Abstract
Venous malformation (VM) is the most common type of congenital vascular malformation (CVM). They are present at birth and are often symptomatic, causing morbidity and pain. VMs can be challenging to diagnose and are often confused with hemangioma in terminology as well as with imaging. An accurate clinical history and cross-sectional imaging are critical for diagnosis and for devising management. This manuscript will review imaging approaches to diagnosing VMs and current treatment strategies.
Keywords: Magnetic resonance, sclerotherapy, ultrasound (US), venous malformations (VM)
Introduction
Venous malformation (VM) is the most common type of congenital vascular malformation (CVM) with an incidence of 1 to 2 in 10,000 and a prevalence of 1% (1,2). They can cause significant morbidity, pain and discomfort to patients as they can lead to serious local and systemic complications. Although present at birth, they are not always clinically evident until later in life and tend to grow in concert with the child and without spontaneous regression (3). VMs are composed of ectatic venous channels found usually in the head, neck, limbs, and trunk and are thought to be sporadic in most cases, though familial inheritance patterns exist (4). Accurate diagnosis has been a limiting factor in VM management (5). An increased emphasis has been placed on creating comprehensive classification systems for diagnostic and therapeutic purposes of this chronic condition. Doppler ultrasound (US) and magnetic resonance imaging (MRI) are key imaging methods used to characterize and diagnose VMs. Treatment options include surgery, sclerotherapy, and ablative therapies. Here, we will review imaging approaches to diagnosing VMs and common strategies used today in their treatment.
Classification systems
The International Society for the Study of Vascular Anomalies (ISSVA) Classification System places an emphasis on the pathologic and hemodynamic features of vascular anomalies and was recently updated in 2014 to incorporate newly identified anomalies and genes (6). The ISSVA system first distinguishes CVMs between vascular tumors and vascular malformations. This difference is especially important as vascular malformations are frequently misdiagnosed as hemangiomas, the most common vascular tumor (7). Up to 71.3% of hemangioma cases are misclassified, resulting in a suboptimal treatment in up to 20.6% of these patients (8,9). Terms such as “rectal hemangioma”, “vertebral body hemangioma” and “hepatic hemangioma” that are rife in the world’s literature may not be hemangioma but VMs in the rectal wall, intraosseous within a vertebral body, and within the liver, the most common benign tumor of the liver routinely seen on US.
The Hamburg Classification, developed by Prof. Stefan Belov MD, can also be used to classify congenital vascular anomalies and was most recently updated in 2013 (5,10). This system classifies vascular malformations into five main categories: arterial, venous, arterio-venous, lymphatic, and combined vascular malformations. The Hamburg system’s key feature is that it further divides these main categories into two embryological based subcategories: extratruncular or truncular lesions. Extratruncular malformations occur in earlier embryonic stages, while truncular anomalies form during the later stages of embryonic development. The Hamburg Classification is based primarily at the macroscopic level while the ISSVA System can be utilized to classify lesions at the cellular level distinguishing vascular malformations from vascular tumors.
The ISSVA system establishes criteria to differentiate between hemangiomas (and other vascular tumors). It is especially useful in diagnosis as it characterizes hemodynamic discrepancies, which can easily be distinguished using imaging techniques that provide high specificity and sensitivity for this purpose. The ISSVA Classification System has recently included the useful elements of the Hamburg Classification into its classification system.
Description of vascular malformations
The type of flow can be used to group vascular malformations into low-flow and high-flow malformations according to their vascular hemodynamics (11,12). High-flow malformations are most commonly arteriovenous malformations. Low-flow malformations include lymphatic malformations (LMs), capillary-venulous malformations, and VMs, glomovenous malformations, and non-shunting mixed-lesions (3,13).
VMs
VMs are the most common type of low-flow vascular lesions and comprise up to two-thirds of CVMs. VMs are typically subdivided into sporadic VMs (94%), dominantly inherited cutaneomucosal VMs (1%) and, dominantly inherited and non-inherited glomuvenous malformations (5%) first described by Vikkula et al. (1,14-16).
Clinical findings and characteristics of VMs are shown in Table 1. VM features predispose to stagnant blood flow, which can spontaneously thrombose, and clinically present as pain and swelling (phlebitic syndrome), overlying skin changes, and tissue and limb overgrowth, though the exact symptomatic clinical presentation tends to be location-dependent. Patients with large VMs rarely present with increased D-dimer levels, low platelets, and low fibrinogen levels; an indicator for low-grade intravascular coagulopathy. In large VMs, spontaneous thrombosis within the static venous lake is observed in around 40% of patients (14). With over 40% of VMs occurring in the head and neck region, airway compromise can also be of particular concern if involvement of the aerodigestive system is prominent (21).
Table 1. Positive and negative clinical findings and characteristics of venous malformations (17-20).
| Clinical signs | Clinical findings |
|---|---|
| Positive | Bluish/purple superficial appearance |
| Occasionally palpable phleboliths and spontaneous thrombi | |
| Soft and compressible mass | |
| Swelling | |
| Pain | |
| Well-defined or diffuse and infiltrative | |
| Can involve superficial or deep structures | |
| May enlarge during Valsalva maneuver, puberty or pregnancy | |
| Negative | Hyperemia |
| Pulsatility | |
| Palpable Local Thrill | |
| Characteristics | Occur in childhood or early adulthood |
| Can occur anywhere in the body, but are predominantly located in the head and neck (40% of cases), trunk (20%) and extremities (40%) | |
| Lesions are categorized as: (I) microcystic small diameter locules; (II) macrocystic (large, more translucent subcutaneous masses in the neck, pelvis or axilla); (III) mixed | |
| VMs are post-capillary, by definition, and represent enlarged and engorged structures. These may demonstrate abnormal and sparse smooth muscle cells within the thin vessel wall, absent or insufficient valves, chaotic vascular branching, and are non-functional hemodynamically |
VM, venous malformation.
Recently, various inherited forms of VMs have been shown to have a strong association with a loss-of-function mutation on the angiopoetin receptor gene TIE2/TEK (22). Chromosome 9p (23) or the upregulation of factors such as transforming growth factor beta (TGF-beta) and basic fibroblast growth factor (FGF-beta) (24) have also been identified as alternative pathways. Furthermore, progesterone receptors have been identified in VMs, which could potentially explain their likelihood to grow during hormonal changes (25). Other factors that can exacerbate them include thrombosis, infection, trauma, or even incomplete treatment (14).
Imaging of vascular malformations
Although imaging is not critical for the clinical diagnosis of cutaneous VMs, it is necessary to detect and evaluate deeper lesions. X-rays can image calcified phleboliths and the degree of dystrophic calcification in VMs, which can be useful in suggesting the presence of VMs, as it has been shown that over 1/3 of VMs have bony changes (26). CT does not have significant use in discerning VMs, unless there is intraosseous involvement and may be superior to MR imaging of bone. The imaging evaluation predominantly involves Doppler US and MRI. Magnetic resonance angiography (MRA)/MRV is useful in mapping the venous system and has supplanted the diagnostic role of conventional angiography in many cases (27). Angiography remains an adjunct imaging modality and important for cases requiring therapeutic intervention of VMs. Direct percutaneous puncture of the VM with contrast injection (discussed in ‘Treatment Strategies’) is a key initial imaging technique carried out prior to sclerotherapy.
