Abstract
In this document we discuss the main challenges encountered when producing flexible electrical stimulation implants, and present our approach to solving them for prototype production. We include a study of the optimization of the flexible PCB design, the selection of additive manufacturing materials for the mold, and the chemical compatibility of the different materials. Our approach was tested on a flexible gastro-stimulator as part of the ENDOGES research program.
Key Words: soft encapsulation, flexible electronics, electrical stimulation
Any implantable device requires a protection method, both to protect the body from implant contamination and the implant electronics from corrosion. In the development stages of new applications, stimulation implants produced from printed circuit boards (PCB) protected from the body fluids using soft encapsulation could be more advantageous than rigid devices. This method uses the PCB developed during the circuit testing phase, hence considerably speeding up the production of prototype devices. Further, direct encapsulation of the active circuit removes the need for the costly production of a hermetic package.1 With a few exceptions, such as the micro-packages produced for visual prostheses,2 hermetic packages are most often produced using a rigid titanium shell with metal-in-glass feedthroughs. The overall dimensions of a PCB encapsulated in silicone rubber are likely to be smaller than with a rigid case, and the number of connections can be high. Silicone rubber is permeable to water, hence the encapsulation layer becomes rapidly saturated with water vapor after implantation.3 Therefore, it should strongly adhere to the substrate and occupy every available spaces to prevent the creation of voids that would lead to water condensation over electrical pads. If there is no loss of adhesion between the encapsulant and all the surfaces, there will be no corrosion inducing a potential failure. One drawback of the soft encapsulation is that the device lifetime cannot be predicted from non-destructive tests performed on the sample before implantation. There are no reported methods to assess encapsulation quality suitable to predict implant lifetime.1 For hermetic packages, this test is a helium leak test, which is of limited value for very small internal implant volumes (<1mm3).4 However, for soft encapsulation, only a handful of publications report tests on the long-term underwater adhesion between silicone rubbers and substrates and data provided are mainly empirical.5,7 While lifetime prediction is not currently possible, we can rely on accelerated life tests to estimate the likelihood of a failure for periods of months to years.8 We argue that, in several stages of an active implant development, this is sufficient, and hence soft encapsulation of PCB is a promising prototyping method. Indeed, this method is already used for tests in animal models.9 The appeal of the method would be further enhanced if the implants were flexible, able to adapt to a certain shape or to dynamically follow natural motions of a body part or an internal organ. This paper focuses on the study of the soft encapsulation technique applied to flexible electronics. We discuss the design of a PCB to produce a truly flexible circuit, the characterization of the chemical reactions involved and how the silicone rubber wets the different surfaces of the implant, in light of the importance of avoiding the appearance of voids or trapped gas bubbles in the encapsulant.
Materials and Methods
Silicone Rubbers
The following silicone rubbers have been selected for their high elongation capability and adhesive properties. Note that the MED-6607 contains naphtha as solvent (non-volatile content as given in the technical data sheet = 30 %) and MED-2214 contains xylene as solvent (non-volatile content as given in the technical data sheet = 35 %).
Flexible PCB
Flexible substrate
Flexible substrates are thinner than rigid ones, and lighter, and they can be bent in several configurations to fit a specific area and occupy a given space in 3 dimensions. Flexible substrates are also convenient and more reliable when using multiple rows of connectors and cables.10 In this paper, we report on the development of a truly flexible device, one that will still be able to bend after implantation. Flexible PCBs are however more expensive (without taking into account the possible cost reductions in the assembly phase) and subject to tearing if not properly designed and handled.10 This has to be taken into account in the early stage of the design to ensure a maximum lifetime for the implantable electronics.
Design: tracks and components position
The will to produce a flexible device introduces further considerations as Integrated Circuits (ICs) and Surface Mounted Devices (SMD) are rigid, which leads to the stiffening of the substrate and local loss in flexibility. We propose to spread the rigid ICs across the board and to reserve component-free fold-lines. Increasing the number of fold-lines and spreading the components will improve the actual flexibility of the populated PCB. However, this increases the PCB area. Hence, the design of a PCB for the production of a flexible implant calls for a trade-off between flexibility and overall device dimensions.10 A new risk of failure introduced by the use of flexible circuits is the possible tearing of the thin flexible substrate. Several methods are recommended:
Avoid any concave right-angle corner on the substrate borders. They should be replaced by circular corners, and the larger the radius, the better the toughness against tearing.
