Abstract
Melanoma is a cancer with a rising incidence, and metastatic disease is almost always lethal. We investigated the feasibility of targeting melanin, an intracellular melanocyte pigment, to deliver cytotoxic radiation to human melanoma cells in vivo by using a melanin-binding mAb (6D2). Nude mice bearing MNT1 pigmented human melanoma tumors were treated with mAb 6D2 labeled with 1.5 mCi (1 Ci = 37 GBq) of the β-emitter 188-Rhenium (188Re) and manifested inhibition of tumor growth and prolonged survival. mAb 6D2 bound tumor melanin and demonstrated no crossreactivity with normal melanized tissues in black mice. The mechanism of melanin targeting involved Ab binding to extracellular melanin released during tumor cell turnover or to dying cells with permeable membranes. In this approach, the cytotoxic radiation emanating from labeled Ab bound to melanin is presumably delivered by “crossfire” effect to the adjacent viable tumor cells. Our results establish the feasibility of targeting melanin released from dead melanoma cells in tumors with radiolabeled Abs to achieve a therapeutic effect. In contrast to conventional tumor antigens, melanin is insoluble, resistant to degradation, and can be expected to accumulate in targeted tissues, suggesting that the efficacy of therapy could increase with each subsequent treatment cycle.
Melanoma is one of the few cancers with a rising incidence (1). Malignant melanoma affects ≈40,000 individuals each year in the United States and an estimated 100,000 individuals worldwide (2). Melanoma has a strong economic impact because it disproportionately causes disease among younger individuals. Whereas primary tumors can be successfully removed surgically, there is no satisfactory treatment for metastatic melanoma (3). Immune approaches to the therapy of metastatic melanoma include treating patients with (i) Abs to tumor-associated antigens in association with nonspecific immune stimulants, or (ii) vaccination with tumor cells, tumor cell lysates, peptides, carbohydrates, gene constructs encoding proteins, or antiidiotype Abs that mimic tumor-associated antigens (4, 5). Unfortunately, the prognosis for metastatic melanoma, even with treatment, is dismal, with a median survival time of 8.5 months and a 5-year survival rate of 6% (3). There has been little change in these results over the past 25 years.
Igs labeled with radionuclides can be used for diagnosis and therapy of tumors. Radioimmunoimaging can survey the entire body for metastases in a single study and detect occult lesions. Radioimmunotherapy (RIT) takes advantage of the specificity of the antigen-Ab interaction to deliver lethal doses of radiation to target cells (6). mAbs radiolabeled with 99m-Technetium (99mTc), 111-Indium (111In) or 131-Iodine (131I) have been used extensively for imaging metastatic melanoma: 58 patient trials involving 3,638 patients were recently reviewed in ref. 7. In contrast, and to the best of our knowledge, only two case reports on the use of RIT for melanoma have been described (8), although obtained results both in patients (8) and in preclinical studies (9) were promising. RIT of melanoma may have been discouraged by the perception that melanoma is a radiation-resistant tumor (10), given the inefficacy of radiation therapy of melanoma with external beam sources. In fact, inadequate external radiation therapy can promote metastatic spread in human melanoma (11). In other cancers, RIT is effective by promoting apoptosis in irradiated tumor cells, “bystander” effect (death of adjacent, nonirradiated cells), and cell-cycle arrest (12-15). Another example of the discordance between efficacy of external beam and RIT is found in studies of the human pathogenic fungi Cryptococcus neoformans and Histoplasma capsulatum, which are extremely resistant to external gamma radiation (LD90 = 4,000 Gy), but relatively susceptible to killing by RIT with 188Re- and 213Bi-labeled mAbs (LD90 = 1-4 Gy) (16). Thus, if a suitable antigen and Ab possessing adequate delivery, accessibility, and penetrance properties can be identified, together with an isotope with an optimal half-life and emission range, it may be possible to develop RIT for metastatic melanoma. We have chosen 188Re, a high-energy β-emitter (maximal energy = 2.12 MeV) as an isotope for this “proof-of-principle” study because its has considerable range (several millimeters) in tissue and its relatively short half-life of 16.9 h matches the short plasma half-life of IgMs.
Although several Abs to melanoma cell-surface antigens have been evaluated for imaging and therapy, the majority of studies have targeted high molecular weight melanoma-associated proteoglycan antigen. Targeting melanoma with an Ab to melanin has not been used. This finding is surprising because most human melanomas have melanin, which may contribute to the resistance of these cells to antineoplastic therapy (10, 17, 18). The LD90 for melanotic melanoma cell lines is ≈12-14 Gy (17), which is at least 2 times higher than for other cancer cell lines.
Given that melanin is normally found intracellularly within melanosomes, one might expect that this pigment is not a suitable target for Ab therapy. However, because melanomas are rapidly growing tumors, we hypothesized that cell turnover and lysis would release melanin pigment that could then be targeted for delivery of cytotoxic radiation by melanin-binding radiolabeled Abs. Furthermore, this strategy is attractive because melanin in normal tissues should not be accessible to the Ab by virtue of its intracellular location. To test this hypothesis, we used a murine mAb generated against the melanin produced by the fungus C. neoformans (19). Because both fungal and human melanins have similarities in structure (20, 21), and are negatively charged (22-24), we surmised that an Ab to fungal melanin should also bind to tumor melanin and be suitable for RIT.
Materials and Methods
Abs. The melanin-binding mAb 6D2 (IgM) was produced as described in ref. 19. The IgM mAb 12A1, which binds to C. neoformans capsule (25) and mouse IgM UNLB, binding to lipopolysaccharide (clone 11E10, Southern Biotechnology Associates), were used as irrelevant isotype-matched controls.
