Abstract
The hypothalamic arcuate nucleus (ARC) is a major integration center for energy and glucose homeostasis that responds to leptin. Resistance to leptin in the ARC is an important component of the development of obesity and type 2 diabetes. Recently, we showed that Endospanin1 (Endo1) is a negative regulator of the leptin receptor (OBR) that interacts with OBR and retains the receptor inside the cell, leading to a decreased activation of the anorectic STAT3 pathway. Endo1 is up-regulated in the ARC of high fat diet (HFD)-fed mice, and its silencing in the ARC of lean and obese mice prevents and reverses the development of obesity.
Objective
Herein we investigated whether decreased Endo1 expression in the hypothalamic ARC, associated with reduced obesity, could also ameliorate glucose homeostasis accordingly.
Methods
We studied glucose homeostasis in lean or obese mice silenced for Endo1 in the ARC via stereotactic injection of shRNA-expressing lentiviral vectors.
Results
We observed that despite being leaner, Endo1-silenced mice showed impaired glucose homeostasis on HFD. Mechanistically, we show that Endo1 interacts with p85, the regulatory subunit of PI3K, and mediates leptin-induced PI3K activation.
Conclusions
Our results thus define Endo1 as an important hypothalamic integrator of leptin signaling, and its silencing differentially regulates the OBR-dependent functions.
Keywords: Leptin receptor, OB-RGRP/Endospanin1, Insulin, Obesity, Diabetes
Abbreviations: ARC, arcuate nucleus; Endo1, Endospanin1; OBR, leptin receptor; HFD, high fat diet; CD, chow diet; T2D, type 2 diabetes; DIO, diet-induced obesity; BW, body weight; ip, intraperitoneal; GTT, glucose tolerance test; LIF, leukemia inhibitory factor; PLA, proximity ligation assay
Highlights
-
•
Endospanin1 interacts with p85, the regulatory subunit of PI3K.
-
•
Endospanin1 silencing increases STAT3 but decreases AKT activation mediated by leptin.
-
•
Endospanin1 dissociates leptin-regulated body weight and glucose homeostasis.
-
•
Endospanin1 differentially regulates leptin signaling and biological functions.
1. Introduction
The central nervous system integrates neural, hormonal, and nutrient-related signals into outcomes that correctly maintain energy- and glucose-homeostasis. Afferent hormonal signals from the periphery, such as the adipokine leptin, mediate information regarding the body energy status to the brain in order to control food intake, energy expenditure, fat mass, and glucose homeostasis. Dysfunction along a metabolic neuronal pathway can trigger the development of obesity and type 2 diabetes (T2D).
Leptin is mainly secreted by the adipose tissue and circulates to the brain to activate the long signaling-competent isoform of the leptin receptor (OBR) in the feeding centers of the hypothalamus, particularly the arcuate nucleus (ARC). Deficiencies of leptin (ob/ob mice) or the leptin receptor (db/db mice, fa/fa rats) trigger morbid obesity and T2D in both rodents and humans due to uncontrolled food intake, decreased energy expenditure, and insulin resistance [1], [2]. Leptin-deficient mice and humans can be successfully treated with leptin leading to correction of metabolic abnormalities including T2D [3]. However, most obese people, in proportion to their fat mass, often exhibit elevated circulating leptin levels, to which they are not responsive. This lack of responsiveness is likely a consequence of desensitization to leptin, a phenomenon referred to as “leptin resistance” [4], [5]. Several molecular mechanisms have been hypothesized to account for leptin resistance [6], such as endoplasmic reticulum stress [7], decreased transport of leptin into the mediobasal hypothalamus [8], or a defect in OBR trafficking including diminished exposure of OBR at the neuronal cell surface leading to lower leptin signaling [9], [10]. The amount of OBR at the plasma membrane is crucial since only a small fraction of OBR (5–20%) is exposed at the cell surface [11], [12], where receptor localization is required to trigger a signaling response.
Leptin regulation of energy balance, food intake, and energy expenditure is mainly mediated by the hypothalamus and implicates the Janus Kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) pathway [13], [14]. A large body of evidence also supports an important role of central leptin on glucose homeostasis through the PI3K/AKT pathway and leptin action on ARC neurons is part of this regulation [15], [16], [17]. The PI3K/AKT pathway is also a crucial mediator of insulin receptor (IR) signaling, and crosstalk between insulin and leptin were revealed at this level [18], [19].
We had previously shown that Endospanin1 (Endo1), a small four-transmembrane protein, generated in humans from the same lepr gene as OBR, but with no amino-acid sequence similarity with the receptor [20], [21], [22], behaves as a negative regulator of OBR function [9], [10], [21]. Similar to OBR, Endo1 mRNA was detected in central and peripheral tissues, suggesting ubiquitous expression [21]. Endo1, mainly localized in intracellular compartments, interacts with OBR, retains the receptor inside the cells, and targets it to endosomes for lysosomal degradation [21]. Increasing the expression of Endo1 in cells leads to diminished OBR cell surface exposure, while Endo1 silencing augments the number of OBR at the cell surface, concomitant with enhanced leptin-induced STAT3 phosphorylation [9], [10]. In line with this, Endo1 silencing in the mouse ARC, where its expression level is increased in response to high fat diet (HFD), was sufficient to prevent and reverse the development of obesity in a HFD-Induced Obesity (DIO) mouse model [9], [10]. We therefore defined Endo1 as a valuable therapeutic target against obesity.
Since decreasing obesity often leads to amelioration of T2D [23], [24], in the present work, we investigated whether silencing Endo1 in the hypothalamic ARC, associated with reduced body weight (BW), could also ameliorate glucose homeostasis accordingly. In two different experimental settings (in which Endo1 is silenced either before or after DIO challenge), we reveal that the depletion of Endo1 in the ARC surprisingly worsens glucose homeostasis under conditions of enriched food. Interestingly, at the cellular level, Endo1 silencing has differential effects on leptin signal transduction. The absence of Endo1 increases leptin-induced STAT3 phosphorylation, while its presence is required for leptin-stimulated AKT phosphorylation through its physical interaction with p85, the regulatory subunit of PI3K.
2. Results
2.1. Endo1 downregulation in the ARC of obese mice after DIO decreases body weight but impairs glucose homeostasis
Endo1 silencing in the hypothalamic ARC was achieved by stereotactic bilateral injection of Endo1 shRNA-expressing lentiviral vectors in the ARC (Supplem Figure S1A–D) of male C57/Bl6 mice with fully developed obesity and T2D leading to a downregulation of Endo1 in the ARC (Supplem Figure S1C–D), as previously described [10]. Obese animals treated with Endo1 (versus control shRNA) were kept on HFD (Endo1-HFD-HFD vs CTRL-HFD-HFD) or returned to regular chow diet (CD) conditions (Endo1-HFD-CD vs CTRL-HFD-CD) (Figure 1A). Diminishing Endo1 levels specifically in the ARC of obese mice, in combination with CD, clearly facilitates the recovery from DIO, by allowing leptin to better activate STAT3 phosphorylation in the ARC (Supplem Figure S2A), and to induce sustained weight loss (Figure 1B) with decreased fat mass (Supplem Figure S2B) and food intake (Supplem Figure S2C; see also [10]).
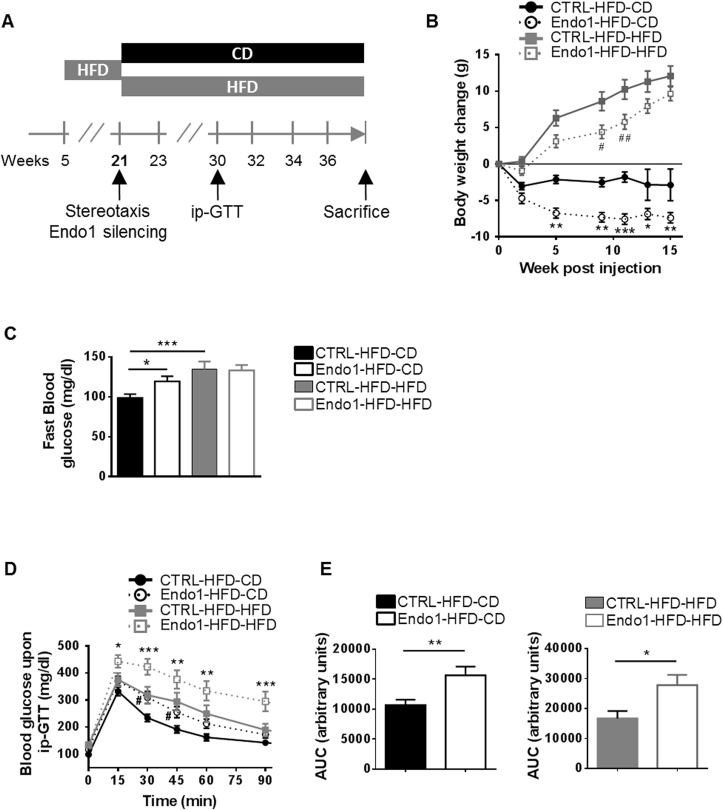
Figure 1.
