Abstract
Objective(s):
Nowadays, cardiovascular diseases (CVDs) are the major risk factors of death globally. One of the most undeniable reasons of CVDs is metabolic syndrome (MetS). MetS is defined as a complex of diseases including insulin resistance, hyperglycemia, obesity, high blood pressure and dyslipidemia. The use of complementary medicine such as traditional herbal species can be effective in treatment of MetS’s complications. Cinnamomum verum (family Lauraceae) is a medicinal global plant which has been used daily by people all over the world. Positive effects of cinnamon in reducing blood pressure, plasma glucose, obesity and ameliorating dyslipidemia which represented in traditional medicine introduced it as probable decreasing MetS’s complications agent. The aim of this review was to investigate the mechanisms of C. verum in reducing the MetS’s complications and CVDs risk factors.
Materials and Methods:
Various databases such as PubMed, Science Direct, Scopus, Web of Science, Google Scholar and Persian Websites such as www.sid.ir with keywords search of cinnamon, cinnamomum, cinnamaldehyde, atherogenic, hypertension, hyperglycemia, insulin resistance, obesity and dyslipidemia have been included in this search.
Results:
Clinical data and mechanisms of action of C. verum and its active ingredients that have been shown in this review indicated that cinnamon has protective effects against MetS’s aspects in various ways.
Conclusion:
The use of this plant can be effective in reducing MetS’s complications and its morbidity and mortality.
Keywords: Cardiovascular disease, Cinnamon, Insulin resistance, Mechanism, Metabolic syndrome, Obesity
Introduction
Metabolic syndrome (MetS) is a condition includes insulin resistance and hyperglycemia, central obesity, high blood pressure, dyslipidemia, lower levels of high density lipoprotein cholesterol (HDL-c) and hypertriglyceridemia (1). Numerous definitions of MetS are exist such as definition based on World Health Organization (WHO) and National Cholesterol Education Program’s Adult Treatment Panel III (NCEP: ATP III) criteria. With WHO criteria each person with type 2 diabetes, glucose/insulin metabolism disturbance or insulin resistance, who are involved with 2 of the following 4 items: [1] hypertension, [2] dyslipidemia, [3] obesity and [4] microalbuminuria, considered to have MetS (Table 1) (2).
Table 1.
Metabolic syndrome: WHO criteria (2)
| BMI > 30 or waist-hip ratio > 0.90 (men) or > 0.85 (women) dyslipidemia: TG >150 mg/dL, HDL-c < 35 (men), or < 39 (women) blood pressure: > 160/90 mm Hg, with or without medication microalbuminuria: AER 20–200 ug/min |
People with at least 2 of above risk factors with type 2 diabetes, impaired glucose tolerance, or insulin resistance are considered to have metabolic syndrome
AER: average albumin excretion rate, BMI: body mass index, HDL-c: high density lipoprotein cholesterol, TG: triglyceride
Body mass index (BMI) and waist circumference (WC) are routine predictive markers of MetS but nowadays waist hip ratio (WHR) and waist height ratio (WHtR) are better separator of MetS risk factors than BMI and WC. Racial variations in predictive markers suggest that the power of each obesity index differ by ethnic group. For example among Korean adults WHR is better predictor of multiple metabolic risk factors and among adult Iranian population WC is superior to BMI and WHR (3). Based on ATPIII criteria WC cut-off value is 102 cm for men and 88 cm for women. Based on WHO criteria WHR cut-off value is 9.0 in men and 8.5 in women as decisive benchmarks for MetS (4).
Based on National Health And Nutrition Examination Survey [NHANES III], 44% of American with at least 70 years old was suffered from MetS and its prevalence rises with increasing age and BMI, which abdominal obesity (53%), hypertension (40%), and hyperglycemia (39%) were the most frequently occurring risk factors for MetS (1, 5).
Nowadays medicinal plants are considered as preventive and curative agents for their properties such as safety, popularity, easy to earn and less side effects (6). Many studies have been shown that herbal drugs are useful in treatment of MetS (7-12).
Cinnamon
Cinnamomum verum (formerly C. zeylanicum) is a medicinal plant belongs to Lauraceae, which is generally called “true cinnamon tree” or “Ceylon cinnamon tree”. C. verum is a small tropical tree that originated in Sri Lanka, East and Middle Asia (13, 14).
Phamacognostical Characteristics
The C. verum tree grows to around 10 m and leaves are leathery, usually opposite, that are lanceolate to ovate, 11 to 16 cm long, with sharp tips. The pallid yellow flowers, which are tubular with 6 lobes, grow in panicles that are as long as the leaves. The fruit is tiny, 1 to 1.5 cm long, and black when ripens (Figure 1) (13).
Figure 1.

Cinnamomum verum
Chemical Composition
Different parts of cinnamon such as leaves, bark, root bark and fruits have various amount of resinous compounds (Table 2). Cinnamaldehyde, cinnamate and cinnamic acid are the main resinous ingredients found in cinnamon that increase in quantity when cinnamon ages (Figure 2). Cinnamaldehyde is responsible for its spicy taste and fragrance. Essential oils, such as trans-cinnamaldehyde, cinnamyl acetate and eugenol are found in cinnamon (15, 16).
Table 2.
Chemical contents of different parts of cinnamon (17)
| Parts of cinnamon | Dominant ingredient (s) |
|---|---|
| Leaves | Eugenol: 70.00 to 95.00% |
| Bark | Cinnamaldehyde: 65.00 to 80.00% |
| Root bark | Camphor: 60.00% |
| Fruit | trans-cinnamyl acetate 42.00 to 54.00% |
| Buds | Terpene hydrocarbons: 78.00% alpha-Bergamotene: 27.38% |
| Flowers | (E)-cinnamyl acetate: 41.98% trans-alpha-bergamotene: 7.97% |
Figure 2.
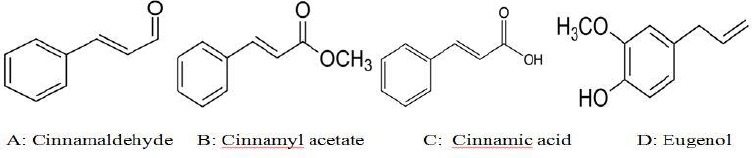
Chemical structure of main ingredients of cinnamon
Therapeutic Uses and Pharmacological Charactristics
Cinnamon is a very popular culinary spice and is also used in candy, incense, toothpaste and perfumes. Its oil is used in medicine as a carminative, antiseptic and astringent. In traditional medicine cinnamon has been used as antitussive, antiarthritis, antimicrobial, antifungal, anti-oxidant, anti-inflammatory agent and used in treatment of sore pain and dental problems. According to the recent studies, cinnamon may prevent or delay diabetes, colon cancer and bleeding time. Recent investigation in United States National Library of Medicine showed that cinnamon is effective in treatment of diarrhea, emesis, muscle cramps, infections, molds, flu and erectile dysfunction. Also cinnamon has been effective against multiple sclerosis, Alzheimer and human immune deficiency (HIV) infection (17-20).
Purposes
The purpose of this study was to evaluate the aspects of MetS and its complications and the effects of cinnamon on prevention and treatment of it in patients. This has been tried to investigate and show the mechanisms involved and the results of cinnamon uses in this field.
Methodology
Various databases such as PubMed, Science Direct, Scopus, Web of Science, Google Scholar and Persian Websites such as www.sid.ir with keywords search of cinnamon, cinnamomum, cinnamaldehyde, athero-genic, hypertension, hyperglycemia, insulin resistance, dyslipidemia, etc have been involved in this research. In this review, selected articles were indicated the effects of cinnamon on MetS conditions in a 20 years period of 1995 to 2015. Attempts have been done to make a comparison of related mechanisms and most important of them was highlighted.
Metabolic syndrome and cardiovascular risk
One of the most undeniable reasons of cardiovascular diseases (CVD) is MetS (21). Individuals with MetS were at increased risk for long-term CV outcomes (22). The MetS defined according to the ATPIII criteria was associated with a 2-fold increase in risk of CVD, CV mortality, myocardial infarction and stroke, and a 1.5-fold increase in risk of all-cause mortality. CVDs are the number 1 cause of death globally. It was estimated that 17.5 million people died from CVDs in 2012, representing 31% of all global deaths (23). In Over a 7-year period study, CV mortality was 12% in those with MetS and 2.2% in those without it (22).
Important liable mechanisms for MetS related to CVDs are summarized in Table 3.
Table 3.
