Abstract
Objective(s):
This paper aims to investigate synergistic inhibition of polysaccharide from Sepia esculenta ink (SIP), a newly isolated marine polysaccharide in our laboratory, on breast cancer MDA-MB-231 cells exposed to cisplatin.
Materials and Methods:
Cell viability of MDA-MB-231 cells was determined by CCK 8 assay. Median-effect concentration was analyzed using Chou-Talalay method that was also subjected to determine cell inhibition ratio and combined index, as well as interaction between SIP and cisplatin. Proliferation and migration abilities were detected with plate colony formation assay and cell wound scratch assay, respectively. Expression of MMP-2 and MMP-9 proteins was measured with Western blot assay.
Results:
Data showed that SIP not only suppressed proliferation and migration of MDA-MB-231 cells, and expression of MMP-2 and MMP-9 proteins, also promoted inhibition of cisplatin on proliferation, migration and MMPs expression of MDA-MB-231 cells, which indicates synergy inhibition of drug combination of SIP and cisplatin on breast cancer cells. The median-effect concentrations of cisplatin and SIP were 4.9 and 1659.6 μg/ml, respectively. Whereas the concentration of combination drug was 158.5 μg/ml. The data indicated that drug combination can decrease dosages of the two single agents, especially the usual dosage of cisplatin.
Conclusion:
This research demonstrated that SIP repressed proliferation and metastasis of MDA-MB-231 cells and promoted anticancer effect of cisplatin on the breast cancer cells. The data suggested that SIP is a potential natural drug that can be used as an auxiliary medicine alongside chemotherapy in treating breast cancer.
Keywords: Chou-Talalay method, Cisplatin, MDA-MB-231 cells, Sepia esculenta ink
Introduction
Breast cancer, most common cancer in women, is treated with initial surgical resection and adjuvant chemotherapy, although the undesirable event is generally hard to avoid (1). As a common chemotherapeutic drug, cisplatin has been proved to be effective in treating metastatic breast cancer, used alone or combined with other anticancer drugs (2, 3). Despite the positive roles of cisplatin in destroying breast cancer cells, cytotoxicity also occurs on normal cells, mediating nuclear DNA to inter- and intra-strand cross-linkage and eventually resulting in apoptosis (4). Reduction of cisplatin dosage is adopted to impair toxicity on normal tissues/organs, which should decrease the killing effects on breast cancer cells as a result.
Squid ink polysaccharide (SIP) has been proved to be a multifunctional marine active substance. Besides antioxidation (5-7), UV resistance (5), antimutagenesis (8), antitumor (9-11), and immune-potentiation (11), chemopreventative activity was also found in previous reports. Our findings showed that SIP could effectively attenuate the toxicity of cyclophosphamide on testes, protecting reproductive ability of mice via activation of the Nrf2/ARE pathway (6, 7). Also protection of SIP towards intestine of mice exposed to cyclophosphamide was reported elsewhere (12-14).
Furthermore Zong et al (11) found that SIP could enhance the antitumor activity of cyclophosphamide, which indicates a synergistic enhancing effect through the combination of anticancer agent and SIP.
Drug combination, commonly called cocktail therapy, is widely used to treat cancer. The therapeutic method can not only achieve synergistic therapeutic effect, but also reduce dosage and toxicity of antitumor agents, as well as reduce or delay the induction of drug resistance (15). The objectives should be based on optimal dose proportions of the drugs used, an important and necessary problem to be solved before therapy. The Chou-Talalay method for drug combination based on the median-effect equation is an analytical method, used in vitro, that can be used to quantify antagonism, synergism, and additive effect among the drugs (16). To take full advantage of the antitumor activities of cisplatin and SIP, as well as the chemopreventive activity of SIP, this study investigated the inhibitory effect of a combination drug of cisplatin and SIP on the proliferation and metastasis of breast cancer MDA-MB-231 cells. SIP used in this study is a newly isolated polysaccharide, with a different primary structure from the reported SIPs, from Sepia esculenta ink in our laboratory (17).
