Abstract
Objective(s):
Vascular calcification is one the major characteristics in patients with various types of chronic inflammatory disorders. MiRNAs have been shown to be involved in many normal biological functions as well as diseases; however, their role in vascular calcification has not received much attention.
Materials and Methods:
In the current study, we built a vascular calcification rat model using vitamin D3 plus nicotine and analyzed miRNA expression profile by miRNA chip assay. Potential target of one selected miRNA with sharpest variation in expression were predicted by both PicTar and TargetScan. The impact of the selected miRNA on the expression of the potential target on both mRNA and protein levels were measured by RT-PCR and Western blot, respectively.
Results:
Our results identified 16 dysregulated miRNAs, among which miR-297a showed the sharpest variation. Further analysis focusing on miR-297a revealed that fibroblast growth factor 23 (FGF23) was a potential target of miR297a. Measurement of FGF23 and its regulator Klotho on both mRNA and protein levels demonstrated that FGF23 was significantly increased while Klotho was decreased in rats with vascular calcification.
Conclusion:
Our results indicated that FGF23 was target of miR-297a and decreased miR-297a in vascular calcification lead to the increase of FGF23, which together with Klotho might enhance vascular calcification. The findings of this study could provide valuable information for the understanding of mechanisms underlying miR-dependent vascular calcification as well as potential treatment target for the disease.
Keywords: Fibroblast growth factor 23, MicroRNA-297a, Chronic inflammatory disorder, Vascular calcification, Klotho
Introduction
Vascular calcification, one of the major features in patients with chronic inflammatory disorders including type 2 diabetes mellitus, chronic kidney disease and atherosclerosis, is usually associated with significant adverse events and even mortality (1, 2). Vascular calcification is a complicated biological process which includes significant expression variations in alkaline phosphatase (ALP), osteocalcin (OC), bone morphogenetic protein 2 (BMP-2) and osteogenesis of transcription factor Runx2 etc (2-5). However, the precise mechanisms underlying vascular calcification still remain elusive till now.
MicroRNAs (miRs) are a large class of non-coding small RNAs with 17-25 nucleotides (6). MiRs are important regulators of gene expression on post-transcriptional level and participate in various normal physiological processes, whereas miR dysregulation could result in impaired cellular function and disease progression (7). The associations of miRs with a variety of diseases have been reported, including cardio-vascular diseases, cancers and autoimmune diseases, however, the role of miRs in vascular calcification has been not extensively investigated and evidence for miRs modulation in vascular calcification is very limited (8-13). Till now, only a few miRs were identified to be associated with the pathogenesis of vascular calcification, such as miR-125b targeting SP7 and miR-204 targeting Runx2 (14, 15).
Using Vitamin D3 plus nicotine induced rat aortic calcification model, we analyzed the miR expression profile in vascular calcification. Moreover, our research also revealed for the first time that miR-297a was down-regulated in rats with vascular calcification, which consequently increased the level of its target fibroblast growth factor 23 (FGF23) and enhanced calcification. The findings in our study could provide valuable information for the understanding of mechanisms underlying miR-dependent vascular calcification as well as potential treatment target for the disease.
Materials and Methods
Animals and ethical statements
Seven-week old, specific antigen free (SPF) male Sprague Dawley (SD) rats weighing around 250 g were purchased from Shanghai slack laboratory animal Co, LTD and hosted in SPF environment with food and water supplied. All protocols involving animals were reviewed by the institutional ethical review board and performed with accordance to the provincial guidelines on animal experimentation.
Vascular calcification model construction and sampling
Rat vascular calcification model was built as previously described with modifications (16, 17). In brief, rats were received 300,000 IU/kg vitamin D3 (Sigma-Aldrich) once a day intramuscularly and 5 mg/kg nicotine (Sigma-Aldrich) twice a day orally for a continuous 4 weeks. For control rats, equal volumes of saline solution were administrated through the same routes for the time periods. The body weight and blood pressure of each rat were measured at day 3, 5, 7, 15 and 20. At the end of week 4, rats were anesthetized and blood samples were taken and sera were isolated and aliquoted and stored at -80 °C. Intact aortas were also harvested and stored at -80 °C till use.
