Abstract
Background
Iron overload, diagnosed by means of magnetic resonance imaging (MRI), is an increasingly recognized disorder in hemodialysis patients. Specific MRI protocols have been shown to provide a reliable estimation of tissue iron content in non-renal patient populations but have not been validated in dialysis patients. Such validation studies require liver biopsy for histological comparison, but this invasive and risky procedure raises ethical concerns, especially regarding frail patients with end-stage renal disease.
Materials and methods
We compared in a pilot study Scheuer’s histological classification and Deugnier and Turlin’s histological classification of iron overload (Perls staining) with signal-intensity-ratio MRI values obtained with the Rennes University algorithm in 11 hemodialysis patients in whom liver biopsy was formally indicated for their medical follow-up.
Results
For Scheuer’s histological classification, the Wilcoxon non-parametric matched-pairs test showed no significant difference in the ranking of iron overload by the two methods eg histology and MRI (sum of ranks = 1.5; p = 1). The MRI and Scheuer’s histological classifications were tightly correlated (rho = 0.866, p = 0.0035, Spearman’s coefficient), as were the absolute liver iron concentrations (LIC) at MRI (rho = 0.860, p = 0.0013, Spearman’s coefficient). The absolute liver iron concentrations at MRI were also highly correlated with Deugnier and Turlin’s histological scoring (rho = 0.841, p = 0.0033, Spearman’s coefficient).
Conclusions
This pilot study shows that liver iron determination based on signal-intensity-ratio MRI (Rennes University algorithm) very accurately identifies iron load in hemodialysis patients, by comparison with liver histology.
Keywords: Medicine, Internal medicine, Medical imaging
1. Introduction
During the past three decades, routine use of recombinant erythropoiesis-stimulating agents (ESA) has enabled anemia to be corrected in most patients with end-stage renal disease (ESRD), transforming their quality of life and clinical outcomes [1, 2]. As the use of ESA is frequently associated with true or functional iron deficiency, almost all hemodialysis patients on ESA currently receive parenteral iron to ensure efficient erythropoiesis [1, 2]. Intravenous (IV) iron therapy has gained popularity in the nephrology community in the last fifteen years because of its convenience (infusion during the dialysis sessions), its superiority over oral preparations for treating true iron deficiency, and its ability to overcome functional iron deficiency, which is common in this setting. Furthermore, IV iron products enable cost savings of about 20%–30% on expensive ESA molecules [1, 3, 4]. Until recently, it was widely considered that iron overload among dialysis patients was more prevalent during the pre-ESA era, when blood transfusion was frequently used to treat anemia and when intravenous iron therapy was given without concomitant ESA administration. Iron overload used to be considered exceptional among hemodialysis patients in the ESA era but is now an increasingly recognized clinical problem [3, 4, 5, 6, 7, 8, 9, 10].
The liver is the main iron storage site in humans, and the liver iron concentration (LIC) correlates closely with total body iron stores in patients with secondary hemosideroses such as thalassemia major, sickle cell disease and genetic hemochromatosis [11]. Hepatic magnetic resonance imaging (MRI) is now considered the gold standard method for estimating and monitoring iron stores in patients with secondary hemosideroses and genetic hemochromatosis, as it permits “serial radiological biopsy”. MRI is believed to be one of the major contributors to the improvement in knowledge and patient care in this setting [11, 12, 13]. There are currently three main MRI methods for determining LIC: signal-intensity-ratio MRI (Rennes University algorithm), R2 relaxometry, and R2* relaxometry, all of which have been validated in cohorts of non-renal patients with secondary hemosiderosis, genetic hemochromatosis or other liver diseases, who underwent liver biopsy for biochemical iron assay [14, 15, 16].