Important liable mechanisms for metabolic syndrome related to cardiovascular diseases
| Hypertension | Dyslipidemia | Proinflammatory cytokines | Insulin resistance | Microalbuminuria |
|---|---|---|---|---|
| Insulin resistance stimulates sympathetic nervous system | Enhancement of lipolysis in adipocytes | Elevation of plasma concentrations of IL-6, TNF-α, C-reactive protein, and resistin | Overabundance of circulating fatty acids by lipolysing of triacylglycerol by insulin | Glomerular hyperfiltration |
| Insulin resistance mediates hyperadrenergic state | Insulin drives lipogenesis in the liver | Reduction of anti-inflammatory adipokines such as adiponectin | Inhibition of antilipolytic effect of insulin by circulating fatty acids | Over-production of ROS |
| Insulin resistance stimulates renal sodium absorption | Increase CETP activity and lipolysis of HDL-c | Impairment activation of protein kinase Ce-_ and protein kinase C-by Fatty acids | Insulin resistance, inflammation and altered renal hemodynamics | |
| Insulin can cause upregulation of angiotensin II type I receptors | Enhancement of triglyceride synthesis in the liver | Defect in insulin stimulated IRS-1 and IRS-2 tyrosine phosphorylation | ||
| Low levels of plasma natriuretic peptides | Activation of protein kinase Ce-_ and c-Jun N-terminal kinase-1.41 | |||
| Insulin resistance increases endothelin 1impairment of NO-mediated vasodilation | Fatty acids increase hepatic glucose production and diminish inhibition of glucose production by insulin | |||
| Hyperuricemia | Defect in mitochondrial oxidative phosphorylation | |||
| Production of endogenous digoxin-like factor | Deficient in the endoplasmic reticulum X-box binding protein-1, hyperactivation of c-Jun N-terminal kinase-1 increases serine phosphorylation of IRS-1 |
NO: nitric oxide, CETP: cholesteryl ester transfer protein, HDL-c: high density lipoprotein cholesterol, IL-6: interlukin 6, TNF-α: tumor necrosis factor α, ROS: reactive oxygen species, IRS: insulin receptor substrate
Cinnamon and metabolic syndrome
Based on traditional medicine and recent scientific based evidence cinnamon and its active ingredients such as cinnamaldehyde, cinnamate, cinnamic acid and eugenol in the forms of aqueous and alcoholic extracts have a variety of therapeutic effects. Different aspects of MetS including high blood glucose, dyslipidemia, obesity and high blood pressure are ameliorated with cinnamon extracts. Investigations about cinnamon showed that this plant is a cardiovascular protective agent and has a potential effect in reducing MetS complications due to its anti-diabetic, anti-oxidant, anti-inflammatory and beneficial effects in lipid profile (24-26) (Figure 3 and Table 4).
Figure 3.
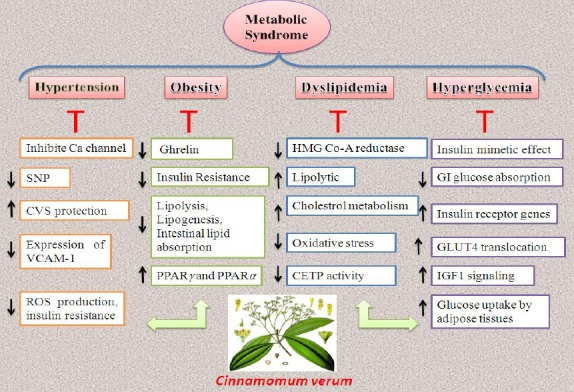
Schematic description for protective mechanisms of Cinnamomum verum in ameliorating metabolic syndrome complications
Table 4.
Most important mechanisms of cinnamon in reducing metabolic syndrome complications
| Effect | Mechanisms | Reference |
|---|---|---|
| Anti-diabetic | Insulin mimetic effect Activating glycogen synthase, inhibiting glycogen synthase kinase 3β Reducing glucose absorption in the small intestine through increasing in glucosidase enzymes and inhibition of intestinal ATPase Up-regulating the expression of insulin receptor genes Activating the PPAR γ and AMP kinase Activating IGF1 signaling in fibroblasts Modulating mitochondrial physiology and elevation of cellular metabolism Modulating the insulin and IGF signaling pathways such as mTOR, Cyclic-AMP signaling and autophagy Inhibition of Alph-amylase Up-regulation of GLUT4 translocation in muscle and adipose tissues Phosphorylation of AMPK and acetyl-CoA carboxylase and inhibition of AMPK intended to reduction in glucose uptake by adipose tissues Anti-AGEs formation |
(28) (29) (30) (37) (40) (42) (43) (43) (45) (46) (46) (47) |
| Anti-oxidant | Increasing conjugate dienes and glutathione Free radical scavenging activity Increasing SOD, GPX, and CAT Decreasing lipoperoxidation Inhibiting expression of inducible nitric oxide |
(55,57) (58) (62) (63) (67) |
| Antihypertensive | Reducing in free radicals production and insulin resistance Reducing sympathetic nerve traffic Activation of the chemosensory cation channel Release of calcitonin gene-related peptide Restoration of normal Ca2+ influx and vasorelaxation Reducing plasma uric acid Cardiovascular protective effects Blocking of thromboxane A2 receptors inhibition of platelet aggregation, alongside [3H] inositol monophosphate formation, thromboxane B2 content and Intracellular Ca2+ prostaglandin increasing in PE1 formation Activating hemeoxygenase enzyme Reducing the expression of VCAM-1 |
(58) (72) (75) (77) (80) (81) (83) (84) (85) (86) (87) |
| Anti-inflammatory | Inhibition of NF-κB activity Reducing the activation of Src/spleen-tyrosine kinase Inhibition of TNF-α gene, IL-2, IL-4, and IFNγ Induction of sirtuin expression Decrease IL1β concentration Inhibition of 5-LPO, COX2 and iNOS enzymes |
(93) (94) (95) (97) (98) (99) |
| Antihyperlipidemic | Inhibiting hepatic HMG Co-A reductase enzyme, lipolytic activity and increasing hepatic anti-oxidant enzymes activity Inhibition of activity against copper-mediated LDL-c oxidation and potent CETP inhibitory activity |
(110) (111) |
| Antiobesity | Agonist of TRPA1 and reduction in ghrelin secretion Activation of both PPARγ and PPARα receptors Reduction in insulin resistance Inhibition of lipolysis Inhibition of lipogenesis and fatty acid oxidation Inhibition of intestinal lipid absorption Antagonist at cannabinoid receptors |
(37) (114) (1,104) (115) (116) (117) (118) |
Insulinotropic and anti-diabetic effects
As mentioned in previous section in Table 3, the main mechanism of MetS complications is insulin resistance. Insulin resistance is a fundamental key role for other MetS complications including obesity, dyslipidemia and hypertension that summarized in Table 3 (1).
Surely the main characteristic of cinnamon is an insulin mimetic effect (27, 28). The evaluation of beneficial effects of cinnamon on treatment of Type 2 diabetes and insulin resistance began almost 20 years ago. In this time, Khan et al extracted an unidentified component from cinnamon and named it as insulin potentiating factor (27). Cinnamon extracts can activate the insulin-receptor-kinases and inhibit insulin-receptor-phosphatases as enhancer of insulin-receptor function and inhibitor of the enzyme that blocks insulin-receptor attachment, respectively. This state causes phosphorylation of the insulin receptors and enhancing its effects (28). The major active components in cinnamon for its anti-diabetic effects are in aqueous extract as water soluble doubly-linked procyanidin type – A polymers. They were able to upregulate glucose uptake, increase glycogen synthesis by activating glycogen synthase and inhibiting glycogen synthase kinase 3β (29), and reducing glucose absorption in the small intestine through increasing in glucosidase enzymes and inhibition of intestinal ATPase (30, 31).
In in vitro study performed by Anderson et al in 3T3-L1 adipocytes, methylhydroxychalcone polymer (MHCP), the bioactive compound isolated from cinnamon, stimulated the autophosphorylation of the insulin receptor and all pathways listed above were increased by MCHP (32). In vivo and in vitro studies performed by Qin et al showed that aqueous cinnamon extract at dose of 30 and 300 mg/kg body weight of rats for 3 weeks potentiate the insulin effect through up-regulation of the glucose uptake in adipocytes and induced glucose utilization, increased insulin-receptor substrate (IRS)-1 tyrosine phospho-rylation levels, skeletal-muscle insulin receptors stimulation and IRS-1 association with phospha-tidylinositol (PI) 3-kinase (33). Other polyphenolic compounds of cinnamon like rutin, catechin, quercetin and kaempferol have insulin like activity (34). The polyphenolic content of cinnamom extract is important to induce anti-diabetic activity. The aqueous extract of C. zeynalicum containing 45 and 75% gallic acid equivalents (GAE) of polyphenol content was higher officious in lowering blood glucose activity than extract containing 15% GAE in streptozotocin-induced diabetic rats (35). The anti-diabetic effects of cinnamon have been shown in many studies (36-39).