Measurement of serum ALP, phosphate and calcium
The levels of ALP, phosphate and calcium in serum samples were measured using commercial colorimetric kits according to the manufacturer’s instructions (all kits were purchased from Abcam, ab83369 for ALP, ab102505 for calcium and ab65622 for phosphate respectively).
MiR chip assay
Total RNA was prepared using mirVana miRNA isolation kit (mirVana AM1561) according the manufacturer’s instructions and labeled with Cy3. MiRNA chip was purchased from Signosis (AP-0003) and performed with accordance to the manufacturer’s instructions. Microarray was scanned with GeneChipR Scanner 3000 and data was analyzed with miRNA QC Tool software.
RT-PCR
Total RNA was prepared as described in the miR chip assay. For detection of miR-297a, stem-loop RT-PCR was performed. The primers for miR-297a and internal control U6 amplification were purchased from Ribobio Inc. For detection of FGF23 and Klotho expression, regular RT-PCR was adopted and GAPDH was used as an internal control. Primers were listed in Table 1. The relative mRNA expression was calculated using 2-ΔΔCT formula.
Table 1.
Primer pairs used in RT-PCR
| Gene | Primer (5’-3’) |
|---|---|
| FGF23 | For: ATGCTAGGGACCTGCCTTAGA |
| Rev: GGAGCCAAGCAATGGGGAA | |
| Klotho | For: GGGACACTTTCACCCATCACT |
| Rev: ACGTTGTTGTAACTATCGCTGG | |
| GAPDH | For: GAAGGTGAAGGTCGGAGTC |
| Rev: GAAGATGGTGATGGGATTTC |
For, forward; Rev, reverse
Western blot
Tissue was first homogenized on ice and centrifuged at 13000 g for 10 min at 4 °C. Superna-tants were collected and protein concentration was determined using Protein Assay Kit (Beyotime). Equal amount of samples were then isolated by 12% SDS-PAGE and transferred onto a 0.45 μm PVDF membrane (Millipore). Subsequently, membrane was first blocked with 5% non-fat milk for 1 hr at room temperature and then incubated with primary antibodies and corresponding HRP-conjugated secondary antibodies for 2 hr and 1hr at room temperature, respectively. After incubation, the membrane was extensively washed and immune-bands on the membrane were visualized using ECL substrate (Beyotime) under a CCD camera (Alpha Innotech). The gray-scale of the bands was analyzed by Quantity-one v4.62 software. The relative expression of FGF23 and Klotho was normalized to that of the internal control GAPDH. Primary antibodies anti-FGF23, Klotho and GAPDH were all purchased from Santa Cruz. HRP-conjugated secondary antibodies were purchased from Boster.
Statistical analysis
All data were expressed as mean±standard deviation (SD). For comparisons between two groups, student’s t test was adopted. A P<0.05 was considered statistically significant. All statistical analyses were performed with SPSS 11.5 (SPSS. Inc).
Results
Construction of vascular calcification (VC) rat model
VC rat model was constructed using vitamin D3 and nicotine. As shown in Figure 1, VC rats demonstrated significantly slower weight gain comparing to control rats (Figure 1A). Moreover, blood pressure of VC rats continuously increased as time increased, while that of control rats remained at a relatively constant level (Figure 1B). The concentration of calcium, phosphate and activity of ALP are indicators of VC. Measurement of calcium and phosphate concentrations as well as ALP activities in serum samples 4 weeks after initial drug administration further revealed that these three elements in serum were also significantly increased (Figure 1C and D). At last, a pathological assay (Von Kossa staining) was conducted to evaluate VC more directly. As shown in Figure 1E, calcium spots were dramatically increased in VC group than in control group. Taken together, our results indicated that the VC rat model was successfully constructed.