Endo1 downregulation in the ARC of obese mice after DIO decreases body weight but impairs glucose homeostasis. (A) Experimental Protocol for the “Obesity reversal experiment”. (B) Body weight of obese mice after stereotactic injection of lentiviral vectors expressing control (CTRL) or Endo1 shRNA in the ARC and kept on HFD (CTRL or Endo1-HFD-HFD) or switched to CD (CTRL or Endo1-HFD-CD). CTRL-HFD-CD vs Endo1-HFD-CD: *, p < 0.05; **, p < 0.01; ***, p < 0.005. CTRL-HFD-HFD vs Endo1-HFD-HFD: #, p < 0.05; ##, p < 0.01 (n = 15–20 per group). (C) Fasting blood glucose at week 32. *, p < 0.05; ***, p < 0.005 (n = 15–20 per group). (D) Intraperitoneal Glucose Tolerance Test (ip-GTT) (2 g/kg). CTRL-HFD-CD vs Endo1-HFD-CD: #, p < 0.05. CTRL-HFD-HFD vs Endo1-HFD-HFD: *, p < 0.05; **, p < 0.01; ***, p < 0.005 (n = 15–20 per group). (E) Area Under the Curve of (D). *, p < 0.05; **, p < 0.01. Data are presented as means ± SEM.
As expected, mice with continuous HFD (both control (CTRL-HFD-HFD) and Endo1-silenced (Endo1-HFD-HFD) animals) showed more elevated fasting blood glucose than control animals switched to CD (CTRL-HFD-CD) (Figure 1C). Unexpectedly, when switched to CD, Endo1 silencing in Endo1-HFD-CD mice was incapable of normalizing fasting glycemia, which was more elevated than in control animals (CTRL-HFD-CD) (Figure 1C). In line with this, the intraperitoneal (ip) glucose tolerance test (ip-GTT) revealed higher glucose intolerance in Endo1-silenced mice compared to controls in both diets (Figure 1D–E). Altogether, these data reveal that mice silenced for Endo1 in the ARC, despite eating less and being leaner than control mice, showed altered glucose homeostasis resulting in increased fasting plasma glucose levels and glucose intolerance.
2.2. Endo1 downregulation in the ARC of obese mice after DIO impairs sympathoadrenal activity and leads to decreased glucose-induced insulin secretion
To investigate the underlying mechanism of the altered glucose homeostasis, insulin secretion was monitored in response to glucose challenge. CTRL-HFD-CD mice robustly responded to glucose stimulation by increasing insulin secretion upon an ip challenge of glucose (Figure 2A). As expected, this response was largely diminished in obese mice kept on HFD (CTRL-HFD-HFD), an experimental condition known to induce β-cell alteration [25]. However, as opposed to CTRL-HFD-CD, Endo1-HFD-CD mice exhibited only a weak increase in plasma insulin at a magnitude similar to mice kept on HFD, suggesting that Endo1 silencing in the ARC is responsible for altered insulin secretion (Figure 2A). This is further illustrated by the diminished amount of insulin released upon glucose injection in Endo1-silenced mice both on CD and HFD (Figure 2B). While insulin secretion is impaired, pancreatic insulin levels are not significantly different in all groups (Figure 2C) suggesting that the disturbance in insulin secretion does not come from alteration of islets integrity.
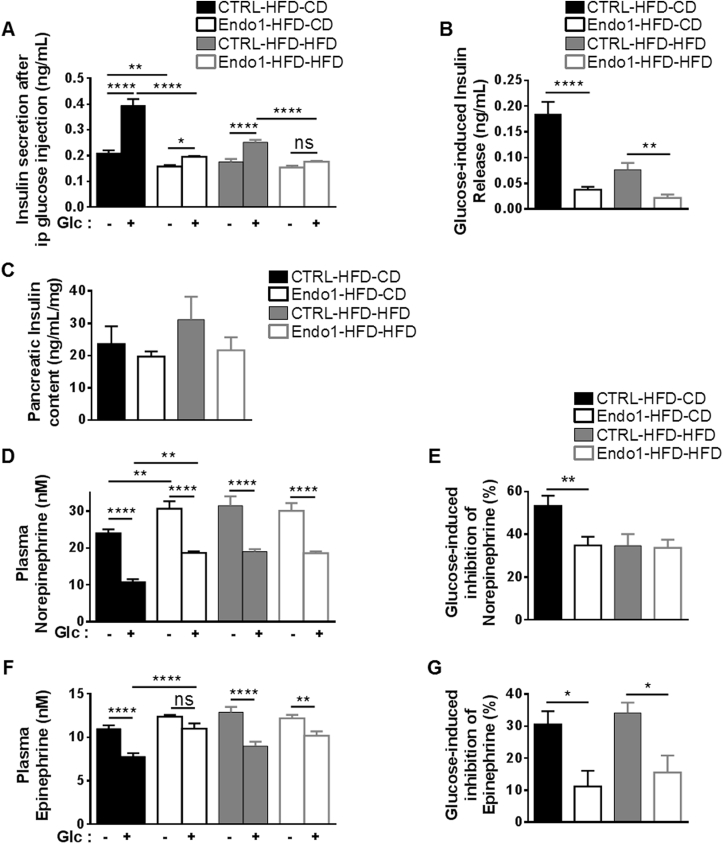
Figure 2.
Endo1 downregulation in the ARC of obese mice after DIO impairs sympathoadrenal activity and leads to decreased glucose-induced insulin secretion. (A) Plasma insulin monitored 20 min after ip glucose injection (2 g/kg). ns, p > 0.05; *, p < 0.05; **, p < 0.01; ****, p < 0.001 (n = 15–20 per group). (B) Glucose-induced insulin release calculated from (A). **, p < 0.01; ****, p < 0.001. (C) Insulin content from acid/ethanol pancreas extract (n = 8 per group). (D) Plasma norepinephrine monitored 20 min after ip glucose injection (2 g/kg). **, p < 0.01; ****, p < 0.001 (n = 15–20 per group). (E) Glucose-induced inhibition of norepinephrine calculated from (D). **, p < 0.01. (F) Plasma epinephrine monitored 20 min after ip glucose injection (2 g/kg). ns, p > 0.05; **, p < 0.01; ****, p < 0.001 (n = 15–20 per group). (G) Glucose-induced inhibition of epinephrine calculated from (F). *, p < 0.05. Data are presented as means ± SEM.
The sympathoadrenal system is a well-known regulator of insulin secretion as exemplified by the direct inhibitory effect of catecholamines on insulin release by β-cells [26], [27], [28]. Given the impairment of insulin secretion in Endo1-silenced mice, plasma catecholamines were quantified. As opposed to CTRL-HFD-CD mice, we observed that Endo1-HFD-CD mice exhibited higher plasma epinephrine and norepinephrine levels, suggesting an enhanced sympathoadrenal activity (Figure 2D–F). Indeed, glucose-induced inhibition of plasma epinephrine and norepinephrine was significantly reduced in Endo1-silenced mice (Figure 2E–G). These data suggest that the dysregulation in sympathoadrenal tone, as reflected by abnormal increase of plasma catecholamine observed in silenced mice, participates in the reduction of insulin secretion and altered glucose homeostasis observed upon Endo1 silencing in the ARC of obese mice.
2.3. Endo1 silencing in the ARC of mice prior to DIO prevents obesity but impairs glucose homeostasis when mice are challenged with a HFD
The alteration in glucose homeostasis upon Endo1 silencing in the ARC could arise from the inability of this treatment to reverse the diabetic phenotype already installed in obese mice after 3 months on HFD. To investigate this possibility, we silenced Endo1 in the ARC of naïve and lean C57Bl/6 mice prior to any HFD treatment by bilateral stereotactic injection of Endo1 shRNA-expressing lentiviral vectors (Figure 3A). As expected, mice silenced for Endo1 in the ARC were protected against the development of obesity after 10 weeks of HFD (Endo1-CD-HFD) (Figure 3B; see also [9]), with reduced fat mass (Supplem Figure S3A), lower food intake (Supplem Figure S3B), and better leptin-induced STAT3 phosphorylation in the ARC (Supplem Figure S3C).
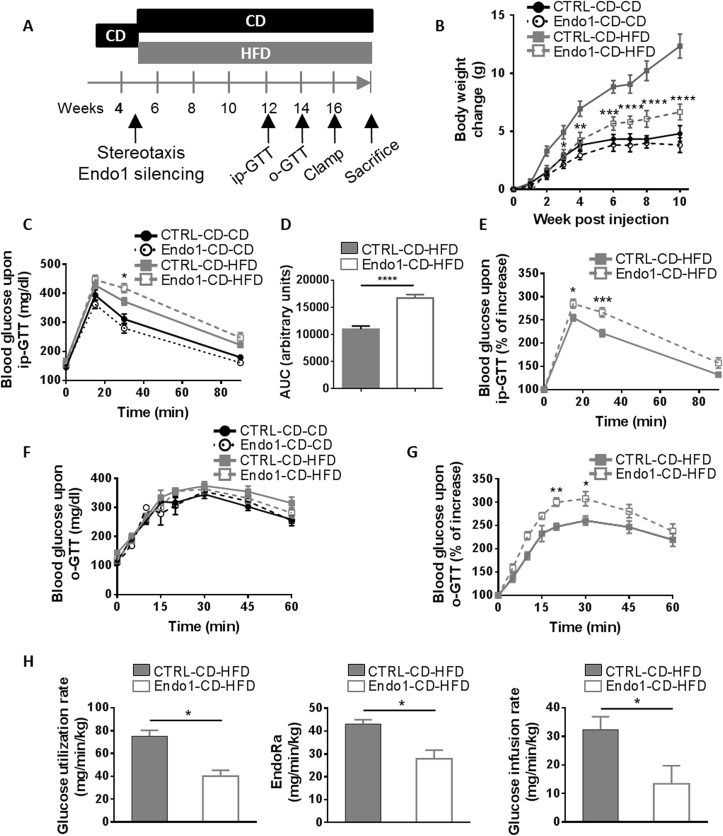
Figure 3.
