Abstract
Objective(s):
In order to grow cells in a three-dimensional (3D) microenvironment, self-assembling peptides, such as PuraMatrix, have emerged with potential to mimic the extracellular matrix. The aim of the present study was to investigate the influence of the self-assembling peptide on the morphology, survival, proliferation rate, migration potential, and differentiation of human meningioma stem-like cells (hMgSCs).
Materials and Methods:
The efficacy of a novel method for placing hMgSCs in PuraMatrix (the injection approach) was compared to the encapsulation and surface plating methods. In addition, we designed a new method for measurement of migration distance in 3D cultivation of hMgSCs in PuraMatrix.
Results:
Our results revealed that hMgSCs have the ability to form spheres in stem cell culture condition. These meningioma cells expressed GFAP, CD133, vimentin, and nestin. Using the injection method, a higher proliferation rate of the hMgSCs was observed after seven days of culture. Furthermore, the novel migration assay was able to measure the migration of a single cell alone in 3D environment.
Conclusion:
The results indicate the injection method as an efficient technique for culturing hMgSCs in PuraMatrix. Furthermore, the novel migration assay enables us to evaluate the migration of hMgSCs.
Keywords: Brain tumor, Cell culture, Cell migration, RADA16-I, Stem cells
Introduction
The main objective of tissue engineering is to provide a three-dimensional (3D) environment in order to mimic the extracellular matrix, facilitate nutrients, and exchange biochemical substances (1). Among several biomaterials, self-assembly peptide nanofiber scaffold (SAPNS) indicates a new promising biomaterial with ideal properties, such as proper biocompatibility, biodegradability, and safety (2, 3). A 3D scaffold is typically created by SAPNS in the presence of physiological fluids, which allows fibers to form stable β-sheet structures with a diameter of about 10 nm and pore diameter of 5 to 200 nm (3). BD™ PuraMatrix™ peptide hydrogel (PM), a member of SAPNS, is a 16 amino acid synthetic peptide (RADA16; AcN-RADARADARADARADA-CONH2), which is widely used for 3D culture. PM mimics several properties of the natural extracellular matrix in which cells can easily proliferate, differentiate, and migrate (4, 5). Different cells, such as osteoblast cells, cartilage cells, cancer cells, human fetal neural progenitor cells, dental pulp stem cells, human umbilical vein endothelial cells, adult mouse neural stem cells (NSCs), and human fetal Schwann cells have been studied by 3D cell culture using PM (6-14).
Meningioma, one of the most common intracranial brain tumors, arises from arachnoid cells (14, 15). Recent studies have focused on isolating and characterizing the tumor stem-like cells from human meningioma (16, 17). However, obtaining human meningioma stem like cells (hMgSCs) and establishing an efficient method for meningioma cell cultures are needed to be improved. Development and establishment of a 3D in vitro model may establish the cultivation of meningioma cells and enhance cell proliferation and migration.
The encapsulation and surface plating methods were used to create PM-3D culture systems. In a recent study, we introduced a new method for placing adult rat neural stem/progenitor cells into PM called as the injection method. This novel method created a well 3D culture and maintained the cell survival (18). In the present study, we evaluated the morphology, cell viability, proliferation, and differentiation of hMgSCs cultivated with PM by the novel injection method and compared that to the classic procedures. Furthermore, we designed a new method to assess migration of the single cells in the hMgSCs cultivated in PM. This novel approach may be used as a disease modelling platform.
Materials and Methods
Study design
Firstly, human tumor stem-like cells were isolated and cultured and their characteristics were assessed by immunocytochemistry. To evaluate the viability and proliferation, the cells obtained from 5th passage were cultured in 2D culture (without any adhesive agent), monolayer culture (using laminin) or 3D cultures using the injection, surface plating, and encapsulation approaches (using PM). To study the migration capacity, the cells was cultivated in different concentrations of PM by the injection method. Ethical approval was obtained from the Ethics Committee of Shefa Neuroscience Research Center, Tehran, Iran.
Isolation of brain tumor stem-like cells
The sample was collected during tumor resection surgery from a 38-year-old woman (meningioma, right occipital lobe) and transferred to the laboratory in a microtube containing oxygenated cold phosphate buffer saline (PBS). Tissue was mechanically dissociated using scalpel and repeated pipetting and then enzymatically dissociated with trypsin (0.05%, Gibco, Germany) for 5 min at 37 °C. Fetal bovine serum (FBS) was added as a trypsin inhibitor. After centrifugation, the supernatant was discarded and cells were transferred into T-25 untreated flask in DMEM/F12 medium (Gibco, Germany) plus 1% N2 supplement (Gibco, Germany), 1% B27 supplement (Gibco, Germany), 1% glutamine (Gibco, Germany), 20 ng/ml epidermal growth factor (Sigma, Germany), 20 ng/ml basic fibroblast growth factor (Sigma, Germany) as well as 1% penicillin/streptomycin solution (Gibco, Germany) and maintained in a humid 37 °C incubator with 5% CO2 in air. The cells were passaged every 5 days.