Materials and Methods
Cell culture
Breast cancer MDA-MB-231 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) and Ham F-12 (DMEM/F12 = 1:1) supplemented with 10% foetal bovine serum (FBS) at 37 °C, 5 % CO2 in a humidified atmosphere. Cells were harvested and seeded at a density of 1×106 cells/ml.
Proliferation and viability analysis of MDA-MB-231 cells
Cells (1×104 cells/ml) were seeded in 96-well cell culture plate, at 100 μl in each well, and subjected to determine cell viability when treated with cisplatin and/or SIP using cell counting kit 8 (CCK 8), 10 μl of CCK 8 solution were used in each well. At the end of this treatment, optical density values were read at a wavelength of 450 nm. Cell growth inhibiting rate (fa) was calculated as follows:
fa= (1-OD450nm in drug treatment cells/OD450 nm in vehicle treatment cells)×100%
Results determination using median-effect principle
Results were determined on the basis of median-effect equation fa/fu = (D/Dm)m that was reduced to the best-fit equation y = bx + a. Thereinto, a = -m lg Dm, b = m, x = lg D, y = lg (fa/fu), fa expresses the drug inhibitory rate, fu is 1 – fa, D represents the applied drug dosage, m is the slope of the line, and Dm is used to indicate median-effect concentration. Based on the equation, calculating the median-effect concentration and the required concentration each effective time-point of the two drugs was done to find the combination index (CI) of the two drugs. CI < 1, CI = 1, or CI > 1 denoted synergistic, additive, or antagonistic effect, respectively (16).
Plate colony formation assay
Logarithmic growth phase MDA-MB-231 cells were harvested and reseeded in 96-well cell culture plates, at 500 cells per well. After incubation for 24 hr, cells were treated with vehicle, SIP (800 μg/ml), cisplatin (3 μg/ml) and SIP (800 μg/ml) plus cisplatin (3 μg/ml), respectively. After 7 days, medium-free cells were fixed with 10% paraformaldehyde for 10 min and stained for 2 to 3 min with Giemsa stain, and then washed three times with phosphate buffered saline. The formed cell clones were counted and used to calculate colony forming efficiency as follows:
Colony forming efficiency = (number of cell clone / seeded cell count) × 100 %.
Cell wound scratch assay
MDA-MB-231 cells in their logarithmic growth phase were harvested and transferred into 6-well cell culture plates at density of 1×105 cells/ml. While cells grew to 90% confluence, the well-bottom of the cell plate was scratched with a sterile pipette tip and then we measured the scratch width and recorded it as T0. Cells were incubated for 15 min in drug-free medium followed by stimulated culture for 24 hr with vehicle, SIP and/or cisplatin. Finally scratch widths T24 were measured and used to calculate migration rate as follows:
Migration rate = (T0 – T24) / T0 × 100 %
Western blotting assay
Protein lysate from cells lysed at 4 °C in non-denaturing cell lysis buffer was denatured in protein sample buffer for 5 min in boiling water, and subjected to SDS-polyacrylamide gel electrophoresis. Following transferring to nitrocellulose membranes, the target proteins were probed with antibodies. A chemiluminescence detection system was used to visualize the membranes. The target protein content was normalised with β-actin protein content.
Statistical analysis
Data were analysed using SPSS 19.0 software. One-way analysis of variance and the post hoc Tukey HSD testing were used to evaluate differences between groups, P<0.05 and P<0.01 were considered significant.
Results
Cisplatin and/or SIP inhibited viability of MDA-MB-231 cells
Data (Table 1) from CCK 8 assay system showed that cisplatin (1, 2, 4, 8, 16 μg/ml) and/or SIP (100, 200, 400, 800, 1600 μg/ml) suppressed proliferation of the breast cancer cells to a significant extent, and the inhibition was observed to behave in a dose-dependent manner. Besides stronger inhibitory effect was visualized in combination drug treated cells, compared with the cells exposed to SIP or cisplatin.
