Abstract
Objective
Celastrol was recently identified as a potential novel treatment for obesity. However, the effect of Celastrol on nonalcoholic fatty liver disease (NAFLD) remains elusive. The aim of this study is to evaluate the role of Celastrol in NAFLD.
Methods
Functional studies were performed using wild-type C57BL/6J (WT) mice and liver specific Sirt1-deficient (LKO) mice. The molecular mechanism was explored in primary mouse liver and primary hepatocytes.
Results
When WT mice receiving a high-fat diet (HFD) were treated with Celastrol, reductions in body weight, subcutaneous and visceral fat content, and liver lipid droplet formation were observed, along with reduced hepatic intracellular triglyceride and serum triglyceride, free fatty acid, and ALT concentrations. Furthermore, Celastrol decreased hepatic sterol regulatory element binding protein 1c (Srebp-1c) expression, enhanced the phosphorylation of hepatic AMP-activated protein kinase α (AMPKα), and increased the expression of hepatic serine–threonine liver kinase B1 (LKB1). Additionally, Celastrol treatment improved glucose tolerance and insulin sensitivity in WT mice fed the HFD. Celastrol administration also improved the anti-inflammatory and anti-oxidative status by inhibiting nuclear factor kappa B (NFκB) activity and the mRNA levels of proinflammatory cytokines and increasing mitochondrial DNA copy number and anti-oxidative stress genes expression in WT mice liver, in vivo and in vitro. Moreover, Celastrol induced hepatic Sirt1 expression in WT mice, in vivo and in vitro. These Celastrol-mediated protective effects in WT mice fed a HFD were abolished in LKO mice fed a HFD. It was more interesting that Celastrol aggravated HFD-induced liver damage in LKO mice fed a HFD by inhibiting the phosphorylation of AMPKα and boosting the translocation of NFκB into the nucleus, thereby resulting in the increase of Srebp-1c expression and the mRNA levels of liver proinflammatory cytokines.
Conclusions
Celastrol ameliorates NAFLD by decreasing lipid synthesis and improving the anti-oxidative and anti-inflammatory status. And Sirt1 has an important role in Celastrol-ameliorating liver metabolic damage caused by HFD.
Keywords: Nonalcoholic fatty liver disease, Celastrol, Sirt1, Lipid metabolism, Chronic inflammation, Oxidative stress
Highlights
-
•
Celastrol alleviates high-fat diet-induced hepatic steatosis in wild type mice.
-
•
Celastrol improves anti-inflammatory and antioxidant status in wild type mice.
-
•
Celastrol induces the expression of hepatic Sirt1 in wild type mice.
-
•
Celastrol promotes AMPKα phosphorylation in liver specific Sirt1-deficient mice.
-
•
Celastrol increases the activity of NFκB in liver specific Sirt1-deficient mice.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD), characterized histologically by hepatic steatosis, is currently the most common chronic liver disease worldwide. NAFLD is not caused by alcohol consumption or viruses, is not congenital, and does not display autoimmune liver disease markers. NAFLD is emerging as a risk factor for diabetes and cardiovascular disease [1]. The pathologic characteristics of NAFLD comprise a wide spectrum of liver damage, ranging from simple steatosis to nonalcoholic steatohepatitis (NASH) and cirrhosis [2]. The pathogenesis of NAFLD is complex and some risk factors including lipid metabolic disorder, chronic inflammation, and oxidative stress have been shown to play a key role in the pathogenesis of NAFLD [3]. There are some theories to explain the pathological features of NAFLD, such as the 'two hit' model [4]. The first hit is that insulin resistance causes lipid accumulation in hepatocytes, and the second hit is that cellular insults result in inflammation and fibrosis from oxidative stress, mitochondrial dysfunction, lipid peroxidation, and cytokine activity [5]. Additionally, hepatocyte death caused by the defect of hepatocyte regeneration and oxidative stress inhibiting the replication of mature hepatocytes represents the proposed ‘third hit’ in NAFLD pathogenesis [6]. However, another view has been proposed that free fatty acids can also directly cause toxicity by increasing oxidative stress and activating inflammatory pathways [6].
Silent mating type information regulation 2 homolog 1 (Sirt1) plays a crucial role in the DNA damage response, carcinogenesis, and the regulation of lifespan, which are all mediated by its nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase activity. Sirt1 and its activators play a key role in lipid and glucose homeostasis and insulin sensitivity via regulating mitochondrial biogenesis and β-oxidation, and improving anti-inflammatory activities [7]. Previous studies indicated that the activation of Sirt1 in the liver protects against high-fat diet (HFD)-induced metabolic damage [7], [8], [9], [10].
Celastrol, also known as tripterine, was originally identified from traditional Chinese medicine and used for the treatment of cancer and some inflammatory diseases. In addition, Celastrol has been shown to be a very potent inhibitor of lipid peroxidation in mitochondria by direct radical scavenging. Moreover, it can prevent the attack of oxygen radicals on the inner mitochondrial membrane by increasing its negative surface charge [11]. Recently, Celastrol was identified as a leptin sensitizer and potential novel treatment for obesity [12], [13], and Celastrol-treated mice exhibited a decrease in liver weights due to reduced hepatic steatosis and intrahepatic triglycerides accumulation [13]. However, whether Sirt1 mediates the effect of Celastrol on NAFLD has remained elusive.
In this study, we report that Celastrol ameliorates NAFLD by decreasing lipid synthesis and improving the anti-oxidative and anti-inflammatory status. However, Celastrol aggravates liver metabolic damage in liver specific Sirt1-deficient mice fed a HFD through inhibiting the phosphorylation of AMP-activated protein kinase α (AMPKα) and boosting the translocation of nuclear factor kappa B (NFκB) into the nucleus, thereby increasing expression of Srebp-1c and the mRNA levels of liver proinflammatory cytokines.