In comparison between cinnamic acid and cinnamaldehyde, Hafizure et al showed that cinnamic acid was able to improve glucose tolerance at dose of 10 mg/kg in rats comparable to glibenclamide with dose of 5 mg/kg, but in vitro study showed that glucose-stimulated insulin secretion with cinnamic acid was very higher than cinnamaldehyde (36). Cinnamaldehyde was able to up-regulate expression of insulin receptor genes (37). Cinnamaldehyde increases the expression levels of peroxisome proliferator-activated receptor γ (PPARγ) and activates AMP kinase that induces insulin sensitivity (40, 41). Cinnamon can activate insulin-like growth factor 1 (IGF1) signaling in fibroblast, that tend to lowering insulin resistance and improvement in glycemic control but it can down-regulate insulin signaling in adipocytes (42). Modulating mitochondrial physiology and elevation of cellular metabolism are another anti-diabetic mechanism of cinnamon (43). Cinnamon by modulating the insulin and IGF1 signaling pathways such as mTOR, Cyclic-AMP signaling and autophagy can exhibit anti-diabetic action (44). Alph-amylase inhibition of cinnamon is another anti-diabetic mechanism of it. Hydro-alcoholic extract of cinnamon can reduce activity of pancreatic α-amilase activity in both in vivo and in vitro study performed by Beejmohon et al. This effect was occured without stimulating of insulin secretion (45). Up-regulation of glucose transporter 4 (GLUT4) and translocation in muscle and adipose tissues by cinnamon extract was proved by Shen et al. In this study cinnamon stimulated the phosphorylation of AMPK and acetyl-CoA carboxylase. Inhibition of AMPK intended to reduction in glucose uptake by adipose tissues and cinnamon is an AMPK activatore (46). Flavonoid and phenolic compounds such as epicatechin, catechin, and procyanidin B2 isolated from cinnamon. These ingredients have antioxidant activity and entrapping properties of reactive carbonyl species, such as methylglyoxal (MGO), an intermediate reactive carbonyl of advanced glycated end products (AGEs) formation (a marker responsible for diabetic complications) can reduce the risk of progression of diabetes (47).
Above mechanisms were confirmed in Ranasinghe’s study. They evaluated the effect of cinnamon on glucose metabolism in a meta-analysis of 16 in vitro and in vivo studies (48). A study consist of 109 patients with type 2 diabetes was done in a period of 3 month. 1 g of cinnamon was fed to treated group. After this period hemoglobin A1c (HbA1c) was lower significantly in treated group (49).
Many human and cellular studies showed anti-diabetic, insulinotropic and insulin resistance lowering effect of cinnamon (50, 51). Insulin resistance is important basis of MetS and fundamental aspects of growing its complications. Reducing insulin resistance is the most important mechanism of ameliorating MetS’s complications by cinnamon.
Anti-oxidant activity
Free radicals and oxidative stress are main causes that involved in multiple conditions like MetS. Normal physiological conditions of body systems require balance between oxidant/anti-oxidant mechanisms. Production of reactive oxygen species (ROS) may have a pivotal role in pathogenesis of many disorders such as diabetes, hypertension and CVDs that are main complications of MetS (52, 53). Anti-oxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH), as a none-enzymatic anti-oxidant have a significant role in contrasting ROS and oxidative stress condition. Anti-oxidant enzyme and non-enzymatic anti-oxidant molecules activity in many inflamed conditions were reduced (54). Thus, diminishing these states with anti-oxidant enhancing systems is useful to decrease in MetS complications.
Dietry anti-oxidant such as polyphenols, vitamins A, B, C, and E neutralize the oxidation process by binding to free radicals, chelating catalytic metals and acting as oxygen scavengers (55, 56).
C. zeylanicum has 65.3% anti-oxidant activity and also a very strong free radical scavenging activity and have been shown for many extract such as alcoholic, aqueous and etheric of many parts of plant (57, 58). A recent study compared the anti-oxidant potentiality of several plants, including cinnamon, spinach, chard, Jerusalem artichoke, and red cabbage. Boga et al found that extracts of cinnamon had the most potent anti-oxidant effects (59). Phenolic compounds are found in almost all parts of the plants that responsive for anti-oxidant activity of cinnamon and a potent scavenger of hydrogen peroxide, nitric oxide, and lipid peroxide free radicals (60). Cinnamon’s essential oil and its component eugenol both show anti-oxidant activity (61). Use of 75 mg/kg of C. zeylanicum for 4 weeks in rats, as an anti-oxidant in food increased SOD, GPX, and CAT that leads to the elimination of ROS as well as decreasing lipoperoxidation (LPO) level and the apoptotic index (62). In addition cinnamon has anti-LPO in vegetable oil that inhibited malondialdehyde (MDA, as a marker of LPO) production at 500, 1000 and 2000 ppm concentration of cinnamon (63). The essential oils and some of the major compounds present in cinnamon, including (E)-cinnamaldehyde, eugenol, and linalool, were investigated in reference to peroxynitrite induced nitration and LPO. Different flavonoids isolated from cinnamon have free-radical-scavenging activities and anti-oxidant properties (64).
Cinnamon oil exhibited SOD like activity that evaluated by measuring the inhibition of pyrogallol autoxidation that is catalyzed by the superoxide radical (65). Cardiac and hepatic anti-oxidant enzymes, lipid conjugate dienes, and glutathione (GSH, as non-enzymatic anti-oxidant protein) of rats increased after 90 days of administration of the bark powder of C. verum (10%) (57).
In vitro and in vivo anti-oxidant studies of C. tamala showed that cinnamaldehyde as major constituent of plant can decrease MDA level and increased GSH that be reduced (66). Cinnamaldehyde has potential activity against the production of nitric oxide and it can inhibit the expression of inducible nitric oxide as an enzyme that has a role in oxidative stress (67).
In comparison between the anti-oxidant activities of cinnamon extracts, Roussel et al showed that etheric (0.69 mg), methanolic (0.88 mg) and aqueous (0.44 mg) extracts, inhibited the oxidative process in 68%; 95.5% and 87.5% respectively. The butylated hydroxytoluene (BHT) control inhibited 80% oxidation (68). In Pandey study, ethanolic and aqueous extract of C. verum showed almost equal capacity to inhibit free radicals in 2,2-Diphenyl-1-picrylhydrazyl test (DPPH) with IC50=13.53 µg/ml and 13.3, respectively. In this study scavenging superoxide radical by ethanolic extract was found to be more potent (IC50=119.7 µg/ml) than aqueous extract and ascorbic acid with IC50=197.8 µg/ml and 237.1, respectively (69). The uses of the aqueous extract of cinnamon in duration of 6 weeks in patients with impaired fasting blood glucose can decrease MDA level and increased plasma thiol content (70).
About anti-oxidant activity of cinnamon 178 articles were found in period of year 1995 to 2015. In all of them that consisting of in vitro, in vivo and food industry studies, cinnamon showed anti-oxidant activity as increasing in anti-oxidant enzyme activity such as SOD, CAT and GOX, decreasing in MDA, LPO, ROS production and total oxidant index value. These mechanisms can decrease oxidative stress and finally secondary effects of it to all parts of the body.
Effects on cardiovascular diseases and antihyper-tensive activity
Resistance to the metabolic actions of insulin caused by proinflammatory and pro-oxidative milieu that created by visceral obesity could diminish endothelium functions and induction of vascular rarefaction, reductions in vascular relaxation, and impaired vascular remodeling (71). As mentioned befors insulin resistance is main demonstration of MetS and has system-wide implications for other tissue such as the kidney that affects blood pressure regulation. In addition, the additional autocrine and paracrine activities of adipose tissue, another feature of MetS, contribute to inappropriate activation of the renin-angiotensin-aldosterone system and the sympathetic nervous system that promote kidney microvascular remodeling, stiffness, and sodium (Na+) retention that in turn promote hypertension (72). Thus, insulin resistance and obesity are two basic causes of hypertension and must be considered in treatment of hypertension.
Short term use of cinnamon can significantly reduce blood pressure especially among those who are prediabetic or type 2 diabetic. In a meta-analyzed performed in 2012 the results of the three clinical trials studies were shown that average drop in systolic blood pressure was 5.39 mmHg, while average drop in diastolic blood was 2.6 mmHg (73). Treatment of 59 subjects who had type-2 diabetes with 1,200 mg of cinnamon per day was shown that systolic blood pressure reduced by 3.4 mmHg on average after twelve weeks (74). Vasorelaxation and decrease in blood pressure are important actions on cardiovascular system by cinnamon. The activation of the chemosensory cation channel (TRPA1) and L-type currents were more potent in ventricular cardiomyocytes (VCM) than in vascular smooth muscle cells (VSMC). This effect may contribute to its vasorelaxing action (75). These effects were confirmed by Nyadjeu et al (76). Ankyrin (A) transient receptor potential channel has unitary conductance and slight selectivity for Ca2+ versus Na+. In peripheral arteries TRPA1 agonist such as cinnamon can stimulate these channels and then release of calcitonin gene-related peptide was occurred and vasodilation was seen. Another mechanism was seen in cerebral circulation that TRPA1 in endothelium beds induce hyperpolarization and then vasodilation (77). Renin–angiotensin–aldosterone system and increased free radical formation are two main consequences of insulin resistance state. These phenomena in CVS lead to development of endothelial dysfunction and hypertension (73).
It is a close association between glycemic indicators (fasting plasma glucose or HbA1c) and systolic and diastolic blood pressure levels (24, 74). Also, decrease in level of MDA by cinnamon linked with blood pressure regulation (68). The reduction of blood pressure by 12.5%, 26.6% and 30.6% in rats at the doses of 5, 10 and 20 mg/kg of C. zeylanicum methanolic extract, respectively was seen in Nvadjeu’s study. In this study increase in NO tissue’s concentration was the main mechanism of antihypertensive effects of extract (78).
