Abstract
Objective
Fibroblast-growth factor 21 (FGF21) is thought to be important in metabolic regulation. Recently, low protein diets have been shown to increase circulating FGF21 levels. However, when energy contribution from dietary protein is lowered, other macronutrients, such as carbohydrates, must be increased to meet eucaloric balance. This raises the possibility that intake of a diet rich in carbohydrates may induce an increase in plasma FGF21 levels per se. Here we studied the role of dietary carbohydrates on the levels of circulating FGF21 and concomitant physiologic effects by feeding healthy men a carbohydrate rich diet without reducing protein intake.
Methods
A diet enriched in carbohydrates (80 E% carbohydrate; CHO) and a eucaloric control diet (CON) were provided to nine healthy men for three days. The energy intake during the CHO diet was increased (+75% energy) to ensure similar dietary protein intake in CHO and CON. To control for the effect of caloric surplus, we similarly overfed (+75% energy) the same subjects for three days with a fat-rich diet (78 E% fat; FAT), consisting of primarily unsaturated fatty acids. The three diets were provided in random order.
Results
After CHO, plasma FGF21 concentration increased 8-fold compared to CON (329 ± 99 vs. 39 ± 9 pg ml−1, p < 0.05). In contrast, after FAT only a non-significant tendency (p = 0.073) to an increase in plasma FGF21 concentration was found. The increase in FGF21 concentration after CHO correlated closely (r = 0.88, p < 0.01) with increased leg glucose uptake (62%, p < 0.05) and increased hepatic glucose production (17%, p < 0.01), indicating increased glucose turnover. Plasma fatty acid (FA) concentration was decreased by 68% (p < 0.01), supported by reduced subcutaneous adipose tissue HSL Ser660 phosphorylation (p < 0.01) and perilipin 1 protein content (p < 0.01), pointing to a suppression of adipose tissue lipolysis. Concomitantly, a 146% increase in the plasma marker of hepatic de novo lipogenesis C16:1 n−7 FA (p < 0.01) was observed together with 101% increased plasma TG concentration (p < 0.001) in association with CHO intake and increased plasma FGF21 concentration.
Conclusion
Excess dietary carbohydrate, but not fat, led to markedly increased FGF21 secretion in humans, notably without protein restriction, and affected glucose and lipid homeostais.
Keywords: FGF21, Diet, Carbohydrates, Lipolysis, Liver
Abbreviations: ATGL, adipose triglyceride lipase; AMPK, AMP-activated kinase; BCA, bicinchoninic acid; BM, body mass; BMI, body mass index; CHO, carbohydrate-rich diet; ChREBP, carbohydrate-responsive element binding protein; CON, control diet; FA, fatty acid; FAT, fat-rich diet; FGF21, fibroblast growth factor 21; GLUT4, glucose transporter 4; HSL, hormone sensitive lipase; LM, leg mass; VO2peak, maximal oxygen consumption; PKA, protein kinase A; Ra, rate of appearance; TG, triacylglycerol; VLDL, very low density lipoprotein
Highlights
-
•
Dietary carbohydrate excess induces circulating FGF21 8-fold in humans.
-
•
Increased FGF21 was associated with increased hepatic glucose production and lipogenesis.
-
•
The induction of FGF21 was associated with increased leg glucose uptake.
-
•
The induction of FGF21 was accompanied by indices of lower adipose tissue lipolysis.
1. Introduction
The hormone fibroblast growth factor 21 (FGF21) was early reported to improve glucose homeostasis and to lower circulating triglycerides in obese and insulin resistant mice [1] and later observed to be important in the metabolic adaptations to fasting and ketosis [2], [3]. Now, FGF21 has received increasing interest due to its role in the regulation of energy homeostasis and its potential antidiabetic and lipid-lowering properties [4], [5]. Therefore, FGF21 holds promise as a therapeutic target, although intriguingly being elevated in obesity and type 2 diabetes [6], [7]. In mice, restriction of essential amino acids [8], [9], [10] as well as non-essential amino acids [11] has been shown to increase plasma FGF21 levels. In humans, 28 days restriction of dietary protein content to 6 E%, compared to 15 E% in the baseline diet, was shown to increase plasma FGF21 concentration by 171% [12]. This was, however, under hypercaloric (+39 E%) conditions resulting in a weight gain (+3.16 kg on average). Furthermore, the reduced protein was compensated by increasing the fat intake from 25 to 52 E% and decreasing the carbohydrate intake from 60 to 42 E% [13]. Thus, it is not clear whether the increase in circulating FGF21 obtained in the study by Laeger et al. resulted from protein restriction per se, alterations in carbohydrate and fat intake, or as a result of the weight gain. Our recent human study demonstrated that 7 days of a eucaloric protein diluted diet (9.0 E% protein) increased plasma FGF21 concentration by 500% compared to a control diet with 20.2 E% dietary protein [11]. Together, these findings have led to speculations that dietary protein restriction induces FGF21 secretion and thereby enhances metabolic health [12], [14].
Studies in rodents have also implied a role of carbohydrates in FGF21 induction. Hence, 12 h carbohydrate feeding (provided after 24 h of fasting) was shown to induce FGF21 mRNA in rat liver [15]. Furthermore, monosaccharide-induced increases in FGF21 gene expression in hepatocytes and mouse liver were mediated via the transcription factor ChREBP [16], [17], [18]. This is supported by the observation that FGF21 content in plasma was not increased in ChREBP knockout mice in response to various sugars [19]. In addition, a recent study in mice, providing 25 diets with varying macronutrient composition, showed an additive effect of high-carbohydrate feeding to the induction of circulating FGF21 with low-protein feeding [20]. Even though it has been shown in humans that single monosaccharide intake briefly increased plasma FGF21 concentration [21], the role of dietary carbohydrates in an ordinary diet (including mainly polysaccharides, but also di- and monosaccharides) without protein restriction in humans is not known.
Despite reports demonstrating that starvation and ketogenic diets induce FGF21 in mice, an increase in circulating FGF21 concentration is not detected until after 7–10 days of starvation in humans [22], [23], and the reported effects of ketogenic diets are inconsistent [22], [24]. Hence, in the context of dietary interventions the regulation of FGF21 may vary between mouse and man.