Table 1.
Inhibition of the two drugs used on proliferation of breast cancer cells in a dose-dependent manner
| Cisplatin | SIP | Cisplatin + SIP | |||
|---|---|---|---|---|---|
| C (μg/ml) | fa (Dx1) | C (μg/ml) | fa (Dx2) | C (μg/ml) | fa (Dx1.2) |
| 1 | 0.1698 ± 0.015Aa | 100 | 0.2191 ± 0.028Aa | 1 + 100 | 0.3753 ± 0.021Aa |
| 2 | 0.2885 ± 0.012Bb | 200 | 0.3555 ± 0.036Bb | 2 + 200 | 0.6276 ± 0.035Bb |
| 4 | 0.4636 ± 0.018Cc | 400 | 0.3922 ± 0.029BBbc | 4 + 400 | 0.7053 ± 0.048Bc |
| 8 | 0.6261 ± 0.211Dd | 800 | 0.4529 ± 0.041BCcd | 8 + 800 | 0.8418 ± 0.057Cd |
| 16 | 0.7568 ± 0.256Ee | 1600 | 0.4964 ± 0.039Cd | 16 + 1600 | 0.8770 ± 0.061Cd |
Effect evaluation of the two drugs based on median-effect principle
According to the inhibitory effects of the two drugs on the cancer cells, linear equations describing the behaviour of the two drugs were fitted using the median-effect principle, respectively, and used to calculate m, Dm and r. The data are presented in Table 2. According to the Chou-Talalay method, the median-effect concentrations of cisplatin and SIP were 4.9 and 1659.6 μg/ml, respectively. Whereas the concentration of combination drug was 158.5 μg/ml, far lower than the sum of the two drugs. The data indicated that drug combination can reduce concentrations of the two single agents, especially the usual dosage of cisplatin.
Table 2.
Evaluation of the effects of two drugs based on median-effect principle
| Drugs | Linear equations | Median effect concentration (Dm, μg/ml) | R2 |
|---|---|---|---|
| Cisplatin | y = 0.9901x – 0.6816 | 4.9 | 0.9988 |
| SIP | y = 0.2865x – 0.9227 | 1659.6 | 0.9963 |
| Cisplatin+SIP | y = 0.8552x – 1.8801 | 158.5 | 0.9871 |
Note: y = lg (fa/fu), x = lg D
Combination index of cisplatin and SIP with different effect
Based on the data in Table 2, a combination index (CI) was deduced from different effects (fa) of combination of cisplatin and SIP, which was used to evaluate the interaction between the two combined drugs with their different effects (Figure 1). At fa< 0.3, CI>1 indicated antagonism between cisplatin and SIP. Whereas fa>0.4 showed CI<1, representing a synergetic effect of the two drugs.
Figure 1.
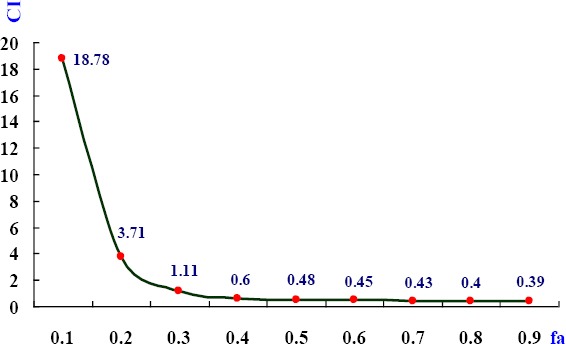
Combination indices of cisplatin and SIP with different effects. Under the combination of cisplatin and SIP with the scheduled concentrations, (cisplatin + SIP, 1 + 100, 2 + 200, 4 + 400, 8 + 800, and 16 + 1600, μg/ml), different effects (fa) on viability of MDA-MB-231 cells produced the corresponding combination indices (CI) of the two drugs
For ensuring to achieve better results in the following work, concentration of cisplatin and SIP were selected to be 3 μg/ml and 800 μg/ml, respectively.