The effect of cinnamon on blood pressure was greater with higher baseline systolic blood pressure. Cinnamon is independently associated with blood pressure levels than sodium intake (74). Cinnamon with dose of 50 mg/kg for 2 weeks was given to dogs. Among this period systolic blood pressure and heart rate in treated dogs was lower significantly than normal group. QT and PR interval was longer in treated group. R wave amplitude in treated group was shorter and may contribute to lowest blood pressure in this group. In this study decrease in blood pressure was hypothesized on decrease in vascular resistance and decrease in cardiac stroke volume (79).
The normalization of vascular contractility via restoration of normal Ca2+ influx parallel to its insulinotropic effect are main mechanisms to prevent development of hypertension in patients with insulin deficiency and insulin resistance (80).
Hyperuricemia is one of the causes of an increased risk for incident hypertension. 450 mg/kg or higher doses of C. cassia oil reversed serum and hepatic levels of uric acid to the same level as normal in control mice by inhibiting those liver enzymes responsible for converting purines to uric acid (81, 82). Insulin resistance in MetS condition induces directly and indirectly increasing in markers of adrenergic state such as resting tachycardia, increased sympathetic nerve traffic and high plasma norepinephrine values (72). Thus, the effects of cinnamon on any of the above issues resulted in hypotensive effects of it. The insulinotropic effect of cinnamon was discussed already.
The CVS protective effects of cinnamon were shown in many studies (18, 83). The antiathrosclerotic effects and preventive vascular diseases of cinnamon resulted from inhibition of vascular smooth muscle cell proliferation through blockade of thromboxane A2 (TXA2) receptors mediated proliferation by cinnamon (84). Cinnamophilin, a lignin isolated from C. philippinense, inhibited in vitro human platelet aggregation, alongside [3H] inositol monophosphate formation, thromboxane B2 (TXB2) content and intracellular Ca2+ was decreased and prostaglandin E1 (PE1) formation was increased. Cinnamophilin was a potent TX inhibitor. These results were shown in rats and guinea pigs (85).
The induction of hemeoxygenase (HO) by 2-methoxycinnamaldehyde (2-MCA), a component isolated from C. cassia, amended the ischemia/reperfusion (I/R) injury. 2-MCA also can decrease the expression of vascular cell adhesion molecule-1 (VCAM-1) in TNF-activated endothelial cells. This effect was shown in rats precisely (86, 87).
Effects of 200 mg/kg cinnamon bark extract on myocardial hemodynamic parameters in rats was investigated by Badalzadeh et al. In this study, hemodynamic parameters such as left ventricular systolic and diastolic pressures, ventricular contraction and relaxation, left ventricular developed pressure, work index of the heart and coronary flow were measured during the 8 weeks period of training. In this study, exhausted animals were compared in these parameters with animals receiving cinnamon. The results showed that treated group had better consequences with enhanced cardiac force and contractility, positive inotropic effect, improved heart performance, increased coronary flow, better myocardial contractility and cardiac work (88).
Anti-inflammatory activities
Inflammation and rise in proinflammatory cytokines are common features of the MetS. Adipocytes and macrophages within fat secrete numerous hormones and cytokines that may contribute to the characteristic pathophysiological changes seen in the MetS, and local inflammation within adipose tissue may be the sentinel event that causes systemic insulin resistance and systemic inflammation, two of the cardinal features of the MetS (24). Circulating cytokines have similar metabolic effects on muscle, liver, and endothelium. Adipose tissue-derived cytokines, such as interlukin 6 (IL-6) and leptin induce endothelial cell activation and inflammation that causes atherosclerosis in the vascular beds and tend to mortality (89).
The anti-inflammatory activities of cinnamon and its essential oils indicated in many studies (90, 91). The inhibition of nuclear factor kappa B (NF-κ) was seen by Lee et al by 2’-hydroxycinnamaldehyde isolated from C. cassia bark that tended to inhibition of production of nitric oxide (92). Also NF-κ suppression by cinnamaldehyde was seen in Muhammad et al study. In this in vitro study on AGS/MKN-45 cells, cinnamaldehyde inhibited production of IL-8 secretion/expression from Helicobacter pylori-infected cells and degradation of I-κB was reduced by use of cinnamaldehyde (93). Reducing the activation of Src/spleen-tyrosinekinase- (Src/Syk-) as an inflammatory signaling cascades, is another anti-inflammatory mechanism of cinnamon with its ethanolic extract (94). Tumor necrosis factor-α (TNFα) levels were decreased with aqueous extract of cinnamon in in vivo and in vitro model of lipopolysaccharide-induced TNFα rising. In vitro inhibition of TNF-α gene by cinnamon water extract was seen in Lee’s study via the modulation of JNK, p38, and ERK1/2 activation and IκBα degradation (95). In another study performed by Hong et al cinnamon water extract (CWE) inhibited expression of TNF-α in in vitro and in vivo model. In this study degradation of IκBα and MAP kinase phosphorylation induced by lipopolysaccharide in macrophages was strongly inhibited by the polyphenol-rich CWE fraction. The results of this process tended to inhibition of TNF-α and IL-6 production. This strong anti-inflammatory effect of CWE was related to high polyphenolic content of extract. Procyanidins, catechin, epicatechin and ellagic acid are the main ingredients of CE with anti-inflammatory effect (96). Also, CWE prevented anti-CD3-stimulated T cells from secreting IFN-γ (95). Cinnamon polyphenols induced the expression of the pro-apoptotic protein, Bax and suppressed the expression of the anti-apoptotic protein, Bcl-xl, in OGD treated cells. Cinnamon polyphenols reduced OGD-induced inflammatory factors including TNF-α and phospho-NF-κB p65 and also induced sirtuin1 expression as a negative regulator of NF-κB activity through the deacetylation of the p65 lysine 310 (97). Interleukin-1 beta (IL-1β) suppressing production was showed with Chao et al. In this study eugenol was the main ingredient of cinnamon to do this (98). Eugenol could also inhibit 5-lipoxygenase enzyme in polymorphonuclear leukocytes and it can inhibit inducible nitric oxide synthesis (iNOS), cyclooxygenase-2 (COX-2), and nitric oxide (NO) production (99). Inhibition of NO production by E-cinnamaldehyde and o-methoxycinnamaldehyde with IC50 values with RAW 264.7 cells of 55±9 μM and 35±9 μM, respectively; was shown in another study performed by Gunawardena et al (100). Cinnamon extract with doses of 50, 100, and 200 mg/kg was used for evaluation of anti-inflammatory effects on rats. All aspects of models of inflammation such as paw volume, weight loss, and paw edema and cotton pellet-induced granuloma were ameliorated and significant reduction in elevated serum TNF-α concentration was seen. Also cinnamon inhibited cytokines (IL-2, IL-4, and IFNγ) release from concanavalin-stimulated lymphocytes in in vitro (101). Inhibition of angiogenesis by cinnamon extract through blocking of vascular endothelial growth factor 2 (VEGF2) signaling and diminishing of endothelial cell proliferation, migration and tube formation which seen in in vitro study are the other main anti-inflammatory mechanisms of cinnamon (102).
Effects on dyslipidemia
Insulin resistance and obesity are two factors that each one can stimulates another one in the progression of MetS. Obesity and increased adipocyte mass are accompanied by angiotensinogen, TNF-α, leptin, resistin, and plasminogen activator inhibitor 1 (PAI-1) elevation in plasma. Plasma concentration of adiponectin is actually decreased in obesity, as well as in the type 2 diabetes mellitus. TNF-α and resistin are correlated with insulin-resistant state (103, 104). The role of insulin resistance in dyslipidemia was mentioned in Table 3.
Cholesterol- and lipid-lowering effects of cinnamon were shown in many studies (105, 106). In Khan et al study, cinnamon with doses of 1, 3, and 6 g per day caused a reduction triglyceride (TG), total cholesterol, and LDL-c cholesterol levels in humans (107). In streptozotocin-induced diabetic rats fed with 5% cinnamon for 8 weeks HDL-c significantly increased, while cholesterol, LDL-c and TG were significantly decreased. Also increase in adiponectin and decrease in leptin were seen (108). The lipid lowering effect of cinnamon was evaluated by Javed et al. In this study C. zeylanicum bark powder at doses of 0.50 g/kg, 0.75 g/kg and methanol extract equivalent to 0.75 g/kg powder produced a reduction in triglycerides total cholesterol LDL-c and increase in HDL-c (109).
Inhibiting hepatic HMG Co-A reductase enzyme is the main hypolipidemic mechanism of cinnamon. Reduction in oxidative stress by cinnamon through inhibition of 5-lipoxygenase enzyme is another mechanism that reduces lipid peroxidation. Cinnamon extracts have lipolytic activity. The enhancement of hepatic antioxidant enzyme activity is a critical role in hypolipidemic characteristics of cinnamon (110).
Cinnamon has the strongest inhibition of activity against copper-mediated LDL-c oxidation, LDL-c phagocytosis by macrophages and has potent cholesteryl ester transfer protein (CETP) inhibitory activity (111).