The aim of the present study was to evaluate the role of dietary carbohydrates in the regulation of circulating FGF21, without restricting protein intake. We furthermore aimed to investigate whether a potential dietary regulation of circulating FGF21 would have an impact on glucose and lipid homeostasis in humans. For this purpose, we performed a highly controlled randomized three day cross-over dietary intervention study in healthy men. In order to maximize substrate provision and hence metabolic flux, in particular to the liver but also in peripheral tissues, and even more importantly to avoid a decrease in protein intake, the carbohydrate-rich diet was provided in excess of eucaloric energy requirements. To control for the effect of caloric excess, subjects also ingested a similar hypercaloric fat-rich diet in which fat consisted primarily of unsaturated fatty acids.
2. Methods
2.1. Subjects and diets
Nine men were recruited for the study, which was approved by the Copenhagen Ethics Committee (KF 01 261127) and performed in accordance with the Declaration of Helsinki. Informed written consent was received from each participant prior to study inclusion. All subjects were healthy, moderately physically active, and with no family history of diabetes. This study is part of a larger project aiming at elucidating the effects of different types of diets on metabolism in nine male volunteers (unpublished). In the present study, these subjects ingested a hypercaloric carbohydrate-rich diet (CHO) (80 E% carbohydrate, 11 E% protein, 9 E% fat) and a eucaloric control diet (CON) (62 E% carbohydrates, 14 E% protein, 24 E% fat), reflecting their habitual diet. A hypercaloric high-fat diet, consisting primarily of unsaturated FA (FAT) (10 E% carbohydrate, 12 E% protein, 78 E% fat) was also provided in order to serve as control for the caloric excess. The macronutrient composition of CHO, FAT, and CON are summarized in Supplemental Table S1. All diets were provided for three days in a randomized cross-over design, with each intervention separated by at least three weeks. Before each three-day intervention, all subjects consumed the eucaloric control diet for 5 days to ensure the same conditions before each intervention. All food items were weighed and prepared in the metabolic kitchen. The daily menus were delivered to the subject, who ingested them at home. The CHO diet consisted of carbohydrate-rich food items as bread, pasta, cereals, corn, jam, and juice, with high to moderate glycemic index. The diet was mainly comprised of polysaccharides, with 19% refined sugar and the ratio between glucose and fructose was 1:1 (Supplemental Table S3 and S4). In the FAT diet, mono- and polyunsaturated fatty acids both comprised 34 E% (Supplemental Table S1).
To evaluate the training status of the participants, maximal oxygen uptake was measured by an incremental exercise test on a Monark Ergomedic 839E bicycle ergometer (Monark, Sweden). Body composition and leg mass (LM) were determined using dual-energy X-ray absorptiometry (Lunar DPX-IQ DEXA Scanner, Lunar Corporation, USA) after 4 h of fasting. The nine male subjects were 23 ± 3 (mean ± SD) years old, with a body mass index (BMI) of 23.7 ± 1.7 kg m−2 and maximal oxygen uptake (VO2peak) of 52 ± 4 ml kg−1 min−1.
The daily energy requirements were individually determined from weighed dietary registrations and calculations of energy requirements (WHO/FAO/UNU). The three days CHO and FAT diets were provided in excess of eucaloric energy requirements, with a +75% increase in daily energy intake. Hence, the subjects ingested 13.7 ± 0.2 MJ during the CON diet and according to the hypercaloric energy provision 24.0 ± 0.4 MJ during the experimental diets (Table S1).
2.2. Experimental protocol
After three days on the experimental diets or the control diet, subjects ingested a light standardized breakfast (1.6 MJ) at 5 A.M at home (Supplemental Table S5). Subjects arrived at the institute at 7.30 A.M, and, after a period of supine rest, teflon catheters were inserted in the femoral artery and vein under local anesthesia. Then, a [6,6-2H2] glucose tracer infusion (0.055 mg kg−1 min−1) was administered in order to measure hepatic glucose production. The infusion was initiated by a bolus injection of [6,6-2H2] glucose (3.203 mg kg−1). After 120 min, blood samples were obtained from the femoral artery and vein. Femoral arterial blood flow was determined simultaneously with the arterio-venous blood sampling by a laser ultra-sound Doppler technique (Philips iU22, ViCare Medical, Denmark) [25]. In order to study molecular metabolism in adipose tissue, biopsies were obtained from the subcutaneous periumbilical adipose tissue, immediately frozen in liquid nitrogen and stored at −80 °C. All blood and tissue samples were collected 6 h after the breakfast meal, when subjects were in the post-absorptive state.
2.3. Plasma analyses
Plasma glucose concentration was measured on an ABL615 (Radiometer Medical A/S, Denmark), and insulin concentration was measured by an enzyme-linked immunosorbent assay (ELISA) (ALPCO, USA). The plasma concentrations of FA (NEFA C kit, Wako Chemicals GmbH, Germany) and triacylglycerol (TG) (GPO-PAP kit, Roche Diagnostics, Germany) were measured using enzymatic colorimetric methods (Hitachi 912 automatic analyzer, Boehringer, Germany). Plasma cholesterol concentration was measured by a fluorometric method (Roche Diagnostics, Germany). Plasma epinephrine and norepinephrine concentrations were determined by radioimmunoassay (2-CAT 125I RIA kit, Labor Diagnostika, Germany). Plasma adiponectin concentration was measured on an AutoDELFIA (Perkin Elmer, USA) automated analyzer. Plasma FGF21 concentration was measured by ELISA (FGF21 Quantikine ELISA kit, R&D Systems, USA), in which the standard curve range for the assay was 10–2000 pg ml−1. FGF21 was analyzed in both arterial and venous plasma, with no difference obtained in FGF21 concentration. Plasma enrichments of the stable glucose isotope were measured using liquid chromatography mass spectrometry (ThermoQuest Finnegan AQA, USA) as previously described [26]. For analysis of palmitoleic acid concentration in plasma, lipids were extracted using chloroform/methanol (2:1, v/v) and filtered prior to saponification and methylation using 12% BF3 in methanol. Methylated FA were separated as described in detail elsewhere [27]. The respective FA species were identified by retention time using standard mixtures of methyl esters (Nu-Chek, USA).