Inhibition of cisplatin and/or SIP on the clonogenic capacity of MDA-MB-231 cells
In Figure 2, the three colony forming efficiencies were all markedly lower than the vehicle-treated cells. Moreover obvious difference was observed between co-treated and single-treated cells. In addition colony forming efficiency of SIP-exposed cells was significantly higher than that of cisplatin-stimulated cells.
Figure 2.
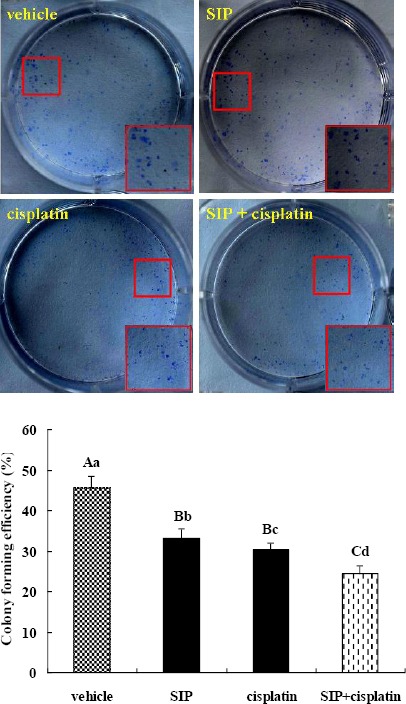
Inhibition of SIP and/or cisplatin on colony forming efficiency of MDA-MB-231 cells. Triple-negative breast cancer MDA-MB-231 cells were exposed to SIP (800 μg/ml), cisplatin (3 μg/ml), or a combination thereof (800 μg/ml of SIP + 3 μg/ml of cisplatin). While the colony number of combination-treated cells was more than 50, incubation of all groups of cells was stopped and cells were fixed, stained, and counted. abcdP<0.05, ABCP<0.01
Inhibition of cisplatin and/or SIP on migration of MDA-MB-231 cells
From Figure 3, it can be seen that both SIP and cisplatin clearly inhibited migration of cultured MDA-MB-231 cells, and the inhibition of the combination of the two drugs was more significant than their single use. Migration rates of SIP, cisplatin or combinations thereof were 28.6%, 19.6%, or 11.7%, respectively. Although cisplatin’s suppression on migration of the breast cancer cells was more marked than that of SIP, the natural polysaccharide resulted in a significant reduction in cell mobility. Also, a more obvious inhibition was observed in the wells co-treated with SIP and cisplatin, the decreased degree of mobility rate was extremely significant and was larger than that with both cisplatin and SIP.
Figure 3.
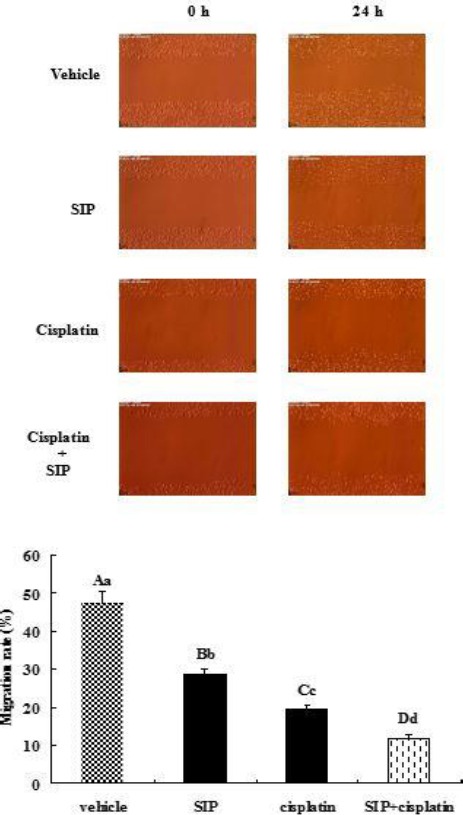
Inhibition of SIP and/or cisplatin on migration of MDA-MB-231 cells. MDA-MB-231 cells were treated with SIP (800 μg/ml), cisplatin (3 μg/ml), or a thereof (800 μg/ml of SIP + 3 μg/ml of cisplatin), respectively, for 24 hr. Scratch width at 0 or 24 hr was measured to calculate the migration rate of differently treated cells. abcdP<0.05, ABCDP<0.01
Inhibition of cisplatin and/or SIP on expression of MMP proteins in MDA-MB-231 cells
Figure 4 shows that cisplatin, SIP, and combinations thereof, all downregulated matrix metalloproteinase 2 (MMP-2) protein contents in the cells, and combinations of the drugs induced a more obvious reduction than the two single agents. From the data presented in Figure 4, the MMP-9 protein content was decreased in co-treated cells compared with vehicle-, SIP- or cisplatin-treated cells. However both SIP and cisplatin failed to inhibit expression of the MMP-9 protein in MDA-MB-231 cells.
