Abstract
Objective
Zfp423 is a multi zinc-finger transcription factor expressed in preadipocytes and mature adipocytes in vivo. Our recent work has revealed a critical role for Zfp423 in maintaining the fate of white adipocytes in adult mice through suppression of the beige cell thermogenic gene program; loss of Zfp423 in mature adipocytes of adult mice results in a white-to-beige phenotypic switch. However, the exact requirements of Zfp423 in the fetal stages of early adipose development in vivo have not been clarified.
Method
Here, we utilize two models that confer adipose-specific Zfp423 inactivation during fetal adipose development (Adiponectin-Cre; Zfp423loxP/loxP and Adiponectin-rtTA; TRE-Cre; Zfp423loxP/loxP). We assess the impact of fetal adipose Zfp423 deletion on the initial formation of adipose tissue and evaluate the metabolic consequences of challenging these animals with high-fat diet feeding.
Results
Deletion of Zfp423 during fetal adipose development results in a different phenotype than is observed when deleting Zfp423 in adipocytes of adult mice. Inactivation of Zfp423 during fetal adipose development results in arrested differentiation, specifically of inguinal white adipocytes, rather than a white-to-beige phenotypic switch that occurs when Zfp423 is inactivated in adult mice. This is likely explained by the observation that adiponectin driven Cre expression is active at an earlier stage of the adipocyte life cycle during fetal subcutaneous adipose development than in adult mice. Upon high-fat diet feeding, obese adipose Zfp423-deficient animals undergo a pathological adipose tissue expansion, associated with ectopic lipid deposition and systemic insulin resistance.
Conclusions
Our results reveal that Zfp423 is essential for the terminal differentiation of subcutaneous white adipocytes during fetal adipose tissue development. Moreover, our data highlight the striking adverse effects of pathological subcutaneous adipose tissue remodeling on visceral adipose function and systemic nutrient homeostasis in obesity. Importantly, these data reveal the distinct phenotypes that can occur when adiponectin driven transgenes are activated in fetal vs. adult adipose tissue.
Keywords: Adipogenesis, Zfp423, Pparg, Subcutaneous adipocytes, Preadipocytes, Obesity, Insulin resistance
Highlights
-
•
Fetal adipose deletion of Zfp423 disrupts terminal differentiation of white adipocytes.
-
•
Inguinal WAT of Zfp423-deficient mice undergoes a pathological expansion in obesity.
-
•
Pathological expansion of subcutaneous adipose triggers systemic insulin resistance.
1. Introduction
White adipocytes are the primary cell type in mammals for safe energy storage in the form of triglyceride. These cells are characterized by their large single (unilocular) lipid-droplet appearance and the presence of the enzymatic machinery to synthesize triglyceride for storage in times of energy surplus. White adipocytes also produce hormones and circulating cytokines (termed adipokines) that regulate food intake and nutrient homeostasis [1]. Brown and beige adipocytes are heat-producing, or “thermogenic,” adipocytes that are characterized by their abundance of mitochondria and ability to burn stored energy to produce heat [2], [3]. This thermogenic capacity is mostly mediated by uncoupling protein 1 (Ucp1). Ucp1 is a transport protein that sits within the inner mitochondrial membrane and facilitates a proton leak [4].
The importance of white adipose tissue is highlighted by two seemingly disparate conditions. An absence of adipose tissue, or “lipodystrophy,” results in the loss of protective adipokines (e.g. adiponectin and leptin) and ectopic lipid accumulation in non-adipose tissues such as the liver, muscle, and pancreas. The latter is deleterious to nutrient homeostasis, as excessive accumulation of lipid species can trigger insulin resistance and cardiovascular disease [5]. Increased adiposity, as seen in the condition of obesity, also confers significant risk for developing the same chronic disorders, including insulin resistance, diabetes mellitus, cardiovascular disease, and cancer. However, a significant portion of obese individuals appears relatively resistant to acquiring metabolic syndrome, at least for a period of time [6], [7], [8]. This suggests that additional factors, outside of increased adiposity per se, determine metabolic health in obesity.
One clear predictor of metabolic disease in the setting of obesity is the cellularity of adipose depots [9], [10]. Adipose tissue expansion in obesity, in principle, can occur through increased lipid-loading into existing adipocytes, leading to increased cell size, or “adipocyte hypertrophy.” Adipocyte hypertrophy is characteristic of adipose tissue in obese individuals with metabolic syndrome. The prevailing theory is that engorged fat cells outstrip their vascular supply, leading to local hypoxia, fibrosis, adipocyte dysfunction, and cell death [11], [12], [13], [14], [15], [16]. Similar to what is observed in lipodystrophy, excess lipid is deposited in peripheral non-adipose tissues that are incapable of safely storing excessive amounts of triglyceride.
Increases in fat cell number, or “adipocyte hyperplasia,” can also occur in obese individuals, resulting in the accumulation of more numerous adipocytes at the expense of large hypertrophic adipocytes. “Healthy-obese” individuals, in comparison to BMI-matched individuals with metabolic syndrome, often exhibit an adipose phenotype characterized by smaller and more numerous adipocytes. This strongly correlates with low inflammation, fibrosis, and preservation of circulating levels of the insulin-sensitizing hormone adiponectin [17], [18], [19]. The storage of triglyceride over numerous adipocytes is believed to help limit the loss of adipocyte function. These observations imply that adipocyte recruitment is protective in obesity and that a pathological hypertrophic adipose expansion in obesity effectively creates a “partial lipodystrophic” phenotype.
Clinical studies also point to the location of excess adipose accumulation as one of the best predictors of metabolic health. Individuals who preferentially store excess adiposity in the visceral compartment are at a greater risk than those who accumulate adipose tissue in the subcutaneous region [20]. In fact, subcutaneous adipose tissue appears protective against insulin resistance [21]. Some engineered mouse models preferentially accumulate subcutaneous WAT and exhibit a metabolically healthy obese phenotype [22], [23]. Thus, the preservation of functional subcutaneous WAT is widely believed to be critical for the maintenance of whole body metabolic homeostasis. However, the precise requirements of functional subcutaneous WAT in vivo have not been directly assessed, in large part because genetic tools to manipulate WAT in a depot-selective manner have been lacking.
The main transcriptional machinery that governs adipogenesis has been carefully elucidated, largely using cell culture models [24], [25], [26]. The nuclear hormone receptor, Pparγ, represents the key regulator of adipocyte differentiation [27]. We have previously identified the multi-C2H2 zinc-finger transcription factor, Zfp423, as a regulator of multiple stages of the adipocyte life cycle. Zfp423 expression identifies committed peri-endothelial preadipocytes that reside in the adipose tissue vasculature and contribute to white adipocyte hyperplasia associated with high-fat diet feeding [28], [29]. In cells, Zfp423 regulates preadipocyte commitment and differentiation, at least in part through regulation of preadipocyte levels of Pparγ [30]. Zfp423 is also expressed in fully differentiated adipocytes; however, expression is higher in white adipocytes as compared to brown adipocytes. In mature white adipocytes, Zfp423 functions to suppress the thermogenic gene program, thereby maintaining the white adipocyte identity of these cells [31].
Genetic evidence supporting a critical role for Zfp423 in maintaining the white adipose lineage comes from our recent study in which we utilized a doxycycline-inducible system for Cre-mediated inactivation of Zfp423 in adult adipocytes (Adiponectin-rtTA; TRE-Cre; Zfp423loxP/loxP; “Zfp423-iAKO” mice) [31]. Inducible adipocyte-specific inactivation of Zfp423 in adult mice leads to a robust conversion of mature white adipocytes into thermogenic, beige-like cells. These data revealed that Zfp423 is required in differentiated white adipocytes to suppress the thermogenic gene program and maintain the white adipocyte identity. However, clear genetic evidence supporting a role for Zfp423 in the initial formation of adipocytes has been lacking. Germline inactivation of Zfp423 (Zfp423−/−) in mice results in malformation of the central nervous system and perinatal death [32], [33], [34]. Analysis of Zfp423−/− embryos (embryonic day 18.5) revealed a severe defect in interscapular brown adipose tissue (BAT) formation and a reduction in the numbers of nascent subdermal white adipocytes present at this stage [30]. However, the importance of Zfp423 to white adipose depot development could not be fully clarified given the other confounding phenotypes in this model.
In this study, we reveal that the loss of adipose Zfp423 during fetal WAT development leads to arrested terminal differentiation of inguinal white adipocytes by the time of birth. Upon high-fat diet feeding as adults, Zfp423-deficient animals become obese but exhibit a metabolic phenotype similar to that of partial lipodystrophy, characterized by pathological inguinal WAT expansion, hepatic steatosis, and systemic insulin resistance. This developmental defect can be rescued by administering the Pparγ ligand, rosiglitazone, specifically during the fetal and early postnatal period. All together, these data define Zfp423 as a critical regulator of adipose development and illustrate the systemic metabolic consequences of pathological subcutaneous adipose tissue expansion in obesity.
2. Materials and methods
2.1. Animals
The Pdgfrα-Cre (stock# 013148), Adiponectin-Cre (stock# 010803), and Rosa26RloxP-stop-loxP-tdtomato transgenic strains were from Jackson lab. Zfp423loxP/loxP mice were a generous gift from Dr. S. Warming (Genentech, Inc.) [34]. AdiponectinrtTA and TRE-Cre transgenic mice have been described previously [35]. Mice were maintained with a 12-hour light/dark cycle and free access to food and water. All animal experiments were performed according to procedures approved by the UTSW Institutional Animal Care and Use Committee.