US
Duplex US is a useful, non-invasive imaging technique and should be used as the first modality when investigating the presence of a vascular malformation, especially for superficial lesions or those in the extremities (5). Other benefits are its ubiquity in clinical settings, low-cost and its lack of ionizing radiation, which is an important aspect in young patients (26). Conversely depth and spatial resolution is low, resulting in a narrow field of vision. Limitations in discerning the involvement of nearby structures including nerves and bone are inherent with the use of US (26,28).
Priority should be given to identify the nature of an anomaly and if a vascular malformation is detected, to ascertain the type of malformation. To this end, 2D (B-mode) US is particularly useful in being able to discern tumors/hemangiomas from vascular malformations. B-mode ultrasonography reveals the mass, borders, and size of a lesion. Soft tissue solid mass indicates a tumor while, vascular channels with minimal soft tissue points to a VM. The presence of heterogenous and hypo- or anechoic vascular spaces typically represents compressible subcutaneous or intra-muscular vascular spaces which are the hallmarks of VMs on B mode or high-resolution grey-scale US (5,29). Range in appearance includes purely solid to multicystic, localized and well-defined to infiltrative, or cavitary (most common) to dysplastic lesions. MR is superior in this regard as it readily images blood-fluid levels. Doppler mode, conversely, is better used to discern hemodynamics, identifying high or low flow lesions (5). Flow has been demonstrated in 84% of lesions, with monophasic and biphasic flow seen in 78% and 6%, respectively. In particular flow is apparent upon augmentation when using spectral analysis and power Doppler. Up to 16% reveal no detectible flow, largely due to the low-flow and static state within VMs (30). Tubular, tortuous, anechoic formations are sometimes observed in the surrounding subcutaneous fat, muscles, tendons and other tissues. The presence of acoustically shadowing phleboliths post application of compression is a strong diagnostic clue for venous lesions (31). Valsalva or manual compression can be required at times to induce visible flow (17,18). US findings should be corroborated by MRI, and in the case of deep intramuscular lesions, MRI should be the first choice imaging modality to ensure that the lesion is accurately localized.
MRI
The advent of MRI has led to huge advances in the noninvasive investigation of vascular malformations. Lesions and soft tissue depiction is vastly enhanced in particular when compared to CT. Non-ionizing radiation exposure is also avoided and 3D reconstruction is superior to CT. Contrast-enhanced MRI and MRA are therefore the preferred imaging modalities for pre-procedure diagnosis and interventional planning as well as post-procedure evaluation of the vascular malformation. Conventional MRI has 100% sensitivity and 24% to 33% specificity in differentiating VMs from non-VMs (32). Dynamic contrast MR angiography increases specificity to 95%. The high spatial and temporal resolution of MRI allows for an improved visualization of the lesion’s extent, especially with the use of newer blood pool contrast agents such as Ablavar® (gadofosveset trisodium, Lantheus Medical Imaging, North Billerica, MA, USA) which have an extended vascular half-life. Ablavar chelates gadolinium intravenously and binds reversibly to serum albumin thereby enabling a prolonged optimal intravascular simultaneous enhancement of both the arterial and venous system as the flow transitions from the arterial phase to the venous phase (33-35). In particular the lesion’s anatomic relationship to adjacent structures can be defined and is consequently a vital aide in planning of therapy. Intraoperative MRI has also been used to facilitate accurate needle placement and image guidance during percutaneous therapy of VMs is well-established (28).
Images should be acquired in at least two orthogonal planes (18). VMs typically display as septated lobulated masses without mass effect. They appear hypo-or isointense on T1-weighted (W) images, although they can appear more hyperintense if the lesion contains fat. Thrombi can also appear hyperintense while, small low signal intensity points can denote phleboliths; a hallmark of VMs. In cases of hemorrhage or thrombosis, a heterogeneous signal can be observed on T1-sequences. On T1-W fluid sensitive sequences VMs can be hyperintense and indeed similar to fluid and the extension of the malformation can be ascertained using this sequence setting.
VMs, LMs, capillary VMs, and pediatric hemangioma are best imaged using short T1 inversion recovery (STIR) sequences and T2-W imaging with fat suppression (FS) (Figure 1) (16,18,26,28,30).
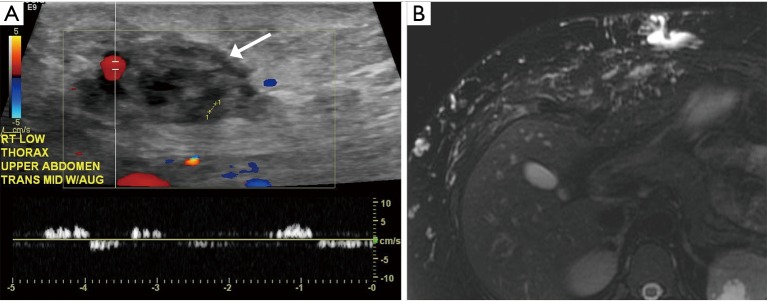
Figure 1.
25 years old with extensive venous malformation in the right abdominal wall. (A) Color Doppler tubular venous channels within the soft tissue (white arrow) of the abdominal wall that demonstrate low flow; (B) MRI image with extensive T2 hyperintense signal within tubular structures in the subcutaneous tissue of the abdominal wall. MRI, magnetic resonance imaging.
The STIR and T2-W FS examination protocol captures sequences for defining the lesion itself and its relationship to the involved anatomic architecture. Hemosiderin, dystrophic calcification, or phleboliths can then be demonstrated with the use of gradient echo T2-W sequences. VMs consistently display a high signal on T2-W FS, no flow voids in spin-echo images and infiltrate across tissue planes. There may be surrounding edema if a spontaneous thrombosis is present, no arterial or early venous enhancement, slow gradual peripheral enhancement, and diffuse enhancement on delayed images (18,26,28,30). Pre-gadolinium and post-gadolinium fat saturated T1-W imaging can be carried out to evaluate the perfusion of the malformation (16,18,26,28).
Several MR imaging-based morphology classification schemes correlate imaging observations to treatment pathways by providing a structure to link lesion morphology with the ideal treatment and outcome. Lesion size and lesion definition are key criteria utilized to differentiate lesions into a grading linked to outcomes (see Table 2) (14,19,36).