2.4. Western blotting
Samples of subcutaneous adipose tissue were homogenized (Tissue Lyzer II, Qiagen, Germany) in ice-cold buffer as described previously [28]. Homogenates were rotated end-over-end for one hour, and lysate supernatants were collected by centrifuging 20 min at 16,000 g at 4 °C. The upper fat layer was carefully removed before the lysate supernatant was collected. Protein concentrations were determined in triplicates using the bicinchoninic acid (BCA) method (Pierce Biotechnology BCA, USA). Samples were heated (96 °C) in Laemmli buffer before subjected to SDS-PAGE and semi-dry blotting. The primary antibodies used are described in Supplemental Table S2. Secondary antibodies were from Dako Cymation (Denmark). Membranes were probed with enhanced chemiluminescence (ECL+; Amersham Biosciences, USA), and immune complexes were visualized using BioRad ChemiDoc™ MP Imaging System (Hercules, USA). Signals were quantified (Image Lab, Life Sciences, USA) and expressed as arbitrary units.
2.5. Calculations
Leg glucose uptake was calculated as the arterial-venous difference multiplied by blood flow in accordance with Fick's principle and related to leg mass (LM). Glucose rate of appearance (Ra) was calculated from the last 30 min of the 120 min tracer infusion period using Steele's steady state equations [29].
2.6. Statistical analyses
All data are expressed as means ± SEM, except characteristics of the subjects (means ± SD). Data from each experimental trial were compared to the control trial by paired t-tests, to test for effect of the respective experimental diets. The strength of association between parameters was analyzed using Pearson correlation analysis.
3. Results and discussion
3.1. Dietary carbohydrate but not caloric excess increases circulating FGF21
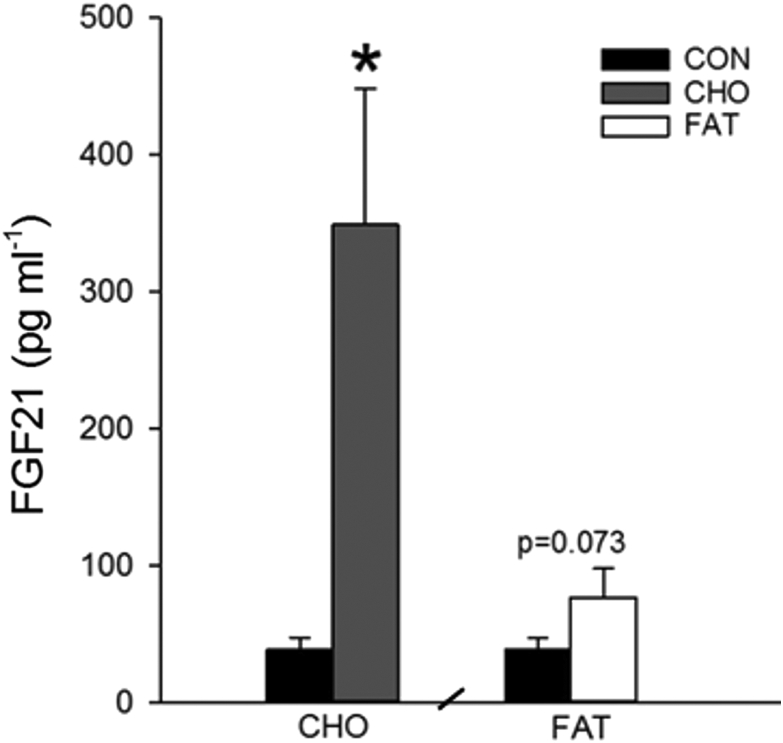
Here we demonstrate in healthy male volunteers that three days consumption of a hypercaloric carbohydrate-rich diet (80 E%) increased the plasma FGF21 concentration 803% compared with CON (p < 0.05) (Figure 1). In contrast, the concentration of FGF21 in plasma only tended (p = 0.073) to be increased after three days consumption of a hypercaloric unsaturated high-fat diet (78 E% fat) (Figure 1). These data underscore that dietary carbohydrates potently induce FGF21 during conditions of non-restricted protein intake, highlighting a novel and important effect of dietary carbohydrates in the regulation of circulating FGF21 in humans. The fact that the FAT diet did not appreciably increase plasma FGF21 concentration indicates that caloric excess per se does not induce FGF21 secretion.
Figure 1.
Effect of the dietary interventions on arterial plasma FGF21 concentration. Data are means ± SEM. Paired t-tests were used. CON: eucaloric control diet, CHO: hypercaloric carbohydrate-rich diet, FAT: hypercaloric high-fat diet. *p < 0.05 compared with CON. n = 9, one subject did not complete the CHO trial, hence n = 8 in CHO. Data were obtained in the post-absorbtive state 6 h after a small 1.6 MJ breakfast (5 A.M).
In mice, FGF21 mRNA is reported to be predominantly expressed in pancreas and liver [30], with markedly lower FGF21 mRNA levels present in human skeletal muscle [31], [32] and subcutaneous and visceral adipose tissue [6], [33]. Elevated circulating levels of FGF21 in mice and humans have mainly been linked to increased FGF21 synthesis in the liver [34], [35]. Thus, the observed increase in plasma FGF21 concentration in the present human study was likely related to induction of FGF21 in the liver.
3.2. Lipid and glucose homeostasis in the context of excess carbohydrate intake and FGF21 induction
Next we aimed to investigate the metabolic effects of the CHO diet and whether the dietary upregulation of circulating FGF21 was associated with changes in glucose and lipid homeostasis.