Badalzadeh et al study showedthat 200 mg/kg CBE significantly decreased serum levels of total cholesterol, LDL-c, and increased HDL-c level and HDL-c/LDL-c ratio as compared to control group in an 8 weeks test period (83).
Effects on obesity
One of the most important causes of CVD is obesity. Obesity is a source of proinflammatory cytokines and increase in oxidative stress condition (112). Insulin resistance is a major cause of the obesity while it can also be caused by obesity. Alteration in endocrine and paracrine hormones such as ghrelin is another cause of obesity (113).
Cinnamaldehyde as an agonist of TRPA1 in epithelial mouse stomach cells reduced cumulative food intake and gastric emptying rates. Also, it up-regulated expression of TRPA1 and insulin receptor genes in parallel with increase in insulin sensitivity was seen in cinnamaldehyde in vitro incubation model. Reduction in ghrelin secretion was another consequence of this in vitro model (37). Cinnamon water extract in Sartorius et al study reduced insulin resistance, lowered blood glucose, and serum lipid level and ameliorated obesity-related type 2 diabetes in mice due to activation of both PPARγ and PPARα (114).
Polyphenolic compounds with anti-obesogenic effects are abundant in cinnamon species. In vitro studies showed that differentiation of adipocyte could be inhibited by polyphenolic compounds; also they inhibited lipolysis (115), lipogenesis (116) or intestinal lipid absorption (117) that they tend to lowering weight. Polyphenolic compounds are inducers of fatty acid oxidation (116) or antagonist at cannabinoid receptors (118) and attenuate the inflammatory changes (119).
In a double blind, randomized, placebo controlled clinical trial led on 44 patients with type 2 diabetes, Vafa et al showed that consumption of 3 g/day cinnamon after 8 weeks could decreased significantly the levels of fasting blood glucose, HbA1c, weight, triglyceride, body fat mass and BMI in contrast to the baseline but these differences were not significant when compared with placebo groups (120).
In summary and according to the contents expressed above, the antiobesity effect of cinnamon was due to its insulin sensitivity, cardiovascular protection and immunomodulatory effects.
Conclusion
This review article expressed the main aspects of metabolic syndrome and protective mechanisms of cinnamon and its active ingredients in reducing and ameliorating complications of metabolic syndrome. Features of metabolic syndrome including dyslipide-mia, hyperglycemia, hypertension and obesity are under the influence by cinnamon that be proven by in vivo and in vitro studies that be shown in this article. It has been concluded that cinnamon has potential therapeutic use in metabolic syndrome and can prevent morbidity and mortality due to cardiovascular diseases.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- 1.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 2.JAMA. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 3.Bener A, Yousafzai MT, Darwish S, Al-Hamaq AO, Nasralla EA, Abdul ghani M. Obesity index that better predict metabolic syndrome: body mass index, waist circumference, waist hip ratio, or waist height ratio. J Obes. 2013:269038. doi: 10.1155/2013/269038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srikanthan P, Seeman TE, Karlamangla AS. Waist-hip-ratio as a predictor of all-cause mortality in high-functioning older adults. Annals Epidemiol. 2009;19:724–731. doi: 10.1016/j.annepidem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Razavi BM, Hosseinzadeh H. A review of the effects of Nigella sativa L. and its constituent, thymoquinone, in metabolic syndrome. J Endocrinol Invest. 2014;37:1031–40. doi: 10.1007/s40618-014-0150-1. [DOI] [PubMed] [Google Scholar]
- 6.Tabatabai SM, Dashti S, Doosti F, Hosseinzadeh H. Phytotherapy of opioid dependence and withdrawal syndrome: a review. Phytother Res. 2014;28:811–830. doi: 10.1002/ptr.5073. [DOI] [PubMed] [Google Scholar]
- 7.Hosseinzadeh H, Nassiri-Asl M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J Endocrinol Invest. 2014;37:783–788. doi: 10.1007/s40618-014-0096-3. [DOI] [PubMed] [Google Scholar]
- 8.Hassani FV, Shirani K, Hosseinzadeh H. Rosemary (Rosmarinus officinalis) as a potential therapeutic plant in metabolic syndrome: a review. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:931–949. doi: 10.1007/s00210-016-1256-0. [DOI] [PubMed] [Google Scholar]
- 9.Hosseini A, Hosseinzadeh H. A review on the effects of Allium sativum (Garlic) in metabolic syndrome. J Endocrinol Invest. 2015;38:1147–1157. doi: 10.1007/s40618-015-0313-8. [DOI] [PubMed] [Google Scholar]
- 10.Razavi BM, Hosseinzadeh H. Saffron: a promising natural medicine in the treatment of metabolic syndrome. J Sci Food Agric. 2016 doi: 10.1002/jsfa.8134. In Press. [DOI] [PubMed] [Google Scholar]
- 11.Akaberi M, Hosseinzadeh H. Grapes (Vitis vinifera) as a potential candidate for the therapy of the metabolic syndrome. Phytother Res. 2016;30:540–556. doi: 10.1002/ptr.5570. [DOI] [PubMed] [Google Scholar]
- 12.Hosseini A, Hosseinzadeh H. A review on the effects of Allium sativum (Garlic) in metabolic syndrome. J Endocrinol Invest. 2015;38:1147–57. doi: 10.1007/s40618-015-0313-8. [DOI] [PubMed] [Google Scholar]
- 13.Shu Z, Xiwen L, Jie L, Van der Werff H. CINNAMOMUM Schaeffer, Bot. Exped. 74. 1760, nom. cons. Flora. China. 2008;7:166–187. [Google Scholar]
- 14.Jayaprakasha GK, Rao LJ. Chemistry, biogenesis, and biological activities of Cinnamomum zeylanicum. Crit Rev Food Sci Nutr. 2011;51:547–562. doi: 10.1080/10408391003699550. [DOI] [PubMed] [Google Scholar]
- 15.Singh G, Maurya S, DeLampasona MP, Catalan CA. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem Toxicol. 2007;45:1650–1661. doi: 10.1016/j.fct.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Senanayake UM, Lee TH, Wills RBH. Volatile constituents of cinnamon (Cinnamomum zeylanicum) oils. J Agric Food Chem. 1978;26:822–824. [Google Scholar]
- 17.Rao PV, Gan SH. Cinnamon: a multifaceted medicinal plant. Evid Based Complement Alternat Med. 2014:642942. doi: 10.1155/2014/642942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranasinghe P, Pigera S, Premakumara GA, Galappaththy P, Constantine GR, Katulanda P. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): a systematic review. BMC Complement Altern Med. 2013;13:275. doi: 10.1186/1472-6882-13-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aneja K, Joshi R, Sharma C. Antimicrobial activity of dalchini (Cinnamomum zeyancum bark) extracts on some dental caries pathogens. J Pharm Res. 2009;2:1387–1390. [Google Scholar]
- 20.Mathew S, Abraham TE. In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food Cheml Toxcol. 2006;44:198–206. doi: 10.1016/j.fct.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Miranda PJ, De Fronzo RA, Califf RM, Guyton JR. Metabolic syndrome: Definition, pathophysiology, and mechanisms. Am Heart J. 2005;149:33–45. doi: 10.1016/j.ahj.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissén M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 23.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk, a systemic reviewe and meta-analysis. J Am Coll Cardiol. 2010;56:1113–1138. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 24.Ziegenfuss TM, Hofheins JE, Mendel RW. Effects of water soluble cinnamon extracts on body composition and features of the metabolic syndrome in prediabetic men and women. J Int Soc Sports Nutr. 2006;3:45–53. doi: 10.1186/1550-2783-3-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Couturier K, Batandier C, Awada M, Hininger-Favier I, Canini F, Anderson RA, et al. Cinnamon improves insulin sensitivity and alters the body composition in an animal model of the metabolic syndrome. Arch Biochem Biophys. 2010;501:158–161. doi: 10.1016/j.abb.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 26.Shen Y, Jia LN, Honma N, Hosono T, Ariga T, Seki T. Beneficial effects of cinnamon on the metabolic syndrome, inflammation, and pain, and mechanisms underlying these effects-a review. Tradit Complement Med. 2012;2:27–32. doi: 10.1016/s2225-4110(16)30067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan A, Bryden NA, Polansky MM, Anderson RA. Insulin potentiating factor and chromium content of selected foods and spices. Biol Trace Elem Res. 1990;24:183–188. doi: 10.1007/BF02917206. [DOI] [PubMed] [Google Scholar]
