Abstract
Objective(s)::
Granulocyte-colony stimulating factor (G-CSF) is used in clinical practice for the treatment of neutropenia and to stimulate generation of hematopoietic stem cells in bone marrow donors. In the present study, the ability of G-CSF in mobilizing exogenous bone marrow stem cells (BMSCs) from peripheral blood into the brain was tested. We for the first time injected a small amount of BMSCs through the tail vein.
Materials and Methods:
We choose 25 male Wistar rats (200–250 g) were lesioned by 6-OHDA injected into the left substantia nigra, pars compacta (SNpc). G-CSF (70 µg/kg/day) was given from the 7th day after lesion for five days. The BMSCs (2×105) were injected through the dorsal tail vein on the 7th day after lesion.
Results:
The number of rotations was significantly lower in the stem cell therapy group than in the control group. In the third test in the received G-CSF and G-CSF+stem cells groups, animals displayed significant behavioral recovery compared with the control group (P<0.05). There was a significant difference in the average of dopaminergic neurons in SNpc between the control group and G-CSF and G-CS+stem cells groups. We didn’t detect any labeling stem cells in SNpc.
Conclusion:
G-CSF can’t mobilize low amounts of exogenous BMSCs from the blood stream to injured SNpc. But G-CSF (70 µg/kg) is more neuroprotective than BMSCs (2×105 number[w1] of BMSCs). Results of our study suggest that G-CSF alone is more neuroprotective than BMSCs.
Keywords: Bone marrow stem cells, Dopaminergic neurons, Granulocyte colony stimulating -, factor, Parkinson
Introduction
Parkinson’s disease (PD), is a common progressive neurodegeneration disorder characterized by the degeneration of dopaminergic (DA) neurons, within the substantia nigra pars compacta (SNpc) and the accumulation of protein aggregates in the brain (1). PD affects 0.5–1% of people older than 65 years of age(2). In PD degeneration of midbrain DA neurons in the SNpc, reduce the dopamine level in the striatum (3-5), as a result it causes clinical symptoms such as rigidity, tremor resting and bradykinesia and abnormal reflex (6). Current therapeutic options for PD are L-dopa, dopamine agonists, enzyme inhibitors and deep brain stimulation in the thalamus, subthalamic nucleus, and globus pallidus (7). The therapeutic methods for PD patients are all symptomatic, but not essential and regenerative (8). A new treatment for PD is to transplant embryonic stem cells (ES) to the striatum, and results appear promising (9-11). One of the problems that must be solved is the formation of teratomas that can occur after transplantation of ES cell-derived midbrain neurons (12). Mesenchymal stem cells (MSCs) are derived from bone marrow and can differentiate into many types of cells like adipocyte, osteocyte, chondrocyte, neuron, and myocardium (13-17). One of the benefits of using bone marrow stem cells (BMSC), in PD is that if they can give rise to DA neurons, there is little possibility that immune rejection will occur because these cells can be easily derived from patients. Moreover, the use of an autograft solves the ethical problems related to ES cells (12). [w2] In many studies, it has been reported that hematopoietic cytokines like granulocyte macrophage-colony stimulating factor (GM-CSF), granulocyte-colony stimulating factor (G-CSF), or erythropoietin had neuroprotective effects and recovered neurologic functions after central nervous system (CNS) injury (18-23).
G-CSF is a growth factor that affects hematopoietic stem cell (CD34+) to regulate neutrophil progenitor proliferation and differentiation. G-CSF is used to mobilize stem cells for transplantation in patient with hematological malignancy (24). Adult neural stem cells express the G-CSF receptor. G-CSF can penetrate the intact blood-brain barrier (25) and plays a noticeable role in CNS (26). Many studies indicate that G-CSF protects against neurodegeneration in a number of neurological disease models such as PD(27, 28), Huntington’s disease (29), and cerebral ischemia (30). Interestingly, G-CSF has been shown to be able to cause neurogenesis in vitro and in the rodent brain (25). Therefore, G-CSF can be a novel regeneration therapy in stem cell therapy in treating neurodegenerative disease. However, there have been no reports on the effect of G-CSF on the transplanted BMSC in a PD model.
In order to survey this, in this study, after generating the PD model by 6-hydroxydopamin (6-OHDA), we evaluated the ability of G-CSF to migrate transplanted BMSC to SNpc and proliferate and differentiate these cells to DA neurons and restore nigrostriatal function.
