Abstract
Levels of obesity have reached epidemic proportions on a global scale, which has led to considerable increases in health problems and increased risk of several diseases, including cardiovascular and pulmonary diseases, cancer and diabetes mellitus. People with obesity consume more food than is needed to maintain an ideal body weight, despite the discrimination that accompanies being overweight and the wealth of available information that overconsumption is detrimental to health. The relationship between energy expenditure and energy intake throughout an individual’s lifetime is far more complicated than previously thought. An improved comprehension of the relationships between taste, palatability, taste receptors and hedonic responses to food might lead to increased understanding of the biological underpinnings of energy acquisition, as well as why humans sometimes eat more than is needed and more than we know is healthy. This Review discusses the role of taste receptors in the tongue, gut, pancreas and brain and their hormonal involvement in taste perception, as well as the relationship between taste perception, overeating and the development of obesity.
Introduction
In societies where calorie-dense foods are readily available and where little, if any, work is required to obtain calories, many individuals find it difficult to control their food intake in order to maintain a healthy body weight. Huge progress has been made in the identification of genes associated with risk of obesity, as well as those involved in the endocrinology of food intake, satiety, food digestion and food absorption;1 however, the obesity and diabetes epidemics persist and seem to be accelerating.2 A report released by the Centers for Disease Control revealed that, as of 2012, there are 29 million people with diabetes mellitus and 86 million people with pre-diabetes in the USA. The vast majority of the affected patients have type 2 diabetes mellitus, which is associated with obesity or being overweight.3
Mammalian physiological systems have developed such that an animal’s drive to feel good is paramount.4 Palatable foods contain fat, sweet chemicals and a little salt; if such foods are readily available they will be eaten to excess, precisely because they taste good and make one feel good.5 The molecular signals from the gut that dictate satiety are easily manipulated by sentient humans, as are adiposity signals and visual cues that indicate body fat content.4,6,7 For example, as an individual progresses from being overweight to having obesity, circulating levels of leptin (a hormonal indicator of total body adiposity) increase, but expression of the leptin receptor is downregulated.8,9 In general, gut hormones that regulate satiety, such as glucagon-like peptide 1 (GLP-1) and cholecystokinin (CCK), have very short half-lives because they are rapidly degraded. Therefore, the influence of these hormones on hunger and satiety can be modulated by leaving a short gap between food intake and by eating frequently.1 Repeated exposures to palatable, processed and inexpensive foods, especially those containing sugars, reinforces the pleasure of the food and leads to overeating.10
For a comprehensive appreciation of how hormones regulate gustatory responses it is necessary to understand the anatomy, physiology and biochemistry that underlie taste perception. Outstanding research has yielded information on the roles of neurotransmitters, both classic and nonclassic small molecules, in shaping taste responses, and this topic has been extensively reviewed elsewhere.11–13 This Review discusses the biological mechanisms that underpin taste perception, the hormones produced in taste buds and their involvement in taste perception, as well as taste receptors in the gut and pancreas. Additionally, the neuronal response to ingestion of sugar is addressed.
Chemosensory processes in the tongue
Taste bud cell types and taste perception
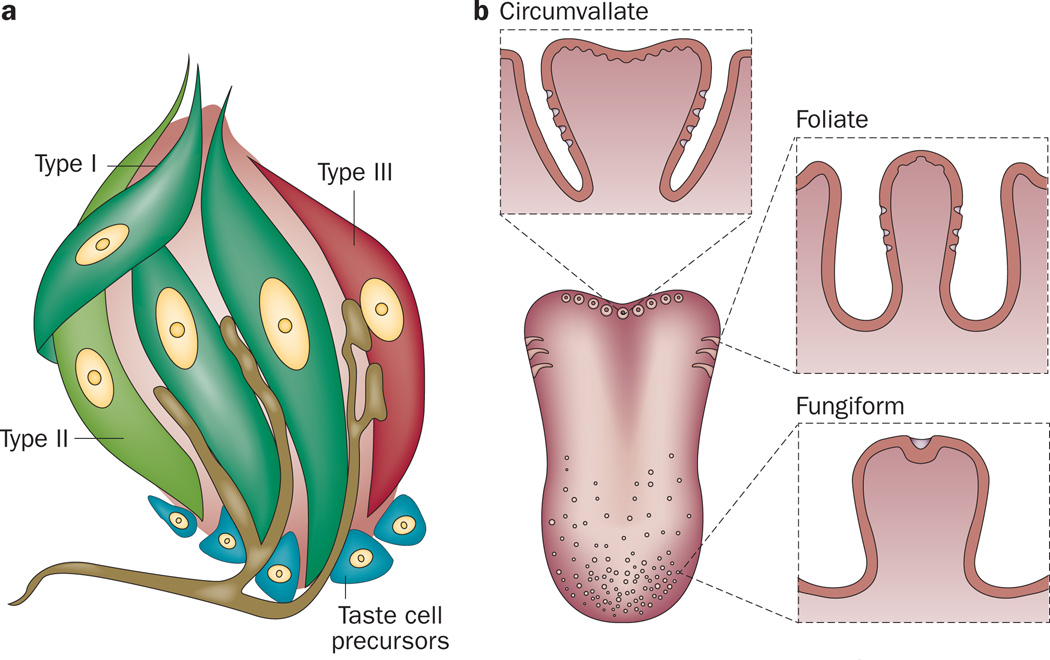
Taste, or gustation, is one of the five primary senses. Taste perception is triggered by chemicals when they come in contact with taste bud cells (TBCs) of the tongue; subpopulations of TBCs synthesize hormones (such as GLP-1 and ghrelin) that are also found in the gut and brain.14,15 Five basic tastes are recognized by most animals (sweet, umami, bitter, salty and sour) and there is growing evidence that fat can also be tasted.16,17 Taste buds have a structure similar to that of a garlic bulb (Figure 1a) and they primarily reside within taste papillae located in circumvallate, foliate and fungiform papillae of the tongue (Figure 1b). Some isolated taste buds and chemosensory cells that express G-protein-coupled taste receptors (taste GPCR) are present on the palate, epiglottis, pharynx, larynx and the nasoincisor duct of rats.18,19 Each taste bud contains ~50–100 TBCs (assembled as ‘cloves’ in the garlic-bulb-like structure) that are of epithelial origin, unlike olfactory receptor cells that arise from neurogenic precursors.20 TBCs are classified into four subtypes and all taste buds contain cells of all four subtypes regardless of their anatomical location (Figure 1a).
Figure 1.
Localization and structure of taste buds in the human tongue. a | Schematic representation of a taste bud and intragemmal nerve fibres. By convention, four subtypes of taste bud cells are present in taste buds. Of the four subtypes, only type III taste bud cells form recognizable synapses with the afferent nerve fibres. b | Localization of taste papillae. Circumvallate (back of tongue), foliate (sides of tongue) and fungiform (middle and front of tongue).