Figure 1.
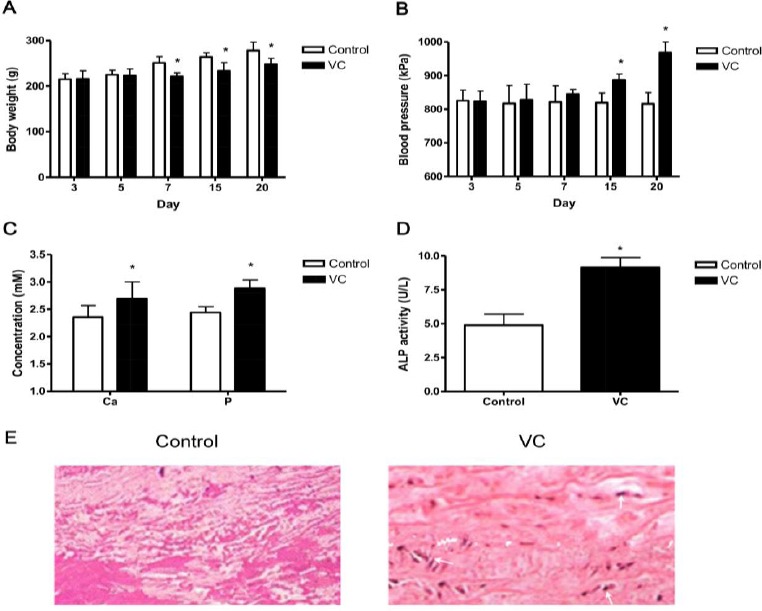
Evaluation of rat vascular calcification model (n=15). Rats were received vitamin D3 and nicotine (VC) or saline solution (control) daily for 4 weeks. (A and B) The body weight (A) and blood pressure (B) were measured on day 3, 5, 7, 15 and 20. (C and D) Four weeks after the initial administration of vitamin D3 and nicotine, rats were sacrificed and concentrations of Ca and Phosphate (C) as well as ALP activity (D) were measured. Data shown are mean±SD of three independent experiments (n=15). *, P<0.05. (E) VC identification by Von Kossa staining. Representative result is shown
miR expression profile difference between VC and control groups
Using miRNA chip assay, we next analyzed the expression difference of miRNAs between VC and control rats. As shown in Table 2, our assay detected a total number of 16 miRs differently expressed in VC rats. Among them, 10 miRs were up-regulated while the rest 6 were down-regulated. Of all the 16 miRs, miR-297a showed the sharpest expression change, consequently, this miR was chosen for more detailed mechanism analysis.
Table 2.
Expression profile difference of miRNAs in vascular calcification rats
| ProbeSet Name | Fold change | Regulation | Median CV (%) |
|---|---|---|---|
| mmu-miR-126-3p | 3.2317 | Up | 4.32 |
| mmu-miR-23b | 3.6404 | Up | 6.02 |
| mmu-miR-187-3p | 3.0996 | Up | 6.34 |
| mmu-miR-125b-5p | 2.0147 | Up | 5.48 |
| mmu-miR-497 | 3.1366 | Up | 7.11 |
| mmu-miR-145-3p | 2.0654 | Up | 6.04 |
| mmu-miR-32-5p | 2.3452 | Up | 6.23 |
| mmu-miR-30a-5p | 2.0639 | Up | 5.99 |
| mmu-miR-33-5p | 2.0660 | Up | 6.10 |
| mmu-miR-126-3p | 2.1254 | Up | 5.48 |
| mmu-miR-133a-3p | 3.6332 | Down | 7.46 |
| mmu-miR-2861 | 2.4365 | Down | 8.20 |
| mmu-miR-210-3p | 2.1334 | Down | 6.09 |
| mmu-miR-674-5p | 2.7562 | Down | 7.13 |
| mmu-miR-18a-3p | 2.085 | Down | 6.27 |
| mmu-miR-297a | 5.331 | Down | 5.12 |
Decreased miR-297a lead to FGF-23 expression increase in VC rats
First, we confirmed the miR279a expression level in VC and control rats using stem-loop RT-PCR and result was consistent with miR chip assay (Figure 2A). Next, we analyzed the potential target gene of miR-297a using two programs PicTar (pictar.mdc-berlin.de/) and TargetScan (http://www.targetscan.org/mmu_61/). Results of both program indicated that FGF23 could be the target gene of miR-297a. FGF23 is a member of the fibroblast growth factor family and participates in phosphate metabolism (18). Within the FGF23 signaling pathway, Klotho is one of the most important regulators (19). Consequently, we next analyzed the change of FGF23 and Klotho in VC and control rats on both mRNA and protein levels. RT-PCR analysis revealed that the mRNA level of FGF23 was significantly increased in VC rats while that of Klotho was on the other hand decreased (Figure 2B). The measurement of FGF23 and Klotho expression on protein level using Western blot demonstrated similar tendencies (Figure 2C and D).