Endo1 silencing in the ARC of mice prior to DIO prevents obesity but impairs glucose homeostasis on HFD. (A) Experimental Protocol for the “Obesity prevention experiment”. (B) Body weight after stereotactic injection of lentiviral vectors expressing control (CTRL) or Endo1 shRNA in the ARC and kept on CD (CTRL or Endo1-CD-CD) or switched to HFD (CTRL or Endo1-CD-HFD). CTRL-CD-HFD vs Endo1-CD-HFD: *, p < 0.05; **, p < 0.01; ***, p < 0.005; ****, p < 0.001. (n = 8 per group). (C) Intraperitoneal Glucose Tolerance Test (ip-GTT) (2 g/kg). CTRL-CD-HFD vs Endo1-CD-HFD: *, p < 0.05 (n = 15–18 per group). (D) Area Under the Curve (AUC) of (C). ****, p < 0.001. (E) Intraperitoneal Glucose Tolerance Test (ip-GTT) normalized to fasting blood glucose of HFD mice. *, p < 0.05; ***, p < 0.005 (n = 15–18 per group). (F) Oral Glucose Tolerance Test (o-GTT) (3 g/kg) (n = 8 per group). (G) Oral Glucose Tolerance Test (o-GTT) normalized to fasting blood glucose of HFD mice. *, p < 0.05; **, p < 0.01 (n = 8 per group). (H) Glucose utilization rate, endogenous glucose production (EndoRa), and glucose infusion rate required to maintain euglycemia in euglycemic hyperinsulinemic clamp. *, p < 0.05 (n = 4 per group). Data are presented as means ± SEM.
When glucose homeostasis was investigated, we observed that silencing of Endo1 in lean mice on CD did not trigger obvious glucose intolerance in ip (Figure 3C) or oral glucose tolerance test (Figure 3F, Supplem Figure S3D) compared to the controls. Those results suggest that a low level of Endo1 in the ARC does not have a significant impact on glucose homeostasis when mice are fed with a CD. Conversely, Endo1-silenced mice put on HFD (Endo1-CD-HFD), despite being leaner, exhibited a lower glucose tolerance compared to CTRL-CD-HFD controls (Figure 3C–G, Supplem Figure S3D). Euglycemic hyperinsulinemic clamps of HFD mice were performed to further assess insulin sensitivity on obesigenic diet. Endo1-CD-HFD mice are characterized by a significant fall in overall glucose uptake (GUR), a lower liver glucose production (EndoRa), and an overall decrease in insulin sensitivity compared to CTRL-CD-HFD, as suggested by the lower glucose infusion rate (Figure 3H). Altogether, the data indicate that Endo1 silencing in the ARC does not have an impact on CD-fed mice but leads to molecular modifications that worsen the status of diabetes of silenced mice challenged with a HFD, despite the prevention of obesity.
2.4. Endo1 silencing in the ARC prior to DIO impairs sympathoadrenal activity and leads to defective glucose-stimulated insulin secretion and to higher glucagon levels on HFD
Since Endo1 silencing in obese mice after DIO was characterized by an alteration of insulin secretion, this was later investigated in response to glucose administration in mice silenced for Endo1 prior to DIO. Endo1-CD-CD and CTRL-CD-CD mice exhibited analogous insulin secretion profiles (Figure 4A) along with a similar glucose tolerance, supporting no obvious alteration in glucose homeostasis of Endo1-silenced mice on CD. In contrast, on HFD, while CTRL-CD-HFD exhibited the expected compensatory increase in insulin secretion in the glucose tolerance test, Endo1-CD-HFD animals exhibited a strongly diminished insulin response (Figure 4A). This result suggests a lack of adaptation in insulin secretion of Endo1-CD-HFD in regard to insulin resistance evidenced in clamp studies, in which an increase in insulin secretion would have been expected to compensate for insulin resistance (Figure 3H). Similarly, Endo1-animals on HFD (Endo1-CD-HFD) were characterized by higher plasma glucagon (Figure 4B). Analysis of pancreatic content of insulin and glucagon revealed no significant changes in pancreatic insulin (Figure 4C) and glucagon levels (Figure 4D) of mice on the same diets.
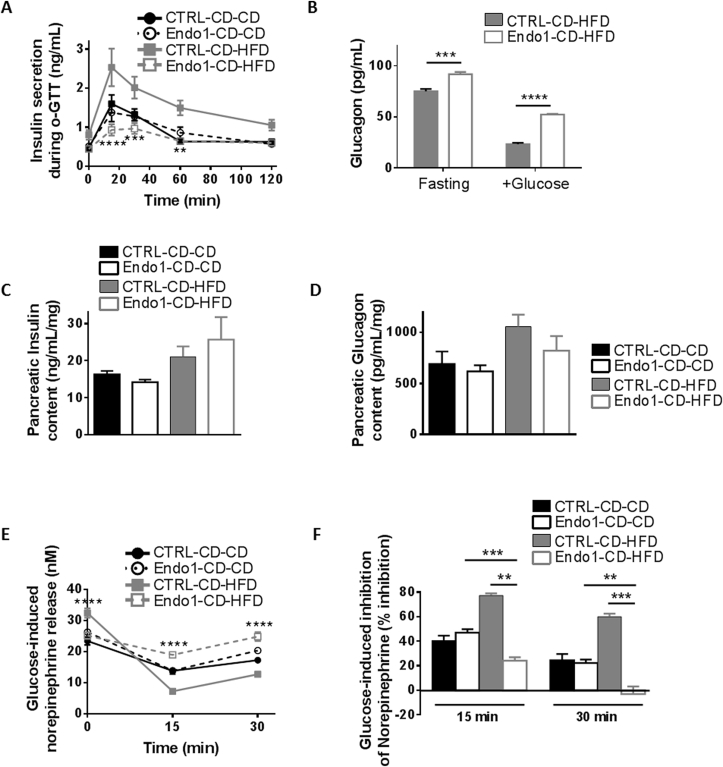
Figure 4.
Endo1 downregulation in the ARC of mice prior to DIO impairs sympathoadrenal activity and leads to decreased glucose-induced insulin secretion and higher glucagon release when challenged a HFD. (A) Plasma insulin monitored during oral Glucose Tolerance Test (o-GTT). CTRL-CD-HFD vs Endo1-CD-HFD: **, p < 0.01; ***, p < 0.005; ****, p < 0.001 (n = 5–8 per group). (B) Plasma glucagon monitored at fasting and 15 min during o-GTT. ***, p < 0.005; ****, p < 0.001 (n = 4 per group). (C) Insulin content from acid/ethanol pancreas extract (n = 4 per group). (D) Glucagon content from acid/ethanol pancreas extract (n = 4 per group). (E) Plasma norepinephrine monitored during o-GTT after oral glucose administration. CTRL-CD-HFD vs Endo1-CD-HFD: ****, p < 0.001 (n = 8 per group). (F) Glucose-induced inhibition of norepinephrine calculated from (E). **, p < 0.01; ***, p < 0.005 (n = 8 per group). Data are presented as means ± SEM.
In agreement with the known inhibitory effect of catecholamines on insulin secretion [26], [27], [28], [29], the time course of glucose-stimulated insulin release was inversely correlated to plasma norepinephrine levels (Figure 4E). Norepinephrine levels remained high in Endo1-CD-HFD mice, paralleling the low insulin release in response to glucose. Indeed, Endo1-CD-HFD mice are characterized by markedly attenuated inhibition of plasma norepinephrine upon glucose administration (Figure 4F). Here again, alteration of the sympathoadrenal system could account for the abnormal insulin and glucagon secretion of Endo1-CD-HFD mice during glucose administration.
Collectively, our data show that Endo1 silencing in the ARC, before and after DIO, i) alters norepinephrine release leading to aberrant insulin and glucagon secretion in response to glucose, and ii) decreases insulin sensitivity promoting a diabetic phenotype, when mice have been once fed a HFD. The molecular mechanism leading to impaired glucose homeostasis in Endo1-silenced mice cannot be intuitively explained by known functions of Endo1.
2.5. Endo1 interacts with p85, the regulatory subunit of PI3K
To understand the molecular mechanism underlying the impact of Endo1 silencing on glucose metabolism, we determined the interactome of Endo1 with the Ultimate Y2H™ yeast-two-hybrid assay (Hybrigenics). Among the top proteins interacting with Endo1, p85, the regulatory subunit of the class I phosphoinositide 3-kinase (PI3K), was assigned with a confidence score as “Good Confidence in the interaction.” p85 protein caught our attention as the PI3K pathway has been shown to be responsible for the effect of leptin on glucose homeostasis in the hypothalamus [16], [17], [30].