Type I cells
Approximately 50% of the total number of TBCs are type I cells that maintain the supporting structure of the taste buds. These cells are characterized by distinct electrophysiological features. Type I TBCs have small voltage-gated outward K+ and inward Na2+ currents but no voltage-gated Ca2+ currents.21 Amiloride-sensitive sodium channel subunit α (commonly known as epithelial sodium channel subunit α [α-ENaC]) is expressed on type I cells and is considered to be the major mediator of perception of low salt (for example, NaCl or KCl).22,23 TBC-specific deletion of α-ENaC in mice caused complete loss of behavioural attraction to salt;23 however, the downstream signalling mechanisms that are activated when a low-salt tastant engages type I cells and how these cells communicate with nerve fibres are unknown. In addition to expressing α-ENaC, these cells express membrane- bound ATPase on the cell surface that degrades ATP released from neighbouring cells. Type I cells have extensive lamellar processes that wrap around the other cell types within the taste bud structure, which probably function to control the dissipation of cell signalling molecules throughout the taste bud and isolate ion fluctuations to specific areas of the taste bud.24,25
Type II cells
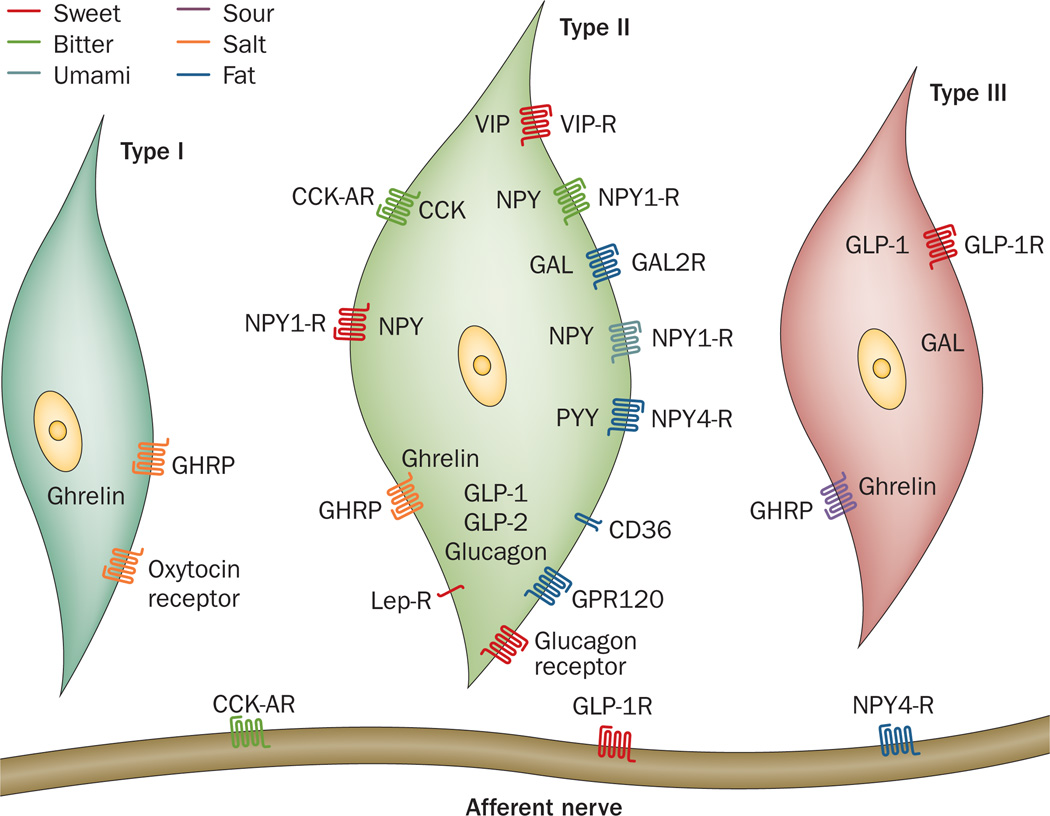
Type II cells, often referred to as receptor cells, express receptors for sweet, umami and bitter tastants.26–28 Sweet and umami tastants are sensed by heterodimeric GPCR comprising a family of three receptors (TAS1Rs): taste receptor type 1 member 1 (TAS1R1), taste receptor type 1 member 2 (TAS1R2) and taste receptor type 1 member 3 (TAS1R3). Heterodimeric receptors of TAS1R1 and TAS1R3 subunits are activated by umami tastants (for example, glutamate, broth, mushrooms, meat and l-amino acids)29–31 and heterodimeric receptors of TAS1R2 and TAS1R3 subunits are activated by sweet tastants (for example, sucrose, fructose and glucose, as well as artificial sweeteners such as sucralose).30,32–34 Mice that lack TAS1R3 have diminished responses to both sweet and umami tastes, which indicates that although these heterodimeric complexes might be the major mediators for these tastes, other mechanisms for sweet and umami taste perceptions exist.35 Bitter tastants (such as caffeine, quinine and denatonium benzoate) are sensed by GPCR of the type 2 taste receptor (TAS2R) family, which has ~30 members.36–38 Each type II TBC expresses either the TAS1Rs or specific members of the TAS2R family (each bitter-tasting cell can co-express 4–11 TAS2Rs) and, therefore, responds exclusively to either sweet and umami, or bitter tastants. Type II cells contain the bulk of the different hormones synthesized by TBCs, as well as their cognate receptors (Figure 2).14,15,39–44 Expression of fat sensors, such as free fatty acid receptor 4 (GPR120) and platelet glycoprotein 4 (CD36), which detect long-chain fatty acids (LCFAs), in type II TBCs has been reported (Figure 2).16,17
Figure 2.
Expression of hormones and their receptors in the three subtypes of taste bud cells that perceive the five prototypic tastants, as well as fat. The specific taste perceptions are represented as sweet, bitter, umami, sour, salt and fat. Abbreviations: CCK, cholecystokinin; CCK-AR, cholecystokinin-A receptor; CD36, platelet glycoprotein 4; GAL, galanin; GAL2-R, galanin receptor type 2; GHRP, ghrelin receptor; GLP-1, glucagon-like peptide 1; GLP-1R, glucagon-like peptide 1 receptor; GLP-2, glucagon-like peptide 2; GPR120, free fatty acid receptor 4; Lep-R, leptin receptor; NPY, neuropeptide Y; NPY1-R, neuropeptide Y receptor type 1; NPY4-R, neuropeptide Y receptor type 4; PYY, peptide tyrosine tyrosine; VIP, vasoactive intestinal peptide; VIP-R, vasoactive intestinal peptide receptor.
Type III cells
Presynaptic cells (type III cells) are the only type of TBCs that form conventional neuronal synapses with sensory afferent intragemmal nerve fibres. Similar to neurons, these cells contain voltage-gated Ca2+ channels and release vesicular serotonin, acetylcholine, norepinephrine and γ-aminobutyric acid (GABA) when depolarized.45 These cells also express polycystic kidney disease 2-like 1 protein (PKD2L1) and polycystic kidney disease 1-like 3 protein (PKD1L3) channels, which together are involved in perception of sour (acid) taste.46 The absence of PKD2L1-expressing type III cells in mice caused either complete abolition of the response or reduced sensitivity to acidic chemicals (such as citric acid).47,48 High salt concentrations are aversive and activate bitter sensing in type II cells and sour sensing in type III cells.49
Taste cell precursors
The final subtype of TBCs (previously referred to as type IV cells) comprise a small heterogeneous group of cells located toward the base of the taste bud structure. These cells were initially thought to be the exclusive progenitor cells for the differentiated TBC types;50 however, it is no longer thought that the TBC stem cell niche is located solely at the base of the taste bud.51–53 Sonic hedgehog protein (SHH) regulates the differentiation of TBCs. SHH-expressing cells within taste buds signal to SHH-responsive cells, which are located outside of the taste bud and express the transcription factors zinc finger protein GLI1 and patched domain-containing 1 (known as patched 1). Multiple areas of SHH-responsive cells surround taste buds in the adult mouse tongue.52 Using lineage-tracing experiments, SHH-expressing cells within taste buds were shown to be immediate precursors of the other three cell types.51 Additionally, another study confirmed that the progenitors of TBCs are located outside of the taste bud itself by showing that very few (<10%), if any, cells proliferate within taste buds.53 Thus, the small cells present at the base of taste buds are of two different categories, quiescent precursor cells and immature taste cells, neither of which are progenitor cells. Therefore, the term type IV cell is no longer commonly used to describe a particular TBC type.
Signalling mechanisms of taste perception
Given that type III cells are the only taste cells with conventional neuronal synapses (Figure 1a), type II and type III cells were previously thought to communicate via gap junctions to elicit activation of taste nerves to convey information to the brain regarding the nature of the tastant.54,55 In transgenic mice that express a fluorescently labelled trans-synaptic protein (wheat germ agglutinin; WGA) under the control of the TAS1R3 promoter, some WGA expression was found in serotonin- positive cells (presumably type III cells), which implied direct communication and passage of proteins from type II cells.56 However, two subsequent publications did not find lateral transfer of non-fluorescently labelled WGA linked to TAS1R3 in type III cells.57,54 Correspondingly, transgenic mice expressing WGA under the control of the PKD1L3 promoter, which is only expressed in type III cells, did not have any WGA present in type II cells.55 These data suggest that no direct transfer of protein occurs between type II and type III cells. Furthermore, genetic ablation of type III cells from mice does not result in disruption of sweet, bitter or umami perception, which demonstrated that these cells are not required for transmission of taste information from type II cells.47
Studies published in the past decade have shown that type II cells directly communicate with taste nerves via ATP release and activation of purinergic receptors on nerve fibres, thereby negating the need for direct transfer of molecules between the two cell types.12,24 In addition to ATP, type II cells release locally produced hormones for communicating information to neighbouring cells (paracrine effect). These hormones can also alter taste perception in an autocrine fashion, by modifying taste signalling mechanisms in the hormone-expressing cells.58 In addition, the afferent nerve fibres in taste buds contain receptors for locally produced hormones, such as GLP-1 and neuropeptide Y (NPY),14,59 thereby enabling modulation of the intensity of the taste signal that acts as an ‘on switch’ for downstream cell signalling after exposure to a particular tastant (Figure 2).