Recent studies of quantitative MRI to estimate LIC in hemodialysis patients have suggested a strong link between the infused iron dose and the risk of iron overload, and have challenged the reliability of current iron biomarker cutoff values and clinical guidelines, especially regarding recommended iron doses [7, 8, 9, 10]. These MRI studies contributed greatly to the organization of the recent KDIGO (Kidney Disease Improvement of Global Outcomes) controversies conference on iron management in chronic kidney disease (CKD), which recognized in its final statement the “iron overload entity” in hemodialysis patients and called for an agenda of research on this topic, especially MRI techniques [17]. Moreover, in June 2015 the Dialysis Advisory Group of the American Society of Nephrology published an aggiornamento on the policy of high “blind” usage of intravenous iron products in hemodialysis patients, especially in view of recent MRI data on iron overload [18].
Validation of MRI-based liver iron determination in dialysis patients, by comparison with liver biopsy, is of paramount scientific importance but is hindered by ethical concerns due to the invasiveness and risks of biopsy, especially in frail ESRD patients.
Here we studied 11 hemodialysis patients with a formal indication for liver biopsy, and compared the histological classifications of iron overload by Perls’ coloration (Scheuer’s classification and Deugnier and Turlin’s classsification) and by signal-intensity-ratio MRI using the Rennes University algorithm.
2. Materials and methods
2.1. Patients and dialysis
After receiving the patients’ written informed consent, as well as technical and ethical approval from the Drug, Devices and Clinical Trials Committee of our institution (COMEDIMS Claude Galien, 9 December 2004), hemodialysis patients free of overt inflammation or malnutrition and undergoing chronic intermittent bipuncture bicarbonate hemodialysis three times a week at Claude Galien’s dialysis unit, with ultrapure dialysate and single-use biocompatible membranes, were enrolled in this prospective, cross-sectional and longitudinal study, starting on 31 January 2005 (aimed at studying iron stores by MRI and with planned end date 31 January 2020). The patients were treated for anemia according to European best practice guidelines. The inclusion and exclusion criteria of the study, and the treatment of anemia, are described in depth in our first report on this cohort [10]. This study is registered under International Standard Randomized Controlled Trial Number (ISRCTN) 80100088. In early 2013, after publication of our original work revealing the high prevalence of iron overload in dialysis patients [10], discussions with ethicists in our institution led us to consider that a prospective study comparing MRI and liver biopsy in frail dialysis patients would be unethical, especially given the vast amount of relevant MRI data in patients with genetic hemochromatosis, secondary hemosiderosis and various liver diseases. The ethicists advised us to compose in a pilot study a prospective cohort of patients who required liver biopsy or liver surgery for their medical care and to perform closely a quantitative hepatic MRI. Hepatic MRI is routinely performed in our institution and is fully reimbursed by the national social insurance system in France. It is non invasive, brief (less than 30 min), well tolerated, and devoid of physical and psychological side effects (excepting claustrophobic patients and other standard contraindications).
2.2. MRI estimation of hepatic iron stores
We used a signal-intensity-ratio method based on T1 and T2* contrast imaging without gadolinium, as established by Gandon and coworkers at Rennes University and validated in a cohort of 191 patients who underwent liver biopsy for biochemical iron assay [14]. Patients on iron therapy received their iron dose at least one week before MRI. MRI measurements were made by a senior radiologist (YC) who was unaware of the patients’ medical history and iron biochemistry values. According to Rennes University, LIC values below 40 μmol/g of dry liver are normal, whereas values between 41 and 100 μmol/g represent mild iron overload, 101–200 μmol/g moderate iron overload, and >200 μmol/g severe iron overload (Table 1) [14]. These MRI categories of iron overload have been shown to reflect an increasing risk of complications in iron overload disorders (Table 1)[11, 14]. Briefly, a Sigma MRI unit (GE Medical Systems, Milwaukee, WI, USA) operating at a field strength of 1.5 Tesla was used. Five weighted gradient-recalled-echo sequences of the liver (GRE T1, PD, T2, T2+ and T2 + +) were acquired with a repetition time of 120 ms. Measurements were made in five regions of interest (ROI) larger than 1 cm2 (usually 3 ROI on the right liver and 2 ROI on paraspinal muscles on the same slice) to calculate the liver-to-muscle ratio. The software algorithm provided by Rennes University (http://www.radio.univ-rennes1.fr) was used to determine hepatic iron content.