Melanoma Cells. Human lightly pigmented melanoma cells SK-MEL-28 (American Type Culture Collection) were grown in complete growth medium (American Type Culture Collection) supplemented with 10% FBS with or without 110 μM l-tyrosine to promote melanin formation. Highly pigmented human melanoma MNT1 cells (26) were grown in MEM/20% FBS medium. The percentage of viable cells in the samples was determined to be 96 ± 1% by a standard blue dye exclusion assay.
Radioisotopes and Radiolabeling of the Abs. In some nontherapy experiments, we used the diagnostic radioisotopes 99mTc and 111In for labeling of mAbs. Diagnostic isotopes with chemistries similar to those of therapeutic isotopes are often used for imaging procedures and quality control. 99mTc was used as “a matching pair” isotope for 188Re (27, 28) and 111In was used as a matching pair for 213Bi (29). 111In and 99mTc were purchased from commercial vendors. 188Re in the form of sodium perrhenate Na188ReO4 was eluted from a 188W/188Re generator (Oak Ridge National Laboratory, Oak Ridge, TN). For labeling with 111In, the IgMs were conjugated to N-[2-amino-3-(p-isothiocyanatophenyl)propyl]-trans-cyclohexane-1,2-diamine-N,N′,N″,N‴,N″″-pentaacetic acid as in ref. 30, and the average number of chelates per mAb was determined by the yttrium-arsenazo III spectrophotometric method (31). The 75 ligand to 1 mAb molar ratio in the conjugation reaction resulted in 4.5-5 ligands per mAb molecule. The mAbs were labeled with 188Re and 99mTc “directly” through binding of reduced 188Re or 99mTc to the generated SH groups on the Ab as in ref. 32.
Immunoreactivity Determination. The reactivity of mAb 6D2 labeled with 188Re or 111In for fungal melanin was determined by immunofluorescence using melanin particles isolated from melanized C. neoformans as in ref. 33.
Binding of Radiolabeled mAbs to SK-MEL-28 and MNT1 Cells. The binding of 111In-6D2 and 111In-12A1 to SK-MEL-28 cells was evaluated by incubating 2 μg/ml mAb (specific activity, 1 mCi/mg; 1Ci = 37 GBq) with 0.2-2.0 × 106 whole cells of the human lightly pigmented melanoma cells SK-28-MEL grown with or without 110 μM l-tyrosine, which promotes melanin formation. After incubation for 1 h at 37°C, the cells were collected by centrifugation, the supernatant was removed, the cell pellet was washed with PBS, the pellet and the supernatant were counted in a γ-counter, and the percentage binding to the cells was calculated. The binding of 188Re-6D2 and 188Re-12A1 (specific activity, 1 mCi/mg) to MNT1 cells was studied as above except that both whole cells and cells lysed with water were used. The cells were lysed before addition of radiolabeled mAbs.
Immunofluorescence of Live MNT1 Cells. To elucidate the mechanism of mAb 6D2 binding to melanoma cells in vitro, binding to live MNT1 cells was analyzed by immunofluorescence as in ref. 33. Approximately 106 melanoma cells were blocked for nonspecific binding by incubation in SuperBlock (Pierce) for 1 h at 37°C. Melanin-binding mAb 6D2, tetramethylrhodamine B isothiocyanate-conjugated GAM IgM, and negative control mAb 5C11, which binds mycobacterial lipoarabinomannan (34), were used for staining. The slides were viewed with an Olympus AX70 microscope (Melville, NY) equipped with a tetramethylrhodamine B isothiocyanate filter.
Transmission Electron Microscopy (TEM) with ImmunoGold Labeling. ImmunoGold TEM was performed as in ref. 19 to confirm the binding of mAb 6D2 to melanin in MNT1 tumors from a nude tumor-bearing mouse. The samples were examined by using a 100CX transmission electron microscope (JEOL, Tokyo).
Immunohistochemistry. Tissues containing tumor from mice injected with MNT1 melanoma cells were embedded in paraffin and sectioned. Melanin-binding mAb 6D2, tetramethylrhodamine B isothiocyanate-conjugated GAM IgM, and negative control mAb IgM UNLB were used for staining as described in refs. 19 and 33.
Biodistribution of Radiolabeled mAbs in Nude Mice. All animal studies were carried out in accordance with the guidelines of the Albert Einstein College of Medicine Institute for Animal Studies. Nude female mice (The Jackson Laboratory) were injected i.v. with 25 μCi (100 μg) 188Re-6D2 or 188Re-IgM (specific activity, 0.25 mCi/mg). At 5 and 24 h after injection, animals were killed, and their major organs were removed, blotted to remove excess blood, weighed, and counted in a γ-counter. Three animals were used for each time interval.
Scintigraphic Imaging of Tumor-Bearing Mice with 99mTc-6D2. Tumors were induced by injecting 5 × 106 MNT1 human melanoma cells into the right flank of female nude mice. The imaging studies were initiated 4 weeks after implantation, when tumors reached 0.6-1.0 cm in diameter. Tumor-bearing mice were imaged with 0.4 mCi (100 μg) 99mTc-6D2 (i.v. injection; specific activity, 4 mCi/mg) to ascertain tumor localization. At different times, the animals were imaged for 5 min with a Dyna 4C gamma camera (Picker International, Highland Heights, OH), and images were quantified by using the program nuclear mac 3.01 (Scientific Imaging, Littleton, CO).
Calculation of Doses to the Tumor and Other Organs. The estimated dose delivered to the tumor was calculated by using the formula D = 1.44 × Ao × Te × S, where Ao is the administered activity, Te is the effective half-life of the radiopharmaceutical, and S is absorbed dose per unit cumulated activity. Te was determined by quantifying tumor uptake at 3 and 24 h after injection, and S for 188Re for a tumor with a mass of 1 g was obtained by using the program mirdose 3.