Figure 4.
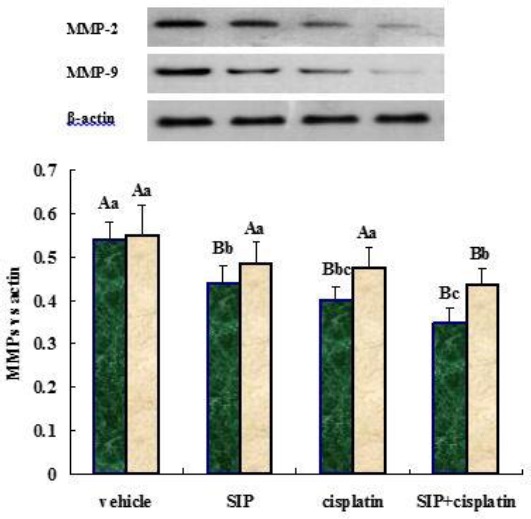
Inhibition of SIP and/or cisplatin on expression of MMPs proteins in MDA-MB-231 cells. MDA-MB-231 cells were treated with SIP (800 μg/ml), cisplatin (3 μg/ml), or a combination thereof (800 μg/ml of SIP + 3 μg/ml of cisplatin), respectively. Proteins in nitrocellulose membrane were probed using specific antibodies against MMP-2, MMP-9 and β-actin. The secondary antibodies were peroxidase-conjugated polyclonal antibodies. Darker and light-coloured bars represent MMP-2 or MMP-9 proteins, respectively. abcP<0.05, ABP<0.01
Discussion
Breast cancer has become one of the most dangerous cancers and accounts for 30% of all the female cancers (1). It is divided into three types according to different receptors, including human epidermal growth factor receptor-2 (HER-2) positive, hormone receptor (HR) positive and triple negative breast cancer (TNBC). TNBC cells cannot express HER-2, PR (progesterone receptor) and ER (oestrogen receptor). Clinical features of TNBC include earlier onset age, poor prognosis and high metastasis. Metastasis is diagnosed in 70% of patients, which is a main death cause of breast cancer (18, 19). The MDA-MB-231 cell derived from TNBC was used to undergo treatment by combination drug therapy using cisplatin and SIP.
Apart from surgical resection, chemotherapy is an important treatment among the therapies used towards the cancer. As a widely used chemotherapeutic agent, cisplatin can achieve a good therapeutic effect that should be the main basis for its clinical application for many years. Being a non-specific and cell cycle specific antitumor agent, the toxicity of cisplatin towards kidney, ear, and nerve increases gradually along with elevation of cisplatin content in serum (20, 21). The inevitable toxicity of the antitumor agent for its mechanisms is responsible for decreasing clinical dosages and resultantly declined treatment effects. In addition, resistance decreases the therapeutic effect of cisplatin on cancer (22, 23). So screening a low-toxicity agent to strengthen the antitumor activity of low-dosage cisplatin would be an effective method of curing cancer; SIP could be a potential anticancer agent that can be used as an adjunctive drug to strengthen the effects of cisplatin.