2.2. Histological analysis
Tissues were dissected and fixed in 4% paraformaldehyde overnight. Paraffin processing, embedding, sectioning, and standard hematoxylin and eosin (H&E) staining were performed at the Molecular Pathology Core Facility at UTSW. Bright-field and fluorescent images were acquired using Keyence BZ-X710 microscope. Adipocyte size analysis was conducted following published protocols with minor modifications [36]. Keyence BZ-X Analyzer software was used for analysis based on bright-field images of H&E-stained paraffin sections. >300 adipocytes were quantified in each individual animal.
2.3. Indirect immunofluorescence
The following antibodies and concentrations were used: guinea pig anti-perilipin 1:1500 (Fitzgerald 20R-PP004); rabbit anti-F4/80 1:500 (Santa Cruz M-300); donkey anti-rabbit Alexa 647 1:200 (Invitrogen); and goat anti-guinea pig Alexa 488 1:200 (Invitrogen). Paraffin sections were dewaxed and hydrated in Xylene and 100%–95%–80%–70%–50% Ethanol and ddH2O. Slides were placed in chambers containing 1× R-Buffer Buffer A pH 6.0 solution and antigen retrieval was done using Antigen Retriever 2100 (Electron Microscopy Sciences) for 2 h. Following PBS wash for 5 min, Fx Signal Enhancer (Invitrogen) was added to the slides for 30 min at room temperature. Slides were then blocked for 30 min in PBS containing 10% normal goat serum at room temperature. Primary antibodies were then diluted in PBS containing 10% normal goat serum and added to paraffin sections overnight at 4 °C. Following overnight incubation, slides were washed in PBS and incubated with secondary antibodies diluted in PBS containing 10% normal goat serum for 2 h at room temperature. Washed slides were mounted with Prolong Anti-Fade mounting medium containing DAPI (Invitrogen) before images were acquired for analysis.
2.4. In vivo insulin stimulation
After 6-hour fasting, animals were anesthetized and injected with insulin (1 U/kg for chow-fed mice; 2 U/kg for HFD-fed mice) through the portal vein. 5 min after the injection, iWAT, gWAT, and liver were quickly snap-frozen for subsequent analysis. Samples harvested before insulin injection were used as untreated controls.
2.5. Immunoblotting and antibodies
Protein extracts from cells or tissues were prepared by homogenization in RIPA lysis buffer (Santa Cruz). Protein extracts were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto PVDF membrane (Millipore). After incubation with the indicated primary antibodies at 4 °C overnight, the blots were incubated with IR Dye-coupled secondary antibodies (LI-COR) and visualized by the LI-COR Odyssey infrared imaging system. The anti-pSer473-Akt (#9271) and anti-Akt (#9272) antibodies were from Cell Signaling. The anti-Adiponectin antibody was from Dr. P.E. Scherer (UTSW).
2.6. Isolation of adipose stromal vascular fraction (SVF) and in vitro differentiation
White fat tissues dissected from mice (4–5 weeks of age) were washed in PBS, minced thoroughly, and digested in vacuum filtered digestion buffer (100 mM Hepes PH 7.4, 120 mM NaCl, 50 mM KCl, 5 mM glucose, 1 mM CaCl2, 1.5% bovine serum albumin, and 1 mg ml−1 collagenase D) for 2 h at 37 °C with shaking. Digested tissues were then filtered through 100 μM cell strainers to remove undigested tissues. The flow-through was centrifuged for 5 min at 600 g to pellet the stromal vascular cells. The floating adipocyte layer was discarded and the stromal vascular cells were re-suspended in growth media containing DMEM/F12 (Invitrogen) plus 10% FBS. For in vitro differentiation, SVF cells isolated by collagenase digestion were plated onto collagen-coated dishes and cultured in 10% CO2 at 37 °C to reach confluency. Confluent cultures were stimulated with adipogenic cocktail (growth media supplemented with 5 μg ml−1 insulin, 1 μM dexamethasone, 0.5 mM isobutylmethyxanthine, and 1 μM rosiglitazone when indicated) for 48 h. Subsequently, cells were maintained in growth media supplemented with 5 μg ml−1 insulin until harvest.
2.7. Flow cytometry analysis
Stromal vascular fraction cells from either inguinal or gonadal WAT depots were first incubated on ice for 20 min in 2% FBS/PBS containing anti-mouse CD16/CD32 Fc Block (clone 2.4G2) (1:200). Cells were then incubated with primary antibody (anti-CD31 clone 390 1:200, anti-CD45 clone 30-F11 1:200, anti-Pdgfrα clone 16A1 1:100), incubated rotating at 4 °C for 30 min, washed three times with 2% FBS/PBS, and then analyzed using a FACSCalibur™ flow cytometer at UT Southwestern Medical Center Flow Cytometry Core Facility. All antibodies were obtained commercially from BioLegend (San Diego, CA USA).
2.8. In vitro and ex vivo lipolysis assay
In vitro lipolysis was performed on SVF-derived adipocytes at day 8 after differentiation. For ex vivo lipolysis assay, inguinal or gonadal fat pads were surgically removed from Zfp423-AKO mice or control littermates and diced into ∼100 mg pieces. Differentiated cells or diced fat pads were then washed with PBS and pre-incubated in 5% CO2 at 37 °C for 1 h in DMEM plus 2% fatty acid free bovine serum albumin (BSA). After the pre-incubation, cells or diced fat pads were incubated in KRBH buffer (Sigma) plus 2% fatty acid free BSA with the presence of vehicle or 10 μg/ml CL316243 (Sigma) in 5% CO2 at 37 °C for 2 h. Free glycerol content in the medium was measured using Free Glycerol Determination Kit (Sigma). Glycerol release from each sample was normalized to the total protein content.
2.9. Oil Red O staining
In vitro differentiated cells were fixed in 10% formalin for 10 min at room temperature. Following fixation, the cells were washed with deionized water twice and incubated in 60% isopropanol for 5 min. Then the cells were completely air dried at room temperature before Oil Red O working solution (2 g l−1 Oil Red O in 60% isopropanol) was added. After incubation at room temperature for 10 min, the Oil Red O solution was removed and the cells were washed with deionized water for 4 times before the images were acquired for analysis.
2.10. Gene expression analysis
Total RNA from tissue or cells was extracted and purified using the TRIzol reagent (Invitrogen) and the RNeasy Mini Kit (Qiagen). cDNA was synthesized with M-MLV reverse transcriptase (Invitrogen) and random hexamer primers (Invitrogen). Relative expression of mRNAs was determined by quantitative PCR using SYBR Green PCR system (Applied Biosystems) and values were normalized to Rps18 levels using the ΔΔ−Ct method. All primer sequences used in the study are provided in Supplementary Table 1.
2.11. Glucose and insulin tolerance test
For glucose tolerance tests (GTT), mice were injected i.p. with glucose (Sigma) at the dosage of 1 g per kg body weight after an overnight fast. For insulin tolerance test (ITT), mice were injected i.p. with 0.75 U human insulin (Eli Lilly) per kg body weight after a fast of 6 h. Blood was collected by venous bleeding from the tail vein at 0, 15, 30, 60, 90, and 120 min post injection. Glucose concentrations were measured using Bayer Contour glucometers.
2.12. Statistical analysis
All data are presented as the mean ± s.e.m. Statistical analysis was performed by unpaired two-tailed Student's t-test or two-way ANOVA analysis with Bonferroni's post-test using Microsoft Excel or Graph Pad Prism 7.0. p values < 0.05 were considered to be statistically significant. No statistical methods were used to predict sample sizes. No randomization or blinding was performed to allocate the samples.
3. Results
3.1. Inactivation of Zfp423 in the Pdgfrα lineage leads to growth defects including arrested development of adipose tissue
Recent insight into the identity and molecular characteristics of adipose precursor populations has begun to inform strategies to target the adipose lineage at early stages of tissue development. Expression of platelet-derived growth factor receptor α-chain (Pdgfrα) identifies a pool of cells within the adipose tissue stroma that contains adipose precursors. Adipose precursors and all adipocytes in the major adipose depots appear to descend from cells with a history of Pdgfrα expression [37]. Despite the widespread expression of endogenous Pdgfrα in mesenchymal populations throughout the mouse, it is reported that one strain of the Pdgfrα-Cre mice efficiently and specifically targets the adipose lineage and can be used for gene ablation at the level of adipose progenitors [37].
Therefore, in order to investigate the role of Zfp423 at the earliest stages of adipogenesis in vivo, we developed a mouse strain in which Zfp423 is inactivated specifically in the Pdgfrα-lineage (PdgfrαLin) by crossing Pdgfrα-Cre transgenic mice with mice carrying conditional (loxP-flanked) Zfp423 alleles (Pdgfrα-Cre; Zfp423loxp/loxp animals, denoted as “PdgfrαLin-Zfp423-KO” mice). The PdgfrαLin-Zfp423-KO mice were born in the expected Mendelian ratio; however, knockout mice were noticeably smaller in size (Supplementary Figure 1a) and most were not able to survive beyond 4–5 weeks of age. A significant reduction in white adipose depot mass was observed in Zfp423-deficient mice compared to control littermates (Supplementary Figure 1b). mRNA levels of adipocyte-selective genes in both inguinal and gonadal WAT were significantly lower in knockout animals as compared to controls (Supplementary Figures 1c and d). These data are consistent with the hypothesis that Zfp423 is required for the formation of white adipose tissue. However, the striking growth defects in the animals suggest that Cre-mediated recombination of Zfp423 occurs outside the adipose lineage, thus confounding any further analysis with this model.
