016/j.ympev.2008.04.029. [DOI] [PubMed] [Google Scholar]
- Giasson L., Specht C.A., Milgrim C. Cloning and comparison of A alpha mating-type alleles of the Basidiomycete Schizophyllum commune. Molecular and General Genetics. 1989;218:72–77. doi: 10.1007/BF00330567. [DOI] [PubMed] [Google Scholar]
- Gilbertson R.L. Wood-rotting fungi of North America. Mycologia. 1980;72:1–49. [Google Scholar]
- Glaeser J.A., Smith K.T. Decay fungi of oaks and associated hardwoods for western arborists. Western Arborist. 2010;Winter 2010:32–46. [Google Scholar]
- Glenn J.K., Gold M.H. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Archives of Biochemistry and Biophysics. 1985;242:329–341. doi: 10.1016/0003-9861(85)90217-6. [DOI] [PubMed] [Google Scholar]
- Graeme K.A. Mycetism: a review of the recent literature. Journal of Medical Toxicology. 2014;10:173–189. doi: 10.1007/s13181-013-0355-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granado J.D., Kertesz-Chaloupková K., Aebi M. Restriction enzyme-mediated DNA integration in Coprinus cinereus. Molecular and General Genetics. 1997;256:28–36. doi: 10.1007/s004380050542. [DOI] [PubMed] [Google Scholar]
- Grell M.N., Linde T., Nygaard S. The fungal symbiont of Acromyrmex leaf-cutting ants expresses the full spectrum of genes to degrade cellulose and other plant cell wall polysaccharides. BMC Genomics. 2013;14:928. doi: 10.1186/1471-2164-14-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith G.W., Nicholson J., Nenninger A. Witches' brooms and frosty pods: two major pathogens of cacao. New Zealand Journal of Botany. 2003;41:423–435. [Google Scholar]
- Gross A., Holdenrieder O., Pautasso M. Hymenoscyphus pseudoalbidus, the causal agent of European ash dieback. Molecular Plant Pathology. 2014;15:5–21. doi: 10.1111/mpp.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaumin J.J., Legrand P. Armillaria root rots. In: Gonthier P., Nicolotti G., editors. Infectious forest diseases. CABI; Boston: 2013. pp. 159–177. [Google Scholar]
- Guillaumin J.J., Mohammed C., Anselmi N. Geographical distribution and ecology of the Armillaria species in western Europe. Forest Pathology. 1993;23:321–341. [Google Scholar]
- Gusse A.C., Miller P.D., Volk T.J. White-rot fungi demonstrate first biodegradation of phenolic resin. Environmental Science & Technology. 2006;40:4196–4199. doi: 10.1021/es060408h. [DOI] [PubMed] [Google Scholar]
- Guzmán G. J. Cramer; Liechtenstein: 1983. The genus Psilocybe: a systematic revision of the known species including the history, distribution and chemistry of the hallucinogenic species. [Google Scholar]
- Guzmán G. Species diversity in the genus Psilocybe (Basidiomycotina, Agaricales, Strophariaceae) of world mycobiota, with special attention to hallucinogenic properties. International Journal of Medicinal Mushrooms. 2005;7:305. [Google Scholar]
- Guzmán G. The hallucinogenic mushrooms: diversity, traditions, use and abuse with special reference to the genus Psilocybe. In: Misra J.K., Deshmukh S.K., editors. Fungi from different environments. Science Publishers; New Hampshire: 2009. pp. 256–277. [Google Scholar]
- Guzmán G., Allen J.W., Gartz J. A worldwide geographical distribution of the neurotropic fungi, an analysis and discussion. Annali del Museo Civico di Rovereto: Sezione Archeologia, Storia, Scienze Naturali. 1998;14:189–280. [Google Scholar]
- Habijanic J., Berovic M., Boh B. Submerged cultivation of Ganoderma lucidum and the effects of its polysaccharides on the production of human cytokines TNF-α, IL-12, IFN-γ, IL-2, IL-4, IL-10 and IL-17. New Biotechnology. 2015;32:85–95. doi: 10.1016/j.nbt.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Hailei W., Ping L., Yu Q. Removal of phenol in phenolic resin wastewater by a novel biomaterial: the Phanerochaete chrysosporium pellet containing chlamydospore-like cells. Applied Microbiology and Biotechnology. 2016;100:5153–5164. doi: 10.1007/s00253-016-7353-7. [DOI] [PubMed] [Google Scholar]
- Hallen H.E., Luo H., Scott-Craig J.S. Gene family encoding the major toxins of lethal Amanita mushrooms. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19097–19101. doi: 10.1073/pnas.0707340104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa Y., Hirano Y., Watabe H. First isolation of Schizophyllum commune in a harbor seal (Phoca vitulina) Medical Mycology. 2016;54:492–499. doi: 10.1093/mmy/myw008. [DOI] [PubMed] [Google Scholar]
- Harmsen M.C., Schuren F.H., Moukha S.M. Sequence analysis of the glyceraldehyde-3-phosphate dehydrogenase genes from the basidiomycetes Schizophyllum commune, Phanerochaete chrysosporium and Agaricus bisporus. Current Genetics. 1992;22:447–454. doi: 10.1007/BF00326409. [DOI] [PubMed] [Google Scholar]
- Hartley A.J., de Mattos-Shipley K., Collins C.M. Investigating pleuromutilin-producing Clitopilus species and related basidiomycetes. FEMS Microbiology Letters. 2009;297:24–30. doi: 10.1111/j.1574-6968.2009.01656.x. [DOI] [PubMed] [Google Scholar]
- Heneghan M.N., Burns C., Costa A.M. Functional analysis of Agaricus bisporus serine proteinase 1 reveals roles in utilization of humic rich substrates and adaptation to the leaf-litter ecological niche. Environmental Microbiology. 2016 doi: 10.1111/1462-2920.13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneghan M.N., Costa A.M., Challen M.P. A comparison of methods for successful triggering of gene silencing in Coprinus cinereus. Molecular Biotechnology. 2007;35:283–296. doi: 10.1007/BF02686014. [DOI] [PubMed] [Google Scholar]
- Heneghan M.N., Porta C., Zhang C. Characterization of serine proteinase expression in Agaricus bisporus and Coprinopsis cinerea by using green fluorescent protein and the A. bisporus SPR1 promoter. Applied and Environmental Microbiology. 2009;75:792–801. doi: 10.1128/AEM.01897-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbett D.S. A phylogenetic overview of the Agaricomycotina. Mycologia. 2006;98:917–925. doi: 10.3852/mycologia.98.6.917. [DOI] [PubMed] [Google Scholar]
- Hibbett D.S., Binder M. Evolution of marine mushrooms. The Biological Bulletin. 2001;201:319–322. doi: 10.2307/1543610. [DOI] [PubMed] [Google Scholar]
- Hikino H., Konno C., Mirin Y. Isolation and hypoglycemic activity of ganoderans A and B, glycans of Ganoderma lucidum fruit bodies. Planta Medica. 1985;51:339–340. [PubMed] [Google Scholar]
- Hildén K., Mäkelä M.R., Lankinen P. Agaricus bisporus and related Agaricus species on lignocellulose: production of manganese peroxidase and multicopper oxidases. Fungal Genetics and Biology. 2013;55:32–41. doi: 10.1016/j.fgb.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Hirano T., Honda Y., Watanabe T. Degradation of bisphenol A by the lignin-degrading enzyme, manganese peroxidase, produced by the white-rot basidiomycete, Pleurotus ostreatus. Bioscience, Biotechnology, and Biochemistry. 2000;64:1958–1962. doi: 10.1271/bbb.64.1958. [DOI] [PubMed] [Google Scholar]
- Hodgin L.A., Högenauer G. The mode of action of pleuromutilin derivatives. European Journal of Biochemistry. 1974;47:527–533. doi: 10.1111/j.1432-1033.1974.tb03721.x. [DOI] [PubMed] [Google Scholar]