SIP was a naturally bioactive polysaccharide with a unique primary structure. Presently derived from squid ink, a discarded material in the course of squid processing. Presently, three laboratories have reported two types of SIP with different primary structures (8, 24, 25), but similar biofunctions, such as chemoprevention and antitumor activity (6, 7, 9-14). Based on our investigations over nearly ten years, a novel SIP with a different primary structure consisted of two major monosaccharides, galactosamine and arabinose, from those reports (8, 24, 25), was isolated from Sepia esculenta ink (17) and was proved to protect ovary of mouse from cyclophosphamide mediated cytotoxicity through regulating apoptosis and autophagy, via activation of PI3K/Akt and p38 MAPK pathways (17).
In this work we used the Chou-Talalay method to investigate the inhibitory effects of combination drug therapy using cisplatin and SIP on the proliferation and metastasis of breast cancer MDA-MB-231 cells. Combined, or single use of cisplatin and SIP inhibited proliferation of the breast cancer cells, furthermore, inhibition of combination drug therapy was superior to that arising from use of the two single agents and was so in a dose-dependent manner. Both cisplatin and SIP suppressed MDA-MB-231 cells with the median-effect concentrations of them were 4.9 μg/ml and 1659.6 μg/ml, respectively, However, the two agents were combined, the median-effect concentration of the drug combination was far lower than the sum of those as single agents, which indicated that a lower dosage of the combination of drugs could achieve higher inhibitory effects and that resistance and toxicity of cisplatin could be impaired through dose reduction of single usage. Based on cell growth inhibiting rate (fa) and combination index (CI) of the two drugs used here, it could be found that CI<1 corresponded to fa>0.4 implying a synergistic effect between cisplatin and SIP, and CI>1 (fa<0.3) indicated an antagonistic relationship between the drugs.
Inhibition of SIP on tumors was found in the few published papers (9-11), as was demonstrated once again by this study. Chen et al (9) reported that SIP did not affect HepG2 cell proliferation, but inhibited invasion and migration. Zong et al (10, 11) found that SIP inhibited tumor growth and metastasis in S180-bearing mice via inducing tumor cell apoptosis, inhibited metastasis of B16F10 cells, and caused angiogenesis. Although SIPs used in these trials had different primary structures, their inhibitory effects on tumour were similar. Our newly isolated SIP exhibited its inhibition on TNBC MDA-MB-231 cells, including proliferation and metastasis, which was similar to these reports (9-11). More than that, this study revealed that a combination of cisplatin and SIP showed a better inhibitory effect on colony formation ability and migration rate of MDA-MB-231 cells compared with cisplatin or SIP. Meanwhile, two key proteins promoting tumor metastasis, MMP-2 and MMP-9 (26), were both down-regulated more significantly in combination drug therapy on exposed cells than in two single-drug-treated cells. These data suggested that SIP strengthened antitumor activity of cisplatin on TNBC cells in a synergistic manner.
Conclusion
This work demonstrated that SIP can effectively promote the antitumor activity of cisplatin, a commonly used chemotherapeutic drug, on triple-negative breast cancer MDA-MB-231 cells. Some reports, including our previous research, have discovered that SIP can weaken cyclophosphamide-induced cytotoxicity in normal tissues/organs of model animals through regulating apoptosis and autophagy via activation Nrf2/ARE, PI3K/Akt, and p38 MAPK pathways. Therefore, a valuable hypothesis may be postulated as to whether or not, SIP impairs the killing effects of chemotherapeutic agents on cancer cells. Published research, and results in this paper, revealed that the speculation is false. SIP has been proved to be a double-faced functional natural bioactive polysaccharide towards chemotherapeutic drug-induced cytotoxicity, giving chemoprevention effects on normal cells and synergy enhancement on cancer cells. The other is that, based on the median-effect principle, SIP can reduce the clinical dosage of chemotherapeutic drugs to harvest two inspiring outcomes, strengthening therapeutic effects and reducing cytotoxicity and resistance of anticancer agents. Consequently SIP is a potential natural, marine drug for cancer treatment.