- 28.Sangal A. Role of cinnamon as beneficial antidiabetic food adjunct: a review. Adv Appl Sci Res. 2011;2:440–450. [Google Scholar]
- 29.Couturier K, Qin B, Batandier C, Awada M, Hininger-Favier I, Canini F, et al. Cinnamon increases liver glycogen in an animal model of insulin resistance. Metabolism. 2011;60:1590–1597. doi: 10.1016/j.metabol.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Adisakwattana S, Lerdsuwankij O, Poputtachai U, Minipun A, Suparpprom C. Inhibitory activity of cinnamon bark species and their combination effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Plant Foods Hum Nutr. 2011;66:143–148. doi: 10.1007/s11130-011-0226-4. [DOI] [PubMed] [Google Scholar]
- 31.Kreydiyyeh SI, Usta J, Copti R. Effect of cinnamon, clove and some of their constituents on the Na(+)-K(+)-ATPase activity and alanine absorption in the rat jejunum. Food Chem Toxicol. 2000;38:755–762. doi: 10.1016/s0278-6915(00)00073-9. [DOI] [PubMed] [Google Scholar]
- 32.Anderson RA, Broadhurst CL, Polansky MM, Schmidt WF, Khan A, Flanagan VP, et al. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J Agric Food Chem. 2004;52:65–70. doi: 10.1021/jf034916b. [DOI] [PubMed] [Google Scholar]
- 33.Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Cinnamon extract (traditional herb) potentiates in vivo insulin-regulated glucose utilization via enhancing insulin signaling in rats. Diabetes Res Clin Pract. 2003;62:139–148. doi: 10.1016/s0168-8227(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 34.Anderson RA, Broadhurst CL, Polansky MM, Schmidt WF, Khan A. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J Agric Food Chem. 2004;52:65–70. doi: 10.1021/jf034916b. [DOI] [PubMed] [Google Scholar]
- 35.Im K, Issac A, Ninan E, Maliakel B, Kuttan R. Effects of the polyphenol content on the anti-diabetic activity of Cinnamomum zeylanicum extracts. Food Funct. 2014;5:2208–2220. doi: 10.1039/c4fo00130c. [DOI] [PubMed] [Google Scholar]
- 36.Blevins SM, Leyva MJ, Brown J, Wright J, Scofield RH, Aston CE. Effect of cinnamon on glucose and lipid levels in non-insulin-dependent type 2 diabetes. Diabetes Care. 2007;30:2236–2237. doi: 10.2337/dc07-0098. [DOI] [PubMed] [Google Scholar]
- 37.Hafizur RM, Hameed A, Shukrana M, Raza SA, Chishti S, Kabir N, et al. Cinnamic acid exerts anti-diabetic activity by improving glucose tolerance in vivo and by stimulating insulin secretion in vitro. Phytomedicine. 2015;22:297–300. doi: 10.1016/j.phymed.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Camacho S, Michlig S, De Senarclens-Bezençon C, Meylan J, Meystre J, Pezzoli M, et al. Antiobesity and anti-hyperglycemic effects of cinnamaldehyde via altered ghrelin secretion and functional impact on food intake and gastric emptying. Sci Rep. 2015;21:7919. doi: 10.1038/srep07919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mang B, Wolters M, Schmitt B, Kelb K, Lichtinghagen R, Stichtenoth DO, et al. Effects of a cinnamon extract on plasma glucose, HbA, and serum lipids in diabetes mellitus type 2. Eur J Clin Invest. 2006;36:340–344. doi: 10.1111/j.1365-2362.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 40.Huang B, Yuan HD, Kimdo Y, Quan HY, Chung SH. Cinnamaldehyde prevents adipocyte differentiation and adipogenesis via regulation of peroxisome proliferator-activated receptor-gamma (PPARgamma) and AMP-activated protein kinase (AMPK) pathways. J Agric Food Chem. 2011;59:3666–3673. doi: 10.1021/jf104814t. [DOI] [PubMed] [Google Scholar]
- 41.Sheng X, Zhang Y, Gong Z, Huang C, Zang YQ. Improved insulin resistance and lipid metabolism by cinnamon extract through activation of peroxisome proliferator-activated receptors. PPAR Res. 2008:581348. doi: 10.1155/2008/581348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takasao N, Tsuji-Naito K, Ishikura S, Tamura A, Akagawa M. Cinnamon extract promotes type I collagen biosynthesis via activation of IGF-I signaling in human dermal fibroblasts. J Agric Food Chem. 2012;60:1193–200. doi: 10.1021/jf2043357. [DOI] [PubMed] [Google Scholar]
- 43.Usta J, Kreydiyyeh S, Bajakian K, Nakkash-Chmaisse H. In vitro effect of eugenol and cinnamaldehyde on membrane potential and respiratory chain complexes in isolated rat liver mitochondria. Food Chem Toxicol. 2002;40:935–940. doi: 10.1016/s0278-6915(02)00071-6. [DOI] [PubMed] [Google Scholar]
- 44.Schriner SE, Kuramada S, Lopez TE, Truong S, Pham A, Jafari M. Extension of Drosophila lifespan by cinnamon through a sex-specific dependence on the insulin receptor substrate chico. Exp Gerontol. 2014;60:220–230. doi: 10.1016/j.exger.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beejmohun V, Peytavy-Izard M, Mignon C, Muscente-Paque D, Deplanque X, Ripoll C, et al. Acute effect of Ceylon cinnamon extract on postprandial glycemia: alpha-amylase inhibition, starch tolerance test in rats, and randomized crossover clinical trial in healthy volunteers. BMC Complement Altern Med. 2014;14:351–359. doi: 10.1186/1472-6882-14-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen Y, Honma N, Kobayashi K, Jia LN, Hosono T, Shindo K, et al. Cinnamon extract enhances glucose uptake in 3T3-L1 adipocytes and C2C12 myocytes by inducing LKB1-AMP-activated protein kinase signaling. PLoS One. 2014;9:e87894. doi: 10.1371/journal.pone.0087894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng X, Ma J, Chao J, Sun Z, Chang RC, Tse I, et al. Beneficial effects of cinnamon proanthocyanidins on the formation of specific advanced glycation endproducts and methylglyoxal-induced impairment on glucose consumption. J Agric Food Chem. 2010;58:6692–6696. doi: 10.1021/jf100538t. [DOI] [PubMed] [Google Scholar]
- 48.Ranasinghe P, Jayawardana R, Galappaththy P, Constantine GR, Gunawardana DV, Katulanda P. Efficacy and safety of ‘true’ cinnamon (Cinnamomum zeylanicum) as a pharmaceutical agent in diabetes: a systematic review and metaanalysis. Diabet Med Dec. 2012;29:1480–1492. doi: 10.1111/j.1464-5491.2012.03718.x. [DOI] [PubMed] [Google Scholar]
- 49.Crawford P. Effectiveness of cinnamon for lowering hemoglobin A1C in patients with type 2 diabetes: a randomized, controlled trial. J Am Board Fam Med. 2009;22:507–512. doi: 10.3122/jabfm.2009.05.080093. [DOI] [PubMed] [Google Scholar]
- 50.Cao H, Polansky MM, Anderson RA. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch Biochem Biophys. 2007;459:214–222. doi: 10.1016/j.abb.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 51.Vanschoonbeek K, Thomassen BJ, Senden JM, Wodzig WK, van Loon LJ. Cinnamon supplementation does not improve glycemic control in postmenopausal type 2 diabetes patients. J Nutr. 2006;136:977–980. doi: 10.1093/jn/136.4.977. [DOI] [PubMed] [Google Scholar]
- 52.Valko M, Leibfritz D, Moncola J, Cronin MTD, Mazura M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Mu T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. 2010;31:2741–2749. doi: 10.1093/eurheartj/ehq396. [DOI] [PubMed] [Google Scholar]
- 54.Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev. 2009;89:27–71. doi: 10.1152/physrev.00014.2008. [DOI] [PubMed] [Google Scholar]
- 55.Cadenas E. Basic mechanisms of antioxidant activity. Biofactors. 2009;6:391–397. doi: 10.1002/biof.5520060404. [DOI] [PubMed] [Google Scholar]
- 56.Halliwell B. Free radicals and antioxidants—quo vadis? Trends Pharmacol Sci. 2011;32:125–130. doi: 10.1016/j.tips.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 57.Dhuley JN. Anti-oxidant effects of cinnamon (Cinnamomum verum) bark and greater cardamon (Amomumsubulatum) seeds in rats fed high fat diet. Indian J Exp Biol. 1999;37:238–242. [PubMed] [Google Scholar]
- 58.Mancini-Filho J, van-Koiij A, Mancini DAP, Cozzolino F, Torres RP. Antioxidant activity of cinnamon (Cinnamomum zeylanicum breyne) extracts. Bollettino Chimico Farmaceutico. 1998;137:443–447. [PubMed] [Google Scholar]