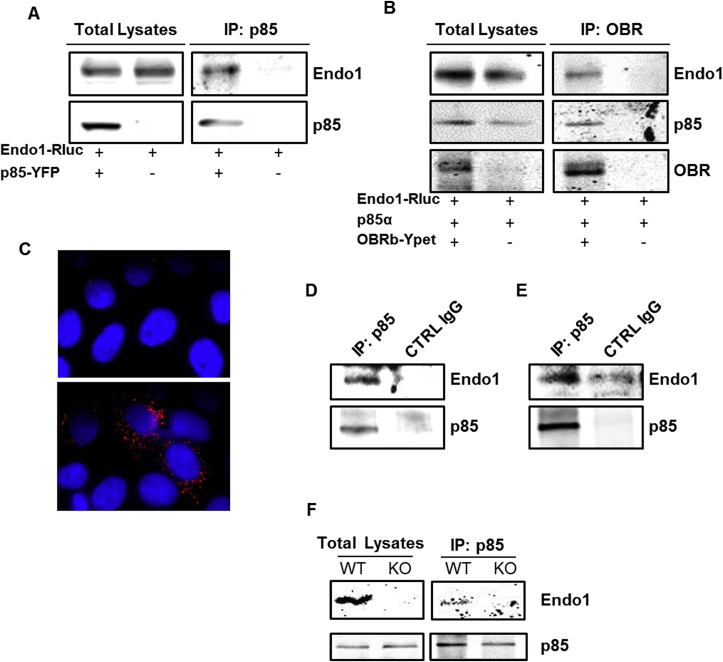
Co-immunoprecipitation experiments in transfected Human Embryonic Kidney (HEK293) cells confirmed the interaction between Endo1 and p85 (Figure 5A) and showed that Endo1 and p85 co-immunoprecipitated along with OBR (Figure 5B), suggesting the formation of a tricomplex. Furthermore, Endo1 and p85 colocalized in transfected HeLa cells as revealed by proximity ligation assay (Figure 5C). Importantly, the interaction between endogenous Endo1 and endogenous p85 was validated in HEK293 (Figure 5D) and in N46 hypothalamic cells (Figure 5E). We further investigated the existence of the protein complex in mouse tissues and showed that Endo1 interacts with p85 in the hypothalamus of wild type (WT) mice but not of Endo1 knock-out (KO) mice as a control (Figure 5F).
Figure 5.
Endospanin1 interacts with p85, the regulatory subunit of PI3K. (A) Representative western blot of Endo1 co-immunoprecipitated with p85 in HEK293TSA cells, transiently expressing p85-YFP and Endo1-Rluc. Cell lysates were submitted to p85 immunoprecipitation with anti-GFP antibody (n = 4). (B) Representative western blot of Endo1 and p85 co-immunoprecipitated with OBR in HEK293TSA cells, transiently expressing OBRb-Ypet, p85 and Endo1-Rluc. Cell lysates were submitted to OBR immunoprecipitation with anti-GFP antibody (n = 3). (C) Microscopy images of p85/Endo1 interaction by in situ Proximity Ligation Assay (PLA) in HeLa cells transiently expressing p85 alone (top), or p85 and 6Myc-Endo1 (bottom). The nuclei were stained with DAPI. (D–E) Representative western blot of endogenous Endo1 co-immunoprecipitated with endogenous p85 in HEK293TSA cells (D) or hypothalamic N46 cells (E). Cell lysates were submitted to p85 immunoprecipitation, with anti-p85 antibody, or control IgG for negative control (n = 3–7). (F) Endo1 is co-immunoprecipitated with p85 from hypothalamus lysates from WT mice but not from Endo1 KO mice.
Collectively, these results suggest that Endo1, by interacting with p85, regulates leptin-stimulated PI3K pathway and plays a role in the control of glucose homeostasis.
2.6. Endo1 silencing increases leptin-induced STAT3 activation but inhibits PI3K/AKT signaling
Our finding that Endo1 is involved in the PI3K pathway is in line with a very recent study defining the function of Endo1 in insects. The latter suggested that Endo1 silencing in Helicoverpa armigera larvae was correlated with a decrease in the transcript levels of PI3K in larvae and an increased level of the Forkhead transcription factor FoxO, known to be regulated by the PI3K signaling pathway [31]. However, unlike in insects, the absence of Endo1 in mammals does not impact on the protein levels of p85 (PI3K) or FoxO as investigated in the mouse hypothalamus, cortex, and brainstem of Endo1 KO mice compared to WT, and in human HeLa cells silenced for Endo1 (Supplem Figure S4).
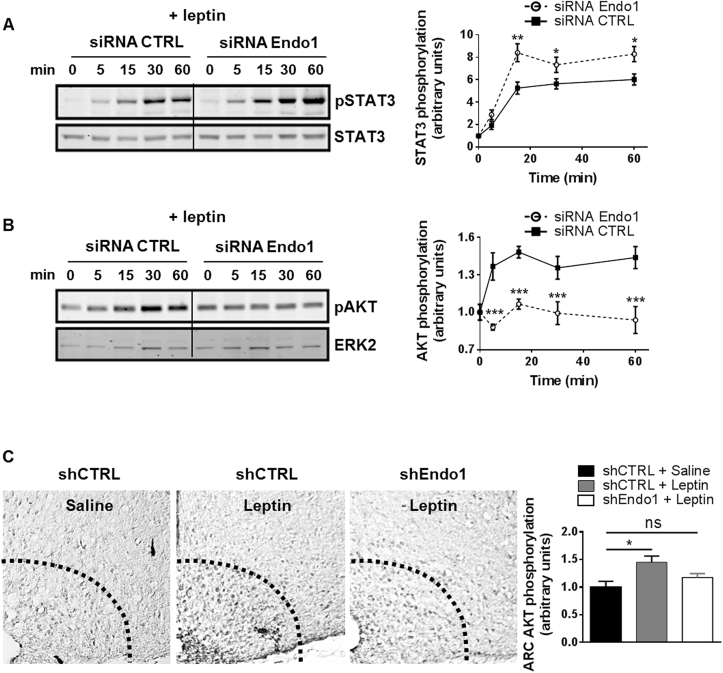
On the other hand, considering the crucial role of leptin-regulated PI3K in glucose homeostasis [16], [17], in combination with the altered glucose homeostasis seen in Endo1-silenced mice, suggested a defective leptin-mediated PI3K activation. We therefore hypothesized that the interaction between Endo1 and the regulatory subunit of PI3K, p85, would be required to convey leptin-mediated activation of PI3K. To investigate this possibility, we assessed the effect of Endo1 silencing on the phosphorylation of AKT, also known as protein kinase B (PKB), which is a major downstream target of PI3K. The impact of Endo1 silencing on leptin signaling was assessed in cellular models, with siRNA achieving 80% Endo1 silencing at the mRNA (Supplem Figure S5A) and protein levels (Supplem Figure S5B). Endo1 silencing enhanced leptin-induced STAT3 phosphorylation in a HEK293 cell line stably expressing the signaling-competent long isoform of the leptin receptor, OBRb, confirming our previous work [9], [10] (Figure 6A). In contrast, Endo1 downregulation inhibited leptin-stimulated AKT phosphorylation in the same cells (Figure 6B). In line with this, leptin-mediated AKT phosphorylation was attenuated in a cellular model where Endo1 expression is totally absent, i.e. primary hepatocytes from Endo1 KO mice compared to WT primary cells (Supplem Figure S5C). Importantly, the effect of Endo1 on receptor signaling seemed to be OBRb-specific since Endo1 silencing in cells did not alter the ability of insulin or the leukemia inhibitory factor (LIF) to activate the phosphorylation of AKT (Supplem Figure S6).
Figure 6.
Endospanin1 is necessary for leptin-induced PI3K activity. (A–B) Representative western blot of 10 nM leptin-stimulated STAT3 (A) and AKT (B) phosphorylation following 2 consecutive transfections of HEK293-OBRb with Endo1 or control siRNA for 4 days. Densitometry analysis of (A) and (B) is presented on the right panel. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (n = 3–5). (C) Representative images showing phospho-AKT (Ser473) immunoreactivity in the ARC of mice 8 weeks after stereotactic injection with CTRL shRNA or Endo1 shRNA-expressing lentiviral vectors. CTRL-CD-CD or Endo1-CD-CD mice received for 15 min intraperitoneal injections of either vehicle (saline) or leptin (2 mg/kg). Densitometry analysis is presented on the right panel. Leptin increased the number of phospho-AKT (Ser473)-immunoreactive cells in the ARC, while Endo1 silencing significantly abrogated this effect. ns, p > 0.05; *, p < 0.05 (n = 3–6). Data are presented as means ± SEM.