2. Materials and methods
2.1. Animal protocols
Wild-type C57BL/6J (WT) male mice, approximate 6 weeks of age were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). Mice with floxed alleles of Sirt1 were crossed with mice that express Cre recombinase driven by the albumin promoter (Jackson Laboratory) in the liver to generate liver specific Sirt1-deficient (LKO) mice, which express a Sirt1 mutant protein in liver. The LKO mice bear a conditional deletion of Sirt1 exon 4, which encodes 51 amino acids of the conserved Sirt1 catalytic domain [14]. All mice were housed in individually ventilated cages and maintained on a 12 h light-dark cycle. The mice were fed a HFD (60% fat; Research Diets) ad libitum with free access to water. The WT and LKO male mice fed a HFD were intraperitoneally injected with Celastrol (200 μg/kg, ≥98% (HPLC), Cat. No. C0869; Sigma, St. Louis, MO) or DMSO (control) at 14-weeks-old every two days for 4 weeks. All animal experiments were conducted under protocols approved by the Animal Research Committee of the Institute of Laboratory Animals, Chinese Academy of Medical Sciences and Peking Union Medical College. Serum and liver concentrations of triglyceride (TG) and total cholesterol (TC) were determined using an automated Monarch device (Peking Union Medical College Hospital, Beijing, China). To assess Lipopolysaccharide (LPS) susceptibility, mice were injected intraperitoneally with 20 mg/kg of Escherichia coli-derived LPS serotype 0111:B4 (Sigma). All surgeries were performed under sodium pentobarbital anesthesia, and all precautions were taken to minimize the suffering.
2.2. Isolation and culture of mouse primary hepatocytes
Mouse primary hepatocytes were isolated and cultured as previously reported [15]. Hepatocytes were harvested after incubation for different concentration of Celastrol treatment for indicated time.
2.3. Histological analysis
For H&E staining, liver tissues were fixed in 10% neutral-buffered formalin, embedded in paraffin, and cut into 4-μm sections. For Oil Red O staining, liver tissues were frozen in liquid nitrogen and cut into 8-μm sections. Sections were stained and analyzed at 200× magnification using a microscope.
2.4. RNA isolation and quantitative RT-PCR
Total RNA was extracted from mouse liver or primary hepatocytes using a TRIzol-based method (Roche Molecular Biochemicals, Indianapolis, IN). Approximately 2 μg of total RNA was reverse-transcribed into a first-strand cDNA pool using SuperScript™ reverse transcriptase and random primers (Abcam). Q-PCR was performed using the SYBR Green I Q-PCR kit (Promega) with a Bio-Rad CFX system. All gene expression data were normalized to GAPDH expression levels. The specific primer sequences are presented in Supplemental Table S1.
2.5. Western blotting
Protein was extracted from frozen liver samples or cultured hepatocytes in cell lysis buffer. In total, 40–60 μg of protein was loaded onto a 10% SDS-polyacrylamide gel, and separated proteins were transferred to PVDF membranes. Western blot assays were performed using specific antibodies. Anti-Sirt1 antibody was purchased from Millipore (Cat. No. 7131, Millipore, Schwalbach, Germany), and anti-p-AMPKα and AMPKα antibodies were purchased from Cell Signaling Technologies (Cat. No. 2535 and 5831, Beverly, MA, USA). Anti-Srebp-1 antibody was obtained from Santa Cruz Biotechnology (Cat. No. sc-13551, Santa Cruz, CA, USA). Anti-Gapdh and α-tubulin antibodies were obtained from Abmart (Cat. No. CW0100A and CW0098A, Arlington, MA, USA). Anti-Sod2, NFκb and LKB1 antibodies were purchased from ABclonal Technology (Cat. No. A1340, A2771 and A2122, Abclonal, Wuhan, China). Anti-HDAC3 antibody was obtained from Abcam (Cat. No. ab7030, Abcam, Cambridge, UK).
2.6. Tolerance test
The glucose tolerance test (GTT) and insulin tolerance test (ITT) were performed after 4 weeks of Celastrol administration. For GTT, mice fasted for 16 h received an intraperitoneal injection of glucose (1.5 g/kg). For and ITT, mice fasted for 6 h received an intraperitoneal injection of human insulin (0.75 IU/kg). Blood glucose concentrations were measured from tail blood at the indicated times using a One-Touch Ultra® glucometer (LifeScan Inc., Milpitas, CA).
2.7. Mitochondrial DNA copy number
The mitochondrial DNA (mtDNA) copy number was used as a marker for mitochondrial density using qPCR as previously reported [16]. Briefly, total DNA was isolated from liver or hepatocytes using a Universal Genomic DNA Extraction Kit (Tiangen) according to the manufacturer's instructions. The mitochondrial DNA copy number was calculated from the ratio of COX II (mitochondrial-encoded gene)/β-actin (nuclear-encoded gene). The primer sequences of COX II and β-actin are presented in Supplemental Table S1.
2.8. Measurement of mitochondrial ROS accumulation
Mitochondrial reactive oxygen species (ROS) accumulation was measured as previously reported [17]. In brief, intracellular production of mitochondrial ROS was measured using MitoSOX Red (Molecular Probes). For this assay, primary hepatocytes were plated in six-well plates. Cells were treated with Celastrol or DMSO. After 24 h, cells were trypsinized, harvested, and loaded with MitoSOX Red for 10 min in the dark at 37 °C. Next, cells were washed twice with PBS, and fluorescence was measured using flow cytometry (excitation at 510 nm, emission at 580 nm). A minimum of 10,000 cells was acquired for each sample.
2.9. Immunofluorescence staining
Primary hepatocytes were fixed in 4% PFA, permeabilized with PBS, 0.2% Triton X-100, and incubated with anti-p65 monoclonal antibodies (ABclonal), followed by incubation with a TRITC-conjugated anti-mouse antibody. Nuclei were stained with DAPI.
2.10. Statistical analysis
The quantitative data are presented as the mean ± SD or SEM of three independent experiments. For the statistical analysis, the differences among means values were analyzed by nonparametric independent-sample test (SPSS), and p < 0.05 was considered statistically significant. *p < 0.05, **p < 0.01, ***p < 0.001.
