Abstract
The status of lymph node involvement is an important prognostic factor for breast cancer. However, the presence of intratumoral lymphatic vessels in primary tumor lesions and the relationship between lymphatic vessel density (LVD) and lymph node metastasis (LNM) have not been firmly established. Therefore, we performed a meta-analysis study to investigate these issues. According to the pre-established inclusion and exclusion criteria, 13 studies, involving 1029 breast cancer patients, were included in this study. Using immunohistochemical staining, intratumoral lymphatic vessels were detected in 40.07% of breast cancer patients (240/599), and peritumoral lymphatics were detected in 77.09% (397/515). All studies demonstrated that peritumoral LVD was higher than intratumoral LVD, with a pooled standard mean difference and 95% confidence interval (95% CI) of 1.75 (1.28 to 2.21). Both intratumoral LVD and peritumoral LVD positively correlated with LNM, with correlation coefficients of 0.14 (95% CI 0.05 to 0.23) and 0.31 (95% CI 0.13 to 0.49), respectively. In summary, our study reports the overall detection rate of intratumoral lymphatics and demonstrates the associations between intratumoral LVD, peritumoral LVD, and LNM in breast cancer. Additionally, controlled studies with a larger number of subjects are needed to establish these relationships.
Breast cancer is the most common malignant tumor in females. Although great efforts have been made in the field of early diagnosis and adjuvant therapy, the incidence and overall mortality of breast cancer continues to increase1. Since breast tumor cells commonly infiltrate into the lymphatic system, lymph node status is routinely used to identify a patient’s prognosis, tumor stage, and treatment modality2,3. Inhibition of lymph node metastasis (LNM) is a promising way to prevent distant metastasis, which has been proved by many studies4,5. However, the relationship between lymphangiogenesis and LNM remains ambiguous.
Due to the lack of specific markers, the detection of lymphatic vessels has been hampered in previous studies. Intratumoral lymphatic vessels were considered to be rare and nonfunctional due to mechanical compression6. With the identification of specific markers, such as podoplanin/D2–40, vascular endothelial growth factor receptor-3 (VEGFR-3), lymphatic vessel endothelial hyaluronan receptor -1 (LYVE-1) and Prox-1, many experimental and clinicopathological studies have demonstrated the existence of intratumoral lymphatics. The intratumoral lymphatics are considered to be undergoing dynamic changes that can facilitate tumor metastasis7. The entry of tumor cells into lymphatic vessels is promoted by lymphangiogenesis and lymphatic enlargement8,9. Therefore, lymphatic vessel density (LVD), a representation of lymphangiogenesis, can serve as an indicator of early lymphogenous spread.
Some studies have suggested that LVD is associated with an increased risk of LNM10,11; however, this conclusion is not supported by all of the published studies12,13. The evidence is limited because the published studies are observational studies and included relatively small sample sizes, which could have led to confounding factors and selection bias. Moreover, the different LVD counting methods and the varied dilutions of antibodies could have affected the conclusions. With the accumulating evidence, we conducted a meta-analysis study to investigate the overall detection rate of intratumoral lymphatics and to estimate the relationships between intratumoral LVD, peritumoral LVD and LNM in breast cancer.