- Hofmann A., Heim R., Brack A. Psilocybin und Psilocin, zwei psychotrope Wirkstoffe aus mexikanischen Rauschpilzen. Helvetica Chimica Acta. 1959;42:1557–1572. [Google Scholar]
- Hofrichter M. Review: lignin conversion by manganese peroxidase (MnP) Enzyme and Microbial Technology. 2002;30:454–466. [Google Scholar]
- Hood I.A., Petrini L.E., Gardner J.F. Colonisation of woody material in Pinus radiata plantations by Armillaria novae-zelandiae basidiospores. Australasian Plant Pathology. 2008;37:347. [Google Scholar]
- Hseu R.S., Wang H.H., Wang H.F. Differentiation and grouping of isolates of the Ganoderma lucidum complex by random amplified polymorphic DNA-PCR compared with grouping on the basis of internal transcribed spacer sequences. Applied and Environmental Microbiology. 1996;62:1354–1363. doi: 10.1128/aem.62.4.1354-1363.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E.L., Aylward F.O., Kim Y.-M. The fungus gardens of leaf-cutter ants undergo a distinct physiological transition during biomass degradation. Environmental Microbiology Reports. 2014;6:389–395. doi: 10.1111/1758-2229.12163. [DOI] [PubMed] [Google Scholar]
- Hudson H.J. Cambridge University Press; Cambridge: 1986. Fungal biology. [Google Scholar]
- Irshad M., Asgher M. Production and optimization of ligninolytic enzymes by white rot fungus Schizophyllum commune IBL-06 in solid state medium banana stalks. African Journal of Biotechnology. 2011;10:18234–18242. [Google Scholar]
- Isaac S. Springer; Netherlands: 1991. Fungal-plant interactions. [Google Scholar]
- Iwase K. XIII. Cultivation of mycorrhizal mushrooms. Food Reviews International. 1997;13:431–442. [Google Scholar]
- James T.Y., Lee M., van Diepen L.T. A single mating-type locus composed of homeodomain genes promotes nuclear migration and heterokaryosis in the white-rot fungus Phanerochaete chrysosporium. Eukaryot Cell. 2011;10:249–261. doi: 10.1128/EC.00212-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James T.Y., Srivilai P., Kues U. Evolution of the bipolar mating system of the mushroom Coprinellus disseminatus from its tetrapolar ancestors involves loss of mating-type-specific pheromone receptor function. Genetics. 2006;172:1877–1891. doi: 10.1534/genetics.105.051128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings D.H., Bravery A.F. Wiley; New York: 1991. Serpula lacrymans: fundamental biology and control strategies. [Google Scholar]
- Jess S., Bingham J. Biological control of sciarid and phorid pests of mushroom with predatory mites from the genus Hypoaspis (Acari: Hypoaspidae) and the entomopathogenic nematode Steinernema feltiae. Bulletin of Entomological Research. 2004;94:159–167. doi: 10.1079/ber2003286. [DOI] [PubMed] [Google Scholar]
- Jiang J., Grieb B., Thyagarajan A. Ganoderic acids suppress growth and invasive behavior of breast cancer cells by modulating Ap-1 and Nf-Kappa B signaling. International Journal of Molecular Medicine. 2008;21:577. [PubMed] [Google Scholar]
- Jin X., Ruiz Beguerie J., Sze D.M. Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database of Systematic Reviews. 2012;4:Cd007731. doi: 10.1002/14651858.CD007731.pub2. [DOI] [PubMed] [Google Scholar]
- Johnson J. Phylogenetic relationships Lepiota sensu lato based on morphological and molecular data. Mycologia. 1999;91:443–458. [Google Scholar]
- Jones K. Reishi mushroom: ancient medicine in modern times. Alternative and Complementary Therapies. 1998;4:256–266. [Google Scholar]
- Jones R.N., Fritsche T.R., Sader H.S. Activity of retapamulin (SB-275833), a novel pleuromutilin, against selected resistant gram-positive cocci. Antimicrobial Agents and Chemotherapy. 2006;50:2583–2586. doi: 10.1128/AAC.01432-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamzolkina O.V., Volkova V.N., Kozlova M.V. Karyological evidence for meiosis in the three different types of life cycles existing in Agaricus bisporus. Mycologia. 2006;98:763–770. doi: 10.3852/mycologia.98.5.763. [DOI] [PubMed] [Google Scholar]
- Kano R., Oomae S., Nakano Y. First report on Schizophyllum commune from a dog. Journal of Clinical Microbiology. 2002;40:3535–3537. doi: 10.1128/JCM.40.9.3535-3537.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karston P.A. Enumeratio Boletinearum et Polyporearum Fennicarum, systemate novo dispositarum. Revue mycologique, Toulouse. 1881;3:16–19. [Google Scholar]
- Kauserud H., Knudsen H., Högberg N. Evolutionary origin, worldwide dispersal, and population genetics of the dry rot fungus Serpula lacrymans. Fungal Biology Reviews. 2012;2:84–93. [Google Scholar]
- Kauserud H., Svegarden I.B., Saetre G.P. Asian origin and rapid global spread of the destructive dry rot fungus Serpula lacrymans. Molecular Ecology. 2007;16:3350–3360. doi: 10.1111/j.1365-294X.2007.03387.x. [DOI] [PubMed] [Google Scholar]
- Kavanagh F., Hervey A., Robbins W.J. Antibiotic substances from Basidiomycetes. VIII. Pleurotus mutilus (Fr.) Sacc. and Pleurotus passeckerianus pilat. Proceedings of the National Academy of Sciences of the United States of America. 1951;37:570–574. doi: 10.1073/pnas.37.9.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh F., Hervey A., Robbins W.J. Antibiotic substances from Basidiomycetes IX. Drosophila subatrata. Proceedings of the National Academy of Sciences of the United States of America. 1952;38:555–560. doi: 10.1073/pnas.38.7.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay S.M., Brown A.E. Colonization by Psilocybe semilanceata of roots of grassland flora. Mycological Research. 1990;94:49–56. [Google Scholar]
- Kemppainen M., Circosta A., Tagu D. Agrobacterium-mediated transformation of the ectomycorrhizal symbiont Laccaria bicolor S238N. Mycorrhiza. 2005;16:19–22. doi: 10.1007/s00572-005-0008-7. [DOI] [PubMed] [Google Scholar]
- Kerrigan R.W. Global genetic resources for Agaricus breeding and cultivation. Canadian Journal of Botany. 1995;73:973–979. [Google Scholar]
- Kerrigan R.W. McGraw-Hill Education; 2013. Breeding the button mushroom (Agaricus bisporus) [Google Scholar]
- Kerrigan R.W., Imbernon M., Callac P. The heterothallic life cycle of Agaricus bisporus var. burnettii and the inheritance of its tetrasporic trait. Experimental Mycology. 1994;18:193–210. [Google Scholar]
- Kerrigan R.W., Royer J.C., Baller L.M. Meiotic behavior and linkage relationships in the secondarily homothallic fungus Agaricus bisporus. Genetics. 1993;133:225–236. doi: 10.1093/genetics/133.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilaru S., Collins C.M., Hartley A.J. Establishing molecular tools for genetic manipulation of the pleuromutilin-producing fungus Clitopilus passeckerianus. Applied and Environmental Microbiology. 2009;75:7196–7204. doi: 10.1128/AEM.01151-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilaru S., Collins C.M., Hartley A.J. Investigating dominant selection markers for Coprinopsis cinerea: a carboxin resistance system and re-evaluation of hygromycin and phleomycin resistance vectors. Current Genetics. 2009;55:543–550. doi: 10.1007/s00294-009-0266-6. [DOI] [PubMed] [Google Scholar]