Table 1.
Magnetic Resonance Imaging grading of liver iron storage according to Rennes University.
| LIC ≤ 40 μmol/g of dry weight | Normal |
| 40 < LIC ≤ 100 μmol/g | Mild iron overload |
| 100 < LIC ≤ 200 μmol/g | Moderate iron overload |
| LIC > 200 μmol/g | Severe iron overload |
LIC : Liver iron content.
MRI Classification according : see Ref. [14].
2.3. Liver histology
Liver specimens obtained by transjugular biopsy, wedge biopsy or partial hepatectomy were fixed in neutral-buffered formalin, zinc chloride Zenker’s or Hollande’s fixative, and embedded in paraffin. Hematoxylin-eosin, Masson’s trichrome, and Perls’ stains were available for each specimen [19, 20]. Each biopsy was read by the same hepatopathologist for the presence of specific pathological features [20]. Hepatic iron was assessed (by ML) by light microscopy on Perls-stained slides and firstly graded on a scale of 0 to 4 according to Scheuer et al. [19]. The normal grade of stainable liver iron is 0 or 1, while grade 4 is the degree seen in fully developed untreated hemochromatosis (Table 2) [19, 20]. Hepatic iron was also secondly graded (by ML) according to Deugnier and Turlin (total iron score: 0 to 60) after determining hepatocyte iron score (0 to 36), sinusoidal iron score (0 to 12) and portal iron score (0 to 12) (Table 3) [20].
Table 2.
Histological grading of liver iron storage according to Scheuer.
| 0 | Granules absent or barely discernable at magnification x 400 |
| 1 | Granules barely discernable at x 250 magnification and easily confirmed at x 100 |
| 2 | Discrete granules resolved at x 100 magnification |
| 3 | Discrete granules resolved at x 25 magnification |
| 4 | Masses visible at magnification x 10 or at naked eye |
According to Scheuer et al. [19].
Table 3.
Histological grading of liver iron storage according Deugnier and Turlin.
| Hepatocytic iron score (HIS) | 0, 3, 6, 9, or 12 according to granules size in each Rappaport area | 0–36 |
| Sinusoidal iron score (SIS) | 0, 3, 6, 9, or 12 according to granules size in each Rappaport area | 0–12 |
| Portal iron score (PIS) | 0, 1, 2, 3, or 4 according to the percentage of iron overloaded macrophages, biliary cells, and vascular walls | 0–12 |
| Total iron score (TIS) | 0–60 |
According to Deugnier Y and Turlin B [20].
In order to avoid hemorrhagic complications after liver biopsy or liver surgery, patients had dialysis sessions for one month using heparin-coated membranes (Evodial from Hospal-Gambro-Baxter) plus low-dose anticoagulation of the extracorporeal circuit with 1000 IU of unfractionated heparin as a bolus at outset and mid-session; heparin was antagonized at the outset of the dialysis session with its specific antidote protamine (2000 IU). Hemoglobin was assayed at each dialysis session during the month following liver biopsy or surgery.
2.4. Statistical analyses
As advocated by Sheskin, for the comparison of two sets of ordinal data (e.g. MRI and Perls’ categories according Scheuer’s classification), we used Wilcoxon’s non-parametric matched-pairs signed-rank test. Correlations between the MRI and histological categories, and between absolute MRI LIC values and scores at the histological classifications, were analyzed with Spearman’s rank-order correlation coefficient. We also constructed a scatterplot of ranks for MRI categories and Perls’ categories according Scheuer’s classification [21].
To permit formal comparison between the MRI scale (4 categories) and the corresponding Perls’ scale according Scheuer, categories 0 and 1, which indicate normal liver iron content, were combined for analysis. Thus, the modified Perls-Scheuer scale also comprised 4 counterpart categories.
