Abstract
Objective(s):
Parkinson’s disease (PD) is a progressive neurological disorder associated with motor disabilities and cognitive dysfunction as well. Evidence indicates that PD occurs less frequently in women than men, confirming a role for steroid hormones in protection of dopaminergic nigrostriatal neurons. It is reported that soy genistein, an estrogen agonist phytoestrogen, display neuroprotective effects against neuronal death. In this study we evaluated the effect of genistein in animal models of Parkinsonism (P) and Parkinsonism + ovariectomized (OP).
Materials and Methods:
The experiments were carried out on the control, P and OP animals. Learning and memory abilities were evaluated using Morris water maze. The latency and speed of locating the platform were measured as cognitive indices. Motor behaviors were assessed by testing the animals in rota rod and the latency to fall from the rod was scored.
Results:
We found that Parkinsonism leads to the cognitive and motor disabilities; ovariectomy intensified these disorders. Whereas genistein treatment improved the maze performances in both P and OP animals it failed to influence the kinetic problems. Genistein displayed a neuroprotective effect on dopaminergic neurons.
Conclusion:
Positive impact of genistein on the spatial learning and memory may reflect its effects on the nigrostriatal pathway and striatum. Nevertheless, ineffectiveness of genistein on the motor disorders, despite its neuroprotective impacts, led us to conclude that the cognitive improvement by genistein may also contribute to its effects in other areas of brain.
Keywords: Genistein, Learning, Memory, Motor disorders, Ovariectomy, Parkinson
Introduction
Parkinson’s disease (PD) is a progressive neuro-pathological disease which is known as the second most frequent type of neurodegenerative disorder (1). PD is characterized by degeneration of dopaminergic neurons in substantia nigra pars compacta (SNpc) with a subsequent depletion of dopamine in striatum (2). A well-known hallmark of the disease is motor deficits as a result of loss of inhibitory circuit’s function (3). The motor symptoms cause both hypo- and hyperkinesia including muscular rigidity, brady-kinesia, resting tremor and postural instability (4-6). Progressed conditions of the disease, however, is also accompanied with cognitive deficiencies (7). Severity of disease and age underlie the cognitive capacity in Parkinsonism. The pathogenetic mechanisms of PD are broadly described, however, inflammation, apoptosis, aging, mitochondrial dys-function and oxidative stress are known as key factors contributed in the nigrostriatal pathway neuronal death (2-8-9).
A large body of evidence indicates neuroprotective effects of estrogens (10, 11). The role of estrogens in dopaminergic transmission in basal ganglia is well proved. It is shown that estrogen reduces dopamine depletion of striatum in the animal models of PD (12). However, positive effects of estrogen therapy in treatment of PD have been covered by unwanted side effects of the hormone such as breast and uterine cancer (13).
Phytoestrogens have been considered as appropriate replacements for synthetic estrogens. They are plant-derived non-steroidal compounds that resemble the effects of endogenous estrogen both structurally and functionally without undesired side effects. Along with estrogens, phytoestrogens are also found to display neuroprotective effects (14, 15). It is reported that soy phytoestrogen genistein display neuroprotective effects against neuronal death and decreased dopamine release in striatum (16).
This study was designed to assess if the phyto-estrogen genistein underlies cognitive impairments as well as motor deficits in animal models of Parkinsonism and also replace estrogen in ovariectomized animals with Parkinsonism.
Materialsnd Methods
Animals
Thirty two female Wistar rats (170-200 g) were obtained from animal breeding center of Kashan University of Medical Sciences. Animals were kept in room under standard conditions with a 12 hr light/dark cycle, temperature 22 °C and air humidity of 55—60%. Efforts were made to minimize the number of animals used and their suffering.
Nigrostriatal pathway lesion
6-hydroxydopamine hydrochloride was used for lesion of the nigrostriatal pathway (17). The neurotoxin provides useful animal models of PD by inducing degeneration of the dopaminergic neurons in the substantia nigra pars compacta (SNpc) (18). Neurotoxic effects of 6-OHDA occur through a two-step mechanism involving accumulation of the toxin into catechola-minergic neurons, followed by alteration of cellular homeostasis and finally neuronal damage (19).
The animals were anesthetized with intraperi-toneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg IP) and fixed in a stereotaxic frame. Bilateral lesion was made by injection of 6-OHDA (10 µg/µl in normal saline containing 0.2% ascorbate) via a 25 µl Hamilton syringe into the SNpc at the coordinates: AP, -5.3 mm from bregma; ML, 1.6 mm; DV, -8 mm (20). Fourteen days after injection of 6-OHDA the rats were entered experiments.