Figure 2.
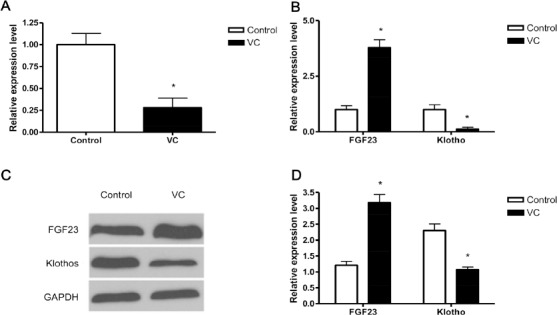
Fibroblast growth factor 23 (FGF23) is the target of miR-297a in VC. Rats were received vitamin D3 and nicotine (VC) or saline solution (control) daily for 4 weeks. Four weeks after the initial administration of vitamin D3 and nicotine, rats were sacrificed and miR-297a was determined by stem-loop RT-PCR (A). The expression of FGF23 and Klotho were also determined by RT-PCR (B) and Western blot (C and D), respectively. (D) The gray scale of the immunobands was quantified and relative expression of FGF23 and Klotho was calculated using GAPDH as an internal control. (A, B and D) Data shown are mean±SD of three independent experiments (n=15). *, P<0.05. (C) Representative results are shown.
Taken together, our results here indicated that miR-297a was decreased in VC rats, which consequently increased the expression of its regulation target FGF23. The varied FGF23 and its regulator Klotho together might result in further enhancement on vascular calcification.
Over the past years, our understanding to vascular calcification has been significantly improve-ed. However, the precise mechanism still remains elusive. MiRs have been discovered to be important regulators in both normal biological functions and various abnormalities. MiRs have been massively investigated in many diseases like cancers, and autoimmune diseases, but studies on their importance in vascular calcification are still very limited (14, 15). In the current study, we identified 16 abnormally expressed miRs using a vascular calcification rat model. Among these 16 miRs, 10 were up-regulated while the rest 6 were down-regulated. Our further study by focusing on one of down-regulated miR (miR-297a) revealed that it was positive regulator in vascular calcification by targeting FGF23. In recent studies, miR-297a expression variation has also been described in many types of diseases including cerebral ischemia, S. japonicum infection and lung tumor (20-22). However, this is the first study that has identified the target of miR-297a.
FGF23, a secreted protein by osteocytes, participates in blood phosphorus metabolism. Previous study has revealed that FGF23 is positively correlated to VC (23). Klotho is an important protein in the FGF23 signaling pathway and the expression of FGF23 could affect the expression of Klotho in a negative manner (24). Moreover, in the condition of Klotho knock-out or suppression, FGF23 could enhance hyperphosphate-induced VC (25, 26). In our current study, we revealed that miR-297a was down-regulated in VC, which consequently enhanced the expression of its target FGF23. The abnormal up-regulation of FGF23 might then result in the decrease of Klotho and VC.