Comparative Scintigraphic Imaging of Black and White Mice with 188Re-6D2 mAb. To find out whether mAb 188Re-6D2 reacted with normal melanocytes, we performed comparative imaging of C57BL/6 black mice and BALB/c white mice. Notably, C57BL/6 mice have black hair, black eyes, and melanized skin on their tails. Six C57BL/6 and six BALB/c mice were injected i.v. with the same activity used in therapy experiments: 1.5 mCi 188Re-6D2 (100 μg; specific activity, 15 mCi/mg). Mice were imaged on a gamma camera 3 and 24 h after injection.
Therapy of MNT1 Tumor-Bearing Mice with mAb 188Re-6D2. For therapeutic studies, mice were injected in the right flank with 5 × 106 highly pigmented MNT1 human melanoma cells. Four weeks after injection with the tumor cells, the mice were divided into four groups of seven to eight animals and treated i.v. with (i) 1.5 mCi 188Re-6D2 mAb, (ii) 1.5 mCi 188Re-IgM irrelevant mAb UNLB, (iii) 100 μg of unlabeled mAb 6D2, or (iv) were left untreated. The size of the tumor was measured with calipers in three dimensions and the tumor volume was calculated as the product of these measurements multiplied by 0.5.
Histology. Tumors and the kidneys from randomly selected MNT1 tumor-bearing treated and control mice were removed, formalin-fixed, paraffin-embedded, stained with hematoxylin/eosin, and analyzed histologically. To determine whether radiation damage was induced by mAb 188Re-6D2 in melanin-containing normal tissues, six C57BL/6 mice received 1.5 mCi 188Re-6D2 as described. At 2 and 4 weeks after administration of the 188Re-6D2, three injected mice and three untreated littermates were killed. The eyes and melanized skin from the tails were removed and analyzed histologically as above.
Statistics. The Wilcoxon rank sum test was used to compare organ uptake in biodistribution studies. A two-tailed Student's t test for unpaired data was used to analyze differences in Ab cell binding during in vitro studies. The log-rank test was used to assess the course of animal survival. Differences were considered statistically significant when P values were <0.05.
Results
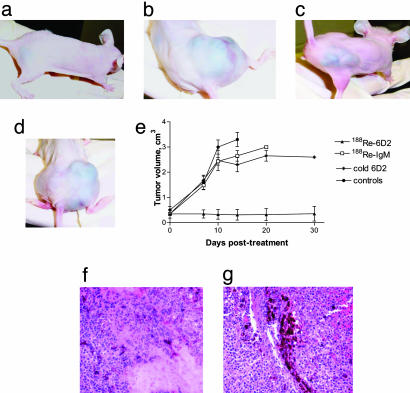
Therapy of MNT1 Melanoma in Nude Mice with 188Re-6D2. To examine the effect of radiolabeled Abs to melanin on melanoma tumors, four groups of seven to eight nude mice bearing MNT1 human pigmented melanoma tumors were treated i.v. with (i) 1.5 mCi 188Re-6D2 mAb, (ii) 1.5 mCi 188Re-IgM irrelevant mAb UNLB, (iii) 100 μg unlabeled mAb 6D2, or (iv) were left untreated. The diameter of the tumors on the day of treatment was 0.6-1.0 cm. Growth was completely inhibited in the group treated with 188Re-6D2 (Fig. 1a), and tumor regression occurred in animals with smaller initial tumors (0.6-0.7 cm in diameter). Residual thin (≈1 mm thickness) melanin plaques remained in mice with regressed tumors when they were killed at day 30 after treatment. During the observation period, no deaths occurred in mice treated with 188Re-6D2. In contrast, tumors continued to grow aggressively in mice treated with the irrelevant 188Re-IgM (Fig. 1b) or unlabeled 6D2 (Fig. 1c), and in untreated mice (Fig. 1d). Although there was no statistically significant difference in tumor size between unlabeled 6D2 and 188Re-IgM groups (P = 0.4), there was a difference (P < 0.05) between untreated controls and unlabeled 6D2 starting from day 14 after treatment (Fig. 1e). By day 20 after treatment all control mice, except for one in the unlabeled 6D2 group, had died. The dose delivered by 188Re-6D2 to the MNT1 tumor was calculated to be ≈20 Gy, the dose to the kidneys was ≈5 Gy.
Fig. 1.
Therapy of MNT1 pigmented melanoma tumors in nude mice. (a) 188Re-6D2-treated mouse, day 27 after treatment. (b) 188Re-IgM-treated mouse, day 10 after treatment (killed). (c) The last surviving mouse, treated with unlabeled 6D2 mAb, day 27 after treatment. (d) Untreated mouse, day 14 (killed). (e) Progression of tumors in treated and control mice. Bars are 1 SD. (f) Micrograph (hematoxylin/eosin staining) of a tumor from a mouse treated with 188Re-6D2. (g) Micrograph of a tumor from an untreated mouse.
Histological analysis of the tumors from the mice treated with one dose of mAb 188Re-6D2 showed considerably more necrotic and fibrosed tissue (Fig. 1f) than of the tumors from control mice (Fig. 1g). Malignant cells that appeared viable were present in treated and control tumors, but the control mice had significantly more cancerous cells with some melanoma infiltrating into tissue (Fig. 1g). No evidence of any acute tubular or glomerular injury to the kidneys of the animals treated with radiolabeled Ab was seen (data not shown).