Immunofluorescence assay
The spheres obtained from the second passage were dissociated and cultured as monolayer in 96-well treated plates in the presence of the above mentioned medium for 10 days. For immunocytochemistry, the culture medium was discarded and the cells were rinsed with PBS and fixed with 4% paraformaldehyde. Permeabilization was done by Triton X-100 solution and normal horse serum was used as a blocker. Then cells were incubated with CD133 (1:200, mouse polyclonal, Abcam), vimentin (1:250, mouse polyclonal, Abcam), glial fibrillary acidic protein (GFAP; 1:250, mouse polyclonal, Abcam) or nestin (1:500, mouse polyclonal, Abcam) primary antibodies overnight. Subsequently, secondary antibody (FITC goat anti-mouse; Molecular Probes, Invitrogen) was used. Finally, cells were counterstained with DAPI (1:1000, Santa Cruz) to detect cell nuclei. Negative controls were incubated only with secondary antibodies.
Monolayer culture of the cells using laminin
According to the manual instruction, laminin (Sigma, Germany) was prepared. Briefly, laminin was diluted at 10 µg/ml with PBS and added (75 ml) to 96-well untreated plates, before incubated at 37 °C for 1-2 hr. Next, the plates were washed 3 times with sterile PBS and finally the cells (3×104 cells/well) were cultured.
3D cultures
In the injection and surface plating methods, 50 µl of 0.15% PM (BD Bioscience, Germany) solution were added to 96-well untreated plates. To adjust the pH of PM, 150 µl of DMEM/F12 was added and then changed for 3 times per hour at 37 °C. After that, cells (3×104 cells/well) were injected into PM or placing in the surface of it (5). In the encapsulation method, 25 µl of PM was mixed with 25 µl of DMEM/F12 containing 3×104 cells and cultured in 96-well untreated plates.
Proliferation assay
To determine the proliferation rate of brain tumor stem-like cells in PM, the cells were cultured for 7 days by different methods (3 samples for each group) before (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; MTS) assay (Cell-Titer 96® AQueous One Solution, Promega) was performed. 80-µl-culture medium was discarded and added 20-µl-MTS solution for 3 hr in 37 °C. Afterward, absorbance was measured by an ELISA microplate reader at 490 nm.
Cell viability assay
Viability of cells was analyzed by LIVE/DEAD Viability/Cytotoxicity kit (Molecular Probe) at days 3 and 7 after culturing. In this two-color fluorescence cell viability assay, calcein AM and ethidium homodimer (EthD-1) are optimal dyes. According to the manual instruction, stain solution was prepared by 2 µm of calcein AM and 4 µm of EthD-1 in PBS. The culture medium was discarded, and 100 µl of the stain solution was added for 30 min in 37 °C. Finally, the stain solution was removed and the cells washed with PBS.
Data were analyzed by Image J software and the percentage of live cells was calculated according to the following formula:
Migration assay
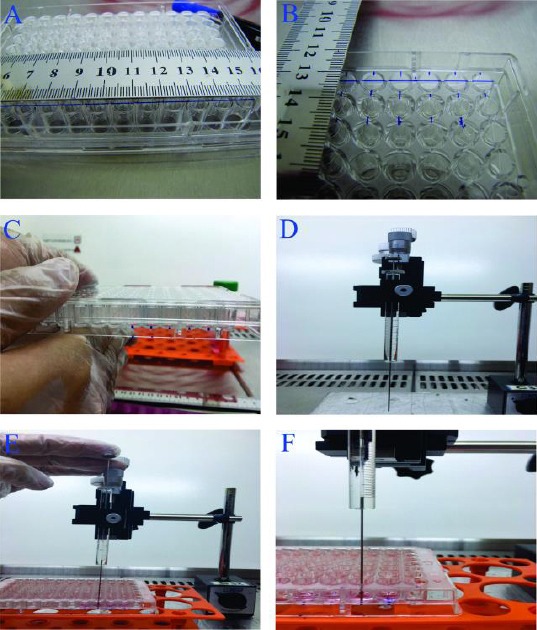
To evaluate cell migration, we designed a new method in 3D culture using the injection method. The new migration method is a high throughput testing and can be automated. Each well of 96-well untreated plates was divided into two equal parts by drawing a line on the bottom of the plate (Figure 1A). To detect injection site, a point was marked in the middle of each well (Figure 1B, C). To improve the accuracy of the method, Hamilton syringe was fixed on a static base (Figure 1D) and therefore all injections were done on the same specified point and at the same time point (Figure 1E, F). Briefly, 50 µl of 0.15%, 0.3%, 0.5% and 1% PM was injected into the well. Subsequently, scaffold was created by adding 100 µl of medium to the PM and its pH was adjusted by changing the medium every 1 hr for 3 hr. 2×103 cells/well, injected in one side of the scaffold by a 26 gage Hamilton syringe with the rate of 0.5 μl/min. We also used laminin as a 2D control so that 2×103 cells/well were injected into one side of a 96-well plate treated by laminin.
