Abstract
Objective(s):
Staphylococcus aureus is an important bacterial pathogen responsible for a variety numbers of nosocomial and community acquired infections. Biofilm formation is regarded as an important factor in the establishment of S. aureus infection. The contribution of the genetic background of S. aureus to biofilm formation is poorly understood. The aim of the present work was to genotype S. aureus strains associated to biofilm based on the coagulase and protein A genes and to evaluate the association between the genetic background and the biofilm forming ability of clinical S. aureus isolates.
Materials and Methods:
A total number of 100 S. aureus were isolated from nosocomial infections and biofilm formation capability was investigated using phenotypic assay and molecular detection of biofilm associated genes. The strains were genotyped based on coagulase (coa) and protein A (spa) gene polymorphisms using restriction fragments length polymorphism-polymerase chain reaction (RFLP-PCR).
Results:
RFLP-PCR of coa gene generated two types and three subtypes. Amplification of spa gene resulted in two banding patterns and their restriction digestion generated three subtypes. The combined coa and spa RFLP patterns generated nine genotypes (G1-G9). The genotypes G4 and G1 were the most prevalent (32.1% and 24.3%, respectively).
Conclusion:
High clonal diversity of S. aureus strains able to produce biofilm was observed. Biofilm formation correlates with the spa and coa clonal lineage in our population and testing for multiple gene polymorphisms could be employed for local epidemiologic purposes.
Keywords: Adhesion molecules, Biofilm, Coagulase, RFLP, S. aureus, Spa typing
Introduction
Staphylococcus aureus is recognized worldwide as an important bacterial pathogen causing nosocomial infections and is regarded as a major world health threat owing to its versatility, drug resistance, virulent factors and biofilm formation (1-3).
Biofilm is a community of microorganisms adhering to each other on a surface. These adherent cells are surrounded by a matrix of extracellular polymers. Biofilm formation is initiated by bacterial adherence to the surface via adhesions, followed by multiplication and cell aggregation to form multilayered cell clusters encased within a slimy matrix (4). It has been found that the aggregation step is regulated by the intracellular adhesion (ica) operon which encodes the proteins mediating the synthesis of polysaccharide intercellular adhesion (PIA) molecules (5).
Bacteria embedded in a biofilm are more resistant to antimicrobials due to the reduction of antimicrobial penetration, slower bacterial metabolic state as well as easier exchange of resistance genes among cells (1, 6).
Association between biofilm formation ability to genetic background of S. aureus isolates is not well understood. In a recent study the differences in biofilm formation was suggested to be due to the staphylococcus protein A (spa) lineage (7). In addition, coa gene has also been recently employed for subtyping of S. aureus isolates (8). A large number of molecular methods have been developed for typing of S. aureus strains. DNA fingerprinting by Pulsed-Field Gel Electrophoresis (PFGE) is considered to be one of the most reliable, discriminatory, and reproducible typing procedures to allow the detection of high degree DNA polymorphism (9), but it is technically demanding, time-consuming and expensive. Thus, the aim of the current study was to genotype S. aureus strains isolated from nosocomial infections by molecular typing of spa and coa genes using RFLP-PCR and to evaluate any possible association between the genetic lineage to presence of icaA and icaD genes as well as biofilm formation capability. Another objective was to determine prevalence of the genes encoding intracellular adhesion molecules (icaA and icaD) among isolated strains.
Materials and Methods
Bacterial isolates
A total number of 100 S. aureus strains were collected from patients suffering from nosocomial infections between October 2014 and November 2015. The bacteria were isolated from urine, blood, sputum, wound and skin infections from different hospitals in Rasht (Iran). Bacterial identification was performed using Gram staining, tube coagulase and catalase assays, manitol salt agar and DNase agar plates (10).
Bacterial DNA extraction
Extraction of genomic DNA was performed using the method described previously with minor modification (11). Briefly, 5 ml of bacterial suspensions grown in Luria–Bertani (LB) broth were centrifuged (10 min at 6000×g) and the pellets were re-suspended in Tris-EDTA (TE) buffer. Then, 30 μl of 10% SDS and 3 μl of 20 mg/ml proteinase-K were added to each tube and incubated for 60 min at 37 °C. Then, 600 µl of (25:24:1) phenol/chloroform/isoamyl alcohol solution was added and centrifuged at 6000×g for 5 min. Finally, one volume of cold ethanol was added to the supernatant and DNA was precipitated after centrifugation (10 min at 8000 ×g). The DNA yield was re-suspended in TE buffer and stored at -20 °C for subsequent analysis.