3. Results
3.1. Celastrol alleviates high-fat diet-induced hepatic steatosis in wild type mice
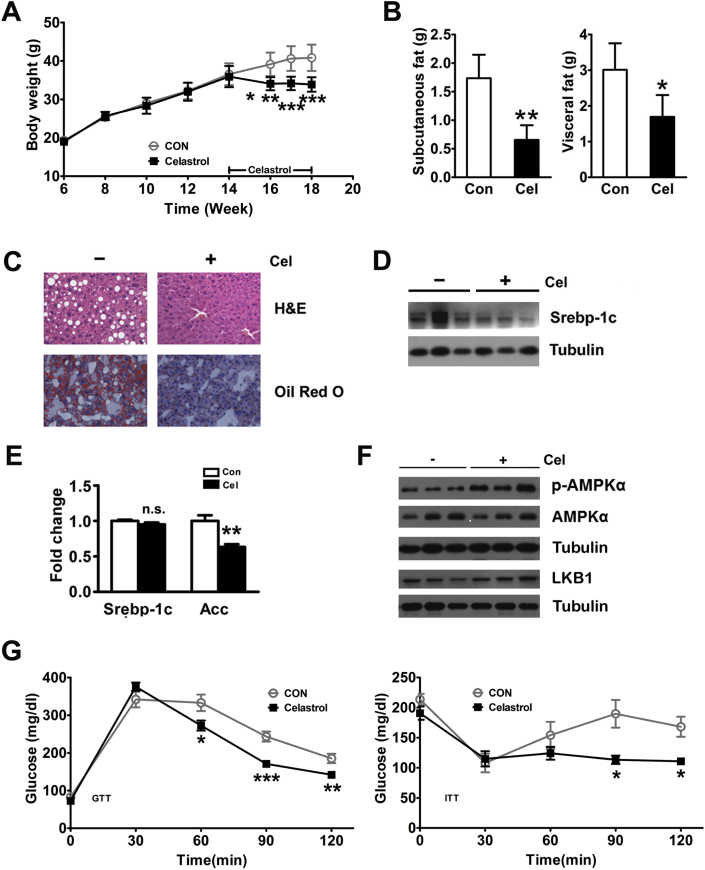
As previously reported [12], Celastrol treatment for 4 weeks decreased the body weight of WT mice fed the HFD by 17% (p < 0.05) (Figure 1A). In addition, we also tested daily food intake for two weeks after treatment in WT mice fed the HFD. The daily food intake for the control WT mice fed the HFD treated by DMSO was 2.30 ± 0.03 g during the first two weeks of trial (Figure S1A). In the Celastrol-treated group, the daily food intake was reduced to 1.80 ± 0.06 g in WT mice fed the HFD (Figure S1A). The Celastrol-induced change in body weight was also manifested by a reduction in subcutaneous and visceral fat in the WT mice (Figure 1B). Furthermore, we also detected the role of Celastrol in liver steatosis. We found the liver weights of the Celastrol-treated WT mice fed the HFD were lower compared to the control group (Figure S1B). However, the liver/body weight ratio was not significantly different between the Celastrol-treated WT mice fed the HFD and those in the control group (Figure S1C). Chronic HFD exposure causes apparent hepatosteatosis, including the massive accumulations of large lipid droplets and the ballooning degeneration of liver cells, which were clearly reversed by Celastrol treatment in WT mice fed the HFD (Figure 1C). The biochemical analysis revealed that Celastrol also reduced hepatic TG and serum TG, FFA, and ALT concentrations of WT mice fed the HFD (Table 1). Srebp-1c has been reported to be an important transcription factor involved in liver fat metabolism by regulating the expression of genes involved in fatty acid synthesis [18]. In this study, Celastrol decreased the hepatic protein expression of Srebp-1c in WT mice fed the HFD in vivo and in vitro (Figure 1D and Figure S1D). In addition, Celastrol also reduced the hepatic mRNA levels of the Srebp-1c target gene, acetyl-CoA carboxylase (Acc), in WT mice fed the HFD and the cellular mRNA levels of Srebp-1c and Acc in primary hepatocytes (Figure 1E and Figure S1E). AMPKα is an energy sensor that regulates cellular metabolism including lipid metabolism via down-regulation of Srebp-1c [19], [20]. In WT mice fed the HFD, Celastrol promoted phosphorylation of AMPKα but did not increase AMPKα protein levels (Figure 1F). Furthermore, Celastrol increased the hepatic expression of serine–threonine liver kinase B1 (LKB1), a principal upstream kinase for AMPK (Figure 1F). To further investigate whether Celastrol plays a role in systemic glucose and insulin sensitivity, we performed the GTT and ITT. Compared to the control group, Celastrol had no obvious effect on basal glucose levels but improved glucose tolerance and insulin sensitivity in WT mice fed on the HFD (Figure S1F and Figure 1G).
Figure 1.
Celastrol protects against high-fat diet-induced hepatic steatosis. Effects of Celastrol treatment in WT mice fed a HFD. Reduction in body weight (A) and subcutaneous and visceral fat weight (B). (C) Reversal of HFD-induced hepatosteatosis as indicated by H&E and Oil Red O staining (200×). (D) Decreased protein expression of hepatic Srebp-1c. (E) Decreased hepatic mRNA levels of Srebp-1c and its target gene, Acc. (F) Celastrol treatment increased hepatic AMPKα phosphorylation and LKB1 expression in WT mice fed a HFD. (G) Glucose tolerance tests and insulin tolerance tests. The wild type mice were treated with DMSO (n = 8) or Celastrol (n = 8, intraperitoneally injected with 200 μg/kg at 14-weeks-old every two days for 4 weeks). The quantitative data are presented as the mean ± SD or SEM of three independent experiments. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, n.s., not significant.
Table 1.
Metabolic profiles of WT and LKO mice fed a high-fat diet.
| WT |
LKO |
|||
|---|---|---|---|---|
| Con | Celastrol | Con | Celastrol | |
| Serum TG (mg/dL) | 278.9 ± 19.46 | 228.7 ± 4.226* | 141.7 ± 16.08 | 135.8 ± 5.197 |
| Serum TC (mg/dL) | 191.3 ± 14.46 | 184.2 ± 8.969 | 144.4 ± 10.24 | 151.5 ± 7.038 |
| Serum FFA (mg/dL) | 1.390 ± 0.0715 | 1.091 ± 0.0498** | 1.073 ± 0.0692 | 1.039 ± 0.0740 |
| Serum ALT (U/L) | 84.06 ± 8.813 | 50.94 ± 3.084** | 68.25 ± 7.170 | 62.58 ± 14.84 |
| Serum AST (U/L) | 167.6 ± 11.01 | 160.8 ± 6.589 | 176.1 ± 8.902 | 136.6 ± 18.89 |
| Liver TG (mg/g liver) | 140.9 ± 14.03 | 88.30 ± 11.44* | 133.4 ± 7.181 | 182.9 ± 5.495** |
| Liver TC (mg/g liver) | 8.017 ± 0.2653 | 8.564 ± 0.5792 | 9.618 ± 0.5686 | 11.29 ± 0.9825 |
The data are presented as the mean ± SEM. (n = 6-8 per group), ∗ p < 0.05, ∗∗ p < 0.001, compared with the control group.