Next, we verified the effect of Endo1 silencing on leptin signaling in the ARC. As previously shown, STAT3 phosphorylation upon leptin injection was increased in the hypothalamic ARC punches of Endo1-silenced mice vs controls (Supplem Figures S2A and S3C, see also [9], [10]). However, whereas leptin increased phospho-AKT immunoreactivity in the ARC of brain sections of control mice as expected, this effect was abrogated in Endo1-silenced mice (Figure 6C). These results suggest that i) Endo1 is essential for the leptin-induced activation of the PI3K/AKT pathway, and ii) Endo1 exerts differential effects on OBR signaling.
Altogether, our data show that, under HFD conditions or when mice have been challenged with HFD, Endo1 silencing in the hypothalamic ARC triggers a leaner phenotype (see also [9], [10]) but elevated levels of plasma catecholamines, a lower glucose-stimulated insulin secretion, a higher plasma glucagon, a diminished glucose uptake in tissues, and an attenuated insulin sensitivity, all ultimately leading to altered glucose homeostasis. At the molecular level, Endo1 silencing enhances leptin-induced STAT3 signaling, but impairs the activation of PI3K/AKT in response to leptin.
3. Discussion
In the present study, we designed two experimental protocols to study the influence of Endo1 downregulation on glucose homeostasis. Endo1 negatively regulates the leptin-induced activation of the JAK2/STAT3 pathway, while, by interacting with p85, Endo1 is required for leptin-induced activation of the PI3K pathway, demonstrating that Endo1 is an important integrator of leptin signaling. This central role on leptin function is further supported by the observation that the downregulation of Endo1, which is up-regulated in the ARC upon HFD [10], is sufficient to prevent [9] and reverse DIO [10] but not T2D.
3.1. Endo1 silencing leads to dissociation between obesity and diabetes
It is commonly accepted that T2D is a consequence of overweight and obesity and that the majority of T2D are overweight or obese. However, the obese phenotype is not necessarily linked to and can be separated from the diabetic phenotype. Indeed, the prevalence of pre-diabetes and T2D is around 40% in obese patients [23]. The mechanisms underlying the dissociation between obesity and T2D have been elusive, but several studies attempted to understand this divergence and proposed a functional involvement of peripheral tissues such as the adipose tissue [32], [33]. Other studies suggested that BW and glucose metabolism could be separately controlled via central regulation. s/s mice mutated for STAT3 docking sites on OBRb, in which leptin was therefore unable to activate STAT3, are hyperphagic and obese, similar to OBRb-deficient db/db mice, but their glucose homeostasis is relatively unaltered compared to db/db mice [15]. Interestingly, Bates and colleagues also reported dissociation between leptin-regulated control of energy homeostasis and reproductive functions, which could be discriminated at the level of JAK2/STAT3 pathway [13]. Furthermore, genetically engineered mice, in which OBRb expression was selectively restored in the ARC, were found to exhibit only modest effects on food intake and BW, while they showed markedly improved hyperinsulinemia and normalized blood glucose levels [16]. Inversely, the deletion of both insulin receptor and OBR from POMC neurons resulted in impaired glucose tolerance and insulin resistance, despite maintaining normal BW [34]. Our current results on Endo1 corroborate those studies and bring new light on the molecular mechanism. Endo1 silencing in the ARC corrects obesity (see also [9], [10]) but not hyperglycemia or glucose intolerance in response to HFD.
From a mechanistic point of view, the differential effect of Endo1 on STAT3 and PI3K/AKT pathways constitutes the molecular basis underlying the dissociation between obesity and diabetes. Interestingly, a very recent study established a link between Endo1 and PI3K signaling in insect larvae, suggesting that Endo1 regulation of PI3K may be conserved during evolution although through different mechanisms from insects to mammals [31].
3.2. Endo1 regulates differentially leptin receptor signaling
A noteworthy and unexpected finding of the present study is that the effect of Endo1 on OBR signaling is pathway-specific, defining Endo1 as a mediator of biased OBR signaling. Indeed, Endo1 downregulation increases STAT3 signaling but abrogates PI3K/AKT signaling promoted by leptin. Endo1 silencing has been shown to increase OBR cell surface exposure and leptin-induced JAK2/STAT3 signaling, most likely by decreasing lysosomal degradation of OBR and increasing its recycling back to the plasma membrane [9], [10], [21]. In the present study, despite the expected increase of OBR cell surface expression, we show that leptin-induced activation of the PI3K/AKT pathway is largely diminished in the absence of Endo1. This suggests an additional mechanism of action of Endo1 on OBR signaling, that is independent from its effect on the number of OBR exposed at the plasma membrane. The interaction between Endo1 and p85, the regulatory subunit of PI3K could provide a physical basis for the specific effect of Endo1 on PI3K activation. The leptin-induced recruitment of IRS4 on OBRb pY1077 has been shown to mediate the interaction of p85 on OBRb [35], the latter being associated with Endo1. In this signaling complex, Endo1, by also interacting with p85, could be required for the conformational change within PI3K (p85–p110), alleviating the inhibitory repression mediated by p85 on p110, the catalytic subunit of PI3K [36]. A similar hypothesis has been proposed for the endosomal Rab5 effector, which, through its interaction with p85, is required for insulin-mediated activation of PI3K [37]. Hence, Endo1 is a regulatory molecule that is not only a trafficking molecule directing OBR from endosomes to lysosomes but also directly participates in the regulation of OBR signaling. Proteins such as Endo1 support the importance of accessory molecules in determining receptor function.
3.3. Endospanin1 silencing in the ARC leads to altered glucose homeostasis
Previous studies suggested a clear link between central leptin-mediated PI3K and control of glucose homeostasis [17], [19], [38]. Restoring OBRb expression in the ARC of OBR-deficient rats increased insulin sensitivity in a manner dependent on central PI3K signaling [17]. Herein, we identified multiple outcomes resulting from an alteration of leptin-mediated PI3K pathway associated with an enhanced activation of STAT3 to the diabetic phenotype following the central silencing of Endo1.
We gathered strong evidence, suggesting that silencing of Endo1 in the ARC could lead to a diminished pancreatic capacity to secrete insulin and to inhibit glucagon release in response to glucose. Excessive stimulation of sympathoadrenal activity reflected by increased production of epinephrine and norepinephrine was shown to lead to the inhibition of insulin secretion [26], [27] and the increase of glucagon release [39], [40], [41]. A similar situation exists in mice silenced for Endo1 in the ARC, in which elevated epinephrine/norepinephrine levels were elevated. Likewise, the impairment of insulin secretion in response to glucose in silenced mice could also additively promote hyperglucagonemia [42]. Multiple studies suggested that hypothalamic leptin is indeed involved in the inhibitory effect of pancreatic insulin release [43], [44], [45], [46]. In our study, under conditions of Endo1 downregulation in the ARC, impairment of the fine tuning between important leptin-dependent signaling pathways would trigger aberrant regulation by central leptin of peripheral insulin/glucagon secretion in response to glucose. Modifications of leptin signaling in the ARC upon Endo1 downregulation disturb the molecular signaling balance at a site crucial for the homeostatic control resulting in metabolic alterations.
Furthermore, the central action of leptin is known to regulate glucose metabolism in skeletal muscle, the main tissue taking up circulating glucose. Indeed, central administration of leptin in wild type animals increases glucose uptake in peripheral tissues including skeletal muscle [47], [48]. The intracerebroventricular administration of leptin in lean rats [38] and in obese leptin-deficient (ob/ob) mice [19] improved glucose metabolism in muscle in a hypothalamic PI3K dependent manner [38]. More precisely, leptin-mediated hypothalamic activation of PI3K potentiates muscle insulin sensitivity, promoting glucose uptake and fatty acid β-oxidation [38], [49], [50]. The alteration of central leptin-induced PI3K is foreseen to alter glucose uptake by skeletal muscle. In line with this, overall insulin sensitivity is impaired in mice silenced for Endo1 demonstrated by a decrease of glucose utilization rate and glucose infusion rate as evidenced in clamp studies.
3.4. Importance of Endo1 expression levels
Our results raise the question of whether Endo1 contributes to leptin resistance. The observed increase of Endo1 in the ARC of HFD fed mice [10] presumably diminishes OBR at the cell surface and favors its degradation, thus leading to a decrease in OBR signaling and contributing to leptin resistance. On the other hand, low levels of Endo1 alter leptin-stimulated PI3K signaling favor development of T2D. Because of its dual impact on OBR trafficking and signaling, a fine balance of Endo1 expression in the hypothalamus would be required to preserve an intact metabolic physiology. Genetic epidemiology should give answers on the involvement of Endo1 in the development of diabetes in humans, primarily by looking at Endo1 variants in lean diabetic individuals where we could expect that Endo1 expression or function is dramatically diminished.
Endo1 silencing in the ARC does not seem to impact BW or glucose homeostasis when mice are fed a normal diet. However, decreasing Endo1 levels in HFD worsens glucose homeostasis, even after having removed HFD. Since Endo1 is necessary for leptin-mediated PI3K/AKT signaling, but not STAT3 signaling, this suggests that the increase of Endo1 expression upon obesigenic diet is foreseen to contribute in counteracting HFD-mediated deleterious effects on glucose homeostasis, to the detriment of BW regulation. Indeed, the role of Endo1 in HFD would be protective if improving glucose homeostasis by facilitation of the PI3K activation, which could be considered more important than abolishing the anorectic action of leptin through the STAT3 pathway. This Endo1-mediated biased signaling is in agreement with the concept of selective leptin resistance [51].