As the upper 95% of LIC in healthy adults is 32 μmol/g of dry eight liver, the upper limit of MRI normality for statistical analyses was thus set at 32 μmol/g and values between 32 and 100 μmol/g at MRI represent here mild iron overload [11, 14].
Prism 6 software (GraphPad, San Diego, USA) was used for all tests, and p values < 0.05 were considered to denote statistical significance [21].
3. Results
3.1. Characteristics of the dialysis patients
We recruited 11 patients (4 women and 7 men) from 26 March 2013 to 15 April 2016. Their clinical characteristics are shown in Table 4. Their median dialysis vintage was 18 months (range 4 to 140). Nine of them had transjuglar biopsy (performed by RB), while one (patient #1) had wedge biopsy and one (patient #2) had partial hepatectomy. No complications occurred, including hemorrhagic events.
Table 4.
Characteristics of the patients studied.
| Number | Age (Years) | Original Nephropathy | Sex (female: F; Male: M) | Dialyse vintage (months) | Number of days between MRI and histology | LIC at MRI micromol/g of dry liver | Grade of Perls staining according Scheuer | Hepatic iron score according Deugnier and Turlin | Type of liver biopsy | Purpose of liver histology | Final diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | Chronic pyelonephritis | M | 48 (4 years) | 7 | 20 | 0 | 0 | Wedge | Hepatitis C- liver enzyme anomalies- Liver and spleen enlargement at echography | Left nephrectomy for pyonephrosis on underlying lithiasis- Portal fibrosis |
| 2 | 50 | Genetic segmental glomerulosclerosis | F | 100 (8 years and 3 months) | 60 | 55 | 2 | 6 | Partial hepatectomy | Maintenance on the waiting list - Hepatic tumor- Hepatitis C | Sclerosed hemangioma associated with fibrosteatosis |
| 3 | 49 | Late congenital renal hypoplasia | F | 20 | 84 | 198 | 2 | 21 | Transjugular | Inscription on the waiting list (second transplantation)- Hepatic dysmorphia at echography | Hepatic hemosiderosis with mixed pattern |
| 4 | 70 | Diabetic nephropathy | M | 18 | 36 | 30 | 2 | 3 | Transjugular | Inscription on the waiting list- Hepatic dysmorphia at echography | Mild macrovacuolar steatosis related to aziathopurine given for ulcerative colitis |
| 5 | 54 | Renal dysplasia | F | 29 | 13 | 35 | 2 | 3 | Transjugular | Maintenance on the waiting list- Liver enlargement at echography with liver enzyme anomalies | Cardiac liver related to heart failure due to fistulae hyperflow |
| 6 | 66 | ANCA +, anti-MPO + vasculitis | F | 7 | 55 | 154 | 3 | Not applicable- No portal space | Transjugular | Search for immune hepatitis | Hepatic hemosiderosis with mixed pattern |
| 7 | 59 | Diabetic nephropathy | M | 4 | 60 | 55 | 2 | 6 | Transjugular | Inscription on the waiting list- Liver enzyme anomalies- Steatosis at echography- Alcohol addiction | Severe alcoholic fibrosteatosis |
| 8 | 59 | Hypertensive nephropathy | M | 12 | 34 | 20 | 1 | 6 | Transjugular | Inscription on the waiting list- Liver enzyme anomalies- Steatosis at echography- Alcohol addiction | Alcoholic fibrosteatosis |
| 9 | 58 | Renal unilateral agenesia | M | 4 | 20 | 25 | 0 | 0 | Transjugular | Inscription on the waiting list- Hepatic dysmorphia at echography | Congenital hepatic hemiatrophy associated with renal agenesis |
| 10 | 67 | Diabetic nephropathy | M | 140 (11 years and 8 months) | 3 | 200 | 3 | 30 | Transjugular | Maintenance on the waiting list - Abnormal hepatic echography | Large cell hepatic dysplasia |
| 11 | 44 | Hypertensive nephropathy | M | 16 | 4 | 108 | 3 | 18 | Transjugular | Maintenance on the waiting list - Abnormal hepatic echography | Hepatic hemosiderosis with reticulo-endothelial pattern |
In most cases, MRI was performed less than two months before scheduled liver biopsy (Table 4). In three patients with iron overload (patients #2, #3 and #6), because of a lag period greater than 6 weeks, the MRI LIC value at the time of liver biopsy was extrapolated from the slope of the decline between the first MRI and a second MRI performed 3 to 6 months later, based on the kinetics reported in iron-overload hemodialysis patients [10].