3.2. Celastrol increases hepatic anti-inflammation and antioxidant capacity
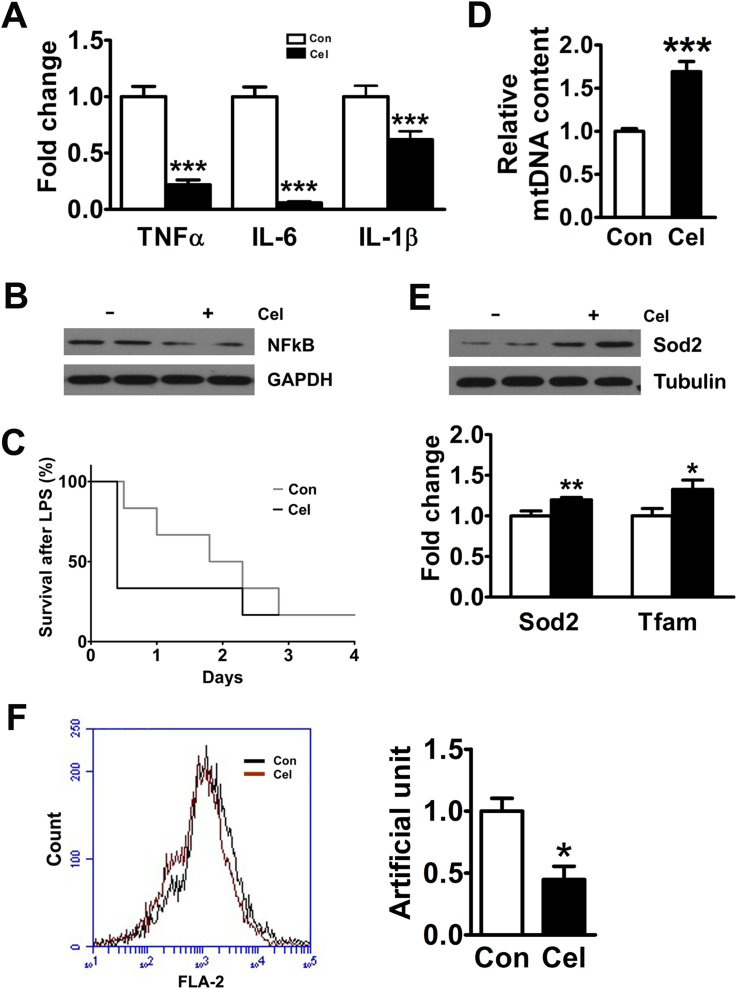
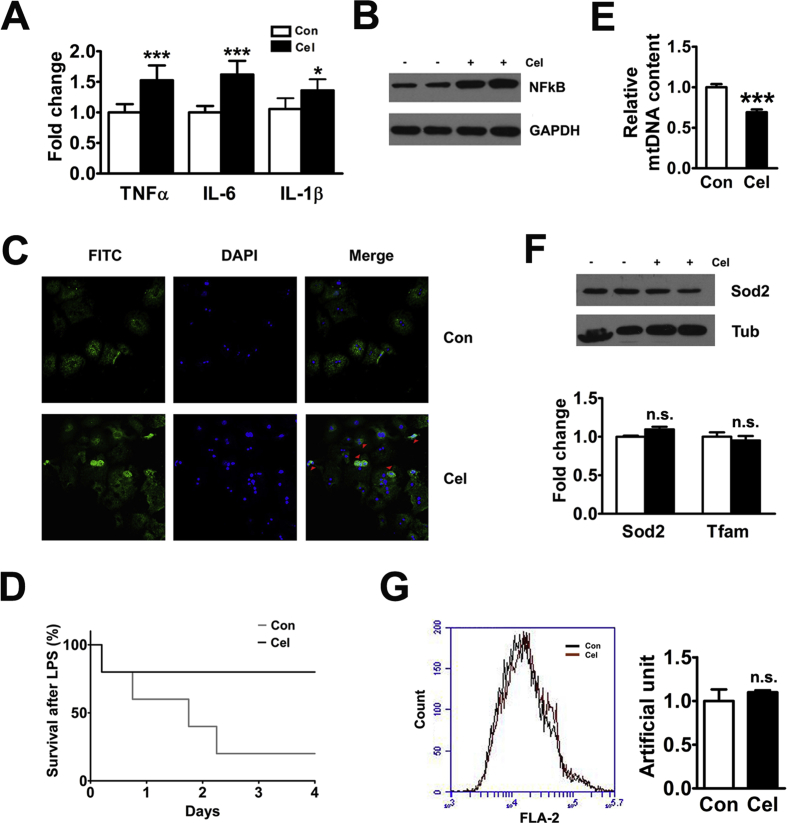
HFD-induced obesity leads to chronic inflammation, which is believed to be a key feature of obesity [21]. Previous reports have indicated that the mRNA expression of interleukin 6 (IL-6) and tumor necrosis factor α (TNFα) was significantly increased in the livers of the WT mice fed a HFD [8]. Our results showed that Celastrol inhibited the hepatic mRNA expression of TNFα, IL-6, and IL-1β in vivo and in vitro (Figure 2A and Figure S2A). Because IL-6 and TNFα are regulated by NFκB, we tested the effect of Celastrol on the p65 subunit of NFκB complex. The results showed that Celastrol inhibited hepatic NFκB expression in vivo and in vitro (Figure 2B and Figure S2B). LPS is an inflammatory agent that activates a wide range of responses partly mediated by NFκB. In this study, WT mice fed a standard diet treated by Celastrol had a marked LPS hypersensitivity compared with the controls (Figure 2C). Collective studies have suggested that oxidative stress may also contribute to clinical progression from simple fatty liver to NASH [3]. Mitochondria are now widely recognized to have numerous complex functions, including the regulation of oxidative stress and inflammation [22]. In this study, Celastrol increased hepatic mtDNA copy number (Figure 2D). Moreover, Celastrol promoted the expression of the antioxidant-related genes Sod2 and Tfam, in vivo and in vitro (Figure 2E and Figure S2C). In addition, Celastrol inhibited mitochondrial reactive oxygen species (ROS) production in WT primary hepatocytes (Figure 2F).
Figure 2.