Animal groups
The animals were randomly divided into 4 groups with 8 rats in each. Parkinsonism was induced in three groups of animals (as just mentioned). The control (CON) animals and one 6-OHDA injected group (P) received vehicle. Also two groups of rats were subjected to bilateral ovariectomy and then received 6-OHDA. These two groups were pretreated with either genistein (OPG) or genistein solvent dimethyl sulfoxide (OP). The daily treatments with genistein were occurred for one week after ovariectomy.
Rota rod test
Rota rod test is a suitable method to check the motor coordination (21). In this study the animals were introduced to the rota rod test for evaluation of their locomotor function. The animals were trained three trials/day with 30 min intertrial interval for two consecutive days (22). Each trial lasted 5 min. The acceleration of rod was firstly set at 5 rpm and gradually increased to 45 rpm during 120 sec. The experiment continued for a total duration of 5 min with the same acceleration. The latency to fall from the rod was scored.
Learning and memory performance
All groups were tested for their ability of spatial learning by performing Morris water maze behavior-ral experiment. A circular galvanized tank (140 cm in diameter × 60 cm in depth) with four quadrants was used. Water was added to the maze up to 20 cm below the rim. Water temperature was maintained at approximately 22 °C. The pool was randomly divided into four equal quadrants named northeast, southeast, southwest and northwest. The escape platform (10 cm diameter) was submerged 1.5 cm below the water surface and placed at the midpoint of southeast quadrant. The position of the platform was unchanged throughout experiments. The room walls were provided with visual cues as spatial hallmarks. In the training session the rats were received four spatial trials/day for 4 consecutive days. On each trial animals were released facing the wall of the pool, in a pseudorandomly selected start position. If it failed to find the platform within 90 sec, it was guided to the platform and allowed to rest on it for 20 sec. Then the animal was gently dried and moved to a holding cage. In order to determine the capability of the animals to retrieve and retain information, the probe trial was conducted on day 5, 24 hr after the final training session. The platform was removed and the animal was released into the quadrant diagonally opposite to the quadrant containing the platform and allowed to swim for 30 sec. Performance on the training and probe trial phases was measured by the escape latency and the speed (to find the submerged platform), and the percent of time passed in the target quadrant, respectively. A video camera connected to an image analysis system was placed above the center of the water maze and data collected by a personal computer equipped with commercially available water maze software (Radiab 7, IR Iran) for subsequent behavioral analysis.
Histological procedures
After the behavioral testing, the animals were anesthetized by chloral hydrate (0.5 ml/100 g). Brains were perfused transcardinally with a neutral-buffered formalin fixative solution (NBF10%, pH=7.4). Then, the brains were removed from the skull and stored in the same solution at 4 °C overnight. Next, the brains were transferred into a tissue processor for 17.5 hr. Finally, the tissues were frozen and the coronal sections at 5 µM thick were prepared using cryostat technique. Cresyl violet (Nissl) staining was performed for assessment of the extent of histological lesion in the nigrostriatal pathway (23). The coronal sections of brains were stained with %1 cresyl violet, dehydrated in graded series of ethanol, immersed in xylene and mounted in Entellan. The data were not included for analyzing if degeneration of the nigrostriatal pathway was not confirmed.
Cell counting
Counting was done blind to the treatments received. Cell numbers were counted at a high power (×200) magnification. All sections were digitally photographed and Nissl-stained cell were counted by a blind reader with a Nikon microscope (Nikon, Japan) and expressed per square millimeter (mm2). Neurons were counted only when their nuclei were clearly visualized within one focal plane. Number of SNpc neurons was expressed as the total counts obtained from the representative sections (24).
Statistical evaluations
The data pooled from both the training phase of the Morris water maze and rota rod experiments were analyzed using repeated measures analysis of variance (ANOVA). Three-way ANOVA was applied to the values resulted from the probe trials and the cell counting. The ANOVA analyses were followed by LSD post hoc test if were significant. The statistical variations were considered significant if P<0.05. All data are reported as mean±SEM.
Results
Assessment of spatial learning and memory performances
Navigation in the water maze was selected as a special tool for evaluation of the cognitive aspects of Parkinsonism. The escape latency as well as the speed to locate the hidden platform and duration of time spent in the correct quadrant were considered as indices of spatial memory capabilities.