Materials and Methods
Experimental protocol
A neurotoxin, 6-OHDA was injected into left SNpc of adult male Wistar rats. Rats were divided into 5 groups (n=5) : group 1, DMEM vehicle group, group 2, 6-OHDA lesion group, group3, 6-OHDA lesion followed by G-CSF treatment, group 4, 6-OHDA lesion followed by BMSC injection, group 5, 6-OHDA lesion followed by BMSC injection and G-CSF treatment. Group 3, was treated by G-CSF for seven days 7, days after 6-OHDA lesion. Group 4 were injected 2×105 BMSCs via the tail vein and group 5, were injected BMSC through the vena caudalis, 7 days after 6-OHDA and were injected 70 µg/kg intraperitoneally.
Animals
Adult male Wistar rats (200–250 g body weight) were provided by the Anatomy Department, Experimental Center of Semnan Medical University . All animals were maintained under temperature- and light-controlled conditions (20–23°C, 12-hr-light/12-hr-dark cycles) with free access to food and water. All procedures were carried out in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals that was authorized by the Ethics Committee of Semnan Medical University Semnan, Iran.
Hydroxydopamine lesion
All rats were anesthetized with 100 mg/kg ketamine and 20 mg/kg xylazine (IP) and placed in a stereotaxic instrument (Stoelting, USA). 2 µl of 6-OHDA (8 µg/µl of 6-OHDA dissolved in saline containing 0.2 mg/ml ascorbic acid) (Sigma-Aldrich), was injected into the left SNpc, using a 28-gauge Hamilton syringe, into the following coordinates: -4.8 mm anterior to the bregma, -1.6 mm lateral to the sagittal suture, and 8.2 mm dorsoventral to the surface of the brain with tooth-bar set at 3.3 mm (Paxinos and Watson, 1998). The injection rate was 1 µl/min, and the syringe was left in place for an additional 5 min before being retracted slowly (1 mm/min).
Behavioral test
The first week after the surgery was chosen for PD model estimation by scoring the rotational behavior. All rats were tested with apomorphine (Sigma, 2.5 mg/kg, IP). The number of contralateral rotations were counted 5 min after the injection and assessed for 30 min. Rats that performed more than seven times per min contralateral were considered to be adequate PD rats.
The behavioral test was repeated three and five weeks after the surgery.
BMSC culture
BMSCs were cultured according to a modification of the Sanchez-Ramos method (31). BMSCs were obtained in sterile condition from adult male Wistar rat tibias and femurs, by using a syringe with a 21 G needle and flushing the bones. The cells were cultured into each 75 cm2 culture flask in DMEM containing 20% fetal bovine serum (FBS) 100 U penicillin per millimeter and 100 U streptomycin per millimeter. Cells were seeded at 37 °C, in an atmosphere of 5% CO2. The medium was changed after 48 hr and every 3–4 days to remove the non-adherent cells, when the flask approached 80% confluence, the cells were detached by Incubation with 0.25% trypsin and 1 mM EDTA at 37 °C for 4-5 min and re-plated into 75 cm2 culture flasks.
The third generation of BMSCs was incubated in 3 µg/ml BrdU (sigma, USA) at 37 °C for 72 hr. The cells that were incubated with BrdU were washed 3 times with PBS after 72 hr to remove unconjugated BrdU and harvested with 0.25% tyrosine and 1 mM EDTA treatment (37 °C, 5% humidified CO2) and then centrifuged at 1000 rpm for 5 min. the pellets were washed 3 times with PBS. The number of cells for injection was 2×105, which was dissolved in 0.5 cc DMEM. The cells were injected through the vena caudalis in groups 4 and 5, nine days after surgery.
G-CSF injection schedule
G-CSF was diluted in its solvent to obtain a final concentration of 70 µg/ml and stored at 2–8 °C. G-CSF treatment started in groups 3 and 4, nine days after surgery by intraperitoneal injection at 70 µg/kg (32) for five consecutive days.
Immunohistochemical and histological study
In order to investigate DA neurons, the brains were fixed 24-48 hr in 2.5 paraformaldehyde. Coronal sections of 7 µm were cut using a microtome and processed as free-floating. The sections were first blocked in 10% methanol and H2O2 for 10 min in darkness. The slides were incubated in citrate buffer (pH=6) at 98°C for 10–15 min. The sections were blocked in 10% normal goat serum, 1% BSA, 0.3% Triton X-100 in PBS for 2 hr at room temperature. The sections were incubated with primary antibody anti-tyrosine hydroxylase (Abcam 6211) dissolved in 0.3% TBS with 1% BSA (1:500) overnight at 4°C. The sections were incubated in secondary antibody HRP (Abcam 6221) dissolved with 1% BSA (1:500) for 1 hr at room temperature. The sections were incubated with streptavidin-peroxidase polymer, ultrasensitive dissolved in PBS (1:200) for 30 min at room temperature. Chromogen 1% DAB was added to sections for 10 min in darkness. The sections were stained with 1% cresyl fast violet for 1 min. The immune positive cell bodies were counted by an observer blinded to treatment history. The slides were washed after all incubation stages. The picture of each section was taken by an Olympus AX70 microscope and DP11 digital camera under 40× magnification. An area of 10,000 μm² was measured randomly in the region of SNc in ten separate microscopic fields. Every fourth section throughout the entire SNpc was counted using NIH Image J software. To avoid double counting of neurons, immune positive cells were counted only when their nuclei were optically visible. All double-labeling was confirmed by rotating the image along each axis to ensure that the signals were localized within the same cell rather than separate cells in the close vicinity.