- Kilaru S., Hoegger P.J., Kües U. The laccase multi-gene family in Coprinopsis cinerea has seventeen different members that divide into two distinct subfamilies. Current Genetics. 2006;50:45–60. doi: 10.1007/s00294-006-0074-1. [DOI] [PubMed] [Google Scholar]
- Kilaru S., Hoegger P.J., Majcherczyk A. Expression of laccase gene lcc1 in Coprinopsis cinerea under control of various basidiomycetous promoters. Applied Microbiology and Biotechnology. 2006;71:200–210. doi: 10.1007/s00253-005-0128-1. [DOI] [PubMed] [Google Scholar]
- Kim K.-H., Kang Y.M., Im C.H. Identification and functional analysis of pheromone and receptor genes in the B3 mating locus of Pleurotus eryngii. PLoS ONE. 2014;9:e104693. doi: 10.1371/journal.pone.0104693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjalke M., Andersen M.B., Schneider P. Comparison of structure and activities of peroxidases from Coprinus cinereus, Coprinus macrorhizus and Arthromyces ramosus. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology. 1992;1120:248–256. doi: 10.1016/0167-4838(92)90244-8. [DOI] [PubMed] [Google Scholar]
- Klironomos J.N., Hart M.M. Food-web dynamics. Animal nitrogen swap for plant carbon. Nature. 2001;410:651–652. doi: 10.1038/35070643. [DOI] [PubMed] [Google Scholar]
- Kniep H. Uber morphologische and physiologische Geschlechtsdifferenzierung (Ubtersuchungen an Basidiomyzeten) Verhandlungen der Physisch-Medische GesellschaftWurzburg. 1920;46:1–18. [Google Scholar]
- Kohler A., Kuo A., Nagy L.G. Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nature Genetics. 2015;47:410–415. doi: 10.1038/ng.3223. [DOI] [PubMed] [Google Scholar]
- Kooij P.W., Aanen D.K., Schiøtt M. Evolutionarily advanced ant farmers rear polyploid fungal crops. Journal of Evolutionary Biology. 2015;28:1911–1924. doi: 10.1111/jeb.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij P.W., Liberti J., Giampoudakis K. Differences in forage-acquisition and fungal enzyme activity contribute to niche segregation in panamanian leaf-cutting ants. PLoS ONE. 2014;9:e94284. doi: 10.1371/journal.pone.0094284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij P.W., Poulsen M., Schiøtt M. Somatic incompatibility and genetic structure of fungal crops in sympatric Atta colombica and Acromyrmex echinatior leaf-cutting ants. Fungal Ecology. 2015;18:10–17. doi: 10.1016/j.funeco.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij P.W., Rogowska-Wrzesinska A., Hoffmann D. Leucoagaricus gongylophorus uses leaf-cutting ants to vector proteolytic enzymes towards new plant substrate. The ISME journal. 2014;8:1032–1040. doi: 10.1038/ismej.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen K. Infertility and clonal size in the Armillariella mellea complex. Karstenia. 1978;18:31–42. [Google Scholar]
- Korhonen K., Hintikka V. Cytological evidence for somatic diploidization in dikaryotic cells of Armillariella mellea. Archives of Microbiology. 1974;95:187–192. [Google Scholar]
- Korripally P., Timokhin V.I., Houtman C.J. Evidence from Serpula lacrymans that 2, 5-dimethoxyhydroquinone is a lignocellulolytic agent of divergent brown rot basidiomycetes. Applied and Environmental Microbiology. 2013;79:2377–2383. doi: 10.1128/AEM.03880-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosowska-Shick K., Clark C., Credito K. Single- and multistep resistance selection studies on the activity of retapamulin compared to other agents against Staphylococcus aureus and Streptococcus pyogenes. Antimicrobial Agents and Chemotherapy. 2006;50:765–769. doi: 10.1128/AAC.50.2.765-769.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothe E. Mating types and pheromone recognition in the Homobasidiomycete Schizophyllum commune. Fungal Genetics and Biology. 1999;27:146–152. doi: 10.1006/fgbi.1999.1129. [DOI] [PubMed] [Google Scholar]
- Krauss U., Hidalgo E., Bateman R. Improving the formulation and timing of application of endophytic biocontrol and chemical agents against frosty pod rot (Moniliophthora roreri) in cocoa (Theobroma cacao) Biological Control. 2010;54:230–240. [Google Scholar]
- Kreisel H. Pilze aus Pilzgärten von Atta insularis in Kuba. Zeitschrift für Allgemeine Mikrobiologie. 1972;12:643–654. [PubMed] [Google Scholar]
- Kringstad K.P., Lindstroem K. Spent liquors from pulp bleaching. Environmental Science & Technology. 1984;18:236A–248A. doi: 10.1021/es00126a714. [DOI] [PubMed] [Google Scholar]
- Kropp B.R. Inheritance of the ability for ectomycorrhizal colonization of Pinus strobus by Laccaria bicolor. Mycologia. 1997;89:578. [Google Scholar]
- Kues U. Life history and developmental processes in the basidiomycete Coprinus cinereus. Microbiology and Molecular Biology Reviews. 2000;64:316–353. doi: 10.1128/mmbr.64.2.316-353.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kues U. From two to many: multiple mating types in Basidiomycetes. Fungal Biology Reviews. 2015;29:126–166. [Google Scholar]
- Kües U., Navarro-González M. How do Agaricomycetes shape their fruiting bodies? 1. Morphological aspects of development. Fungal Biology Reviews. 2015;29:63–97. [Google Scholar]
- Kukor J.J., Martin M.M. Acquisition of digestive enzymes by siricid woodwasps from their fungal symbiont. Science. 1983;220:1161–1163. doi: 10.1126/science.220.4602.1161. [DOI] [PubMed] [Google Scholar]
- Kwon O., Park Y.-J., Kim H.-I. Taxonomic position and species identity of the cultivated Yeongji ‘Ganoderma lucidum’ in Korea. Mycobiology. 2016;44:1–6. doi: 10.5941/MYCO.2016.44.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal G.A., Gomes L.H., Albuquerque P.S.B. Searching for Moniliophthora perniciosa pathogenicity genes. Fungal Biology. 2010;114:842–854. doi: 10.1016/j.funbio.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Lee N., Bakkeren G., Wong K. The mating-type and pathogenicity locus of the fungus Ustilago hordei spans a 500-kb region. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:15026–15031. doi: 10.1073/pnas.96.26.15026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler K.B., Fox D.S., Fraser J.A. Mating-type locus of Cryptococcus neoformans: a step in the evolution of sex chromosomes. Eukaryotic Cell. 2002;1:704–718. doi: 10.1128/EC.1.5.704-718.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.-J., Zhang D.-H., Yue T.-H. Improved polysaccharide production in a submerged culture of Ganoderma lucidum by the heterologous expression of Vitreoscilla hemoglobin gene. Journal of Biotechnology. 2016;217:132–137. doi: 10.1016/j.jbiotec.2015.11.011. [DOI] [PubMed] [Google Scholar]
- Li J., Zhang J., Chen H. Complete mitochondrial genome of the medicinal mushroom Ganoderma lucidum. PLoS ONE. 2013;8:e72038. doi: 10.1371/journal.pone.0072038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Liu J., Zhao Y. Possible mechanism underlying the antiherpetic activity of a proteoglycan isolated from the mycelia of Ganoderma lucidum in vitro. Journal of Biochemistry and Molecular Biology. 2005;38:34–40. doi: 10.5483/bmbrep.2005.38.1.034. [DOI] [PubMed] [Google Scholar]
