Abstract
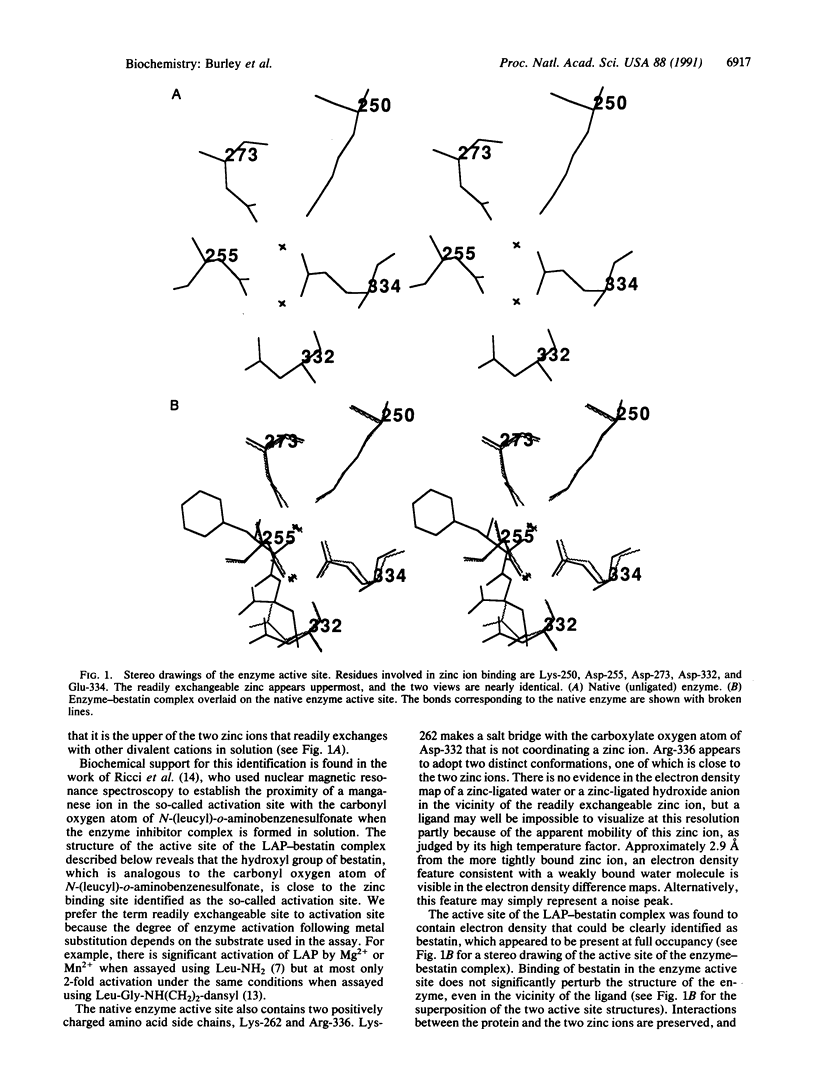
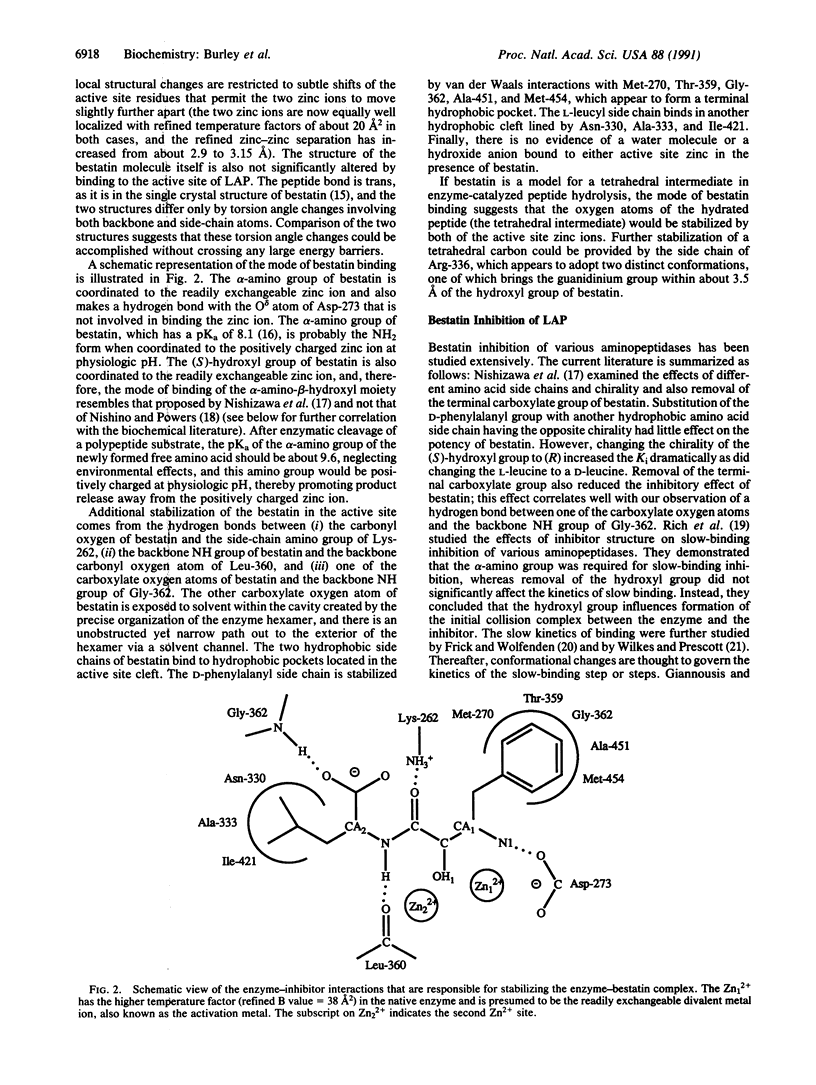
The three-dimensional structures of native bovine lens leucine aminopeptidase (EC 3.4.11.1) and its complex with bestatin, a slow-binding inhibitor, have been solved and exhaustively refined. The mode of binding of bestatin to leucine aminopeptidase may be similar to that of a tetrahedral intermediate that is thought to form during peptide bond hydrolysis. Bestatin binds in the active site with its alpha-amino group and hydroxyl group coordinated to the zinc ion located in the readily exchangeable divalent cation binding site. Its phenylalanyl side chain is stabilized by van der Waals interactions with Met-270, Thr-359, Gly-362, Ala-451, and Met-454, which appear to form a terminal hydrophobic pocket. The leucyl side chain binds in another hydrophobic cleft lined by Asn-330, Ala-333, and Ile-421. Hydrogen bonds involving active site residues Lys-262, Asp-273, Gly-360, and Leu-362 are responsible for stabilizing the backbone nitrogen and oxygen atoms of bestatin. The mode of bestatin inhibition of leucine aminopeptidase is discussed and correlated with biochemical studies of bestatin analogues. In addition, features of a mechanism of catalysis of peptide hydrolysis by leucine aminopeptidase are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burley S. K., David P. R., Taylor A., Lipscomb W. N. Molecular structure of leucine aminopeptidase at 2.7-A resolution. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6878–6882. doi: 10.1073/pnas.87.17.6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter F. H., Harrington K. T. Intermolecular cross-linking of monomeric proteins and cross-linking of oligomeric proteins as a probe of quaternary structure. Application to leucine aminopeptidase (bovine lens). J Biol Chem. 1972 Sep 10;247(17):5580–5586. [PubMed] [Google Scholar]