Bromodeoxyuridine (BrdU) staining
For BrdU staining, sections were first denatured in 2 N HCl for 30 min at 37°C washed extensively in 0.1 M borate buffer (pH=8.5) and rinsed well in PBS to bring pH back to 7.4. The sections were then exposed to primary mouse anti-BrdU (1:500). After immune staining, the sections were mounted on silane-coated microscope slides (Fisher Scientific) with Prolong Gold antifade reagent containing DAPI, a nuclear counterstain. Fluorescent signals were detected using n motic fluorescent microscope.
Statistical analysis
All data were expressed as mean±SEM. Data were analyzed using one-way ANOVA followed by Turkey’s post hoc test for DA neuron counts and optical density analysis. Two-way repeated measures ANOVA was used for in vivo microdialysis data and behavioral test. A value of P<0.05 was considered to be statistically significant.
Results
BMSCS result
The BMSCS derived from the femurs and tibias of adult rats. These cells comprised heterogeneous groups. After culturing the BMSCs, the initial plating of the adherent cells displayed a small rounded shape, a spindle shape, or large flattened morphology (Figure 1A). Following subculture, they adhered rapidly and expanded without visible changes in either their proliferation patterns or morphology (Figure 1B). The rounded cells disappeared after consecutive passages and fibroblast-like cells became enriched (Figure 1C). In the 3rd passage, the fibroblast-like cells became morphologically homogeneous (Figure 1D).
Figure 1.
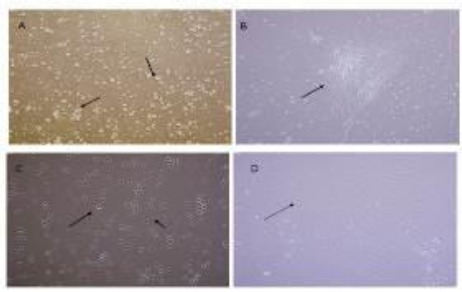
Cultured bone marrow cells. (A) In passage 0 stage adherent cells showed small round, spindle-shape (arrow) (x10). (B) Formation colony (arrow shows colony) (C). Most cells grew and showed fibroblast-like morphology (arrow) (x10). (D) In passage 3 the fibroblast-like cells became morphologically homogeneous (x10) Scale bar: 100 µm
Changes of rotational test
Toledo-Aral et al suggested rotational behavior in animal model of PD is significantly associated with DA neuron content in the striatum (33). The effects of treatment were examined in 6-OHDA lesioned rats by quantification of rotations in response to apomorphine. In the second test, the number of rotations was significantly lower in the stem cell therapy group than the control group. In the third test in the received G-CSF and G-CSF+stem cells groups, animals displayed significant behavioral recovery compared with the control group (P<0.05). Figure 2 shows the changes of the rotational test in the apomorphine administered group.
Figure 2.
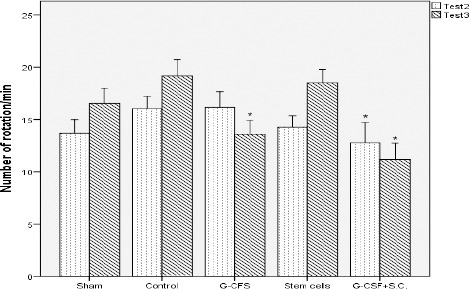
The result of behavioral tests, show statistically significant differences between the control group and stem cell, G-CSF, and G-CSF + BMSCs groups. In the second test, 3 weeks after surgery, in the G-CSF + BMSCs group the number of rotations significantly decrease. In the third test, 5 weeks after surgery, in G-CSF and G-CSF + BMSCs groups the number of rotations significantly decrease. * compared with the control group (P<0.05, All values are mean±SEM)
Immunohistochemistry
6-OHDA caused decreased number of dopaminergic neurons in SNpc. To investigate which of our treatments were more neuroprotective, we counted TH-immunoreactive dopaminergic neurons in SNpc. Representative image of neurons in SNpc of 6-OHDA (Figure 3A), G-CSF-treated (Figure 3B), stem cells (Figure 3C), G-CSF+stem cells (Figure 3D) groups are shown. The number of TH+ cells in the SNpc of the control group was 58.67±5.5, 107.00±7.2 in the G-CSF group, 85.33±4.9 in the stem cells group, and 111.00±7.3 in the G-CSF+stem cells group (P<0.05). There were significant differences in the averages of TH+ neurons in SNpc among the control, G-CSF, and G-CS+stem cells groups (Figure 4).