Phenotypic screening of biofilm formation
Biofilm formation capability of the S. aureus strains was investigated using the cultivation of the isolates on Congo Red Agar (CRA) (12). Briefly, bacterial isolates were inoculated onto CRA and incubated at 37 °C for 24 hr followed by subsequent storage at room temperature. After 48-72 hr the strains producing black colonies were regarded as biofilm producers, whereas, strains producing red colonies were considered as biofilm non-producers.
Molecular detection of icaA and icaD genes
Polymerase chain reaction (PCR) was employed to detect icaA and icaD genes among all isolates. The master mix for the PCR was prepared as follows: 3 μl of 10⊆ PCR buffer, 1 μl of 25 mm MgCl2, 3 μl of 10 mm dNTP mix, 0.5 μl of Taq DNA polymerase, 12.5 μl of MilliQ water and 1 μl of each of the forward and reverse primers. Finally, 3 µl of each DNA template was added in the corresponding tubes to make up the final reaction volume of 25 μl. The PCR primer pairs used in this study were presented in Table 1 (13).
Table 1.
Primer pairs used for amplification of target genes
| Target gene | Primers (5’-3’) | Reference |
|---|---|---|
| icaA | F: CCT AAC TAA CGA AAG GTA G | (13) |
| R: AAG ATA TAG CGA TAA GTG C | ||
| icaD | F: AAA CGT AAG AGA GGT GG | (13) |
| R: GGC AATATG ATC AAG ATA C | ||
| spa | F: ATC TGG TGG CGT AAC ACC TG | (14) |
| R: CGC TGC ACC TAA CGC TAA TG | ||
| coa | F: CGA GAC CAA GAT TCA ACA AG | (14) |
| R: AAA GAA AAC CAC TCA CAT CA |
The reaction mix was incubated at 94 °C for 10 min followed by 30 cycles of PCR, denaturation for 50 sec at 94 °C, primer annealing at 49 °C for both icaA and icaD and polymerization step at 72 °C for 1 min, was conducted. The polymerization was concluded by an extension period of 10 min at 72 °C. Then, PCR products were mixed with 3 µl DNA stain (PowerLoad ™) and were visible after electrophoresis in a 1.5% agarose gel in TBE buffer and under UV trans-illumination.
Molecular typing of the isolates
Restriction fragments length polymorphism PCR (RFLP-PCR) was employed for genotyping of the isolates. Amplification of coa and spa genes was performed using the primers presented in Table 1 (14). The amplification reactions consisted of an initial DNA denaturation at 95 °C for 5 min followed by 30 cycles of denaturation at 95 °C for 50 sec, annealing at 55 °C and 56 °C for coa and spa genes, respectively for 50 sec and extension at 72 °C for 60 sec, followed by a final extension at 72 °C for 7 min.
Restriction digestion fragments of coa and spa genes were prepared using HaeIII and Bsp 1431 restriction enzymes (ThermoFisher™), respectively. Briefly, 10 units of the respect enzymes were added to 8 µl of each gene amplicon and incubated 6 hr at 37 °C. The restriction fragments were visualized following electrophoresis in a 1.5% agarose gel and under UV trans-illumination. The interpretation criteria for identifying different strains were a single band difference. Unique PCR-RFLP patterns were assigned a genotype (14).
Statistical analysis
SPSS 18.0 software was used for statistical analysis. Difference in the prevalence of different genotypes of S. aureus strains was evaluated using the t test and a P-value of 0.05 was considered as statistically significant.
Results
Biofilm formation capability
The isolated S. aureus were evaluated for biofilm formation capability using phenotypic screening as well as molecular detection of icaA and ica D genes. Cultivation of the isolates on CRA showed that 60 isolates (60%) were able to form biofilm (Figure 1). In addition, all isolated strains were investigated for biofilm associated genes, icaA and icaD. Molecular investigation revealed that both ica genes were present in the 78 isolates (Figure 2a & b). Thus, molecular typing was performed on the isolates which harbored icaA, D genes.