Figure 1.

Cell migration assay in 3D culture
The bottom of the plate was divided into two parts (A)
The injection points were specified by a marker (B and C) Then Hamilton syringe was fixed on a static base (D)
All injections were done on the specified point at the same time (E and F)
Images were taken at predetermined time points (days 1, 2, 3, 6, 7 and 9 after cell injection) and analyzed by infinity software. To calculate the average daily distance was traveled by the cells, the following method was used (Figure 2 and 3):
Figure 2.
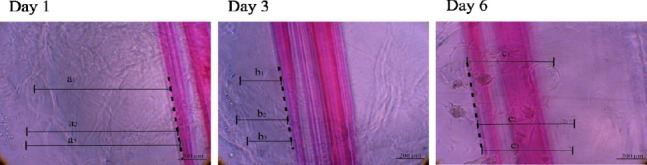
Phase-contrast images of hMgSCs are shown at 1, 3 and 6 days after cultivation. Measurement of hMgSCs migration distance after injection into PuraMatrix is shown. Migration distance at day 3=[(a1+a2+a3)/3]–[(b1+b2+b3)/3]. Migration distance at day 6=[(a1+a2+a3)/3]+ [(c1+c2+c3)/3]
Figure 3.
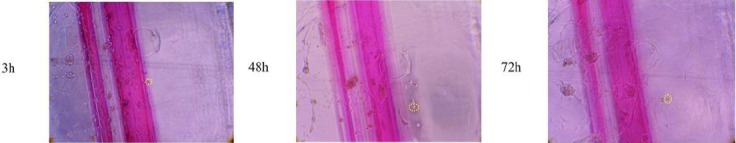
Phase-contrast images of hMgSCs are shown at 3, 48 and 72 hr after injection in PM. A single cell was marked by yellow circles. Migration was obviously seen at predetermined time
i) If the cells did not reach the red line, migration distance in a defined day=the mean distances of three pioneer cells to the red line at the defined day + the mean distances of three pioneer cells to the red line at the first day.
ii) If the cells crossed the red line, migration distance in a defined day=the mean distances of three pioneer cells to the red line at the defined day + the mean distances of three pioneer cells to the red line at the first day.
Statistical analysis
Data are presented as mean ± standard deviation (SD) of the mean. To determine the significance, one-way analysis of variance (ANOVA) was used. P<0.05 was considered statistically significant.
Results
Histopathology of meningioma
Gross examination of meningioma showed that the specimen consists of multiple irregular tan brown soft tissue pieces measuring 7x5x2 cm in aggregate. Histopathological results revealed a benign-appearing meningeal origin neoplasia composed of ovaloid and spindle cells with whirling growth and rare psammomatous calcification (meningioma, transitional variant, WHO grade I; Figure 4).
Figure 4.
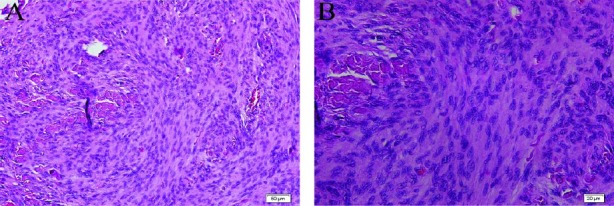
Histopathology of specimen resected during surgery. H&E staining shows cells of tumor, composed of spindle cells arranging in whorl patterns (A). H&E staining shows ovaloid and spindle cells of tumor with plentiful cytoplasm and psammomatous calcification (B)
Spheres culture of meningioma cells
Using combined mechanical and enzymatic methods, meningioma tissue was dissociated into single cells. Cells were cultured under neurosphere culture conditions. To determine self-renewal and proliferation capacity, the tumor stem-like cells were passaged for 5 times (each time as they reached a confluence of -70%). Results showed that single cells produced round and smooth spheres after 5 days and these spheres could be passaged for 5 times after each passage, indicating that meningioma cells in spheres were capable of self-renewal (Figure 5).
Figure 5.