Adding extra copper on the corner, which acts locally as a mechanical reinforcement, increases the substrate resistance at that weak spot.
When needed, use holes in slit…
Or recessed hole
Or drilled holes
Mold Design
While molds for working with silicone rubber are most commonly made of Polytetrafluoroethylene (PTFE), 3D printing is attractive, in terms of availability and prototyping time. Particular attention should however be paid to the choice of the mold material. Indeed, the presence of some chemicals can lead to unwanted reactions between the mold and the silicone rubber, resulting in a failure of the reticulation process. Moreover, a material having a low adhesion to silicone rubber should be selected in order to ease the unmolding process. As it is relatively low cost, we have tested the well-known and often-used Polylactic Acid (PLA) with the silicone rubbers selected (II.A) to evaluate if chemical reactions inhibit the cure.
Cleaning Procedure
When using soft encapsulation, the cleaning process is a crucial step to limit the number of particles on the circuit and the mold as they can lead to a loss of adhesion at the silicone rubber to substrate interface which in turn may contribute to the formation of a conductive fluid over the substrate. This could, after implantation, cause a functional failure of the implant.8 A similar cleaning procedure is thus applied on the samples used for the tests described in the next sections (II.E and II.F) so that the samples are in the same state as they would be for the encapsulation of a device. The cleaning protocol consists of several steps: all parts of the implant (the populated substrate) are cleaned twice with a brush dipped in chloroform, then immersed for 3 minutes in 15 cm of deionized water (DI-water) continuously refreshed by a stream of fresh DI-water. The components are then immersed between 3 to 5 minutes in a cleaning solution (500 mL of DI-water, 5mL of Teepol, 125 gr of Na3PO4 and DI-water again to reach a 1 L volume) then rinsed in DI-water. The components are finally immersed again in the same container continuously refreshed by a stream of fresh DI-water. The immersion is stopped once the conductivity values of incoming and outgoing water are equivalent (76 +/- 1 microSiemens/cm). The components are then dried in an oven at 40°C for 60 minutes and at room temperature for 90 minutes. The components are then stored in a sealed container before further experimentation.
Silicone wetting through contact angle measurements
Encapsulation of the implant requires good wetting, by the silicone rubber, of the surface of each individual component which is part of the final implant. This wetting is characterized by the contact angles of the silicone over the different components. Thus, contact angles between liquid uncured silicone rubber and individual implant components were measured with a Krüss DSA-100 tensiometer placed in a clean room to avoid contamination. Droplets of the uncured silicone elastomer were deposited on the implant components with a pipette. Wetting of the substrates by the silicone rubber droplets were video recorded and analyzed with the Krüss DSA-100 tensiometer analysis software. The contact angles were measured when the droplets were stable (after 11 seconds contact time with the substrates).
Chemical reactions
Good wetting of the surfaces is crucial to ensure that no voids or bubbles will be trapped during the encapsulation process, yet another cause of trapped air bubbles could be chemical reactions occurring between the system components and the silicone rubber. The reactions of the Si-H bond with alcohols or water produce hydrogen according to the equations hereunder,11 which can lead to the creation of bubbles in the uncured silicone rubber.
To test the occurrence of those reactions, part A and part B of the low consistency silicone elastomer MED4-4220 were degassed for 30 minutes. No gas bubbles nor microbubbles could be visually observed in neither part. Pairs of samples representative of all the separate components used for the elaboration of the PCB were then immersed for a few seconds in part A or part B (one of each pair of samples in each) of the MED4-4220, then removed and suspended to a drying rack to allow the excess of viscous liquid to flow. After the dip coating, each individual component covered by either part A or part B of MED4-4220 was visually observed. All the components were then placed in an oven at 40°C for 90 minutes, to accelerate the targeted chemical reactions. After 24 hours, the coated samples were microphotographed by AVT Prosilica GX1910 to evaluate and conclude about the presence of bubbles.
Results and Discussion
Electronics and mold design
Fig.1 shows the design of the circuit in Altium Designer 3D view. The mold has been designed with a 3D CAD software and printed with a Makerbot 3D printer.
Fig 1.