- 59.Boğa M, Hacıbekiroğlu I, Kolak U. Antioxidant and anticholinesterase activities of eleven edible plants. Pharm Biol. 2011;49:290–295. doi: 10.3109/13880209.2010.517539. [DOI] [PubMed] [Google Scholar]
- 60.Aravind R, Aneesh T, Bindu A, Bindu K. Estimation of phenolics and evaluation of antioxidant activity of Cinnamomum malabatrum (Burm F) Blume Asian J Res Chem. 2012;5:628–632. [Google Scholar]
- 61.Chericoni S, Prieto JM, Iacopini P, Cioni P, Morelli I. In vitro activity of the essential oil of Cinnamomum zeylanicum and eugenol in peroxynitrite-induced oxidative processes. J Agric Food Chem. 2005;53:4762–4765. doi: 10.1021/jf050183e. [DOI] [PubMed] [Google Scholar]
- 62.Khaki A. Effect of Cinnamomum zeylanicumon on Spermatogenesis. Iran Red Crescent Med J. 2015;17:e18668. doi: 10.5812/ircmj.18668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keshvari M, Asgary S, Jafarian-dehkordi A, Najafi S, Ghoreyshi-Yazdi S. M. Preventive effect of cinnamon essential oil on lipid oxidation of vegetable oil. ARYA Atheroscler. 2013;9:280–286. [PMC free article] [PubMed] [Google Scholar]
- 64.Okawa M, Kinjo J, Nohara T, Ono M. DPPH (1,1-diphenyl-2-Picrylhydrazyl) radical scavenging activity of flavonoids obtained fromsomemedicinal plants. Biol Pharm Bullet. 2001;24:1202–1205. doi: 10.1248/bpb.24.1202. [DOI] [PubMed] [Google Scholar]
- 65.Kim SJ, Han D, Moon KD, Rhee JS. Measurement of superoxide dismutase-like activity of natural antioxidants. Biosci Biotechnol Biochem. 1995;59:822–826. doi: 10.1271/bbb.59.822. [DOI] [PubMed] [Google Scholar]
- 66.Kumar S, Vasudeva N, Sharma S. GC-MS analysis and screening of antidiabetic, antioxidant and hypolipidemic potential of Cinnamomum tamala oil in streptozotocin induced diabetes mellitus in rats. Cardiovasc Diabetol. 2012;11:1–11. doi: 10.1186/1475-2840-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee HS, Kim BS, Kim MK. Suppression effect of Cinnamomum cassia bark-derived component on nitric oxide synthase. J Agric Food Chem. 2002;50:7700–7703. doi: 10.1021/jf020751f. [DOI] [PubMed] [Google Scholar]
- 68.Roussel AM, Hininger I, Benaraba R, Ziegenfuss TN, Anderson RA. Antioxidant effects of a cinnamon extract in people with impaired fasting glucose that are overweight or obese. J Am Coll Nutr. 2009;28:16–21. doi: 10.1080/07315724.2009.10719756. [DOI] [PubMed] [Google Scholar]
- 69.Pandey M, Chandra DR. Evaluation of Ethanol and Aqueous extracts of Cinnamomum verum Leaf Galls for Potential Antioxidant and Analgesic activity. Indian J Pharm Sci. 2015;77:243–247. doi: 10.4103/0250-474x.156630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moselhy SS, Ali HK. Hepatoprotective effect of cinnamon extracts against carbon tetrachloride induced oxidative stress and liver injury in rats. Biol Res. 2009;42:993–998. [PubMed] [Google Scholar]
- 71.Villela NR, Kramer-Aguiar LG, Bottino DA, Wiernsperger N, Bouskela E. Metabolic disturbances linked to obesity: the role of impaired tissue perfusion. Arq Bras Endocrinol Metab. 2009;53:238–245. doi: 10.1590/s0004-27302009000200015. [DOI] [PubMed] [Google Scholar]
- 72.Mancia G, Bousquet P, Elghozi JL, Esler M, Grassi G, Julius S, et al. The sympathetic nervous system and the metabolic syndrome. J Hypertens. 2007;25:909–920. doi: 10.1097/HJH.0b013e328048d004. [DOI] [PubMed] [Google Scholar]
- 73.Akilen R, Pimlott Z, Tsiami A, Robinson N. Effect of short-term administration of cinnamon on blood pressure in patients with prediabetes and type 2 diabetes. Nutrition. 2013;29:1192–1196. doi: 10.1016/j.nut.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 74.Akilen R, Tsiami A, Devendra D, Robinson N. Glycated haemoglobin and blood pressure-lowering effect of cinnamon in multi-ethnic Type 2 diabetic patients in the UK: a randomized, placebo-controlled, double-blind clinical trial. Diabet Med. 2010;27:1159–1167. doi: 10.1111/j.1464-5491.2010.03079.x. [DOI] [PubMed] [Google Scholar]
- 75.Alvarez-Collazo J, Alonso-Carbajo L, López-Medina AI, Alpizar YA, Tajada S, Voetes T, et al. Cinnamaldehyde inhibits L-type calcium channels in mouse ventricular cardiomyocytes and vascular smooth muscle cells. Pflugers Arch. 2014;466:2089–2099. doi: 10.1007/s00424-014-1472-8. [DOI] [PubMed] [Google Scholar]
- 76.Nyadjeu P, Dongmo A, Nguelefack TB, Kamanyi A. Antihypertensive and vasorelaxant effects of Cinnamomum zeylanicum stem bark aqueous extract in rats. J Complement Integr Med. 2011 doi: 10.2202/1553-3840.1490. doi:10.2202/1553-3840.1490. [DOI] [PubMed] [Google Scholar]
- 77.Earley S. TRPA1 channels in the vasculature. Br J Pharmacol. 2012;167:13–22. doi: 10.1111/j.1476-5381.2012.02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nyadjeu P, Nguelefack-Mbuyo EP, Atsamo AD, Nguelefack TB, Dongmo AB, Kamanyi A. Acute and chronic antihypertensive effects of Cinnamomum zeylanicum stem bark methanol extract in L-NAME-induced hypertensive rats. BMC Complement Altern Med. 2013;31:13–27. doi: 10.1186/1472-6882-13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaffash Elahi R. The effect of the cinnamon on dog’s heart performance by focus on Kortkoff sounds. J Animal Veterinary. 2012;11:3604–3608. [Google Scholar]
- 80.El-Bassossy HM, Fahmy A, Badawy D. Cinnamaldehyde protects from the hypertension associated with diabetes. Food Chem Toxicol. 2011;49:3007–3012. doi: 10.1016/j.fct.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 81.Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2011;63:102–110. doi: 10.1002/acr.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rao A, Pandya V, Whaley-Connell A. Obesity and insulin resistance in resistant hypertension: implications for the kidney. Adv Chronic Kidney Dis. 2015;22:211–217. doi: 10.1053/j.ackd.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 83.Badalzadeh R, Shaghaghi M, Mohammadi M, Dehghan G, Mohammadi Z. The effect of cinnamon extract and long-term aerobic training on heart function, biochemical alterations and lipid profile following exhaustive exercise in male rats. Adv Pharm Bull. 2014;4:515–520. doi: 10.5681/apb.2014.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ko FN, Yu SM, Kang YF, Teng CM. Characterization of the thromboxane (TP-) receptor subtype involved in proliferation in cultured vascular smooth muscle cells of rat. Br J Pharmacol. 1995;116:1801–1808. doi: 10.1111/j.1476-5381.1995.tb16666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu SM, Wu TS, Teng CM. Pharmacological characterization of cinnamophilin, a novel dual inhibitor of thromboxane synthase and thromboxane A2 receptor. Br J Pharmacol. 1994;111:906–912. doi: 10.1111/j.1476-5381.1994.tb14824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hwa JS, Jin YC, Lee YS, Ko YS, Kim YM, Shi LY, HJ Kim, JH Lee, TM Ngoc, YS Kim, KC Chang, et al. 2-Methoxycinnamaldehyde from Cinnamomum cassia reduces ratmyocardial ischemia and reperfusion injury in vivo due to HO-1 induction. J Ethnopharmacol. 2012;139:605–615. doi: 10.1016/j.jep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 87.Song F, Li H, Sun J, Wang S. Protective effects of cinnamic acid and cinnamic aldehyde on isoproterenol-induced acute myocardial ischemia in rats. J Ethnopharmacol. 2013;150:125–130. doi: 10.1016/j.jep.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 88.Badalzadeh R, Shaghaghi M, Mohammadi M, Dehghan G, Mohammadi Z. The effect of cinnamon extract and long-term aerobic training on heart function, biochemical alterations and lipid profile following exhaustive exercise in male rats. Adv Pharm Bull. 2014;4:515–520. doi: 10.5681/apb.2014.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rega G, Kaun C, Demyanets S, Pfaffenberger S, Rychli K, Kastl SP, et al. Vascular endothelial growth factor Is induced by the inflammatory cytokines interleukin-6 and oncostatin M in human adipose tissue In vitro and in murine adipose tissue In vivo. Arterioscler Thromb Vasc Biol. 2007;27:1587–1595. doi: 10.1161/ATVBAHA.107.143081. [DOI] [PubMed] [Google Scholar]
- 90.Muhammad JS, Zaidi SF, Shaharyar S, Refaat A, Usmanghani K, Saiki I, et al. Anti-inflammatory effect of cinnamaldehyde in Helicobacter pylori induced gastric inflammation. Biol Pharm Bull. 2015;38:109–115. doi: 10.1248/bpb.b14-00609. [DOI] [PubMed] [Google Scholar]