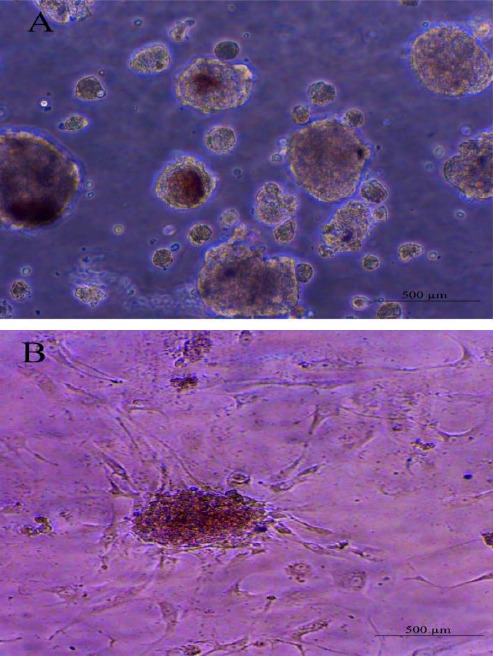
Photomicrographs of hMgSCs at early passage. Note sphere formation and self-renewal capacity of hMgSCs. Neurospheres were formed after 5 days of primary culture (A). Adherent monolayer cells and a neurosphere at day 5 after the first passage (B)
Characterization of the cells Using immunofluore-scence assay
Immunofluorescence analysis was used to investigate the expression of stem cell markers in the meningioma cells. The majority of the meningioma cells expressed vimentin, the first marker to be expressed during cell differentiation. In addition, the expression of GFAP as well as nestin was detected in the majority of these cells whereas CD133 was partly expressed (Figure 6).
Figure 6.
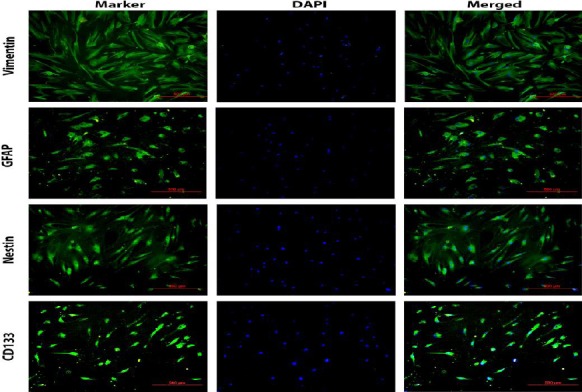
Characterization of human meningioma stem-like cells immunostaining of vimentin, GFAP, nestin, and CD133. Nuclei were counterstained with DAPI. The merged images are shown in the right column
Proliferation assay
To evaluate the proliferative capacity of the meningioma cells cultured by different methods, MTS test was performed. The proliferation rate in the injection group was significantly higher than that in the surface plating group at day 7 (P<0.05, Figure 4). However, there were no differences in cell proliferation features of meningioma cells between 2D, laminin, and the encapsulation methods (Figure 7).
Figure 7.
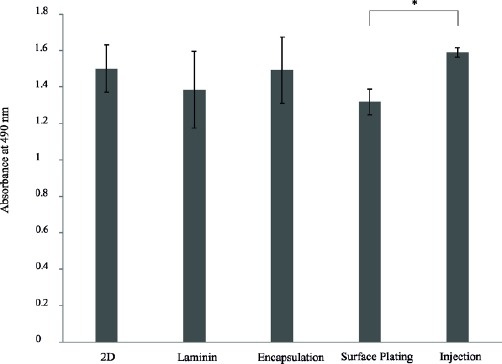
MTS cell proliferation assays of human meningioma stem-like cells after 7 days of culture. Cells were injected in PuraMatrix. *P<0.05 between the surface plating and injection groups
Cell viability
The percentage of live cells in each group at day 3 is presented in Figure 8. The results showed that the percentage of survival cells in the encapsulation group (76.5±6.1%) was not different from other groups after 3 days (P=0.4). At day 7, the percentage of live cells in the laminin group (89.3±2%) was similar to the other groups (P=0.12, Figure 9).
Figure 8.
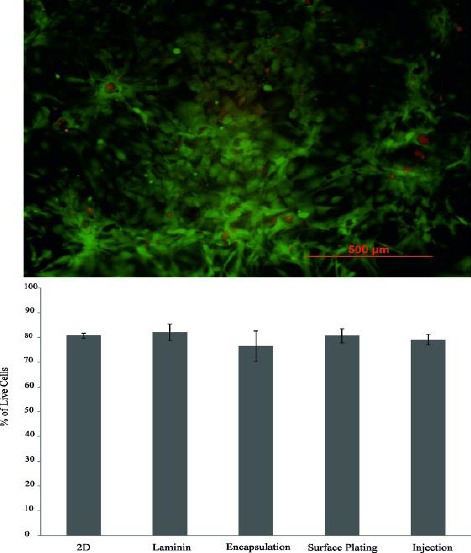
Live/Dead fluorescence image of human meningioma stem-like cells injected in PM after 3 days of culture. Red fluorescent nuclei indicate dead cells and live cells exhibit green fluorescent. Quantification of the staining by manual counting of living and dead cells of 2D, laminin, and 3D cultures are shown. There was no difference among the groups in terms of cell viability after 3 days of culture
Figure 9.