The importance of Endo1 in the regulation of energy homeostasis in the ARC raises the question of its specific role in POMC and AgRP metabolic neurons. The phenotype of Endo1-silenced mice would be compatible with higher activation of STAT3 in AgRP neurons (leanness, [52]). However, in the latter, an impairment of the leptin-induced PI3K/AKT activation, subsequent to Endo1 silencing, would prevent leptin-mediated inhibition of AgRP neurons [53]. In line with this, we previously observed an unexpected increase of AgRP mRNA levels in the ARC of Endo1-silenced mice [10]. This suggests that a change in AgRP is not necessarily correlated with a modulation of food intake, as previously observed by others [52], [54]. On the other hand, mice with inhibition of PI3K through deletion of p110β in AgRP neurons also display a lean phenotype with reduced adiposity, lower food intake, and resistance to diet-induced obesity [55], similar to the phenotype of Endo1-silenced mice in the ARC. In the same line, increased leptin-induced STAT3 activation in Endo1-silenced POMC neurons would be expected to trigger greater stimulation of the melanocortin system known to mediate leptin effect on energy intake [56]. Furthermore, leptin signaling in POMC neurons is also known to have a key role in the regulation of glucose homeostasis [57], [58]. The alteration of glucose control with Endo1 silencing suggests the involvement of Endo1 in mediating leptin action in POMC neurons for this control. Clearly a synergic regulation between these two neuronal populations via specific signaling pathways is required for an integrated and proper response to leptin, regulation that could be Endo1-dependent.
Finally, the role of Endo1 in the whole body where OBR is also largely expressed (other brain regions and peripheral tissues) warrants further investigations. The examination of ubiquitous or tissue-specific mouse KO models for Endo1 would give insight into the impact of this protein on the control of energy homeostasis.
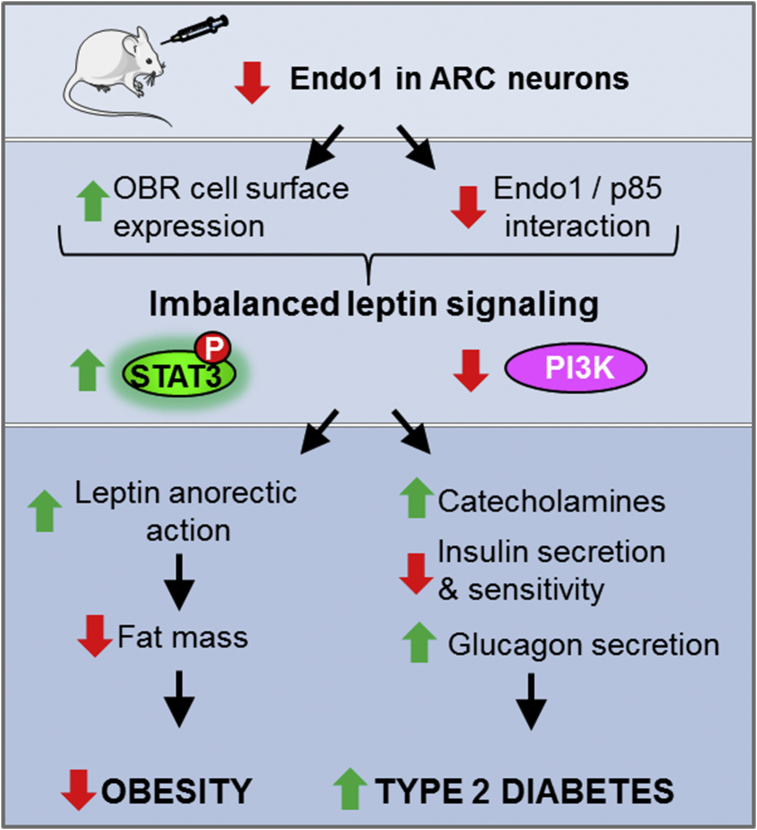
In conclusion, this work, combined with our previous studies, shows that Endo1 is up-regulated in the ARC of obese mice, and its silencing in the ARC of lean and obese mice subjected to HFD prevents and reverses obesity development, respectively, but impairs glucose homeostasis. These opposite effects result from a differential regulation of OBR signaling pathways in an Endo1-dependent manner (Figure 7).
Figure 7.
Scheme summary. The differential effect of Endo1 silencing on leptin signaling contributes to the dissociation between obesity and type II diabetes.
4. Research design and methods
4.1. Animals
Animal use and procedures were approved by the French ethics committee. C57BL/6J male mice (Charles River) were housed in specific pathogen-free biosafety level 2 animal facility in a standard 12-hr on/off light cycle, according to institutional guidelines. Mice were fed a standard rodent diet or high fat diet (D12451; Research Diets) and were provided with water and food ad libitum. BW was monitored weekly. For more details, see SI Experimental Procedures.
4.2. Lentivirus production and intracerebral stereotactic injection
Lentiviral production and stereotactic injection were performed as previously described [9]. For more details, see SI Experimental Procedures.
4.3. RNA extraction and quantitative real-time RT-PCR
For more details, see SI Experimental Procedures.
4.4. Generation of Endo1 KO mice
Conversely to humans, the mouse Endo1 gene localized on chromosome 4 contains four exons and is not genetically linked to the OBR gene, allowing for the generation of transgenic mice with specific Endo1 gene disruption without affecting OBR expression. The procedure for the generation of Endo1 KO mice is described in SI Experimental Procedures.
4.5. Measures of glucose tolerance
Glucose tolerance tests were performed by intraperitoneal d-glucose (2 g/kg) Injection following a 16 h fast. For oral glucose tolerance test, 3 g/kg d-glucose was orally administered. Blood glucose levels were determined from the tail vein at 0, 15, 30, 60, 90, and 120 min using a hand-held glucometer (One Touch Ultra, LifeScan).
4.6. Plasma assays
Plasma was obtained from whole blood collected from tail vein on heparin capillaries and centrifuged at 5000 g for 5 min. Plasma immunoreactive insulin and glucagon levels were determined by ELISA (Crystal Chem Inc., Chicago). Plasma epinephrine and norepinephrine was determined by HPLC.
4.7. Pancreatic insulin and glucagon content
The whole pancreas was dissected free from fat immediately after the mouse was killed. After the measurement of the wet weight, the pancreas was homogenized with ULTRA-TURRAX and acid-ethanol extracted (0.18 M HCl in 70% ethanol) for 16 h at 4 °C. Insulin and glucagon concentration in clarified supernatant (after centrifugation at 4000 g) were determined by Ultrasensitive Mouse Insulin kit (Crystal Chem).
4.8. Euglycemic-hyperinsulinemic clamps
Mice were catheterized 7 days before the experiment as previously described [59]. Briefly, after being fasted for 6 h, awake animals were placed unrestrained in their home cage for the duration of the clamp experiment. After a bolus of 80 mU/kg insulin, glucose (15%) infusion started, whereas insulin infusion was kept constant at 3.3 mU/kg/min. Blood glucose levels were determined from tail blood samples (5 μl) at t = 0 and then every 15 min (glucose analyzer Accu-check, Roche). Glucose infusion rate was changed in order to maintain euglycemia. Steady state was ascertained when glucose measurements were constant for at least 20 min at a fixed glucose infusion rate and was achieved within 50–80 min.
4.9. Yeast-two hybrid screen
A yeast-two-hybrid screen against a human brown adipose tissue cDNA library was performed by Hybrigenics (Paris, France) using human Endo1 intracellular loop (amino-acid residue 51–70) as bait. Protein–protein interactions were individually assigned a statistical confidence score (from A to F) where categories A, B, and C represent positive interactions at different levels of confidence [60]. The positive clones encoding p85 were computed with a confidence score of C (i.e. “Good Confidence in the interaction”).
4.10. Co-immunoprecipitation
For detailed information, see SI Experimental Procedures. Briefly, hypothalamic tissues were dissected from fasted 12-week old Endo1 KO and WT mice. OBRb-Ypet and p85-YFP transfected cells or dissected hypothalami were lysed with CHAPS 10 mM (Sigma). Cleared lysates were subjected to p85 or OBR immunoprecipitation with Protein G Sepharose® (Sigma) and anti-GFP antibody (Roche) or anti-p85 (Millipore) as specified for each experiment. For endogenous experiments, control rabbit IgG (Santa Cruz Biotechnology) was used as negative control. Protein complexes were denatured in Laemmli buffer supplemented with 50 mM DTT, separated by SDS-PAGE and analyzed by WB.
4.11. Proximity ligation assay (PLA)
Transfected HeLa cells, plated onto glass coverslips, were fixed with 4% PFA (PolySciences Europe) for 15 min at RT. Fixed cells were permeabilized with 0.1% Triton X-100 for 10 min, followed by blocking in PBS containing 10% horse serum for 1 h at RT. Cells were incubated with rabbit anti-p85 (1:1000, Millipore) and mouse anti-Myc (1:200, Santa Cruz Biotechnology) antibodies overnight at 4 °C. PLA was conducted using Duolink® In Situ-Fluorescence kit (Sigma), following the instructions of the manufacturer. Images were captured using a Zeiss Axioskop Observer Z.1 microscope equipped with epifluorescence, with laser excitation filters at 548–572 nm and emission lines at 590–624 nm. Deconvoluted images were further analyzed with ImageJ software (NIH, Bethesda).