Median liver iron concentration at MRI was 55 μmol/g of dry weight (range: 20–200 μmol/g) Four patients had normal LIC values (less than 32 μmol/g of dry liver), while three patients had mild iron overload (LIC between 32 and 100 μmol/g) and four patients had moderate iron overload (LIC from 101 to 200 μmol/g) (Table 4).
3.2. Quantitative MRI accurately estimates iron overload in dialysis patients, by comparison with liver histology
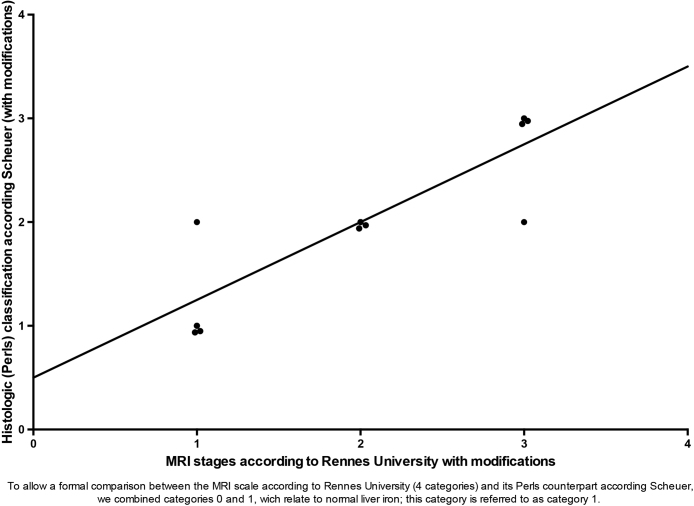
The overall agreement between MRI and histology was very good (82%), as reflected by the scatterplot of ranks (Fig. 1). Comparison of the two datasets (MRI and Perls-Scheuer’s histological classification) with the Wilcoxon matched-pairs test showed no statistical difference in the ranking (sum of the ranks = 1.5; p = 1).
Fig. 1.
Scatterplot of ranks of the MRI and histologic Perls-Scheuer classifications.
The MRI and Scheuer’s histological classification were tightly correlated (rho = 0.866, p = 0.0035, Spearman’s correlation rank order coefficient), as were the absolute liver iron concentrations at MRI (rho = 0.860, p = 0.0013, Spearman’s correlation rank order coefficient).
The absolute liver iron concentrations at MRI were also highly correlated with Deugnier and Turlin’s histological scoring (rho = 0.841, p = 0.0033, Spearman’s correlation rank order coefficient).
4. Discussion
In this pilot study, we found excellent agreement between LIC values determined by signal-intensity-ratio MRI (Rennes University algorithm) and histological estimation of iron load in dialysis patients. It should be noted that this study respected ethical requirements in these frail patients, and that no hemorrhagic complications occurred after liver biopsy, which was required for their medical management.
Because of the design of this pilot study, the time period between MRI and biopsy varied greatly among the patients. Moreover, in three patients (#2, #3 and #6), because of a lag period greater than 6 weeks, to avoid spurious results, the MRI LIC value at the time of liver biopsy was extrapolated from the slope of the decline between the first MRI and a second MRI performed 3 to 6 months later, based on the liver iron efflux kinetics previously reported [10].