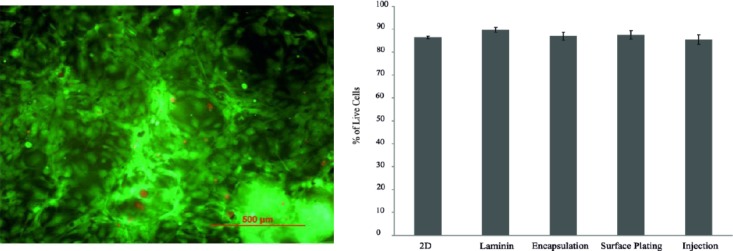
Live/Dead assay of human meningioma stem-like cells injected in PuraMatrix after 7 days of culture. The cell viability didn’t differ among the groups at day 7
Migration assay
The migration capacity of the meningioma cells was investigated in different concentrations of PM as well as in laminin in days 2, 3, 6, 7 and 9 of cell culture. The migration distance of meningioma cells cultured on laminin was higher compared to different PM concentration at different time points. In contrary, the lowest distance of cell traveling was observed in cell culture with 1% PM (Figure 10).
Figure 10.
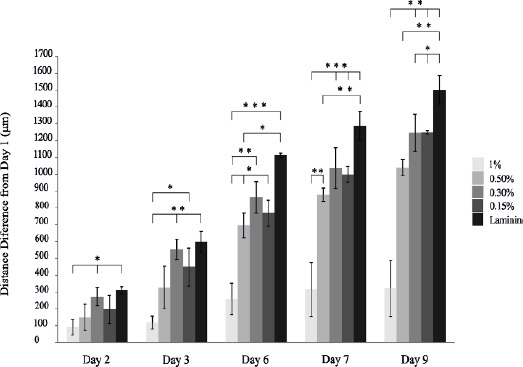
Migration capacity of human meningioma stem-like cells (hMgSCs) injected as a single cells within PuraMatrix (PM) at different periods after cell culture. Data indicated different migration capacity in laminin as well as different concentrations of PM (0.15%, 0.3%, 0.5%, and 1%). *, **, and *** indicate P<0.05, P<0.01, and P<0.001, respectively
At day 2, cells in the laminin group traveled a longer distance compared to the cells in 1% PM group (P<0.05). Among different 3D groups, the migration distance in 0.3% PM was significantly higher than that in 1% PM in the second day (P<0.05). At day 3, the cells cultured on laminin migrated a longer distance compared to the cells cultivated in 1% PM (P<0.01). Moreover, the migration distance of the cells in 0.15% and 0.3% PM was significantly longer than that in 1% PM (P<0.01 and P< 0.05, respectively). At day 6, the traveled distance in the laminin group was higher than that in 0.5% and 1% PM groups (P<0.01 and P<0.05, respectively). Moreover, the migration distance of cells in 0.15%, 0.3%, and 0.5% PM was significantly higher than that in 1% PM (P<0.05).
At days 7, the cells in the laminin group traveled a longer distance compared to cells in 0.5% and 1% PM groups (P<0.001 and P<0.01, respectively). This index in 0.5% (P<0.001), 0.3%, and 0.15% (P<0.01) PM was significantly higher than that in 1% PM group. At day 9, the cells cultivated on laminin traveled a longer distance compared to cells in 1% PM (P<0.001), 0.5% (P<0.01), 0.3%, and 0.15% PM groups (P<0.05). In addition, the migration distance in 0.15%, 0.3% and 0.5% PM was significantly higher than that in 1% PM (P<0.001).
Discussion
In this study, we isolated and cultured tumor stem-like cells from human meningioma tissue. Our results showed that neurospheres could be derived from meningioma tissue under neurosphere culture conditions and expressed NSCs, tumor stem cells, and astrocyte markers. 3D culture of these cells with PM by different methods showed that the viability of cells was lower in the encapsulation group compared to the other methods at day 3. The proliferation of the meningioma cells in the injection method was significantly higher than other approaches. In addition, we designed a new method to evaluate migration distance of tumor stem-like cells in a 3D culture using different concentrations of PM. The results indicated that the migration distance of the cells was affected by the concentration of PM.
In the present study, meningioma sphere cells were isolated and cultivated from human meningioma tissue. Meningioma tissue contains a lot of interstitial matrixes/fibers; makes it hard to digestion (16). Long digestion by using enzyme and/or mechanical methods leads to cell death and reduces the number of living cells. As the majorities of meningioma cells are benign and cannot easily spread, one of the main problems in meningioma tissue culture is low cell growth. A defined system for the cultivation of meningioma cells has not been introduced. Tang et al established a digestion method that consisted of three enzyme cocktail recipes (collagenase/dispase/DNase) and a four-step digestion method (16). In our study, we used 0.05% trypsin for enzymatic digestion followed by mechanical intervention (repeated pipetting). Using this inexpensive and comfortable method, we obtained a large number of single cells. According to previous studies, the proliferation rate of meningioma cells in monolayer culture was less than that in sphere culture (16, 19). To enhance cell growth, we cultured meningioma cells under suspended spheres status. Our neurosphere medium contained B27 supplement, N2 supplement, bFGF, and EGF. Previous investigations used conditional NSCs medium containing progesterone and androstenedione at hypoxic condition for culture of meningioma cells (16, 20). In our cultivation methods, cells are not manipulated with hormones and oxygen deprivation.