Figure 3.

Tyrosine hydroxylase immune histochemistry staining of substantia nigra of rat images: (a) control group, (b) G-CSF group, (c) stem cell group, (d) G-CSF+ stem cell group. Arrows show TH+ dopaminergic neurons (the brown spots)
Figure 4.
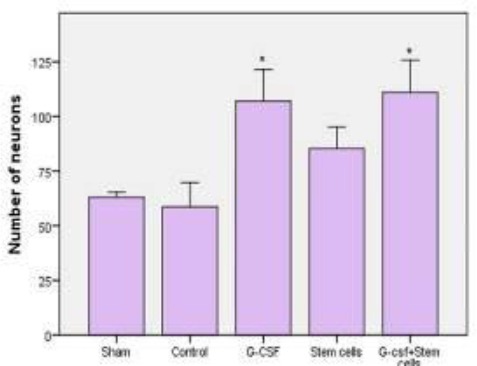
Treatment with G-CSF and G-CSF + BMSCs surviving dopaminergic neurons in substantia nigra. *compared with the control group (P<0.05, All values are mean±SEM)
Therefore G-CSF and G-CSF+stem cells can protect dopaminergic neurons against the toxic effects of 6-OHDA.
BrdU labeling
To detect migrated stem cells into SNpc by G-CSF, we performed BrdU staining as described before. After staining of brain sections, we did not detect any labeling stem cells in SNpc. We concluded G-CSF can’t mobilize 2×105 exogenous BMSCs from bloodstream to injured brain.
Discussion
In the present study, we investigated whether G-CSF is able to migrate low amounts of exogenous BMSCs from the bloodstream into SNpc. BMSCs have several characteristics which make them attractive for a novel therapeutic option for neurodegenerative disease including PD. BMSCs is able to self-renew and generate other types of cells, like oligodendrocyte, astrocyte, and neurons. Other characteristics of BMSCs are the ability to secrete a variety of neurotrophic factors. The current treatment just could decrease PD symptoms, therefore regenerative medicine with stem cell is a hopeful way to treat PD. In this paper, we tried to differentiate BMSCs to DA neurons by G-CSF. Most studies focus on in situ injection of BMSCs into lesion brain. This procedure is incisive and may cause infection. G-CSF is able to mobilize hematopoietic stem cells into the bloodstream, and stimulate them to differentiate to granulocytes. G-CSF can mobilize BMSCs in the peripheral blood (PB), and PB-BMSCs can integrate into injured cerebral tissue and differentiate into neural cells (34). We proposed if G-CSF can mobilize endogenous BMSCs into peripheral blood, perhaps it could mobilize exogenous BMSCs into the blood stream. One disadvantage of intravenous injection is embolism, therefore we chose small amounts of BMSCs for injection. We injected 2×105 BMSCs through the dorsal vein. This amount of BMSCs is used for in-situ injection in the brain. Park et al injected 106 BMSCs trough the tail vein and 1.7% of total cells penetrated into the injured brain (35). After BrdU staining we didn’t observe any labeled BMSCs in SNpc, and concluded G-CSF couldn’t mobilize 2×105 into SNpc. We suggest using the cells trapped in other organs such as the spleen, liver, and even the lungs. We assume G-SCF must be injected in higher doses with BMSCs. In higher doses perhaps G-CSF mobilizes exogenous BMSCs into the injured brain, or injection of more BMSCs via the tail vein is necessary. In this study injected BMSCs recovered the rotational behavior in the primary stage of the disease, but wasn’t statistically significant. It shows that BMSCs can produce a variety of factors which are neuroprotective and can survive DA neurons that result to recover rotational behavior. In BMSCs, the number of neurons was more than in the control group but wasn’t statically significant. In the third test, BMSCs couldn’t recover the rotational test. Therefor BMSCs in the blood stream with a low number are neuropro-tective and recover symptoms in the primary stage. BMSCs might have neuroprotective effects, but after immunohistochemistry staining, we observed they couldn’t keep pathway of SN, and TH+ is spread in lesion tissue (Figure 3c). In G-CSF and G-CSF + BMSCs groups, the pathway of SN was kept. Therefore G-CSF is the main factor that kept the pathway. In vitro studies suggest it can inhibit cells participating in the immune response and release a variety of the immunosuppressive factors (36-38). MSCs have neuroprotective effects on dopaminergic neurons with anti-inflammatory mechanism mediated by modulation of microglial activity (39). Therefore, we suggest the recovery motor dysfunction is that BMSCs secret neurotrophic factors into the bloodstream.