3.2. Inguinal adipose tissue development is impaired in Adiponectin-Cre; Zfp423loxP/loxP mice
Hong et al. recently identified a population of proliferating Pdgfrα+ preadipocytes that emerge in the inguinal WAT depot at E16.5 of development [38]. Interestingly, by E18.5, these cells express adipose progenitor markers (e.g. CD34, CD29, CD24) but also express perilipin and adiponectin; the latter two genes are largely restricted to mature adipocytes after birth. In support of this observation, the authors demonstrate that the Adiponectin-Cre transgene targets a subset of adipose stromal vascular cells in fetal inguinal WAT. These targeted stromal cells likely represent actively differentiating or highly committed preadipocytes. This stands in contrast to the expression pattern of Cre in adult Adiponectin-Cre mice. In postnatal WAT depots, Cre expression is restricted to fully mature adipocytes [37].
We independently confirmed and extended the results of Hong et al. by analyzing Cre-dependent reporter expression in Adiponectin-Cre; Rosa26RloxP-stop-loxP-tdtomato mice (Supplementary Figure 2a). We isolated the stromal vascular fraction (SVF) from inguinal WAT of E18.5 embryos and analyzed the adipose progenitor pool (Pdgfrα+; CD45−; CD31−) by flow cytometry (Supplementary Figure 2b). Consistent with the results described by Hong et al., a subset of the Pdgfrα+ population was tdTomato+ (Supplementary Figure 2d). Moreover, a vast majority of tdTomato+ cells actively expressed Pdgfrα (Supplementary Figure 2e). Not all Pdgfrα+ cells were targeted by Adiponectin-Cre at this particular stage; however, Hong et al. have demonstrated that these Adiponectin-Cre targeted cells proliferate and are highly adipogenic [38]. Of note, Adiponectin-Cre targeted precursors were only found in fetal inguinal WAT; tdTomato+ precursors were not found in SVF of adult WAT (Supplementary Figures 2f and g) or in the SVF of developing gonadal WAT at postnatal day 6 (P6) (Supplementary Figure 2h). These data indicate that during fetal inguinal WAT development, the adiponectin promoter is active at an earlier stage of the differentiation program than previously recognized. In contrast, adiponectin expression after birth is restricted to mature lipid-laden adipocytes.
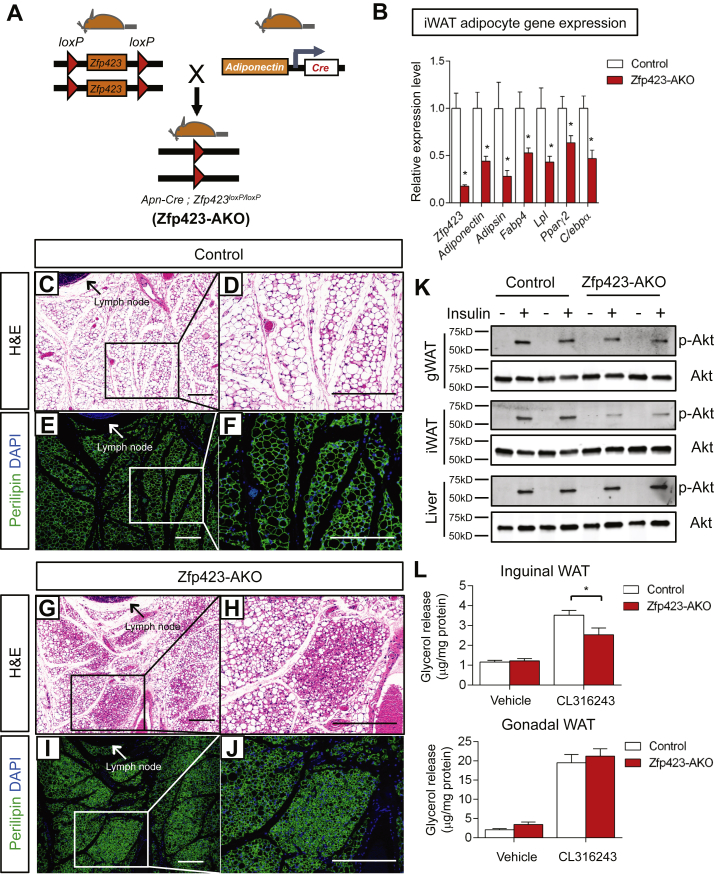
Based on this observation and aforementioned published results, we reasoned that the adiponectin-Cre transgene would target floxed alleles in either the fetal precursor stage or as cells are actively undergoing differentiation. This would allow for targeted disruption of Zfp423 in the developing inguinal WAT depot and provide a tool to investigate the function of Zfp423 during the terminal differentiation of adipocytes and fetal development of inguinal WAT. Thus, we bred adiponectin-Cre transgenic mice with Zfp423loxP/loxP animals (Adiponectin-Cre; Zfp423loxp/loxp animals, denoted herein as Zfp423-AKO mice) (Figure 1A). Zfp423-AKO mice were born in the expected Mendelian ratio and exhibited similar growth rate in comparison with control littermates. However, mRNA levels of Pparγ2 and other adipocyte-selective genes in both inguinal WAT and fractionated inguinal adipocytes were significantly lower in Zfp423-AKO mice (Figure 1B; Supplementary Figure 3a). Histological analysis of inguinal fat depots isolated from Zfp423-AKO animals revealed the appearance of patches of adipocytes, which were smaller in size, loaded with less lipid, and lacked the signature unilocular white adipocyte morphology (Figure 1C–J). This phenotype differs significantly from the inducible model in which adipocyte-specific deletion of Zfp423 induced in adult mice leads to a robust white-to-beige adipocyte conversion (∼200-fold increase in Ucp1 mRNA), without impacting the fat cell phenotype per se [31]. In sharp contrast, the expression of thermogenic genes Ucp1, Cidea, and Prdm16 in iWAT of Zfp423-AKO mice was only marginally higher (<2-fold) than the expression found in controls (Supplementary Figures 3a and b). The lower expression of Pparγ and other adipocyte-selective genes in purified adipocytes suggests that fetal inguinal adipocytes fail to fully undergo terminal differentiation in the absence of Zfp423. These data suggest that indeed Cre activity occurs at an earlier stage of the adipocyte life cycle than it does in adult mice and that the formation of a fully mature adipocyte is needed before Zfp423-deficiency can trigger an induction of the thermogenic gene program.
Figure 1.
Inguinal adipose tissue development is impaired in Adiponectin-Cre; Zfp423loxP/loxP mice. (A)Adiponectin-Cre; Zfp423loxP/loxP (Zfp423-AKO) mice are generated by breeding Adiponectin-Cre transgenic mice to animals carrying floxed Zfp423 alleles (Zfp423loxP/loxP). (B) Relative mRNA levels of Zfp423 and indicated adipocyte genes in fractionated inguinal white adipose tissue (iWAT) adipocytes from 8 weeks-old control and Zfp423-AKO mice. * denotes p < 0.05 from student's t-test. n = 5 mice. (C–F) Representative H&E staining (c, d) and perilipin immunofluorescence staining (e, f) of iWAT sections obtained from 8 weeks-old control mice. Scale bar, 200 μm. (G–J) Representative (g, h) H&E staining and (i, j) perilipin immunofluorescence staining of iWAT sections obtained from 8 weeks-old Zfp423-AKO mice. Scale bar, 200 μm. (K) Western blot analysis of phosphorylated-Akt (p-AKt) and total Akt protein levels in tissue extracts of gonadal WAT (gWAT), iWAT, and liver of 8 weeks-old old control and Zfp423-AKO mice administrated with insulin (1 U/kg). (L) β3-adrenergic receptor induced lipolysis (measured as glycerol release) in ex vivo diced iWAT and gWAT isolated from 8 weeks-old control and Zfp423-AKO mice. *p < 0.05 from two-way ANOVA. n = 6 mice.
Next, we assessed the overall functionality of the inguinal WAT depot in control and Zfp423-AKO mice. First, we tested the responsiveness of adipose depots to insulin stimulation, as reflected by the activation of its downstream signal transducer, Akt (phosphorylated-AKT). As compared to control animals, insulin-induced Akt phosphorylation was significantly impaired in the inguinal WAT of Zfp423-AKO mice (Figure 1K). Moreover, the lipolytic capacity of Zfp423-deficient adipose tissue was impaired. Isolated inguinal WAT depots from Zfp423-AKO mice released significantly less glycerol as compared to control inguinal WAT upon stimulation by the β3-adrenergic receptor agonist CL316243 (Figure 1L). These data provide functional evidence that inguinal WAT depots of Zfp423-AKO mice are not fully mature and developed.
Levels of Zfp423 were significantly lower in the gonadal WAT depot as well as the interscapular brown adipose tissue depot of Zfp423-AKO mice (Supplementary Figures 3c and d), consistent with the known activity of the adiponectin-Cre transgene in all adipose depots. However, none of the abnormalities observed in the inguinal WAT depots of Zfp423-AKO mice was found in the gonadal WAT depot or interscapular brown adipose tissue (BAT) depot (Supplementary Figuers 3c–h). This could imply a depot-selective requirement of Zfp423 during adipose tissue development. However, based on the differential activity of the adiponectin-Cre transgene in precursors of different depots, Cre-mediated deletion of Zfp423 likely occurred later in the differentiation program of gonadal white adipocytes and interscapular brown adipocytes. In fact, the expression of the thermogenic gene program was elevated in the gWAT of Zfp423-AKO mice (Supplementary Figure 3c), similar to the gonadal WAT phenotype found in the previously reported inducible adult adipocyte-specific Zfp423 knockout model. Baseline levels of these same thermogenic genes in the BAT were not impacted by the loss of Zfp423 (Supplementary Figure 3d).