Using immunostaining, we assessed the expression of tumor and NSCs markers in the spheres derived from human meningioma tissue cultured in neurosphere medium. Our results revealed that nestin and vimentin expressed in stem-like cells derived from human meningioma cells. Intermediate filament bundles are formed by gathering of nestin and vimentin in neuroepithelial cells during neural embryogenesis (21). Nestin is detected in most of the gliomas and glioblastomas (22). In contrary to our findings, using a different isolation and cultivation methods, a lack of immunoreactivity for nestin was reported in meningioma sphere cells (16). GFAP, a structural protein which is the predominant component of glial intermediate filaments, is accepted as a reliable marker for normal mature and developing, and reactive and neoplastic astrocytes (23). A few cases of meningioma with triple expression of GFAP, vimentin and cytokeratin have been reported previously (24, 25). It has been revealed that the cells in the original and recurrent meningioma tumors were positive for vimentin and GFAP (26). In keeping with our results, it has been also reported that GFAP is expressed in most of astrocytoma and glioma tumors (27). Our study indicated the expression of CD133 in some hMgSCs. Immunofluorescence studies suggested that the expression of CD133 in human glioma and NSCs are positively correlated with higher proliferation capacity of meningioma cells (16, 17, 28).
In the present study, PM as a self-assembling peptide was used for creating a supporting scaffold for hMgSCs. PM is a nontoxic, biodegradable, and biocompatible hydrogel, which was used to induce neuritis outgrowth and synapse formation in pheochromocytoma 12 cells (29). It has been reported that proliferation of NSCs and migration of hippocampal neural cells were amplified by cultivation in PM (5, 30). Cultivation in PM has also been used to enhance osteoblast proliferation and migration (6). These data are in line with our results indicating that PM could change the tumor microenvironments and amplify the cell proliferation. However, it should be noted that cultivation in RADA16-I has been reported to inhibit the growth of human leukemia cells in vitro and in vivo (31).
Recently, we have found a significant increase in cell viability and proliferation after injection and surface plating of cultivation of neural stem/progenitor cells in PM compared to the encapsulation method (18). In the present study, although there was no difference in survival of hMgSCs among different methods of cell cultivation in PM, the proliferation rate was markedly improved using the injection method. Mechanical factors and pH have significant impacts on regulating stem cell proliferation (32). It seems that the injection method, especially at low concentration of PM, provides a better environment for cell proliferation compared to the surface plating and encapsulation approaches. The injection method has been suggested to provide a scaffold with proper pH and creates a well 3D environment, possibly by facilitation of fresh medium turn-over and adjusting pH to the physiological level (18).
To date, several approaches were used to evaluate the cell migration. In one of the previous methods, cell migration assays were determined by using trans-well migration chambers with membranes (pore size of 8 µm), which is a modified Boyden chamber (33). In this method, cells were seeded in the upper chamber of a trans-well, and allowed to migrate on the lower side of the inserts. After predetermined times, the migrating cells were stained, and then optical density or cell count were performed (33-36). One of the main disadvantages of this method is inability to measure the distance of cell migration. Another method was designed to evaluate the migration and development of fetal human neural stem cells cell in PM (10). It has been shown that PM (0.25%) had low toxicity and preserved several crucial properties of human fetal neural stem cells, such as migration and neuronal differentiation (10). This method measures the migration distance of the cell that traveled from the edge of each control sphere and sphere embedded in PM. However, this approach is not able to measure the migration of a single cell alone. To evaluate migration capacity of hMgSCs and in order to overcome most of these problems, we designed a method in 3D culture using injection method. We injected single cells in one side of PM at different concentrations and assessed single cell migration. Our results indicated that the migration of hMgSCs seems to be dependent on the scaffold concentration, so that the higher migration rates are obtained with lower PM concentrations with the optimal concentration of 0.3%. In this method, migration distance of a single cell could be evaluated.
Conclusion
Preparing a 3D culture with PM, especially using the injection method, provides an outstanding condition for evaluation of hMgSCs behavior. In addition, by using the injection method and migration assay, an appropriate approach is available to use in the investigations targeting cell migration.
Acknowledgment
This work was supported by the Shefa Neuroscience Center, Tehran, Iran grant related to Doctoral-Thesis 31528 and Iran National Science Foundation, Tehran, Iran (INSF).