Another goal of this study was to evaluate the therapeutic ability of G-CSF in treating PD by using the 6-OHDA rat model of PD. Most motor symptoms of PD are the result of loss of dopaminergic neurons in the SNpc (40). We found G-CSF can decrease rotational behavior in the 4th week after beginning of PD progress. In second test BMSCs could reduce rotation test, therefore we conclude G-CSF present neuroprotective effects in passing time.
The highest number of neurons was counted in G-CSF+BMSCs (Figure 4). We suggest G-CSF is the certain factor for surviving neurons because the number of neurons in the BMSCs group was not statically significant, but was significant in the G-CSF group. One reason is that loss of DA neurons is result of inflammatory events and apoptosis cascade, therefore by suppression of inflammatory reaction and apoptosis cascade probably symptoms of PD decrease. G-CSF exerts its effects by stimulating secretion of anti-inflammatory factors and the active anti-apoptotic pathway. G-CSF has anti-inflammatory and anti-apoptotic effects, many studies demonstrated G-CSF protects against cell death in many animal models (41, 42).
Other investigations demonstrated that G-CSF decreases the level of inteleukin-1β, interluekin-6, and interluekin-8, which modulate inflammatory response (43-45). The activation of ERK pathway is the essential mechanism responsible for G-CSF-induced neuroprotection (40). Bad protein is a proapoptotic member of Bcl-2 family which displaces Bax from binding to Bcl-2 and Bcl-XL, resulting in cell death (46, 47). Overexpression of Bcl-2 in neurons makes these cells resistant to injury by 6-OHDA (48). G-CSF and its receptor (G-CSFR) were expressed in dopaminergic neurons in SN (40). Binding of G-CSF to G-CSFR causes receptor dimerization followed by the activation of associated JAK tyrosine kinases. JAK tyrosine kinases phosphorylate the G-CSFR and activate STAT transcriptional factors (49, 50). JAK and STAT pathway influence dopaminergic neuron viability (51). Anti-apoptotic BCL-2 family, Bcl-2, and Bcl-xl suppress the production of oxygen radicals and inhibit caspase activation in apoptosis cascade (52). Cao et al suggest increased expression of Bcl-2 and decreased expression of Bax, perhaps important mechanisms in protective effects of G-CSF against MPTP-induced cell death (27). 6-OHDA produces reactive oxygen species (ROS) (53). ROS-induced apoptosis is mediated by cytochrome C release and subsequently caspase-3 (54). G-CSF inhibits caspase-3 by stimulation of the ERK pathway (40).
Conclusion
The present study indicates that G-CSF is more neuroprotective than the low amount of BMSCs and saves neurons due to its neuroprotective effects, but can mobilize these exogenous cells to SNpc. We suggest in other studies, G-CSF is better used by more exogenous BMSCs because the present study and other studies demonstrate that G-CSF is neuro-protective in several doses, but G-CSF potential of mobilizing exogenous BMSCs from the bloodstream into the injured brain is not investigated. If G-CSF could mobilize exogenous BMSCs in low numbers from the bloodstream into the brain it could prevent embolism. From a therapeutic point of view, G-CSF alone is enough to protect dopaminergic neurons. The present results suggest injecting 106 - 3×103 BMSCs via the tail vein.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgment
This work was supported by a grant from the Nervous System Stem Cells Research Center, Department of Anatomy, Semnan University of Medical Sciences, Semnan, Iran. The results reported in this paper were part of a student thesis.