- Carpenter F. H., Vahl J. M. Leucine aminopeptidase (Bovine lens). Mechanism of activation by Mg 2+ and Mn 2+ of the zinc metalloenzyme, amino acid composition, and sulfhydryl content. J Biol Chem. 1973 Jan 10;248(1):294–304. [PubMed] [Google Scholar]
- Frick L., Wolfenden R. Mechanistic implications of the inhibition of peptidases by amino aldehydes and bestatin. Biochim Biophys Acta. 1985 Jul 1;829(3):311–318. doi: 10.1016/0167-4838(85)90238-9. [DOI] [PubMed] [Google Scholar]
- Giannousis P. P., Bartlett P. A. Phosphorus amino acid analogues as inhibitors of leucine aminopeptidase. J Med Chem. 1987 Sep;30(9):1603–1609. doi: 10.1021/jm00392a014. [DOI] [PubMed] [Google Scholar]
- Gordon E. M., Godfrey J. D., Delaney N. G., Asaad M. M., Von Langen D., Cushman D. W. Design of novel inhibitors of aminopeptidases. Synthesis of peptide-derived diamino thiols and sulfur replacement analogues of bestatin. J Med Chem. 1988 Nov;31(11):2199–2211. doi: 10.1021/jm00119a023. [DOI] [PubMed] [Google Scholar]
- Harbeson S. L., Rich D. H. Inhibition of arginine aminopeptidase by bestatin and arphamenine analogues. Evidence for a new mode of binding to aminopeptidases. Biochemistry. 1988 Sep 20;27(19):7301–7310. doi: 10.1021/bi00419a019. [DOI] [PubMed] [Google Scholar]
- Henson H., Frohne M. Crystalline leucine aminopeptidase from lens (alpha-aminoacyl-peptide hydrolase; EC 3.4.11.1). Methods Enzymol. 1976;45:504–520. doi: 10.1016/s0076-6879(76)45045-0. [DOI] [PubMed] [Google Scholar]
- Liang J., Lipscomb W. N. Binding of sulfonamide and acetamide to the active-site Zn2+ in carbonic anhydrase: a theoretical study. Biochemistry. 1989 Dec 12;28(25):9724–9733. doi: 10.1021/bi00451a028. [DOI] [PubMed] [Google Scholar]
- Lin W. Y., Lin S. H., Van Wart H. E. Steady-state kinetics of hydrolysis of dansyl-peptide substrates by leucine aminopeptidase. Biochemistry. 1988 Jul 12;27(14):5062–5068. doi: 10.1021/bi00414a017. [DOI] [PubMed] [Google Scholar]