TAS1Rs (umami and sweet), TAS2Rs (bitter) and fat receptors share downstream signalling pathways, albeit in different subtypes of TBCs, that ultimately lead to release of ATP and hormones. Specifically, when a tastant (sweet, bitter, umami or LCFAs) binds to its specific taste receptor or sensor, PLCβ2 is activated and second messengers such as IP3 are generated. IP3 causes release of intracellular Ca2+ that gates TRPM5 (transient receptor potential cation channel subfamily M member 5; encoded by Trpm5) channels, which results in cellular depolarization.60–62 The action potentials generated in the TBCs lead to release of non-vesicular ATP through voltage-gated CALMH1 (calcium homeostasis modulator 1) channels63,64 that engage purinergic receptors on the sensory nerve fibres, as well as on type II and type III cells.12 In type II cells, activation of purinergic receptors potentiates increased release of ATP, whereas in type III cells, voltage-gated Ca2+ channels are activated, which causes release of classic neurotransmitters.12,65 Knockout of Trpm5 in mice abolished sweet, bitter and umami discrimination66 and Trpm5 was also found to be required for LCFA-mediated depolarization of TBCs and a preference in mice for fat over non-fat- containing tastants.61,67,68 Knockout of CALHM1 in mice led to severely impaired perception of sweet, umami and bitter tastes, as well as a strong reduction in release of ATP from type II TBCs in response to tastants.64
Together, these observations suggest that the major line of communication of taste information from type II cells to the brain occurs via release of ATP, which interacts directly with the taste nerves to convey information to higher order neurons. Importantly, the ATP that is released from type II cells is degraded by membrane-bound ATPases on type I cells, which generates ADP that prevents purinergic receptor desensitization on afferent fibres.69 Non-specific ectopeptidases degrade some ADP molecules to adenosine that can act on adenosine receptor A2B, which is present on a subset of TAS1R–expressing cells and enhances ATP release (and presumably also increases hormone release) in response to activation of TAS1Rs by sweet-tasting ligands.70
Sour taste is perceived when protons enter type III cells, causing cellular acidification. This proton influx results in closure of resting K+ channels, membrane depolarization and release of classic neurotransmitters.71
Hormones expressed in taste buds
CCK and Y-family peptides
CCK and NPY were the first hormones to be described in rat TBCs.41,72 CCK functions in an autocrine fashion through the cholecystokinin receptor type A (CCK-AR) and inhibits activation of both delayed rectifier and inward rectifier K+ channels, as well as increasing intracellular Ca2+ by as much as 30% upon stimulation of TAS2Rs.41 Given that K+ channels are needed for membrane repolarization in TBCs, these cells remain in a depolarized state for an increased period of time, which results in prolonged bitter signalling.58 Additionally, rats that lack CCK-AR have enhanced lick responses to sweet substances, including artificial sweeteners, which demonstrates that absence of this receptor alters taste perception.73
Expression of NPY in TBCs overlaps almost entirely with that of CCK; similarly, NPY is also released from TBCs upon membrane depolarization.58 The Y family of peptides comprises pancreatic polypeptide, NPY and peptide tyrosine tyrosine (PYY), which are involved in regulation of energy metabolism via a family of GPCR known as NPY receptors (NPY1-R, NPY2-R, NPY4-R, NPY5-R and NPY6-R).74 NPY4-R is expressed on taste nerve fibres and NPY1-R is expressed on type II TBCs that express TAS1Rs, as well as microvilli projections.58,59 In TBCs, NPY functions antagonistically to CCK and enhances inwardly rectifying K+ currents, which results in downregulation of signalling in response to sweet and umami tastants.72,75 This hierarchical signalling arrangement, which amplifies perception of bitter taste, enables bitter-tasting toxins not to be overshadowed by simultaneous presentation of sweet stimuli and their accompanying hedonic sensations. The ability to interrogate sweet and bitter tastants when presented side by side in the wild enables primates to differentiate between bitter (poisonous or rotten vegetation) and sweet (ripe and usually non-poisonous vegetation) is evolutionarily critical to survival.76 This process is only one of several adaptive mechanisms to ensure that bitter taste is paramount.77
PYY, which engages NPY1-R and NPY4-R, is secreted from L-cells and is also produced in taste buds.43 Rodents express two forms of the protein: PYY(1–36), which is relatively non-selective for all NPY receptors, and PYY (3–36), which is a product of dipeptidyl peptidase 4 (DPP4) enzyme activity, is the most abundant form of the protein found in circulation and preferentially activates NPY2-R.78 TBCs do not express DPP4;14 therefore, PYY(1–36) is likely to be the most abundant form of the protein present in taste buds. Mice lacking PYY demonstrated considerably reduced preferences for fat taste.43,59 Moreover, mice lacking CD36 (a lipid sensor that belongs to the scavenger receptor family and binds saturated and unsaturated LCFAs)79,80 showed no taste preference for LCFA-enriched solutions and solid food, which indicates that CD36 function is an absolute requirement for oral discrimination of LCFAs.81 PYY might be involved in regulating CD36 expression and/or function; however, this possibility has not yet been studied.
Glucagon, GLP-1 and GLP-2
Glucagon, GLP-1 and glucagon-like peptide 2 (GLP-2) are cleavage products generated from a single pro-glucagon peptide encoded by GCG.82 GLP-1 and GLP-2 are the major protein products found in enteroendocrine L-cells, and glucagon is the main peptide generated in α-cells in islets of Langerhans in the pancreas, although GLP-1 and GLP-2 are also produced in these cells.83 The glucagon-like hormones are present in TAS1R–expressing type II cells and some type III cells and their cognate receptors are expressed on adjacent afferent fibres (Figure 2).14 GLP-2 is a potent trophic factor in the gut;84 however, the role of this hormone in TBCs is not known. By comparison, GLP-1, which is insulinotropic in islets of Langerhans, contributes to sweet tasting.82
Mice deficient for GLP-1-receptor (GLP-1R) have dramatically reduced taste responses to both calorie-containing (sucrose) and artificial (sucralose) sweeteners and have increased sensitivity to umami tastants.14,85 Sucrose, artificial sweeteners and umami stimuli elicit secretion of GLP-1 and NPY from TBCs in mouse circumvallate papillae—an effect not present in TAS1R3 null mice.86 This finding illustrates the reliance on GLP-1 for downstream signalling, cell depolarization and signal transmission to GLP-1Rs on sensory afferents for complete perception of a given tastant. Sour and high salt concentrations increase NPY, but not GLP-1, secretion from TBCs.87 Studies in both humans and mice have shown that LCFAs increase GLP-1 secretion from TBCs in addition to reinforcing the preference for sucrose in a GLP-1R dependent manner, probably by interacting with GPR120.88,89 Given that DPP4, an enzyme that rapidly degrades active GLP-1, is not present in taste buds, it is possible that GLP-1R signalling is increased owing to a high local concentration of GLP-1.14 Expression of GLP-1 and NPY in TBCs is sufficient for differentiation between sweet, umami, bitter and sour (aversive) tastes.87 Glucagon and its receptor are present in a subpopulation of type II cells (Figure 2),42 and either pharmacological or genetic disruption of glucagon signalling in TBCs led to a reduction in only sweet taste signalling.42 Paracrine glucagon signalling through its specific receptor enables enhancement of sweet signalling in a local circuit. However, unlike the effects of sweet tastants on GLP-1 release, glucagon secretion decreases in response to sweet tastants, which indicates that glucagon is expressed in a different population of type II TBCs than those that express GLP-1 and GLP-2.
VIP peptides
VIP is synthesized in a subpopulation of cells that express TAS1R3 and TAS2R in addition to the VIP receptors, VIP-R-1 and VIP-R-2.40 Mice that are deficient for VIP have enhanced perception of sweet and bitter tastants. Thus, activation of the GLP-1 signalling pathway results in opposing effects on sweet taste perception compared with that of VIP signalling. Interestingly, the number of cells expressing GLP-1 and the overall expression levels of this hormone were substantially increased in the TBCs of mice lacking VIP, which suggests that an intrinsic balance in the number of cells that express either GLP-1 or VIP exists.40
Ghrelin
Widely studied in the context of appetite regulation, ghrelin is secreted from X/A-like cells in the stomach and, along with its receptor, GHRP, is reported to be expressed in all four TBC subtypes.15 However, expression of the enzyme that acylates and activates ghrelin, ghrelin–O-acyltransferase (GOAT), seems to be restricted to a subset of ghrelin-expressing cells.15,90,91 Mice lacking GHRP have considerably reduced perception of salty and sour tastes; however, there might be receptors other than GHRP and ligands in addition to acylated ghrelin that drive these responses in TBCs.92,93 Mice lacking either GOAT or ghrelin showed slightly different phenotypes with respect to taste perception. Similar to PYY-deficiency, lack of either GOAT or ghrelin resulted in decreased responsiveness to LCFAs (which suggests this pathway might act in synergy with that of PYY). However, perception of salt taste was reduced in mice lacking ghrelin but enhanced in GOAT-deficient mice.15
Oxytocin
Although TBCs and the nerve fibres that surround the TBCs seem to lack oxytocin expression, the oxytocin receptor is present on type I (low-salt sensing) TBCs (Figure 2). Exposure of type I TBCs to oxytocin resulted in Ca2+ mobilization that was blocked by an oxytocin receptor antagonist.94 These histological and cellular findings support the known role for oxytocin as a hormone that regulates salt appetite and natriuresis.95
Galanin
Found predominantly in the central nervous system and gut,96 galanin is involved in regulating food intake, as well as gut motility and hormone secretion.97 This hormone is expressed in type II and type III TBCs; mRNA transcripts that code for galanin receptor type 2 (GAL2-R) have been detected in taste buds (Figure 2).98 Mice that overexpressed galanin had a 55% increase in consumption of fat-rich diets but showed no increase in preference for sweet or bitter tastes;99 conversely, mice that were deficient for galanin had reduced preferences for fatty diets compared with wild-type mice.100 These results strongly support a role for galanin in the regulation of fat consumption, possibly by modulating the lipid sensor signalling machinery (via CD36 and/or GPR120) in type II TBCs.88,101
Leptin
Leptin is a key hormone that is involved in regulating energy expenditure, body weight, fat mass and feeding behaviour. Levels of circulating leptin are highly correlative with total body adiposity.44 The leptin receptor is expressed on type II cells (Figure 2) and its activation at room temperature (24 °C) hyperpolarizes TBCs to sweet stimuli by activating outward K+ currents.102 However, when sweet stimuli were applied to mouse tongues at 35 °C, after administration of leptin, the magnitude of neuronal responses through the chorda tympani branch of cranial nerve VII (facial nerve), which transmits taste information from TBCs in the front of the tongue, was increased.103 Although the experimental conditions of the studies differed, it is known that TRPM5 channels have increasing activation with increasing temperatures104 and, therefore, these findings support a role for leptin in augmenting activity of TRPM5 channels, which leads to increased ATP release and increased activity through sensory nerves with increasing temperatures.