Acknowledgment
This work was supported by the Natural Science Foundation of Guangdong Province, China (2016A030313753), the National Natural Science Foundation of China (31171667), and the Opening Project of Guangxi Key Laboratory of Buffalo Genetics, Reproduction and Breeding (SNKF-2014-01). The results described in this paper were part of student thesis.
Conflict of interest
The authors declare there are no conflicts of interest regarding the publication of this paper.
References
- 1.Smith L, Welham KJ, Watson MB, Drew PJ, Lind MJ, Cawkwell L. The protemic analysis of cisplatin resistance in breast cancer cells. Oncol Res. 2007;16:497–506. doi: 10.3727/096504007783438358. [DOI] [PubMed] [Google Scholar]
- 2.Martin M. Platinum compounds in the treatment of advanced breast cancer. Clin Breast Cancer. 2001;2:190–208. doi: 10.3816/CBC.2001.n.022. [DOI] [PubMed] [Google Scholar]
- 3.Vassilomanolakis M, Koumakis G, Barbounis V, Demiri M, Panopoulos C, Chrissohoou M, et al. First-line chemotherapy with docetaxel and cisplatin in metastatic breast cancer. Breast. 2005;4:136–141. doi: 10.1016/j.breast.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Garand C, Guay D, Sereduk C, Chow D, Tsofack SP, Langlois M, et al. An integrative approach to identify YB-1-interacting proteins required for cisplatin resistance in MCF-7 and MDA-MB-231 breast cancer cells. Cancer Sci. 2011;102:1410–1417. doi: 10.1111/j.1349-7006.2011.01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo P, Liu HZ. Antioxidant ability of squid ink polysaccharides as well as their protective effects on DNA damage in vitro. Afr J Pharm Pharmacocl. 2013;7:1382–1388. [Google Scholar]
- 6.Le XY, Luo P, Gu YP, Tao YX, Liu HZ. Squid ink polysaccharide reduces cyclophosphamide-induced testicular damage via Nrf2/ARE activation pathway in mice. Iran J Basic Med Sci. 2015;18:827–831. [PMC free article] [PubMed] [Google Scholar]
- 7.Le XY, Luo P, Gu YP, Tao YX, Liu HZ. Interventional effects of squid ink polysaccharide on cyclophos-phamide-associated testicular damage in mice. Bratisl Med J. 2015;116:334–339. doi: 10.4149/bll_2015_063. [DOI] [PubMed] [Google Scholar]
- 8.Liu C, Li X, Li Y, Feng Y, Zhou S, Wang F. Structural characterization and antimutagenic activity of a novel polysaccharide isolated from Sepiella maindroni ink. Food Chem. 2008;110:807–813. doi: 10.1016/j.foodchem.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Chen S, Wang J, Xue C, Li H, Sun B, Xue Y, et al. Sulfation of a squid ink polysaccharide and its inhibitory effect on tumor cell metastasis. Carbohyd Polym. 2010;81:560–566. [Google Scholar]
- 10.Zong A, Liu Y, Zhang Y, Song X, Shi Y, Cao H, et al. Anti-tumor activity and the mechanism of SIP-S: A sulfated polysaccharide with anti-metastatic effect. Carbohyd Polym. 2015;129:50–54. doi: 10.1016/j.carbpol.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Zong A, Zhao T, Zhang Y, Song X, Shi Y, Cao H, et al. Anti-metastatic and anti-angiogenic activities of sulfated polysaccharide of Sepiella Maindroni ink. Carbohyd Polym. 2013;91:403–409. doi: 10.1016/j.carbpol.2012.08.050. [DOI] [PubMed] [Google Scholar]
- 12.Zuo T, Cao L, Xue C, Tang QJ. Dietary squid ink polysaccharide induces goblet cells to protect small intestine from chemotherapy induced injury. Food Funct. 2015;6:981–986. doi: 10.1039/c4fo01191k. [DOI] [PubMed] [Google Scholar]
