Abstract
Impaired regeneration and consequent muscle wasting is a major feature of muscle degenerative diseases. Nutritional interventions such as adjuvant strategy for preventing these conditions are recently gaining increasing attention. Ingestion of n3-polyunsaturated fatty acids has been suggested as having a positive impact on muscle diseases. We recently demonstrated that a diet enriched with plant derived n3-fatty acid, α-linolenic acid (ALA), exerts potent beneficial effects in preserving skeletal muscle regeneration in models of muscle dystrophy. To better elucidate the underlying mechanism we here investigate on the expression level of the anti- and pro-apoptotic proteins, as well as caspase-3 activity, in C2C12 myoblasts challenged with pathological levels of tumor necrosis factor-α (TNF). The results demonstrated that ALA protective effect on C2C12 myoblasts was associated with a decrease in caspase-3 activity and an increase of the Bcl-2/Bax ratio. Indeed, the effect of ALA was directed to rescuing Bcl-2 expression and to revert Bax translocation to mitochondria both affected in an opposite way by TNF, a major pro-inflammatory cytokine expressed in damaged skeletal muscle. Therefore, ALA counteracts inflammatory signals in the muscle microenvironment and may represent a valuable strategy for ameliorating skeletal muscle pathologies.
Key Words: myoblasts, apoptotic proteins, muscle wasting, inflammation, phytochemicals
Tumor necrosis factor-α (TNF) is known to be an inflammatory cytokine and one of the key regulators of skeletal muscle homeostasis.1 A sustained high level of TNF is associated with chronic inflammatory disease and has been identified to play a significant role in mechanisms of muscle pathology.2 Indeed, TNF is a major pro-inflammatory cytokine that is expressed in damaged skeletal muscle; increased TNF levels have been found in the plasma and muscles of dystrophic animals and humans.1 High TNF plasma levels (0.5-10 ng/ml) have been associated with myoblast and myocyte apoptosis, inhibition of myogenic differentiation and muscle wasting.3 Supplementation with marine-derived n3-polyunsaturated fatty acids (n3-Pufas) has been suggested to have a positive impact on muscle, increasing muscle mass and improving function in elderly individuals,4 and in patients with cancer.5 In contrast, only a few studies have been performed to elucidate the effects of plant-derived n3-PUFAs, such as α-linolenic acid (ALA), on muscle. Our previous findings have demonstrated that flaxseed, rich in ALA, preserves dystrophic skeletal muscle regeneration in an animal model characterized by increased plasma levels of tumor necrosis factor-α.6 Indeed, we observed, among other effects, a reduction of apoptotic frequency in myoblasts exposed to by high levels of TNF. The sustained TNF stimulation is mediated primarily through the activation of type I receptor inducing multiple cell death pathways (extrinsic and intrinsic) that contribute to progressive cell apoptosis.7 The initial stage of apoptosis involves the expression of ligands for ‘death receptors’ and altered levels of Bcl-2 family proteins,8 such as pro-apoptotic Bax and anti-apoptotic Bcl-2. Noteworthy, Bax upregulation and/or translocation to mitochondria is one of the early markers of apoptotic process.9 Indeed, Bax, when in excess over the anti-apoptotic Bcl-2 proteins, translocases from the cytosol to the mitochondrial membrane, where it promotes pores formation. Because of that, the mitochondrial intrinsic apoptotic pathway is activated, with the release of pro-apoptotic factors and activation of the final effector caspases.10 Cells become committed to cell death and the proteolytic activation of caspases triggers the downstream apoptotic events (DNA fragmentation, nuclear condensation, cell shrinkage, membrane blebbing, etc.). Understanding the mechanisms of these events and how ALA might modulate them is of fundamental importance in order to conceive any new therapeutic approach related to bioactive nutrient supplements as ALA. Thus, to better elucidate the mechanisms we investigate here the expression levels of anti-apoptotic and pro-apoptotic proteins and Bax compartmentalization in C2C12 myoblasts challenged with TNF.