Training phase
The escape latency
The data pooled from four days training in the Morris water maze appeared a general significant difference between the four groups of animals (F3, 508=16.854; P<0.0001). The post test analysis showed that the P group had a lower performance compared to their CON counterparts (P=0.0001). Although both groups improved their maze steering, however, the CON animals showed a more pronounced improvement compared to the P group (about 25% and 70% difference between the two groups in the first and last days, respectively). Ovariectomy further decreased the maze learning in where the OP group displayed the worst behavior in learning the task (P=0.002, compared to P group). The estrogen agonist genistein significantly improved the maze learning so that the OPG groups showed a performance close to the CON animals (P=0.166). Figure 1 depicts how Parkinsonism, ovariectomy and the genistein treatment underlie the maze learning.
Figure 1.
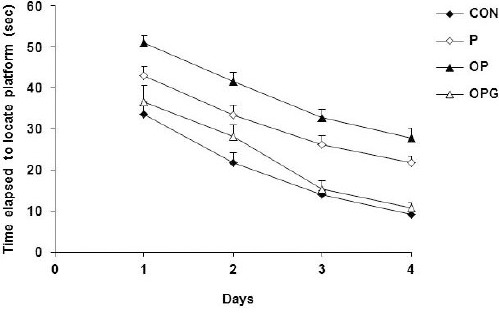
Illustration of escape latencies required to locate the hidden platform. While 6-OHDA injection led to a lower performance in the P animals ovariectomy further deteriorated the maze navigation in the OP group. The OPG group found the maze target in a decreased latency compared to their OP counterparts. Each point indicates average of data pooled from 4 trials. Data are shown as mean±SEM
The maze navigating speed
Cognitive strategies in Morris water maze considerably depends on normal motor activity. Because of deteriorated activity in the P, OP and OPG groups we also considered the speed of animals’ performances in the maze. Analysis of variance showed that the differences between the testing groups was statistically significant (F3,508=16.459; P<0.0001). The CON animals were considerably superior to the P rats and the difference was increased as the experiments proceeds (P=0.0001). Ovariectomy worsen the maze performance so that the OP group showed a significant decreased speed compared to P group (P=0.0001). The OPG animals receiving genistein had a higher speed during the maze steering when compared to the OP group (P=0.0001). Figure 2 illustrates the speed of the different groups in the water maze.
Figure 2.
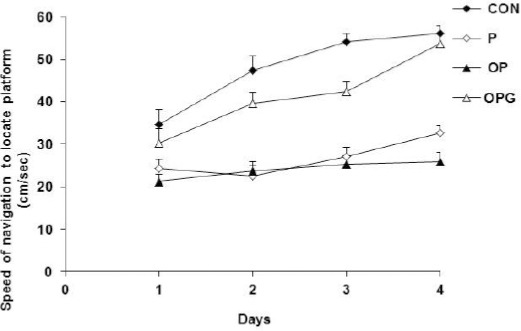
The speed by which the testing groups locate the hidden platform. The CON and OP displayed the highest and lowest speed in the maze navigation, respectively. Genistein had a positive effect on the performance of the OPG rats. Each point indicates average of data pooled from 4 trials. Data are shown as mean±SEM
Probe trial test
The animals were introduced to the probe test to elucidate how they can retrieve the learned task in the water maze. A general statistical difference was evident between all testing groups (F3,28=36.985; P<0.0001). The P rats navigated the correct quadrant with a duration of about half compared to the CON rats (P=0.0001). Ovariectomy further decreased the memory consolidation so that OP animals steered the target quadrant much shorter (by about one third) in comparison to the P group (P=0.034). The OPG animals treated with genistein displayed an almost similar retrieval test compared to their CON counterparts (Figure 3).
Figure 3.
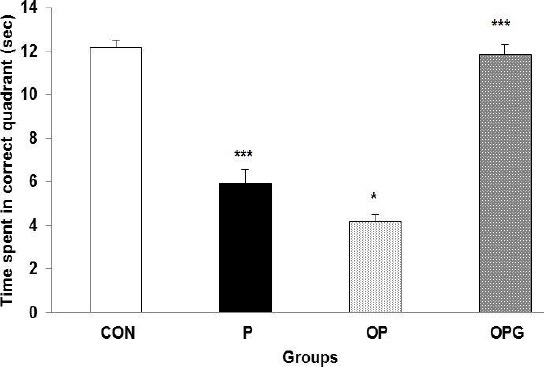
Histograms illustrate mean time elapsed in the correct quadrant as an index of memory consolidation test. The P and specially the OP animals spent a significant shorter time in the target quadrant. Genistein improved the probe testing in the Parkinson-ovariectomized groups. Data are shown as Mean ± SEM.