Binding of Anti-Melanin mAb 6D2 to MNT1 Melanoma Cells in Vitro. To prove that antifungal melanin mAb 6D2 could also bind to tumor-derived melanin and to elucidate the mechanism of mAb 6D2 binding to MNT1 cells, we studied the binding of this mAb to MNT1 cells by immunofluorescence (Fig. 2). The mAb 6D2 bound only to nonviable melanoma cells (Fig. 2, arrowhead), which comprised 3-5% of the total number of cells in culture as measured by the dye exclusion assay. Nonviable cells apparently released their melanin or had permeable cell membranes that allowed Ab access to melanin (Fig. 2, Right). No binding was observed to viable cells with intact cell membranes (Fig. 2, arrow). Control IgM did not bind to either viable or dead MNT1 cells (data not shown).
Fig. 2.
Immunofluorescence of viable (arrow) and nonviable (arrowhead) MNT1 melanoma cells in vitro. (Original magnification, ×250.) (Left and Right) Light microscopy and immunofluorescence of MNT1 melanoma cells stained with mAb 6D2, respectively. Note that only nonviable cells are stained, which is consistent with the fact that cellular disruption is needed for mAb access to cellular melanin.
Binding of Anti-Melanin mAb 6D2 to Tumor Melanin. ImmunoGold TEM experiments were performed to establish at the ultrastructural level whether mAb 6D2 could interact with tumor melanin. TEM of the MNT1 tumor from a nude mice made cytoplasmic and extracellular melanin accessible to Ab when the tissue was sectioned (Fig. 3a). No association of gold balls with melanin was observed when the tumor tissue was stained with irrelevant IgM (data not shown).
Fig. 3.
Binding of 6D2 antifungal melanin Ab to tumor and fungal melanin. (a) ImmunoGold TEM of MNT1 melanoma cell stained with mAb 6D2. (Original magnification, ×20,000.) C, cytoplasm; E, extracellular space; N, cell nucleus. White and black arrows indicate gold balls labeling intracellular and extracellular melanin, respectively; two different melanoma cells are shown. There are multiple melanin particles associated with gold balls and the arrows indicate representative ones. (b) Immunohistochemistry of MNT1 melanoma tumor. Light microscopy (Upper) and immunofluorescence (Lower) of MNT1 melanoma tumor stained with mAb 6D2, respectively. Melanin debris in the extracellular space are visible adjacent to the amelanotic cell. (c) Melanin particles (“ghosts”) isolated from C. neoformans yeast cells (Left) and stained with 188Re-labeled melanin-binding mAb 6D2 (Right). (Original magnification, ×250.) (d) Binding of 111In-6D2 and 111In-12A1 to SK-MEL-28 whole cells. (e) Binding of 188Re-6D2 to MNT1 whole and lysed cells (cells were lysed before addition of radiolabeled mAbs).
Immunohistochemical analysis also revealed that the melanin-binding mAb labeled the extracellular melanin debris infiltrated within the tissue (Fig. 3b). As described above, the intracellular melanin was made accessible by sectioning of the tumor cells. No labeling occurred with controls.
Immunoreactivity of mAb 6D2 After Labeling with Radionuclides. Direct labeling of mAb 6D2 with 99mTc and 188Re through generation of thiol groups resulted in 93 ± 5% radioactivity binding to mAb, and labeling of N-[2-amino-3-(p-isothiocyanatophenyl)propyl]-trans-cyclohexane-1,2-diamine-N,N′,N″,N‴,N″″-pentaacetic acid-6D2 with 111In, in 90 ± 4%. Subsequent purification gave products with radiochemical purity of 97 ± 1%. Both 188Re- (Fig. 3c) and 111In-labeled (data not shown) mAb 6D2 retained their ability to bind to fungal melanin as shown by immunofluorescence.
Binding of Radiolabeled mAb 6D2 to Human Melanoma Cells SK-28-MEL and MNT1. To investigate the dependence of Ab binding on the amount of melanin in the cells, cell binding of 111In-6D2 and the irrelevant IgM 111In-12A1 was evaluated by incubating radiolabeled mAbs (specific activity, 1 mCi/mg) with whole SK-28-MEL cells, grown with and without l-tyrosine (Fig. 3d). No binding of irrelevant mAb to the cells was observed (Fig. 3d). Cell binding of 111In-6D2 was significantly higher for the melanoma cells grown with l-tyrosine, suggesting melanin-specific binding. Almost 100% of 111In-6D2 was cell-bound when the number of cells per well reached 2 × 106. At this concentration, 3-5% of the cells were not viable (6 × 104 to 105 cells per well), had permeable membranes, and provided enough melanin to ensure almost 100% binding of the Ab. To investigate whether there was a correlation between the amount of extracellular melanin and the Ab binding, we used whole and lysed naturally melanized human melanoma MNT1 cells. The binding 188Re-labeled mAbs 6D2 and 12A1 (1 mCi/mg) to the cells was assessed for this purpose (Fig. 3e). The melanin released from lysed cells was spun down with the cell debris and no melanin particles were left behind in the supernatant, thus increasing the radioactivity of the pellet in comparison with the pellet of intact cells. Whereas no 188Re-12A1 bound to the cells, almost 100% of the 188Re-6D2 bound to whole MNT1 cells at the concentration of 2 × 106 cells per well. Significantly higher binding was observed for lysed cells where saturation binding was reached at 106 cells per well.
Biodistribution of 188Re-6D2 and 188Re-IgM in Nude Mice. To assess the stability of radiolabeled mAb in vivo, we studied the biodistribution of 188Re-6D2 in comparison with 188Re-IgM in tumor-free nude mice (Fig. 4a). There was less 188Re-6D2 versus 188Re-IgM in the blood, liver, and muscle at both 5 and 24 h, possibly due to the significantly higher kidney uptake of 188Re-6D2, which may have served as a “sink” organ in this case. Both Abs were rapidly cleared from the blood with an ≈5-fold decrease within 24 h. Low uptake in the stomach for both Abs is consistent with the stability of the 188Re radiolabel on the labeled Abs because 188Re released from the Ab molecule would be quickly converted into perrhenate  , which targets stomach and thyroid (35). A striking difference was observed in kidney uptake at 5 h, with 188Re-6D2 uptake being almost 2-fold higher than that of 188Re-IgM. By 24 h, however, the kidney uptake of 188Re-6D2 fell dramatically to levels close to those of 188Re-IgM with no redistribution of activity, pointing at the hepatobilliary excretion route.