Material and Methods
Experimental model
Murine C2C12 myoblasts (American Type Culture Collection) were cultured in growth media (GM) consisting of Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 15% Fetal Bovine Serum (FBS) and 50 mg/ml gentamicin (Sigma-Aldrich, St. Louis, MO) and seeded at a density of 104 cell/cm2 onto multiwell plates or flasks. To induce differentiation, when at 70% confluence, cells were shifted to a differentiating medium (DM) consisting of DMEM supplemented with 2% fetal horse serum and 50 mg/ml gentamicin. Cell cultures were treated with 10 ng/ml recombinant mouse TNF (Endogen, Rockford, USA) added to DM in order to mimic the in vivo inflammation environment in the presence or absence of ALA (10 µM). ALA (Sigma Aldrich) was preliminarily complexed with fatty acid free bovine serum albumin (BSA fraction V, Sigma-Aldrich, fatty acid/BSA molar ratio 4:1). For the mock group, the cells were incubated with DM only.
Caspase-3 activity
Caspase-3 activity was measured by a fluorimetric assay kit (Sigma-Aldrich) following the manufacturer’s instructions. Briefly, cell lysates were incubated for 1 h at 37 °C, in a solution containing caspase-3 substrate acetyl-Asp-Glu-Val-Asp-7-amido-4-methylcoumarin (Ac-DEVD-AMC). The hydrolysis of the Ac-DEVD-AMC by caspase-3 resulted by the release of the fluorescent 7-amino-4-methylcoumarin (AMC) moiety. The release of fluorescent AMC, index of caspase-3 activity, was assessed by a spectrofluorimeter (PerkinElmer Inc. USA) at 360 nm (excitation) and 460 nm (emission).
Western blot analysis
C2C12 were treated with a lysis buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 2 mM orthovanadate) and a proteinase inhibitor cocktail (Sigma-Aldrich). Cell lysates were centrifuged at 13,000 xg at 4° C for 15 min, and the protein content was quantified by the Bradford method (Amresco, Inc. Solon, Oh, USA). Protein extracts were resolved by 10% SDS-polyacrylamide gel electrophoresis (PAGE) followed by their transfer onto polyvinylidene difluoride membranes. Membranes were blocked with PBS/bovine serum albumin 5% for 1h at room temperature and probed with specific antibodies against cleaved caspase-3 (Cell Signaling Technology Inc, Beverly, MA), Bcl-2 and Bax, (Sigma-Aldrich) for 1 h at room temperature. α-tubulin protein (Sigma-Aldrich) was used as the internal control. Membranes were then incubated with peroxidase-linked appropriate secondary antibody (Vector Laboratories, Inc.) and, after treatment with ECL reagent (Amersham Biosciences, GE Healthcare, USA), exposed to Hyperfilm ECL to visualize immunoreactive proteins. For densitometric quantification, the protein expression levels were analyzed by the Scion Image software.
Subcellular fractionation
C2C12 cells were homogenized in ice-cold buffer containing 50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 250mM sucrose, 1 mM DTT, 0.5 mM PMSF, 20 mg/ml leupeptin, 20 mg/ml aprotinin, and 20 mg/ml trypsin inhibitor, using a Dounce homogenizer. After centrifugation at 800 g for 15 min at 4 °C the supernatant, free of debris and nonhomogenized cells, was further centrifuged at 10 000 g for 30 min at 4 °C to yield the mitochondrial pellet. The remaining supernatant was called cytosolic supernatant. Pellets were resuspended in lysis buffer and protein concentration of the fractions was estimated. Protein fraction extracts were resolved by 15% SDS-polyacrylamide gel electrophoresis and western blot assays were performed as described before. Membranes were incubated with specific antibody against Bax, (Cell Signaling Technology Inc, Beverly, MA). Cross contamination between fractions was assessed by western blot analysis using anti-COX IV (Abcam) as mitochondrial marker.