- Lima J.O., dos Santos J.K., Pereira J.F. Development of a transformation system for Crinipellis perniciosa, the causal agent of witches' broom in cocoa plants. Current Genetics. 2003;42:236–240. doi: 10.1007/s00294-002-0358-z. [DOI] [PubMed] [Google Scholar]
- Linder D.H. The genus Schizophyllum. I. Species of the western hemisphere. American Journal of Botany. 1933;20:552–564. [Google Scholar]
- Ling C., Fu L., Gao S. Design, synthesis, and structure–activity relationship studies of novel thioether pleuromutilin derivatives as potent antibacterial agents. Journal of Medicinal Chemistry. 2014;57:4772–4795. doi: 10.1021/jm500312x. [DOI] [PubMed] [Google Scholar]
- Litten W. The most poisonous mushrooms. Scientific American. 1975;232:90–101. doi: 10.1038/scientificamerican0375-90. [DOI] [PubMed] [Google Scholar]
- Liu D., Gong J., Dai W. The genome of Ganoderma lucidum provides insights into triterpenes biosynthesis and wood degradation [corrected] PLoS ONE. 2012;7:e36146. doi: 10.1371/journal.pone.0036146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone D. Sabilillah Publications; USA: 2015. Transhumanism: the history of a dangerous idea. [Google Scholar]
- Lo V.C., Ren Q., Pham C.L. Fungal hydrophobin proteins produce self-assembling protein films with diverse structure and chemical stability. Nanomaterials. 2014;4:827–843. doi: 10.3390/nano4030827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucic E., Fourrey C., Kohler A. A gene repertoire for nitrogen transporters in Laccaria bicolor. New Phytologist. 2008;180:343–364. doi: 10.1111/j.1469-8137.2008.02580.x. [DOI] [PubMed] [Google Scholar]
- Lugones L.G., Scholtmeijer K., Klootwijk R. Introns are necessary for mRNA accumulation in Schizophyllum commune. Molecular Microbiology. 1999;32:681–689. doi: 10.1046/j.1365-2958.1999.01373.x. [DOI] [PubMed] [Google Scholar]
- Ma B., Mayfield M.B., Gold M.H. The green fluorescent protein gene functions as a reporter of gene expression in Phanerochaete chrysosporium. Applied and Environmental Microbiology. 2001;67:948–955. doi: 10.1128/AEM.67.2.948-955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison A.C., Holt J., Jeger M.J. Spatial dynamics of a monocyclic disease in a perennial crop. Ecological Modelling. 1996;88:45–52. [Google Scholar]
- Manzi P., Gambelli L., Marconi S. Nutrients in edible mushrooms: an inter-species comparative study. Food Chemistry. 1999;65:477–482. [Google Scholar]
- Martin F., Aerts A., Ahrén D. The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature. 2008;452:88–92. doi: 10.1038/nature06556. [DOI] [PubMed] [Google Scholar]
- Martin F., Selosse M.-A. The Laccaria genome: a symbiont blueprint decoded. New Phytologist. 2008;180:296–310. doi: 10.1111/j.1469-8137.2008.02613.x. [DOI] [PubMed] [Google Scholar]
- Meinhardt L.W., Rincones J., Bailey B.A. Moniliophthora perniciosa, the causal agent of witches' broom disease of cacao: what's new from this old foe? Molecular Plant Pathology. 2008;9:577–588. doi: 10.1111/j.1364-3703.2008.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelot D., Melendez-Howell L.M. Amanita muscaria: chemistry, biology, toxicology, and ethnomycology. Mycological Research. 2003;107:131–146. doi: 10.1017/s0953756203007305. [DOI] [PubMed] [Google Scholar]
- Miele A., Giardina P., Sannia G. Random mutants of a Pleurotus ostreatus laccase as new biocatalysts for industrial effluents bioremediation. Journal of Applied Microbiology. 2010;108:998–1006. doi: 10.1111/j.1365-2672.2009.04505.x. [DOI] [PubMed] [Google Scholar]
- Mihail J.D. Bioluminescence patterns among North American Armillaria species. Fungal Biology. 2015;119:528–537. doi: 10.1016/j.funbio.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Mikheyev A.S., Mueller U.G., Abbot P. Comparative dating of Attine ant and Lepiotaceous cultivar phylogenies reveals coevolutionary synchrony and discord. The American Naturalist. 2010;175:E126–E133. doi: 10.1086/652472. [DOI] [PubMed] [Google Scholar]
- Misiek M., Braesel J., Hoffmeister D. Characterisation of the ArmA adenylation domain implies a more diverse secondary metabolism in the genus Armillaria. Fungal Biology. 2011;115:775–781. doi: 10.1016/j.funbio.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Nishijima M. Studies on fungal polysaccharides 27. Structural examination of a water-soluble, anti-tumor polysaccharide of Ganoderma lucidum. Chemical & Pharmaceutical Bulletin. 1981;29:3611–3616. doi: 10.1248/cpb.29.3611. [DOI] [PubMed] [Google Scholar]
- Möller A. G. Fischer; Harvard: 1893. Die Pilzgarten einiger südamerikanischer Ameisen. [Google Scholar]
- Mondego J.M.C., Carazzolle M.F., Costa G.G.L. A genome survey of Moniliophthora perniciosa gives new insights into Witches' Broom Disease of cacao. BMC Genomics. 2008;9:548. doi: 10.1186/1471-2164-9-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin E., Kohler A., Baker A.R. Genome sequence of the button mushroom Agaricus bisporus reveals mechanisms governing adaptation to a humic-rich ecological niche. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17501–17506. doi: 10.1073/pnas.1206847109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton O.C., Hirsch P.R., Kerry B.R. Infection of plant-parasitic nematodes by nematophagous fungi – a review of the application of molecular biology to understand infection processes and to improve biological control. Nematology. 2004;6:161–170. [Google Scholar]
- Mueller G.M. Field Museum of Natural History; Chicago, Ill.: 1992. Systematics of Laccaria (Agaricales) in the continental United States and Canada, with discussions on extralimital taxa and descriptions of extant types. [Google Scholar]
- Mueller G.M., Gardes M. Intra-and interspecific relations within Laccaria bicolor sensu lato. Mycological Research. 1991;95:592–601. [Google Scholar]
- Mueller U.G., Gerardo N.M., Aanen D.K. The evolution of agriculture in insects. Annual Review of Ecology, Evolution, and Systematics. 2005;36:563–595. [Google Scholar]
- Müller A., Volmer K., Mishra-Knyrim M. Growing poplars for research with and without mycorrhizas. Frontiers in Plant Science. 2013;4:332. doi: 10.3389/fpls.2013.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Rivas A., Specht C.A., Drummond B.J. Transformation of the basidiomycete, Schizophyllum commune. Molecular & General Genetics. 1986;205:103–106. doi: 10.1007/BF02428038. [DOI] [PubMed] [Google Scholar]
- Murrill W.A. Luminescence in the fungi. Mycologia. 1915;7:131–133. [Google Scholar]
- Musshoff F., Madea B., Beike J. Hallucinogenic mushrooms on the German market – simple instructions for examination and identification. Forensic Science International. 2000;113:389–395. doi: 10.1016/s0379-0738(00)00211-5. [DOI] [PubMed] [Google Scholar]
- Mwenje E., Ride J.P. Purification and characterization of an endo-polygalacturonase (PG1) from a Zimbabwean species of Armillaria. Physiological and Molecular Plant Pathology. 1999;55:131–139. [Google Scholar]
- Mwenje E., Ride J.P., Pearce R.B. Distribution of Zimbabwean Armillaria groups and their pathogenicity on cassava. Plant Pathology. 1998;47:623–634. [Google Scholar]