Results
Study selection process
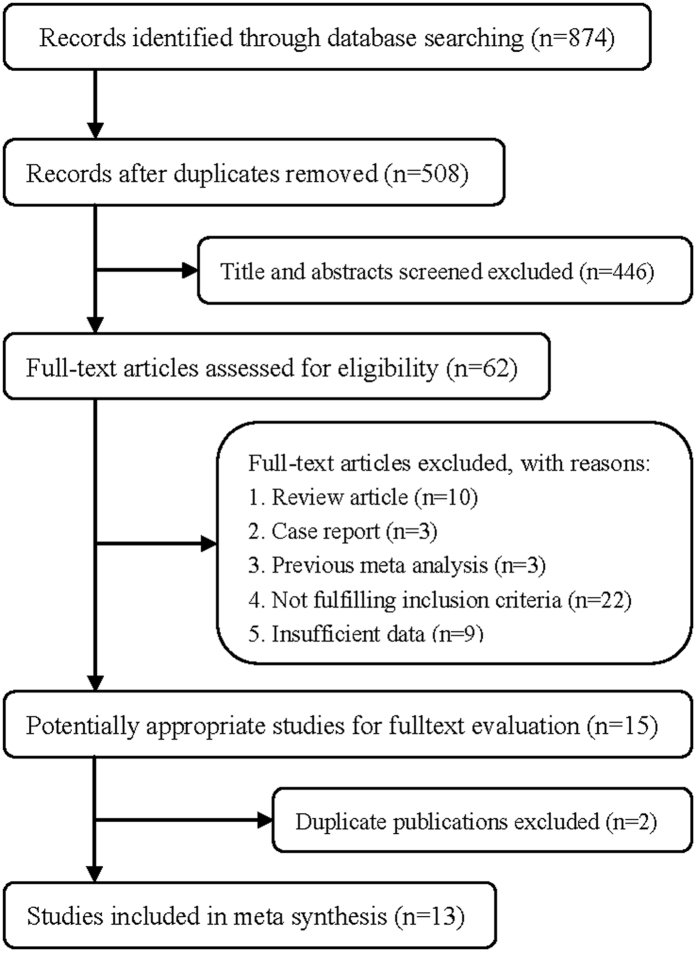
The flow chart of the article selection process is presented in Fig. 1. PubMed and Embase databases were searched to identify the relevant studies. We initially obtained 874 potential papers from the two databases, including 420 from PubMed and 454 from Embase. After screening the titles and abstracts, most of them were excluded, either because of duplicate publications, if they were letters or reviews, or did not distinguish between intratumoral LVD and peritumoral LVD. Finally, 13 papers were adopted according to the inclusion criteria.
Figure 1. Process of study selection for the meta-analysis.
Characteristics of the included studies
The details of the included studies are exhibited in Table 110,11,12,13,14,15,16,17,18,19,20,21,22. The publication years ranged from 2005 to 2014. A total of 1,029 breast cancer patients, ranging from 26 to 89 years old (except two studies that did not indicate the age19,20), were included in this study. All patients underwent surgical treatments and immunohistochemical examinations with D2–40/podoplanin antibodies. Intratumoral LVD and peritumoral LVD were determined by counting the number of lymphatic vessels using the high magnification field under a microscope. All studies reported sufficient sample sizes, ranging from 25 to 177 patients.
Table 1. Main characteristics and results of the included studies.
| Author, Year, Country | Size | Age | Tumor type | Antibody and dilution | Intratumoral LVD and detection rate | Peritumoral LVD and detection rate |
|---|---|---|---|---|---|---|
| Doric, 2014, Bosnia and Herzegovina14 | 75 | 59 (37–87)a | invasive BC | D2–40 (1:100) | 1.9 ± 1.7 (27/75) | 3.9 ± 1.0 (75/75) |
| Raica, 2013, Italy15 | 55 | 26–81b | ductal invasive BC | D2–40 (NG) | 1.89 ± 2.35 (NG) | 6.85 ± 3.55 (NG) |
| Ciobanu, 2013, Romania16 | 25 | 58 (45–69)c | lobular invasive BC | D2–40 (1:100) | 3.40 ± 2.55 (18/25) | 8.68 ± 5.64 (19/25) |
| Zhao, 2012, China12 | 73 | 53.8 (29–75)a | ductal invasive BC | D2–40 (1:25) | 5.47 ± 2.03 (NG) | 8.77 ± 3.30 (NG) |
| Kandemir, 2012, Turkey17 | 69 | 54.8 (39–85)a | ductal invasive BC | D2–40 (1:50) | 16.3 ± 9.7 (18/69) | 66.3 ± 20.5 (25/69) |
| Ding, 2012, China18 | 75 | 52.1 (42–63)a | ductal invasive BC and Paget disease | D2–40 (NG) | 2.06 ± 2.93 (NG) | 12.99 ± 7.97 (NG) |
| Mohammed, 2009, UK11 | 177 | 57 (32–70)c | invasive BC | D2–40 (1:100) | 0.26 ± 0.51 (73/177) | 1.02 ± 0.76 (177/177) |
| Liu, 2009, China19 | 91 | NG | invasive BC | D2–40 (1:100) | 5.12 ± 2.69 (NG) | 8.22 ± 3.21 (NG) |
| EI-Gohary, 2008, USA10 | 48 | 64 (27–89)a | invasive BC | D2–40 (1:50) | 3.7 ± 6.1 (24/48) | 8.8 ± 6.8 (46/48) |