4.12. Signaling in Endo1-silenced cells
Protein Endo1 downregulation was efficiently performed by transfecting cells with either CTRL or Endo1 siRNA (SI Experimental Procedures). 16 h-starved cells were stimulated with 10 nM leptin (PLR Ltd), or 10 ng/mL LIF (Sigma) or insulin in dose response (Sigma) before being lysed in Laemmli buffer with phosphatase inhibitors and analyzed in WB.
4.13. Leptin signaling in HEK cells, hepatocyte primary culture and hypothalamic ARC
Hepatocytes were isolated from liver (25–30 g) of fed 12-week old Endo1 KO and WT mice, by collagenase. Hypothalamic ARC was recovered by micropunches of 200 μm frozen brain sections. For more details, see SI Experimental Procedures.
4.14. Western blot (WB)
WB protocols and antibodies used are described in SI Experimental Procedures.
4.15. Immunohistochemistry
After dissection, the brains of perfused animals were fixed, dehydrated, and sectioned throughout the hypothalamus on a cryostat. Fixed brain sections were incubated with the anti-phospho-AKT Ser473, IHC-specific (Cell Signaling Technology) and with a biotinylated secondary goat anti-rabbit antibody and then treated with ABC (Vector Laboratories) solution according to manufactures instructions. For more details, see SI Experimental Procedures.
4.16. Statistics
Data were analyzed by two-tailed unpaired Student's t-test, one sample t-test, or by one-way or two-way ANOVA (corrected for repeated measures if required) followed by Fisher's LSD, Tukey or Sidak's multiple comparisons tests, using Graphpad software (California). Results are presented as means ± SEM, and differences were considered significant if p < 0.05.
Acknowledgements
Author Contribution: J.D. and R.J. wrote the manuscript; V.V., C.R., M.F., S.M., and C.M. reviewed the manuscript and contributed to discussion; J.D and R.J. designed experiments and managed the project; V.V., C.R., P.C., J.D., and S.M. performed research and analyzed data, C.S. and J.M. helped designing research, T.H., K.O., and Y.R. contributed to valuable tools, J.M.L. performed blood sample analysis. This work was supported by grants from the European Union's Seventh Framework Programme (FP7/2007–2013) under grant agreement n° 241592 (JD, RJ), the Agence Nationale de la Recherche ANR-12-JSV1-0011 (JD), Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS), a PhD fellowship from the « Ministère de l'Education Nationale et de la Recherche en Technologie » (MNERT, ED419) (CR), a research fellowship from the « Fondation pour la Recherche Médicale » (VV, CR). We would like to thank the Cochin Institute's Morphology and Histology facility for technical assistance. We thank Marlene Freyburger for helping with the stereotactic injection. cDNAs for p85α and p85α fused to the yellow variant of the green fluorescent protein (p85α-YFP) were kindly provided by Dr T. Issad and Dr G. Bismuth (Insitut Cochin, Paris), respectively. Anti-p85 and anti-Endo1 antibodies were generous gifts from Dr F. Verdier (Insitut Cochin, Paris) and Dr Y. Rouillé (Institut Pasteur, Lille), respectively. The authors declare no competing financial interests.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.10.009.
Conflict of interest
All authors declare that there are no known conflict of interest associated with this publication.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Tartaglia L.A., Dembski M., Weng X., Deng N., Culpepper J., Devos R. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 3.Coppari R., Bjorbaek C. Leptin revisited: its mechanism of action and potential for treating diabetes. Nature Reviews Drug Discovery. 2012;11:692–708. doi: 10.1038/nrd3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorbaek C. Central leptin receptor action and resistance in obesity. Journal of Investigative Medicine. 2009;57:789–794. doi: 10.231/JIM.0b013e3181bb0d49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers M.G., Cowley M.A., Munzberg H. Mechanisms of leptin action and leptin resistance. Annual Review of Physiology. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 6.Roujeau C., Jockers R., Dam J. New pharmacological perspectives for the leptin receptor in the treatment of obesity. Frontiers in Endocrinology (Lausanne) 2014;5:167. doi: 10.3389/fendo.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozcan L., Ergin A.S., Lu A., Chung J., Sarkar S., Nie D. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metabolism. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Balland E., Dam J., Langlet F., Caron E., Steculorum S., Messina A. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metabolism. 2014;19:293–301. doi: 10.1016/j.cmet.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couturier C., Sarkis C., Seron K., Belouzard S., Chen P., Lenain A. Silencing of OB-RGRP in mouse hypothalamic arcuate nucleus increases leptin receptor signaling and prevents diet-induced obesity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19476–19481. doi: 10.1073/pnas.0706671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vauthier V., Swartz T.D., Chen P., Roujeau C., Pagnon M., Mallet J. Endospanin 1 silencing in the hypothalamic arcuate nucleus contributes to sustained weight loss of high fat diet obese mice. Gene Therapy. 2014;21:638–644. doi: 10.1038/gt.2014.36. [DOI] [PubMed] [Google Scholar]
- 11.Belouzard S., Delcroix D., Rouille Y. Low levels of expression of leptin receptor at the cell surface result from constitutive endocytosis and intracellular retention in the biosynthetic pathway. Journal of Biological Chemistry. 2004;279:28499–28508. doi: 10.1074/jbc.M400508200. [DOI] [PubMed] [Google Scholar]
- 12.Diano S., Kalra S.P., Horvath T.L. Leptin receptor immunoreactivity is associated with the Golgi apparatus of hypothalamic neurons and glial cells. Journal of Neuroendocrinology. 1998;10:647–650. doi: 10.1046/j.1365-2826.1998.00261.x. [DOI] [PubMed] [Google Scholar]
- 13.Bates S.H., Stearns W.H., Dundon T.A., Schubert M., Tso A.W., Wang Y. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 14.Buettner C., Pocai A., Muse E.D., Etgen A.M., Myers M.G., Jr., Rossetti L. Critical role of STAT3 in leptin's metabolic actions. Cell Metabolism. 2006;4:49–60. doi: 10.1016/j.cmet.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bates S.H., Kulkarni R.N., Seifert M., Myers M.G., Jr. Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell Metabolism. 2005;1:169–178. doi: 10.1016/j.cmet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Coppari R., Ichinose M., Lee C.E., Pullen A.E., Kenny C.D., McGovern R.A. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metabolism. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Morton G.J., Gelling R.W., Niswender K.D., Morrison C.D., Rhodes C.J., Schwartz M.W. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metabolism. 2005;2:411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Berthou F., Rouch C., Gertler A., Gerozissis K., Taouis M. Chronic central leptin infusion differently modulates brain and liver insulin signaling. Molecular and Cellular Endocrinology. 2011;337:89–95. doi: 10.1016/j.mce.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Koch C., Augustine R.A., Steger J., Ganjam G.K., Benzler J., Pracht C. Leptin rapidly improves glucose homeostasis in obese mice by increasing hypothalamic insulin sensitivity. Journal of Neuroscience. 2010;30:16180–16187. doi: 10.1523/JNEUROSCI.3202-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailleul B., Akerblom I., Strosberg A.D. The leptin receptor promoter controls expression of a second distinct protein. Nucleic Acids Research. 1997;25:2752–2758. doi: 10.1093/nar/25.14.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seron K., Couturier C., Belouzard S., Bacart J., Monte D., Corset L. Endospanins regulate a postinternalization step of the leptin receptor endocytic pathway. Journal of Biological Chemistry. 2011;286:17968–17981. doi: 10.1074/jbc.M111.224857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vauthier V., Jaillard S., Journel H., Dubourg C., Jockers R., Dam J. Homozygous deletion of an 80 kb region comprising part of DNAJC6 and LEPR genes on chromosome 1P31.3 is associated with early onset obesity, mental retardation and epilepsy. Molecular Genetics and Metabolism. 2012;106:345–350. doi: 10.1016/j.ymgme.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 23.Neeland I.J., Turer A.T., Ayers C.R., Powell-Wiley T.M., Vega G.L., Farzaneh-Far R. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308:1150–1159. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribaric G., Buchwald J.N., McGlennon T.W. Diabetes and weight in comparative studies of bariatric surgery vs conventional medical therapy: a systematic review and meta-analysis. Obesity Surgery. 2014;24:437–455. doi: 10.1007/s11695-013-1160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee Y., Hirose H., Ohneda M., Johnson J.H., McGarry J.D., Unger R.H. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Debuyser A., Drews G., Henquin J.C. Adrenaline inhibition of insulin release: role of cyclic AMP. Molecular and Cellular Endocrinology. 1991;78:179–186. doi: 10.1016/0303-7207(91)90121-8. [DOI] [PubMed] [Google Scholar]