References
- 1.Ali F, Stott SR, Barker RA. Stem cells and the treatment of Parkinson’s disease. Exp Neurol. 2014;260:3–11. doi: 10.1016/j.expneurol.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Toulouse A, Sullivan AM. Progress in parkinson’s disease-where do we stand? Prog Neurobiol. 2008;85:376–392. doi: 10.1016/j.pneurobio.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Prakash N, Wurst W. Development of dopaminergic neurons in the mammalian brain. Cell Mol Life Sci. 2006;63:187–206. doi: 10.1007/s00018-005-5387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vernier P, Moret F, Callier S, Snapyan M, Wersinger C, Sidhu A. The degeneration of dopamine neurons in Parkinson’s disease: insights from embryology and evolution of the mesostriatocortical system. Ann N Y Acad Sci. 2004;1035:231–249. doi: 10.1196/annals.1332.015. [DOI] [PubMed] [Google Scholar]
- 5.Badban L, Safari M, Sameni HR, Bandegi AR, Vafaei AA, Rashidy-Pour A, et al. Protective effects of water extract of propolis on dopaminergic neurons, brain derived neurotrophic factor and stress oxidative actors in the rat model of parkinson’s disease. Int J Pharmacol. 2015;11:300–308. [Google Scholar]
- 6.Sethi KD. Clinical aspects of Parkinson disease. Curr Opin Neurol. 2002;15:457–460. doi: 10.1097/00019052-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Lindvall O, Kokaia Z. Prospects of stem cell therapy for replacing dopamine neurons in Parkinson’s disease. Trends Pharmacol Sci. 2009;30:260–267. doi: 10.1016/j.tips.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Kadota T, Shingo T, Yasuhara T, Tajiri N, Kondo A, Morimoto T, et al. Continuous intraventricular infusion of erythropoietin exerts neuroprotective/rescue effects upon Parkinson’s disease model of rats with enhanced neurogenesis. Brain Res. 2009;1254:120–127. doi: 10.1016/j.brainres.2008.11.094. [DOI] [PubMed] [Google Scholar]
- 9.Redmond DE, Jr, Sladek JR, Spencer DD. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;345:146–147. doi: 10.1056/NEJM200107123450214. [DOI] [PubMed] [Google Scholar]
- 10.Dunnett SB. Transplantation of embryonic dopamine neurons: what we know from rats. J Neurol. 1991;238:65–74. doi: 10.1007/BF00315683. [DOI] [PubMed] [Google Scholar]
- 11.Piccini P, Brooks DJ, Bjorklund A, Gunn RN, Grasby PM, Rimoldi O, et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson’s patient. Nat Neurosci. 1999;22:1137–1140. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- 12.Kim HJ. Stem cell potential in Parkinson’s disease and molecular factors for the generation of dopamine neurons. Biochim Biophys Acta. 2011;1812:1–11. doi: 10.1016/j.bbadis.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 14.Priller J, Persons DA, Klett FF, Kempermann G, Kreutzberg GW, Dirnagl U. Neogenesis of cerebellar Purkinje neurons from gene-marked bone marrow cells in vivo. J Cell Biol. 2001;155:733–738. doi: 10.1083/jcb.200105103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Bone marrow stem cells regenerate infarcted myocardium. Pediatr Transplant. 2003;3:86–88. doi: 10.1034/j.1399-3046.7.s3.13.x. [DOI] [PubMed] [Google Scholar]
- 16.Jadidi M, Biat SM, Sameni HR, Safari M, Vafaei AA, Ghahari L. Mesenchymal stem cells that located in the electromagnetic fields improves rat model of Parkinson’s disease. Iran J Basic Med Sci. 2016;19:741. [PMC free article] [PubMed] [Google Scholar]
- 17.Safari M, Jadidi M, Baghian A, Hasanzadeh H. Proliferation and differentiation of rat bone marrow stem cells by 400μT electromagnetic field. Neurosci Lett. 2016;612:1–6. doi: 10.1016/j.neulet.2015.11.044. [DOI] [PubMed] [Google Scholar]
- 18.Park HC, Shim YS, Ha Y, Yoon SH, Park SR, Choi BH, et al. Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Engin. 2005;11:913–922. doi: 10.1089/ten.2005.11.913. [DOI] [PubMed] [Google Scholar]
- 19.Grasso G, Sfacteria A, Passalacqua M, Morabito A, Buemi M, Macri B, et al. Erythropoietin and erythropoietin receptor expression after experimental spinal cord injury encourages therapy by exogenous erythropoietin. Neurosurgery. 2005;56:821–827. doi: 10.1227/01.neu.0000156493.00904.7e. discussion-7. [DOI] [PubMed] [Google Scholar]