, which targets stomach and thyroid (35). A striking difference was observed in kidney uptake at 5 h, with 188Re-6D2 uptake being almost 2-fold higher than that of 188Re-IgM. By 24 h, however, the kidney uptake of 188Re-6D2 fell dramatically to levels close to those of 188Re-IgM with no redistribution of activity, pointing at the hepatobilliary excretion route.
Fig. 4.
Biodistribution and scintigraphic images of radiolabeled 6D2 mAb in nude mice. (a) Biodistribution of 188Re-6D2 and 188Re-IgM in nude mice at 5 and 24 h after i.v. injection. (b) Three-hour image of an MNT1 tumor-bearing mouse given i.v. 99mTc-6D2. (c) The same mouse at 24 h. The bladder had been voided before the image was taken and is not visible; the injection sites are visible on the tails at 3 and 24 h. (d) Three-hour image of a black C57BL/6 mouse given i.v. 188Re-6D2.
Scintigraphic Imaging of Tumor-Bearing Nude Mice and Pigmented Mice with Radiolabeled 6D2 mAb. MNT1 tumor-bearing mice were imaged with 0.4 mCi 99mTc-6D2 (i.v. injection) (Fig. 4 b and c). Excellent localization in the tumor was achieved at 3 h and remained high at 24 h. Significant uptake of 99mTc-6D2 mAb was also observed in the kidneys. No difference in uptake of 188Re-6D2 was observed in the hair follicles, eyes, heads, and melanized tails of C57BL/6 black mice at 3 h (Fig. 4d) and at 24 h (data not shown) after injection in comparison with white BALB/c mice (data not shown). The uptake in the head was 0.30 ± 0.15% injected dose (ID) per organ for black mice and 0.40 ± 0.20% ID per organ for white mice (P = 0.5); no statistical difference in uptake in the tails of black mice (0.22 ± 0.13% ID per organ) was seen in comparison with white mice (0.17 ± 0.10% ID per organ) (P = 0.7). No radiation damage was detected histologically in the eyes and melanized skin of black mice treated with mAb 188Re-6D2 (data not shown).
Discussion
We have investigated the use of a mAb to fungal melanin as a vehicle to deliver cytotoxic radiation to melanoma cells. This study addresses two questions: (i) whether an Ab to fungal melanin binds to mammalian tumor melanin, and (ii) the feasibility of using melanin, an intracellular pigment, to target radiation to melanoma cells. RIT is currently experiencing a renaissance, and so far has been most effective in the treatment of “liquid” and “semiliquid” malignancies such as lymphoma and leukemia (36).
The cell line MNT1 is highly pigmented with eumelanin (26), which is a dark brown/black pigment (20, 37). The binding of mAb 6D2 to this type of melanin was established by demonstrating that mAb 6D2 bound to MNT1 cells and to MNT1 tumors in mice. The lightly pigmented SK-MEL-28 human melanoma cells are pigmented with pheomelanin (38), a red/brown pigment consisting of subunits similar to those of eumelanin, but also rich in sulfur. The in vitro cell binding studies with 111In-6D2 suggest that pheomelanin is also targeted by melanin-binding Ab. Thus, melanin-binding Ab can be used for targeting cells from various pigmented melanoma cell lines.
The second problem involved the feasibility of using melanin, an intracellular pigment, to facilitate the delivery of cytotoxic radiation to melanoma cells. We hypothesized that melanin in melanoma tumors could be targeted by a melanin-binding Ab as a result of extracellular release from dead cells or by virtue of greater permeability of dying cells. Malignant tumors contain abnormally permeable degenerating cells, which make feasible targeting of mAbs to intracellular antigens (39, 40). This approach is currently used for detection of prostate cancer, where the intracellular epitope of a prostate-specific membrane antigen is targeted with 111In-labeled 7E11 mAb (ProstaScint; Cytogen, Princeton, NJ) (41). Our approach is to target an abundant tumor-associated pigment that is very resistant to degradation and persists at the site of the tumor (Fig. 1a). Furthermore, melanin is likely to be accessible to Ab only in tumor tissue and its persistence in tissue should allow for multiple treatments with the radiolabeled Ab. In fact, the efficacy of RIT may increase with the number of treatment cycles as pigment released from cells accumulates in tissue. Hence, melanin provides a fundamentally different target than previously described protein targets.
The biodistribution of radiolabeled mAb 6D2 was characterized by fast blood clearance (calculated half-life, 6.5 h), in good agreement with the fact that the half-lives of IgMs are short (42-44) and clearance of an IgM from the blood may be complete in 24 h (43). Immunofluorescence demonstrated that mAb 6D2 bound to nonviable MNT1 cells in vitro, whereas immunohistochemistry and ImmunoGold TEM established mAb binding to nonviable cells and extracellular melanin from in vivo tumors. The isotope selected for treatment of s.c. tumors was 188Re, a high-energy β-emitting therapeutic radionuclide (45, 46), with an optimal tissue range of 23.0-32.0 mm to produce cure probability of 90% (47). Such broad optimal tissue range for 188Re enabled us to treat tumors with different diameters (0.6-1.0 cm). The maximum tolerated activity of 188Re-IgG Abs was reported to be ≈0.8 mCi in non-tumor-bearing BALB/c mice injected i.v. with the Ab (48). Because blood clearance of IgMs, which is an important factor contributing to radiotoxicity of immunoconjugates, is several times faster than that of IgGs, we used almost double that dose in our therapy studies (1.5 mCi).