Table 2. Classification systems with outcomes (8,14,18,19,26,36-38).
| Grading scheme | Modality | Category | Anatomic features and hallmarks | Treatment | Implications for treatment |
|---|---|---|---|---|---|
| Goyal et al. | MRI based | Grade 1 | Well defined, ≤5 cm diameter | Sclerotherapy (as monotherapy) | 71% excellent results, 29% good results, 0% poor results |
| Grade 2A | Well defined, >5 cm | 22% excellent results, 44% good results, 33% poor results | |||
| Grade 2B | Ill defined, ≤5 cm diameter | 26% excellent results, 15% good results, 60% poor results | |||
| Grade 3 | Ill defined, >5 cm | 0% excellent results, 43% good results, 57% poor results | |||
| Fayad et al. | MRI based | Extent | Focal, multifocal, or diffuse | Sclerotherapy | Multifocal treatments and multiple sessions are required |
| Tissue layer involvement | Skin/subcutaneous/muscle/tendon/bony cortex/marrow | Risk of skin or nerve injury is considered; muscular involvement; risk of contracture; bone or marrow involvement; risk of fracture |
|||
| Connection | Deep venous system | Increased risk of deep venous thrombosis | |||
| Dubois | DPP based | Cavitary | Cavities with late venous drainage without abnormal veins | Sclerotherapy | Improved results |
| Spongy | Small, ‘honeycomb’ cavities and venous drainage | Difficult to treat, particularly when the VM is intramuscular | |||
| Dysmorphic | Rapid opacification of dysmorphic veins | Improved results, however recurrence is likely | |||
| Dubois/Puig | DPP based | Type I | Isolated, well circumscribed without visible venous drainage | Sclerotherapy | Highest rate of cure or satisfactory result, 92.3% complication-free predictive value |
| Type II | Malformation draining into normal veins | High rate of cure or satisfactory result, 93.8% complication-free predictive value | |||
| Type III | Malformation draining into ectatic dysplastic veins | 50% exclusion rate, higher risk of complications | |||
| Type IV | Venous ectasia | 60% exclusion rate & higher risk of complications | |||
| Berenguer et al. | Direct contrast venogram | Lobulated | Rounded clusters of vascular spaces | Sclerotherapy (multiple therapy sessions, 1–10) | 27% in all three categories rated outcome as near normal, 38% reported marked improvement, 13% reported slight improvement, and 4% reported no change |
| Varicose | Irregular dilated channels | No significant difference in outcome could be shown between categories | |||
| Combined | Combination of the above | ||||
| Boston Children’s | Based on imaging and clinical features | Spongiform | Large sponge-like singular lesion | – | Most common type |
| Phlebectatic | Large diameter combined vascular malformation | Increased risk of deep vein thrombosis and pulmonary embolism | |||
| Aneurysmatic | Involves large veins such as inferior vena cava | Increased risk of deep vein thrombosis and pulmonary embolism | |||
| Reticular | Small veins, spider-like appearance, combined vascular syndromes | 50% have spontaneous Tie-2 mutations (unlike the autosomal dominant inheritance pattern found in familial mucocutaneous VMs) |
VM, venous malformation.
Post therapy follow up assessment is vital in a clinical setting. A significant percentage of patients will suffer from their VMs again after treatment. In particular female patients who undergo pregnancy or younger patients undergoing puberty as hormonal changes trigger recurrence or proliferation. MRI is most suited to evaluate clinical outcomes after successful sclerotherapy. The reduced size of the lesion is noted and treated segments have an elevated heterogeneity, diminished STIR and T2 signal intensity and decreased contrast enhancement. This information allows planning a future procedure for the untreated sections (37,39).
Treatment strategies
Prior to commencing treatment, it is pertinent to review the lesion’s pathophysiology, etiology, and consequences of a procedure. There may be instances when it is prudent to delay intervention in favor of observation, or to avoid intervention if there are no significant symptoms and risks. Interventionalists are advised to take a multi-disciplinary approach consulting dermatologists, surgeons as well as other specialists to determine if treatment is warranted, and to construct the method of intervention. Generally, lesions with severe symptoms and potential complications should be treated. In particular, extratruncular malformations usually display significantly worse symptoms and higher recurrence than truncular forms, and thus this subtype is more likely to require treatment (5).
When treatment is not required or there is an interim period before definitive treatment can be given, associated and secondary complications, such as pain or anemia caused by bleeding, should be treated. Patients with VMs in the extremities should be given compression therapy using compression stockings to minimize symptoms like swelling and thrombophlebitis forcing venous blood from the stagnant VMs into the deep venous system. Low dose aspirin may also be beneficial in reducing painful VM related blood clots, though reports of its efficacy in the literature are still sparse (40). Some institutions include severe pain, joint involvement, severe craniofacial implications (seeing, hearing, eating, breathing) and hemorrhage as absolute indications for moving forward with the development of a therapeutic regimen (see Table 3) (41).
Table 3. Indications for the treatment of venous malformations.
| Bleeding from below skin to intramuscular or retroperitoneal hematoma, hematuria, rectal bleeding, hematemesis, hemoptysis, or intracerebral or intraspinal bleeding |
| Lesions are in close proximity to important structures or obstruct inflow and outflow of important structures |
| Lesions are in life-threatening areas or in areas with high probabilities of complications |
| Lesions cause excessively adverse hemodynamic effects |
| Disabling pain |
| Functional impairment |
| Excessive cosmetic implications |
| Recurrent thrombosis |
When additional treatment is necessary, a number of modalities can be used, including surgery, various forms of sclerotherapy, and laser therapy. Surgical intervention was traditionally considered to be the initial form of treatment if the lesion could be completely resected and had minimal anatomic and functional consequences. However, the emergence of sclerotherapy as a viable yet cost-effective and minimally invasive technique has spurred its use as mono-therapy or in conjunction with surgery (5,26). As excision of complex lesions remains difficult due to secondary intraoperative bleeding, the favored approach is now sclerotherapy. Those lesions classified as extratruncular malformations, are diffusely infiltrative and involve multiple muscles or layers of fascia, making them less amenable to any surgical intervention. Truncular VMs typically involve large, localized venous formations and aneurysms, and have minimal chances of recurrence post-resection as a result of their embryonic nature. Thus these types of lesions are suited for surgical therapy with or without adjunct sclerotherapy. Pre-operative sclerosant therapy or N-butyl cyanoacrylate (nBCA) injections can often be carried out 24–48 hours prior to excision (3).
Direct percutaneous puncture with contrast injection
Direct percutaneous puncture with contrast injection or phlebography (DPP) is the fine-needle puncture of the VM with subsequent contrast injection under fluoroscopy. It is the gold standard diagnostic tool for specificity when confirmation of a VM is required after alternative imaging approaches have not yielded definitive results, in cases when treatment planning is required or when a neoplasm must be ruled out. DPP is a component of the initial work-up of a VM prior to sclerotherapy. The patient usually requires sedation with a possible regional block or alternatively general anesthesia. Prior to injecting the sclerosant, VM architecture, nearby anatomy, flow rate, type of venous drainage, and volume of contrast distribution relative to the known appearance of the VM image is assessed (see Figures 2 and 3) (26). DPP morphologic classifications have been designed based on the venous drainage pattern to assist in real-time decision making during a sclerotherapeutic procedure, post-procedure management, and prediction of outcomes (see Table 4) (14,17,42). Real-time MRI image guidance for sclerotherapy of VMs is an alternative new approach. For instance, fast imaging steady-state procession (FISP) MR imaging fluoroscopy has shown good results for patients treated with ethanolamine or sodium tetradecyl sulfate (STS) sclerotherapy (26).