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Zhang S, Gelain F, Zhao X. Designer self-assembling peptide nanofiber scaffolds for 3D tissue cell cultures. Elsevier: Seminars in cancer biology; 2005. [DOI] [PubMed] [Google Scholar]
- 2.Holmes TC, de Lacalle S, Su X, Liu G, Rich A, Zhang S. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc Natl Acad Sci. 2000;97:6728–6733. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhan X, Gao M, Jiang Y, Zhang W, Wong WM, Yuan Q, et al. Nanofiber scaffolds facilitate functional regeneration of peripheral nerve injury. Nanomed Nanotechnol Biol Med. 2013;9:305–315. doi: 10.1016/j.nano.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Zhang S, Ellis-Behnke R, Zhao X, Spirio L. PuraMatrix: self-assembling peptide nanofiber scaffolds. In: Elisseeff PX, Ma J, editors. Scaffolding in Tissue Engineering. Boca Raton (FL): CRC; 2005. pp. 217–238. [Google Scholar]
- 5.Gelain F, Bottai D, Vescovi A, Zhang S. Designer self-assembling peptide nanofiber scaffolds for adult mouse neural stem cell 3-dimensional cultures. PloS One. 2006;1:e119. doi: 10.1371/journal.pone.0000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horii A, Wang X, Gelain F, Zhang S. Biological designer self-assembling peptide nanofiber scaffolds significantly enhance osteoblast proliferation, differentiation and 3-D migration. PloS One. 2007;2:e190. doi: 10.1371/journal.pone.0000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Horii A, Zhang S. Designer functionalized self-assembling peptide nanofiber scaffolds for growth, migration, and tubulogenesis of human umbilical vein endothelial cells. Soft Matter. 2008;4:2388–2395. [Google Scholar]
- 8.Kumada Y, Zhang S. Significant type I and type III collagen production from human periodontal ligament fibroblasts in 3D peptide scaffolds without extra growth factors. PLoS One. 2010;5:e10305. doi: 10.1371/journal.pone.0010305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moradi F, Bahktiari M, Joghataei MT, Nobakht M, Soleimani M, Hasanzadeh G, et al. BD PuraMatrix peptide hydrogel as a culture system for human fetal Schwann cells in spinal cord regeneration. J Neurosci Res. 2012;90:2335–2348. doi: 10.1002/jnr.23120. [DOI] [PubMed] [Google Scholar]
- 10.Thonhoff JR, Lou DI, Jordan PM, Zhao X, Wu P. Compatibility of human fetal neural stem cells with hydrogel biomaterials in vitro. Brain Res. 2008;1187:42–51. doi: 10.1016/j.brainres.2007.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavalcanti BN, Zeitlin BD, Nör JE. A hydrogel scaffold that maintains viability and supports differentiation of dental pulp stem cells. Dent Mater. 2013;29:97–102. doi: 10.1016/j.dental.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mi K, Wang G, Liu Z, Feng Z, Huang B, Zhao X. Influence of a self-assembling peptide, RADA16, compared with collagen I and matrigel on the malignant phenotype of human breast-cancer cells in 3D cultures and in vivo. Macromol Biosci. 2009;9:437–443. doi: 10.1002/mabi.200800262. [DOI] [PubMed] [Google Scholar]
- 13.Cheng TY, Chen MH, Chang WH, Huang MY, Wang TW. Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering. Biomaterials. 2013;34:2005–2016. doi: 10.1016/j.biomaterials.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 14.Dolecek TA, Dressler EVM, Thakkar JP, Liu M, Al-Qaisi A, Villano JL. Epidemiology of meningiomas post-Public Law 107-206: The Benign Brain Tumor Cancer Registries Amendment Act. Cancer. 2015;121:2400–2410. doi: 10.1002/cncr.29379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mawrin C, Kalamarides M. Meningiomas Molecular Pathology of Nervous System Tumors. Springer; 2015:225–238. [Google Scholar]
- 16.Tang H, Gong Y, Mao Y, Xie Q, Zheng M, Wang D, et al. Cd133-positive cells might be responsible for efficient proliferation of human meningioma cells. Int J Mol Sci. 2012;13:6424–6439. doi: 10.3390/ijms13056424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hueng DY, Sytwu HK, Huang SM, Chang C, Ma HI. Isolation and characterization of tumor stem-like cells from human meningiomas. J Neuro-oncol. 2011;104:45–53. doi: 10.1007/s11060-010-0469-1. [DOI] [PubMed] [Google Scholar]
- 18.Aligholi H, Rezayat SM, Azari H, Mehr SE, Akbari M, Mousavi SMM, et al. Preparing neural stem/progenitor cells in PuraMatrix hydrogel for transplantation after brain injury in rats: A comparative methodological study. Brain Res. 2016;1642:197–208. doi: 10.1016/j.brainres.2016.03.043. [DOI] [PubMed] [Google Scholar]