*** difference between CON v. P (P=0.0001); * difference between P v. OP (P=0.05); *** difference between $$OP v. OPG (P=0.0001)
Assessment of motor behaviors
The animals were introduced to rota rod apparatus to evaluate how the Parkinsonism affected the motor activity. Analysis of variance confirm a substantial difference between all groups entered the experiment (F3,28=6.785; P<0.0001). The duration of remaining on the rota rod was significantly longer in the control animals compared to the P subjects (P=0.020). Ovariectomy further disturbed the motor activity where the OP rats stayed less time in the rota rod compared to the P ones (P=0.036). As demonstrated in Figure 4, compared to the other testing groups, the CON animals displayed a higher improvement in the motor activity as the experiments proceed. The estrogen receptor agonist genistein not effectively underlined the behavior of the OPG rats (P=0.686).
Figure 4.
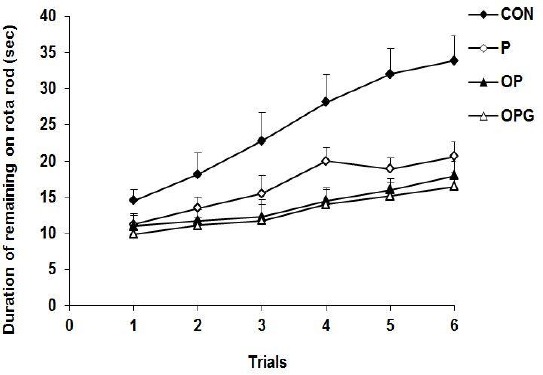
The latency to fall from the rota rod. The longest latency was obtained by the CON rats. The P group injected with 6-OHDA significantly decreased the remaining time on the rota rod. Ovariectomy intensified the disability in the motor behavior in the OP animals. The OPG animals treated with genistein behaved similar to the OP group
Histological findings
We also asked if ovariectomy or genistein treatment affect density of the neuronal loss in the nigrostriatal pathway. Statistical evaluations indicated that the counted neurons in the different testing groups were varied significantly (F3,104 =16.23; P<0.0001). Number of the neurons in the SNpc of the P group was considerably decreased in comparison to the control group (P=0.0001). Ovariectomy led to a further decrease in the dopaminergic neurons where the counted neurons were significantly lower in the OP rats compared to their P counterparts (P=0.004). The SNpc in the genistein treated animals displayed to contain substantially more dopaminergic neurons compared to the P (P=0.0001) and OP (P=0.0001) rats (Figure 5A). The sections taken from the OPG group showed an almost similar neuronal density as in the CON group (P=0.081). Figure 5B represents density of the dopaminergic neurons in the histologically processed sections of the SNpc area stained by Nissl method.
Figure 5A.
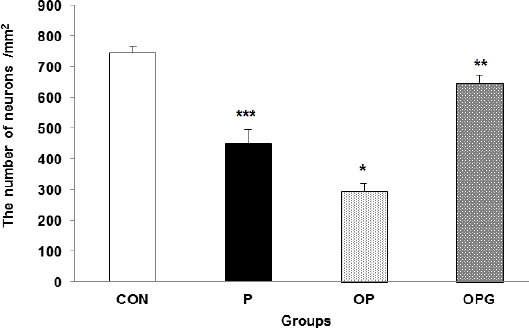
The number of neurons in a square millimeter area of the SNpc. Data are shown as Mean±SEM
*** difference between CON v. P (P =0.0001); * difference between P v. OP (P=0.01); ** difference between OP v. OPG (P=0.001)
Figure 5B.
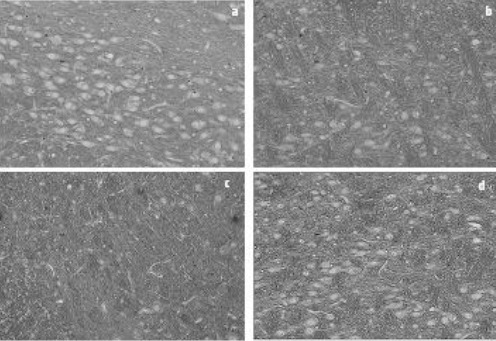
Photomicrographs of coronal sections (5 micrometers) of the SNpc stained with Nissl. a: control (CON); b: 6-OHDA injected (P); c: ovariectomized 6-OHDA injected (OP); d: OP receiving of genistein (OPG). Reduced number of the dopaminergic neurons is visible in the P group compared to the CON rats. Ovariectomy further decreased the nigrostriatal neurons in the OP. Genistein treatment prevented decrease in the dopaminergic neurons so that the slide taken from the OPG rats has a perspective close to that from the CON animals
Discussion
Probable role of phytoestrogens on Parkinson associated motor and cognitive problems were under focus of this study. Our findings indicated that bilateral injection of 6-OHDA in the SNpc leads to both memory impairment and motor disabilities. Ovariectomy exacerbates the cognitive and motor deficits. Whereas the estrogen receptor agonist genistein elicited a substantial improvement in the spatial learning and memory it was failed to influence the motor behaviors.