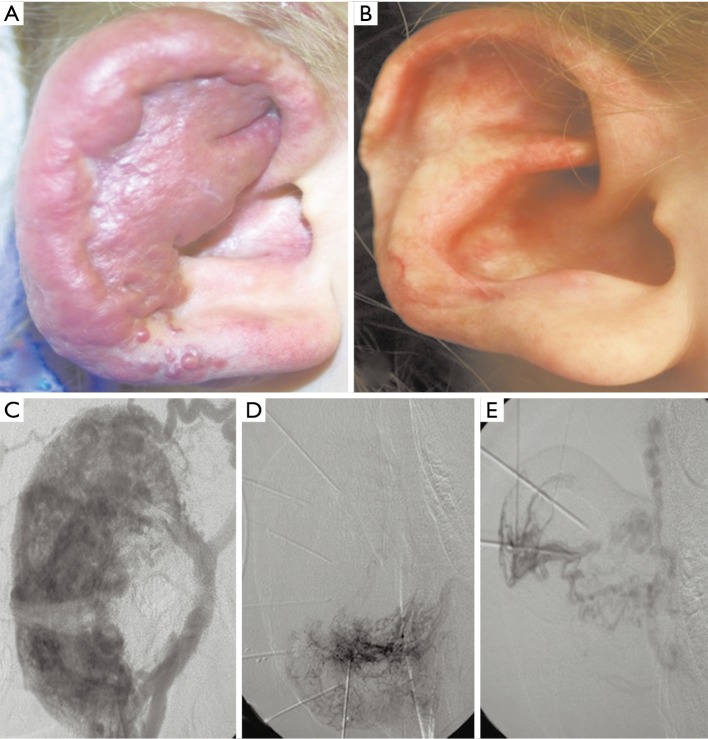
Figure 2.
A 4-year-old female with right ear venous malformation. (A) Pre-treatment image demonstrates a soft and compressible focal disfiguring lesion with bluish/purple appearance and significant enlargement of the ear; (B) post-treatment image with improved cosmesis of the ear; (C) post-contrast digital subtraction angiography (DSA) image demonstrates the extent of the venous malformation; (D,E) DSA images during the intervention demonstrate segmental obliteration of the venous malformation.
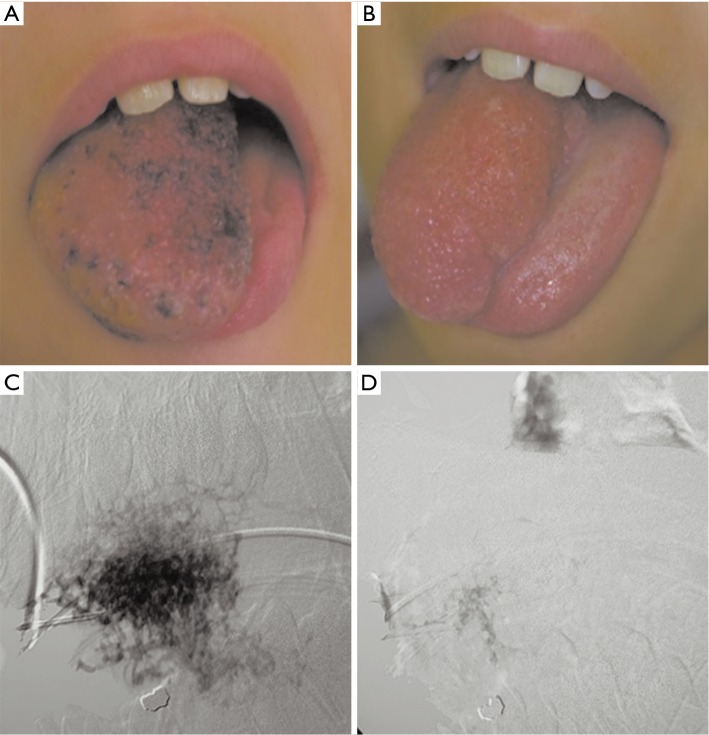
Figure 3.
9 years old with asymmetrically enlarged tongue secondary to lingual venous malformation. (A) Pre-treatment image shows an enlarged right side of the tongue with superficial, diffuse lesions with bluish discoloration on the dorsum of the tongue; (B) post-treatment image demonstrates reduction in lingual asymmetry and improvement in the cosmetic appearance of the tongue; (C) the lesion was percutaneously accessed using a 21 gauge needle and absolute alcohol mixed with contrast was instilled into the lesion; (D) obliteration of the venous malformation after treatment as demonstrated by lack of contrast opacification.
Table 4. Pre and post treatment VM features for ultrasound and MRI (16,18,26).
| Imaging modality | Differential imaging characteristics categories | Pre-treatment features | Post-treatment features |
|---|---|---|---|
| Ultrasound | Architecture | Heterogeneous, tubular, torturous, acoustically shadowed phleboliths | Decreased size, involution of VM, absence of phleboliths |
| Echogenicity | Hypo- or anechoic vascular space and formations | Hypo- or anechoic | |
| Flow | Low rate monophasic flow, though some can appear without any flow | Decreased flow | |
| MRI | Architecture | Can appear septated with lobulated margins, infiltrates tissue planes and reveals full extent of infiltration at margins of lesion, surrounding edema possible, lower SI or signal voids represent dystrophic calcification or phleboliths, thrombi are hyperintense | Evaluation post 6 months shows decreased lesion size |
| T1 imaging | Iso- or hypointense | Diminished signal intensity post 6 months | |
| T2 imaging | High signal intensity | Diminished signal intensity post 6 months | |
| Enhancement | Can be diffuse, hetero-, or homogenous | Early evaluation demonstrates heterogeneous high signal intensity in treated areas. Post 6 months enhancement is decreased | |
| Flow measurement | Low | In cases of extensive VM, gadolinium enhanced imaging is used to demonstrate residual perfusion of VM |
VM, venous malformation; MRI, magnetic resonance imaging.
Sclerotherapy
Ethanol sclerotherapy
Ethanol sclerotherapy offers a low-cost, convenient, and effective modality of treatment. It has shown curative potential for several types of vascular malformations and is the most common and most potent sclerosant used to treat VMs (5,43). The exact mechanism by which ethanol acts as an in vivo sclerosant is to denude the endothelial cell from the vascular wall and precipitate its protoplasm then cause fractures to the level of the internal elastic lamina. Platelet aggregation on the denuded surface then embolizes the vessel from the periphery to the center (43,44). Ethanol is believed to preferentially displace water molecules at the membrane surface, thereby penetrating the lipid bilayer and compromising the structural integrity of the cell membrane (44). This triggers necrosis and apoptosis of the treated tissue, inducing intravascular thrombosis and intense inflammatory response (45).