- 13.Zuo T, He X, Cao L, Xue C, Tang QJ. The dietary polysaccharide from Ommastrephes bartrami prevents chemotherapeutic mucositis by promoting the gene expression of antimicrobial peptides in Paneth cells. J Funct Food. 2015;12:530–539. [Google Scholar]
- 14.Zuo T, Cao L, Li X, Zhang Q, Xue C, Tang Q. The squid ink polysaccharide protect tight junctions and adherens junctions from chemotherapeutic injury in the small intestinal epithelium of mice. Nutr Cancer. 2015;67:364–371. doi: 10.1080/01635581.2015.989369. [DOI] [PubMed] [Google Scholar]
- 15.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 16.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay Method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 17.Liu HZ, Tao YX, Luo P, Deng CM, Gu YP, Yang L, et al. Preventive effects of a novel polysaccharide from Sepia esculenta ink on ovarian failure and its action mechanisms in cyclophosphamide-treated mice. J Agr Food Chem. 2016;64:5759–5766. doi: 10.1021/acs.jafc.6b01854. [DOI] [PubMed] [Google Scholar]
- 18.Dawood S. Triple-negative breast cancer: Epidemiology and management options. Drugs. 2010;70:2247–2258. doi: 10.2165/11538150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Ressler S, Mlineritsch B, Greil R. Triple negative breast cancer. Memo. 2010;3:185–189. [Google Scholar]
- 20.Hatzopoulos S, Di Stefano M, Campbell KM, Falgione D, Ricci D, Rosignoli M, et al. Cisplatin ototoxicity in the Sprague Dawley rat evaluated by distortion product otoacoustic emissions. Audiology. 2001;40:253–264. [PubMed] [Google Scholar]
- 21.Liedert B, Materna V, Schadendorf D, Thomale J, Lage H. Overexpression of cMOAT (MRP2/ABCC2) is associated with decreased formation of platinum-DNA adducts and decreased G2-arrest in melanoma cells resistant to cisplatin. J Invest Dermatol. 2003;121:172–176. doi: 10.1046/j.1523-1747.2003.12313.x. [DOI] [PubMed] [Google Scholar]
- 22.Jia Y, Zhang C, Zhou L, Xu H, Shi Y, Tong Z. Micheliolide overcomes KLF 4-mediated cisplatin resistance in breast cancer cells by downregulating glutathione. OncoTargets Ther. 2015;8:2319–2327. doi: 10.2147/OTT.S88661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radin D, Lippa A, Patel P, Leonardi D. Lifeguard inhibition of Fas-mediated apoptosis: a possible mechanism for explaining the cisplatin resistance of triple-negative breast cancer cells. Biomed Pharmacother. 2016;77:161–166. doi: 10.1016/j.biopha.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 24.Takaya Y, Uchisawa H, Narumi F, Matsue H. Illexins A, B, and C from squid ink should have a branched structure. Biochem Biophys Res Commun. 1996;226:335–338. doi: 10.1006/bbrc.1996.1357. [DOI] [PubMed] [Google Scholar]
- 25.Chen SG, Xu J, Xue CH, Dong P, Sheng WJ, Yu GL, et al. Sequence determination of a non-sulfated glycosaminoglycan-like polysaccharide from melanin-free ink of the squid Ommastrephes bartrami by negative-ion electrospray tandem mass spectrometry and NMR spectroscopy. Glycoconjugate J. 2008;25:481–492. doi: 10.1007/s10719-007-9096-2. [DOI] [PubMed] [Google Scholar]
- 26.Thorns V, Walter GF, Thorns C. Expression of MMP-2, MMP-7, MMP-9, MMP-10 and MMP-11 in human astrocytic and oligodendroglial gliomas. Anticancer Res. 2003;23:3937–3944. [PubMed] [Google Scholar]