Statistical analysis
Results are expressed as the mean ± SD. The analysis of variance was performed (ANOVA) for comparisons between more than two groups, whereas the two-tailed unpaired Student’s t-test was used for comparisons of the mean differences between two groups. A suitable post-hoc test was used in combination with ANOVA to test for significant differences between groups. Differences were considered statistically significant when P< 0.05. (SPSS for Windows, SPSS, Inc., Chicago, IL).
Results
ALA inhibits TNF-induced activation of caspase-3 in C2C12 myoblasts
In C2C12 myoblasts TNF (48 h) induced a five-fold increase in cleaved caspase-3 as shown by western blot analysis (Figure 1A). After ALA+TNF incubation, the expression of active caspases was repressed. Quantitative data are reported in Figure 1B. As evidenced in the figure 1 C, the protease activity of caspase-3, was increased after 48 h exposure to TNF, compared to mock C2C12 cells. ALA reverted TNF effect reducing significantly the caspase-3 activity.
Fig. 1.
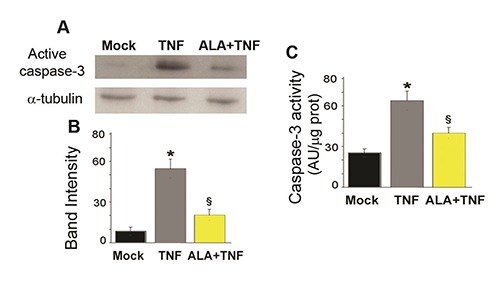
ALA reduces caspase-3 activity in C2C12 myoblasts. A) The representative Western bolt shows cleaved caspase-3 expression as assessed in C2C12 cells incubated for 48h in DM (differentiation medium) alone (Mock) or with TNF (tumor necrosis factor-α) or TNF and ALA (α-linolenic acid). α-tubulin was used as a loading control. B) Bands intensities of the western blot signals in (A). Values are expressed as arbitrary units. C) Bars are representative of caspase-3 activity measured by the fluorimetric assay. Fluorescence values, expressed as arbitrary units (AU), were normalized to protein content.
Data derived from three separate experiments are presented as the means±SD. * P<0.05 compared with Mock cells; § P<0.05 compared with the TNF group.
ALA up-regulates Bcl-2 /Bax ratio
The western blotting analysis of the anti-apoptotic Bcl-2 and the pro-apoptotic Bax proteins was carried out. The Bcl-2 and Bax intracellular levels are illustrated in Figure 2A. In 48 h TNF-treated C2C12 cells, the expression of the anti-apoptotic protein Bcl-2 decreased whereas the pro-apoptotic Bax increased. Conversely, TNF +ALA treatment determined an increase of the anti-apoptotic Bcl-2 expression. As evidenced in the Figure 2B the ratio Bcl-2/Bax was significantly reduced in cells treated with TNF compared to mock cells. Conversely, addition of ALA in the medium counteracted dramatically the effect of TNF.
Fig. 2.
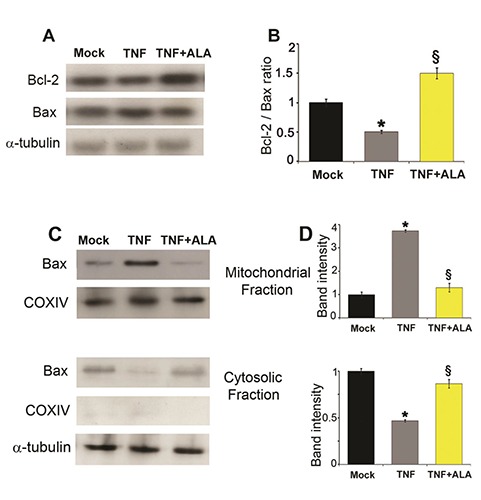
ALA regulates the expression levels of Bcl-2 family proteins and Bax traslocation. A) Representative Western blots of the protein content of Bcl-2 and Bax. α-tubulin was used as loading control. B) The ratio of Bax/Bcl-2 protein content was estimated on the basis of densitometric measurements of the Western blot signals. C) Western blots of the Bax protein in enriched mitochondrial and cytosolic fractions of C2C12 cells. COX IV and /?/-tubulin were used as mitochondrial and cytosolic marker respectively. D) Relative expression of Bax protein in mitochondria and cytosol. Values are expressed as arbitrary units.