Figure 1.
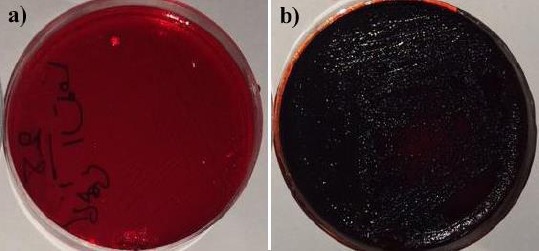
Biofilm formation assay on Congo Red Agar (CRA). a) red colonies of non-biofilm producing Staphylococcus aureus, b) black colonies of biofilm producing Staphylococcus aureus
Figure 2.
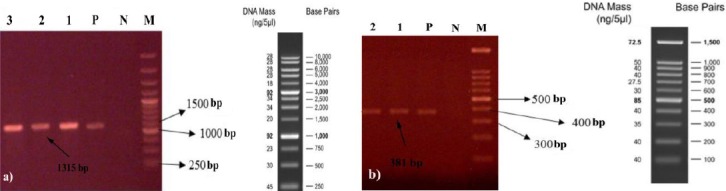
PCR detection of ica AD genes in Staphylococcus aureus clinical isolates. a) icaA, b) icaD. M: molecular size marker, N: negative control, P: positive control, 1-3: S. aureus isolates
Molecular typing of coagulase gene
PCR amplification of coa gene resulted in two different patterns following electrophoresis on 1.5% agarose gel (coaT1 and coaT2). The coaT1 isolates displayed single PCR band of 891 bp with the frequency of 65.3% (51/78), while the remaining 27/78 strains (type coaT2) showed double PCR bands including 810 and 405 bp bands with the frequency of 34.7% (Figure 3a).
Figure 3.
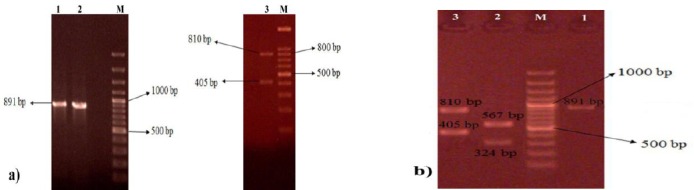
a) PCR amplification of coa gene in Staphylococcus aureus clinical isolates. b) restriction digestion of coa gene PCR product using HaeIII. M: molecular size marker (100bp), N: negative control, P: positive control, 1-3: samples
Restriction digestion of coa gene PCR products using HaeIII resulted in three different subtypes. According to the results, 64.7% (33/78) of the strains belonged to the subtype coaT1a which showed two RFLP bands of 567 and 324 bp while, PCR products of 18/78 strains belonging to the subtype coaT1b were not digested with the restriction enzyme. Restriction digestion of coaT2 strains resulted in one subtype with three RFLP bands of 405 and 810 bp. According to the results, the majority of the isolates belonged to the subtype coaT1a and the lowest frequency was observed for coaT1b strains (Figure 3b).
Molecular typing of spa gene
The PCR products of spa genes were observed in all isolates. The majority of the isolates (68/78), which had one PCR product of 1200 bp, were designed as spaT1 strains and the remaining 10/78 strains showed two PCR products with 1296 and 240 bp bands (spaT2) (Figure 4a). RFLP restriction of spa gene using Bsp 1431 resulted in three distinct subtypes including spaT1a spaT1b and spaT2 with the frequency of 38.5% (30/78), 48.7% (38/78) and 12.8% (10/78), respectively. The spaT1a strains displayed four restriction fragments of 600, 353, 150 and 97 bp while restriction digestion of spaT1b resulted in three fragments of 953, 150 and 97 bp. RFLP pattern of spaT2 strains generated three bands of 796, 500 and 240 bp (Figure 4b).
Figure 4.