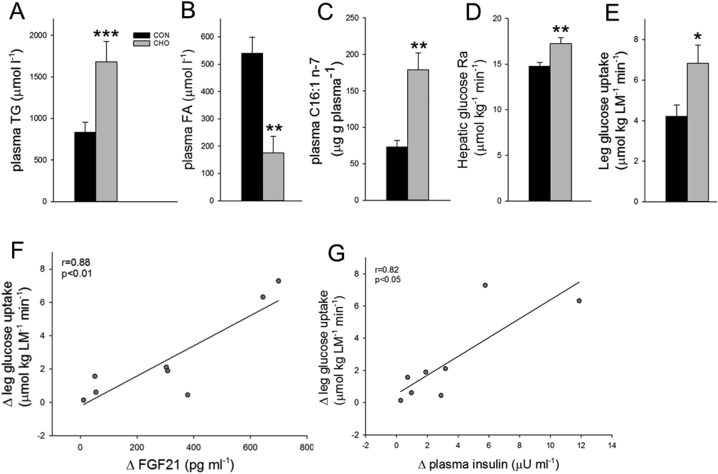
After CHO, the plasma TG concentration was increased by 101% compared with CON (p < 0.001) (Figure 2A) at the same time as the plasma FA concentration was decreased by 68% (Figure 2B). Hence, the increase in plasma TG concentration does not seem to be ascribed to increased hepatic FA supply from plasma. It has been shown in another study in healthy men that 4 days hypercaloric provision of carbohydrates (enterally) increases VLDL-TG secretion rate markedly, in part due to increased secretion of de novo synthesized FA in the liver [36]. Extending these observations to the present study, the obtained increase in plasma TG concentration likely reflected increased VLDL-TG secretion after the several days of high carbohydrate intake. Along this line, we observed a 146% increase in plasma C16:1 FA concentration after CHO (Figure 2C), a marker of de novo lipogenesis [37], indicating an increased hepatic de novo lipogenesis. Interestingly, positive correlations were observed between the increase in plasma FGF21 concentration after CHO and the increase in C16:1 n−7 (r = 0.79, p < 0.05) and the increase in plasma TG (r = 0.70, p < 0.05). Together these findings suggest that the carbohydrate load from the CHO diet caused substantial de novo lipogenesis in the liver. This may contribute to the observed increase in FGF21 secretion, as cross-sectional studies have demonstrated that plasma FGF21 concentration is positively associated with intrahepatic lipid content [24], [38], [39], which is further associated with VLDL-TG secretion rate in healthy subjects [40], thus supporting a relationship between hepatic lipid synthesis and FGF21 secretion. Notably, we observed that total cholesterol (p < 0.01) and LDL-cholesterol (p < 0.01) concentrations in plasma were reduced (Table 1), indicating an improved lipoprotein profile even in the face of the increase in plasma TG concentrations approaching mild hypertriglyceridemia. This is in line with the observation of reduced plasma LDL-cholesterol concentrations in obese humans after FGF21 administration [41].
Figure 2.
Systemic and peripheral lipid and glucose homeostasis after the control diet (CON) and the carbohydrate-rich diet (CHO). A. Arterial plasma triacylglycerol (TG) concentration. B. Arterial fatty acid (FA) concentration. C. Arterial palmitoleic acid concentration. D. Hepatic glucose rate of appearance (Ra). E. Leg glucose uptake expressed per kg leg mass (LM). F + G. Scatter plots illustrating the associations between the change in plasma FGF21 (F) or plasma insulin (G) after CHO and the change in leg glucose uptake. Data are means ± SEM. Paired t-tests were used in A–E. Pearsons correlation analysis was applied in F + G. *p < 0.05, **p < 0.01, ***p < 0.001 compared with CON. n = 9 in CON, one subject did not complete the CHO trial, hence n = 8 in CHO. Data were obtained in the post-absorptive state 6 h after a small 1.6 MJ breakfast (5 A.M).
Table 1.
Arterial plasma parameters. Data are means ± SEM and obtained in the late post-absorptive state 6 h after a small 1.6 MJ breakfast. *p < 0.05, **p < 0.01 compared to CON. n = 9 in CON and n = 8 in CHO. Paired t-tests were applied to test for effect of the CHO diet.
| Arterial plasma parameters | CON | CHO |
|---|---|---|
| Glucose, mmol l−1 | 5.7 ± 0.1 | 5.6 ± 0.0 |
| Insulin, μU ml−1 | 5.0 ± 0.9 | 7.9 ± 1.4* |
| Epinephrine, nmol l−1 | 0.3 ± 0.1 | 0.2 ± 0.1 |
| Norepinephrine, nmol l−1 | 1.3 ± 0.5 | 1.6 ± 0.6 |
| Adiponectin, μg ml−1 | 7.1 ± 1.5 | 7.4 ± 1.1 |
| Total cholesterol, mmol l−1 | 4.2 ± 0.3 | 3.6 ± 0.2** |
| HDL-cholesterol, mmol l−1 | 1.2 ± 0.1 | 1.1 ± 0.1* |
| LDL-cholesterol, mmol l−1 | 2.4 ± 0.3 | 1.8 ± 0.2** |
After CHO, hepatic glucose production was increased by 17% compared with CON (p < 0.01) (Figure 2D), despite slightly increased plasma insulin levels (p < 0.05) (Table 1). The increase in plasma FGF21 after CHO was positively correlated to the increase in hepatic glucose production (r = 0.71, p < 0.05), suggesting a link between FGF21 and hepatic glucoregulation. Our data are supported by findings in mice, in which 8 days administration of FGF21 increased basal hepatic glucose production [42].