- 20.Gibson CL, Jones NC, Prior MJ, Bath PM, Murphy SP. G-CSF suppresses edema formation and reduces interleukin-1beta expression after cerebral ischemia in mice. J Neuropathol Exp Neurol. 2005;64:763–769. doi: 10.1097/01.jnen.0000179196.10032.dd. [DOI] [PubMed] [Google Scholar]
- 21.Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci U S A. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouhy D, Malgrange B, Multon S, Poirrier AL, Scholtes F, Schoenen J, et al. Delayed GM-CSF treatment stimulates axonal regeneration and functional recovery in paraplegic rats via an increased BDNF expression by endogenous macrophages. FASEB J. 2006;20:1239–1241. doi: 10.1096/fj.05-4382fje. [DOI] [PubMed] [Google Scholar]
- 23.Ghahari L, Safari M, Joghataei MT, Mehdizadeh M, Soleimani M. Effect of combination therapy using hypothermia and granulocyte colony-stimulating factor in a rat transient middle cerebral artery occlusion model. Iran Biomed J. 2014;18:239. doi: 10.6091/ibj.13852.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sprigg N, Bath PM, Zhao L, Willmot MR, Gray LJ, Walker MF, et al. Granulocyte-colony-stimulating factor mobilizes bone marrow stem cells in patients with subacute ischemic stroke: the Stem cell Trial of recovery EnhanceMent after Stroke (STEMS) pilot randomized, controlled trial (ISRCTN 16784092) Stroke. 2006;37:2979–2983. doi: 10.1161/01.STR.0000248763.49831.c3. [DOI] [PubMed] [Google Scholar]
- 25.Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115:2083–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider A, Kuhn HG, Schabitz WR. A role for G-CSF (granulocyte-colony stimulating factor) in the central nervous system. Cell Cycle. 2005;4:1753–1757. doi: 10.4161/cc.4.12.2213. [DOI] [PubMed] [Google Scholar]
- 27.Cao XQ, Arai H, Ren YR, Oizumi H, Zhang N, Seike S, et al. Recombinant human granulocyte colony-stimulating factor protects against MPTP-induced dopaminergic cell death in mice by altering Bcl-2/Bax expression levels. J Neurochem. 2006;99:861–867. doi: 10.1111/j.1471-4159.2006.04125.x. [DOI] [PubMed] [Google Scholar]
- 28.Meuer K, Pitzer C, Teismann P, Kruger C, Goricke B, Laage R, et al. Granulocyte-colony stimulating factor is neuroprotective in a model of Parkinson’s disease. J Neurochem. 2006;97:675–686. doi: 10.1111/j.1471-4159.2006.03727.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee ST, Park JE, Kim DH, Kim S, Im WS, Kang L, et al. Granulocyte-colony stimulating factor attenuates striatal degeneration with activating survival pathways in 3-nitropropionic acid model of Huntington’s disease. Brain Res. 2008;1194:130–137. doi: 10.1016/j.brainres.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 30.Lu CZ, Xiao BG. Neuroprotection of G-CSF in cerebral ischemia. Front Biosci. 2007;12:2869–2875. doi: 10.2741/2278. [DOI] [PubMed] [Google Scholar]
- 31.Ye M, Chen S, Wang X, Qi C, Lu G, Liang L, et al. Glial cell line-derived neurotrophic factor in bone marrow stromal cells of rat. Neuroreport. 2005;16:581–584. doi: 10.1097/00001756-200504250-00013. [DOI] [PubMed] [Google Scholar]
- 32.Prakash A, Chopra K, Medhi B. Granulocyte-colony stimulating factor improves Parkinson’s disease associated with co-morbid depression: an experimental exploratory study. Indian J Pharmacol. 2013;45:612–615. doi: 10.4103/0253-7613.121374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toledo-Aral JJ, Mendez-Ferrer S, Pardal R, Lopez-Barneo J. Dopaminergic cells of the carotid body: physiological significance and possible therapeutic applications in Parkinson’s disease. Brain Res Bull. 2002;57:847–853. doi: 10.1016/s0361-9230(01)00771-7. [DOI] [PubMed] [Google Scholar]
- 34.Deng J, Zou ZM, Zhou TL, Su YP, Ai GP, Wang JP, et al. Bone marrow mesenchymal stem cells can be mobilized into peripheral blood by G-CSF in vivo and integrate into traumatically injured cerebral tissue. Neurol Sci. 2011;32:641–651. doi: 10.1007/s10072-011-0608-2. [DOI] [PubMed] [Google Scholar]
- 35.Park HJ, Lee PH, Bang OY, Lee G, Ahn YH. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson’s disease. J Chem. 2008;107:141–151. doi: 10.1111/j.1471-4159.2008.05589.x. [DOI] [PubMed] [Google Scholar]