An s.c. model with highly melanized MNT1 cells was chosen to evaluate the potential of RIT because this model would provide sufficient amounts of melanin for determining a therapeutic effect. The MNT1 tumors contained ≈0.3 mg of melanin per g of tumor (E.D., J.D.N., A.D.S., and A.C., unpublished observations). Assuming 10% of cells in the tumor were nonviable, the amount of accessible melanin was 30 μg per g of tumor, which proved to be sufficient for the binding of 188Re-6D2 mAb and the delivery of tumoricidal doses of radiation. The histological evaluation of treated and untreated tumors suggested that beta radiation caused death of melanoma cells through necrosis. The dose delivered by 188Re-6D2 to the MNT1 tumor was calculated to be ≈20 Gy, which is in the tumoricidal range for solid tumors (12). These results dispel the notion that melanoma cells are radiation-resistant. RIT can be effective through such mechanisms as the induction of apoptosis in irradiated cells, bystander effect, and cell-cycle arrest (12-16). Furthermore, Ab properties such as the ability to catalyze the synthesis of oxidants (49) and to activate complement may also contribute to the therapeutic effect. In fact, these effects may have been responsible for the delay in tumor growth observed in the group of mice treated with unlabeled 6D2.
A noticeable accumulation of radiolabeled Abs was observed in the kidneys during biodistribution experiments. Because both melanin (24) and renal tubular cells (50) have negative charges, this accumulation may reflect electrostatic interactions between the variable region of the melanin-binding Abs and kidney cells. Alternatively, the kidneys may preferentially clear damaged radiolabeled molecules of mAb 6D2 with a molecular weight of <50,000. It is also possible that antigen-Ab complexes are being deposited in the kidneys. Because of the kidney uptake and the calculated dose of ≈5 Gy delivered to the kidneys by 188Re-6D2, we evaluated the histology of kidney tissue in the treated animals for signs of radioactive damage. No acute tubular or glomerular damage was apparent as a result of the treatment. Also our data with 188Relabeled melanin-binding peptides (51) indicate that doses of 12 Gy to the kidneys in nude mice did not cause any long-term renal toxicity (E.D., J.D.S., T. Moadel, R. Bryan, and A.C., unpublished observations). If the accumulation of the Ab in the kidneys proves to be a concern in the clinic, this problem may be circumvented by the administration of positively charged amino acids, such as d-lysine, to protect the kidneys (50, 52, 53). However, the available evidence suggests higher maximum tolerated doses for radionuclide therapy than the 21-27 Gy maximum tolerated dose (54) for external beam radiation.
We did not observe uptake of 188Re-6D2 in melanized skin on the tails, or other melanin-containing tissues of black C57BL/6 mice, which means that any uptake in these organs was below the detection limit of the gamma camera (≈0.1% of the therapeutic dose used in this study). There was no evidence of radiation damage by histological examination of the skin, hair follicles, and eyes of these animals. The absence of mAb 6D2 binding to intact melanized tissue is consistent with our observation that the Ab binds only to damaged or sectioned MNT1 cells. These findings suggest that crossreactivity of melanin-binding Abs with normal tissues would be minimal, because melanin in healthy tissue should be intracellular and thus inaccessible to melanin-binding Ab.
Also, because melanin is not soluble, it is unlikely that melanin-Ab complexes exist in circulation. If melanin particles separate from the tumor, they will most likely be sequestered by healthy macrophages in the liver and spleen, thus becoming intracellular and not accessible to the anti-melanin Ab. In this regard, systemic injection of melanin particles has shown that the pigment persists in mouse tissues including the liver (ref. 55 and J.D.N. and A.C., unpublished results).
It may be possible to enhance the efficacy of melanin-directed RIT by combining this modality with conventional chemotherapy, which would increase the number of dead cells and consequently the amount of melanin for targeting. The results also suggest that rapidly growing aggressive tumors with a high degree of cellular turnover would be most susceptible to this therapeutic approach. We recognize that RIT with melanin-binding Abs may only be useful against melanotic melanomas. However, these Abs constitute the majority of tumors and are the most resistant to chemotherapy and radiation therapy. Besides, “amelanotic” melanomas often contain small quantities of melanin and many of them may also be amenable to RIT. We observed binding of radiolabeled 6D2 to very lightly melanized SK-MEL-28 cells. This result is significant because melanoma metastases can be less melanized than primary tumor and such cells are capable of spreading the cancer in the body. We do not anticipate that targeting melanin would select for the evolution of a melanotic melanoma to an amelanotic tumor because amelanotic variants in a normal tumor would still be susceptible to killing by the “crossfire” effect of radiation emanating from the Ab bound to melanin in the tumor mass. We note that the promising therapeutic results in our model were obtained with a single injection of radiolabeled IgM mAbs, which are very large molecules with poor tissue penetration and a short serum half-life. However, fast blood clearance of IgMs may be actually beneficial for minimizing radiotoxicity of RIT. We anticipate that significantly better results may be obtained in future studies of melanoma therapy by using repeated doses of humanized radiolabeled IgG or Fab′ fragments, which may display better tissue penetration. This work establishes an approach to treatment of human metastatic melanoma based on targeting melanin, an intracellular pigment that is released from the dead cells in the tumors, with radiolabeled Abs.
Acknowledgments
We thank Dr. V. Hearing (National Institutes of Health, Bethesda) for a gift of MNT1 cells. This work was supported by Albert Einstein College of Medicine Cancer Center Grant 9526-9223 (to E.D.) and in part by National Institutes of Health Grants AI01489 (to J.D.N.) and AI33774, AI13342, AI052733, and HL59842 (to A.C.).