| Van der Schaft, 2007, Netherlands13 | 121 | 61.4 ± 12.2d | ductal invasive BC | Podoplanin (NG) | 0.35 ± 1.29 (12/121) | 4.68 ± 3.98 (55/121) |
| Li, 2006, Japan20 | 80 | NG | ductal invasive BC | D2–40 (1:100) | 1.93 ± 0.43 (NG) | 5.41 ± 0.85 (NG) |
| Agarwal, 2005, USA22 | 55 | 53 (35–72)a | invasive BC | D2–40 (1:40) | 0.3 ± 0.5 (NG) | 2.31 ± 0.97 (NG) |
| Van der Auwera, 2005, Belgium21 | 85 | 25.6–83.2b | inflammatory and non-inflammatory BC | D2–40 (1:20) | 5,24 ± 4.90 (68/84) | 8.25 ± 5.72 (NG) |
Note: a: mean (range); b: range; c: median (range); d: mean ± SD; BC: breast cancer; LVD: lymphatic vessel density; NG: not given.
Data analysis
Among the 13 studies included, seven10,11,13,14,16,17,21 reported the detection rate of intratumoral lymphatics, with an overall rate of 40.07% (240/599). Six of the included studies10,11,13,14,16,17 reported the detection rate of peritumoral lymphatics, with an overall rate of 77.09% (397/515). All 13 studies were used to assess the differences between intratumoral LVD and peritumoral LVD. The values of intratumoral and peritumoral LVD and the pooled SMD value with 95% CI are presented in Fig. 2. Despite significant heterogeneity (P < 0.05, I2 = 95%), all studies indicated that peritumoral LVD values were higher than intratumoral LVD, with a pooled SMD of 1.75 (95% CI 1.28 to 2.21). The random-effects model was used to combine the SMD values because of significant heterogeneity.
Figure 2. Forest plot of the standard mean differences between peritumoral LVD and intratumoral LVD in breast cancer.
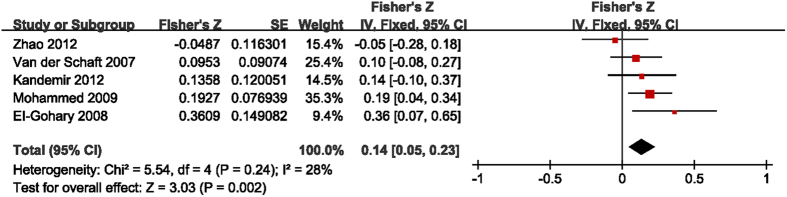
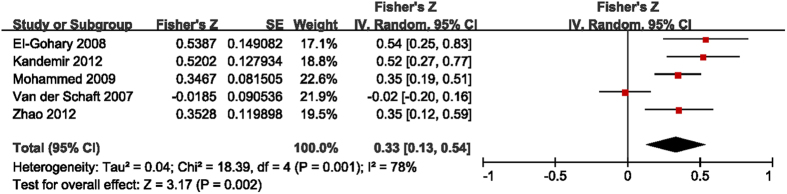
The studies that include detailed data of intratumoral and peritumoral LVD and the presence of LNM were selected to investigate the interrelationships between them10,11,12,13,17. The results presented with means and standard deviations, or two by two frequency tables, were transformed to obtain the r values. The Fisher’s Z transformation was used to convert r values to Z values. The main outcomes are summarized in Figs 3 and 4. The pooled Fisher’s Z values for the relationships between intratumoral LVD and LNM and between peritumoral LVD and LNM were 0.14 (95% CI 0.05 to 0.23, I2 = 28%, P = 0.002, Fig. 3) and 0.33 (95% CI 0.13 to 0.54, I2 = 78%, P = 0.002, Fig. 4), respectively. Finally, the pooled Fisher’s Z values were converted back to r values by inverse Fisher’s Z transformation. Both intratumoral LVD (r = 0.14, 95% CI 0.05 to 0.23) and peritumoral LVD (r = 0.31, 95% CI 0.13 to 0.49) were positively correlated with LNM in breast tumors.