- Nakazawa T., Ando Y., Kitaaki K. Efficient gene targeting in ΔCc.ku70 or ΔCc.lig4 mutants of the agaricomycete Coprinopsis cinerea. Fungal Genetics and Biology. 2011;48:939–946. doi: 10.1016/j.fgb.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Namekawa S.H., Iwabata K., Sugawara H. Knockdown of LIM15/DMC1 in the mushroom Coprinus cinereus by double-stranded RNA-mediated gene silencing. Microbiology. 2005;151:3669–3678. doi: 10.1099/mic.0.28209-0. [DOI] [PubMed] [Google Scholar]
- Naraian R., Kumari S., Ram S. Pleurotus as an exclusive eco-friendly modular biotool. In: Gupta V., Sharma G., Tuohy M., editors. The handbook of microbial bioresources. CABI; Boston: 2016. pp. 140–158. [Google Scholar]
- Nef H.M., Möllmann H., Hilpert P. Apical regional wall motion abnormalities reminiscent to Tako-Tsubo cardiomyopathy following consumption of psychoactive fungi. International Journal of Cardiology. 2009;134:e39–e41. doi: 10.1016/j.ijcard.2007.12.064. [DOI] [PubMed] [Google Scholar]
- Niculita-Hirzel H., Labbé J., Kohler A. Gene organization of the mating type regions in the ectomycorrhizal fungus Laccaria bicolor reveals distinct evolution between the two mating type loci. New Phytologist. 2008;180:329–342. doi: 10.1111/j.1469-8137.2008.02525.x. [DOI] [PubMed] [Google Scholar]
- Niederpruem D.J., Wessels J.G. Cytodifferentiation and morphogenesis in Schizophyllum commune. Bacteriological Reviews. 1969;33:505–535. doi: 10.1128/br.33.4.505-535.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak R. Are pleuromutilin antibiotics finally fit for human use? Annals of the New York Academy of Sciences. 2011;1241:71–81. doi: 10.1111/j.1749-6632.2011.06219.x. [DOI] [PubMed] [Google Scholar]
- Ohm R.A., Aerts D., Wösten H.A.B. The blue light receptor complex WC-1/2 of Schizophyllum commune is involved in mushroom formation and protection against phototoxicity. Environmental Microbiology. 2013;15:943–955. doi: 10.1111/j.1462-2920.2012.02878.x. [DOI] [PubMed] [Google Scholar]
- Ohm R.A., de Jong J.F., Berends E. An efficient gene deletion procedure for the mushroom-forming basidiomycete Schizophyllum commune. World Journal of Microbiology and Biotechnology. 2010;26:1919–1923. doi: 10.1007/s11274-010-0356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohm R.A., de Jong J.F., de Bekker C. Transcription factor genes of Schizophyllum commune involved in regulation of mushroom formation. Molecular Microbiology. 2011;81:1433–1445. doi: 10.1111/j.1365-2958.2011.07776.x. [DOI] [PubMed] [Google Scholar]
- Ohm R.A., de Jong J.F., Lugones L.G. Genome sequence of the model mushroom Schizophyllum commune. Nature Biotechnology. 2010;28:957–963. doi: 10.1038/nbt.1643. [DOI] [PubMed] [Google Scholar]
- Oluba O.M., Olusola A.O., Eidangbe G.O. Modulation of lipoprotein cholesterol levels in Plasmodium berghei malarial infection by crude aqueous extract of Ganoderma lucidum. Cholesterol. 2012;2012:1–6. doi: 10.1155/2012/536396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paim V.R.L.d.M., Luz E.D.M.N., Pires J.L. Sources of resistance to Crinipellis perniciosa in progenies of cacao accessions collected in the Brazilian Amazon. Scientia Agricola. 2006;63:572–578. [Google Scholar]
- Palfreyman J.W. The domestic dry rot fungus, Serpula lacrymans, its natural origins and biological control. 2001. http://www.arcchip.cz/w08/w08_palfreyman2.pdf Ariadne workshop:
- Papa I.A., Zulaybar T.O., Raymundo A.K. Increasing pleuromutilin activity of Clitopilus passeckerianus by chemical mutagenesis and improvement of production medium. The Philippine Agricultural Scientist. 2006;89:20–33. [Google Scholar]
- Paracer S., Ahmadjian V. Oxford University Press; Oxford, United Kingdom: 2000. Symbiosis: an introduction to biological associations. [Google Scholar]
- Passie T., Seifert J., Schneider U. The pharmacology of psilocybin. Addiction Biology. 2002;7:357–364. doi: 10.1080/1355621021000005937. [DOI] [PubMed] [Google Scholar]
- Paszczyński A., Huynh V.-B., Crawford R. Enzymatic activities of an extracellular, manganese-dependent peroxidase from Phanerochaete chrysosporium. FEMS Microbiology Letters. 1985;29:37–41. [Google Scholar]
- Percival G.C., Smiley E.T., Fox R.T.V. Root collar excavation with Trichoderma inoculations as a potential management strategy for Honey Fungus (Armillaria Mellea) Arboricultural Journal. 2011;33:267–280. [Google Scholar]
- Pereira D.I.P., Botton S.A., Azevedo M.I. Isolation and molecular characterization of symbiotic fungus from Acromyrmex ambiguus and Acromyrmex heyeri ants of Rio Grande do Sul State, Brazil. Ciência Rural. 2015;45:1256–1261. [Google Scholar]
- Periera J., Figueiredo A., Almeida O. First occurrence of witches' broom disease in the principal cocoa-growing region of Brazil: Centro de Pesquisas do Cacau, Itabuna, BA (Brasil) Tropical Agriculture. 1990;67:188–189. [Google Scholar]
- Perkins J.H., Gordon S.A. Morphogenesis in Schizophyllum commune. II. Effects of monochromatic light. Plant Physiology. 1969;44:1712–1716. doi: 10.1104/pp.44.12.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips-Mora W., Ortiz C.F., Aime M.C. Proceedings 15th International Cocoa Research Conference, San José, Costa Rica. Cocoa Producers' Alliance; 2006. Fifty years of frosty pod rot in Central America: chronology of its spread and impact from Panama to Mexico.http://www.worldcocoafoundation.org/wp-content/uploads/files_mf/phillips2006.pdf [Google Scholar]
- Pilato L. Springer; Heidelberg: 2010. Phenolic resins: a century of progress. [Google Scholar]
- Plaza D.F., Lin C.-W., van der Velden N.S.J. Comparative transcriptomics of the model mushroom Coprinopsis cinerea reveals tissue-specific armories and a conserved circuitry for sexual development. BMC Genomics. 2014;15:492. doi: 10.1186/1471-2164-15-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plett J.M., Daguerre Y., Wittulsky S. Effector MiSSP7 of the mutualistic fungus Laccaria bicolor stabilizes the Populus JAZ6 protein and represses jasmonic acid (JA) responsive genes. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:8299–8304. doi: 10.1073/pnas.1322671111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plett J.M., Gibon J., Kohler A. Phylogenetic, genomic organization and expression analysis of hydrophobin genes in the ectomycorrhizal basidiomycete Laccaria bicolor. Fungal Genetics and Biology. 2012;49:199–209. doi: 10.1016/j.fgb.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Plett J.M., Kemppainen M., Kale S.D. A secreted effector protein of Laccaria bicolor is required for symbiosis development. Current Biology. 2011;21:1197–1203. doi: 10.1016/j.cub.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Podila G.K., Zheng J., Balasubramanian S. Fungal gene expression in early symbiotic interactions between Laccaria bicolor and red pine. Plant and Soil. 2002;244:117–128. [Google Scholar]
- Prince W.T., Ivezic-Schoenfeld Z., Lell C. A phase II clinical study of BC-3781, a pleuromutilin antibiotic, in the treatment of patients with acute bacterial skin and skin structure infection. Antimicrobial Agents and Chemotherapy. 2013;57:2087–2094. doi: 10.1128/AAC.02106-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodorutti D., Vanblaere T., Gobbin D. Genetic diversity of Armillaria spp. infecting highbush blueberry in northern Italy (Trentino region) Phytopathology. 2009;99:651–658. doi: 10.1094/PHYTO-99-6-0651. [DOI] [PubMed] [Google Scholar]