VMs are best treated early, with DPP of the malformation under US or fluoroscopic guidance (46). Ethanol injection can be painful; thereby requiring general anesthesia. Prior to the procedure, contrast should be used to gauge the size of the lesion in its dilated state, identify connections and drainage into physiologically important veins, and to determine the appropriate ethanol volume to inject. Ethanol exposure should last for approximately 10–20 minutes depending on the extent of the lesion, with subsequent aspiration of excess ethanol to reduce collateral damage. The volume of ethanol that can be injected safely is generally suggested to be 0.15–1 mL/kg every 10 minutes and no Swan-Ganz monitoring is necessary (47-50). Physicians are advised to use minimum amounts within the suggested range that will achieve the desired outcome and to ensure that the delivery point does not affect normal tissue. Accidental collateral tissue or skin necrosis, nerve damage, paresthesias, vascular spasms, ischemia, acidosis, hypoglycemia, and secondary intravascular hemolysis are some complications that can arise as a result of treatment. Other complications that can arise include allergic responses, skin pigment change, cardiovascular collapse, hypotension and cardiac arrest (51,52). However, cardiovascular collapse has been obviated by limiting ethanol injected volumes to 0.15 mL/kg ideal body weight (49,53,54).
Figures 2 and 3 demonstrate the effectiveness of treatment with absolute ethanol sclerotherapy. If required optimal spacing between treatments of the same area ranges between 3–4 weeks. Follow-up imaging is necessary to evaluate treatment outcomes. US can be performed in between sessions and after 1 month of the last ethanol therapy (55). MRI evaluation should occur annually after the final ethanol treatment in order to allow inflammation to subside and provide an accurate assessment of the treatment’s effectiveness and growth of any remaining VMs, although some advocate MRI follow-up as early as 1 to 3 months (37). Difficult lesions may benefit from intermittent ethanol sclerotherapy and medication in lieu of surgical intervention. Post-sclerotherapy, NSAIDs or stronger pain medications if appropriate, can be used to treat local pain and swelling. Vigilant treatment planning and practitioner-technique remains crucial to procedural success. Accordingly, clinical outcomes may vary greatly based on institutional expertise and approach. Often serial sclerotherapies may be necessary due to high recurrence rates as the untreated veins can dilate over time (28,38,51,56).
Other sclerosants and therapies
Surfactant agents are viable alternatives that act with a similar mechanism to ethanol but have lower toxicities. Polidocanol is a popular surfactant agent that is relatively effective, painless, and incites minimal endothelial damage. New research has investigated the use of its foam variant in conjunction with iopromide, a radiopaque contrast agent, which allows for continuous detection of sclerosant flow, better measurements, and decreased removal from the target site via blood flow (57). While foam has shown superior results compared to liquid sclerosants, sclerosant leakage from the target site can still cause complications. Thus additional research on radiopaque foam is warranted. STS is another surfactant agent commonly used as a sclerotherapeutic for varicose veins. It is best suited for treating smaller lesions with lower resistance. Bleomycin is an antibiotic drug with chemotherapeutic properties that disrupts the tight junctions in the endothelium which is also frequently used (58). It rarely causes inflammation and thrombosis, and has few other complications, making it well suited to treating the air passages or orbit (59).
Research on novel ablative techniques has shown promise. Endovenous thermal ablation may have an adjunctive role in the therapy of large truncular VMs, while radiofrequency ablation has demonstrated good results in limited cases for patients unable to undergo sclerotherapy or have failed prior interventions (5,60). van Breugel et al. have described the efficacy of non-invasive magnetic resonance-guided high intensity focused US ablation in the treatment of vascular malformations (61). This technique has been successfully used to treat uterine fibroids and breast cancer (62,63). Preliminary results in its application towards vascular malformations are encouraging, however further clinical testing is necessary. A lesser explored modality is irreversible electroporation which has been used for tumor, nerve, and smooth muscle cell ablation (64). This technique may hold promise for VM treatment.
Neodymium yttrium-aluminium-garnet (Nd:YAG) laser therapy has been increasingly used when venous lesions are small, located in difficult anatomical situations, and have not responded to other treatments with good control of VMs (5,65). It is usually administered via a flexible optic cable and causes the photocoagulation of blood vessel tissue (66). Its effects are highly localized which can cause adverse effects, especially if important structures, such as nerves are too close to the area of action. Nonetheless, with appropriate precautions, it is a fast, safe, and easily administered modality (65).
Treatment effectiveness
Sclerotherapy is the established gold standard, first-line treatment for VMs (67). The reported effectiveness of bleomycin is between 43–82.7% (68-72). Zhang et al. conducted a statistically significant 138 patient series that stated a 95% effective treatment with ethanol, while only 65% of treatments were effective in the bleomycin group, additionally few cases of skin necrosis was seen in both groups (73). This could indicate superior results with ethanol and numerous other studies have published similar findings (50,56,73-78).
However, systematic literature reviews, including a Cochrane review examining 35 published studies evaluating effectiveness of VM treatments identified a significant level of bias and the existence of methodological flaws in study designs (79). Treatment success rates in studies were between 71–100% and 65–94% in another. They concluded that the lack of evidence precludes them from being able to ascertain if one treatment option is superior to another; however, they all corroborated that multiple treatment sessions are warranted (67,80). Ethanol complications can be as high as 18% while, for other agents it is as low as 6% (67,79). Thus sclerotherapy with STS or polidocanol is advised in terms of lower adverse effects by some studies (67). However, ethanol is more commonly used and deemed to be more effective, with better outcomes, although operator experience and high patient volume is crucial (50).