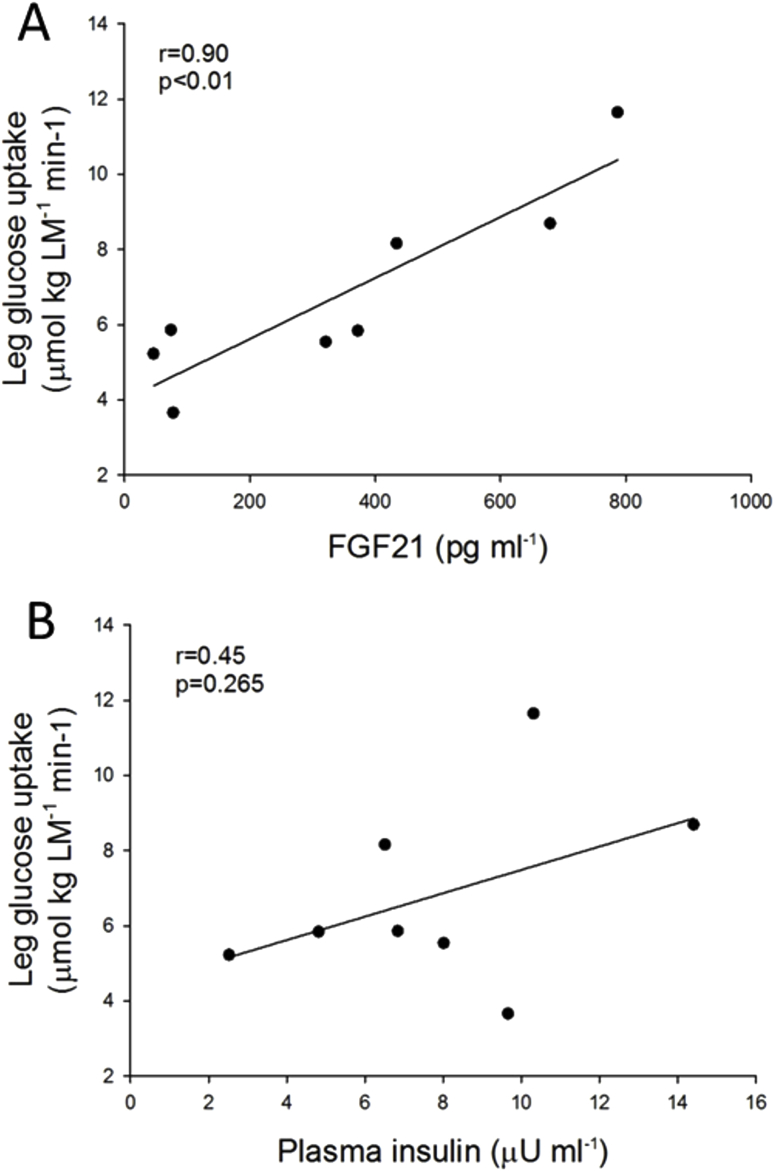
Notably, after CHO the increase in circulating FGF21 was accompanied by a 62% increase in leg glucose uptake compared to CON (p < 0.05) (Figure 2E). This increase in leg glucose uptake was strongly correlated to the increase in plasma FGF21 concentration (r = 0.88, p < 0.01) (Figure 2F). This may imply that FGF21 has direct effects on muscle glucose uptake, in agreement with FGF21 treatment increasing basal glucose uptake in human myotubes [43]. Concomitantly, a small increase in plasma insulin concentration after CHO compared to CON was obtained (7.9 ± 1.4 versus 5.0 ± 0.9 μU ml−1, Table 1), which was also correlated to the increase in leg glucose uptake (r = 0.82, p < 0.05) (Figure 2G). The plasma insulin concentration reported to half-maximally stimulate leg glucose uptake is ∼50 μU ml−1 [44], [45]. Thus, it can be questioned to what extent the 2.9 μU ml−1 increase in plasma insulin, with insulin levels on the lower flat part of the dose-response curve, was responsible for the significant increase in leg glucose uptake after the CHO diet. However, it is possible that the apparent effect of insulin on leg glucose uptake is due to potentiation of the insulin effect by FGF21, as FGF21 has been shown to potentiate the effect of insulin on glucose uptake in human myotubes and mouse muscle [43]. Notably, when the absolute plasma concentrations of FGF21 and insulin after the CHO diet were correlated with leg glucose uptake, in contrast to the delta-values when compared to CON in Figure 2F, G, the plasma FGF21 concentration correlated closely with leg glucose uptake (r = 0.9, p < 0.01), while plasma insulin did not (r = 0.45, p = 0.265) (Supplemental Figure S1). This may suggest that FGF21 rather than insulin is the major regulator of the increase in leg glucose uptake observed in the present study.
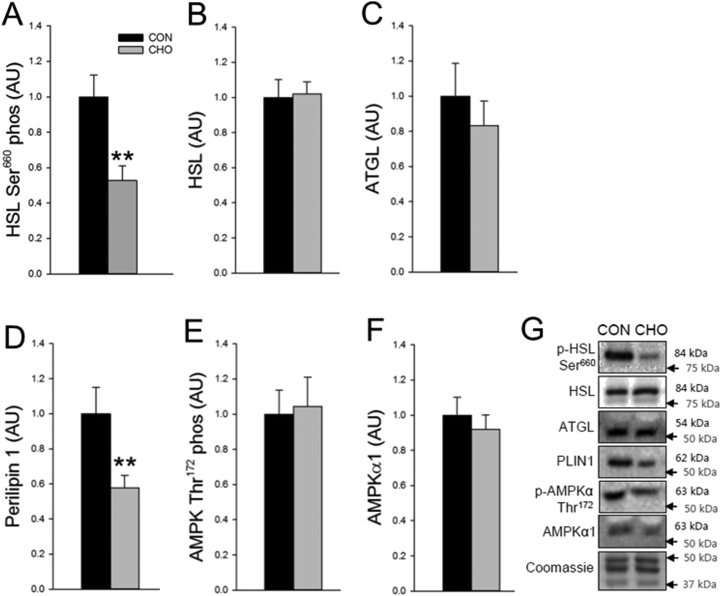
FGF21 has also been suggested to regulate basal glucose uptake in human adipocytes [1], 3T3-L1 adipocytes [46] and mouse adipose tissue [47] via an adiponectin-AMPK dependent pathway [48], [49]. However, the present data provide no indications of an adiponectin-AMPK axis activated in human adipose tissue in response to increased circulating FGF21 (Table 1 and Figure 3E–F).
Figure 3.
Molecular metabolism in subcutaneous adipose tissue after the control (CON) and the carbohydrate-rich diet (CHO). A. Hormone-sensitive lipase (HSL) Ser660 phosphorylation. B. HSL protein content. C. Adipose triglyceride lipase (ATGL) protein content. D. Perilipin 1 protein content. E. AMP-activated protein kinase (AMPK) Thr172 phosphorylation. F. AMPKα1 protein content. G. Representative blots. Data are means ± SEM. Paired t-tests were used in A–F. **p < 0.01 compared with CON. n = 9 in CON, one subject did not complete the CHO trial, hence n = 8 in CHO. Data were obtained in the post absorptive state 6 h after a small 1.6 MJ breakfast (5 A.M).