Consistent to our results are findings indicating impaired cognitive performances in 6-OHDA injected animals (25). Plenty evidence prove a link between estrogen and development of Parkinson related disorders. It is demonstrated that ovariectomy negatively affects learning and memory (26, 27). Parkinsonism worsens in women during the premenstrual period, when estrogen and progesterone levels are presumably at their nadir (28).
It is reported that post-menopausal reduction of estrogen leads to a reduced human cognition (29). Accordingly, women who had taken postmenopausal estrogen were less likely to develop PD (30). Gatto et al. found that PD less frequently occur in women than men, prompting investigation into whether estrogen protects dopaminergic neurons (31). Protective effect of estrogen against 6-OHDA induced dopaminergic degeneration has been reported in the ovariectomized animals (32). Also, Rugbjerg et al. (2013) reported a protective effect of estrogen on the risk for PD in women.
A relevancy between phytoestrogens and memory has been considered in some studies (14). In ovariectomized female rats phytoestrogen treatments resulted in a dose-dependent improvement of learning and memory (33). The OP rats fed with food riched with soya bean, consisting high amount of phytoestrogens, improve their spatial navigation in Morris water maze (34).
Our histological analysis verifies the behavioral results where the number of neurons in the SNpc was decreased in both the P and OP animals; more pronounced in the latter group. Phytoestrogens, as estrogen agonists (35-36), are known to play a significant role in reducing nerve damage and losing neurons through counteracting oxidative stresses (37-38). Having most active compound of isoflavonoids, genistein bands with both types of α and β estrogen receptors, with a more affinity to the latter (39-40). Beta-type estrogen receptors have been reported to be present in the dopaminergic neurons of the SNpc (40). Consistently, genistein considerably prevent the reduction in number of neurons in the SNpc in PD models of rats (16).
It is reported that phytoestrogens affect cognitive damage resulted from cholinergic deficits in Parkinson’s disease through the impact on cholinergic system and consequently decreased neuronal damage (41). This may be due to the presence of acetylcholine transferase mRNA in the frontal cortex of brain that protects and enhances cognitive performance (42-44). Through antioxidant activities (45) and reducing free radicals (46) genistein play a neuroprotective role on nerve cells damage in Parkinson’s patients. It is suggested that genistein, with antioxidant properties similar to estradiol, can be used to prevent or treat central neurodegeneration in postmenopausal period (47).
Further it is demonstrated that phyto-estrogens significantly ifluences the brain calcium-binding protein calbindin and plays an important role in mediating cell proliferation, apoptosis, and neurotoxicity (13). Phytoestrogens alter the expression of proteins involved in neural protection and inflammation in rats (35).
Our data indicated that genistein is ineffective on motor disorders in either P or OP groups. The effect of estrogen on motor behaviors in Parkinsonism has been controversial. Estrogen has a limited efficacy in the treatment of dyskinetic disorders (48). Reported that while estrogen had no effect on PD patients’ motor functioning, the use of progesterone worsened the symptoms of Parkinsonism (49). Moreover, others have concluded that estrogen increases disease severity (28-50).
Conclusion
We found that genistein treatment effectively restores the impaired spatial learning and memory in the P and OP animals. On the other hand, the phytoestrogen was failed to improve the disturbed motor activities. Accordingly, it seems that genistein substitutes estrogen in the ovariectomized rats and further prevents 6-OHDA induced neuronal loss. Both actions of genistein may be involved in the improved spatial learning and memory in the P and OP animals. However, other possible mechanisms such as antioxidant properties, positive impact on the cholinergic transmission and anti-inflammatory effects by genistein cannot be ruled out. Irrelevancy of the genistein actions with the dyskinesia observed in this study suggests that the cognitive improvement by genistein may also contribute to effect of the phytoestrogen on some areas of brain other than the striatum.
Acknowledgment
This study was supported by grant No.9302 from Kashan University of Medical Sciences to M. Salami. Special thanks to Dr H Haghdoost for providing 6-OHDA, Dr A Heidari for his helpful comments on the manuscript and Dr A Azami for assistance in histological procedures. The results appeared in this paper were part of student thesis (E Arbabi).