3.3. Developmental defects caused by Zfp423-deficiency are cell autonomous and can be rescued by Pparγ agonism
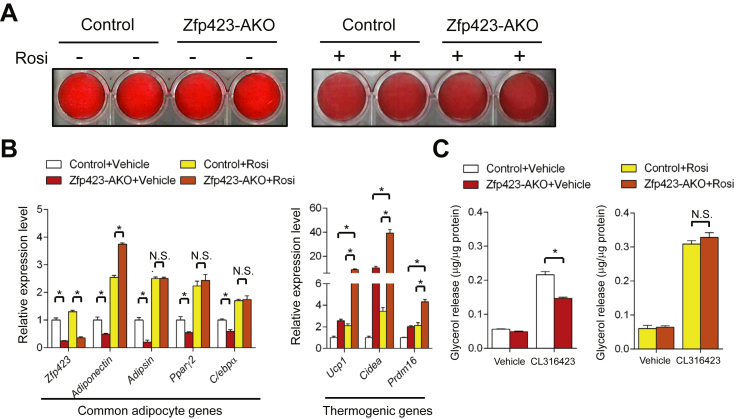
Next, we evaluated whether the observed defects in inguinal WAT development and function were largely due to a cell autonomous role for Zfp423 in adipocyte differentiation. We isolated and established cultures of the stromal vascular fraction (SVF) of inguinal WAT of control and Zfp423-AKO mice. In vitro adipocyte differentiation from cultured cells was induced using the standard 3T3-L1 adipogenic cocktail containing dexamethasone, IBMX and insulin. Differentiated cultures from both genotypes appeared equally capable of accumulating lipid, as indicated by Oil Red O staining (Figure 2A). However, mRNA levels of Pparγ2 and other genes selective to mature adipocytes (adiponectin, adipsin, and C/ebpα) were consistently reduced in the Zfp423-deficient cells (Figure 2B). These data suggest that the loss of Zfp423 expression after the onset of adiponectin/Cre expression leads to arrested terminal differentiation. Consistent with the observations made in vivo, β3-adrenergic agonist-induced glycerol release was attenuated in Zfp423-deficient cells, suggesting Zfp423-deficient cells were functionally defective in terms of lipolytic ability (Figure 2C). Collectively, these results provide evidence that Zfp423 is required for the terminal differentiation of inguinal white adipocytes and the fetal development of this WAT depot.
Figure 2.
Developmental defects caused by Zfp423-deficiency are cell autonomous and can be rescued by Pparγ agonism. (A) Oil Red O staining of in vitro derived adipocytes differentiated from cultures of the inguinal stromal vascular fraction of control and Zfp423-AKO mice. Cultures were treated with vehicle (−) or rosiglitazone (+) throughout differentiation. (B) Relative mRNA levels of common adipocyte genes and thermogenic genes in adipocytes cultures differentiated as shown in (a). * denotes p < 0.05 from two-way ANOVA. N.S. denotes not statistically significant. n = 4 for all conditions. (C) β3-adrenergic receptor induced lipolysis in in vitro derived control and Zfp423-AKO white adipocytes treated with vehicle or rosiglitazone during differentiation. * denotes p < 0.05 from two-way ANOVA. N.S. denotes not statistically significant. n = 3 mice.
We previously reported that Zfp423 regulates Pparγ expression in 3T3 fibroblast cell lines [30]. Consistent with these data, Pparγ2 expression was lower in freshly isolated or in vitro derived Zfp423-deficient adipocytes from Zfp423-iAKO mice (Figure 1, Figure 2). However, Pparγ expression in Zfp423-deficient adipocytes was not completely absent. Thus, we asked whether agonism of the remaining Pparγ in Zfp423 knockout adipocytes is sufficient to rescue the defects in white adipocyte differentiation and function. First, we performed in vitro differentiation assays with the Pparγ agonist rosiglitazone added to the differentiation media. Under this condition, adipocyte cultures from both groups appeared equally differentiated (Figure 2A); no statistically significant differences in mRNA levels of adipocyte-selective genes were observed (Figure 2B). Moreover, the CL316423-induced lipolytic capacity of Zfp423-deficient cells was restored by rosiglitazone treatment (Figure 2C), suggesting that the rescue of Pparγ activity by its agonist was largely sufficient to correct the differentiation defects caused by Zfp423-deficiency. Consistent with this observation, the expression of the thermogenic gene program in rescued Zfp423-deficient adipocyte cultures is strongly induced (Figure 2B). This phenotype is now similar to adipocyte cultures in which Zfp423 is inactivated post-differentiation.
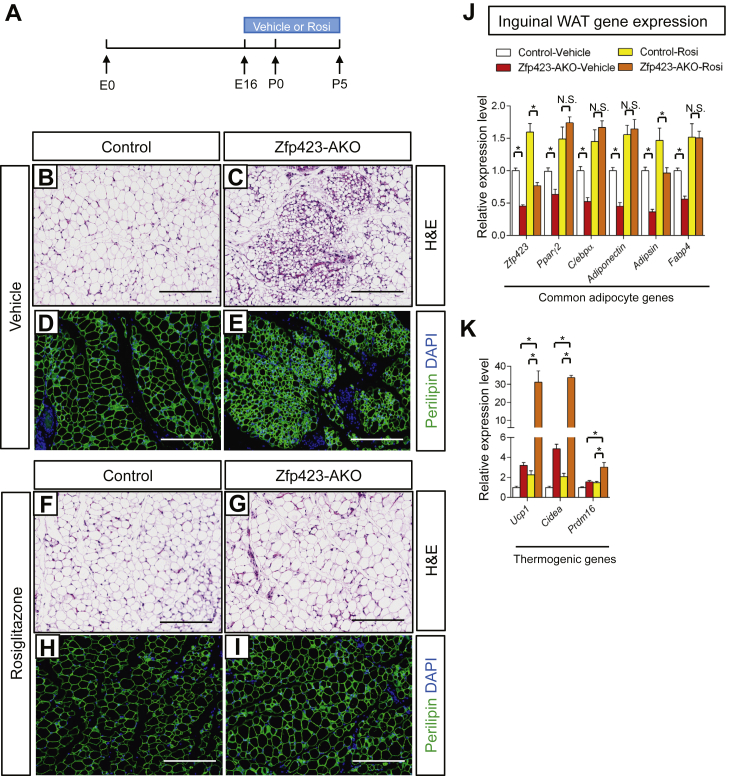
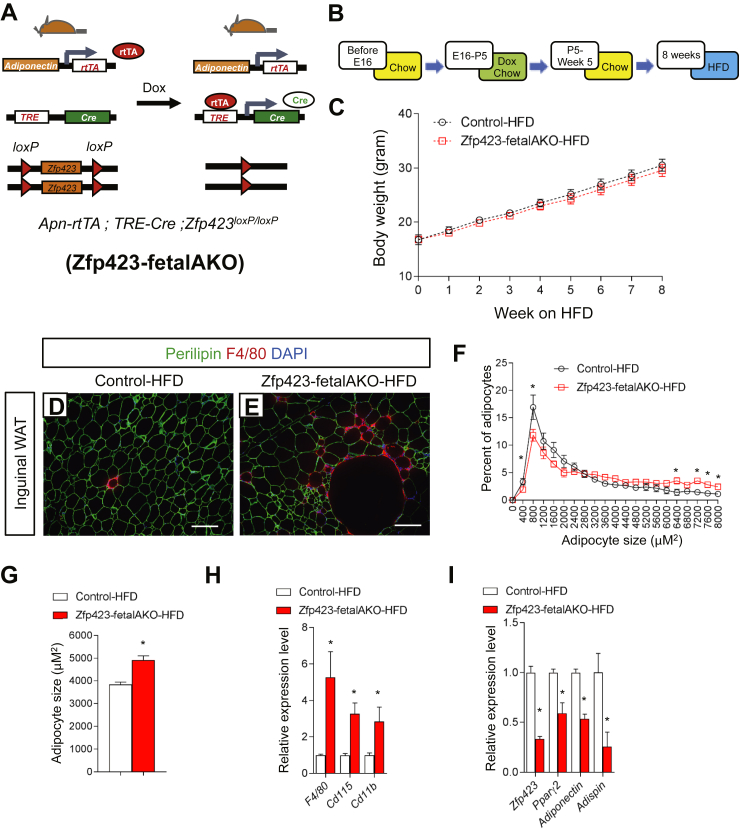
We also tested the ability of rosiglitazone to rescue the observed defects in inguinal WAT development in vivo. Inguinal adipocytes begin to appear around embryonic day 16 (E16) and most adipocytes are formed by postnatal day 5 (P5) [35]. Thus, we administered vehicle or rosiglitazone to control and Zfp423-AKO animals from E16 to P5 via gavage of pregnant and lactating mothers (Figure 3A). At P5, iWAT from vehicle-treated Zfp423-AKO pups appeared poorly differentiated; numerous patches of small, lipid-devoid cells were apparent (Figure 3B–E), and levels of all adipocyte-selective genes were lower than in vehicle-treated control mice (Figure 3J). However, none of these developmental defects was observed in Zfp423-AKO pups exposed to rosiglitazone during the peri-natal period. Compared to iWAT from rosiglitazone-treated control mice, iWAT from rosiglitazone-treated knockout mice exhibits a normal morphology (Figure 3F–I) and expresses comparable levels of adipocyte-selective genes (Figure 3J). Thus, we reasoned that these rescued animals are now essentially similar to the aforementioned model in which Zfp423 is inactivated in adipocytes of adult mice. Indeed, the thermogenic gene program was strongly induced in the rescued Zfp423-AKO mice (Figure 3K). Altogether, these data provide genetic evidence that Zfp423 is a critical regulator of the fetal development of inguinal white adipose tissue, serving as an upstream activator of Pparγ expression.