Figure 3. Forest plot of the Fisher’s Z values for the correlation between intratumoral LVD and LNM in breast cancer.
Figure 4. Forest plot of the Fisher’s Z values for the correlation between peritumoral LVD and LNM in breast cancer.
Sensitivity analysis and publication bias
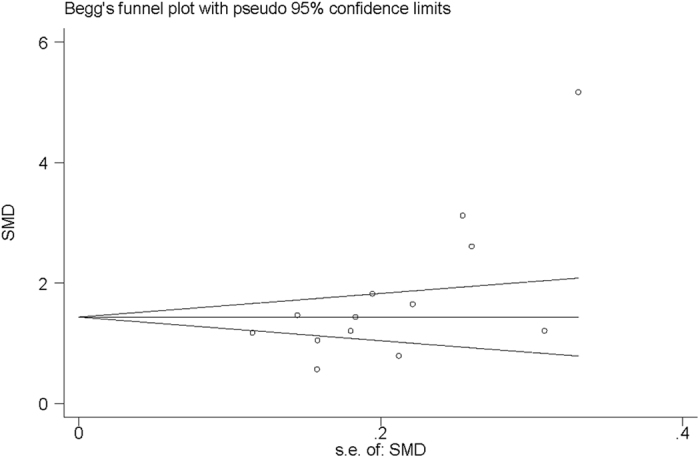
To evaluate the stability of the results, a sensitivity analysis was performed using the random-effects model. Sensitivity analysis, by repeatedly analyzing the data after removing individual studies in turn, demonstrated that no studies were responsible for the disproportionate influence on the pooled estimate (Fig. 5). Begg’s funnel plot of the SMD against the standard error of SMD showed substantial asymmetry (Fig. 6). Egger’s regression test showed evidence of publication bias (P = 0.017).
Figure 5. Plot of the included studies for sensitivity analysis.
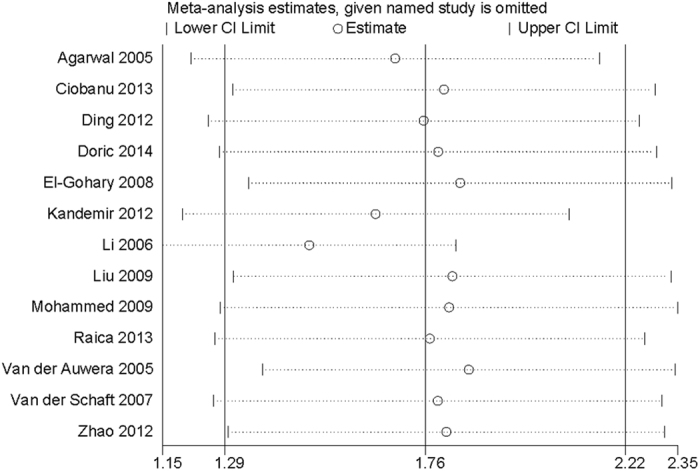
Figure 6. Begg’s funnel plot of the included studies for publication bias.