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008;119:1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skaper SD. The brain as a target for inflammatory processes and neuroprotective strategies. Ann N Y Acad Sci. 2007;1122:23–34. doi: 10.1196/annals.1403.002. [DOI] [PubMed] [Google Scholar]
- 3.Lau Y-S, Patki G, Das-Panja K, Le W-D, Ahmad SO. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson’s disease with moderate neurodegeneration? Eur J Neurosci. 2011;33:1264–1274. doi: 10.1111/j.1460-9568.2011.07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helmich RC, Hallett M, Deuschl G, Toni I, Bloem BR. Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain. 2012;135:3206–3226. doi: 10.1093/brain/aws023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Earhart GM. Dance as therapy for individuals with Parkinson disease. Eur J Phys Rehab Med. 2009;45:231–238. [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor K, Cook J, Counsell C. Heterogeneity in male to female risk for Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:905–906. doi: 10.1136/jnnp.2006.104695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aarsland D, Bronnick K, Fladby T. Mild cognitive impairment in Parkinson’s disease. Curr Neurol Neurosci Rep. 2011;11:371–378. doi: 10.1007/s11910-011-0203-1. [DOI] [PubMed] [Google Scholar]
- 8.Hu LF, Lu M, Tiong CX, Dawe GS, Hu G, Bian JS. Neuroprotective effects of hydrogen sulfide on Parkinson’s disease rat models. Aging Cell. 2010;9:135–146. doi: 10.1111/j.1474-9726.2009.00543.x. [DOI] [PubMed] [Google Scholar]
- 9.Vijitruth R, Liu M, Choi DY, Nguyen XV, Hunter RL, Bing G. Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson’s disease. J Neuroinflammation. 2006;3:1–16. doi: 10.1186/1742-2094-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McFarland K, Price DL, Davis CN, Ma JN, Bonhaus DW, Burstein ES, et al. AC-186, a selective nonsteroidal estrogen receptor beta agonist, shows gender specific neuroprotection in a Parkinson’s disease rat model. ACS Chem Neurosci. 2013;4:1249–1255. doi: 10.1021/cn400132u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70:200–209. doi: 10.1212/01.wnl.0000280573.30975.6a. [DOI] [PubMed] [Google Scholar]
- 12.Datla KP, Murray HE, Pillai AV, Gillies GE, Dexter DT. Differences in dopaminergic neuroprotective effects of estrogen during estrous cycle. Neuroreport. 2003;14:47–50. doi: 10.1097/00001756-200301200-00009. [DOI] [PubMed] [Google Scholar]
- 13.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, Hubbell FA, Ascensao J, Rodabough RJ, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350:991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 14.Bagheri M, Joghataei MT, Mohseni S, Roghani M. Genistein ameliorates learning and memory deficits in amyloid beta(1-40) rat model of Alzheimer’s disease. Neurobiol Learn Mem. 2011;95:270–276. doi: 10.1016/j.nlm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Huang YH, Zhang QH. Genistein reduced the neural apoptosis in the brain of ovariectomised rats by modulating mitochondrial oxidative stress. Br J Nutr. 2010;104:1297–1303. doi: 10.1017/S0007114510002291. [DOI] [PubMed] [Google Scholar]
- 16.Bagheri M. Neuroprotective Effect of Genistein: Studies in Rat Models of Parkinson’s and Alzheimer’s Disease. 2012 [Google Scholar]
- 17.Dexter DT, Holley AE, Flitter WD, Slater TF, Wells FR, Daniel SE, et al. Increased levels of lipid hydroperoxides in the parkinsonian substantia nigra: an HPLC and ESR study. Mov Disorders. 1994;9:92–97. doi: 10.1002/mds.870090115. [DOI] [PubMed] [Google Scholar]
- 18.Marti MJ, Saura J, Burke RE, Jackson-Lewis V, Jimenez A, Bonastre M, et al. Striatal 6-hydroxydopamine induces apoptosis of nigral neurons in the adult rat. Brain Res. 2002;958:185–191. doi: 10.1016/s0006-8993(02)03694-6. [DOI] [PubMed] [Google Scholar]
- 19.Windle WF, Rhines R, Rankin J. A Nissl method using buffered solutions of thionin. Biotech Histochem. 1943;18:77–86. [Google Scholar]
- 20.Paxinos GaCW., editor. The rat brain in stereotaxic coordinates. Academic press; 2004. [Google Scholar]