The recent KDIGO conference on iron management controversies in CKD acknowledged that specific MRI protocols provide a reliable estimate of tissular iron content in non-renal patients, and that liver iron content appears to be elevated in the majority of unselected hemodialysis patients and markedly elevated in about one-third of cases [10, 17]. The same KDIGO conference identified a knowledge gap in the validation of MRI techniques for quantifying iron content in ESRD patients [17]. Here, we provide strong evidence that signal-intensity-ratio MRI, using the Rennes University algorithm, accurately estimates liver iron content in dialysis patients. This is of major importance, as quantitative MRI has recently changed the landscape of iron metabolism studies and iron therapy in ESRD patients, and has been proposed as a tool for determining the toxic dose of parenteral iron, diagnosis of iron overload, and routine monitoring of iron stores in dialysis patients [22, 23].
Perls’ stain has been used for more than a century and continues to be the standard method for visualizing non-heme iron, because of its specificity, simplicity and low cost [24]. Perls’ method is based on the formation of insoluble Prussian blue in tissue sections pretreated with ferrocyanide acid [24]. Prussian blue can be enhanced by using chromogens, 3,3-diaminobenzidine (DAB) being the most widely used and sensitive reagent [24]. Besides its role in diagnosing and classifying iron overload disorders, non-heme iron histochemistry has proved to be a powerful tool for experimental and pathological investigations of the role of non-heme iron in tissue degenerative changes [24].
For more than half a century, Scheuer’s classification of Perls’ staining, initially proposed in genetic hemochromatosis, has been the mainstay for diagnosis and monitoring of both genetic hemochromatosis and secondary hemosiderosis, although more sophisticated classifications are now used, especially that of Barton for epidemiological studies [25] and that of Deugnier and Turlin for hepatic pathophysiology [26]. Because of the design of our study, the Scheuer’s histological classification seems a very valuable tool since it appears as the counterpart of MRI classification of iron overload. Today, Deugnier and Turlin’s classification of Perls’ staining is the best-validated biochemical method for determining liver iron in tissue specimens, in both hemochromatotic and nonhemochromatotic iron-overload disorders, but it is still mainly used for research purposes because of its complexity [20, 26, 27, 28]. Validation of Scheuer’s classification by comparison with biochemical determination of liver iron content was, until recently, based mainly on a report published in the 1990s by Scheuer’s group in thalassemia major patients treated with the chelator desferrioxamine, but recent publications have also shown its validity in alcoholic liver disease and sickle cell disease [29, 30, 31]. It is also noteworthy that recent publications on the epidemiology of iron-overload diseases in African-American and cirrhotic explants of alpha1-antitrypsin deficiency used Perls’ histological grading as the mainstay of their research [32, 33].
For decades, staining and biochemical analysis of deparaffinated tissue were complementary methods for liver histopathology, being especially relevant when the iron distribution is heterogeneous, as in the cirrhotic liver [20]. Recently, the advent of MRI techniques allowing non-invasive quantification of liver iron content, together with HFE genetic testing, has narrowed the indications of liver biopsy, and biochemical determination of liver iron content has become a research tool available only in a few university hospitals [12, 13, 14, 15, 16].
5. Conclusion
This pilot study specifically shows that, by comparison with liver histology, quantitative determination of liver iron content by signal-intensity-ratio MRI (Rennes University algorithm) is very accurate in dialysis patients.
Declarations
Author contribution statement
Guy Rostoker: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mireille Laroudie: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Raphael Blanc, Bernard Galet, Yves Cohen: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Clémentine Rabaté: Contributed reagents, materials, analysis tools or data.