The hypothesis that leptin regulates sweet taste perception is supported by the observation that when leptin was administered to normal mice and to mice genetically lacking leptin, their behavioural testing responses to sucrose and saccharin were substantially decreased; however, no behavioural taste changes occurred after leptin administration to mice lacking leptin receptor.44 Consequently, in leptin-sensitive states, leptin might function to prevent gorging on sweet-containing foods. However, in leptin-resistant states such as obesity, this mechanism of food intake regulation might be blunted. Given that mice lacking VIP have increased sweet perception and increased numbers of GLP-1-expressing TBCs but also have reduced levels of circulating leptin, these data support the existence of homeostatic and functional interactions between VIP, GLP-1 and leptin activity in taste buds.40
TBCs have similarities to islets of Langerhans
Taste buds and islets of Langerhans (endocrine cells in the pancreas) share many phenotypic similarities. Structurally, both are endocrine organs embedded in epithelial tissue, they each have a specialized function that differs from that of the surrounding parenchyma and both have their own blood supply.105, 106 Additionally, analogous to islets of Langerhans, some TBCs co-express the cognate receptors for the specific hormone with which they are associated.107 By contrast, islets and taste buds regulate intake and processing of nutrients at different stages of the digestive process. Taste buds are integrators of information sensed from the various components in food, meaning that they contribute to the preabsorptive responses to food components;108 whereas islet hormone secretion is primarily regulated by the postabsorptive products of food consumption, thereby functioning to regulate energy utilization and glucose metabolism.109
The gut as a chemosensory organ
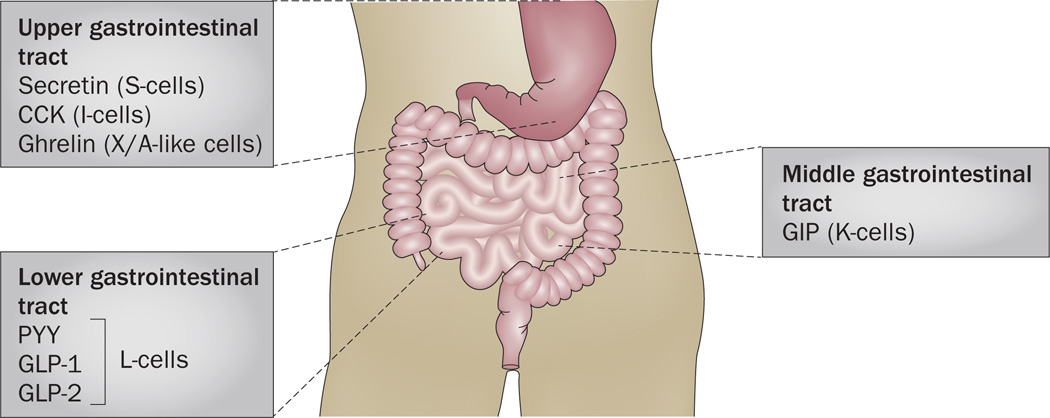
The gut is the largest hormone-producing organ in the body in terms of both number of cells and diversity of hormones; however, enteroendocrine cells represent only 1% of the entire intestinal epithelium.110 Gastrointestinal epithelial cells that function as molecular sensors are involved in multiple processes related to food intake and digestion.111 Interestingly, many of the hormones identified in the gut (Figure 3) are also expressed in TBCs.
Figure 3.
Localization of selected hormones along the gut. Hormones that are expressed in the gut and that are also present in the taste bud cells within taste buds. CCK and ghrelin are found in the upper gastrointestinal tract; CCK is secreted by I-cells and ghrelin is secreted by X/A-like cells. In the middle gastrointestinal tract, K-cells secrete GIP. In the lower gastrointestinal tract, L-cells secrete PYY, GLP-1 and GLP-2. Abbreviations: CCK, cholecystokinin; GIP, gastric inhibitory polypeptide; GLP-1, glucagon-like peptide 1; GLP-2, glucagon-like peptide 2; PYY, peptide tyrosine tyrosine.
Sweet taste receptors
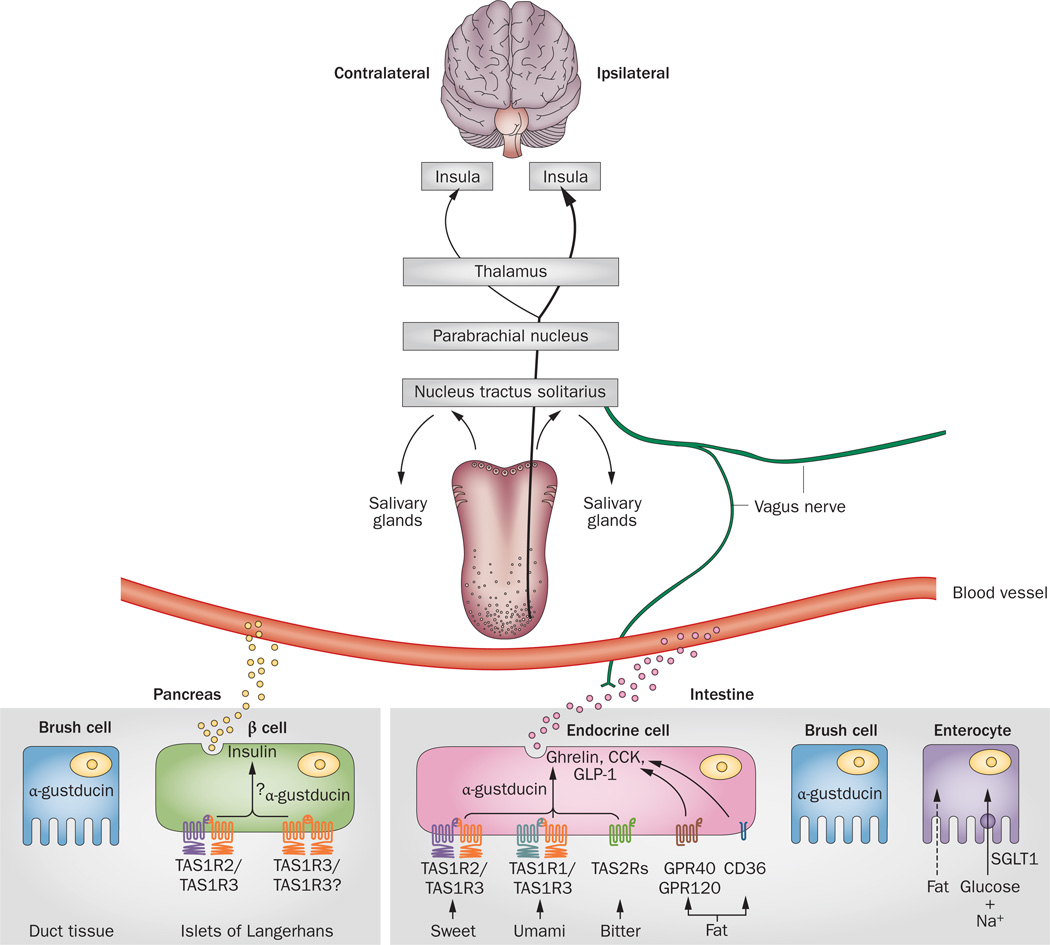
Given that nutrient sensing in the gut is not only important for digestion but also for controlling food intake, research efforts have focused on understanding the taste-gut-brain axis and how it functions to regulate food intake. TAS1Rs, which are directly activated by nutrients, have been detected in brush cells, K-cells, L-cells, K/L enteroendocrine cells and X/A-like cells of the stomach.112 Exposure to glucose or sucralose results in increased secretion of GLP-1 and GLP-2 from L-cells in mouse small intestine, an effect that can be counteracted by gurmarin (a sweet taste inhibitor).113 Intestinal TAS1Rs are associated with secretion of gut hormones and mice deficient for guanine nucleotide-binding protein G(t) subunit α-3 (which is a specific GTP-binding α-subunit expressed in type II TBCs; commonly known as α-gustducin) have reduced glucose- mediated GLP-1 release,114 which indicates that intestinal TAS1Rs could be glucose sensors (Figure 4). TAS1R2/TAS1R3 heterodimers and α-gustducin are present in K/L-cells that express both GLP-1 and gastric inhibitory polypeptide, as well as L-cells that express GLP-1 and GLP-2.115,116 Glucose-mediated GLP-1 release is regulated by several mechanisms: the classic two being glucose-sensing machinery, as in pancreatic β cells, and the sodium-coupled glucose uptake mediated by sodium/glucose transporters (SGLTs).117 SGLT-1 protein is the major route for transport of dietary sugars from the intestinal lumen into enterocytes. TAS1Rs have a substantial role in the upregulation of SGLT-1 in response to both luminal sugars and sweeteners. Mice lacking either TAS1R3 or α-gustducin are unable to increase expression of SGLT-1 protein in the intestine in response to carbohydrate-containing foods entering the small bowel; sodium– glucose-dependent transport after sugar ingestion is also impaired in these animals.115
Figure 4.