- 21.Atlante A, Bobba A, Paventi G, Pizzuto R, Passarella S. Genistein and daidzein prevent low potassium-dependent apoptosis of cerebellar granule cells. Biochem Pharmacol. 2010;79:758–767. doi: 10.1016/j.bcp.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Moazedi A, Ghotbeddin Z, Parham G. Comparison of the effects of dose-dependent zinc chloride on short-term and long-term memory in young male rats. Pak J Biol Sci. 2007;10:2704–2708. doi: 10.3923/pjbs.2007.2704.2708. [DOI] [PubMed] [Google Scholar]
- 23.Yamada K, Takayanagi M, Kamei H, Nagai T, Dohniwa M, Kobayashi K, et al. Effects of memantine and donepezil on amyloid beta-induced memory impairment in a delayed-matching to position task in rats. Behav Brain Res. 2005;162:191–199. doi: 10.1016/j.bbr.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 24.Azami Tameh A, Clarner T, Beyer C, Atlasi MA, Hassanzadeh G, Naderian H. Regional regulation of glutamate signaling during cuprizone-induced demyelination in the brain. Ann Anat. 2013;195:415–423. doi: 10.1016/j.aanat.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Zheng H, Kangas L, Harkonen PL. Comparative study of the short-term effects of a novel selective estrogen receptor modulator, ospemifene, and raloxifene and tamoxifen on rat uterus. Jsteroid Biochem Mol Biol. 2004;88:143–156. doi: 10.1016/j.jsbmb.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Tamtaji O, Taghizadeh M, Takhtfiroozeh S, Talaei S. The Effect of Elaeagnus Angustifolia Water Extract on Scopolamine-Induced Memory Impairment in Rats. Zanjan Univ Med Sci J. 2014;22:101–111. [Google Scholar]
- 27.Pourganji M, Hosseini M, Soukhtanloo M, Zabihi H, Hadjzadeh MA-r. Protective role of endogenous ovarian hormones against Learning and memory impairments and brain tissues oxidative damage induced by lipopolysaccharide. Iran Red Crescent Med J. 2014;16:13954–13962. doi: 10.5812/ircmj.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kompoliti K, Comella C, Jaglin J, Leurgans S, Raman R, Goetz C. Menstrual-related changes in motoric function in women with Parkinson’s disease. Neurology. 2000;55:1572–1575. doi: 10.1212/wnl.55.10.1572. [DOI] [PubMed] [Google Scholar]
- 29.Azizi-Malekabadi H, Hosseini M, Saffarzadeh F, Karami R, Khodabandehloo F. Chronic treatment with the nitric oxide synthase inhibitor, L-NAME, attenuates estradiol-mediated improvement of learning and memory in ovariectomized rats. Clinics. 2011;66:673–679. doi: 10.1590/S1807-59322011000400024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Currie LJ, Harrison MB, Trugman JM, Bennett JP, Wooten GF. Postmenopausal estrogen use affects risk for Parkinson disease. Arch Neurol. 2004;61:886–888. doi: 10.1001/archneur.61.6.886. [DOI] [PubMed] [Google Scholar]
- 31.Gatto N, Deapen D, Stoyanoff S, Pinder R, Narayan S, Bordelon Y, et al. Lifetime exposure to estrogens and Parkinson’s disease in California teachers. Parkinsonism Relat Disord. 2014;20:1149–1156. doi: 10.1016/j.parkreldis.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Perez AI, Borrajo A, Valenzuela R, Lanciego JL, Labandeira-Garcia JL. Critical period for dopaminergic neuroprotection by hormonal replacement in menopausal rats. Neurobiol Aging. 2015;36:1194–1208. doi: 10.1016/j.neurobiolaging.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 33.Pan Y, Anthony M, Clarkson TB. Effect of estradiol and soy phytoestrogens on choline acetyltransferase and nerve growth factor mRNAs in the frontal cortex and hippocampus of female rats. Exp Biol Med. 1999;221:118–125. doi: 10.1046/j.1525-1373.1999.d01-64.x. [DOI] [PubMed] [Google Scholar]
- 34.Sarkaki A, Amani R, Badavi M, Moghaddam AZ, Aligholi H, Safahani M, et al. Pre-treatment effect of different doses of soy isoflavones on spatial learning and memory in an ovariectomized animal model of Alzheimer’s disease. Pak J Biol Sci. 2008;11:1114–1119. doi: 10.3923/pjbs.2008.1114.1119. [DOI] [PubMed] [Google Scholar]
- 35.Lund TD, Lephart ED. Dietary soy phytoestrogens produce anxiolytic effects in the elevated plus-maze. Brain Res. 2001;913:180–184. doi: 10.1016/s0006-8993(01)02793-7. [DOI] [PubMed] [Google Scholar]