Author contributions: E.D., A.C., and J.D.N. designed research; E.D., J.D.N, L.S., A.D.S., A.F., and J.S.N. performed research; E.D. and A.C. analyzed data; J.D.N. and A.C. contributed new reagents/analytical tools; and E.D., A.C., and J.D.N. wrote the paper.
Abbreviations: RIT, radioimmunotherapy; TEM, transmission electron microscopy.
References
- 1.Soengas, M. S., Capodieci, P., Polsky, D., Mora, J., Esteller, M., Opitz-Araya, X., McCombie, R., Herman, J. G., Gerald, W. L., Lazebnik, Y. A., et al. (2001) Nature 409, 207-211. [DOI] [PubMed] [Google Scholar]
- 2.Liu, T. & Soong, S. J. (1996) Surg. Clin. North Am. 76, 1205-1222. [DOI] [PubMed] [Google Scholar]
- 3.Sun, W. & Schuchter, L. M. (2001) Curr. Treat. Options Oncol. 2, 193-202. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg, S. A. (2001) Nature 411, 380-384. [DOI] [PubMed] [Google Scholar]
- 5.Safa, M. M. & Foon, K. A. (2001) Semin. Oncol. 28, 68-92. [DOI] [PubMed] [Google Scholar]
- 6.Waldmann, T. A. (2003) Nat. Med. 9, 269-277. [DOI] [PubMed] [Google Scholar]
- 7.Kang, N. V. & Yong, A. (1998) Surgery (St. Louis) 16, 5-7. [Google Scholar]
- 8.Larson, S. M., Carrasquillo, J. A., McGuffin, R. W., Krohn, K. A., Ferens, J. M., Hill, L. D., Beaumier, P. L., Reynolds, J. C., Hellstrom, K. E. & Hellstrom, I. (1985) Radiology 155, 487-492. [DOI] [PubMed] [Google Scholar]
- 9.Larsen, R. H., Akabani, G., Welsh, P. & Zalutsky, M. R. (1998) Radiat. Res. 149, 155-162. [PubMed] [Google Scholar]
- 10.Rofstad, E. K. (1986) Acta Radiol. Oncol. 25, 1-10. [DOI] [PubMed] [Google Scholar]
- 11.Rofstad, E. K., Mathiesen, B. & Galappathi, K. (2004) Cancer Res. 64, 13-18. [DOI] [PubMed] [Google Scholar]
- 12.Murtha, A. D. (2000) Semin. Radiat. Oncol. 10, 133-138. [DOI] [PubMed] [Google Scholar]
- 13.Knox, S. J., Goris, M. L. & Wessels, B. W. (1992) Radiother. Oncol. 23, 111-117. [DOI] [PubMed] [Google Scholar]
- 14.Xue, L. Y., Butler, N. J., Makrigiorgos, G. M., Adelstein, S. J. & Kassis, A. I. (2002) Proc. Natl. Acad. Sci. USA 99, 13765-13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bishayee, A, Rao, D. V. & Howell, R. W. (1999) Radiat. Res. 152, 88-97. [PMC free article] [PubMed] [Google Scholar]
- 16.Dadachova, E., Howell, R. W., Bryan, R. A., Frenkel, A., Nosanchuk, J. D. & Casadevall, A. (2004) J. Nucl. Med. 45, 313-320. [PubMed] [Google Scholar]
- 17.Kinnaert, E., Morandini, R., Simon, S., Hill, H. Z., Ghanem, G. & Van Houtte, P. (2000) Radiat. Res. 154, 497-502. [DOI] [PubMed] [Google Scholar]
- 18.Hill, H. Z. (1992) BioEssays 14, 49-56. [DOI] [PubMed] [Google Scholar]
- 19.Rosas, A. L., Nosanchuk, J. D., Feldmesser, M., Cox, G. M., McDade, H. C. & Casadevall. A. (2000) Infect. Immun. 68, 2845-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wakamatsu, K. & Ito. S. (2002) Pigm. Cell Res. 15, 174-183. [DOI] [PubMed] [Google Scholar]
- 21.Williamson, P. R., Wakamatsu, K. & Ito, S. (1998) J. Bacteriol. 180, 1570-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosas, A. L., Nosanchuk, J. D., Gomez, B. L., Edens, W. A., Henson, J. M. & Casadevall, A. (2000) J. Immunol. Methods 244, 69-80. [DOI] [PubMed] [Google Scholar]
- 23.Nosanchuk, J. D. & Casadevall, A. (1997) Infect. Immun. 65, 1836-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blower, P. J., Clark, K. & Link, E. M. (1997) Nucl. Med. Biol. 24, 305-310. [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee, J., Nussbaum, G., Scharff, M. D. & Casadevall, A. (1995) J. Exp. Med. 181, 405-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kushimoto, T. A., Basrur, V., Valencia, J., Matsunaga, J., Vieira, W. D., Ferrans, V. J., Muller, J., Appella, E. & Hearing, V. J. (2001) Proc. Natl. Acad. Sci. USA 98, 10698-10703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dadachova, E. & Chapman, J. (1998) Nucl. Med. Commun. 19, 173-181. [PubMed] [Google Scholar]
- 28.Deutsch, E., Libson, K., Vanderheyden, J. L., Ketring, A. R. & Maxon, H. R. (1986) Int. J. Rad. Appl. Instrum. B 13, 465-477. [DOI] [PubMed] [Google Scholar]