Relationship of organ systems with taste-sensing machinery. Nerve fibres that transmit taste information from the taste papillae converge in the NTS in the brainstem; taste perception signals are then routed to the PBN (shown only in rodents), toward the thalamus and terminating in the primary gustatory cortex in the insula. Beyond the pons, one-third of the fibres ascend and crossover in the thalamus such that taste is represented bilaterally in the insulae. Local projections from the NTS mediate salivation from salivary glands and serous secretions from Von Ebner’s glands. α-gustducin is expressed in the brush cells of the pancreatic ducts and intestine; however, its function in these cells in not yet known. α-gustducin and TAS1Rs are also expressed in β cells in islets of Langerhans, where they might be involved in regulating basal insulin secretion. Additionally, TAS1Rs in islets are activated by fructose, which leads to enhanced glucose-mediated insulin secretion. In enteroendocrine cells, receptors for sweet (TAS1R2/TAS1R3 or a putative TAS1R3/TAS1R3 dimer), umami (TAS1R1/TAS1R3), bitter (TAS2R) and long-chain fatty acids (GPR120, GPR40 and CD36) are expressed and are involved in secretion of GLP-1, CCK and ghrelin. Glucose and fat are absorbed into enterocytes: glucose absorption is through sodium-dependent SGLT1 channels and fat molecules freely diffuse across the luminal surface. Abbreviations: CCK, cholecystokinin; CD36; platelet glycoprotein 4; GLP-1, glucagon-like peptide 1; GPR40, free fatty acid receptor 1; GPR120, free fatty acid receptor 4; NTS, nucleus tractus solitarius; PBN, parabrachial nucleus; SGLT1, sodium/ glucose cotransporter 1; TAS1R1, taste receptor type 1 member 1; TAS1R2, taste receptor type 1 member 2; TAS1R3, taste receptor type 1 member 3; TAS2R, taste receptor type 2.
Bitter taste receptors
Multiple members of the TAS2R family are expressed in the mammalian intestine and in the mouse gut-derived enteroendocrine cell line STC-1 (a model system used to study secretory mechanisms in enteroendocrine cells, owing to their ability to synthesize CCK and proglucagon products).111,118 In mice, bitter receptors are localized to a subset of goblet cells in the colon and in the stomach; however, their function, if any, in the colon remains unknown.119
Challenge of wild-type mice with a prototypic TAS2R–agonist mixture (administered by oral gavage) led to an increase in plasma levels of acylated ghrelin with peak levels being achieved by 40 min after gavage. Secretion of acylated ghrelin seems to be regulated by α-gustducin (which is expressed in X/A-like cells) as α-gustducin-deficient mice had blunted responses to challenge with a bitter mixture. Furthermore, in wild-type mice, the increase in levels of secreted acylated ghrelin that resulted from gavage with a bitter mixture translated into an acute increase in food intake followed by a decrease in food intake over 4 h that correlated with a delay in gastric emptying. These effects were not observed in similarly challenged α-gustducin-deficient mice.120 This delay in gastric emptying could prevent potentially toxic chemicals entering the small bowel where they can be ingested. Additionally, retention of the toxin in the stomach likely increases the probability of emesis. Interestingly intragastric infusion of denatonium benzoate in rodents produced flavour aversions that prevented future ingestion of other bitter compounds.121 Bitter receptors in the gut might, therefore, exist to detect toxic compounds that have bypassed olfaction and/or taste protective mechanisms. This idea raises the possibility that agonists of TAS2Rs might be useful to treat obesity as they would potentially prolong the sensation of fullness, increase the time between consuming meals and induce aversion to particular co-administered food stuffs.
Fat-sensing receptors
Owing to their lipophilic nature, LCFAs were previously assumed to diffuse freely across the plasma membrane; however, new evidence of the existence of fat transporters in the intestine suggests that LCFA uptake is an active cellular process. One candidate implicated in the early phase of fat absorption is the scavenger receptor CD36 that is expressed in type II TBCs and differentially expressed along the gut (predominantly in the duodenum, jejunum and ileum and less abundant in the stomach and colon).118 Levels of mRNA transcripts that encode CD36 are considerably reduced in the small intestine of mice after infusion of LCFAs directly into this region of the gastrointestinal tract, which suggests that a saturable mechanism of fatty acid translocation exists122 and is consistent with the reduced levels of CD36 in taste buds observed in the fed state.123 Given that PYY is secreted from L-cells in response to LCFAs, it is possible that this peptide hormone regulates CD36 expression in the gut in a similar manner to that proposed to occur in TBCs. Mice lacking CD36 also demonstrate reduced secretion of secretin and CCK (60% and 50%, respectively) after intragastric loading of LCFAs compared with similarly treated wild-type mice,124 which suggests that activation of CD36 signalling regulates LCFA-mediated hormone secretion (Figure 4).
LCFA-mediated secretion of gut hormones also occurs through a large family of fatty-acid-specific GPCR,125,126 some of which exhibit species and tissue-specific expression. For example, free fatty acid receptor 1 (GPR40) is preferentially expressed in mouse127 and human pancreatic β cells,128,129 and in taste buds of mice,130,131 but is absent in the human tongue.132 By comparison, GPR120 is expressed in type II TBCs.16,17,123 Receptors in this family function as nutrient sensors and are important for the maintenance of energy homeostasis.133
The fatty-acid-specific GPCR are expressed on intestinal enteroendocrine cells (Figure 4) and their functions and mechanisms of action are the focus of ongoing investigations. A decade ago, linoleic acid (α–LA) was shown to stimulate GLP-1 secretion in both SCT-1 cells and in mice, at least in part via GPR120 signalling.89 This finding is consistent with a role of GPR120 signalling in LCFAs-mediated GLP-1 secretion in TBCs.88 Other studies in mice have since provided evidence that GLP-1 secretion seems to be independent of GPR120 signalling, instead suggesting that GPR40 might be the dominant LCFA receptor that drives GLP-1 secretion.134 The discrepancies between the reported findings might be a result of differing expression of the various fatty-acid GPCR in STC-1 cells and in mice.
In addition to CD36, GPR120 and GPR40 have been reported to be involved in LCFA-mediated secretion of CCK in the gut;135,136 however, the extent of their involvement in secretion of CCK from TBCs is unclear. One study performed in SCT-1 cells showed that GPR120 is the predominant mediator of LCFA-mediated CCK secretion;135 however, a study using isolated CCK-eGFP-positive I-cells from mice lacking GPR40 demonstrated that α-LA-mediated secretion of CCK was reduced by 50% when compared with equivalent cells isolated from wild-type animals.136
Endocannabinoid signalling
Anandamide (a fatty-acid ethanolamine) and 2-arachidonoyl-sn-glycerol (2-AG) are endogenous ligands of the GPCR cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2).137 CB1 is expressed in type II TBCs, where it functions to enhance perception of sweet taste.138 The central endocannabinoid system, specifically CB1-mediated signalling, regulates food intake; however peripheral endocannabinoid mechanisms could also be involved. Levels of anandamide in the limbic forebrain and the hypothalamus are increased after fasting;139 peripheral administration of anandamide (and other CB1 agonists) resulted in a considerable increase in food intake in partially satiated rats.140 Surprisingly, administration of oral fat and a complete liquid meal to sham-feeding animals for 5 days also increased the levels of anandamide and 2-AG in the upper gastrointestinal tract, while sucrose and protein administration did not. Accumulation of endocannabinoid in gut tissue was abolished when the vagus nerve was severed, which suggests that endocannabinoid mobilization from the gut requires vagal stimulation.141,142 In addition, some putative fat-sensing orphan GPCR are abundantly expressed in the small intestine. G-protein coupled receptor 55 and G-protein coupled receptor 119 are considered to be endocannabinoid receptors (despite having little homology with CB1 and CB2) and could have important roles in the mobilization of endocannabinoids from the gut; however, whether or not they function to modulate food intake remains unknown.143
Role of the gut microbiota in food sensing
Obesity and metabolic disorders are associated with alterations in the composition and ecology of the gut microbiota.144 The composition of the gut microbiota is highly variable among individuals but stable across healthy individuals. Mice that lack gut microbiota (termed germ-free mice) are substantially leaner than normal mice despite consuming more calories,145 which supports the hypothesis that the microbiota contributes to regulation of energy homeostasis in the host. Whether or not germ-free mice have alterations in TBC morphology or hormone content in tongue papillae has not yet been investigated. However, expression analysis of the genes that code for TAS1R, α-gustducin and SGLT-1 showed increases in the gut in germ-free mice compared with control animals, whereas no differences were observed in expression of the genes encoding TAS1R and α-gustducin in lingual tissues.146 Furthermore, although germ-free mice demonstrated unaltered sweet preferences, they consumed more of a high-sucrose solution than control mice,145 which correlates with the upregulation of expression of genes encoding taste-sensing molecules and glucose transporters in the gut. This upregulation might also be a homeostatic adaptation in response to reduced circulating blood glucose levels (reflecting an energy deficit) in the lean mice. Therefore, continuous monitoring of glucose levels under fed and fasting states in germ-free mice could help to improve understanding of glucose regulation in this model.