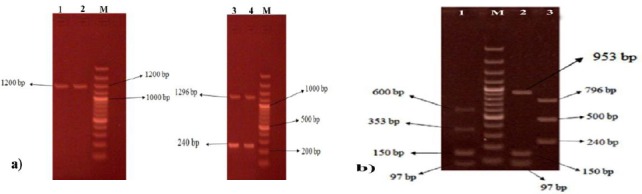
a) PCR amplification of spa gene in Staphylococcus aureus clinical isolates. b) restriction digestion of spa gene PCR product using Bsp 1431. M: molecular size marker (100bp), N: negative control, P: positive control, 1-4: samples
Molecular typing based on the combined coa and spa RFLP-PCR
The combination of coa and spa genes RFLP-PCR resulted in nine different genotypes which were classified as G1-G9. The genotypes G4 and G1 were the most frequent (32.1% and 24.3%, respectively) while the least prevalence belonged to the genotype G8 which only included one isolate (Table 2).
Table 2.
Genotyping of biofilm associated Staphylococcus aureus strains based on the combined coa type/RFLP pattern and spa type/RFLP pattern
| Genotype | coa type/RFLP pattern | spa type/RFLP pattern | Combined (%) |
|---|---|---|---|
| G1 | coa T1a | spa T1a | 19* (24/3) |
| G2 | coa T1a | spa T1b | 6 (7.7) |
| G3 | coa T1a | spa T2 | 5 (6.5) |
| G4 | coa T1b | spa T1a | 25* (32.1) |
| G5 | coa T1b | spa T1b | 4 (5.1) |
| G6 | coa T1b | spa T2 | 10 (12.8) |
| G7 | coa T2 | spa T1a | 5 (6.4) |
| G8 | coa T2 | spa T1b | 1 (1.3) |
| G9 | coa T2 | spa T2 | 3 (3.8) |
*Statistical significance was defined as P< 0.05
Discussion
The ability of bacterial pathogens to produce biofilm is regarded as a major cause of persistent infections and responsible for higher mortality and morbidity. Several observations have supported the link between the clonal lineage and biofilm formation (1). For S. aureus, two possible biofilm formation mechanisms are the bacterial production of polysaccharide slime and the presence of adhesions for the host matrix proteins (5). Previous studies reported a contribution between presence of ica genes and slime production (1, 15).
Clinical isolates of S. aureus were investigated for biofilm formation using phenotypic and molecular assays and any possible association between biofilm formation and bacterial genetic lineage was investigated. Out of 100 bacterial isolates, biofilm formation was observed in 60% of isolates by CRA method, while molecular detection of biofilm associated genes, icaA and icaD, revealed that both genes were present in 78% of isolates. The co-existence of icaA and icaD genes in S. aureus isolates has been reported previously (5, 13). High prevalence of icaA and icaD genes among biofilm associated S. aureus has been reported by several authors (5, 16). The high prevalence of ica genes and their association with biofilm formation indicates the important role of biofilm in the pathogenetic mechanisms of infection caused by S. aureus. In addition, biofilm formation results in higher antibiotic resistance which complicates therapeutic approaches against S. aureus associated infections (6).
Variation between phenotypic and genotypic methods for detection of biofilm producing S. aureus has been reported earlier (15, 16). The variation could possibly be due to the point mutations in the locus and/or other yet unidentified factors that negatively regulate synthesis of PIA molecules or influence biofilm formation (13). In addition, development of biofilm-negative, ica-positive clones has been reported (5).
A total number of 78 S. aureus strains which harbored icaA, D genes were genotyped on the basis of coagulase and protein A genes polymorphism. Coagulase, which is produced by all strains of S. aureus, is regarded to be a major virulence factor (17). Coagulase production is the principal criterion used by the clinical microbiology laboratory for the identification of S. aureus isolates from human infections. The 3’ end of coa gene of S. aureus strains contains different numbers of an 81 bp tandem short sequence repeats (9, 18). The coagene was present in all our isolates and thus, could be regarded as a good candidate for genotyping of S. aureus strains.