Mireille Griuncelli: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Hörl W.H. Clinical aspects of iron use in the anemia of kidney disease. J. Am. Soc. Nephrol. 2007;18(2):382–393. doi: 10.1681/ASN.2006080856. [DOI] [PubMed] [Google Scholar]
- 2.Eschbach J.W., Egrie J.C., Downing M.R. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin: Results of a combined phase I and II clinical trial. N. Engl. J. Med. 1987;316(2):73–78. doi: 10.1056/NEJM198701083160203. [DOI] [PubMed] [Google Scholar]
- 3.Vaziri N.D. Epidemic of iron overload in dialysis population caused by intravenous iron products: a plea for moderation. Am. J. Med. 2012;125(10):951–952. doi: 10.1016/j.amjmed.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Rostoker G., Vaziri N.D., Fishbane S. Iatrogenic iron overload in dialysis patients at the beginning of the 21st century. Drugs. 2016;76(7):741–757. doi: 10.1007/s40265-016-0569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali M., Rigolosi R., Fayemi A.O. Failure of serum ferritin levels to predict bone-marrow iron content after intravenous iron-dextran therapy. Lancet. 1982;1(8273):652–655. doi: 10.1016/s0140-6736(82)92204-8. [DOI] [PubMed] [Google Scholar]
- 6.Eschbach J.W., Adamson J.W. Iron overload in renal failure patients: changes since the introduction of erythropoietin therapy. Kidney Int. 1999;55(Suppl. 69):S35–S43. doi: 10.1046/j.1523-1755.1999.055suppl.69035.x. [DOI] [PubMed] [Google Scholar]
- 7.Canavese C., Bergamo D., Ciccone G. Validation of serum ferritin values by magnetic susceptometry in predicting iron overload in dialysis patients. Kidney Int. 2004;65(3):1091–1098. doi: 10.1111/j.1523-1755.2004.00480.x. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari P., Kulkarni H., Dheda S. Serum iron markers are inadequate for guiding iron repletion in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011;6(1):77–83. doi: 10.2215/CJN.04190510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghoti H., Rachmilewitz E.A., Simon-Lopez R. Evidence for tissue iron overload in long-term hemodialysis patients and the impact of withdrawing parenteral iron. Eur. J. Haematol. 2012;89(1):87–93. doi: 10.1111/j.1600-0609.2012.01783.x. [DOI] [PubMed] [Google Scholar]
- 10.Rostoker G., Griuncelli M., Loridon C. Hemodialysis-associated hemosiderosis in the era of erythropoiesis-stimulating agents: a MRI study. Am. J. Med. 2012;125(10):991–999. doi: 10.1016/j.amjmed.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Barton J.C., Edwards C.Q., Phatak P.D., Britton R.S., Bacon B.R. Cambridge University Press; 2010. Handbook of iron overload disorders. [Google Scholar]
- 12.Brissot P., Troadec M.B., Bardou-Jacquet E. Current approach to hemochromatosis. Blood Rev. 2008;22(4):195–210. doi: 10.1016/j.blre.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Angelucci E., Barosi G., Camaschella C. Italian Society of Hematology practice guidelines for the management of iron overload in thalassemia major and related disorders. Haematologica. 2008;93(5):741–752. doi: 10.3324/haematol.12413. [DOI] [PubMed] [Google Scholar]
- 14.Gandon Y., Olivié D., Guyader D. Non-invasive assessment of hepatic iron stores by MRI. Lancet. 2004;363(9406):357–362. doi: 10.1016/S0140-6736(04)15436-6. [DOI] [PubMed] [Google Scholar]
- 15.St Pierre T.G., Clark P.R., Chua-Anusorn W. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105(2):855–861. doi: 10.1182/blood-2004-01-0177. [DOI] [PubMed] [Google Scholar]