Figure 3.
Fetal Inguinal WAT development in Adiponectin-Cre; Zfp423loxP/loxP mice is corrected by perinatal administration of the Pparγ agonist, Rosiglitazone. (A) Vehicle or the Pparγ agonist, rosiglitazone (Rosi), was delivered to pregnant/lactating mothers (10 mg kg−1/day) from embryonic day (E) 16 (E16) to postnatal (P) day 5 (P5). Offspring were harvested for analysis at P5. (B, E) Representative H&E staining (B, C) and perilipin immunofluorescence staining (D, E) of iWAT sections obtained from 5 days-old control and Zfp423-AKO pups treated with vehicle. Scale bar, 200 μm. (F, I) Representative H&E staining (F, G) and perilipin immunofluorescence staining (H, I) of iWAT sections obtained from 5 days-old control and Zfp423-AKO pups treated with rosiglitazone. Scale bar, 200 μm. (J) Relative mRNA levels of common adipocyte genes in iWAT from 5 days-old control and Zfp423-AKO pups treated with vehicle or rosiglitazone. * denotes p < 0.05 from two-way ANOVA. N.S. denotes not statistically significant. n = 6 mice. (K) Relative mRNA levels of thermogenic genes in iWAT from 5 days-old control and Zfp423-AKO pups treated with vehicle or rosiglitazone. * denotes p < 0.05 from two-way ANOVA. n = 6 mice.
3.4. Inguinal WAT of Zfp423-deficient mice undergoes a pathological adipose tissue expansion upon high-fat diet feeding
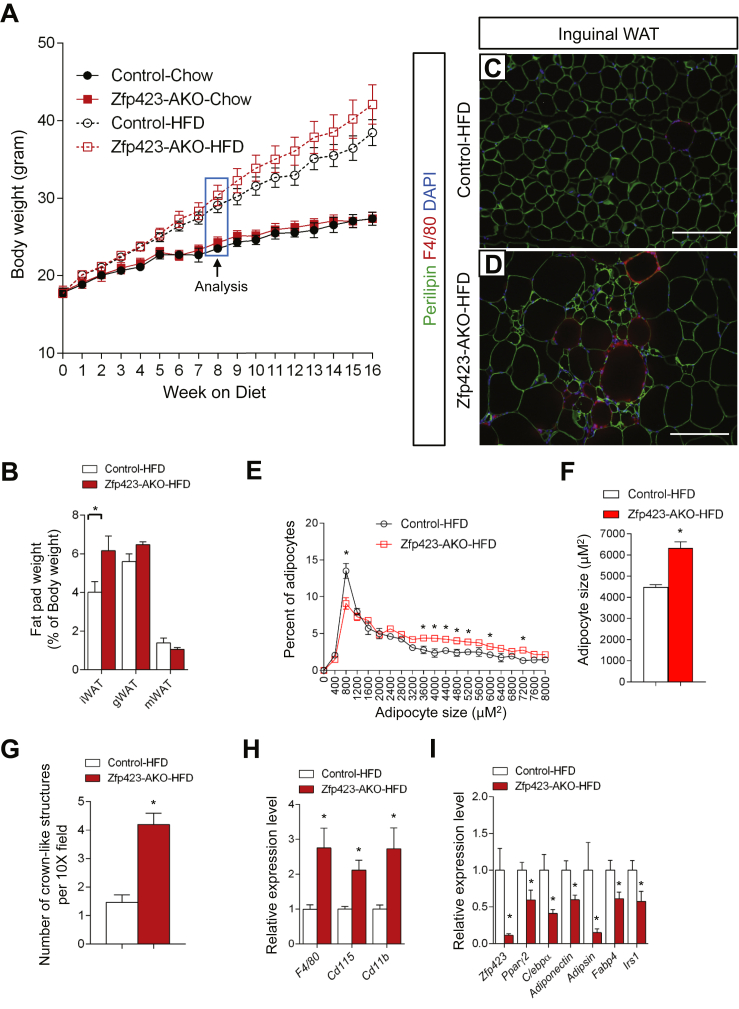
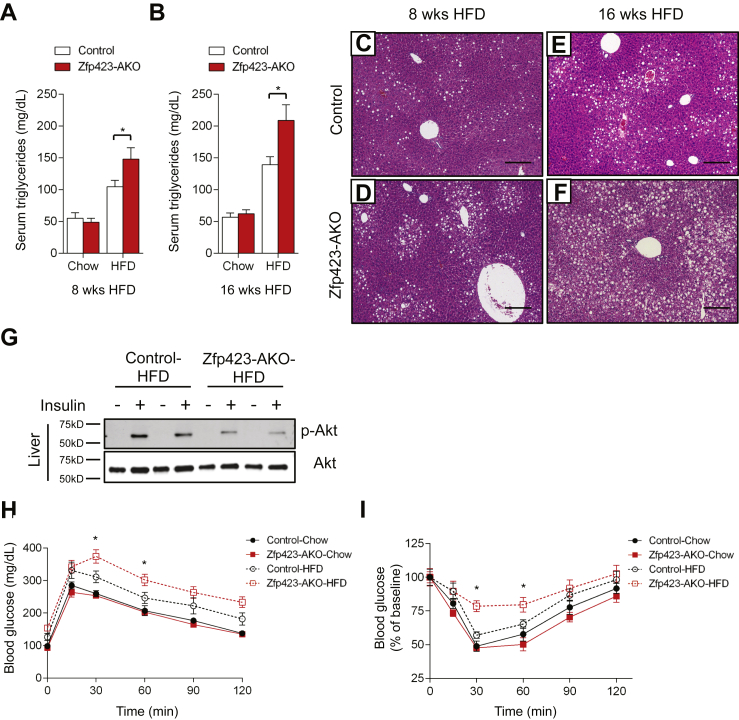
In the setting of caloric excess, a lack of functional adipocytes can lead to inappropriate lipid handling in adipose tissue and/or altered adipokine production. This can subsequently lead to an accumulation of lipids in non-adipose organs. Ectopic storage of lipids can be deleterious (“lipotoxicity”) and trigger the development of insulin resistance [39]. The poor development of the inguinal WAT depot in Zfp423-AKO mice prompted us to challenge the animals with an obesogenic high-fat diet (60% kcal from fat). Control and littermate male Zfp423-AKO mice were administered high-fat diet feed beginning from 5 weeks of age. The control and Zfp423-deficient mice equally gained weight over the first 8 weeks of the high-fat diet regimen (Figure 4A). Afterwards, Zfp423-deficient animals gained slightly more weight than controls; however, by the end of the 16 week period, these differences in weight measurements did not reach statistical significance (Figure 4A).
Figure 4.
High-fat diet feeding leads to a pathological inguinal WAT expansion in Adiponectin-Cre; Zfp423loxP/loxP mice. (A) Weekly body weight measurements of control and Zfp423-AKO mice fed chow or high-fat diet (HFD) for 16 weeks following weaning. n = 6–8 mice. (B) Adipose depot mass (normalized to body weight) of control and Zfp423-AKO mice after 8 weeks of HFD feeding. *p < 0.05 from student's test. n = 6–8 mice. (C, D) Representative immunofluorescence images of Perilipin (green) and F4/80 (red) expression in iWAT paraffin sections obtained from control and Zfp423-AKO mice after 8 weeks of HFD feeding. Scale bar, 200 μm. (E) Distribution of adipocyte size in control and Zfp423-AKO iWAT after 8 weeks of HFD feeding. * denotes p < 0.05 from two-way ANOVA. n = 6–8 mice. (F) Average adipocyte size of control and Zfp423-AKO iWAT after 8 weeks of HFD feeding. * denotes p < 0.05 from student's test. n = 6–8 mice. (G) Number of crown-like structures (F4/80 positive) in control and Zfp423-AKO iWAT after 8 weeks of HFD feeding. * denotes p < 0.05 from student's test. n = 6–8 mice. (H–I) Relative mRNA levels of macrophage markers (H) and adipocyte-selective genes (I) in control and Zfp423-AKO iWAT after 8 weeks of HFD feeding. * denotes p < 0.05 from student's test. n = 6 mice.
Analysis of Zfp423 knockout mice at 8 weeks of age revealed differences in adipose tissue size and morphology. Weights of inguinal, but not gonadal or mesenteric, fat depots of Zfp423-deficient mice were higher than the weights of control inguinal adipose tissues (Figure 4B). Histological analysis revealed a striking inguinal WAT phenotype with hallmarks of unhealthy adipose expansion (Figure 4C and D). Many small adipocytes devoid of lipids were observed in knockout animals, similar to what was observed in depots from chow-fed animals. However, the frequency of large, hypertrophic adipocytes was greater in the Zfp423-AKO mice (Figure 4E). Overall, the average adipocyte size was approximately 40% higher than found in controls (Figure 4F). This hypertrophic adipose phenotype was accompanied by increased inflammation, as assessed by the increased presence of crown-like immune structures (Figure 4G). Moreover, higher expression levels of immune cell selective genes and relatively lower expression of adipocyte-selective genes were detected in total inguinal WAT tissue (Figure 4H and I). After 8 weeks of high-fat diet feeding, the gonadal WAT appeared similar in mass to corresponding depots from control animals (Figure 4B). No significant differences in gonadal adipocyte cell size were observed between the two groups (Supplementary Figure 4a). However, there was an abundance of crown-like structures in this depot of Zfp423-AKO mice (Supplementary Figures 4b and c), correlating with increased expression of immune cell transcripts in whole gonadal white adipose tissue (Supplementary Figure 4d). Chronic adipose inflammation is typically associated with adipose tissue dysfunction. Indeed, in Zfp423-AKO mice, insulin-stimulated AKT phosphorylation was blunted in both inguinal and gonadal WAT depots (Supplementary Figure 4f), and serum levels of adiponectin were significantly lower after 8 and 16 weeks of high-fat diet feeding (Supplementary Figure 4g).