Molecular typing of S. aureus isolates based on coa gene was performed by several researchers (14, 17). Omar et al showed that PCR amplification of coa gene from methicillin resistant S. aureus (MRSA) strains resulted in three band classes and the majority of strains (69.3%) belonged to the 810 bp band class (14). Conversely, in our study only two band classes were observed and 65.3% of strains belonged to the 890 bp band class. The difference of coagulase type could be associated with geographical variation. RFLP pattern of the strains indicated that restriction digestion of 891, 810 and 405 bp bands by HaeIII enzyme was unsuccessful in some strains which results in fewer numbers of genotypes. Omar et al reported the similar finding when studied MRSA (14).
The spa gene contains a polymorphic X region with a varying number of 24 bp repeats, whose number and sequence differ among strains (9, 19). Several authors reported that spa gene is expressed in the majority (but not all) of S. aureus strains (14, 19). However, in our study the spa gene was found in all strains associated with biofilm which shows good potential of this gene for molecular typing.
Molecular typing of coa gene resulted in two genotypes and three subtypes. RFLP digestion of spa gene using Bsp1431 indicated that the majority of the strains (68/78) produced 150 and 97 bp bands which could be regarded as a good indicator in epidemiologic investigations.
Owing to the heterogeneity of coa and spa genes, combination use of coa and spa genes polymorphisms has been indicated to have good discriminative power by several researchers which could be employed for epidemiological studies (14, 20-21). Molecular typing based on the polymorphisms of both coa and spa genes resulted in nine genotypes (G1-G9). The genotypes G4 and G1 were significantly the most prevalent (32.1% and 24.3%, respectively). Several researchers reported that the combination of both coa and spa genes PCR-RFLP patterns results in different number of genotypes (14, 22). The difference could be associated to the geographic variation as well as different restriction enzymes which were employed for RFLP.
Conclusion
High diversity among S. aureus strains, able to produce biofilm, was observed and the combined RFLP-PCR of ica and coa genes indicated that the majority of the biofilm producers were associated to two genotypes. It could be concluded that the molecular typing of S. aureus strains based on protein A and coagulase gene polymorphisms is a valuable tool which could be employed in epidemiological studies. The limitations were the small number of isolates included in the study and lacking an alternative molecular typing method such as PFGE to compare the results.
Acknowledgment
The authors would like to thank all of the participants in this study for their friendly cooperation.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Naicker PR, Karayem K, Hoek KG, Harvey J, Wasserman E. Biofilm formation in invasive Staphylococcus aureus isolates is associated with the clonal lineage. Microb Pathog. 2016;90:41–49. doi: 10.1016/j.micpath.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Cosgrove SE, Fowler VG. Management of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46:S386–S393. doi: 10.1086/533595. [DOI] [PubMed] [Google Scholar]
- 3.Kwiecinski J, Kahlmeter G, Jin T. Biofilm formation by Staphylococcus aureus isolates from skin and soft tissue infections. Currmicrobial. 2015;70:698–703. doi: 10.1007/s00284-014-0770-x. [DOI] [PubMed] [Google Scholar]
- 4.Khoramian B, Jabalameli F, Niasari-Naslaji A, Taherikalani M, Emaneini M. Comparison of virulence factors and biofilm formation among Staphylococcus aureus strains isolated from human and bovine infections. Microb Pathog. 2015;88:73–77. doi: 10.1016/j.micpath.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Arciola CR, Baldassarri L, Montanaro L. Presence of icaA and icaDGenes and slime production in a collection of Staphylococcal strains from catheter-associated infections. J Clin Microbial. 2001;39:2151–2156. doi: 10.1128/JCM.39.6.2151-2156.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khameneh B, Diab R, Ghazvini K, Fazly Bazzaz BS. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb Pathog. 2016;95:32–42. doi: 10.1016/j.micpath.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Atshan SS, Shamsudin MN, Lung T, Than L, Sekawi Z, Ghaznavi-Rad E, Pei Pei C. Comparative characterisation of genotypically different clones of MRSA in the production of biofilms. J Biomed Biotecnol. 2012;2012:417247. doi: 10.1155/2012/417247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosandey A, Boss R, Luini M, Artursson K, Bardiau M, Breitenwieser F, et al. Staphylococcus aureus genotype B and other genotypes isolated from cow milk in European countries. J Dairy Sci. 2016;99:529–540. doi: 10.3168/jds.2015-9587. [DOI] [PubMed] [Google Scholar]
- 9.Stranden A, Frei R, Widmer AF. Molecular typing of methicillin-resistant Staphylococcus aureus: can PCR replace pulsed-field gel electrophoresis? J Clinlmicrobial. 2003;41:3181–3186. doi: 10.1128/JCM.41.7.3181-3186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahon CR, Lehman DC, Manuselis G. Text Book of Diagnostic Microbiology. 3th ed. Philadelphia, PA, USA: 2007. [Google Scholar]
- 11.Das S, Dash HR. Microbial Biotechnology-A Laboratory Manual for Bacterial Systems. Springer; 2014. pp. 1–5. [Google Scholar]
- 12.Freeman DJ, Falkiner FR, Keane CT. New method for detecting slime production by coagulase negative staphylococci. J Clin Pathol. 1989;42:872–874. doi: 10.1136/jcp.42.8.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhanawade NB, Kalorey DR, Srinivasan R, Barbuddhe SB, Kurkure NV. Detection of intercellular adhesion genes and biofilm production in Staphylococcus aureus isolated from bovine subclinical mastitis. Vet Res Commun. 2010;34:81–89. doi: 10.1007/s11259-009-9326-0. [DOI] [PubMed] [Google Scholar]
- 14.Omar NY, Ali HA, Harfoush RA, El Khayat EH. Molecular typing of methicillin resistant staphylococcus aureus clinical isolates on the basis of protein A and coagulase gene polymorphisms. Int J Microbiol. 2014;2014:650328. doi: 10.1155/2014/650328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasudevan P, Nair MK, Annamalai T, Venkitanarayanan KS. Phenotypic and genotypic characterization of bovine mastitis isolates of Staphylococcus aureus for biofilm formation. Vet Microbiol. 2003;92:179–185. doi: 10.1016/s0378-1135(02)00360-7. [DOI] [PubMed] [Google Scholar]
- 16.Yazdani R, Oshaghi M, Havayi A, Pishva E, Salehi R, Sadeghizadeh M, et al. Detection of icaAD gene and biofilm formation in Staphylococcus aureus isolates from wound infections. Iran J Publ Health. 2006;35:25–28. [Google Scholar]
- 17.Hookey JV, Richardson JF, Cookson BD. Molecular typing of Staphylococcus aureus based on PCR restriction fragment length polymorphism and DNA sequence analysis of the coagulase gene. J Clin Microbiol. 1998;36:1083–1089. doi: 10.1128/jcm.36.4.1083-1089.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shopsin B, Gomez M, Waddington M, Riehman M, Kreiswirth BN. Use of coagulase gene (coa) repeat region nucleotide sequences for typing of methicillin-resistant Staphylococcus aureus strains. J Clin Microbiol. 2000;38:3453–3456. doi: 10.1128/jcm.38.9.3453-3456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frenay HM, Theelen JP, Schouls LM, Vandenbroucke-Grauls CM, Verhoef J, Van Leeuwen WJ, et al. Discrimination of epidemic and nonepidemic methicillin-resistant Staphylococcus aureus strains on the basis of protein A gene polymorphism. J Clin Microbiol. 1994;32:846–847. doi: 10.1128/jcm.32.3.846-847.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janwithayanuchit I, Ngam-Ululert S, Paungmoung P, Rangsipanuratn W. Epidemiologic study of methicillin-resistant Staphylococcus aureus by coagulase gene polymorphism. Sci Asia. 2006;32:127–132. [Google Scholar]
- 21.Saei HD, Ahmadi M. Discrimination of staphylococcus aureus isolates on the basis of gene coding protein A using PCR-restriction enzyme analysis. Comp Clin Path. 2012;21:645–652. [Google Scholar]
- 22.Mitani N, Koizumi A, Sano R, Masutani T, Murakawa K, Okamoto Y, et al. Molecular typing of methicillin-resistant Staphylococcus aureus by PCR-RFLP and its usefulness in an epidemiological study of an outbreak. Jpn J Infect Dis. 2005;58:250. [PubMed] [Google Scholar]


