Abstract
Inflammatory myofibroblastic tumors rarely occur in the urinary bladder. These masses follow an indolent course, but due to their histologic similarities to more malignant types of bladder masses, they must be differentiated with immunohistochemical staining. Once diagnosed, the mainstay of treatment for these masses is surgical resection. Due to advancements in robotic surgery, new surgical techniques can be employed to treat these masses with fewer perioperative complications. We report a case of inflammatory myofibroblastic tumor of the urinary bladder in a 29-year-old male treated with robot-assisted partial cystectomy.
Keywords: Inflammatory myofibroblastic tumor, Urinary bladder
Abbreviations: IMT, inflammatory myofibroblastic tumor; ALK, anaplastic lymphoma kinase; TURBT, transurethral resection of the bladder tumor
Introduction
Inflammatory myofibroblastic tumors (IMTs) are proliferative lesions of the submucosal stroma which can arise anywhere in the body.1 Dr. J.A. Roth first described these tumors appearing in the bladder in 1980, and defined them as “unusual pseudosarcomatous lesions.”2 He noted that these tumors have a favorable behavior if totally excised.2 IMTs occurring in the bladder are uncommon and comprise <1% of all bladder tumors. Due to its histologic similarities to other spindle cell neoplasms, such as leiomyosarcoma and sarcomatoid transitional cell carcinoma, definitive diagnosis of these lesions is often difficult.1 Translocation in the anaplastic lymphoma kinase (ALK) gene on band 2p23 is present in 30%–66% of IMTs and immunohistochemical staining can allow for differentiation of IMTs from other spindle cell lesions of the bladder.3 The mainstay of treatment for IMTs of the urinary bladder is local surgical resection, including partial cystectomy and transurethral resection of the bladder tumor (TURBT).4 Recurrence after resection occurs in 10%–25% of patients but usually follows an indolent course.4 Here we present a case of inflammatory myofibroblastic tumor of the bladder in an otherwise healthy young man.
Case report
A 29-year-old Caucasian male with no comorbid conditions presented to an outside clinic after a single episode of painless gross hematuria. Cystoscopy at that time revealed a nodular, sessile bladder tumor measuring 4 cm in size and located at the left lateral wall and dome of the bladder. CT and MRI imaging noted a solitary bladder mass without signs of lymphadenopathy or metastatic spread. Subsequent transurethral biopsy of the mass demonstrated a myofibroblastic neoplasm of low malignant potential. Due to the rarity of this tumor, the patient was referred to the Department of Urology at Texas Tech University Health Sciences Center for further evaluation.
After in-clinic cystoscopy to evaluate tumor burden and bladder capacity, and review of outside imaging to ensure now signs of metastatic disease, the patient was scheduled for a robot-assisted partial cystectomy. In the operating room, patient positioning and port placement followed the standard pattern for robot-assisted laparoscopic approach to the bladder at our institution. A drop-in ultrasound probe was used to visualize the tumor and distinguish its margins (Fig. 1).
Figure 1.
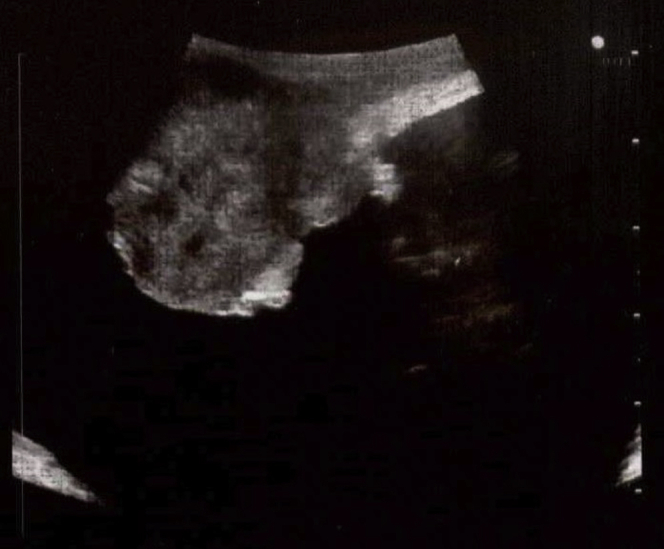
Ultrasound imaging used to determine the margins of the bladder mass.
Concurrent cystoscopy was performed by a bedside assistant for simultaneous intravesical visualization of the tumor. Using these tools, the tumor margins were well-defined. The bladder was scored using robotic monopolar scissors, creating approximately 2 cm margins around the tumor. The bladder was entered and the tumor was excised (Fig. 2). Frozen sections of the surgical margins were sent to pathology and returned as negative. The cystotomy was repaired using running barbed suture and the bladder was filled easily with 300 mL of normal saline to ensure water-tight closure and adequate remaining capacity.
Figure 2.
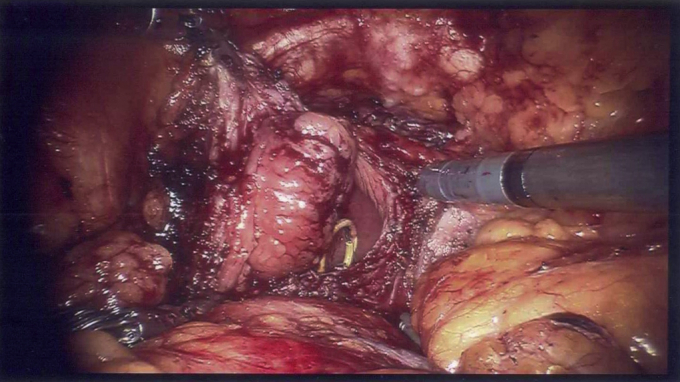
Intracorporeal view of the bladder mass during robot-assisted partial cystectomy.