- Pukkila P.J. Coprinopsis cinerea. Current Biology. 2011;21:R616–617. doi: 10.1016/j.cub.2011.05.042. [DOI] [PubMed] [Google Scholar]
- Purnomo A.S., Mori T., Kamei I. Application of mushroom waste medium from Pleurotus ostreatus for bioremediation of DDT-contaminated soil. International Biodeterioration & Biodegradation. 2010;64:397–402. [Google Scholar]
- Ramírez-Cruz V., Guzmán G., Villalobos-Arámbula A.R. Phylogenetic inference and trait evolution of the psychedelic mushroom genus Psilocybe sensu lato (Agaricales) Botany. 2013;91:573–591. [Google Scholar]
- Ramsbottom J. Dry rot in ships. Essex Naturalist. 1937;25:231–267. [Google Scholar]
- Ramsbottom J. Collins; London: 1953. Mushrooms and toadstools. A study of the activities of fungi. [Google Scholar]
- Raper J.R. Ronald Press Company; New York: 1966. Genetics of sexuality in higher fungi. [Google Scholar]
- Raper J.R., Miles P.G. The genetics of Schizophyllum commune. Genetics. 1958;43:530–546. doi: 10.1093/genetics/43.3.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudaskoski M. Mating-type genes and hyphal fusions in filamentous basidiomycetes. Fungal Biology Reviews. 2015;29:179–193. [Google Scholar]
- Raudaskoski M., Kothe E. Basidiomycete mating type genes and pheromone signaling. Eukaryotic Cell. 2010;9:847–859. doi: 10.1128/EC.00319-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redhead S.A. A biogeographical overview of the Canadian mushroom flora. Canadian Journal of Botany. 1989;67:3003–3062. [Google Scholar]
- Redhead S.A., Moncalvo J.-M., Vilgalys R. (1757) Proposal to conserve the name Psilocybe (Basidiomycota) with a conserved type. Taxon. 2007;56:255–257. [Google Scholar]
- Redhead S.A., Vilgalys R., Moncalvo J.-M. Coprinus Pers. and the disposition of Coprinus species sensu lato. Taxon. 2001;50:203. [Google Scholar]
- Redhead S.A., Vizzini A., Drehmel D.C. Saproamanita, a new name for both Lepidella E.-J. Gilbert and Aspidella E.-J. Gilbert (Amaniteae, Amanitaceae) IMA Fungus. 2016;7:119–129. doi: 10.5598/imafungus.2016.07.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley R., Salamov A.A., Brown D.W. Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:9923–9928. doi: 10.1073/pnas.1400592111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rishbeth J. Species of Armillaria in southern England. Plant Pathology. 1982;31:9–17. [Google Scholar]
- Rittenhouse S., Biswas S., Broskey J. Selection of Retapamulin, a novel Pleuromutilin for topical use. Antimicrobial Agents and Chemotherapy. 2006;50:3882–3885. doi: 10.1128/AAC.00178-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo D.M., Whiting E.C., Elkins R.B. Spatial distribution of Armillaria mellea in pear orchards. Plant Disease. 1998;82:1226–1231. doi: 10.1094/PDIS.1998.82.11.1226. [DOI] [PubMed] [Google Scholar]
- Rolfe R.T., Rolfe F.W. Chapman and Hall; London: 1925. The romance of the fungus world. [Google Scholar]
- Ross-Davis A.L., Stewart J.E., Hanna J.W. Transcriptome of an Armillaria root disease pathogen reveals candidate genes involved in host substrate utilization at the host-pathogen interface. Forest Pathology. 2013;43:468–477. [Google Scholar]
- Rubini M.R., Silva-Ribeiro R.T., Pomella A.W.V. Diversity of endophytic fungal community of cacao (Theobroma cacao L.) and biological control of Crinipellis perniciosa, causal agent of Witches' Broom Disease. International Journal of Biological Sciences. 2005;1:24–33. doi: 10.7150/ijbs.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino C.M., Xue B., Bhavnani S.M. Population pharmacokinetic analyses for BC-3781 using phase 2 data from patients with acute bacterial skin and skin structure infections. Antimicrobial Agents and Chemotherapy. 2015;59:282–288. doi: 10.1128/AAC.02033-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudgard S.A., Maddison A.C., Andebrhan T. Springer; Netherlands: 2012. Disease management in cocoa: comparative epidemiology of witches' broom. [Google Scholar]
- Rühl M., Fischer C., Kües U. Ligninolytic enzyme activities alternate with mushroom production during industrial cultivation of Pleurotus ostreatus on wheat straw-based substrate. Current Trends in Biotechnology and Pharmacy. 2008;2:478–492. [Google Scholar]
- Saini V.K., Naithani S., Thapliyal B.P. Increased delignification rate of Dendrocalamus strictus (Roxburgh) nees by Schizophyllum commune Fr.; Fr. to reduce chemical consumption during pulping process. Songklanakarin Journal of Science and Technology. 2013;35:415–420. [Google Scholar]
- Sánchez C. Cultivation of Pleurotus ostreatus and other edible mushrooms. Applied Microbiology and Biotechnology. 2010;85:1321–1337. doi: 10.1007/s00253-009-2343-7. [DOI] [PubMed] [Google Scholar]
- Santana M.F., de Araújo E.F., de Souza J.T. Development of molecular markers based on retrotransposons for the analysis of genetic variability in Moniliophthora perniciosa. European Journal of Plant Pathology. 2012;134:497–507. [Google Scholar]
- Scholtmeijer K., Wösten H.A.B., Springer J. Effect of introns and AT-rich sequences on expression of the bacterial hygromycin B resistance gene in the basidiomycete Schizophyllum commune. Applied and Environmental Microbiology. 2001;67:481–483. doi: 10.1128/AEM.67.1.481-483.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz T.R., Brady S.G. Major evolutionary transitions in ant agriculture. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5435–5440. doi: 10.1073/pnas.0711024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuren F.H.J., Wessels J.G.H. Highly-efficient transformation of the homobasidiomycete Schizophyllum commune to phleomycin resistance. Current Genetics. 1994;26:179–183. doi: 10.1007/BF00313808. [DOI] [PubMed] [Google Scholar]
- Schützendübel A., Polle A. Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. Journal of Experimental Botany. 2002;53:1351–1365. [PubMed] [Google Scholar]
- Schwarcz R., Hokfelt T., Fuxe K. Ibotenic acid-induced neuronal degeneration: a morphological and neurochemical study. Experimental Brain Research. 1979;37:199–216. doi: 10.1007/BF00237708. [DOI] [PubMed] [Google Scholar]
- Scotti de Carolis A., Lipparini F., Longo V.G. Neuropharmacological investigations on muscimol, a psychotropic drug extracted from Amanita muscaria. Psychopharmacologia. 1969;15:186–195. doi: 10.1007/BF00411168. [DOI] [PubMed] [Google Scholar]
- Selosse M.-A., Jaquot D., Bouchard D. Temporal persistence and spatial distribution of an American inoculant strain of the ectomycorrhizal basidiomycete Laccaria bicolor in a French forest plantation. Molecular Ecology. 1998;7:561–573. [Google Scholar]
- Sena K., Alemanno L., Gramacho K. The infection process of Moniliophthora perniciosa in cacao. Plant Pathology. 2014;63:1272–1281. [Google Scholar]
- Sewell R.A., Halpern J.H., Harrison G.P. Response of cluster headache to psilocybin and LSD. Neurology. 2006;66:1920–1922. doi: 10.1212/01.wnl.0000219761.05466.43. [DOI] [PubMed] [Google Scholar]