- 16.Wood J.C., Enriquez C., Ghugre N. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106(4):1460–1465. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macdougall I.C., Bircher A.J., Eckardt K.U., Obrador G.T., Pollock C.A., Stenvinkel P., Swinkels D.W., Wanner C., Weiss G., Chertow G.M. Conference participants. Iron management in chronic kidney disease: conclusions from a « Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2016;89(1):28–39. doi: 10.1016/j.kint.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Charytan D.M., Pai A.B., Chan C.T. Considerations and challenges in defining optimal iron utilization in hemodialysis. J. Am. Soc. Nephrol. 2015 Jun;26(6):1238–1247. doi: 10.1681/ASN.2014090922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheuer P.J., Williams R., Muir A.R. Hepatic pathology in relatives of patients with haemochromatosis. J. Pathol. Bacteriol. 1962;84:53–64. [PubMed] [Google Scholar]
- 20.Deugnier Y., Turlin B. Pathology of hepatic iron overload. Semin. Liver Dis. 2011;31:260–271. doi: 10.1055/s-0031-1286057. [DOI] [PubMed] [Google Scholar]
- 21.Sheskin D.J. fourth ed. USA: Chapman and Hall, Taylor and Francis Group; Boca Raton: 2007. Handbook of parametric and nonparametric statistical procedures. [Google Scholar]
- 22.Rostoker G., Griuncelli M., Loridon C. Maximal standard dose of parenteral iron for hemodialysis patients: an MRI-Based decision tree learning analysis. Plos One. 2014;9(12) doi: 10.1371/journal.pone.0115096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rostoker G., Griuncelli M., Loridon C. Reassessment of iron biomarkers for prediction of dialysis iron overload: an MRI study. Plos One. 2015;10(7) doi: 10.1371/journal.pone.0132006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meguro R., Asano Y., Odagiri S. Nonheme-iron histochemistry for light and electron microscopy: a historical, theoretical and technical review. Arch. Histol. Cytol. 2007;70(1):1–19. doi: 10.1679/aohc.70.1. [DOI] [PubMed] [Google Scholar]
- 25.Barton J.C., Edwards C.Q., Bertoli L.F. Iron overload in African Americans. Am. J. Med. 1995;99(6):616–623. doi: 10.1016/s0002-9343(99)80248-4. [DOI] [PubMed] [Google Scholar]
- 26.Deugnier Y.M., Turlin B., Powell L.W. Differentiation between heterozygotes and homozygotes in genetic hemochromatosis by means of a histological hepatic iron index: a study of 192 cases. Hepatology. 1993;17(1):30–34. [PubMed] [Google Scholar]
- 27.Deugnier Y.M., Guyader D., Crantock L. Primary liver cancer in genetic hemochromatosis: a clinical, pathological, and pathogenetic study of 54 cases. Gastroenterology. 1993;104(1):228–234. doi: 10.1016/0016-5085(93)90856-8. [DOI] [PubMed] [Google Scholar]
- 28.Turlin B., Deugnier Y. Histological assessment of liver siderosis. J. Clin. Pathol. 1997;50(11):971. doi: 10.1136/jcp.50.11.971-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aldouri M.A., Wonke B., Hoffbrand A.V. Iron state and hepatic disease in patients with thalassemia major: treated with long term subcutaneous desferrioxamine. J. Clin. Pathol. 1987;40(11):1353–1359. doi: 10.1136/jcp.40.11.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karam L.B., Disco D., Jackson S.M. Liver biopsy results in patients with sickle cell disease on chronic transfusions: poor correlation with ferritin levels. Pediatr. Blood Cancer. 2008;50(1):62–65. doi: 10.1002/pbc.21215. [DOI] [PubMed] [Google Scholar]
- 31.Costa Matos L., Batista P., Monteiro N. Iron stores assessment in alcoholic liver disease. Scand. J. Gastroenterol. 2013;48(6):712–718. doi: 10.3109/00365521.2013.781217. [DOI] [PubMed] [Google Scholar]
- 32.Lam M., Torbenson M., Yeh M.M. HFE mutations in alpha-1-antitrypsin deficiency: an examination of cirrhotic explants. Mod. Pathol. 2010;23(5):637–643. doi: 10.1038/modpathol.2010.42. [DOI] [PubMed] [Google Scholar]
- 33.Barton J.C., Bertoli L.F., Alford T.J. Hepatic iron in African Americans who underwent liver biopsy. Am. J. Med. Sci. 2015;349(1):50–55. doi: 10.1097/MAJ.0000000000000389. [DOI] [PMC free article] [PubMed] [Google Scholar]