Celastrol increases hepatic anti-inflammation and antioxidant capacity. (A) Celastrol reduced the mRNA levels of hepatic TNFα, IL-6, and IL-1β in WT mice fed a HFD. (B) Celastrol decreased the protein expression of hepatic NFκB-p65. (C) Kaplan–Meier plot of survival curves of wild type mice on standard diet treatment with Celastrol or DMSO for 4 weeks after injection of 20 mg/kg LPS (n = 6 per group). (D) Celastrol increased the hepatic mitochondrial DNA copy number in WT mice fed a HFD. (E) Celastrol promoted the protein expression of hepatic Sod2 and increased the mRNA levels of hepatic Sod2 and Tfam. (F) Celastrol inhibited mitochondrial ROS production in the primary hepatocytes of WT mice. The quantitative data are presented as the mean ± SEM of three independent experiments. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
3.3. Celastrol induces hepatic Sirt1 expression
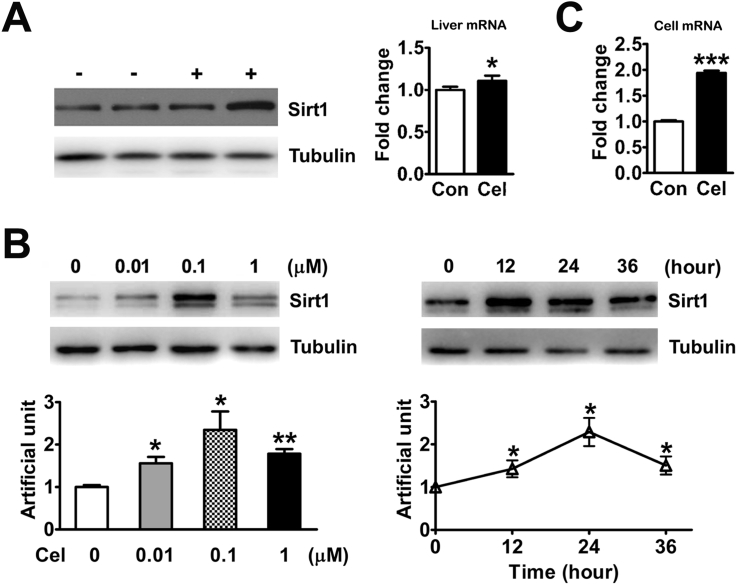
An increasing number of studies have reported that the activation of Sirt1 may affect the pathogenetic molecular cascade and the therapeutic mechanisms of NAFLD [7], [8], [9], [10]. Therefore, we tested the effect of Celastrol on hepatic Sirt1 in vivo. The results showed that Celastrol-induced liver Sirt1 mRNA and protein expression (Figure 3A). Furthermore, we also tested the effect of Celastrol on Sirt1 expression in primary hepatocytes. We found that the protein expression of Sirt1 was induced in the WT primary hepatocytes in response to different concentrations of Celastrol treatment (Figure 3B). In addition, Celastrol also increased the mRNA levels of Sirt1 in WT primary hepatocytes (Figure 3C).
Figure 3.
Celastrol induces hepatic Sirt1 expression. (A) Celastrol induced the protein (left) and mRNA (right) expression of Sirt1 in the livers of WT mice fed a HFD. (B) The protein expression of Sirt1 was induced in WT primary hepatocytes with different concentration of Celastrol for 24 h (left) and with 0.1 μM Celastrol for indicated time (right). (C) The mRNA levels of Sirt1 were increased in the WT primary hepatocytes with 0.1 μM Celastrol for 24 h. The quantitative data are presented as the mean ± SEM of three independent experiments. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
3.4. Alleviation of HFD-induced hepatic steatosis by Celastrol is abolished in liver specific Sirt1-deficient mice
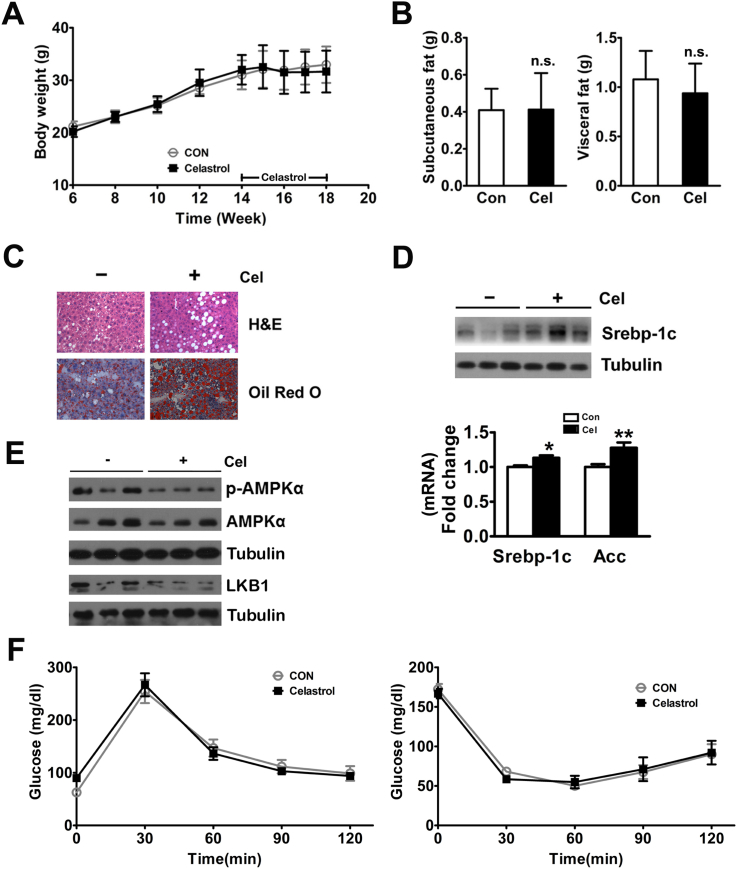
To further evaluate the molecular mechanism of Celastrol-induced protection for NAFLD, we tested the role of Celastrol in LKO mice fed a HFD in vivo. Although liver specific LKO mice bear a conditional deletion of Sirt1 at exon 4, which encodes 51 amino acids of the conserved Sirt1 catalytic domain, the mutant Sirt1 protein migrates slightly faster than WT Sirt1 in Western blot analyses (Figure S3A). Similar to previous reports [23], LKO mice fed the HFD are also protected from body weight gain (Figure S3B). Celastrol had a modest and non-significant effect on the body weights of LKO mice fed a HFD (Figure 4A). In addition, like the role of Celastrol in WT mice on HFD, Celastrol also decreased daily food intake in LKO mice on HFD during the first two weeks of the trial (Figure S4A). Furthermore, the reductions in Celastrol-induced subcutaneous and visceral fat, liver weight, hepatic TG, and serum TG, FFA, and ALT levels in WT mice fed the HFD were abrogated in LKO mice fed the HFD (Figure 4B, Figure S4B and Table 1). Interestingly, Celastrol increased the hepatic lipid droplet formation and TG concentration in LKO mice fed the HFD (Figure 4C and Table 1). In contrast to the WT mice, Celastrol induced the expression of hepatic Srebp-1c in vivo and in vitro (Figure 4D and Figure S4C). And Celastrol increased the mRNA level of hepatic Acc in vivo but not in vitro (Figure 4D and Figure S4C). Furthermore, Celastrol decreased the phosphorylation of AMPKα and inhibited hepatic LKB1 expression in LKO mice fed the HFD (Figure 4E). Celastrol had no effect on glucose tolerance and insulin sensitivity in LKO mice on HFD (Figure 4F).