Discussion
The current meta-analysis included 13 observational studies with an overall population of 1029 breast cancer patients. By immunohistochemical staining with D2–40/podoplanin antibodies, 40.07% of the specimens exhibited intratumoral lymphatics, and 77.09% of the specimens showed peritumoral lymphatics. Peritumoral LVD is significantly higher than intratumoral LVD in breast cancer (P < 0.05). The positive correlation between peritumoral LVD and LNM was moderately stronger than that of intratumoral LVD and LNM. However, there was substantial evidence of heterogeneity among these studies. By conducting a sensitivity analysis, two studies were identified to be the main source of heterogeneity17,20.
Although the existence of peritumoral lymphatics is well recognized, the presence of intratumoral lymphatics is a hotly debated issue in solid tumors, particularly in breast cancer6,23,24. Initial studies reported that breast cancers did not have intratumoral lymphatics25, owing to the increased interstitial pressure created by the proliferating cancer cells26. Williams et al.6 and Vleugel et al.24 failed to detect lymphangiogenesis in breast cancer by using LYVE-1 as a marker of lymphatic vessels. Using the new specific markers of lymphatic vessels, such as D2–40 and podoplanin, recent studies demonstrated that intratumoral lymphatics are detectable11,27. Intratumoral lymphatics were generally detected in 40.07% of breast cancer specimens, and the detection rate of peritumoral lymphatics was 77.09%. Moreover, intratumoral lymphatics are believed to be functional, as tumor cells have been observed to flow within the vessels28.
In addition, our study shows that peritumoral LVD is significantly higher than intratumoral LVD, with a pooled SMD of 1.75 (95% CI 1.28 to 2.21). The result is supported by all included studies. Peritumoral LVD is also reported to be higher than that of normal and benign breast lesions10,12,22. However, the comparison between intratumoral LVD and normal LVD cannot draw a consistent conclusion. Agarwal et al.22 and Van der Auwera et al.21 claimed that intratumoral LVD of breast cancer was lower than that of normal or benign breast lesions. In contrast, other studies indicated there were no differences between them10,12. These contradicting results might be due to the different locations of tumor lymphatic vessels used to define the term of “intratumoral lymphatic vessels”. Van der Auwera et al.21 regarded intratumoral lymphatics as any vessels within the tumor area, either in the inner core or periphery. Another study considered intratumoral lymphatics as vessels present only among tumor cells13. However, in Mohammed et al.’s study11, intratumoral lymphatic vessels referred to the vessels within the inner 2/3 core of the tumor lesion. Due to the lack of studies, the comparisons between intratumoral LVD, peritumoral LVD and the LVD of normal or benign breast lesions were not conducted in this meta-analysis study. In addition, the inconsistent definitions of “intratumoral lymphatic vessels” could also impact the comparison between intratumoral LVD and peritumoral LVD. A larger number of strict standardized studies is needed.
It is well known that blood vessel density, an indicator of tumor angiogenesis, is closely associated with the clinicopathological outcomes of breast cancer29. The methods used for assessing angiogenesis are usually used to measure the lymphangiogenesis of breast cancer as well21,30. Many studies have demonstrated the associations between peritumoral LVD and tumor grade, tumor stage, lymphatic invasion, LNM, and overall survival in breast cancer10,31,32. However, the relationship between intratumoral LVD and clinicopathological behavior is still uncertain. Our study not only demonstrates a positive association between intratumoral LVD and LNM in breast cancer but also reveals that peritumoral LVD has a moderately stronger correlation with LNM than that of intratumoral LVD. These results suggest that peritumoral lymphatic vessels have a more important effect on metastatic dissemination in breast cancer.