- 91.Mashhadi NS, Ghiasvand R, Askari G, Feizi A, Hariri M, Darvishi L, et al. Influence of ginger and cinnamon intake on inflammation and muscle soreness endued by exercise in Iranian female athletes. Int J Prev Med. 2013;4:11–15. [PMC free article] [PubMed] [Google Scholar]
- 92.Lee SH, Lee SY, Son DJ, Lee H, Yoo HS, Song S, et al. Inhibitory effect of 2`-hydroxycinnamaldehyde on nitric oxide production through inhibition of NF-??B activation inRAW264.7 cells. Biochem Pharmacol. 2005;69:791–799. doi: 10.1016/j.bcp.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 93.Muhammad JS, Zaidi SF, Shaharyar S, Refaat A, Usmanghani K, Saiki I, et al. Anti-inflammatory effect of cinnamaldehyde in Helicobacter pylori induced gastric inflammation. Biol Pharm Bull. 2015;38:109–115. doi: 10.1248/bpb.b14-00609. [DOI] [PubMed] [Google Scholar]
- 94.Yu T, Lee S, Yang WS, Jang HJ, Lee YJ, Cho NY. The ability of an ethanol extract of Cinnamomum cassia to inhibit Src and spleen tyrosine kinase activity contributes to its anti-inflammatory action. J Ethnopharmacol. 2012;139:566–573. doi: 10.1016/j.jep.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 95.Lee BJ, Kim YJ, Cho DH, Sohn NW, Kang H. Immunomodulatory effect of water extract of cinnamon on anti-CD3-induced cytokine responses and p38, JNK, ERK1/2, and STAT4 activation. Immunopharmacol Immunotoxicol. 2011;33:714–722. doi: 10.3109/08923973.2011.564185. [DOI] [PubMed] [Google Scholar]
- 96.Hong JW, Yang GE, Kim YB, Eom SH, Lew JH, Kang H. Anti-inflammatory activity of cinnamon water extract in vivo and in vitro LPS-inducedmodels. BMC Complement Altern Med. 2012;12:237. doi: 10.1186/1472-6882-12-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bolin Q, Anderson R. Cinnamon polyphenols increase oxygen-glucose deprivation of decreased PGE2 production by upregulation of SIRT1 and alleviation of the anti-inflammatory effects (830.11) FASEB. 2014;28:830–841. [Google Scholar]
- 98.Chao LK, Hua KF, Hsu HY, Cheng SS, Lin IF, Chen CJ, et al. Cinnamaldehyde inhibits pro-inflammatory cytokines secretion from monocytes/macrophages through suppression of intracellular signaling. Food Chem Toxicol. 2008;46:220–231. doi: 10.1016/j.fct.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 99.Huang SH, Choi YG, Jeong MY, Hong YM, Lee JH, Lim S. Microarray analysis of gene expression profile by treatment of Cinnamomi ramulus in lipopolysaccharide estimulated BV-2 cells. Gene. 2009;443:83–90. doi: 10.1016/j.gene.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 100.Gunawardena D, Karunaweera N, Lee S, Van Der Kooy F, Harman DG, Raju R, et al. Anti-inflammatory activity of cinnamon (C. zeylanicum and C. cassia) extracts –identification of E-cinnamaldehyde and o-methoxy cinnamaldehyde as the most potent bioactive compounds. Food Funct. 2015;6:910–919. doi: 10.1039/c4fo00680a. [DOI] [PubMed] [Google Scholar]
- 101.Rathi B, Bodhankar S, Mohan V, Thakurdesai P. Ameliorative effects of a polyphenolic fraction of Cinnamomum zeylanicum L bark in animal models of inflammation and arthritis. Sci Pharm. 2013;81:567–589. doi: 10.3797/scipharm.1301-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu J, Zhang K, Nam S, Anderson RA, Jove R, Wen W. Novel angiogenesis inhibitory activity in cinnamon extract blocks VEGFR2 kinase and downstream signaling. Carcinogenesis. 2010;31:481–488. doi: 10.1093/carcin/bgp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ross R, Després JP. Abdominal obesity, insulin resistance, and the metabolic syndrome: contribution of physical activity/exercise. Obesity. 2009;17:S1–S2. doi: 10.1038/oby.2009.381. [DOI] [PubMed] [Google Scholar]
- 104.Takemoto K, Deckelbaum RJ, Saito I, Likitmaskul S, Morandi A, Pinelli L, et al. Adiponectin/resistin levels and insulin resistance in children: a four country comparison study. Int J Pediatr Endocrinol. 2015;2015:2. doi: 10.1186/1687-9856-2015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rahman S, Begum H, Rahman Z, Ara F, Iqbal MJ, Abukalam MY. Effect of cinnamon (Cinnamomum cassia) as a lipid lowering agent on hypercholesterolemic rats. J Enam Medical College. 2013;3:94–98. [Google Scholar]
- 106.Kim SH, Choung SY. Antihyperglycemic and antihyperlipidemic action of Cinnamomi cassiae (Cinnamon bark) extract in C57BL/Ks db/db mice. Arch Pharm Res. 2010;33:325–333. doi: 10.1007/s12272-010-0219-0. [DOI] [PubMed] [Google Scholar]
- 107.Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26:3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 108.Shatwan IA, Ahmed LA, Badkook MM. Effect of barley flour, crude cinnamon, and their combination on glycemia, dyslipidemia, and adipose tissue hormones in type 2 diabetic rats. J Med Food. 2013;16:656–662. doi: 10.1089/jmf.2012.0083. [DOI] [PubMed] [Google Scholar]
- 109.Javed I, Faisal I, Rahman Z, Khan MZ, Muhammad F, Aslam B, et al. Lipid lowering effect of Cinnamomum zeylanicum in hyperlipidaemic albino rabbits. Pak J Pharm Sci. 2012;25:141–147. [PubMed] [Google Scholar]
- 110.Lee JS, Jeon SM, Park EM, Huh TL, Kwon OS, Lee MK, et al. Cinnamate supplementation enhances hepatic lipid metabolism and antioxidant defense systems in high cholesterol-fed rats. J Med Food. 2003;6:183–191. doi: 10.1089/10966200360716599. [DOI] [PubMed] [Google Scholar]
- 111.Jin S, Cho KH. Water extracts of cinnamon and clove exhibits potent inhibition of protein glycation and anti-atherosclerotic activity in vitro and in vivo hypolipidemic activity in zebrafish. Food Chem Toxicol. 2011;49:1521–1529. doi: 10.1016/j.fct.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 112.Wronkowitz N, Romacho T, Sell H, Eckel J. Adipose tissue dysfunction and inflammation in cardiovascular disease. Front Horm Res. 2014;43:79–92. doi: 10.1159/000360560. [DOI] [PubMed] [Google Scholar]
- 113.Arslan N, Sayin O, Tokgoz Y. Evaluation of serum xenin and ghrelin levels and their relationship with nonalcoholic fatty liver disease and insulin resistance in obese adolescents. J Endocrinol Invest. 2014;37:1091–1097. doi: 10.1007/s40618-014-0160-z. [DOI] [PubMed] [Google Scholar]
- 114.Sartorius T, Peter A, Schulz N, Drescher A, Bergheim I, Machann J, et al. Cinnamon extract improves insulin sensitivity in the brain and lowers liver fat in mouse models of obesity. PLoS One. 2014;9:e92358. doi: 10.1371/journal.pone.0092358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ogasawara J, Kitadate K, Nishioka H, Fujii H, Sakurai T, Kizaki T, et al. Oligonol, an oligomerized lychee fruit-derived polyphenol, activates the Ras/Raf-1/MEK1/2 cascade independent of the IL-6 signaling pathway in rat primary adipocytes. Biochem Biophys Res Commun. 2010;402:554–559. doi: 10.1016/j.bbrc.2010.10.082. [DOI] [PubMed] [Google Scholar]
- 116.Mercader J, Palou A, Bonet ML. Resveratrol enhances fatty acid oxidation capacity and reduces resistin and retinol-binding protein 4 expression in white adipocytes. J Nutr Biochem. 2011;22:828–834. doi: 10.1016/j.jnutbio.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 117.Uchiyama S, Taniguchi Y, Saka A, Yoshida A, Yajima H. Prevention of diet-induced obesity by dietary black tea polyphenols extract in vitro and in vivo. Nutrition. 2011;27:287–292. doi: 10.1016/j.nut.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 118.Seely KA, Levi MS, Prather PL. The dietary polyphenols trans-resveratrol and curcumin selectively bind human CB1 cannabinoid receptors with nanomolar affinities and function as antagonists/inverse agonists. J Pharmacol Exp Ther. 2009;330:31–39. doi: 10.1124/jpet.109.151654. [DOI] [PubMed] [Google Scholar]
- 119.Overman A, Bumrungpert A, Kennedy A, Martinez K, Chuang CC, West T, et al. Polyphenol-rich grape powder extract (GPE) attenuates inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. Int J Obes (Lond) 2010;34:800–808. doi: 10.1038/ijo.2009.296. [DOI] [PubMed] [Google Scholar]
- 120.Vafa M, Mohammadi F, Shidfar F, Sormaghi MS, Heidari I, Golestan B, et al. Effects of cinnamon consumption on glycemic status, lipid profile and body composition in type 2 diabetic patients. Int J Prev Med. 2012;3:531–536. [PMC free article] [PubMed] [Google Scholar]