Conclusions
VMs are present at birth and grow commensurately with the child and consist of a plethora of abnormal venous vessels that can cause considerable pain and discomfort. US and MRI are the two central imaging techniques in the work-up of VMs. DPP is a gold standard diagnostic tool utilized when other imaging is equivocal. It also plays a central role in aiding decisions during sclerotherapy. Several classification systems have been designed around MRI and DPP that can help decide treatment pathways and also help predict outcomes. Studies indicate that numerous treatment modalities can be combined for best results. Studies on effectiveness of ethanol sclerotherapy have shown the best outcomes, however currently insufficient evidence exists that can associate a single treatment modality to unequivocally superior outcomes. In general multiple therapy sessions are required. A suitable therapy regimen can be implemented through a multidisciplinary approach in liaison with the patient, leading to a manageable, successful treatment of a VM, even in a complex case. In terms of long-term outcomes, further research and follow-up is required to investigate the durability of amelioration of pain and rates of recurrence following treatment.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Vikkula M, Boon LM, Mulliken JB. Molecular genetics of vascular malformations. Matrix Biol 2001;20:327-35. 10.1016/S0945-053X(01)00150-0 [DOI] [PubMed] [Google Scholar]
- 2.Eifert S, Villavicencio JL, Kao TC, et al. Prevalence of deep venous anomalies in congenital vascular malformations of venous predominance. J Vasc Surg 2000;31:462-71. 10.1067/mva.2000.101464 [DOI] [PubMed] [Google Scholar]
- 3.Richter GT, Friedman AB. Hemangiomas and vascular malformations: current theory and management. Int J Pediatr 2012;2012:645678. [DOI] [PMC free article] [PubMed]
- 4.Cox JA, Bartlett E, Lee EI. Vascular malformations: a review. Semin Plast Surg 2014;28:58-63. 10.1055/s-0034-1376263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee BB, Baumgartner I, Berlien P, et al. Diagnosis and Treatment of Venous Malformations. Consensus Document of the International Union of Phlebology (IUP): updated 2013. Int Angiol 2015;34:97-149. [PubMed] [Google Scholar]
- 6.Dasgupta R, Fishman SJ. ISSVA classification. Semin Pediatr Surg 2014;23:158-61. 10.1053/j.sempedsurg.2014.06.016 [DOI] [PubMed] [Google Scholar]
- 7.Nagy M, Brodsky L. Multidisciplinary approach to management of hemangiomas and vascular malformations. Facial Plast Surg Clin North Am 2001;9:551-9. [PubMed] [Google Scholar]
- 8.Clemens RK, Pfammatter T, Meier TO, et al. Vascular malformations revisited. Vasa 2015;44:5-22. 10.1024/0301-1526/a000402 [DOI] [PubMed] [Google Scholar]
- 9.Hassanein AH, Mulliken JB, Fishman SJ, et al. Evaluation of terminology for vascular anomalies in current literature. Plast Reconstr Surg 2011;127:347-51. 10.1097/PRS.0b013e3181f95b83 [DOI] [PubMed] [Google Scholar]
- 10.Lee BB, Laredo J, Lee TS, et al. Terminology and classification of congenital vascular malformations. Phlebology 2007;22:249-52. 10.1258/026835507782655236 [DOI] [PubMed] [Google Scholar]
- 11.Enjolras O, Mulliken JB. Vascular tumors and vascular malformations (new issues). Adv Dermatol 1997;13:375-423. [PubMed] [Google Scholar]
- 12.Lowe LH, Marchant TC, Rivard DC, et al. Vascular malformations: classification and terminology the radiologist needs to know. Semin Roentgenol 2012;47:106-17. 10.1053/j.ro.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 13.Colbert SD, Seager L, Haider F, et al. Lymphatic malformations of the head and neck-current concepts in management. Br J Oral Maxillofac Surg 2013;51:98-102. 10.1016/j.bjoms.2011.12.016 [DOI] [PubMed] [Google Scholar]
- 14.Dompmartin A, Vikkula M, Boon LM. Venous malformation: update on aetiopathogenesis, diagnosis and management. Phlebology 2010;25:224-35. 10.1258/phleb.2009.009041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruder E, Alaggio R, Kozakewich HP, et al. Vascular and perivascular lesions of skin and soft tissues in children and adolescents. Pediatr Dev Pathol 2012;15:26-61. 10.2350/11-11-1119-PB.1 [DOI] [PubMed] [Google Scholar]
- 16.Restrepo R. Multimodality imaging of vascular anomalies. Pediatr Radiol 2013;43 Suppl 1:S141-54. 10.1007/s00247-012-2584-y [DOI] [PubMed] [Google Scholar]
- 17.Dubois J, Garel L. Imaging and therapeutic approach of hemangiomas and vascular malformations in the pediatric age group. Pediatr Radiol 1999;29:879-93. 10.1007/s002470050718 [DOI] [PubMed] [Google Scholar]
- 18.Flors L, Leiva-Salinas C, Maged IM, et al. MR Imaging of Soft-Tissue Vascular Malformations: Diagnosis, Classification, and Therapy Follow-up. RadioGraphics 2011;31:1321-40. 10.1148/rg.315105213 [DOI] [PubMed] [Google Scholar]
- 19.Fayad LM, Hazirolan T, Bluemke D, et al. Vascular malformations in the extremities: emphasis on MR imaging features that guide treatment options. Skeletal Radiol 2006;35:127-37. 10.1007/s00256-005-0057-1 [DOI] [PubMed] [Google Scholar]
- 20.Rak KM, Yakes WF, Ray RL, et al. MR imaging of symptomatic peripheral vascular malformations. AJR Am J Roentgenol 1992;159:107-12. 10.2214/ajr.159.1.1609682 [DOI] [PubMed] [Google Scholar]
- 21.Ohlms LA, Forsen J, Burrows PE. Venous malformation of the pediatric airway. Int J Pediatr Otorhinolaryngol 1996;37:99-114. 10.1016/0165-5876(96)01382-1 [DOI] [PubMed] [Google Scholar]
- 22.Limaye N, Wouters V, Uebelhoer M, et al. Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nat Genet 2009;41:118-24. 10.1038/ng.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boon LM, Mulliken JB, Vikkula M, et al. Assignment of a locus for dominantly inherited venous malformations to chromosome 9p. Hum Mol Genet 1994;3:1583-7. 10.1093/hmg/3.9.1583 [DOI] [PubMed] [Google Scholar]
- 24.Pavlov KA, Dubova EA, Shchyogolev AI, et al. Expression of growth factors in endotheliocytes in vascular malformations. Bull Exp Biol Med 2009;147:366-70. 10.1007/s10517-009-0510-6 [DOI] [PubMed] [Google Scholar]
- 25.Duyka LJ, Fan CY, Coviello-Malle JM, et al. Progesterone receptors identified in vascular malformations of the head and neck. Otolaryngol Head Neck Surg 2009;141:491-5. 10.1016/j.otohns.2009.06.012 [DOI] [PubMed] [Google Scholar]