Although the current meta-analysis study has some definite strengths, some limitations should be considered. All included studies were observational studies with relatively small sample sizes, and several studies6,23 were excluded due to lack of data on intratumoral LVD or peritumoral LVD. Thus, recall bias and selection bias are inevitable. In addition, the unmeasured or inadequately measured factors, such as patient sources, histological types, antibody categories and antibody dilutions, could confound the results. Moreover, different counting methods for lymphatic vessels, such as the number of different hotspots (ten17, three21, and five31), magnification (100×11, 200×31, 400×17), and measuring unit (vessels/mm2 11, vessels/area21), were used in different studies. The values of intratumoral LVD and peritumoral LVD varied notably, resulting in the significant heterogeneity. Therefore, studies with a larger sample size and more standardized methods are required to assess intratumoral LVD and peritumoral LVD.
In conclusion, the study demonstrates the existence of intratumoral lymphatic vessels. Although the overall detection rate of intratumoral lymphatic vessels is lower than that of peritumoral lymphatic vessels, it does not change the fact that they are present and constitute a risk factor for tumor metastasis. Both intratumoral LVD and peritumoral LVD are correlated with the increasing risk of LNM, and peritumoral LVD exhibits a moderately stronger correlation with the increasing risk of LNM than that of intratumoral LVD. It might provide a potential target to prevent lymphangiogenesis and lymphatic metastasis in breast cancer.
Methods
Search strategy
Two independent observers searched the databases of PubMed and Embase. The databases were searched using the following Medical Subject Heading (MeSH) terms or keywords: “breast cancer OR breast carcinoma OR breast neoplasms” AND “lymphatic vessel density OR lymphatic microvessel density OR LVD OR LMVD OR lymphangiogenesis” with no restrictions. All abstracts that indicated LVD assessment in breast cancer, no matter prospective or retrospective, were chosen for further consideration. The reference lists of all selected papers and abstracts were also screened. If it was necessary, we contacted the authors of the original studies for the required data. The search was ended on April 8, 2016.
Inclusion and exclusion criteria
All studies that met the following criteria were included: (1) patients with breast cancer at any age; (2) a sample size larger than 10 patients; (3) no neoadjuvant chemotherapy or radiotherapy administered before the surgical treatment; and (4) specimens stained with the immunohistochemical method. Studies were excluded if they included the following: (1) review articles, case reports, meeting abstracts, or animal studies; (2) an examination of the total LVD of breast tumor lesions without distinguishing intratumoral LVD and peritumoral LVD; or (3) patients previously diagnosed with other diseases that could lead to LNM. Two independent authors followed the above inclusion and exclusion criteria to review the studies. When two or more articles reported duplicated data, we included the study with the most-recent data, the largest dataset, or the most relevant data. In cases of disputes, a third reviewer assessed the study to obtain a consensus.
Data extraction and quality assessment
Two authors independently checked each item mentioned by the publications and discussed the data that was extracted. The information extracted from each study was summarized in a table and included the following items: first author’s name, publication year, country, number of patients (size), age, type of breast cancer involved, antibody and dilution, intratumoral LVD and detection rate, peritumoral LVD and detection rate. Two authors conducted the quality assessment based on the criteria of the Newcastle-Ottawa Quality Assessment scale (NOS)33, which evaluates the methodology in observational studies.
Statistical analysis
Spearman correlation coefficients (r) were used for the meta-analysis because some variables in the original studies were log-transformed before analysis34. The Fisher’s Z transformation was used to convert r values to Z values into a normal distribution34. The standard mean differences (SMDs) and Z values with 95% CIs were combined by RevMan5.3 software. Homogeneity tests were performed with the Q statistic and I2 statistic. A random-effects model or, in the absence of heterogeneity, a fixed-effects model was used to combine the SMDs and Z values with 95% CIs. The pooled r values and 95% CIs were obtained from the inverse Fisher’s Z transformation. Additionally, we conducted a sensitivity analysis by STATA 12.0 software to investigate the influence of a single study on the overall result by omitting each study in turn. Publication bias was detected by Begg’s and Egger’s test. In this study, P < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Zhang, S. et al. Intratumoral and peritumoral lymphatic vessel density both correlate with lymph node metastasis in breast cancer. Sci. Rep. 7, 40364; doi: 10.1038/srep40364 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC) (Nos 81401466 and 81501521).