- 36.Rickard D, Monroe DG, Ruesink T, Khosla S, Riggs B, Spelsberg TC. Phytoestrogen genistein acts as an estrogen agonist on human osteoblastic cells through estrogen receptors αand β. J Cell Biochem. 2003;89:633–646. doi: 10.1002/jcb.10539. [DOI] [PubMed] [Google Scholar]
- 37.Liang HW, Qiu SF, Shen J, Sun LN, Wang JY, Bruce IC, et al. Genistein attenuates oxidative stress and neuronal damage following transient global cerebral ischemia in rat hippocampus. Neurosci Lett. 2008;438:116–120. doi: 10.1016/j.neulet.2008.04.058. [DOI] [PubMed] [Google Scholar]
- 38.Blum-Degen D, Haas M, Pohli S, Harth R, Romer W, Oettel M, et al. Scavestrogens protect IMR 32 cells from oxidative stress-induced cell death. Toxicol Appl Pharmacol. 1998;152:49–55. doi: 10.1006/taap.1998.8503. [DOI] [PubMed] [Google Scholar]
- 39.Arjmandi BH. The role of phytoestrogens in the prevention and treatment of osteoporosis in ovarian hormone deficiency. J Am Coll Nutr. 2001;20:398–402. doi: 10.1080/07315724.2001.10719175. [DOI] [PubMed] [Google Scholar]
- 40.Quesada A, Romeo HE, Micevych P. Distribution and localization patterns of estrogen receptor-βand insulin-like growth factor-1 receptors in neurons and glial cells of the female rat substantia nigra: Localization of ERβand IGF-1R in substantia nigra. J Comp Neurol. 2007;503:198–208. doi: 10.1002/cne.21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee Y-B, Lee HJ, Won MH, Hwang IK, Kang T-C, Lee J-Y, et al. Soy isoflavones improve spatial delayed matching-to-place performance and reduce cholinergic neuron loss in elderly male rats. J Nutr. 2004;134:1827–1831. doi: 10.1093/jn/134.7.1827. [DOI] [PubMed] [Google Scholar]
- 42.Sarkaki A, Amani R, Badavi M, Moghaddam AZ, Aligholi H, Safahani M, et al. Pre-treatment effect of different doses of soy isoflavones on spatial learning and memory in an ovariectomized animal model of Alzheimer’s disease. Pak J Biol Sci. 2008;11:1114–1119. doi: 10.3923/pjbs.2008.1114.1119. [DOI] [PubMed] [Google Scholar]
- 43.Tujioka K, Shi X, Ohsumi M, Tuchiya T, Hayase K, Uchida T, et al. Effect of quantity and quality of dietary protein on choline acetyltransferase and nerve growth factor, and their mRNAs in the cerebral cortex and hippocampus of rats. Amino Acids. 2009;36:13–19. doi: 10.1007/s00726-007-0019-0. [DOI] [PubMed] [Google Scholar]
- 44.Xu J, Zhu J, Shi C, Guo K, Yew DT. Effects of genistein on hippocampal neurodegeneration of ovariectomized rats. J Mol Neurosci. 2007;31:101–112. doi: 10.1007/s12031-007-0010-y. [DOI] [PubMed] [Google Scholar]
- 45.Liang HW, Qiu SF, Shen J, Sun LN, Wang JY, Bruce IC, et al. Genistein attenuates oxidative stress and neuronal damage following transient global cerebral ischemia in rat hippocampus. Neurosci Lett. 2008;438:116–120. doi: 10.1016/j.neulet.2008.04.058. [DOI] [PubMed] [Google Scholar]
- 46.Ma W, Yuan L, Yu H, Ding B, Xi Y, Feng J, et al. Genistein as a neuroprotective antioxidant attenuates redox imbalance induced by β-amyloid peptides 25–35 in PC12 cells. Int J Dev Neurosci. 2010;28:289–295. doi: 10.1016/j.ijdevneu.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Liu LX, Chen WF, Xie JX, Wong MS. Neuroprotective effects of genistein on dopaminergic neurons in the mice model of Parkinson’s disease. Neurosci Res. 2008;60:156–161. doi: 10.1016/j.neures.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Koller W, Barr A, Biary N. Estrogen treatment of dyskinetic disorders. Neurology. 1982;32:547–547. doi: 10.1212/wnl.32.5.547. [DOI] [PubMed] [Google Scholar]
- 49.Strijks E, Kremer JA, Horstink MW. Effects of female sex steroids on Parkinson’s disease in postmenopausal women. Clin Neuropharmacol. 1999;22:93–97. doi: 10.1097/00002826-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Saunders-Pullman R, Gordon-Elliott J, Parides M, Fahn S, Saunders H, Bressman S. The effect of estrogen replacement on early Parkinson’s disease. Neurology. 1999;52:1417–1417. doi: 10.1212/wnl.52.7.1417. [DOI] [PubMed] [Google Scholar]