Unhealthy adipose tissue expansion is often associated with systemic alterations in nutrient handling. Consistent with this notion, we observed higher levels of serum triglycerides in Zfp423-AKO mice than in controls, both after 8 and 16 weeks of high-fat diet feeding (Figure 5A and B). This correlated with a progressive increase in hepatic steatosis (Figure 5C–F) and resistance to insulin-induced AKT activation (Figure 5G) in the knockout animals. Glucose and insulin tolerance tests revealed systemic impairments in glucose homeostasis in Zfp423-AKO mice. Zfp423-AKO mice were less glucose tolerant than control animals and less sensitive to the actions of insulin (Figure 5H and I). All together, the ectopic lipid accumulation, lower serum adiponectin levels, glucose intolerance, and insulin resistance indicate that obese Zfp423-deficient animals exhibit the metabolic profile commonly observed in lipodystrophy.
Figure 5.
Obese Adiponectin-Cre; Zfp423loxP/loxP mice are insulin resistant and have increased hepatic steatosis. (A, B) Serum triglyceride levels in control and Zfp423-AKO mice after 8 (A) or 16 weeks (B) of chow or HFD feeding. * denotes p < 0.05 from two-way ANOVA. n = 6–8 mice. (C, D) Representative H&E staining of livers from control (C) and Zfp423-AKO (D) mice after 8 weeks of HFD feeding. Scale bar, 200 μm. (E, F) Representative H&E staining of livers from control (E) and Zfp423-AKO (F) mice after 16 weeks of HFD feeding. Scale bar, 200 μm. (G) Western blot analysis of phosphorylated-Akt (p-AKt) and total Akt protein levels in liver extracts from 8 week HFD-fed control and Zfp423-AKO mice administrated with insulin (2 U/kg). (H) Glucose tolerance test of control and Zfp423-AKO mice after 8 weeks HFD feeding. * denotes p < 0.05 from Two-way ANOVA. n = 6–8 mice. (I) Insulin tolerance test of control and Zfp423-AKO mice after 8 weeks HFD feeding. * denotes p < 0.05 from Two-way ANOVA. n = 6–8 mice.
3.5. Pathological expansion of subcutaneous WAT is sufficient to trigger gonadal WAT dysfunction and impaired systemic nutrient homeostasis
Defects in adipose development in Zfp423-AKO mice were restricted to the subcutaneous inguinal WAT depot; however, upon high-fat diet feeding, systemic insulin resistance was apparent and gonadal WAT also appears dysfunctional. These data suggest that a pathological remodeling specifically of subcutaneous WAT in obesity is sufficient to drive intra-abdominal WAT dysfunction and systemic nutrient imbalance. In order to test this hypothesis, we utilized our doxycycline-inducible, adipose-specific, Zfp423 knockout model (Zfp423-iAKO mice) (Figure 6A). Wang et al. previously demonstrated that fetal exposure of doxycycline to Adiponectin-rtTA; TRE-Cre; Rosa26RLacZ pups specifically during E16 through P5 of life results in adipose depot-selective gene targeting [35]. The inguinal WAT depot, and to a lesser extent the classic BAT depots, express the Adiponectin-rtTA transgene during this period. However, gonadal and other intra-abdominal WAT depots are not yet formed at this stage and thus are not targeted. Thus, we administered doxycycline-containing chow diet to pregnant/lactating mice carrying control (Adiponectin-rtTA; Zfp423loxP/loxP) and Zfp423-iAKO mice (Adiponectin-rtTA; TRE-Cre; Zfp423loxP/loxP) specifically during E16 to P5 of life and then harvested animals at 5 weeks of age for analysis (Figure 6A). As expected, Zfp423 mRNA levels were significantly reduced in the inguinal WAT depot and interscapular BAT of Zfp423-iAKO mice (Supplementary Figure 5a). However, levels of Zfp423 in the gonadal WAT were not reduced (Supplementary Figure 5b). Similar to the Zfp423-AKO mice described above, the inguinal WAT did not appear fully developed, as revealed by the large regions of undifferentiated cells (Supplementary Figures 5d and e) and the lower expression of adipocyte-selective genes in the tissue (Supplementary Figure 5a). Importantly, brown adipose tissue and gonadal WAT appeared indistinguishable from controls (Supplementary Figure 5c, f–i).
Figure 6.
Transient fetal exposure of Adiponectin-rtTA; TRE-Cre; Zfp423loxP/loxP animals leads to inguinal white adipose depot-selective targeting of Zfp423. (A) Inducible inactivation of Zfp423 in adiponectin-expressing cells is achieved by breeding the adiponectin-rtTA transgenic mice to animals expressing Cre recombinase under the control of the Tet-response element (TRE-Cre) and carrying floxed Zfp423 alleles (Zfp423loxP/loxP). Littermates carrying only adiponectin-rtTA and Zfp423loxP/loxP alleles (i.e. Cre–) were used as the control animals. Exposure of animals to doxycycline from E16 to P5 via feeding of mothers results in a model of selective Zfp423 inactivation in the developing inguinal WAT (Zfp423-fetalAKO mice). (B) Control and Zfp423-fetalAKO mice were exposed to doxycycline from E16 to P5 and then kept on standard chow diet until 5 week old before switching to HFD for another 8 weeks. (C) Weekly body weight measurements of control and Zfp423-fetal AKO mice during 8 weeks of HFD feeding. n = 6 mice. (D, E) Representative immunofluorescence images of perilipin (green) and F4/80 (red) expression in iWAT paraffin sections from control (D) and Zfp423-fetalAKO (E) mice after 8 weeks of HFD feeding. Scale bar, 200 μm. (F) Distribution of adipocyte size in control and Zfp423-fetalAKO iWAT after 8 weeks of HFD feeding. * denotes p < 0.05 from two-way ANOVA. n = 6 mice. (G) Average adipocyte size in control and Zfp423-fetalAKO iWAT after 8 weeks of HFD feeding. * denotes p < 0.05 from student's t-test. n = 6 mice. (H, I) Relative mRNA levels of macrophage markers (H) and adipocyte-selective genes (I) in control and Zfp423-fetalAKO iWAT after 8 weeks of HFD feeding. * denotes p < 0.05 from student's t-test. n = 6 mice.
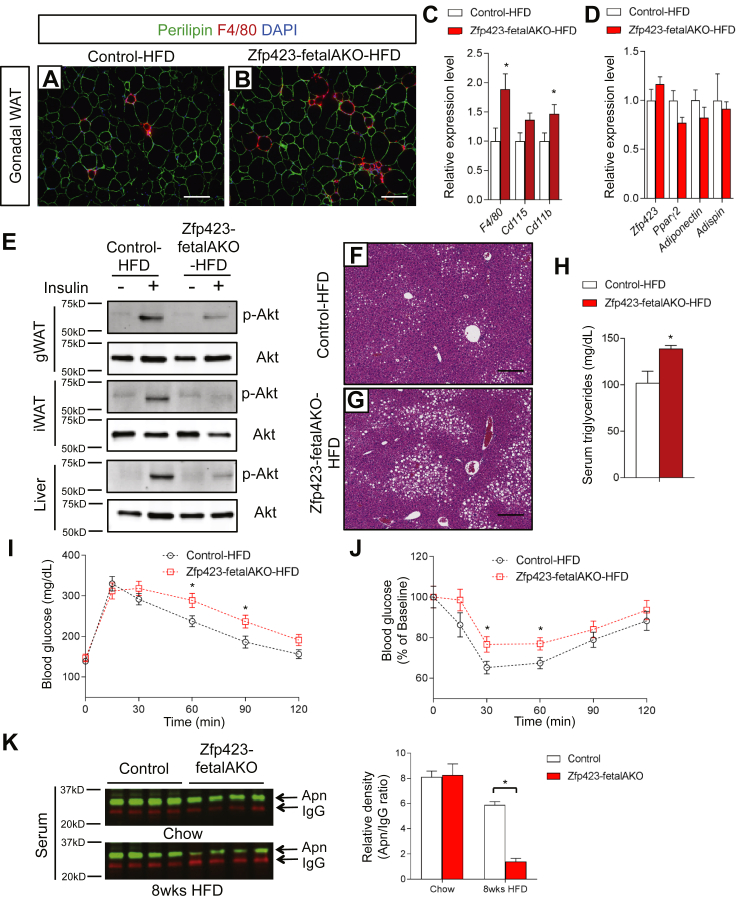
At five weeks of age we challenged the inducible Zfp423 knockout model (herein, “Zfp423-fetalAKO”) to high-fat diet feeding (Figure 6B). After 8 weeks of high-fat diet feeding, many of the phenotypes observed in the obese Zfp423-AKO mice were again apparent. Body weights of Zfp423-fetalAKO mice were indistinguishable from control animals (Figure 6C); however, inguinal WAT had an unhealthy appearance, characterized by adipocyte hypertrophy, increased inflammation, and lower levels of adipocyte-selective transcripts (Figure 6D–I). The expression levels of Zfp423 and other adipocyte genes remained unaltered in Zfp423-fetalAKO gWAT (Figure 7D); however, there was an abundant number of crown-like structures, correlating with the elevated levels of transcripts encoding immune cell markers in the gonadal depot of the knockout mice (Figure 7A–C). Functionally, both inguinal and gonadal WAT of the Zfp423-fetalAKO mice appeared relatively more resistant to the actions of insulin. Insulin-stimulated p-AKT induction was strongly blunted in both depots (Figure 7E). Hepatic insulin resistance was also apparent (Figure 7E), correlating with increased hepatic steatosis and serum triglycerides (Figure 7F–H). Intraperitoneal glucose and insulin tolerance tests revealed that Zfp423-fetalAKO mice were more glucose intolerant and insulin resistant than controls (Figure 7I and J). These phenotypes of Zfp423-fetalAKO mice may be explained, at least in part, by the largely reduced levels of serum adiponectin (Figure 7K). This model of inguinal-selective Zfp423-inactivation largely recapitulated the metabolic phenotype observed in Zfp423-AKO animals. These data strongly suggest that pathological subcutaneous WAT expansion in adult obesity, triggered initially by poor fetal adipocyte differentiation, is sufficient to considerably impair visceral WAT function and systemic glucose and lipid metabolism.