In the present study, the subjects consumed a small and identical breakfast meal after CON and CHO (Supplemental Table S5) 6 h before blood sampling to ensure a similar post-absorptive state. It has been shown in healthy humans that oral administration of monosaccharides in the form of 75 g fructose or glucose acutely increase plasma FGF21 [21]. 75 g fructose increased FGF21 levels by 3.4 fold at 2 h, whereas 75 g glucose induced a more modest 2-fold increase in plasma FGF21 after 3 h. However, in both situations plasma FGF21 levels were back to baseline after approximately 4 h [21]. Considering the transient character of the acute glucose- and fructose-induced increases in circulating FGF21 [21] and that the half-life of recombinant human FGF21 is 1–2 h [46], [50], it is likely that the substantial 8-fold increase in plasma FGF21 concentration obtained in the post-absorptive state in CHO originates from a more persistent secretory regulation. Thus, this study shows the impact of carbohydrate-rich food items, occurring in a normal habitual diet, on FGF21 regulation in humans. Interestingly, genome-wide association studies in human cohorts have shown an association between SNPs related to the FGF21 locus and carbohydrate intake [51], [52]. A link between FGF21 and dietary carbohydrate consumption is further supported by studies in mice and monkeys, which show that FGF21 knockout increases preference for mono- and disaccharide intake. Moreover, FGF21 overexpression or administration of FGF21 analogs decrease the preference for mono- and disaccharides and artificial sweeteners, with no apparent effect on fat or protein intake [19], [53]. Whether the observed higher plasma FGF21 levels after the CHO diet in the present study affected food preference is a possibility, but was not directly evaluated.
3.3. Inhibition of adipose tissue lipolysis
Three days of FGF21 treatment has been reported to inhibit lipolysis in human adipocytes [54]. Supporting these findings, the increased plasma FGF21 concentration was observed at the same time as plasma FA concentration was markedly decreased in the present study after CHO. We observed in subcutaneous adipose tissue that phosphorylation of the activating Ser660 site on hormone sensitive lipase (HSL) was decreased by 47% (p < 0.01) (Figure 3A) and that the protein content of perilipin 1 was decreased by 29% after CHO (p < 0.01) (Figure 3D). Perilipin 1 is a key regulator of both basal and protein kinase A (PKA)-stimulated lipolysis in adipose tissue, and since HSL translocation to the lipid droplets requires a functional perilipin 1 [55] and perilipin 1 activation is required to stimulate HSL-mediated lipolysis [56], these molecular data collectively support that adipose tissue lipolysis was lowered after CHO. Consistent with our observations, Arner et al. found that the FGF21-induced inhibition of lipolysis in the human primary adipocytes was accompanied by a reduced perilipin 1 protein content [54].
In summary, in humans we here employed a dietary strategy by which the effect of short term overfeeding of carbohydrates revealed a paramount and remarkable stimulating effect on FGF21 secretion, without reduction in the dietary protein content. In contrast, energy excess per se, induced by a hypercaloric fat-rich diet, did not induce a detectable increase in circulating FGF21. Dietary carbohydrate excess led to differential responses in the liver and peripheral tissues. The increase in circulating FGF21 after high carbohydrate intake was associated with indices of increased hepatic lipogenesis and glucose production, and this was observed concomitantly with increased peripheral glucose disposal, with a close correlation between the increase in plasma FGF21 concentration and increased leg glucose uptake. This suggests that FGF21 secretion provided a mechanism for peripheral disposal of the carbohydrate load. Furthermore, the FGF21-mediated suppression of adipose tissue lipolysis could be a mechanism to suppress hepatic FA supply under conditions of increased de novo lipogenesis. Accordingly, we here demonstrate an important role of dietary carbohydrates in the regulation of human FGF21 secretion, and our data further point to effects of FGF21 in human glucose and lipid homeostasis.
Author contributions
BK, EAR, JFPW, AML, and KAS designed the study and carried out the experiments. AML, AMF, KAS, LSM, and LM contributed to the results. AML, AMF, and BK wrote the manuscript. All authors contributed to the manuscript and approved the final version of the manuscript.
Acknowledgements
We acknowledge Louise Dalgas Høeg for contributing in performing experiments, and the skilled technical assistance of Irene Bech Nielsen and Betina Bolmgren (University of Copenhagen). The study was supported by grants from the Danish Ministry of Food, Agriculture and Fisheries (3304-FSE-06-0510), the Danish Medical Research Council, The Lundbeck Research Foundation (R17-A1760), The Novo-Nordisk Research Foundation (13303). The University of Copenhagen Excellence Program for Interdisciplinary Research (2016) “Physical Activity and Nutrition for Improvement of Health”. PhD scholarship of Anne-Marie Lundsgaard and post doc fellowship of Andreas Mæchel Fritzen was funded by the Danish Diabetes Academy, supported by the Novo Nordisk Foundation. Kim Sjøberg was funded by a postdoctoral research grant from the Council for Independent Research/Medicine (4092-00309).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.11.001.
Conflicts of interests
The authors have declared that no conflicts of interests exist.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplemental Figure S1.
Scatter plots illustrating the associations between plasma FGF21 concentration (A) and plasma insulin concentration (B) and leg glucose uptake on the experimental day after the CHO diet. Pearsons correlation analyses were applied.