- 27.Peterhoff M., Sieg A., Brede M., Chao C.M., Hein L., Ullrich S. Inhibition of insulin secretion via distinct signaling pathways in alpha2-adrenoceptor knockout mice. European Journal of Endocrinology. 2003;149:343–350. doi: 10.1530/eje.0.1490343. [DOI] [PubMed] [Google Scholar]
- 28.Rojas J.M., Matsen M.E., Mundinger T.O., Morton G.J., Stefanovski D., Bergman R.N. Glucose intolerance induced by blockade of central FGF receptors is linked to an acute stress response. Molecular Metabolism. 2015;4:561–568. doi: 10.1016/j.molmet.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurose T., Seino Y., Nishi S., Tsuji K., Taminato T., Tsuda K. Mechanism of sympathetic neural regulation of insulin, somatostatin, and glucagon secretion. American Journal of Physiology. 1990;258:E220–E227. doi: 10.1152/ajpendo.1990.258.1.E220. [DOI] [PubMed] [Google Scholar]
- 30.Klockener T., Hess S., Belgardt B.F., Paeger L., Verhagen L.A., Husch A. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nature Neuroscience. 2011;14:911–918. doi: 10.1038/nn.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C.X., Zhao A.H. Leptin receptor overlapping transcript (LepROT) gene participates in insulin pathway through FoxO. Gene. 2016;587:64–69. doi: 10.1016/j.gene.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 32.Park Y.J., Kim S.C., Kim J., Anakk S., Lee J.M., Tseng H.T. Dissociation of diabetes and obesity in mice lacking orphan nuclear receptor small heterodimer partner. Journal of Lipid Research. 2011;52:2234–2244. doi: 10.1194/jlr.M016048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalmas E., Toubal A., Alzaid F., Blazek K., Eames H.L., Lebozec K. Irf5 deficiency in macrophages promotes beneficial adipose tissue expansion and insulin sensitivity during obesity. Nature Medicine. 2015;21:610–618. doi: 10.1038/nm.3829. [DOI] [PubMed] [Google Scholar]
- 34.Hill J.W., Elias C.F., Fukuda M., Williams K.W., Berglund E.D., Holland W.L. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metabolism. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wauman J., De Smet A.S., Catteeuw D., Belsham D., Tavernier J. Insulin receptor substrate 4 couples the leptin receptor to multiple signaling pathways. Molecular Endocrinology. 2008;22:965–977. doi: 10.1210/me.2007-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu J., Zhang Y., McIlroy J., Rordorf-Nikolic T., Orr G.A., Backer J.M. Regulation of the p85/p110 phosphatidylinositol 3'-kinase: stabilization and inhibition of the p110alpha catalytic subunit by the p85 regulatory subunit. Molecular and Cellular Biology. 1998;18:1379–1387. doi: 10.1128/mcb.18.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su X., Lodhi I.J., Saltiel A.R., Stahl P.D. Insulin-stimulated Interaction between insulin receptor substrate 1 and p85alpha and activation of protein kinase B/Akt require Rab5. Journal of Biological Chemistry. 2006;281:27982–27990. doi: 10.1074/jbc.M602873200. [DOI] [PubMed] [Google Scholar]
- 38.Roman E.A., Reis D., Romanatto T., Maimoni D., Ferreira E.A., Santos G.A. Central leptin action improves skeletal muscle AKT, AMPK, and PGC1 alpha activation by hypothalamic PI3K-dependent mechanism. Molecular and Cellular Endocrinology. 2010;314:62–69. doi: 10.1016/j.mce.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Ahren B., Veith R.C., Taborsky G.J., Jr. Sympathetic nerve stimulation versus pancreatic norepinephrine infusion in the dog: 1). Effects on basal release of insulin and glucagon. Endocrinology. 1987;121:323–331. doi: 10.1210/endo-121-1-323. [DOI] [PubMed] [Google Scholar]
- 40.Gromada J., Ma X., Hoy M., Bokvist K., Salehi A., Berggren P.O. ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1−/− mouse alpha-cells. Diabetes. 2004;53(Suppl. 3):S181–S189. doi: 10.2337/diabetes.53.suppl_3.s181. [DOI] [PubMed] [Google Scholar]
- 41.Sorenson R.L., Elde R.P., Seybold V. Effect of norepinephrine on insulin, glucagon, and somatostatin secretion in isolated perifused rat islets. Diabetes. 1979;28:899–904. doi: 10.2337/diab.28.10.899. [DOI] [PubMed] [Google Scholar]
- 42.Bansal P., Wang Q. Insulin as a physiological modulator of glucagon secretion. American Journal of Physiology, Endocrinology and Metabolism. 2008;295:E751–E761. doi: 10.1152/ajpendo.90295.2008. [DOI] [PubMed] [Google Scholar]
- 43.Bagnasco M., Dube M.G., Katz A., Kalra P.S., Kalra S.P. Leptin expression in hypothalamic PVN reverses dietary obesity and hyperinsulinemia but stimulates ghrelin. Obesity Research. 2003;11:1463–1470. doi: 10.1038/oby.2003.196. [DOI] [PubMed] [Google Scholar]
- 44.Boghossian S., Dube M.G., Torto R., Kalra P.S., Kalra S.P. Hypothalamic clamp on insulin release by leptin-transgene expression. Peptides. 2006;27:3245–3254. doi: 10.1016/j.peptides.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 45.Hinoi E., Gao N., Jung D.Y., Yadav V., Yoshizawa T., Myers M.G., Jr. The sympathetic tone mediates leptin's inhibition of insulin secretion by modulating osteocalcin bioactivity. Journal of Cell Biology. 2008;183:1235–1242. doi: 10.1083/jcb.200809113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muzumdar R., Ma X., Yang X., Atzmon G., Bernstein J., Karkanias G. Physiologic effect of leptin on insulin secretion is mediated mainly through central mechanisms. FASEB Journal. 2003;17:1130–1132. doi: 10.1096/fj.02-0991fje. [DOI] [PubMed] [Google Scholar]
- 47.Haque M.S., Minokoshi Y., Hamai M., Iwai M., Horiuchi M., Shimazu T. Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes. 1999;48:1706–1712. doi: 10.2337/diabetes.48.9.1706. [DOI] [PubMed] [Google Scholar]
- 48.Kamohara S., Burcelin R., Halaas J.L., Friedman J.M., Charron M.J. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–377. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- 49.Minokoshi Y., Kim Y.B., Peroni O.D., Fryer L.G., Muller C., Carling D. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 50.Toda C., Shiuchi T., Lee S., Yamato-Esaki M., Fujino Y., Suzuki A. Distinct effects of leptin and a melanocortin receptor agonist injected into medial hypothalamic nuclei on glucose uptake in peripheral tissues. Diabetes. 2009;58:2757–2765. doi: 10.2337/db09-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Konner A.C., Bruning J.C. Selective insulin and leptin resistance in metabolic disorders. Cell Metabolism. 2012;16:144–152. doi: 10.1016/j.cmet.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 52.Mesaros A., Koralov S.B., Rother E., Wunderlich F.T., Ernst M.B., Barsh G.S. Activation of Stat3 signaling in AgRP neurons promotes locomotor activity. Cell Metabolism. 2008;7:236–248. doi: 10.1016/j.cmet.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Morrison C.D., Morton G.J., Niswender K.D., Gelling R.W., Schwartz M.W. Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. American Journal of Physiology – Endocrinology and Metabolism. 2005;289:E1051–E1057. doi: 10.1152/ajpendo.00094.2005. [DOI] [PubMed] [Google Scholar]
- 54.Gong L., Yao F., Hockman K., Heng H.H., Morton G.J., Takeda K. Signal transducer and activator of transcription-3 is required in hypothalamic agouti-related protein/neuropeptide Y neurons for normal energy homeostasis. Endocrinology. 2008;149:3346–3354. doi: 10.1210/en.2007-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Qassab H., Smith M.A., Irvine E.E., Guillermet-Guibert J., Claret M., Choudhury A.I. Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons. Cell Metabolism. 2009;10:343–354. doi: 10.1016/j.cmet.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y., Kilroy G.E., Henagan T.M., Prpic-Uhing V., Richards W.G., Bannon A.W. Targeted deletion of melanocortin receptor subtypes 3 and 4, but not CART, alters nutrient partitioning and compromises behavioral and metabolic responses to leptin. FASEB Journal. 2005;19:1482–1491. doi: 10.1096/fj.05-3851com. [DOI] [PubMed] [Google Scholar]
- 57.Huo L., Gamber K., Greeley S., Silva J., Huntoon N., Leng X.H. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metabolism. 2009;9:537–547. doi: 10.1016/j.cmet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berglund E.D., Vianna C.R., Donato J., Jr., Kim M.H., Chuang J.C., Lee C.E. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. Journal of Clinical Investigation. 2012;122:1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Migrenne S., Lacombe A., Lefevre A.L., Pruniaux M.P., Guillot E., Galzin A.M. Adiponectin is required to mediate rimonabant-induced improvement of insulin sensitivity but not body weight loss in diet-induced obese mice. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology. 2009;296:R929–R935. doi: 10.1152/ajpregu.90824.2008. [DOI] [PubMed] [Google Scholar]
- 60.Formstecher E., Aresta S., Collura V., Hamburger A., Meil A., Trehin A. Protein interaction mapping: a Drosophila case study. Genome Research. 2005;15:376–384. doi: 10.1101/gr.2659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.