Figure 4.
Celastrol effects on high-fat diet-induced hepatic steatosis are abolished in liver specific Sirt1-deficient mice. Effects of Celastrol in LKO mice fed a HFD. (A) No effect on the body weight. (B) No effect on the subcutaneous and visceral fat weight. (C) Aggravation of liver metabolic damage as shown via H&E and Oil Red O staining (200×). (D) Increased protein expression of hepatic Srebp-1c and mRNA levels of Srebp-1c and Acc. (E) Celastrol inhibited hepatic AMPKα phosphorylation and LKB1 expression in LKO mice fed a HFD. (F) Glucose tolerance tests and insulin tolerance tests. LKO mice were treated with DMSO (n = 6) or Celastrol (n = 6, intraperitoneally injected with 200 μg/kg at 14-weeks-old every two days for 4 weeks). The quantitative data are presented as the mean ± SD or SEM of three independent experiments. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, n.s., not significant.
3.5. Celastrol inhibits the expression of proinflammatory factors and antioxidant capacity through Sirt1
We further tested the role of Celastrol on chronic inflammation and oxidative stress in LKO mice fed a HFD. In contrast to the WT mice fed a HFD, Celastrol increased the mRNA expression of liver TNFα, IL-6, and IL-1β in LKO mice fed a HFD, but not in the hepatocytes of LKO mice (Figure 5A and Figure S5A). Furthermore, Celastrol also enhanced hepatic NFκB expression in vivo and in vitro and induced NFκB translocation from the cytoplasm to the nucleus (Figure 5B, C, Figure S5B). LKO mice fed a standard diet and treated with Celastrol had reduced LPS hypersensitivity compared with the controls (Figure 5D). In addition, Celastrol decreased the liver mtDNA copy number in LKO mice (Figure 5E). The Celastrol-induced increases in anti-oxidative genes, including Sod2 and Tfam, in WT mice fed the HFD were abolished in LKO mice fed the HFD and the primary hepatocytes of LKO mice (Figure 5F and Figure S5C). Finally, the effect of Celastrol-induced mitochondrial ROS reduction was alleviated in the LKO primary hepatocytes (Figure 5G).
Figure 5.
Celastrol inhibits the expression of proinflammatory factors and antioxidant capacity through Sirt1. Celastrol treatment increased hepatic TNFα, IL-6, and IL-1β mRNA expression (A), induced the protein expression of hepatic NFκB-p65 (B), and promoted the translocation of NFκB-p65 into the nucleus of primary hepatocytes (C). (D) Kaplan–Meier plot of survival curves of wild type mice on standard diet treatment with Celastrol or DMSO for 4 weeks after injection of 20 mg/kg LPS (n = 5 per group). (E) Celastrol reduced hepatic mitochondrial DNA copy number in LKO mice fed a HFD. (F) These Celastrol-induced increases in anti-oxidative genes, observed in WT mice fed a HFD, were abolished in LKO mice fed a HFD and the primary hepatocytes of LKO mice. (G) Celastrol had no effect on mitochondrial ROS production in the primary hepatocytes of LKO mice. The quantitative data are presented as the mean ± SD or SEM of three independent experiments. ∗p < 0.05, ∗∗∗p < 0.001, n.s., not significant.
4. Discussion
Many studies have shown that chronic HFD exposure leads to an increase metabolic damage in the liver of WT mice. Our findings showed that Celastrol administration caused a reduction in body weight, food intake, and subcutaneous and visceral fat in WT mice fed a HFD. Additionally, Celastrol decreased lipid droplet formation in the livers of WT mice fed a HFD. Moreover, Celastrol decreased the liver TG and serum TG, FFA, and ALT concentrations in WT mice fed a HFD. Celastrol treatment also improved glucose tolerance and insulin sensitivity in WT mice fed a HFD. The hallmark of NAFLD is hepatic neutral lipid accumulation, mainly triacylglycerol; thus, Srebp-1c, as a key transcription factor regulating hepatic lipid metabolism, has been proposed to have great potential for NAFLD treatment [18]. Our results showed that Celastrol inhibited the expression of Srebp-1c and its target gene, Acc.
As the most extensively studied Sirtuins, Sirt1 has a prominent role in metabolic tissues, such as the liver, skeletal muscle, and adipose tissues. Some studies have indicated that increased Sirt1 expression in the liver protects against HFD-induced metabolic damage by deacetylating Srebp-1c, thus decreasing its expression [8], [9]. In this study, Celastrol increased Sirt1 expression in the liver and primary hepatocytes. Furthermore, Celastrol had a slight and non-significant effect on the body weights of LKO mice fed a HFD. Work from Liu et al. [12] had indicated that Celastrol is a leptin sensitizer and can suppress food intake by increasing leptin sensitivity. Like the role of Celastrol in WT mice on HFD, Celastrol also decreased daily food intake in LKO mice fed a HFD indicating that Celastrol could increase the hypothalamus leptin sensitivity in both WT and LKO mice. In contrast to the role of Celastrol in WT mice, Celastrol had no effect on subcutaneous and visceral fat, and glucose tolerance and insulin sensitivity in LKO mice fed a HFD. However, Celastrol promoted lipid droplet formation and liver TG concentration in the livers of LKO mice fed a HFD and induced an increase in hepatic Srebp-1c in LKO mice.
Previous studies have indicated an association between chronic inflammation and hepatic steatosis [6]. Our study showed that Celastrol inhibited hepatic proinflammatory factors, such as TNFα, IL-6, and IL-1β. Previously, it was indicated that the NFκB signaling pathway plays an important role in chronic hepatic inflammation [24], and HFD exposure was shown to activate the hepatic NFκB signaling pathway. Therefore, increased NFκB activity is associated with the elevated hepatic expression of TNFα, IL-6, and IL-1β [8], [24]. Sirt1 participates in the inflammatory process by deacetylating NFκB, particularly at its subunit, transcription factor p65 [25]. Resveratrol, a Sirt1 activator, can inhibit the upregulation of inflammatory markers, such as intercellular adhesion molecule 1 and TNFα [26]. Kauppinen and colleagues suggested that the regulation of innate immunity and energy metabolism are connected together through an antagonistic crosstalk between NFκB and Sirt1 signalling pathways [27]. Sirt1 inhibits NFκB signalling directly by deacetylating the p65 subunit of NFκB complex and indirectly by activating AMPK, PPARα, and PGC-1α, further inhibiting NFκB signalling. On the other hand, NFκB signalling down-regulates Sirt1 activity through the expression of miR-34a, IFNγ, and reactive oxygen species. In this study, our results also showed that Celastrol inhibited the expression of hepatic NFκB and enhanced LPS hypersensitivity in WT mice. However, Celastrol enhanced hepatic NFκB expression and induced NFκB translocation from the cytoplasm to the nucleus in LKO mice. And the Celastrol-induced increase in LPS hypersensitivity in WT mice was also reversed. These results indicate that Sirt1 may be an upstream regulator for Celastrol-induced NFκB decrease.