Short-chain fatty acids generated via polysaccharide fermentation by gut microbiota are ligands for the GPCR, which are located in the gut epithelium and are reported to be associated with maintaining energy balance and development of metabolic disorders. In a study that addressed a role for microbiota in recognition of fat, germ-free mice had increased preference for low concentrations of fat and increased calorie intake from fat.145 Interestingly, these animals also had increased expression of CD36 in taste buds, but decreased expression of CD36 in the gut.145 Expression levels of CD36 in taste buds of fed germ-free mice were similar to those of fasting control mice, which suggests that the germ-free animals were in an energy depleted state.16 Similar to the increased sucrose consumption in these mice, the increased preference for fat could be an adaptation to a chronic energy-deficient state, which causes the lean phenotype. Germ-free mice also had reduced protein levels of the satiety peptides CCK, GLP-1 and PYY in the gut that was accompanied by a decrease in expression of GPR40, free fatty acid receptor 3 (GPR41), free fatty acid receptor 2 (GPR43) and GPR120.145 These findings suggest that the gut microbiota is involved in regulating levels of fatty acid receptors in the gut. Levels of circulating leptin and PYY were also reduced in germ-free mice,145 which is consistent with the lean phenotype of these animals. Surprisingly, the germ-free mice had reduced levels of circulating ghrelin, which would be predicted to be elevated under conditions of a chronic energy deficit.
Chemosensory processes in the pancreas
Brush cells in the duct system of the pancreas contain α-gustducin (Figure 4), where it is concentrated in the terminals of interlobular ducts and at the surface of major pancreatic ducts.147 The functional importance of the presence of classic taste receptor molecules in pancreatic ducts is not yet known. However, it seems reasonable to suggest that brush cells sense chemicals in the acinar secretions and/or molecules present in the ductal epithelium itself. The presence of α-gustducin in β cells is well established and early studies used the bitter tastant denatonium benzoate as a tool to elucidate its potential involvement in insulin secretion.148 Although denatonium benzoate increased glucose-mediated insulin secretion from rat islets, its effects could not be attributed to α-gustducin activation; rather, the bitter tastant decreased activity of KATP channels, which led to depolarization of islet β cells and increased Ca2+ influx.
TAS1Rs are also localized in β cells.149 In a mouse β-cell line, exposure to artificial sweeteners (such as sucralose, acesulfame potassium and saccharin) resulted in activation of TAS1R2 and TAS1R3 heterodimers in type II TBCs and stimulation of insulin secretion in a concentration-dependent manner.149 Given that stimulation with artificial sweeteners does not generate ATP (which is a necessity for depolarization of β cells and secretion of insulin under normal physiological conditions), presumably TAS1Rs and downstream signalling pathways are activated by these agents to drive insulin secretion. In isolated mouse pancreatic islets, sucralose was found to enhance insulin secretion in the presence of glucose.149
Fructose is a potent activator of sweet taste receptors and increases glucose-mediated insulin secretion in a sweet receptor-dependent manner;150 however, this effect was not seen in islets isolated from mice deficient in either TAS1R2 or TAS1R3. Intravenous treatment with fructose (1 g per kg body weight) transiently increased levels of circulating insulin in wild-type mice, but in not mice lacking TAS1R2.150 In humans, intravenous administration of fructose had little, if any, effect on insulin secretion under fasting conditions, but when plasma levels of glucose were first increased with intravenous administration of glucose, there was a substantial augmentation of insulin secretion.151 In isolated human islets, fructose (3.0 mM) was shown to increase glucose-induced insulin secretion at high concentrations of glucose (11.0 mM), but not when the concentration of glucose was 5.5 mM; this enhancement was blocked by lactisole, a TAS1R3 receptor allosteric inhibitor.150 A caveat to the data is that fructose concentrations in vivo do not reach the levels used in these studies, even after consumption of drinks rich in fructose or sucrose (which generally have concentrations <500 µM).152 Notably, this effect was also seen for saccharin, which similarly activates TAS1R3.153 Enhancement of insulin secretion by fructose through activation of taste molecules seems to be dependent on PLCβ2, because when its activity was inhibited by an antagonist, the mouse β cells did not respond to fructose.150
In addition, new findings suggest that TAS1Rs are involved in basal (non-glucose stimulated) insulin secretion.154 Islets from mice lacking TAS1R2 hypersecrete insulin at fasting-glucose levels and these mice had lower blood glucose levels, but higher circulating levels of insulin than wild-type mice after 5 h of fasting. However, it is important to note that levels of TAS1R2 in islets are much lower than those of TAS1R3 and knockdown of TAS1R2 did not seem to affect the response to sucralose.155 One possibility that accounts for this discrepancy is that in β cells, TAS1R3 might function as a homodimer (Figure 4). Given the controversy of the available data, much work still needs to be done to fully understand the functions of taste receptors in β cells.
Neuronal control of taste
Taste sensation is bilaterally represented in the brain.156 Cranial nerves VII (facial), IX (glossopharyngeal) and X (vagus) convey taste information through multiple relay stations that ultimately connect to the primary taste cortex (located in the insula, which is overlaid by the opercular cortex). Nerve fibres from the cranial nerves enter the ipsilateral nucleus of the solitary tract (NTS) in the medulla. In rodents, NTS efferent fibres convey taste information to gustatory centres of the parabrachial nucleus (PBN) in the pons that synapse with neurons in the thalamus (Figure 4). In primates, NTS fibres bypass the PBN and synapse directly with thalamic neurons. In both rodents and primates, thalamic afferents project to the insula. In turn, the taste cortex sends projections to the amygdala and onwards to the lateral hypothalamus and nucleus accumbens, where dopamine is released in response to hedonic reward stimuli.157 At or just above the PBN, one-third of the ascending nerve fibres carrying taste perception from the tongue cross and ascend bilaterally to the thalamic taste area, thereby allowing for bilateral taste representation in the brain.157 The NTS is an important site of convergence for gustatory fibres from all three cranial nerves transmitting taste information, autonomic efferent and afferent fibres from the gut, and somatosensory afferent fibres from the face, mouth and tongue via cranial nerve V (the trigeminal nerve).158 In addition, local projections from the NTS control salivation rates within the mouth, which is necessary for mastication and appreciation of food.156 Salivation is highly activated when sour tastants are present in the mouth.159 Von Ebner’s glands, innervated by the glossopharyngeal nerve, secrete serous material containing lipases into the moats of the circumvallate and foliate papillae, which enables release of free fatty acids (the ligands for GPR120 and CD36) from masticated food.160
In experiments using transgenic mice expressing fluorescently labelled WGA driven by either the TAS2R5 or TAS1R3 promoter, bitter-responding neurons were found in the caudal regions and TAS1R3-relaying neurons were identified in the rostral regions of the NTS, PBN, thalamus and gustatory cortex.56 In functional tests using only TAS2R5-WGA transgenic mice, areas in the brain that were labelled with fluorescent WGA showed an increased expression of the immediate early response gene Zif268 (which encodes a product involved in conditioned taste-aversion learning)161 when cycloheximide (a bitter tastant) was applied to the tongue.162 Together, these data seem to support the concept of discrete areas of the brain being dedicated to relaying information pertinent to individual tastes. However, studies that used non- fluorescent WGA did not find definitive labelling of neurons in response to bitter tastants beyond the NTS, possibly because of a lack of sensitivity of the methodology.57,54,163 A comprehensive study using sophisticated in vivo two-photon-calcium imaging and an antegrade tracer to label projections from identified taste- responding cells to the insula demonstrated that taste perceptions of sweet, bitter, umami and salty, but not sour, have discrete topographic segregation in the insula.164 Given that taste-sensory neurons are thought by some neuroscientists to be broadly tuned in the central nervous system, questions on the completeness of the gustatory map remained.165,166 These data have since been substantiated in a detailed follow-up study167 that convincingly defines the afferent neural pathways that relay tastant-specific information from TBCs in the tongue to the dedicated regions in the insula.