- 19.Puduvalli VK, Li JT, Chen L, McCutcheon IE. Induction of apoptosis in primary meningioma cultures by fenretinide. Cancer Res. 2005;65:1547–1553. doi: 10.1158/0008-5472.CAN-04-0786. [DOI] [PubMed] [Google Scholar]
- 20.Hsu DW, Efird JT, Hedley-Whyte ET. Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg. 1997;86:113–120. doi: 10.3171/jns.1997.86.1.0113. [DOI] [PubMed] [Google Scholar]
- 21.Sugawara Ki, Kurihara H, Negishi M, Saito N, Nakazato Y, Sasaki T, et al. Nestin as a marker for proliferative endothelium in gliomas. Lab Invest. 2002;82:345–351. doi: 10.1038/labinvest.3780428. [DOI] [PubMed] [Google Scholar]
- 22.Arai H, Ikota H, Sugawara Ki, Nobusawa S, Hirato J, Nakazato Y. Nestin expression in brain tumors: its utility for pathological diagnosis and correlation with the prognosis of high-grade gliomas. Brain Tumor Pathol. 2012;29:160–167. doi: 10.1007/s10014-012-0081-5. [DOI] [PubMed] [Google Scholar]
- 23.Bonnin JM, Rubinstein LJ. Immunohistochemistry of central nervous system tumors: Its contributions to neurosurgical diagnosis. J Neurosurg. 1984;60:1121–1133. doi: 10.3171/jns.1984.60.6.1121. [DOI] [PubMed] [Google Scholar]
- 24.Budka H. Non-glial specificities of immunocyto-chemistry for the glial fibrillary acidic protein (GFAP) Acta Neuropathol. 1986;72:43–54. doi: 10.1007/BF00687946. [DOI] [PubMed] [Google Scholar]
- 25.Wanschitz J, Schmidbauer M, Maier H, Rössler K, Vorkapic P, Budka H. Suprasellar meningioma with expression of glial fibrillary acidic protein: a peculiar variant. Acta Neuropathol. 1995;90:539–544. doi: 10.1007/BF00294817. [DOI] [PubMed] [Google Scholar]
- 26.Su M, Ono K, Tanaka R, Takahashi H. An unusual meningioma variant with glial fibrillary acidic protein expression. Acta Neuropathol. 1997;94:499–503. doi: 10.1007/s004010050739. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Hu W, Zhang Z, Wei D. Clinical and pathological studies of meningioma-glioma mixed tumor. Surg Sci. 2011;2:140. [Google Scholar]
- 28.Mao XG, Zhang X, Xue XY, Guo G, Wang P, Zhang W, et al. Brain tumor stem-like cells identified by neural stem cell marker CD15. Transl Oncol. 2009;2:247–257. doi: 10.1593/tlo.09136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwasaki M, Wilcox JT, Nishimura Y, Zweckberger K, Suzuki H, Wang J, et al. Synergistic effects of self-assembling peptide and neural stem/progenitor cells to promote tissue repair and forelimb functional recovery in cervical spinal cord injury. Biomaterials. 2014;35:2617–2629. doi: 10.1016/j.biomaterials.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 30.Semino CE, Kasahara J, Hayashi Y, Zhang S. Entrapment of migrating hippocampal neural cells in three-dimensional peptide nanofiber scaffold. Tissue Engin. 2004;10:643–655. doi: 10.1089/107632704323061997. [DOI] [PubMed] [Google Scholar]
- 31.Tang C, Shao X, Sun B, Huang W, Zhao X. The effect of self-assembling peptide RADA16-I on the growth of human leukemia cells in vitro and in nude mice. Int J Mol Sci. 2009;10:2136–2145. doi: 10.3390/ijms10052136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nava MM, Raimondi MT, Pietrabissa R. Controlling self-renewal and differentiation of stem cells via mechanical cues. Biomed Res Int. 2012;2012:797410. doi: 10.1155/2012/797410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rabenstein M, Hucklenbroich J, Willuweit A, Ladwig A, Fink GR, Schroeter M, et al. Osteopontin mediates survival, proliferation and migration of neural stem cells through the chemokine receptor CXCR4. Stem Cell Res Ther. 2015;6:99. doi: 10.1186/s13287-015-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung SY, Kim JM, Min SK, Kim OB, Jang DH, Kang HK, et al. The potential of laminin-2-biomimetic short peptide to promote cell adhesion, spreading and migration by inducing membrane recruitment and phosphorylation of PKCΔ. Biomaterials. 2012;33:3967–3979. doi: 10.1016/j.biomaterials.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Kim JM, Min SK, Kim H, Kang HK, Jung SY, Lee SH, et al. Vacuolar-type H+-ATPase-mediated acidosis promotes in vitro osteoclastogenesis via modulation of cell migration. Int J Mol Med. 2007;19:393–400. [PubMed] [Google Scholar]
- 36.Sugatani T, Alvarez U, Hruska KA. PTEN regulates RANKL-and osteopontin-stimulated signal transduction during osteoclast differentiation and cell motility. J Biol Chem. 2003;278:5001–5008. doi: 10.1074/jbc.M209299200. [DOI] [PubMed] [Google Scholar]