Final pathology of the resected mass revealed negative surgical margins with a well-circumscribed, firm, nodular tumor that involved nearly the full thickness of the bladder wall. It was arranged in fascicles as is common in IMT. The individual tumor cells contained mostly spindled nuclei with abundant amphophilic cytoplasm. Focal areas of necrosis, which are frequently appreciated in IMTs, were also identified in this specimen. On average, there were 4 mitotic figures per 10 high power fields. Immunohistochemical staining showed the tumor cells were positive for ALK-1 (Fig. 3), actin, and pancytokeratin. Focal positivity was also noted for epithelial markers EMA, CAM5.2, and pankeratin. The overall morphology and the immunohistochemical staining positivity of ALK-1 supported the diagnosis of IMT. A second pathology lab outside of our institution's confirmed the tumor to be an IMT.
Figure 3.
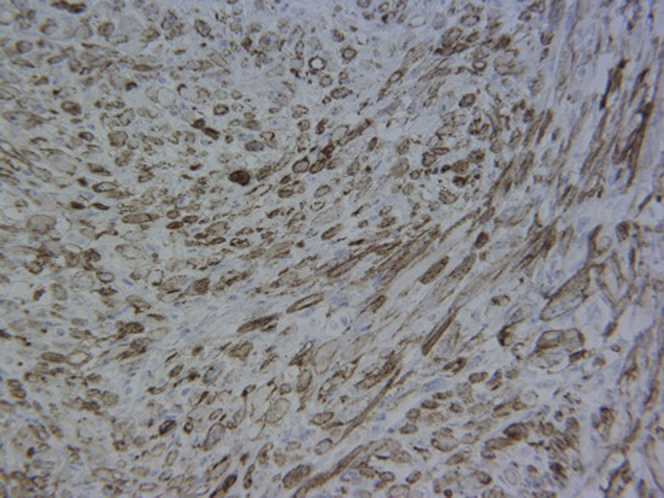
Immunohistochemical staining showing tumor cells positive for ALK-1.
Discussion
Inflammatory myofibroblastic tumors of the bladder are very rare tumors with the average age of presentation being 28 -years-old.2 The etiology of these tumors is still unknown, but two theories have been established. The first theory suggests that an inflammatory state caused by recurrent cystitis, trauma, surgical instrumentation, or other such factors could lead to development of an IMT.2 It is also possible that de novo rearrangement of the ALK gene on band 2p23 leads to spontaneous development of IMTs. Immunohistochemical staining for this gene rearrangement can be used to differentiate IMTs from other more aggressive bladder tumors.3 Currently, no standardized treatment protocol has been established for these bladder masses. Recommended treatment options for these masses include TURBT, partial cystectomy, or radical cystectomy.4
TURBT is often performed for diagnostic purposes, but also serves as a common treatment option with 60.8% of patients electing to treat their IMTs with TURBT.3 Our patient desired complete surgical excision of his tumor over TURBT. Given the tumor's location and size, a partial cystectomy was deemed feasible. While radical cystectomy is often used to treat muscle-invasive bladder tumors of high malignant potential, IMTs can be treated with partial cystectomy. IMTs are generally benign in nature without reported cases of metastasis. Even incomplete resection of IMTs has proven beneficial as remaining IMT cells are noted to remain stable or regress spontaneously.3, 4
Use of robotic assistance for partial cystectomy has several additional benefits over a classic laparoscopic approach. Robot-assisted procedures provide the surgeon with the advantages of three-dimensional viewing, greater degree of freedom of movement, stabilization of surgical movements, and simpler extracorporeal suturing.5 The technical capabilities of the robot system allowed for better delineation of surgical margins and preservation of adequate bladder volume. To our knowledge, this is the first documented IMT resection using robotic assistance.
Conclusion
Inflammatory myofibroblastic tumors of the bladder are ideal for treatment via minimally invasive surgery if the location is favorable. Advances in robotic technology have made partial cystectomy a technically manageable case, thus the approach can be safely employed for amenable bladder masses. Given the benign nature of IMTs, a robot-assisted partial cystectomy represents an excellent treatment option to extirpate this neoplasm while retaining adequate bladder capacity and reducing perioperative and long-term morbidity.
Conflicts of interest
None.
Footnotes
Funding sources: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Roth J.A. Reactive pseudosarcomatous response in urinary bladder. Urology. 1980;16(6):635–637. doi: 10.1016/0090-4295(80)90578-6. [DOI] [PubMed] [Google Scholar]
- 2.Rosado E., Pereira J., Corbusier F. Inflammatory pseudotumor of the urinary bladder. Radiol Case J Radiol Case Rep. 2015;9(1) doi: 10.3941/jrcr.v9i1.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takagi K., Takai M., Kameyama K. ALK gene translocation in inflammatory myofibroblastic tumor of the urinary bladder: A case report. Urol Case Rep. 2015;3(5):138–140. doi: 10.1016/j.eucr.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alam R., Johnson M.H., Caldwell T. Diagnosing and treating inflammatory myofibroblastic tumor of the bladder. Case Rep Urol. 2016;2016:1–3. doi: 10.1155/2016/5724020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allaparthi S., Ramanathan R., Balaji K. Robotic partial cystectomy for bladder Cancer: A single-institutional pilot study. J Endourology. 2010;24(2):223–227. doi: 10.1089/end.2009.0367. [DOI] [PubMed] [Google Scholar]