Glucose sensors are present in the hypothalamus, brainstem, amygdala, septum and cortex, and are particularly abundant in the paraventricular nucleus of the hypothalamus,168–172 a brain region involved in counter-regulatory responses to hypoglycaemia. These neurons are generally classified as either glucose-excited or glucose- inhibited, on the basis of their electrophysio-logical responses to glucose. Although neurons were thought to sense glucose through glucokinase/KATP-dependent pathways in a similar fashion to that of the β cells in islets of Langerhans,172 prototypic sweet-taste receptors and α-gustducin are expressed in multiple areas of the forebrain (including the hypothalamus, hippocampus, the habenula, choroid plexus and cortex).173,174 Moreover, expression levels of the sweet-taste receptors in the brain are regulated by the nutritional status of the animal. In the hypothalamus, nutrient deprivation results in increased expression of sweet-taste receptors, an effect that was reproduced in a mouse hypothalamic cell line grown in low-glucose conditions; interestingly, this effect was reversed by addition of sucralose (a commonly used sweetener that cannot be metabolized) to the cells.175 Together, these findings demonstrate that glucose metabolism and subsequent generation of ATP is not required for regulating the expression of these receptors in hypothalamic neurons.
By comparison, in obese mice (which can be a model for overnutrition and hyperglycaemia), expression levels of the sweet-taste receptors in the hypothalamus were reduced.173 Moreover, hippocampal neurons, which are important for cognitive function, consistently co-express α-gustducin, TAS1R2 and TAS1R3.173,174 A study in which mice were administered the sweetener acesulfame potassium in the drinking water showed reduced levels of TAS1R3 expression in the hippocampus, a phenomenon that was paralleled by changes in prototypic glucose transporter levels and a reduction in cognitive impairment.174 In addition, ablation of TAS1R3 in mice resulted in reduced nutrient-induced activation of mTORC1, which suggests that TAS1Rs function upstream of the cell-signalling mechanisms that gauge nutrient status.176 Given these findings, we propose that taste receptors in the brain are part of a system that underlies long-term sensing of nutritional status, metabolic support and degree of cell turnover.
That tasting and digesting (postabsorption) sugar provides a dual reward is well established. The reward system is initially activated during the tasting of highly palatable foods via a postsynaptic brainstem pathway and reactivated after absorption.177 It seems that, at least in mice, absorption of calorie-rich foods directly influences the reward system independent of taste perception.177–179 For example, Trpm5−/− mice, which cannot transmit sweet, umami or bitter tasting signals from TBCs, develop a robust preference for sucrose drinks.177 Furthermore, drinking sucrose resulted in dopamine release in the nucleus accumbens and ventral striatum in both wild-type and Trpm5−/− mice.180 Disruption of glucose oxidation with administration of 2- deoxyglucose, which is a molecule that prevents cellular glucose utilization, had an inhibitory effect on the intake of artificial sweeteners as mice sought out calorie- containing foods, which illustrates the necessity of glucose oxidation for hedonic reward.181 Conversely, inhibiting the dopamine release that occurs in response to postdigestive effects resulted in reduced glucose intake, even under conditions of glucose deprivation.181 Activation of dopamine neurons synergizes with the taste of sucralose and creates a total stimulus of higher value than sucrose alone.182 Administration of leptin prior to the ingestion of glucose reduced dopamine activation and lowered sugar intake and, therefore, it seems that leptin is an important regulator of the hedonic response to food and is also a regulator of the reward value of nutrients.182
Taste perception and obesity
With the increasing abundance of inexpensive highly palatable calorie-dense processed foods, the prevalence of obesity has increased. Alterations in taste perception are thought to occur as obesity progresses. Studies in mice show that animals fed a high-fat diet have fewer sweet TBCs in taste buds than mice fed a regular chow diet and had reduced responses to sweet tastants and LCFAs, but not umami tastants.183 In physiological terms, mice on a high-fat diet required more sweet and fat tastants than lean mice to evoke similar responses. This modulation of taste perception suggests that a high-fat diet might cause a change in distribution and number of the taste receptor cells. A positive association between liking the taste of fat and BMI in children has been reported.184 Unlike CD36, expression of GPR120 is not downregulated in response to oral ingestion of fat or mixed foods,16 which suggests that this receptor might be a physiological ‘hard drive’ that integrates the amount of food consumed (the calorie equivalent) with taste perception and responses in the gut, liver, adipose tissues and brain. Prior to the introduction of sweetened foods that were scarce before the past millennium, GPR120 was probably an extremely important integrator of the needs of the body for energy that had to be acquired from unprocessed foods. This function is now less important owing to the inclusion of calorie-dense processed foods in modern diets. Mice lacking GPR120 that were fed a high-fat diet developed obesity and glucose intolerance.185 Consistent with the findings in animal models, humans who carry a non-synonymous mutation in GPR120, which results in defective signalling activity, have increased prevalence of obesity.185 Importantly, for healthy individuals, LCFAs consumed alone in the form of fat (without sweet or salt added) are not conducive to overeating as they are not appetising.
Surgical interventions affecting taste and food intake
Weight-loss surgery
Roux-en-Y gastric bypass (RYGB) surgery, in which the majority of the stomach and the proximal intestine are isolated from contact with food, is a widely used procedure for treating obesity. Following RYGB surgery, patients can experience a 60–70% reduction in body weight, which can be maintained for up to 15 years.186,187 In addition, resolution of some obesity- associated metabolic disorders such as type 2 diabetes mellitus can also be achieved with a remission rate of 60–80% within various time frames after surgery.186,187
Studies in rats showed considerable long-term decreases in post-RYGB body weight188–191 that were associated with a reduction of preference for high concentrations of sucrose with no difference in preference for low concentrations of sucrose in operated and control groups.189 Another study found that after RYGB, rats had higher sucrose preference scores than sham-operated rats; however, no differences in citric acid (sour), sodium chloride (low salt) and quinine (bitter) preferences were observed.190
In rodent models of RYBG surgery, increased levels of circulating GLP-1 and PYY, which can influence appetite, have been consistently demonstrated.190–193 However, despite the substantial increases in GLP-1 levels, different animal models of functional GLP-1 deficiency (such as mice treated with GLP-1 receptor antagonist, GLP-1-receptor-deficient mice and α-gustducin-deficient mice) also have reduced body weight and improved glucose homeostasis after RYGB surgery compared with sham-operated animals.192,193
The results from experiments with rodents are consistent with those in humans in which treatment with gastric bypass surgery resulted in decreases in preference for high-caloric foods.194,195 An early study that examined the effects of RYGB surgery (82 individuals) and laparoscopic gastric banding (LGB; 28 individuals) on enjoyment of food after surgery found that many individuals reported a decrease in intensity of taste. In addition, 67% of individuals treated with RYGB and 68% of those who underwent LGB developed aversions to certain foods, such as those tasting bitter and sour, as well as an increased taste for sweet. Moreover, most study participants felt that the change in taste contributed to improved weight loss.196 A study that assessed changes in rewarding properties of a food item after metabolic surgery showed a decreased preference for chocolate accompanied by an increased preference for vegetables in patients who underwent RYGB surgery compared with control individuals.194 Additionally, women who experienced an ~20% reduction in body weight following RYGB surgery reported a rapid shift in sweetness palatability, from pleasant to unpleasant, which caused a decrease in the ingestion of sweet foods.195 Whether metabolic surgery has any effect on taste bud morphology or specific populations of TBCs or their hormones has not yet been studied.
Vagotomy
The main projection sites for vagal input in the brain are also the projection sites for gustatory nerves. Studies of vagotomy surgery (that is, the resection of the vagus nerve in the abdomen) in rodent models have shown that the vagus nerve is required for the gut to regulate aversions and attractions to tasted food.121,178,179,197 Following subdiaphragmatic vagotomy in rats, intake of sucrose and saccharin was decreased; however, water intake remained unchanged.198 Interestingly, food intake and body weight were decreased in rats following vagotomy surgery.199 A complete bilateral truncal vagotomy in rats resulted in a 20% decrease in food intake over 4 weeks following surgery, which was accompanied by decreases in serum levels of leptin and total body fat; however, weight loss did not occur in rats with bilateral splanchnic nerve sectioning.200 In humans, the data on changes in body weight after truncal vagotomy are conflicting, which might be partly as a consequence of incompleteness of vagotomy and/or partial regrowth of vagal fibres. Studies in rat models of vagotomy surgery also report reduced levels of gut-derived endocannabinoids141,142 and it is possible that vagotomy surgery has effects on the release of other humoral factors that influence food intake, such as CCK and GLP-1. Notably, the effects, if any, of vagotomy surgery on expression of sweet, bitter or fat receptors in the gut or taste buds have not been reported.