It is well known that mitochondria are essential for cellular energy metabolism and the control of ROS [28], [29]. Oxidative stress, which arises as an imbalance between the production of ROS and the action of antioxidant defense mechanisms, plays a role in the pathogenesis of NAFLD [30]. The present study showed that Celastrol promoted mitochondrial biogenesis and inhibited mitochondrial ROS production. Chronic inflammatory cytokines are also potent stimulators of ROS production, and ROS can induce oxidative stress, with the subsequent activation of inflammatory pathways and mitochondrial damage [31], [32], [33]. Previous studies have shown that Sirt1 has a regulatory effect on oxidative stress in many tissues through reducing the ROS levels and improving cell survival via this antioxidant effect [34]. The anti-inflammatory activities of Sirt1 and its activators can reduce the oxidative stress and provide beneficial effects in some disorders, such as obesity, hypertension, and endothelial dysfunction [35]. These Celastrol induced increases in mtDNA copy number and anti-oxidative genes and reductions in mitochondrial ROS in WT mice fed a HFD were abolished in LKO mice fed a HFD. Previous studies indicate that Sirt1 can increase the production of ROS-detoxifying enzymes [36]. Additionally, randomized, double-blind, placebo-controlled human studies have shown that resveratrol decreases the levels of ROS [7].
It is well known that AMPK is an energy sensor that regulates cellular metabolism including lipid metabolism [19]. LKB1 is the principal AMPK kinase that catalyzes the phosphorylation of its catalytic α-subunit [37]. Sirt1 can diminish the lysine acetylation of LKB1, resulting in its own activation, and subsequently that of AMPK [38]. The current study showed that Celastrol promoted the phosphorylation of AMPKα and increased hepatic expression of LKB1 in WT mice fed a HFD. In contrast, Celastrol decreased the phosphorylation of AMPKα and inhibited hepatic LKB1 expression in LKO mice fed a HFD. Some findings have indicated that histone deacetylases 3 (Hdac3) is crucial for the maintenance of hepatic lipid homeostasis by co-localizing with a nuclear receptor co-repressor in chromatin near genes in the mouse liver in a circadian pattern [39]. Furthermore, perilipin 2, which stabilizes lipid droplets and controls lipolysis, can be markedly induced following Hdac3 depletion and contributes to both the development of steatosis and improved tolerance to glucose [40]. In the current study, Celastrol inhibited hepatic Hdac3 expression and increased perilipin 2 expression in LKO mice fed the HFD (data not shown). Combined with our above findings that Celastrol increased the expression of NFκB and inhibited the phosphorylation of AMPKα and hepatic Hdac3 expression in LKO mice fed a HFD, this may explain the reason for Celastrol treatment-induced steatosis exacerbation in LKO mice fed a HFD.
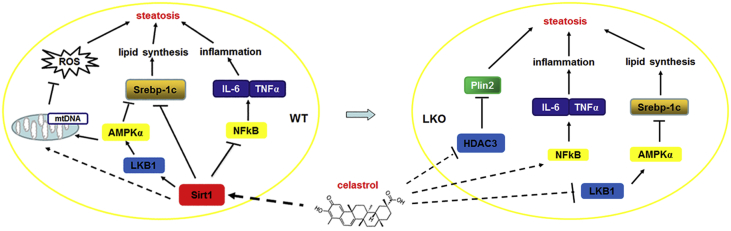
An increasing number of studies have demonstrated that factors including lipid metabolism disorder, chronic inflammation, and oxidative stress played important roles in the pathogenesis of NAFLD [31]. A previous study indicated that Celastrol protects against obesity and metabolic dysfunction through activation of a HSF1-PGC1α transcriptional axis [13]. In the current study, we report that Celastrol can alleviate HFD-induced damage in WT mice fed a HFD by increasing Sirt1 expression, further causing a reduction in Srebp-1c expression and improving the anti-oxidative and anti-inflammatory status (Figure 6 left). However, Celastrol aggravates HFD-induced liver damage in LKO mice fed a HFD by inhibiting the phosphorylation of AMPKα and increasing NFκB activity (Figure 6 right). In summary, Sirt1 plays an important role in Celastrol-ameliorating liver metabolic damage caused by HFD.
Figure 6.
Schematic representation of the action of Celastrol mediated by Sirt1 in liver metabolism.
Acknowledgments
This work was supported by collective grants from the National Natural Science Foundation of China (Grants No. 31371191) and the Major State Basic Research Development Program of China (973 Program 2012CB517504).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.11.002.
Contributor Information
Xiaojun Liu, Email: xiaojunliu@ibms.pumc.edu.cn.
Fude Fang, Email: fangfd@vip.sina.com.