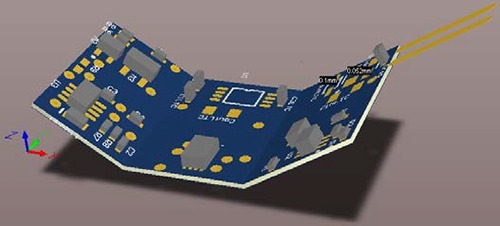
Illustration of the flexible circuit on Altium Designer.
Wetting measures
Freshly mixed MED4-4220 is quite viscous (>20 000 mPa.s). Furthermore, as the hydrosilylation reaction between part A and part B starts right after the mixing step, a buildup of viscosity was observed. For this reason, it was difficult to use the setup to properly measure the contact angle. The only successful attempt was done on the copper and the resulting angle was in the same range as the ones measured with the other silicone rubbers. Contact angles between MED-6607 and MED-2214 and the individual components after 11 seconds contact time could be calculated and are reported in Fig.2. An assumption is made that evaporation of naphtha or xylene solvent in MED-2214 and MED-6607 is not impacting the measurements, as the time scale of the latter is quite short. This set of data clearly shows that the three uncured silicone elastomers are effectively wetting the surface of each individual component of the implants. According to Young’s equation, i.e.
the contact angle θ depends on the solid-gas, the solid-liquid and the liquid-gas interfacial tensions (respectively γSG, γSL, γLG in N/m).12 In our specific case, the surface tension of liquid silicone elastomer is seemingly low enough to induce proper wetting of the different surfaces. The presence of the naphtha or xylene solvent in MED-2214 and MED-6607 is not impacting negatively the wetting ability of the silicone rubber.
Fig 2.
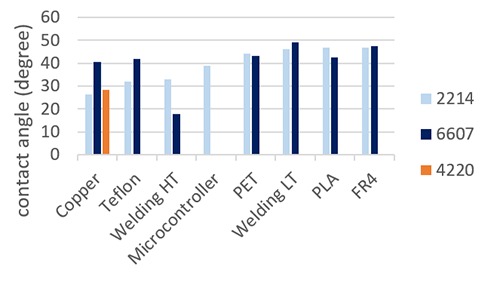
Contact angles of droplets of MED4-4220, MED-6607 and MED-2214 freshly extruded on individual components present in the implant manifacture.
Bubble creation
As specified in II.F, the samples have been analyzed twice. No bubbles nor microbubbles could be observed during both observations, leading to the conclusion that no side chemical reaction between OH groups (from alcohols or water) and Si-H groups is taking place between the silicone rubber and the components. We conclude that the flexible device is suitable to be encapsulated with the selected silicone rubbers.
Encapsulation of the Endoges gastro-stimulator
We have designed and encapsulated a flexible PCB for a gastro-stimulator (see Fig. 3 and Fig. 4). From this first proof of concept, we have identified the following areas for improvements. First, the thickness of silicone has to be adjusted to increase the flexibility. We worked with an encapsulated thickness of 4mm for the implant. This however is too thick to properly bend the device. The next version of the molds could allow for thinner margins around the components and a much thinner layer of silicone at the component-free fold-lines. Then, a bubble is present on the surface of the silicone rubber. This will not lead to any functional failure but it will be critical to avoid that in the future to be able to achieve precise encapsulations with reduced safety margins. Finally, the adhesion between the silicone rubber and the new substrate appeared to be far poorer than the adhesion obtained with a rigid PCB substrate. Adhesion is particularly important in flexible implants, to achieve a sufficient implant lifetime, because the bending motion leads to shear stresses at the silicone rubber to substrate interface. Thus, we will study the adhesion through accelerated life tests in simulated working conditions, including bending the implant as would be expected in the implanted environment. In parallel, we will work on improving the adhesion with the flexible substrate. New silicone rubbers will be selected for their adhesive properties and flexibility. Each silicone rubber will be tested in accelerated life tests with the PET substrate and other flexible substrates such as polyimide to identify the best combination in term of adhesion. If none of those combinations show good adherence properties, the use of pre-processing techniques, such as the application of a primer before the silicone rubber, will be necessary. This solution is not the preferred one because of the complexity it brings to the overall procedure. Although there is room for improvements, we have opened the way to manifacture flexible implants that offer advantages over rigid devices. Future research will focus on assessing and enhancing the adhesion of the encapsulant, since this remains the major issue to ensure that flexible devices will withstand long-term implantations.
Fig 3.