Figure 7.
Pathological expansion of subcutaneous WAT is sufficient to trigger impaired systemic nutrient homeostasis. (A, B) Representative immunofluorescence images of perilipin (green) and F4/80 (red) expression in sections of gWAT from control and Zfp423-fetalAKO mice after 8 weeks of HFD feeding. Scale bar, 200 μm. (C, D) Relative mRNA levels of macrophage markers (C) and adipocyte-selective genes (D) in gWAT of control and Zfp423-fetalAKO mice after 8 weeks of HFD feeding. * denotes p < 0.05 from student's t-test. n = 6 mice. (E) Western blot analysis of phosphorylated-Akt (p-AKt) and total Akt protein levels in tissue extracts of gWAT, iWAT, and liver of HFD-fed control and Zfp423-fetalAKO mice administrated with insulin (2 U/kg). (F, G) Representative H&E staining of livers from control and Zfp423-fetalAKO mice after 8 weeks of HFD feeding. Scale bar, 200 μm. (H) Serum triglyceride levels in control and Zfp423-fetalAKO mice after 8 weeks of HFD feeding. * denotes p < 0.05 from student's t-test. n = 6 mice. (I) Glucose tolerance test of control and Zfp423-fetalAKO mice after 8 weeks HFD feeding. * denotes p < 0.05 from student's t-test. n = 6 mice. (J) Insulin tolerance test of control and Zfp423-fetalAKO mice after 8 weeks HFD feeding. * denotes p < 0.05 from student's t-test. n = 6 mice. (K) Western blot analysis and quantification of serum adiponectin levels in control and Zfp423-fetalAKO mice fed with chow or HFD for 8 weeks starting at 5 weeks of age. For quantification, intensity of adiponectin band is normalized to that of IgG band. * denotes p < 0.05 from two-way ANOVA. n = 4 mice.
4. Discussion
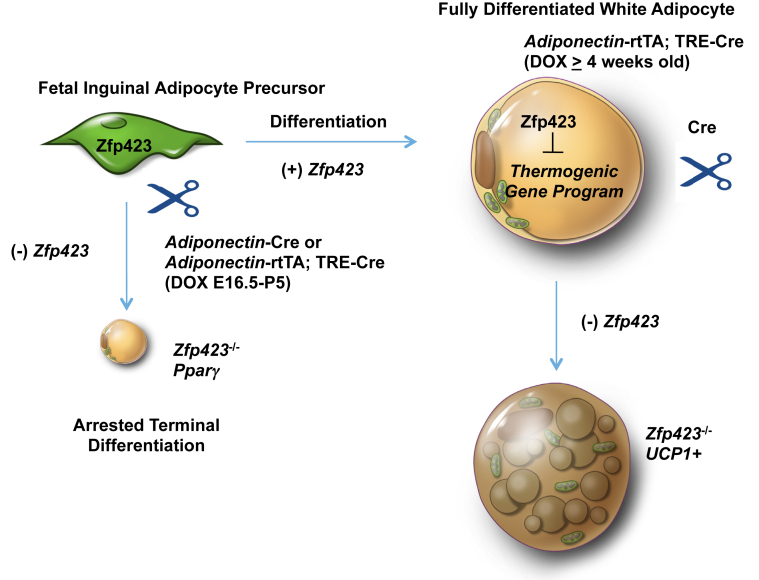
We recently demonstrated that Zfp423 maintains the mature white adipocyte phenotype through suppression of the adipocyte thermogenic gene program. This was demonstrated by targeting mature adipocytes in adult animals through use of the doxycycline-inducible Adiponectin-rtTA transgene. Here, we use the same genetic system or the commonly used constitutively active Adiponectin-Cre allele and observe a vastly different phenotype when gene ablation is induced during fetal adipose development. When Zfp423 is removed from mature adipocytes of adult animals, a white-to-beige phenotypic switch is observed. When Zfp423 is removed from precursors and/or actively differentiating cells, we observe a defect in the terminal differentiation of fetal white adipocytes (Figure 8). This is apparent by the lack of lipid accumulation and lower expression of Pparγ and adipocyte-selective genes. The inability of the thermogenic gene program to activate in these Zfp423-deficient cells is likely due to the reduction in Pparγ expression. This discrepancy in the phenotypes is likely attributable to the fact that Adiponectin-Cre is active during fetal inguinal adipocyte differentiation while only active in fully mature adipocytes in adult animals. These data highlight the emerging notion that fetal adipose precursors appear distinct from adult adipose precursors, highlighted in part by the expression of adiponectin, perilipin, and perhaps other genes [38], [40]. Moreover, these data highlight a critical role for Zfp423 in both the establishment and maintenance of the white adipose lineage.
Figure 8.
Establishment and Maintenance of the White Adipocyte Lineage by Zfp423. Zfp423 expression identifies committed preadipocytes and drives adipocyte differentiation through activation of Pparγ[28], [29], [30]. Cre-mediated inactivation of Zfp423 in developing fetal inguinal WAT precursors results in arrested terminal adipocyte differentiation. In fully differentiated white adipocytes, Zfp423 functions to suppress the thermogenic gene program, thereby maintaining the white adipocyte phenotype. Inducible, Cre-mediated inactivation of Zfp423 in fully differentiated white adipocytes of adult mice leads to a lineage reprogramming in which mature white adipocytes are converted to thermogenic UCP1-expressing beige-like adipocytes [31].
The data presented here highlight some of the pros and cons of the various genetic tools now available for manipulating genes within the adipose lineage. The Pdgfrα-Cre line targets the adipose precursors and descending adipocytes with high efficiency; however, use of these mice as a tool may depend on the expression pattern of the floxed gene of interest. The Adiponectin-Cre line developed by Rosen and colleagues is undoubtedly the most specific and efficient tool for adipose targeting [41]; however, the data here highlight that Cre–mediated recombination in these mice is not entirely confined to fully differentiated adipocytes. During fetal inguinal WAT development, Cre expression occurs in cells that are most likely either highly committed preadipocytes or actively differentiating cells. Further analysis of these cells will be needed to understand their properties. After birth, Cre expression appears limited to mature, lipid-laden adipocytes, at least in the depots examined. Thus, the timing of Cre expression in the different depots of these animals has to be considered when interpreting results. As such, our results can only confirm an important role for Zfp423 in the onset of fetal subcutaneous WAT development. Zfp423 expression marks perivascular adipose precursors in adult mice; however the requirement of this factor in adult adipocyte differentiation in vivo, particularly in intra-abdominal depots, still remains unknown and will require the use of additional models. Importantly, models allowing inducible expression of Cre recombinase, such as the Tet-on system employed here, allow for gene function to be dissected at various stages of life. Furthermore, timing the doxycycline treatment allows white adipose depots to be targeted in a selective manner.
Clinical studies over the past decade have revealed a striking correlation between adipose tissue cellularity and metabolic health in the setting of obesity. The phenotypes of the Zfp423-AKO mice presented here fit well with clinical observations and highlight the importance of adipogenesis to healthy WAT remodeling and nutrient homeostasis in obesity. Poor fetal development of the inguinal WAT depot in Zfp423 knockout mice leads to an expanded subcutaneous depot in obese adults that resemble WAT depots of obese humans with metabolic syndrome. This pathological expansion is characterized by adipocyte hypertrophy, inflammation, and loss of insulin sensitivity. The observed adipocyte hypertrophy may be due to the observed defects in lipolytic capacity of the Zfp423-deficient adipocytes. It is also possible that remaining adipocytes become hypertrophied as an attempt to compensate for the lack of differentiated adipocytes. Clinically, and as observed in our animal models, these adipose phenotypes correlate with low serum adiponectin, high serum triglycerides, and systemic insulin resistance.