References
- 1.Kharitonenkov A., Shiyanova T.L., Koester A., Ford A.M., Micanovic R., Galbreath E.J. FGF-21 as a novel metabolic regulator. Journal of Clinical Investigation. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badman M.K., Pissios P., Kennedy A.R., Koukos G., Flier J.S., Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metabolism. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Inagaki T., Dutchak P., Zhao G., Ding X., Gautron L., Parameswara V. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metabolism. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Kharitonenkov A., Adams A.C. Inventing new medicines: the FGF21 story. Molecular Metabolism. 2014;3:221–229. doi: 10.1016/j.molmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher F.M., Maratos-Flier E. Understanding the physiology of FGF21. Annual Review of Physiology. 2016;78:223–241. doi: 10.1146/annurev-physiol-021115-105339. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X., Yeung D.C., Karpisek M., Stejskal D., Zhou Z.G., Liu F. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 7.Chen W.W., Li L., Yang G.Y., Li K., Qi X.Y., Zhu W. Circulating FGF-21 levels in normal subjects and in newly diagnose patients with Type 2 diabetes mellitus. Experimental and Clinical Endocrinology & Diabetes. 2008;116:65–68. doi: 10.1055/s-2007-985148. [DOI] [PubMed] [Google Scholar]
- 8.Stone K.P., Wanders D., Orgeron M., Cortez C.C., Gettys T.W. Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes. 2014;63:3721–3733. doi: 10.2337/db14-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Sousa-Coelho A.L., Marrero P.F., Haro D. Activating transcription factor 4-dependent induction of FGF21 during amino acid deprivation. Biochemical Journal. 2012;443:165–171. doi: 10.1042/BJ20111748. [DOI] [PubMed] [Google Scholar]
- 10.Pissios P., Hong S., Kennedy A.R., Prasad D., Liu F.F., Maratos-Flier E. Methionine and choline regulate the metabolic phenotype of a ketogenic diet. Molecular Metabolism. 2013;2:306–313. doi: 10.1016/j.molmet.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maida A., Zota A., Sjoberg K.A., Schumacher J., Sijmonsma T.P., Pfenninger A. A liver stress-endocrine nexus promotes metabolic integrity during dietary protein dilution. Journal of Clinical Investigation. 2016 doi: 10.1172/JCI85946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laeger T., Henagan T.M., Albarado D.C., Redman L.M., Bray G.A., Noland R.C. FGF21 is an endocrine signal of protein restriction. Journal of Clinical Investigation. 2014;124(9):3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bray G.A., Smith S.R., de J.L., Xie H., Rood J., Martin C.K. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA. 2012;307:47–55. doi: 10.1001/jama.2011.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller T.D., Tschop M.H. Play down protein to play up metabolism? Journal of Clinical Investigation. 2014;124(9):3691–3693. doi: 10.1172/JCI77508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez J., Palou A., Pico C. Response to carbohydrate and fat refeeding in the expression of genes involved in nutrient partitioning and metabolism: striking effects on fibroblast growth factor-21 induction. Endocrinology. 2009;150:5341–5350. doi: 10.1210/en.2009-0466. [DOI] [PubMed] [Google Scholar]
- 16.Iizuka K., Takeda J., Horikawa Y. Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS Letters. 2009;583:2882–2886. doi: 10.1016/j.febslet.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 17.Benhamed F., Denechaud P.D., Lemoine M., Robichon C., Moldes M., Bertrand-Michel J. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. Journal of Clinical Investigation. 2012;122:2176–2194. doi: 10.1172/JCI41636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uebanso T., Taketani Y., Yamamoto H., Amo K., Ominami H., Arai H. Paradoxical regulation of human FGF21 by both fasting and feeding signals: is FGF21 a nutritional adaptation factor? PLoS One. 2011;6:e22976. doi: 10.1371/journal.pone.0022976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Holstein-Rathlou S., BonDurant L.D., Peltekian L., Naber M.C., Yin T.C., Claflin K.E. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metabolism. 2015 doi: 10.1016/j.cmet.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solon-Biet S.M., Cogger V.C., Pulpitel T., Heblinski M., Wahl D., McMahon A.C. Defining the nutritional and metabolic context of FGF21 using the geometric framework. Cell Metabolism. 2016;24:555–565. doi: 10.1016/j.cmet.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Dushay J.R., Toschi E., Mitten E.K., Fisher F.M., Herman M.A., Maratos-Flier E. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Molecular Metabolism. 2015;4:51–57. doi: 10.1016/j.molmet.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galman C., Lundasen T., Kharitonenkov A., Bina H.A., Eriksson M., Hafstrom I. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metabolism. 2008;8:169–174. doi: 10.1016/j.cmet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Fazeli P.K., Lun M., Kim S.M., Bredella M.A., Wright S., Zhang Y. FGF21 and the late adaptive response to starvation in humans. Journal of Clinical Investigation. 2015;125:4601–4611. doi: 10.1172/JCI83349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dushay J., Chui P.C., Gopalakrishnan G.S., Varela-Rey M., Crawley M., Fisher F.M. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139:456–463. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sjoberg K.A., Rattigan S., Hiscock N., Richter E.A., Kiens B. A new method to study changes in microvascular blood volume in muscle and adipose tissue: real-time imaging in humans and rat. American Journal of Physiology – Heart and Circulatory Physiology. 2011;301:H450–H458. doi: 10.1152/ajpheart.01174.2010. [DOI] [PubMed] [Google Scholar]
- 26.Borno A., Foged L., van H.G. Glucose and glycerol concentrations and their tracer enrichment measurements using liquid chromatography tandem mass spectrometry. Journal of Mass Spectrometry. 2014;49:980–988. doi: 10.1002/jms.3407. [DOI] [PubMed] [Google Scholar]
- 27.Lie O., Lambertsen G. Fatty acid composition of glycerophospholipids in seven tissues of cod (Gadus morhua), determined by combined high-performance liquid chromatography and gas chromatography. Journal of Chromatography. 1991;565:119–129. doi: 10.1016/0378-4347(91)80376-n. [DOI] [PubMed] [Google Scholar]
- 28.Fritzen A.M., Lundsgaard A.M., Jordy A.B., Poulsen S.K., Stender S., Pilegaard H. New nordic diet-induced weight loss is accompanied by changes in metabolism and AMPK signaling in adipose tissue. Journal of Clinical Endocrinology & Metabolism. 2015;100(9):3509–3519. doi: 10.1210/jc.2015-2079. [DOI] [PubMed] [Google Scholar]