Yongsheng Chang, Email: changy@ibms.pumc.edu.cn.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Musso G., Gambino R., Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD) Progress in Lipid Research. 2009;48:1–26. doi: 10.1016/j.plipres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Brunt E.M. Pathology of nonalcoholic fatty liver disease. Nature Reviews Gastroenterology & Hepatology. 2010;7:195–203. doi: 10.1038/nrgastro.2010.21. [DOI] [PubMed] [Google Scholar]
- 3.Krawczyk M., Bonfrate L., Portincasa P. Nonalcoholic fatty liver disease. Best Practice & Research Clinical Gastroenterology. 2010;24:695–708. doi: 10.1016/j.bpg.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Day C.P., James O.F. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 5.Ruhl C.E., Everhart J.E. Epidemiology of nonalcoholic fatty liver. Clinics in Liver Disease. 2004;8:501–519. doi: 10.1016/j.cld.2004.04.008. vii. [DOI] [PubMed] [Google Scholar]
- 6.Dowman J.K., Tomlinson J.W., Newsome P.N. Pathogenesis of non-alcoholic fatty liver disease. QJM. 2010;103:71–83. doi: 10.1093/qjmed/hcp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colak Y., Yesil A., Mutlu H.H., Caklili O.T., Ulasoglu C., Senates E. A potential treatment of non-alcoholic fatty liver disease with SIRT1 activators. Journal of Gastrointestinal and Liver Diseases: JGLD. 2014;23:311–319. doi: 10.15403/jgld.2014.1121.233.yck. [DOI] [PubMed] [Google Scholar]
- 8.Pfluger P.T., Herranz D., Velasco-Miguel S., Serrano M., Tschop M.H. Sirt1 protects against high-fat diet-induced metabolic damage. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponugoti B., Kim D.H., Xiao Z., Smith Z., Miao J., Zang M. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. Journal of Biological Chemistry. 2010;285:33959–33970. doi: 10.1074/jbc.M110.122978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X., Gao Y., Li M., Geng C., Xu H., Yang Y. Sirt1 mediates the effect of the heme oxygenase inducer, cobalt protoporphyrin, on ameliorating liver metabolic damage caused by a high-fat diet. Journal of Hepatology. 2015;63:713–721. doi: 10.1016/j.jhep.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Allison A.C., Cacabelos R., Lombardi V.R., Alvarez X.A., Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer's disease. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2001;25:1341–1357. doi: 10.1016/s0278-5846(01)00192-0. [DOI] [PubMed] [Google Scholar]
- 12.Liu J., Lee J., Hernandez M.A.S., Mazitschek R., Ozcan U. Treatment of obesity with celastrol. Cell. 2015;161:999–1011. doi: 10.1016/j.cell.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma X., Xu L., Alberobello A.T., Gavrilova O., Bagattin A., Skarulis M. Celastrol protects against obesity and metabolic dysfunction through activation of a HSF1-PGC1alpha transcriptional axis. Cell Metabolism. 2015;22:695–708. doi: 10.1016/j.cmet.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Cheng H.L., Mostoslavsky R., Saito S., Manis J.P., Gu Y., Patel P. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto M., Ogawa W., Teshigawara K., Inoue H., Miyake K., Sakaue H. Role of the insulin receptor substrate 1 and phosphatidylinositol 3-kinase signaling pathway in insulin-induced expression of sterol regulatory element binding protein 1c and glucokinase genes in rat hepatocytes. Diabetes. 2002;51:1672–1680. doi: 10.2337/diabetes.51.6.1672. [DOI] [PubMed] [Google Scholar]
- 16.Kong X., Wang R., Xue Y., Liu X., Zhang H., Chen Y. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PloS One. 2010;5:e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukhopadhyay P., Rajesh M., Hasko G., Hawkins B.J., Madesh M., Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nature Protocols. 2007;2:2295–2301. doi: 10.1038/nprot.2007.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed M.H., Byrne C.D. Modulation of sterol regulatory element binding proteins (SREBPs) as potential treatments for non-alcoholic fatty liver disease (NAFLD) Drug Discovery Today. 2007;12:740–747. doi: 10.1016/j.drudis.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Long Y.C., Zierath J.R. AMP-activated protein kinase signaling in metabolic regulation. Journal of Clinical Investigation. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y., Xu S., Mihaylova M.M., Zheng B., Hou X., Jiang B. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metabolism. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 22.Goodpaster B.H. Mitochondrial deficiency is associated with insulin resistance. Diabetes. 2013;62:1032–1035. doi: 10.2337/db12-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen D., Bruno J., Easlon E., Lin S.J., Cheng H.L., Alt F.W. Tissue-specific regulation of SIRT1 by calorie restriction. Genes & Development. 2008;22:1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai D., Yuan M., Frantz D.F., Melendez P.A., Hansen L., Lee J. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nature Medicine. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeung F., Hoberg J.E., Ramsey C.S., Keller M.D., Jones D.R., Frye R.A. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. The EMBO Journal. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csiszar A., Labinskyy N., Podlutsky A., Kaminski P.M., Wolin M.S., Zhang C. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. American Journal of Physiology Heart and Circulatory Physiology. 2008;294:H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kauppinen A., Suuronen T., Ojala J., Kaarniranta K., Salminen A. Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cellular Signalling. 2013;25:1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Brookes P.S., Levonen A.L., Shiva S., Sarti P., Darley-Usmar V.M. Mitochondria: regulators of signal transduction by reactive oxygen and nitrogen species. Free radical Biology & Medicine. 2002;33:755–764. doi: 10.1016/s0891-5849(02)00901-2. [DOI] [PubMed] [Google Scholar]
- 29.Murphy M.P. How mitochondria produce reactive oxygen species. Biochemical Journal. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rolo A.P., Teodoro J.S., Palmeira C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radical Biology & Medicine. 2012;52:59–69. doi: 10.1016/j.freeradbiomed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Asrih M., Jornayvaz F.R. Inflammation as a potential link between nonalcoholic fatty liver disease and insulin resistance. Journal of Endocrinology. 2013;218:R25–R36. doi: 10.1530/JOE-13-0201. [DOI] [PubMed] [Google Scholar]
- 32.Day C.P. Pathogenesis of steatohepatitis. Best Practice & Research Clinical Gastroenterology. 2002;16:663–678. doi: 10.1053/bega.2002.0333. [DOI] [PubMed] [Google Scholar]
- 33.de Lemos E.T., Oliveira J., Pinheiro J.P., Reis F. Regular physical exercise as a strategy to improve antioxidant and anti-inflammatory status: benefits in type 2 diabetes mellitus. Oxidative Medicine and Cellular Longevity. 2012;2012:741545. doi: 10.1155/2012/741545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horio Y., Hayashi T., Kuno A., Kunimoto R. Cellular and molecular effects of sirtuins in health and disease. Clinical Science. 2011;121:191–203. doi: 10.1042/CS20100587. [DOI] [PubMed] [Google Scholar]
- 35.Xu Q., Si L.Y. Resveratrol role in cardiovascular and metabolic health and potential mechanisms of action. Nutrition Research. 2012;32:648–658. doi: 10.1016/j.nutres.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Hori Y.S., Kuno A., Hosoda R., Horio Y. Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PloS One. 2013;8:e73875. doi: 10.1371/journal.pone.0073875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woods A., Johnstone S.R., Dickerson K., Leiper F.C., Fryer L.G., Neumann D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Current Biology. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 38.Ruderman N.B., Xu X.J., Nelson L., Cacicedo J.M., Saha A.K., Lan F. AMPK and SIRT1: a long-standing partnership? American Journal of Physiology Endocrinology and Metabolism. 2010;298:E751–E760. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng D., Liu T., Sun Z., Bugge A., Mullican S.E., Alenghat T. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315–1319. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Z., Miller R.A., Patel R.T., Chen J., Dhir R., Wang H. Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nature Medicine. 2012;18:934–942. doi: 10.1038/nm.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.