- 36.Karussis D, Kassis I, Kurkalli BG, Slavin S. Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci. 2008;265:131–135. doi: 10.1016/j.jns.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Krampera M, Pasini A, Pizzolo G, Cosmi L, Romagnani S, Annunziato F. Regenerative and immunomodulatory potential of mesenchymal stem cells. Curr Opin Pharmacol. 2006;6:435–441. doi: 10.1016/j.coph.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 39.Jin K, Sun Y, Xie L, Mao XO, Childs J, Peel A, et al. Comparison of ischemia-directed migration of neural precursor cells after intrastriatal, intraventricular, or intravenous transplantation in the rat. Neurobiol Dis. 2005;18:366–374. doi: 10.1016/j.nbd.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Huang HY, Lin SZ, Kuo JS, Chen WF, Wang MJ. G-CSF protects dopaminergic neurons from 6-OHDA-induced toxicity via the ERK pathway. Neurobiol Aging. 2007;28:1258–1269. doi: 10.1016/j.neurobiolaging.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 41.Gorgen I, Hartung T, Leist M, Niehorster M, Tiegs G, Uhlig S, et al. Granulocyte colony-stimulating factor treatment protects rodents against lipopolysaccharide-induced toxicity via suppression of systemic tumor necrosis factor-alpha. J Immunol. 1992;149:918–924. [PubMed] [Google Scholar]
- 42.Kitabayashi A, Hirokawa M, Hatano Y, Lee M, Kuroki J, Niitsu H, et al. Granulocyte colony-stimulating factor downregulates allogeneic immune responses by posttranscriptional inhibition of tumor necrosis factor-alpha production. Blood. 1995;86:2220–2227. [PubMed] [Google Scholar]
- 43.Hebert JC, O’Reilly M, Barry B, Shatney L, Sartorelli K. Effects of exogenous cytokines on intravascular clearance of bacteria in normal and splenectomized mice. J Trauma. 1997;43:875–879. doi: 10.1097/00005373-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Heard SO, Fink MP, Gamelli RL, Solomkin JS, Joshi M, Trask AL, et al. Effect of prophylactic administration of recombinant human granulocyte colony-stimulating factor (filgrastim) on the frequency of nosocomial infections in patients with acute traumatic brain injury or cerebral hemorrhage. The Filgrastim Study Group. Crit Care Med. 1998;26:748–754. doi: 10.1097/00003246-199804000-00027. [DOI] [PubMed] [Google Scholar]
- 45.Heard SO, Fink MP. Counterregulatory control of the acute inflammatory response: granulocyte colony-stimulating factor has anti-inflammatory properties. Crit Care Med. 1999;27:1019–1021. doi: 10.1097/00003246-199905000-00051. [DOI] [PubMed] [Google Scholar]
- 46.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 47.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 48.Offen D, Beart PM, Cheung NS, Pascoe CJ, Hochman A, Gorodin S, et al. Transgenic mice expressing human Bcl-2 in their neurons are resistant to 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Proc Natl Acad Sci U S Am. 1998;95:5789–5794. doi: 10.1073/pnas.95.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basu S, Dunn A, Ward A. G-CSF: function and modes of action (Review) Int J Mol Med. 2002;10:3–10. [PubMed] [Google Scholar]
- 50.Boneberg EM, Hartung T. Molecular aspects of anti-inflammatory action of G-CSF. Inflamm Res. 2002;51:119–128. doi: 10.1007/pl00000283. [DOI] [PubMed] [Google Scholar]
- 51.Hagg T, Varon S. Ciliary neurotrophic factor prevents degeneration of adult rat substantia nigra dopaminergic neurons in vivo. Proc Natl Acad Sci U S A. 1993;90:6315–6319. doi: 10.1073/pnas.90.13.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulz JB, Bremen D, Reed JC, Lommatzsch J, Takayama S, Wullner U, et al. Cooperative interception of neuronal apoptosis by BCL-2 and BAG-1 expression: prevention of caspase activation and reduced production of reactive oxygen species. J Neurochem. 1997;69:2075–2086. doi: 10.1046/j.1471-4159.1997.69052075.x. [DOI] [PubMed] [Google Scholar]
- 53.Cohen G, Heikkila RE. The generation of hydrogen peroxide, superoxide radical, and hydroxyl radical by 6-hydroxydopamine, dialuric acid, and related cytotoxic agents. J Biol Chem. 1974;249:2447–2452. [PubMed] [Google Scholar]
- 54.Stridh H, Kimland M, Jones DP, Orrenius S, Hampton MB. Cytochrome c release and caspase activation in hydrogen peroxide- and tributyltin-induced apoptosis. FEBS Lett. 1998;429:351–355. doi: 10.1016/s0014-5793(98)00630-9. [DOI] [PubMed] [Google Scholar]