Of the 16 miRs identified in the current study, many have been reported to be participated in other diseases or normal biological processes. For instance, miR-126 involves in the development of mouse mammary gland and cardiac hypertrophy (27, 28), miR-23 in autoimmune inflammation and cancer metastasis (29, 30) and miR-125b-5p in differential activation of macrophages and inflammation and cutaneous T cell lymphomas (31, 32). Given the complex of the miR regulation network, further research on whether and/or how these miRs participate in vascular calcification is warranted.
Vascular calcification is one the symptoms shared by various chronic inflammatory diseases. Given that each disease has its own uniqueness in pathogenesis, the mechanism in triggering vascular calcification by different diseases might be distinctive. In the current study, all the experimentations were based on a rat vascular calcification model developed by vitamin D3 and nicotine administrations. Therefore, whether this model is suitable for vascular calcification in all kinds of diseases remain to be further defined.
Taken together, our results here indicated that miR-297a was decreased in VC rats, which consequently increased the expression of its regulation target FGF23. The varied FGF23 and its regulator Klotho together might result in further enhancement on vascular calcification. The findings in our study could provide valuable information for the understanding of mechanisms underlying miR-dependent vascular calcification as well as potential treatment target for the disease.
Conclusion
Our results indicated that FGF23 was target of miR-297a and decreased miR-297a in vascular calcification lead to the increase of FGF23, which together with Klotho might enhance vascular calcification. The findings of this study could provide valuable information for the understanding of mechanisms underlying miR-dependent vascular calcification as well as potential treatment target for the disease.
Acknowledgment
This work was supported by a General Project of Science and Technology Bureau in Zhengzhou (N2014S0053). We thank KHE, a professional scientific English-editing agency, for the language editing service.
References
- 1.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu D, Mackenzie NC, Farquharson C, MacRae VE. Mechanisms and clinical consequences of vascular calcification. Front Endocrinol. 2012;6(3):95. doi: 10.3389/fendo.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010;339:189–195. doi: 10.1007/s00441-009-0832-8. [DOI] [PubMed] [Google Scholar]
- 4.Thompson B, Towler DA. Arterial calcification and bone physiology: role of the bone–vascular axis. Nat Rev Endocrinol. 2012;8:529–543. doi: 10.1038/nrendo.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danilevicius C, Lopes J, Pereira R. Bone metabolism and vascular calcification. Braz J Med Biol Res. 2007;40:435–442. doi: 10.1590/s0100-879x2007000400001. [DOI] [PubMed] [Google Scholar]
- 6.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469:336–342. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goretti E, Vausort M, Wagner DR, Devaux Y. Association between circulating microRNAs, cardiovascular risk factors and outcome in patients with acute myocardial infarction. Int J Cardiol. 2013;168:4548–4550. doi: 10.1016/j.ijcard.2013.06.092. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman AL, Wu S. MicroRNAs, cancer and cancer stem cells. Cancer Lett. 2011;300:10–19. doi: 10.1016/j.canlet.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 12.Pauley KM, Cha S, Chan EK. MicroRNA in autoimmunity and autoimmune diseases. J Autoimmunity. 2009;32:189–194. doi: 10.1016/j.jaut.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai R, Ahmed SA. MicroRNA, a new paradigm for understanding immunoregulation, inflammation, and autoimmune diseases. Transl Res. 2011;157:163–179. doi: 10.1016/j.trsl.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goettsch C, Rauner M, Pacyna N, Hempel U, Bornstein SR, Hofbauer LC. miR-125b regulates calcification of vascular smooth muscle cells. Am J Pathol. 2011;179:1594–1600. doi: 10.1016/j.ajpath.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui RR, Li SJ, Liu LJ, Yi L, Liang QH, Zhu X, et al. MicroRNA-204 regulates vascular smooth muscle cell calcification in vitro and in vivo. Cardiovasc Res. 2012;96:320–329. doi: 10.1093/cvr/cvs258. [DOI] [PubMed] [Google Scholar]