- 29.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Annals of the New York Academy of Sciences. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 30.Adams A.C., Coskun T., Cheng C.C., LS OF, Dubois S.L., Kharitonenkov A. Fibroblast growth factor 21 is not required for the antidiabetic actions of the thiazoladinediones. Molecular Metabolism. 2013;2:205–214. doi: 10.1016/j.molmet.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hojman P., Pedersen M., Nielsen A.R., Krogh-Madsen R., Yfanti C., Akerstrom T. Fibroblast growth factor-21 is induced in human skeletal muscles by hyperinsulinemia. Diabetes. 2009;58:2797–2801. doi: 10.2337/db09-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vienberg S.G., Brons C., Nilsson E., Astrup A., Vaag A., Andersen B. Impact of short-term high-fat feeding and insulin-stimulated FGF21 levels in subjects with low birth weight and controls. European Journal of Endocrinology. 2012;167:49–57. doi: 10.1530/EJE-12-0039. [DOI] [PubMed] [Google Scholar]
- 33.Mraz M., Bartlova M., Lacinova Z., Michalsky D., Kasalicky M., Haluzikova D. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clinical Endocrinology (Oxford) 2009;71(3):369–375. doi: 10.1111/j.1365-2265.2008.03502.x. [DOI] [PubMed] [Google Scholar]
- 34.Markan K.R., Naber M.C., Ameka M.K., Anderegg M.D., Mangelsdorf D.J., Kliewer S.A. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63(12):4057–4063. doi: 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen J.S., Clemmesen J.O., Secher N.H., Hoene M., Drescher A., Weigert C. Glucagon-to-insulin ratio is pivotal for splanchnic regulation of FGF-21 in humans. Molecular Metabolism. 2015;4(8):551–560. doi: 10.1016/j.molmet.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aarsland A., Chinkes D., Wolfe R.R. Contributions of de novo synthesis of fatty acids to total VLDL-triglyceride secretion during prolonged hyperglycemia/hyperinsulinemia in normal man. Journal of Clinical Investigation. 1996;98(9):2008–2017. doi: 10.1172/JCI119005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J.J., Lambert J.E., Hovhannisyan Y., Ramos-Roman M.A., Trombold J.R., Wagner D.A. Palmitoleic acid is elevated in fatty liver disease and reflects hepatic lipogenesis. American Journal of Clinical Nutrition. 2015;101:34–43. doi: 10.3945/ajcn.114.092262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H., Fang Q., Gao F., Fan J., Zhou J., Wang X. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. Journal of Hepatology. 2010;53:934–940. doi: 10.1016/j.jhep.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 39.Yilmaz Y., Eren F., Yonal O., Kurt R., Aktas B., Celikel C.A. Increased serum FGF21 levels in patients with nonalcoholic fatty liver disease. European Journal of Clinical Investigation. 2010;40:887–892. doi: 10.1111/j.1365-2362.2010.02338.x. [DOI] [PubMed] [Google Scholar]
- 40.Fabbrini E., Mohammed B.S., Magkos F., Korenblat K.M., Patterson B.W., Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134(2):424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaich G., Chien J.Y., Fu H., Glass L.C., Deeg M.A., Holland W.L. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metabolism. 2013;18(3):333–340. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Berglund E.D., Li C.Y., Bina H.A., Lynes S.E., Michael M.D., Shanafelt A.B. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology. 2009;150(9):4084–4093. doi: 10.1210/en.2009-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mashili F.L., Austin R.L., Deshmukh A.S., Fritz T., Caidahl K., Bergdahl K. Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes and obesity. Diabetes/Metabolism Research and Reviews. 2011;27:286–297. doi: 10.1002/dmrr.1177. [DOI] [PubMed] [Google Scholar]
- 44.Richter E.A., Kiens B., Mizuno M., Strange S. Insulin action in human thighs after one-legged immobilization. Journal of Applied Physiology (1985) 1989;67:19–23. doi: 10.1152/jappl.1989.67.1.19. [DOI] [PubMed] [Google Scholar]
- 45.Wojtaszewski J.F., Nielsen J.N., Richter E.A. Invited review: effect of acute exercise on insulin signaling and action in humans. Journal of Applied Physiology (1985) 2002;93:384–392. doi: 10.1152/japplphysiol.00043.2002. [DOI] [PubMed] [Google Scholar]
- 46.Xu J., Stanislaus S., Chinookoswong N., Lau Y.Y., Hager T., Patel J. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models–association with liver and adipose tissue effects. American Journal of Physiology. Endocrinology and Metabolism. 2009;297(5):E1105–E1114. doi: 10.1152/ajpendo.00348.2009. [DOI] [PubMed] [Google Scholar]
- 47.Emanuelli B., Vienberg S.G., Smyth G., Cheng C., Stanford K.I., Arumugam M. Interplay between FGF21 and insulin action in the liver regulates metabolism. Journal of Clinical Investigation. 2014;124(2):515–527. doi: 10.1172/JCI67353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Z., Tian H., Lam K.S., Lin S., Hoo R.C., Konishi M. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metabolism. 2013;17(5):779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Holland W.L., Adams A.C., Brozinick J.T., Bui H.H., Miyauchi Y., Kusminski C.M. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metabolism. 2013;17(5):790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kharitonenkov A., Wroblewski V.J., Koester A., Chen Y.F., Clutinger C.K., Tigno X.T. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148(2):774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 51.Chu A.Y., Workalemahu T., Paynter N.P., Rose L.M., Giulianini F., Tanaka T. Novel locus including FGF21 is associated with dietary macronutrient intake. Human Molecular Genetics. 2013;22:1895–1902. doi: 10.1093/hmg/ddt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka T., Ngwa J.S., van Rooij F.J., Zillikens M.C., Wojczynski M.K., Frazier-Wood A.C. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. American Journal of Clinical Nutrition. 2013;97:1395–1402. doi: 10.3945/ajcn.112.052183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talukdar S., Owen B.M., Song P., Hernandez G., Zhang Y., Zhou Y. FGF21 regulates sweet and alcohol preference. Cell Metabolism. 2016;23:344–349. doi: 10.1016/j.cmet.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arner P., Pettersson A., Mitchell P.J., Dunbar J.D., Kharitonenkov A., Ryden M. FGF21 attenuates lipolysis in human adipocytes - a possible link to improved insulin sensitivity. FEBS Letters. 2008;582:1725–1730. doi: 10.1016/j.febslet.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 55.Sztalryd C., Xu G., Dorward H., Tansey J.T., Contreras J.A., Kimmel A.R. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. Journal of Cell Biology. 2003;161:1093–1103. doi: 10.1083/jcb.200210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miyoshi H., Souza S.C., Zhang H.H., Strissel K.J., Christoffolete M.A., Kovsan J. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. Journal of Biological Chemistry. 2006;281:15837–15844. doi: 10.1074/jbc.M601097200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.