- Sharma R., Sharma B. Strain improvement in Pleurotus ostreatus using UV light and ethyl methyl sulfonate as mutagens. African Journal of Microbiology Research. 2014;8:432–436. [Google Scholar]
- Shim J.O., Chang K.C., Lee Y.S. The fruiting body formation of Armillaria mellea on oak sawdust medium covered with ground raw carrots. The Korean Society of Mycology. 2006;34:206–208. doi: 10.4489/MYCO.2006.34.4.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa T., Nakamura M., Hayashi N. Production of 2,5-dimethoxyhydroquinone by the brown-rot fungus Serpula lacrymans to drive extracellular Fenton reaction. Holzforschung. 2004;58:305–310. [Google Scholar]
- Silva D.V., Araújo I.S., Branco S.M.J. Analysis of resistance to witches' broom disease (Moniliophthora perniciosa) in flower cushions of Theobroma cacao in a segregating population. Plant Pathology. 2014;63:1264–1271. [Google Scholar]
- Silva S.D.V.M., Matsuoka K. Histology of Crinipellis perniciosa in cacao susceptible and resistant to witches' broom disease. Fitopatologia Brasileira. 1999;24:54–59. [Google Scholar]
- Singh J. E & FN Spon; London: 1994. Building mycology: management of decay and health in buildings. [Google Scholar]
- Siri-in J., Kumla J., Suwannarach N. Culture conditions and some properties of pure culture of ectomycorrhizal fungus, Scleroderma sinnamariense. Chiang Mai Journal of Science. 2014;41:275–285. [Google Scholar]
- Smith M.L., Bruhn J.N., Anderson J.B. The fungus Armillaria bulbosa is among the largest and oldest living organisms. Nature. 1992;356:428–431. [Google Scholar]
- Specht C. Isolation of the B alpha and B beta mating-type loci of Schizophyllum commune. Current Genetics. 1995;28:374–379. doi: 10.1007/BF00326436. [DOI] [PubMed] [Google Scholar]
- Sreenivasan T., Dabydeen S. Modes of penetration of young cocoa leaves by Crinipellis perniciosa. Plant Disease. 1989;73:478–481. [Google Scholar]
- Stajich J.E., Wilke S.K., Ahren D. Insights into evolution of multicellular fungi from the assembled chromosomes of the mushroom Coprinopsis cinerea (Coprinus cinereus) Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11889–11894. doi: 10.1073/pnas.1003391107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankis M.M., Specht C.A., Yang H. The A alpha mating locus of Schizophyllum commune encodes two dissimilar multiallelic homeodomain proteins. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:7169–7173. doi: 10.1073/pnas.89.15.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudheer S., Yeoh W.K., Manickam S. Effect of ozone gas as an elicitor to enhance the bioactive compounds in Ganoderma lucidum. Postharvest Biology and Technology. 2016;117:81–88. [Google Scholar]
- Sudheesh N.P., Ajith T.A., Janardhanan K.K. Ganoderma lucidum (Fr.) P. Karst enhances activities of heart mitochondrial enzymes and respiratory chain complexes in the aged rat. Biogerontology. 2009;10:627–636. doi: 10.1007/s10522-008-9208-9. [DOI] [PubMed] [Google Scholar]
- Sullivan R., Smith J.E., Rowan N.J. Medicinal mushrooms and cancer therapy: translating a traditional practice into Western medicine. Perspectives in Biology and Medicine. 2006;49:159–170. doi: 10.1353/pbm.2006.0034. [DOI] [PubMed] [Google Scholar]
- Taira T., Uusi-Oukari M., Korpi E.R. Early postnatal treatment with muscimol transiently alters brain GABAA receptors and open-field behavior in rat. European Journal of Pharmacology. 1993;230:307–312. doi: 10.1016/0014-2999(93)90566-z. [DOI] [PubMed] [Google Scholar]
- Takemoto T., Nakajima T., Sakuma R. Isolation of a flycidal constituent “Ibotenic acid” from Amanita muscaria and A. pantherina. Yakugaku Zasshi: Journal of the Pharmaceutical Society of Japan. 1964;84:1233–1234. [PubMed] [Google Scholar]
- Tanaka H., Takizawa K., Baba O. Basidiomycosis: Schizophyllum commune osteomyelitis in a dog. The Journal of Veterinary Medical Science. 2008;70:1257–1259. doi: 10.1292/jvms.70.1257. [DOI] [PubMed] [Google Scholar]
- Tang W., Liu J.W., Zhao W.M. Ganoderic acid T from Ganoderma lucidum mycelia induces mitochondria mediated apoptosis in lung cancer cells. Life Sciences. 2006;80:205–211. doi: 10.1016/j.lfs.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Teixeira P.J.P.L., Thomazella D.P.d.T., Pereira G.A.G. Time for chocolate: current understanding and new perspectives on cacao Witches' broom disease research. PLoS Pathogens. 2015;11:e1005130. doi: 10.1371/journal.ppat.1005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira P.J.P.L., Thomazella D.P.d.T., Reis O. High-resolution transcript profiling of the atypical biotrophic interaction between Theobroma cacao and the fungal pathogen Moniliophthora perniciosa. The Plant Cell. 2014;26:4245–4269. doi: 10.1105/tpc.114.130807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomidis T., Exadaktylou E. Effectiveness of cyproconazole to control Armillaria root rot of apple, walnut and kiwifruit. Crop Protection. 2012;36:49–51. [Google Scholar]
- Thorn R., Barron G. Carnivorous mushrooms. Science. 1984;224:76–78. doi: 10.1126/science.224.4644.76. [DOI] [PubMed] [Google Scholar]
- Thorn R.G., Moncalvo J.-M., Reddy C. Phylogenetic analyses and the distribution of nematophagy support a monophyletic Pleurotaceae within the polyphyletic pleurotoid–lentinoid fungi. Mycologia. 2000;92:241–252. [Google Scholar]
- Tiburcio R.A., Costa G.G.L., Carazzolle M.F. Genes acquired by horizontal transfer are potentially involved in the evolution of phytopathogenicity in Moniliophthora perniciosa and Moniliophthora roreri, two of the major pathogens of cacao. Journal of Molecular Evolution. 2010;70:85–97. doi: 10.1007/s00239-009-9311-9. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T.K. Lignin-degrading enzyme from the hymenomycete Phanerochaete chrysosporium burds. Science. 1983;221:661–662. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- Tomoda M., Gonda R., Kasahara Y. Glycan structures of ganoderans B and C, hypoglycemic glycans of Ganoderma lucidum fruit bodies. Phytochemistry. 1986;25:2817–2820. [Google Scholar]
- Tsukagoshi T., Tokiwano T., Oikawa H. Studies on the later stage of the biosynthesis of pleuromutilin. Bioscience, Biotechnology, and Biochemistry. 2007;71:3116–3121. doi: 10.1271/bbb.70547. [DOI] [PubMed] [Google Scholar]
- Tsukatani T., Ogawa H., Anzawa K. Schizophyllum commune-induced allergic fungal rhinosinusitis and sinobronchial mycosis. Medical Mycology Case Reports. 2015;8:10–13. doi: 10.1016/j.mmcr.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyls F., Palenicek T., Horacek J. Psilocybin – summary of knowledge and new perspectives. European Neuropsychopharmacology. 2014;24:342–356. doi: 10.1016/j.euroneuro.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Umar M.H., Van Griensven L.J. Studies on the morphogenesis of Agaricus bisporus: the dilemma of normal versus abnormal fruit body development. Mycological Research. 1999;103:1235–1244. [Google Scholar]
- Vahl M. Flora Danica. 1790;6:1013. [Google Scholar]