Data derived from three separate experiments are presented as the means ±SD. * P<0.05 compared with Mock cells; § P<0.05 compared with the TNF group.
ALA inhibits TNF-Induced Bax translocation to mitochondria
To further clarify the mechanism of ALA anti-apoptotic effects in TNF treated C2C12 cells, the localization of Bax was analyzed by Western blot of mitochondrial and cytosolic fractions. Figure 2C shows that TNF induced Bax translocation to mitochondria while ALA reverted this TNF-induced effect. Quantitative data of the expression of Bax protein in mitochondria and cytosol were shown in Figure 2D. Indeed, after TNF treatment a four-fold increase in Bax mitochondrial expression was observed in C2C12 cells, which was suppressed by ALA stimulation.
Discussion
In the present research, we explicate one of the possible cellular mechanisms involved in the protective effect of ALA against TNF-induced apoptosis on C2C12 cells during differentiation. We here show that ALA interrupted mitochondria-dependent apoptotic pathway triggered by sustained cytokine stimulation. The mitochondria are the main site of action for members of the apoptosis-regulating protein family. Indeed, we observed that ALA significantly reduced apoptosis, decreasing the caspase-3 activity, increasing the anti-apoptotic protein Bcl-2 levels and thus shifting the Bcl-2/Bax ratio to higher levels.
The balance of Bcl-2 and Bax is an important factor of cell apoptosis induction.11 Bax, is a pro-apoptotic protein, found in the cytosol of most normal healthy tissues, in an inactive form in the cells. In apoptotic cells or when Bax is overexpressed, it is targeted to mitochondrial membranes, where the protein increases mitochondrial membrane permeabilization and triggers cytochrome c release, leading to apoptosis.12 Our results revealed that ALA inhibits the mitochondrial translocation of Bax, probably due to its ability to increase Bcl-2. Substantial evidences indicated that Bcl-2 protein prevented apoptosis by blocking the release of mitochondrial interspace proteins and the apoptosis inducing factor.13 Following those events, ALA inhibits cleavage and activation of caspase-3 and downstream apoptotic events.
Previous studies have reported protective effects of ALA against apoptosis in neuronal cells, cardiomyocytes and rat hepatocytes.14-17 By contrast, an opposite effect on mitochondria-dependent apoptotic pathway has been described for 10t, 12c-conjugated linoleic acid in rat hepatoma cells.18 Noteworthy, in this study the fatty acid induced translocation of Bax protein into the mitochondrial membrane leading to apoptosis hepatoma cells. Recently, 10t, 12c-conjugated linoleic acid has been also reported to stimulate mitochondrial biogenesis in C2C12 myoblasts.19 These studies suggest that the cell biological response to different unsaturated fatty acids may depend from their different chemical structure (number and position of double bond and the presence or absence of a cis-9 double bond). In addition, the same fatty acid may have different actions on different cell type.
Apoptosis is one of the main causes of satellite-cell depletion in chronic pathological conditions of muscular diseases.20 Apoptotic nuclei were obseved in dystrophic muscles, particularly in activated satellite cells.21 Furthermore, in the mdx mouse model and in patients affected by Duchenne dystrophy, upregulation of pro-apoptotic proteins such as Bax and caspases were detected in myofibers, suggesting that under pathological conditions, these myofibers undergo apoptosis.22-24 Apoptosis and decreased Bcl-2 expression were detected in other muscular dystrophies such as Limb Girdle MD type 2C,25 and congenital MD type 1A.26
Taken together, these studies indicate that in many muscular dystrophies, apoptosis plays a paramount role in the depletion of satellite cells and consequently in the impaired regeneration. Our findings support the conclusion that ALA leads C2C12 myoblasts to survival instead of undergoing apoptosis TNF-induced directly affecting key proteins involved in balancing survival/death in skeletal muscle.