- Nishino N., Powers J. C. Design of potent reversible inhibitors for thermolysin. Peptides containing zinc coordinating ligands and their use in affinity chromatography. Biochemistry. 1979 Oct 2;18(20):4340–4347. doi: 10.1021/bi00587a012. [DOI] [PubMed] [Google Scholar]
- Nishizawa R., Saino T., Takita T., Suda H., Aoyagi T. Synthesis and structure-activity relationships of bestatin analogues, inhibitors of aminopeptidase B. J Med Chem. 1977 Apr;20(4):510–515. doi: 10.1021/jm00214a010. [DOI] [PubMed] [Google Scholar]
- Ocain T. D., Rich D. H. Synthesis of sulfur-containing analogues of bestatin. Inhibition of aminopeptidases by alpha-thiolbestatin analogues. J Med Chem. 1988 Nov;31(11):2193–2199. doi: 10.1021/jm00119a022. [DOI] [PubMed] [Google Scholar]
- Rich D. H., Moon B. J., Harbeson S. Inhibition of aminopeptidases by amastatin and bestatin derivatives. Effect of inhibitor structure on slow-binding processes. J Med Chem. 1984 Apr;27(4):417–422. doi: 10.1021/jm00370a001. [DOI] [PubMed] [Google Scholar]
- Suda H., Takita T., Aoyagi T., Umezawa H. The structure of bestatin. J Antibiot (Tokyo) 1976 Jan;29(1):100–101. doi: 10.7164/antibiotics.29.100. [DOI] [PubMed] [Google Scholar]
- Taylor A., Sawan S., James T. L. Structural aspects of the inhibitor complex formed by N-(leucyl)-o-aminobenzenesulfonate and manganese with Zn2+-Mn2+ leucine aminopeptidase (EC 3.4.11.1). Studies by NMR. J Biol Chem. 1982 Oct 10;257(19):11571–11576. [PubMed] [Google Scholar]
- Umezawa H., Aoyagi T., Suda H., Hamada M., Takeuchi T. Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes. J Antibiot (Tokyo) 1976 Jan;29(1):97–99. doi: 10.7164/antibiotics.29.97. [DOI] [PubMed] [Google Scholar]
- Wilkes S. H., Prescott J. M. The slow, tight binding of bestatin and amastatin to aminopeptidases. J Biol Chem. 1985 Oct 25;260(24):13154–13162. [PubMed] [Google Scholar]