- van Peer A.F., Park S.-Y., Shin P.-G. Comparative genomics of the mating-type loci of the mushroom Flammulina velutipes reveals widespread synteny and recent inversions. PLoS ONE. 2011;6:e22249. doi: 10.1371/journal.pone.0022249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellinga E.C., de Kok R.P.J., Bruns T.D. Phylogeny and taxonomy of Macrolepiota (Agaricaceae) Mycologia. 2003;95:442–456. [PubMed] [Google Scholar]
- Volk T.J., Burdsall H.H. Fungiflora; Oslo: 1995. A nomenclatural study of Armillaria and Armillariella species. [Google Scholar]
- Wagner R., Mitchell D.A., Sassaki G.L. Current techniques for the cultivation of Ganoderma lucidum for the production of biomass, ganoderic acid and polysaccharides. Food Technology and Biotechnology. 2003;41:371–382. [Google Scholar]
- Waites K.B., Crabb D.M., Duffy L.B. In vitro activity of defaulting against macrolide-susceptible (MSMP) and macrolide-resistant Mycoplasma pneumoniae (MRMP) from the United States, Europe, and China. ASM Microbe 2016 Poster Abstracts. 2016 [Google Scholar]
- Wälti M.A., Villalba C., Buser R.M. Targeted gene silencing in the model mushroom Coprinopsis cinerea (Coprinus cinereus) by expression of homologous hairpin RNAs. Eukaryotic Cell. 2006;5:732–744. doi: 10.1128/EC.5.4.732-744.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton J.D., Hallen-Adams H.E., Luo H. Ribosomal biosynthesis of the cyclic peptide toxins of Amanita mushrooms. Peptide Science. 2010;94:659–664. doi: 10.1002/bip.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Applied Microbiology and Biotechnology. 2011;89:1323–1332. doi: 10.1007/s00253-010-3067-4. [DOI] [PubMed] [Google Scholar]
- Wasser S.P., Weis A.L. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: a modern perspective. Critical Reviews in Immunology. 1999;19:65–96. [PubMed] [Google Scholar]
- Wasson R.G. Harcourt Brace Jovanovich; New York: 1968. Soma – divine mushroom of immortality. [Google Scholar]
- Wasson R.G. The death of Claudius or mushrooms for murderers. Botanical Museum Leaflets, Harvard University. 1972;23:101–128. [Google Scholar]
- Watling R., Kile G.A., Burdsall H.H. Nomenclature, taxonomy and identification. In: Miller O.K., Shaw C.G., Kile G.A., editors. Armillaria root disease agriculture handbook no. 691. USDA; Washington: 1991. pp. 1–9. [Google Scholar]
- Weber N.A. Fungus-growing ants. Science. 1966;153:587–604. doi: 10.1126/science.153.3736.587. [DOI] [PubMed] [Google Scholar]
- Wessels J.G., De Vries O.M., Asgeirsdottir S.A. Hydrophobin genes involved in formation of aerial hyphae and fruit bodies in Schizophyllum. The Plant Cell. 1991;3:793–799. doi: 10.1105/tpc.3.8.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J.S., Fox R.T.V. Stimulation of Armillaria mellea by phenolic fungicides. Annals of Applied Biology. 2002;140:291–295. [Google Scholar]
- Whitehouse H. Multiple-allelomorph heterothallism in the fungi. New Phytologist. 1949;48:212–244. [Google Scholar]
- Wieland T. Modification of actins by phallotoxins. Naturwissenschaften. 1977;64:303–309. doi: 10.1007/BF00446784. [DOI] [PubMed] [Google Scholar]
- Wilcox J.A. Psilocybin and obsessive compulsive disorder. Journal of Psychoactive Drugs. 2014;46:393–395. doi: 10.1080/02791072.2014.963754. [DOI] [PubMed] [Google Scholar]
- Williams L., Northwood J., Crowhurst N. P790 In vitro activity of retapamulin, a novel pleuromutilin, against Staphylococcus spp. (n = 1413) and Streptococcus pyogenes (n = 503) from 26 European centres. International Journal of Antimicrobial Agents. 2007;29:S198. [Google Scholar]
- Wolfe B.E., Tulloss R.E., Pringle A. The irreversible loss of a decomposition pathway marks the single origin of an ectomycorrhizal symbiosis. PLoS ONE. 2012;7:e39597. doi: 10.1371/journal.pone.0039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D.A., Leatham G.F. Lignocellulose degradation during the life cycle of Agaricus bisporus. FEMS Microbiology Letters. 1983;20:421–424. [Google Scholar]
- Woodward C., Hansen L., Beckwith F. Symbiogenics: an epigenetic approach to mitigating impacts of climate change on plants. HortScience. 2012;47:699–703. [Google Scholar]
- Wu G.S., Guo J.J., Bao J.L. Anti-cancer properties of triterpenoids isolated from Ganoderma lucidum – a review. Expert Opinion on Investigational Drugs. 2013;22:981–992. doi: 10.1517/13543784.2013.805202. [DOI] [PubMed] [Google Scholar]
- Wu L., van Peer A., Song W. Cloning of the Lentinula edodes B mating-type locus and identification of the genetic structure controlling B mating. Gene. 2013;531:270–278. doi: 10.1016/j.gene.2013.08.090. [DOI] [PubMed] [Google Scholar]
- Xia Q., Zhang H., Sun X. A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules. 2014;19:17478–17535. doi: 10.3390/molecules191117478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C., Wu Q.P., Cai W. Hypoglycemic effects of Ganoderma lucidum polysaccharides in type 2 diabetic mice. Archives of Pharmacal Research. 2012;35:1793–1801. doi: 10.1007/s12272-012-1012-z. [DOI] [PubMed] [Google Scholar]
- Xu J.-W., Ji S.-L., Li H.-J. Increased polysaccharide production and biosynthetic gene expressions in a submerged culture of Ganoderma lucidum by the overexpression of the homologous α-phosphoglucomutase gene. Bioprocess and Biosystems Engineering. 2015;38:399–405. doi: 10.1007/s00449-014-1279-1. [DOI] [PubMed] [Google Scholar]
- Xu K., Liang X., Gao F. Antimetastatic effect of ganoderic acid T in vitro through inhibition of cancer cell invasion. Process Biochemistry. 2010;45:1261–1267. [Google Scholar]
- Yetis Ü., Özcengiz G., Dilek F.B. Heavy metal biosorption by white-rot fungi. Water Science and Technology. 1998;38:323–330. [Google Scholar]
- You L.-F., Guo L.-Q., Lin J.-F. Overproduction of geranylgeraniol in Coprinopsis cinerea by the expression of geranylgeranyl diphosphate synthase gene. Journal of Basic Microbiology. 2014;54:1387–1394. doi: 10.1002/jobm.201400152. [DOI] [PubMed] [Google Scholar]
- Zanetti R., Zanuncio J., Santos J. An overview of integrated management of leaf-cutting ants (Hymenoptera: Formicidae) in Brazilian forest plantations. Forests. 2014;5:439–454. [Google Scholar]
- Zhang C., Yi Y., Chen J. In vivo efficacy and toxicity studies of a novel antibacterial agent: 14-o-[(2-amino-1,3,4-thiadiazol-5-yl)thioacetyl] mutilin. Molecules. 2015;20:5299–5312. doi: 10.3390/molecules20045299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Jia D., Fei X. An assessment of the genetic diversity within Ganoderma strains with AFLP and ITS PCR-RFLP. Microbiological Research. 2009;164:312–321. doi: 10.1016/j.micres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Zhou J.-S., Ji S.-L., Ren M.-F. Enhanced accumulation of individual ganoderic acids in a submerged culture of Ganoderma lucidum by the overexpression of squalene synthase gene. Biochemical Engineering Journal. 2014;90:178–183. [Google Scholar]
- Zhou L.W., Cao Y., Wu S.H. Global diversity of the Ganoderma lucidum complex (Ganodermataceae, Polyporales) inferred from morphology and multilocus phylogeny. Phytochemistry. 2015;114:7–15. doi: 10.1016/j.phytochem.2014.09.023. [DOI] [PubMed] [Google Scholar]