The upstream mechanisms of these effects may be due to an epigenetic effect on the transcription of genes for these proteins. A great deal of attention is now being dedicated to the use of phytochemicals to modulate important pathways involved in physiological and pathological processes. Basic laboratory research, clinical trials, epidemiological and in silico studies demonstrated that nutrient-rich bioactive foods can induce epigenetic changes and alter genes’ expression by the alteration of the histone structure, DNA methylation and miRs’ modulation.27, 28
The results of this work indicate that plant-derived ALA is able to modulate apoptotic proteins expression by largely rescuing Bcl-2 down expression and in a lesser extent also decreasing Bax levels and inhibiting its mitochondrial translocation in C2C12 myoblasts treated with TNF. TNF is a major pro-inflammatory cytokine that is expressed in damaged skeletal muscle. Thus, counteracting inflammatory signals by bioactive compound such as ALA, in the muscle microenvironment, represents a valuable strategy to ameliorate skeletal muscle pathologies such as dystrophies.
Acknowledgments
The work was supported by ANR (# 13-BSV1-0005), NIH (# 5R01CA180057-02), AFM (# 2012-0773), UPMC Emergence 2011 and IBPS 2015.
Felicia Carotenuto is Visiting Researcher at Diagnostics and Metrology (FSN-TECFIS-DIM), ENEA, C.R. Frascati, Rome 00044, Italy.
Contributor Information
Felicia Carotenuto, Email: Carotenuto:carotenuto@med.uniroma2.it.
Dario Coletti, Email: dario.coletti@upmc.fr.
Paolo Di Nardo, Email: dinardo@uniroma2.it.
References
- 1.Grounds MD, Radley HG, Gebski BL, Bogoyevitch MA, et al. Implications of cross-talk between tumour necrosis factor and insulin-like growth factor-1 signalling in skeletal muscle. Clin Exp Pharmacol Physiol 2008;35:846-51. [DOI] [PubMed] [Google Scholar]
- 2.Langen RC, Schols AM, Kelders MC, Van Der Velden JL, et al. Tumor necrosis factor-alpha inhibits myogenesis through redox-dependent and -independent pathways. Am J Physiol Cell Physiol 2002;283:C714-21. [DOI] [PubMed] [Google Scholar]
- 3.Coletti D, Moresi V, Adamo S, et al. Tumor necrosis factor-alpha gene transfer induces cachexia and inhibits muscle regeneration. Genesis 2005;43:120-8. [DOI] [PubMed] [Google Scholar]
- 4.Smith GI, Atherton P, Reeds DN, et al. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr 2011;93:402-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy RA, Mourtzakis M, Mazurak VC. n-3 polyunsaturated fatty acids: the potential role for supplementation in cancer. Curr Opin Clin Nutr Metab Care 2012;15:246-51. [DOI] [PubMed] [Google Scholar]
- 6.Carotenuto F, Costa A, Albertini MC, et al. Dietary Flaxseed Mitigates Impaired Skeletal Muscle Regeneration: in Vivo, in Vitro and in Silico Studies. Int J Med Sci 2016;13:206-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schutze S, Schneider-Brachert W. Impact of TNF-R1 and CD95 internalization on apoptotic and antiapoptotic signaling. Results Probl Cell Differ 2009;49:63-85. [DOI] [PubMed] [Google Scholar]
- 8.Primeau AJ, Adhihetty PJ, Hood DA. Apoptosis in heart and skeletal muscle. Can J Appl Physiol 2002;27:349-95. [DOI] [PubMed] [Google Scholar]
- 9.Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res 2004;95:957-70. [DOI] [PubMed] [Google Scholar]
- 10.Jurgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, et al. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci U S A 1998;95:4997-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival Science. 1998;281:1322-6. [DOI] [PubMed] [Google Scholar]