- 16.Niederhoffer N, Bobryshev YV, Lartaud-Idjouadiene I, Giummelly P, Atkinson J. Aortic calcification produced by vitamin D3 plus nicotine. J Vasc Res. 1997;34:386–398. doi: 10.1159/000159247. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Chang JR, Duan XH, Yu YR, Zhang BH. Thyroid hormone attenuates vascular calcification induced by vitamin D3 plus nicotine in rats. Calcif Tissue Int. 2015;96:80–87. doi: 10.1007/s00223-014-9934-8. [DOI] [PubMed] [Google Scholar]
- 18.Murer H, Hernando N, Forster I, Biber J. Regulation of Na/Pi transporter in the proximal tubule. Ann Rev Physiol. 2003;65:531–542. doi: 10.1146/annurev.physiol.65.042902.092424. [DOI] [PubMed] [Google Scholar]
- 19.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 20.Hunsberger JG, Fessler EB, Wang Z, Elkahloun AG, Chuang DM. Post-insult valproic acid-regulated microRNAs: potential targets for cerebral ischemia. Am J Transl Res. 2012;4:316–332. [PMC free article] [PubMed] [Google Scholar]
- 21.Han H, Peng J, Hong Y, Zhang M, Han Y, Fu Z, et al. Comparison of the differential expression miRNAs in Wistar rats before and 10 days after Sjaponicum infection. Parasit Vectors. 2013;6:120. doi: 10.1186/1756-3305-6-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izzotti A, Balansky R, D’Agostini F, Longobardi M, Cartiglia C, La Maestra S, et al. Relationships between pulmonary micro-RNA and proteome profiles, systemic cytogenetic damage and lung tumors in cigarette smoke-exposed mice treated with chemopreventive agents. Carcinogenesis. 2013;34:2322–2329. doi: 10.1093/carcin/bgt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukumoto S. Vascular calcification-pathological mechanism and clinical application-regulation of mineral metabolism and mineralization by FGF23. Clin Calcium. 2015;25:687–691. [PubMed] [Google Scholar]
- 24.Razzaque MS. The FGF23–Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5:611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuroo M, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindberg K, Olauson H, Amin R, Ponnusamy A, Goetz R, Taylor RF, et al. Arterial klotho expression and FGF23 effects on vascular calcification and function. PloS One. 2013;8:e60658. doi: 10.1371/journal.pone.0060658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui W, Li Q, Feng L, Ding W. MiR-126-3p regulates progesterone receptors and involves development and lactation of mouse mammary gland. Mol Cell Biochem. 2011;355:17–25. doi: 10.1007/s11010-011-0834-1. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen H, et al. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol. 2007;170:1831–1840. doi: 10.2353/ajpath.2007.061170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu S, Pan W, Song X, Liu Y, Shao X, Tang Y, et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-[alpha] Nat Med. 2012;18:1077–1086. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Hao Y, Yang J, Zhou Y, Li J, Yin S, et al. Genome-wide functional screening of miR-23b as a pleiotropic modulator suppressing cancer metastasis. Nat Commun. 2011;2:554. doi: 10.1038/ncomms1555. [DOI] [PubMed] [Google Scholar]
- 31.Banerjee S, Cui H, Xie N, Tan Z, Yang S, Icyuz M, et al. miR-125a-5p regulates differential activation of macrophages and inflammation. J Biol Chem. 2013;288:35428–35436. doi: 10.1074/jbc.M112.426866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manfe V, Biskup E, Willumsgaard A, Skov AG, Palmieri D, Gasparini P, et al. cMyc/miR-125b-5p signalling determines sensitivity to bortezomib in preclinical model of cutaneous T-cell lymphomas. PloS One. 2013;8:e59390. doi: 10.1371/journal.pone.0059390. [DOI] [PMC free article] [PubMed] [Google Scholar]