Adipose tissue distribution is also a reliable predictor of metabolic health. Preferential expansion of subcutaneous depots is often observed in the “healthy” obese populations. Interestingly, overall inguinal WAT mass was increased in the Zfp423-AKO mice. This suggests that subcutaneous adipose tissue quality is equally, if not more, important than adipose tissue quantity. This may also appear true for visceral adipose tissue. Senol-Cosar et al. recently reported that transgenic expression of tenomodulin in adipose tissue results in a healthy visceral WAT expansion, characterized by increased preadipocyte proliferation and lower inflammation and fibrosis, despite expanded gonadal WAT mass [42]. Our inguinal WAT-selective model of Zfp423 ablation sheds further insight into the importance of healthy subcutaneous WAT by illustrating the systemic metabolic consequences of pathological subcutaneous adipose tissue expansion in obesity. A better understanding of how poor adipogenesis leads to pathological adipose remodeling may lead to novel therapeutic strategies to improve adipose tissue health in obesity.
Acknowledgements
The authors are grateful to members of the UTSW Touchstone Diabetes Center for useful discussions, and Drs. P. Scherer, C. Kusminski, and W. Holland for critical reading of the manuscript. The authors thank the UTSW Animal Resource Center, Metabolic Phenotyping Core, Pathology Core, Live Cell Imaging Core, and Flow Cytometry Core for excellent guidance and assistance with experiments performed here. This study was supported by NIDDK R03 DK099428, R01 DK104789, the Searle Scholars Program, the American Heart Association 15BGIA22460021 to R.K.G, the American Heart Association postdoctoral fellowship 16POST26420136 to M.S, and the NIH NIGMS training grant T32 GM008203 to C.H.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.11.009.
Conflict of interest
All authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Stern J.H., Rutkowski J.M., Scherer P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metabolism. 2016;23(5):770–784. doi: 10.1016/j.cmet.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harms M., Seale P. Brown and beige fat: development, function and therapeutic potential. Nature Medicine. 2013;19(10):1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 3.Kissig M., Shapira S.N., Seale P. SnapShot: brown and beige adipose thermogenesis. Cell. 2016;166(1) doi: 10.1016/j.cell.2016.06.038. pp. 258–258 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Review. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 5.Unger R.H., Clark G.O., Scherer P.E., Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochimica et Biophysica Acta. 2010;1801(3):209–214. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Appleton S.L., Seaborn C.J., Visvanathan R., Hill C.L., Gill T.K., Taylor A.W. Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: a cohort study. Diabetes Care. 2013;36(8):2388–2394. doi: 10.2337/dc12-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voulgari C., Tentolouris N., Dilaveris P., Tousoulis D., Katsilambros N., Stefanadis C. Increased heart failure risk in normal-weight people with metabolic syndrome compared with metabolically healthy obese individuals. Journal of American College of Cardiology. 2011;58(13):1343–1350. doi: 10.1016/j.jacc.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 8.Denis G.V., Obin M.S. ‘Metabolically healthy obesity’: origins and implications. Molecular Aspects of Medicine. 2013;34(1):59–70. doi: 10.1016/j.mam.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee M.J., Wu Y., Fried S.K. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Molecular Aspects of Medicine. 2013;34(1):1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun K., Kusminski C.M., Scherer P.E. Adipose tissue remodeling and obesity. Journal of Clinical Investigation. 2011;121(6):2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kloting N., Bluher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Reviews in Endocrine Metabolic Disorders. 2014;15(4):277–287. doi: 10.1007/s11154-014-9301-0. [DOI] [PubMed] [Google Scholar]
- 12.Gealekman O., Guseva N., Hartigan C., Apotheker S., Gorgoglione M., Gurav K. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123(2):186–194. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy O.T., Perugini R.A., Nicoloro S.M., Gallagher-Dorval K., Puri V., Straubhaar J. Body mass index-independent inflammation in omental adipose tissue associated with insulin resistance in morbid obesity. Surgery for Obesity and Related Disorders. 2011;7(1):60–67. doi: 10.1016/j.soard.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gustafson B., Hedjazifar S., Gogg S., Hammarstedt A., Smith U. Insulin resistance and impaired adipogenesis. Trends in Endocrinology & Metabolism. 2015;26(4):193–200. doi: 10.1016/j.tem.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Gustafson B., Hammarstedt A., Hedjazifar S., Smith U. Restricted adipogenesis in hypertrophic obesity: the role of WISP2, WNT, and BMP4. Diabetes. 2013;62(9):2997–3004. doi: 10.2337/db13-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustafson B., Gogg S., Hedjazifar S., Jenndahl L., Hammarstedt A., Smith U. Inflammation and impaired adipogenesis in hypertrophic obesity in man. American Journal of Physiology, Endocrinology and Metabolism. 2009;297(5):E999–E1003. doi: 10.1152/ajpendo.00377.2009. [DOI] [PubMed] [Google Scholar]
- 17.Kloting N., Fasshauer M., Dietrich A., Kovacs P., Schon M.R., Kern M. Insulin-sensitive obesity. American Journal of Physiology, Endocrinology and Metabolism. 2010;299(3):E506–E515. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- 18.Sun K., Park J., Gupta O.T., Holland W.L., Auerbach P., Zhang N. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nature Communications. 2014;5:3485. doi: 10.1038/ncomms4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy O.T., Czech M.P., Corvera S. What causes the insulin resistance underlying obesity? Current Opinion in Endocrinology, Diabetes and Obesity. 2012;19(2):81–87. doi: 10.1097/MED.0b013e3283514e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kissebah A.H., Krakower G.R. Regional adiposity and morbidity. Physiological Reviews. 1994;74(4):761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- 21.Tran T.T., Yamamoto Y., Gesta S., Kahn C.R. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metabolism. 2008;7(5):410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J.Y., van de Wall E., Laplante M., Azzara A., Trujillo M.E., Hofmann S.M. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. Journal of Clinical Investigation. 2007;117(9):2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kusminski C.M., Holland W.L., Sun K., Park J., Spurgin S.B., Lin Y. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nature Medicine. 2012;18(10):1539–1549. doi: 10.1038/nm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cristancho A.G., Lazar M.A. Forming functional fat: a growing understanding of adipocyte differentiation. Nature Reviews Molecular Cell Biology. 2011;12(11):722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farmer S.R. Transcriptional control of adipocyte formation. Cell Metabolism. 2006;4(4):263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen E.D., Spiegelman B.M. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang F., Mullican S.E., DiSpirito J.R., Peed L.C., Lazar M.A. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARgamma. Proceedings of the National Academy of Science United States of America. 2013;110(46):18656–18661. doi: 10.1073/pnas.1314863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta R.K., Mepani R.J., Kleiner S., Lo J.C., Khandekar M.J., Cohen P. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metabolism. 2012;15(2):230–239. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vishvanath L., MacPherson K.A., Hepler C., Wang Q.A., Shao M., Spurgin S.B. Pdgfrbeta+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metabolism. 2016;23(2):350–359. doi: 10.1016/j.cmet.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta R.K., Arany Z., Seale P., Mepani R.J., Ye L., Conroe H.M. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464(7288):619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao M., Ishibashi J., Kusminski C.M., Wang Q.A., Hepler C., Vishvanath L. Zfp423 maintains white adipocyte identity through suppression of the beige cell thermogenic gene program. Cell Metabolism. 2016 doi: 10.1016/j.cmet.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alcaraz W.A., Gold D.A., Raponi E., Gent P.M., Concepcion D., Hamilton B.A. Zfp423 controls proliferation and differentiation of neural precursors in cerebellar vermis formation. Proceedings of the National Academy of Science United States of America. 2006;103(51):19424–19429. doi: 10.1073/pnas.0609184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng L.E., Zhang J., Reed R.R. The transcription factor Zfp423/OAZ is required for cerebellar development and CNS midline patterning. Developmental Biology. 2007;307(1):43–52. doi: 10.1016/j.ydbio.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warming S., Rachel R.A., Jenkins N.A., Copeland N.G. Zfp423 is required for normal cerebellar development. Molecular and Cell Biology. 2006;26(18):6913–6922. doi: 10.1128/MCB.02255-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q.A., Tao C., Gupta R.K., Scherer P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nature Medicine. 2013;19(10):1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parlee S.D., Lentz S.I., Mori H., MacDougald O.A. Quantifying size and number of adipocytes in adipose tissue. Methods in Enzymology. 2014;537:93–122. doi: 10.1016/B978-0-12-411619-1.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berry R., Rodeheffer M.S. Characterization of the adipocyte cellular lineage in vivo. Nature Cell Biology. 2013;15(3):302–308. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong K.Y., Bae H., Park I., Park D.Y., Kim K.H., Kubota Y. Perilipin+ embryonic preadipocytes actively proliferate along growing vasculatures for adipose expansion. Development. 2015;142(15):2623–2632. doi: 10.1242/dev.125336. [DOI] [PubMed] [Google Scholar]
- 39.Unger R.H., Scherer P.E. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends in Endocrinology & Metabolism. 2010;21(6):345–352. doi: 10.1016/j.tem.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Y., Berry D.C., Tang W., Graff J.M. Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Reports. 2014;9(3):1007–1022. doi: 10.1016/j.celrep.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang S., Kong X., Rosen E.D. Adipocyte-specific transgenic and knockout models. Methods in Enzymology. 2014;537:1–16. doi: 10.1016/B978-0-12-411619-1.00001-X. [DOI] [PubMed] [Google Scholar]
- 42.Senol-Cosar O., Flach R.J., DiStefano M., Chawla A., Nicoloro S., Straubhaar J. Tenomodulin promotes human adipocyte differentiation and beneficial visceral adipose tissue expansion. Nature Communications. 2016;7:10686. doi: 10.1038/ncomms10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.