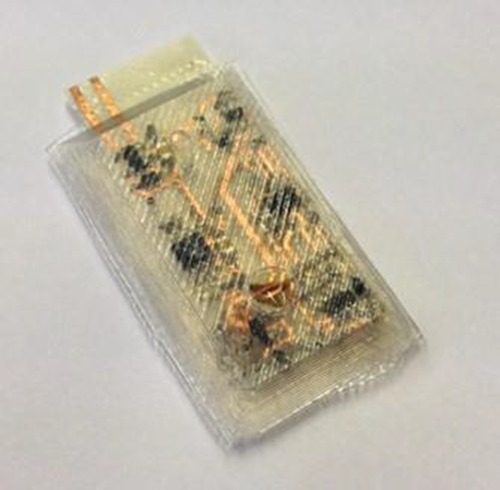
Flexible circuit of our gastro-stimulator encapsulated through vacuum centrifugation
Fig 4.

The encapsulated circuit being bent
Table 1.
Silicone characteristics
| Silicone | Characteristics | ||
|---|---|---|---|
| Type | Curing | Elongation | |
| MED4-4220 | 2 part low consistency elastomer | Platinum | 580% |
| MED-6607 | RTV silicone dispersion coating | Room temperature upon exposure to atmospheric moisture | 600% |
| MED-2214 | Silicone dispersion | Heat cures | 850% |
Acknowledgment
We would particularly like to thank Geoffrey Vanbienne for his fast and efficient support during the whole realization of this project. I also thank Nusil for the help they provided in the selection of the silicone rubbers.
Contributor Information
Laura Hermans, Email: hermanslaura@live.be.
Maxime Bosquet, Email: maxbo11052@gmail.com.
Sam Dehaeck, Email: sam.dehaeck@gmail.com.
Laurent Lonys, Email: llonys@ulb.ac.be.
Benoit Scheid, Email: bscheid@ulb.ac.be.
Antoine Nonclercq, Email: anoncler@ulb.ac.be.
Anne Vanhoestenberghe, Email: a.vanhoest@ucl.ac.uk.
References
- 1.Vanhoestenberghe A, Donaldson N. Corrosion of silicon integrated circuits and lifetime predictions in implantable electronic devices Journal of neural engineering. 2013;10:Xx-Yy. [DOI] [PubMed] [Google Scholar]
- 2.Vanhoestenberghe A, Donaldson N, Lovell NH, et al. Hermetic Encapsulation of an Implantable Vision Prosthesis - Combining Implant Fabrication Philosophies. 13th International FES Society Conference, 2008;187-9. [Google Scholar]
- 3.Traeger RK. Nonhermeticity of Polymeric Lid Sealants vol. 1997;13:147–52. [Google Scholar]
- 4.Vanhoestenberghe A, Donaldson N. The Limits of Hermeticity Test Methods for Micropackages. Artificial Organs 2011;35:242-4. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson PEK, Aylett BJ. Aspects of silicone rubber as encapsulant for neurological prostheses. Part 2: Adhesion to binary oxides. Medical and Biological engineering & Computing 1995;33:289–92. [PubMed] [Google Scholar]
- 6.Donaldson PEK. Aspects of silicone rubber as encapsulant for neurological prostheses. Part 3: Adhesion to mixed oxides. Medical and Biological engineering & Computing. 1995;33:725–7. [DOI] [PubMed] [Google Scholar]
- 7.Donaldson PEK. Aspects of silicone rubber as encapsulant for neurological prostheses. Part 4: Two-part rubbers. Medical and Biological engineering & Computing 1997;35:283–6. [DOI] [PubMed] [Google Scholar]
- 8.Lonys L, Vanhoestenberghe A, Julémont N, et al. Silicone rubber encapsulation for an endoscopically implantable gastrostimulator. Medical and Biological engineering & Computing 2015;4:319-29. [DOI] [PubMed] [Google Scholar]
- 9.Baker S., et al. Transcutaneous Signal Transmission without Breaching the Skin’s Natural Barrier to Infection. Poster for the NC3Rs, 2010. [Google Scholar]
- 10.Fjelstad J. Flexible Circuit Technology, BR Publishing, Inc; 2011;4:49-58. [Google Scholar]
- 11.Noll W. Chemistry and Technology of Silicones. Academic Press; 1968;87. [Google Scholar]
- 12.Ross S, Morrison ID. Colloidal systems and interfaces. John Wiley and Sons, NY, 1988; 89-93. [Google Scholar]