- 26.Legiehn GM, Heran MK. Venous malformations: classification, development, diagnosis, and interventional radiologic management. Radiol Clin North Am 2008;46:545-97, vi. 10.1016/j.rcl.2008.02.008 [DOI] [PubMed] [Google Scholar]
- 27.Herborn CU, Goyen M, Lauenstein TC, et al. Comprehensive time-resolved MRI of peripheral vascular malformations. AJR Am J Roentgenol 2003;181:729-35. 10.2214/ajr.181.3.1810729 [DOI] [PubMed] [Google Scholar]
- 28.Lee BB, Baumgartner I, Berlien HP, et al. Consensus Document of the International Union of Angiology (IUA)-2013. Current concept on the management of arterio-venous management. Int Angiol 2013;32:9-36. [PubMed] [Google Scholar]
- 29.Eivazi B, Fasunla AJ, Hundt W, et al. Low flow vascular malformations of the head and neck: a study on brightness mode, color coded duplex and spectral Doppler sonography. Eur Arch Otorhinolaryngol 2011;268:1505-11. 10.1007/s00405-011-1514-1 [DOI] [PubMed] [Google Scholar]
- 30.Legiehn GM, Heran MK. A Step-by-Step Practical Approach to Imaging Diagnosis and Interventional Radiologic Therapy in Vascular Malformations. Semin Intervent Radiol 2010;27:209-31. 10.1055/s-0030-1253521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubois J, Alison M. Vascular anomalies: what a radiologist needs to know. Pediatr Radiol 2010;40:895-905. 10.1007/s00247-010-1621-y [DOI] [PubMed] [Google Scholar]
- 32.van Rijswijk CS, van der Linden E, van der Woude HJ, et al. Value of dynamic contrast-enhanced MR imaging in diagnosing and classifying peripheral vascular malformations. AJR Am J Roentgenol 2002;178:1181-7. 10.2214/ajr.178.5.1781181 [DOI] [PubMed] [Google Scholar]
- 33.Lewis M, Yanny S, Malcolm PN. Advantages of blood pool contrast agents in MR angiography: a pictorial review. J Med Imaging Radiat Oncol 2012;56:187-91. 10.1111/j.1754-9485.2012.02347.x [DOI] [PubMed] [Google Scholar]
- 34.Milot L, Haider M, Foster L, et al. Gadofosveset trisodium in the investigation of focal liver lesions in noncirrhotic liver: Early experience. J Magn Reson Imaging 2012;36:738-42. 10.1002/jmri.23650 [DOI] [PubMed] [Google Scholar]
- 35.Pressacco J, Papas K. Gadofosveset-enhanced magnetic resonance angiography as a means of evaluating pulmonary arteriovenous malformation: a case report. Magn Reson Imaging 2012;30:886-8. 10.1016/j.mri.2012.02.022 [DOI] [PubMed] [Google Scholar]
- 36.Goyal M, Causer PA, Armstrong D. Venous vascular malformations in pediatric patients: comparison of results of alcohol sclerotherapy with proposed MR imaging classification. Radiology 2002;223:639-44. 10.1148/radiol.2233010025 [DOI] [PubMed] [Google Scholar]
- 37.Dubois J, Soulez G, Oliva VL, et al. Soft-tissue venous malformations in adult patients: imaging and therapeutic issues. Radiographics 2001;21:1519-31. 10.1148/radiographics.21.6.g01nv031519 [DOI] [PubMed] [Google Scholar]
- 38.Berenguer B, Burrows PE, Zurakowski D, et al. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg 1999;104:1-11. 10.1097/00006534-199907000-00002 [DOI] [PubMed] [Google Scholar]
- 39.Legiehn GM, Heran MK. Classification, diagnosis, and interventional radiologic management of vascular malformations. Orthop Clin North Am 2006;37:435-74. 10.1016/j.ocl.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 40.Nguyen JT, Koerper MA, Hess CP, et al. Aspirin therapy in venous malformation: a retrospective cohort study of benefits, side effects, and patient experiences. Pediatr Dermatol 2014;31:556-60. 10.1111/pde.12373 [DOI] [PubMed] [Google Scholar]
- 41.Lee BB. Critical issues in management of congenital vascular malformation. Ann Vasc Surg 2004;18:380-92. 10.1007/s10016-004-0020-y [DOI] [PubMed] [Google Scholar]
- 42.Puig S, Aref H, Chigot V, et al. Classification of venous malformations in children and implications for sclerotherapy. Pediatr Radiol 2003;33:99-103. 10.1007/s00247-002-0838-9 [DOI] [PubMed] [Google Scholar]
- 43.Kim B, Kim K, Jeon P, et al. Long-term results of ethanol sclerotherapy with or without adjunctive surgery for head and neck arteriovenous malformations. Neuroradiology 2015;57:377-86. 10.1007/s00234-014-1483-3 [DOI] [PubMed] [Google Scholar]
- 44.Albanese G, Kondo KL. Pharmacology of sclerotherapy. Semin Intervent Radiol 2010;27:391-9. 10.1055/s-0030-1267848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El-Hakim I, Alyamani A. Management of palatal vascular malformation using absolute ethanol sclerotherapy. Clin Pract 2011;1:e86. 10.4081/cp.2011.e86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ernemann U, Kramer U, Miller S, et al. Current concepts in the classification, diagnosis and treatment of vascular anomalies. Eur J Radiol 2010;75:2-11. 10.1016/j.ejrad.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 47.Lamba S, Keshava SKN, Moses V, et al. Ethanol sclerotherapy for treatment of venous malformations of face and neck— a single centre experience. Eur J Plast Surg 2012;35:345 10.1007/s00238-011-0626-6 [DOI] [Google Scholar]
- 48.Yun WS, Kim YW, Lee KB, et al. Predictors of response to percutaneous ethanol sclerotherapy (PES) in patients with venous malformations: analysis of patient self-assessment and imaging. J Vasc Surg 2009;50:581-9. 10.1016/j.jvs.2009.03.058 [DOI] [PubMed] [Google Scholar]
- 49.Shin BS, Do YS, Cho HS, et al. Effects of Repeat Bolus Ethanol Injections on Cardiopulmonary Hemodynamic Changes during Embolotherapy of Arteriovenous Malformations of the Extremities. J Vasc Interv Radiol 2010;21:81-9. 10.1016/j.jvir.2009.09.026 [DOI] [PubMed] [Google Scholar]
- 50.Yakes WF. Use of Multiple Sclerosant Agents in Vascular Malformation Management: A World in Endovascular Confusion and Chaos. J Vasc Interv Radiol 2015;26:1494-6. 10.1016/j.jvir.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 51.Ali S, Weiss CR, Sinha A, et al. The treatment of venous malformations with percutaneous sclerotherapy at a single academic medical center. Phlebology 2016;31:603-9. 10.1177/0268355516633380 [DOI] [PubMed] [Google Scholar]
- 52.Aronniemi J, Castrén E, Lappalainen K, et al. Sclerotherapy complications of peripheral venous malformations. Phlebology 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 53.Yakes WF, Baker R. Cardiopulmonary collapse: sequelae of ethanol embolotherapy. Radiology 1993;189:145. [Google Scholar]