Footnotes
Author Contributions Z.S., Y.S.H. and Z.L.G. wrote the main manuscript text; Z.D. and G.M.F. prepared all of the figures and tables. C.Y.Q. corrected the draft of the paper and prepared the final version of the manuscript. All authors reviewed the manuscript.
References
- Arranz E. E., Vara J. Á. F., Gámez-Pozo A. & Zamora P. Gene signatures in breast cancer: current and future uses. Translational oncology. 5, 398–403 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad R. S. et al. Lymphatic microvessel density as prognostic marker in colorectal cancer. Modern pathology. 19, 1317–1323 (2006). [DOI] [PubMed] [Google Scholar]
- El-Gendi S. & Abdel-Hadi M. Lymphatic vessel density as prognostic factor in breast carcinoma: relation to clinicopathologic parameters. Journal of the Egyptian National Cancer Institute. 21, 139–149 (2009). [PubMed] [Google Scholar]
- Shayan R., Achen M. G. & Stacker S. A. Lymphatic vessels in cancer metastasis: bridging the gaps. Carcinogenesis. 27, 1729–1738 (2006). [DOI] [PubMed] [Google Scholar]
- Achen M. G. & Stacker S. A. Molecular control of lymphatic metastasis. Annals of the New York Academy of Sciences. 1131, 225–234 (2008). [DOI] [PubMed] [Google Scholar]
- Williams C. S. et al. Absence of lymphangiogenesis and intratumoural lymph vessels in human metastatic breast cancer. The Journal of pathology. 200, 195–206 (2003). [DOI] [PubMed] [Google Scholar]
- Stacker S. A. et al. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nature Reviews Cancer. 14, 159–172 (2014). [DOI] [PubMed] [Google Scholar]
- Stacker S. A. et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nature medicine. 7, 186–191 (2001). [DOI] [PubMed] [Google Scholar]
- Skobe M. et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nature medicine. 7, 192–198 (2001). [DOI] [PubMed] [Google Scholar]
- El-Gohary Y. M. et al. Prognostic significance of intratumoral and peritumoral lymphatic density and blood vessel density in invasive breast carcinomas. American journal of clinical pathology. 129, 578–586 (2008). [DOI] [PubMed] [Google Scholar]
- Mohammed R. A., Ellis I. O., Elsheikh S., Paish E. C. & Martin S. G. Lymphatic and angiogenic characteristics in breast cancer: morphometric analysis and prognostic implications. Breast Cancer Res Treat. 113, 261–273 (2009). [DOI] [PubMed] [Google Scholar]
- Zhao Y. C. et al. Peritumoral lymphangiogenesis induced by vascular endothelial growth factor C and D promotes lymph node metastasis in breast cancer patients. World journal of surgical oncology. 10, 165 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schaft D. W. et al. Absence of lymphangiogenesis in ductal breast cancer at the primary tumor site. Cancer letters. 254, 128–136 (2007). [DOI] [PubMed] [Google Scholar]
- Dorić M. et al. Intratumoural lymphatic vascular density (LVD) correlate with progesterone receptor expression in breast carcinoma. Folia Medica Facultatis Medicinae Universitatis Saraeviensis. 49 (2014). [Google Scholar]
- Raica M., Cimpean A. M., Ceausu R., Ribatti D. & Gaje P. Interplay between mast cells and lymphatic vessels in different molecular types of breast cancer. Anticancer research. 33, 957–963 (2013). [PubMed] [Google Scholar]
- Ciobanu M. et al. Lymphatic microvessels density, VEGF-C, and VEGFR-3 expression in 25 cases of breast invasive lobular carcinoma. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie. 54, 925–934 (2013). [PubMed] [Google Scholar]
- Kandemir N. O., Barut F., Bektas S. & Ozdamar S. O. Can lymphatic vascular density be used in determining metastatic spreading potential of tumor in invasive ductal carcinomas? Pathology oncology research: POR. 18, 253–262 (2012). [DOI] [PubMed] [Google Scholar]