- 12.Sharpe JC, Arnoult D, Youle RJ. Control of mitochondrial permeability by Bcl-2 family members. Biochim Biophys Acta 2004;1644:107-13. [DOI] [PubMed] [Google Scholar]
- 13.Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet 2009;43:95-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang-Lazdunski L, Blondeau N, Jarretou G, et al. Heurteaux C. Linolenic acid prevents neuronal cell death and paraplegia after transient spinal cord ischemia in rats. J Vasc Surg 2003;38:564-75. [DOI] [PubMed] [Google Scholar]
- 15.Yu X, Cui L, Zhang Z, Zhao Q, Li S. alpha-Linolenic acid attenuates doxorubicin-induced cardiotoxicity in rats through suppression of oxidative stress and apoptosis. Acta Biochim Biophys Sin (Shanghai) 2013;45:817-26. [DOI] [PubMed] [Google Scholar]
- 16.Carotenuto F, Minieri M, Monego G, et al. A diet supplemented with ALA-rich flaxseed prevents cardiomyocyte apoptosis by regulating caveolin-3 expression. Cardiovasc Res 2013;100:422-31. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Yang X, Shi H, Dong L, Bai J. Effect of alpha-linolenic acid on endoplasmic reticulum stress-mediated apoptosis of palmitic acid lipotoxicity in primary rat hepatocytes. Lipids Health Dis 2011;10:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamasaki M, Miyamoto Y, Chujo H, et al. Trans10, cis12-conjugated linoleic acid induces mitochondria-related apoptosis and lysosomal destabilization in rat hepatoma cells. Biochim Biophys Acta 2005;1735:176-84. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y, Park Y. Conjugated linoleic acid (CLA) stimulates mitochondrial biogenesis signaling by the upregulation of PPARgamma coactivator 1alpha (PGC-1alpha) in C2C12 cells. Lipids 2015;50:329-38. [DOI] [PubMed] [Google Scholar]
- 20.Jejurikar SS, Kuzon WM., Jr Satellite cell depletion in degenerative skeletal muscle Apoptosis. 2003;8:573-8. [DOI] [PubMed] [Google Scholar]
- 21.Sandri M, Minetti C, Pedemonte M, Carraro U. Apoptotic myonuclei in human Duchenne muscular dystrophy. Lab Invest 1998;78:1005-16. [PubMed] [Google Scholar]
- 22.Tews DS. Muscle-fiber apoptosis in neuromuscular diseases. Muscle Nerve 2005;32:443-58. [DOI] [PubMed] [Google Scholar]
- 23.Coulton GR, Rogers B, Strutt P, Skynner MJ, Watt DJ. In situ localisation of single-stranded DNA breaks in nuclei of a subpopulation of cells within regenerating skeletal muscle of the dystrophic mdx mouse. J Cell Sci 1992;102:653-62. [DOI] [PubMed] [Google Scholar]
- 24.Tews DS, Goebel HH. Apoptosis-related proteins in skeletal muscle fibers of spinal muscular atrophy. J Neuropathol Exp Neurol 1997;56:150-6. [DOI] [PubMed] [Google Scholar]
- 25.Hadj Salem I, Kamoun F, Louhichi N, et al. Impact of single-nucleotide polymorphisms at the TP53-binding and responsive promoter region of BCL2 gene in modulating the phenotypic variability of LGMD2C patients. Mol Biol Rep 2012;39:7479-86. [DOI] [PubMed] [Google Scholar]
- 26.Girgenrath M, Beermann ML, Vishnudas VK, et al. Pathology is alleviated by doxycycline in a laminin-alpha2-null model of congenital muscular dystrophy. Ann Neurol 2009;65:47-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carotenuto F, Albertini MC, Coletti D, et al. How Diet Intervention via Modulation of DNA Damage Response through MicroRNAs May Have an Effect on Cancer Prevention and Aging, an in Silico Study. Int J Mol Sci 2016;17,752;doi:10.3390/ijms17050752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tammen SA, Friso S, Choi SW. Epigenetics: the link between nature and nurture. Mol Aspects Med 2013;34:753-64. [DOI] [PMC free article] [PubMed] [Google Scholar]