- 54.Bisdorff A, Mazighi M, Saint-Maurice JP, et al. Ethanol threshold doses for systemic complications during sclerotherapy of superficial venous malformations: a retrospective study. Neuroradiology 2011;53:891-4. 10.1007/s00234-010-0803-5 [DOI] [PubMed] [Google Scholar]
- 55.Jain R, Bandhu S, Sawhney S, et al. Sonographically guided percutaneous sclerosis using 1% polidocanol in the treatment of vascular malformations. J Clin Ultrasound 2002;30:416-23. 10.1002/jcu.10091 [DOI] [PubMed] [Google Scholar]
- 56.Vogelzang RL, Atassi R, Vouche M, et al. Ethanol embolotherapy of vascular malformations: clinical outcomes at a single center. J Vasc Interv Radiol 2014;25:206-13. 10.1016/j.jvir.2013.10.055 [DOI] [PubMed] [Google Scholar]
- 57.Li K, Liu YR, Chen AW, et al. A new method for using radiopaque sclerosing foam to treat venous malformations. Dermatol Surg 2015;41:726-30. 10.1097/DSS.0000000000000363 [DOI] [PubMed] [Google Scholar]
- 58.Chaudry G, Guevara CJ, Rialon KL, et al. Safety and efficacy of bleomycin sclerotherapy for microcystic lymphatic malformation. Cardiovasc Intervent Radiol 2014;37:1476-81. 10.1007/s00270-014-0932-z [DOI] [PubMed] [Google Scholar]
- 59.Horbach SE, Rigter IM, Smitt JH, et al. Intralesional Bleomycin Injections for Vascular Malformations: A Systematic Review and Meta-Analysis. Plast Reconstr Surg 2016;137:244-56. 10.1097/PRS.0000000000001924 [DOI] [PubMed] [Google Scholar]
- 60.Gao Y, Wang X, Suo W. Management of venous malformations with percutaneous radiofrequency thermal ablation. Br J Dermatol 2012;167:637-42. 10.1111/j.1365-2133.2012.10963.x [DOI] [PubMed] [Google Scholar]
- 61.van Breugel JM, Nijenhuis RJ, Ries MG, et al. Non-invasive magnetic resonance-guided high intensity focused ultrasound ablation of a vascular malformation in the lower extremity: a case report. J Ther Ultrasound 2015;3:23. 10.1186/s40349-015-0042-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ikink ME, Voogt MJ, Verkooijen HM, et al. Mid-term clinical efficacy of a volumetric magnetic resonance-guided high-intensity focused ultrasound technique for treatment of symptomatic uterine fibroids. Eur Radiol 2013;23:3054-61. 10.1007/s00330-013-2915-x [DOI] [PubMed] [Google Scholar]
- 63.Merckel LG, Bartels LW, Kohler MO, et al. MR-guided high-intensity focused ultrasound ablation of breast cancer with a dedicated breast platform. Cardiovasc Intervent Radiol 2013;36:292-301. 10.1007/s00270-012-0526-6 [DOI] [PubMed] [Google Scholar]
- 64.Deipolyi AR, Golberg A, Yarmush ML, et al. Irreversible electroporation: evolution of a laboratory technique in interventional oncology. Diagn Interv Radiol 2014;20:147-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glade R, Vinson K, Richter G, et al. Endoscopic management of airway venous malformations with Nd:YAG laser. Ann Otol Rhinol Laryngol 2010;119:289-93. [DOI] [PubMed] [Google Scholar]
- 66.Vesnaver A, Dovsak DA. Treatment of vascular lesions in the head and neck using Nd:YAG laser. J Craniomaxillofac Surg 2006;34:17-24. 10.1016/j.jcms.2005.07.009 [DOI] [PubMed] [Google Scholar]
- 67.Horbach SE, Lokhorst MM, Saeed P, et al. Sclerotherapy for low-flow vascular malformations of the head and neck: A systematic review of sclerosing agents. J Plast Reconstr Aesthet Surg 2016;69:295-304. 10.1016/j.bjps.2015.10.045 [DOI] [PubMed] [Google Scholar]
- 68.Bai Y, Jia J, Huang XX, et al. Sclerotherapy of Microcystic Lymphatic Malformations in Oral and Facial Regions. J Oral Maxillofac Surg 2009;67:251-6. 10.1016/j.joms.2008.06.046 [DOI] [PubMed] [Google Scholar]
- 69.Hassan Y, Osman AK, Altyeb A. Noninvasive management of hemangioma and vascular malformation using intralesional bleomycin injection. Ann Plast Surg 2013;70:70-3. 10.1097/SAP.0b013e31824e298d [DOI] [PubMed] [Google Scholar]
- 70.Sainsbury DC, Kessell G, Fall AJ, et al. Intralesional bleomycin injection treatment for vascular birthmarks: a 5-year experience at a single United Kingdom unit. Plast Reconstr Surg 2011;127:2031-44. 10.1097/PRS.0b013e31820e923c [DOI] [PubMed] [Google Scholar]
- 71.Yang Y, Sun M, Ma Q, et al. Bleomycin A5 sclerotherapy for cervicofacial lymphatic malformations. J Vasc Surg 2011;53:150-5. 10.1016/j.jvs.2010.07.019 [DOI] [PubMed] [Google Scholar]
- 72.Ul Haq F, Mitchell SE, Tekes A, et al. Bleomycin Foam Treatment of Venous Malformations: A Promising Agent for Effective Treatment with Minimal Swelling. J Vasc Interv Radiol 2015;26:1484-93. 10.1016/j.jvir.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 73.Zhang J, Li HB, Zhou SY, et al. Comparison between absolute ethanol and bleomycin for the treatment of venous malformation in children. Exp Ther Med 2013;6:305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson PL, Eckard DA, Brecheisen MA, et al. Percutaneous ethanol sclerotherapy of venous malformations of the tongue. AJNR Am J Neuroradiol 2002;23:779-82. [PMC free article] [PubMed] [Google Scholar]
- 75.Lee BB, Do YS, Byun HS, et al. Advanced management of venous malformation with ethanol sclerotherapy: mid-term results. J Vasc Surg 2003;37:533-8. 10.1067/mva.2003.91 [DOI] [PubMed] [Google Scholar]
- 76.Lee IH, Kim KH, Jeon P, et al. Ethanol sclerotherapy for the management of craniofacial venous malformations: the interim results. Korean J Radiol 2009;10:269-76. 10.3348/kjr.2009.10.3.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Orlando JL, Caldas JG, Campos HG, et al. Ethanol sclerotherapy of superficial venous malformation: a new procedure. Dermatology 2010;220:376-80. 10.1159/000305525 [DOI] [PubMed] [Google Scholar]
- 78.Su L, Fan X, Zheng L, et al. Absolute ethanol sclerotherapy for venous malformations in the face and neck. J Oral Maxillofac Surg 2010;68:1622-7. 10.1016/j.joms.2009.07.094 [DOI] [PubMed] [Google Scholar]
- 79.van der Vleuten CJ, Kater A, Wijnen MH, et al. Effectiveness of sclerotherapy, surgery, and laser therapy in patients with venous malformations: a systematic review. Cardiovasc Intervent Radiol 2014;37:977-89. [DOI] [PubMed] [Google Scholar]
- 80.Glade RS, Richter GT, James CA, et al. Diagnosis and management of pediatric cervicofacial venous malformations: retrospective review from a vascular anomalies center. Laryngoscope 2010;120:229-35. [DOI] [PubMed] [Google Scholar]