- Ding M. et al. The effect of vascular endothelial growth factor C expression in tumor-associated macrophages on lymphangiogenesis and lymphatic metastasis in breast cancer. Molecular medicine reports. 6, 1023–1029 (2012). [DOI] [PubMed] [Google Scholar]
- Liu H. T., Ma R., Yang Q. F., Du G. & Zhang C. J. Lymphangiogenic characteristics of triple negativity in node-negative breast cancer. International journal of surgical pathology. 17, 426–431 (2009). [DOI] [PubMed] [Google Scholar]
- Li Y. S. et al. Expression of vascular endothelial growth factor-C and its receptor in invasive micropapillary carcinoma of the breast. Pathology international. 56, 256–261 (2006). [DOI] [PubMed] [Google Scholar]
- Van der Auwera I. et al. Tumor lymphangiogenesis in inflammatory breast carcinoma: a histomorphometric study. Clinical cancer research: an official journal of the American Association for Cancer Research. 11, 7637–7642 (2005). [DOI] [PubMed] [Google Scholar]
- Agarwal B., Saxena R., Morimiya A., Mehrotra S. & Badve S. Lymphangiogenesis does not occur in breast cancer. The American journal of surgical pathology. 29, 1449–1455 (2005). [DOI] [PubMed] [Google Scholar]
- Bono P. et al. High LYVE-1-positive lymphatic vessel numbers are associated with poor outcome in breast cancer. Clinical Cancer Research. 10, 7144–7149 (2004). [DOI] [PubMed] [Google Scholar]
- Vleugel M. M. et al. Lack of lymphangiogenesis during breast carcinogenesis. J Clin Pathol. 57, 746–751 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padera T. P. et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science (New York, N.Y.). 296, 1883–1886 (2002). [DOI] [PubMed] [Google Scholar]
- Clarijs R., Ruiter D. J. & de Waal R. M. Lymphangiogenesis in malignant tumours: does it occur? The Journal of pathology. 193, 143–146 (2001). [DOI] [PubMed] [Google Scholar]
- Tezuka K. et al. Clinical significance of intra-tumoral sinusoidal structures showing lympho-endothelial immunoreactivity in breast cancer. Oncology Reports. 20, 25–32 (2008). [PubMed] [Google Scholar]
- Pathak A. P., Bhujwalla Z. M. & Pepper M. S. Visualizing function in the tumor-associated lymphatic system. Lymphatic research and biology. 2, 165–172 (2004). [DOI] [PubMed] [Google Scholar]
- Kato T., Kameoka S., Kimura T., Nishikawa T. & Kobayashi M. The combination of angiogenesis and blood vessel invasion as a prognostic indicator in primary breast cancer. British journal of cancer. 88, 1900–1908 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera I. et al. First international consensus on the methodology of lymphangiogenesis quantification in solid human tumours. British journal of cancer. 95, 1611–1625 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylona E. et al. Lymphatic and blood vessel morphometry in invasive breast carcinomas: Relation with proliferation and VEGF-C and -D proteins expression. Histology and Histopathology. 22, 825–835 (2007). [DOI] [PubMed] [Google Scholar]
- Choi W. W. et al. Angiogenic and lymphangiogenic microvessel density in breast carcinoma: correlation with clinicopathologic parameters and VEGF-family gene expression. Modern pathology. 18, 143–152 (2005). [DOI] [PubMed] [Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 25, 603–605 (2010). [DOI] [PubMed] [Google Scholar]
- Chen L. et al. The correlation between apparent diffusion coefficient and tumor cellularity in patients: a meta-analysis. PloS one. 8, e79008 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]