- 29.Dadachova, E., Nakouzi, A., Bryan, R. & Casadevall, A. (2003) Proc. Natl. Acad. Sci. USA 100, 10942-10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dadachova, E., Mirzadeh, S., Smith, S. V., Knapp, F. F. & Hetherington, E. L. (1997) Appl. Radiat. Isot. 48, 477-481. [DOI] [PubMed] [Google Scholar]
- 31.Pippin, C. G., Parker, T. A., McCurry, T. J. & Brechbiel, M. W. (1992) Bioconjugate Chem. 3, 342-345. [DOI] [PubMed] [Google Scholar]
- 32.Dadachova, E. & Mirzadeh, S. (1997) Nucl. Med. Biol. 24, 605-608. [DOI] [PubMed] [Google Scholar]
- 33.Nosanchuk, J. D., Rosas, A. L. & Casadevall, A. (1998) J. Immunol. 160, 6026-6031. [PubMed] [Google Scholar]
- 34.Glatman-Freedman, A., Martin, J. M., Riska, P. F., Bloom, B. R. & Casadevall, A. (1996) J. Clin. Microbiol. 34, 2795-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dadachova, E., Bouzahzah, B., Zuckier, L. S. & Pestell. R. G. (2002) Nucl. Med. Biol. 29, 13-18. [DOI] [PubMed] [Google Scholar]
- 36.Knox, S. J. & Meredith, R. F. (2000) Semin. Radiat. Oncol. 10, 73-93. [DOI] [PubMed] [Google Scholar]
- 37.Ito, S. & Fujita, K. (1985) Anal. Biochem. 144, 527-536. [DOI] [PubMed] [Google Scholar]
- 38.Rice, L., Wainwright, M. & Phoenix, D. A. (2000) J. Chemother. 12, 94-104. [DOI] [PubMed] [Google Scholar]
- 39.Chen, F. M, Taylor, C. R. & Epstein, A. L. (1989) Cancer Res. 49, 4578-4585. [PubMed] [Google Scholar]
- 40.Welt, S., Mattes, M. J., Grando, R., Thomson, T. M., Leonard, R. W., Zanzonico, P. B., Bigler, R. E., Yeh, S., Oettgen, H. F. & Old, L. J. (1987) Proc. Natl. Acad. Sci. USA 84, 4200-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmes, E. H. (2001) Expert Opin. Investig. Drugs 10, 511-519. [DOI] [PubMed] [Google Scholar]
- 42.Macklis, R. M., Kinsey, B. M., Kassis, A. I., Ferrara, J. L., Atcher, R. W., Hines, J. J., Coleman, C. N., Adelstein, S. J. & Burakoff, S. J. (1988) Science 240, 1024-1026. [DOI] [PubMed] [Google Scholar]
- 43.Borchardt, P. E., Quadri, S. M., Freedman, R. S. & Vriesendorp, H. M. (1998) J. Nucl. Med. 39, 476-484. [PubMed] [Google Scholar]
- 44.Rosenblum, M. G., Levin, B., Roh, M., Hohn, D., McCabe, R., Thompson, L., Cheung, L. & Murray, J. L. (1994) Cancer Immunol. Immunother. 39, 397-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knapp, F. F., Jr. (1998) Cancer Biother. Radiopharm. 13, 337-349. [DOI] [PubMed] [Google Scholar]
- 46.Hoher, M., Wohrle, J., Wohlfrom, M., Hanke, H., Voisard, R., Osterhues, H. H., Kochs, M., Reske, S. N., Hombach, V. & Kotzerke, J. (2000) Circulation 101, 2355-2360. [DOI] [PubMed] [Google Scholar]
- 47.O'Donoghue, J. A., Bardiès, M. & Wheldon, T. E. (1995) J. Nucl. Med. 36, 1902-1909. [PubMed] [Google Scholar]
- 48.Sharkey, R. M., Blumenthal, R. D., Behr, T. M., Wong, G. Y., Haywood, L., Forman, D., Griffiths, G. L. & Goldenberg, D. M. (1997) Int. J. Cancer 72, 477-485. [DOI] [PubMed] [Google Scholar]
- 49.Wentworth, P., Jr., McDunn, J. E., Wentworth, A. D., Takeuchi, C., Nieva, J., Jones, T., Bautista, C., Ruedi, J. M., Gutierrez, A., Janda, K. D., et al. (2002) Science 298, 2195-2199. [DOI] [PubMed] [Google Scholar]
- 50.Behr, T. M., Behe, M., Kluge, G., Gotthardt, M., Schipper, M. L., Gratz, S., Arnold, R., Becker, W. & Goldenberg, D. M. (2002) Eur. J. Nucl. Med. Mol. Imaging 29, 277-279. [DOI] [PubMed] [Google Scholar]
- 51.Nosanchuk, J. D., Valadon, P., Feldmesser, M. & Casadevall, A. (1999) Mol. Cell. Biol. 19, 745-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Behr, T. M., Sharkey, R. M., Juweid, M. E., Blumenthal, R. D., Dunn, R. M., Griffiths, G. L., Bair, H. J., Wolf, F. G., Becker, W. S. & Goldenberg, D. M. (1995) Cancer Res. 55, 3825-3834. [PubMed] [Google Scholar]
- 53.Behr, T. M., Becker, W. S., Sharkey, R. M., Juweid, M. E., Dunn, R. M., Bair, H. J., Wolf, F. G. & Goldenberg, D. M. (1996) J. Nucl. Med. 37, 829-833. [PubMed] [Google Scholar]
- 54.Early, P. J. & Sodee, B. D., eds. (1995) in Principles and Practice of Nuclear Medicine (Mosby, St. Louis), p. 126.
- 55.Rosas, A. L., MacGill, R. S., Nosanchuk, J. D., Kozel, T. R. & Casadevall, A. (2002) Clin. Diagn. Lab. Immunol. 9, 144-148. [DOI] [PMC free article] [PubMed] [Google Scholar]