Conclusions
Bitter, high-salt and sour tastes are aversive and protective against ingesting potentially fatal compounds. Sweet and umami tastes reflect the nutrient content of foods, although with the advent of artificial non-nutrient sweeteners, this relationship is not necessarily always true. Uncoupling taste perception from the postingestive properties of food (the ‘feel good’ aspect) does not result in lower body weights, at least in animal studies, and points to the importance of the hedonic value of food. The addition of sweet, a little salt and a hint of sour to food increases its palatability and its mastication, which together increases its hedonic value. The prevalence of inexpensive high-fructose corn syrup has increased the use of sugar and, coupled with consumption of increased portion sizes and the ease at which food is available, might cause the hedonic value of food to bypass satiety signals over time. A decrease in the amount of work required to prepare foods and obtain adequate calories has also influenced food intake in contemporary societies, further accelerating the pace at which the obesity epidemic has occurred. The possibility that obesity itself influences taste and taste perception also exists; however, the available data regarding taste perception in humans seems to be conflicting. Evidence suggests that individuals who used to have obesity who have lost weight by dieting, as opposed to weight-loss surgery, have a heightened desire for both sweet and high fat foods compared with lean individuals, which might be due to a perceived energy deficit at a central level, thus making it difficult to maintain a calorie-restricted diet.201 Sex differences in taste perception have also been reported,202 which have not always been considered in complex taste studies in humans. In summary, excess energy intake is a multifaceted metabolic condition that involves interactions between taste, hedonic responses and postingestive feedback loops to the brain and periphery; and in order to counter the adverse effects associated with excess energy consumption, novel approaches to intervene in the feedback loop are urgently needed.
Key points.
-
▪
Despite knowing that overeating is harmful, many people who are overweight are unable to control their food intake
-
▪
The satisfaction, or hedonic response, gained from eating overcomes satiety feedback mechanisms
-
▪
Hormones produced in taste cells in the tongue modify the intensity of taste perception; leptin modifies neurological hedonic responses to eating and the intensity of sweet perception
-
▪
Localization of taste receptors is not restricted to taste cells and the roles of these receptors in other physiological functions are being investigated
-
▪
Obesity and/or overnutrition (chronic excess energy states) might affect taste perception; individuals with obesity require increased amounts of tastants to elicit the same intensity of hedonic response as healthy individuals
-
▪
Insight into how metabolic surgery results in weight loss and understanding of the role of gut microbiota in taste perception might reveal how taste perception and obesity are related
Review criteria.
Full text articles were chosen using PubMed and Google Scholar searches using general terms such as “gustatory system”, “taste buds”, “food intake”, “hormone receptors”, “incretins”, “gut hormones”, “hedonic responses”. We have focused the research on articles published between 2005 and 2014, but have included earlier publications that are historically relevant for the topic. Articles selected were full-text, English-language papers.
Acknowledgments
The authors are supported by the Intramural Research Program of the NIA/NIH. The authors wish to thank J. O’Connell, S. Walker, M. Rouse and M. Doyle for assistance with manuscript preparation and I. Gonzalez Mariscal for help with designing the figures.
Footnotes
Competing interests The authors declare no competing interests.
References
- 1.Begg DP, Woods SC. The endocrinology of food intake. Nat. Rev. Endocrinol. 2013;9:584–597. doi: 10.1038/nrendo.2013.136. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC’s Division of Nutrition, Physical Activity, and Obesity. Overweight and obesity [online] 2014 http://www.cdc.gov/obesity/data/adult.html.
- 4.de Graaf C. Effects of snacks on energy intake: an evolutionary perspective. Appetite. 2006;47:18–23. doi: 10.1016/j.appet.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat. Rev. Neurosci. 2011;12:638–651. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- 6.Mattes R. Energy intake and obesity: ingestive frequency outweighs portion size. Physiol. Behav. 2014;134C:110–118. doi: 10.1016/j.physbeh.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Popkin BM, Duffey KJ. Does hunger and satiety drive eating anymore? Increasing eating occasions and decreasing time between eating occasions in the United States. Am. J. Clin. Nutr. 2010;91:1342–1347. doi: 10.3945/ajcn.2009.28962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Considine RV, et al. Serum immunoreactiveleptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell SE, et al. Leptin receptor gene expression and number in the brain are regulated by leptin level and nutritional status. J. Physiol. 2009;587:3573–3585. doi: 10.1113/jphysiol.2009.173328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berthoud H-R, Lenard NR, Shin AC. Food reward, hyperphagia, and obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300:R1266–R1277. doi: 10.1152/ajpregu.00028.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagai T, Kim DJ, Delay RJ, Roper SD. Neuromodulation of transduction and signal processing in the end organs of taste. Chem. Senses. 1996;21:353–365. doi: 10.1093/chemse/21.3.353. [DOI] [PubMed] [Google Scholar]
- 12.Kinnamon SC, Finger TE. A taste for ATP: neurotransmission in taste buds. Front. Cell. Neurosci. 2013;7:264. doi: 10.3389/fncel.2013.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarmolinsky DA, Zuker CS, Ryba NJP. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin Y-K, et al. Modulation of taste sensitivity by GLP-1 signaling. J. Neurochem. 2008;106:455–463. doi: 10.1111/j.1471-4159.2008.05397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin Y-K, et al. Ghrelin is produced in taste cells and ghrelin receptor null mice show reduced taste responsivity to salty (NaCl) and sour (citric acid) tastants. PLoS ONE. 2010;5:e12729. doi: 10.1371/journal.pone.0012729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin C, et al. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS ONE. 2011;6:e24014. doi: 10.1371/journal.pone.0024014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuwatari T, et al. Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett. 1997;414:461–464. doi: 10.1016/s0014-5793(97)01055-7. [DOI] [PubMed] [Google Scholar]
- 18.Roper SD. Taste buds as peripheral chemosensory processors. Semin. Cell Dev. Biol. 2013;24:71–79. doi: 10.1016/j.semcdb.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spector AC, Travers SP, Norgren R. Taste receptors on the anterior tongue and nasoincisor ducts of rats contribute synergistically to behavioral responses to sucrose. Behav. Neurosci. 1993;107:694–702. doi: 10.1037//0735-7044.107.4.694. [DOI] [PubMed] [Google Scholar]
- 20.Barlow LA, Northcutt RG. Embryonic origin of amphibian taste buds. Dev. Biol. 1995;169:273–285. doi: 10.1006/dbio.1995.1143. [DOI] [PubMed] [Google Scholar]
- 21.Medler KF, Margolskee RF, Kinnamon SC. Electrophysiological characterization of voltage-gated currents in defined taste cell types of mice. J. Neurosci. 2003;23:2608–2617. doi: 10.1523/JNEUROSCI.23-07-02608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci. 2008;9:1. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandrashekar J, et al. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finger TE, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 25.Pumplin DW, Yu C, Smith DV. Light and dark cells of rat vallate taste buds are morphologically distinct cell types. J. Comp. Neurol. 1997;378:389–410. doi: 10.1002/(sici)1096-9861(19970217)378:3<389::aid-cne7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 26.DeFazio RA, et al. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J. Neurosci. 2006;26:3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida R, et al. Taste responsiveness of fungiform taste cells with action potentials. J. Neurophysiol. 2006;96:3088–3095. doi: 10.1152/jn.00409.2006. [DOI] [PubMed] [Google Scholar]
- 28.Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J. Neurosci. 2007;27:10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, et al. Human receptors for sweet and umami taste. Proc. Natl Acad. Sci. USA. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H, et al. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc. Natl Acad. Sci. USA. 2004;101:14258–14263. doi: 10.1073/pnas.0404384101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson G, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 32.Max M, et al. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac . Nat. Genet. 2001;28:58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- 33.Nelson G, et al. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 34.Jiang P, et al. The cysteine-rich region of T1R3 determines responses to intensely sweet proteins. J. Biol. Chem. 2004;279:45068–45075. doi: 10.1074/jbc.M406779200. [DOI] [PubMed] [Google Scholar]
- 35.Damak S, et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- 36.Chandrashekar J, et al. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 37.Meyerhof W, et al. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses. 2010;35:157–170. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- 38.Behrens M, Foerster S, Staehler F, Raguse J-D, Meyerhof W. Gustatory expression pattern of the human TAS2R bitter receptor gene family reveals a heterogenous population of bitter responsive taste receptor cells. J. Neurosci. 2007;27:12630–12640. doi: 10.1523/JNEUROSCI.1168-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindemann B. Receptors and transduction in taste. Nature. 2001;413:219–225. doi: 10.1038/35093032. [DOI] [PubMed] [Google Scholar]
- 40.Martin B, et al. Vasoactive intestinal peptide-null mice demonstrate enhanced sweet taste preference, dysglycemia, and reduced taste bud leptin receptor expression. Diabetes. 2010;59:1143–1152. doi: 10.2337/db09-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao F, et al. Expression, physiological action, and co-expression patterns of neuropeptide Y in rat taste-bud cells. Proc. Natl Acad. Sci. USA. 2005;102:11100–11105. doi: 10.1073/pnas.0501988102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elson AET, Dotson CD, Egan JM, Munger SD. Glucagon signaling modulates sweet taste responsiveness. FASEB J. 2010;24:3960–3969. doi: 10.1096/fj.10-158105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.La Sala MS, et al. Modulation of taste responsiveness by the satiation hormone peptide Y Y. FASEB J. 2013;27:5022–5033. doi: 10.1096/fj.13-228064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shigemura N, et al. Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology. 2013;145:839–847. doi: 10.1210/en.2003-0602. [DOI] [PubMed] [Google Scholar]
- 45.Dvoryanchikov G, Huang YA, Barro-Soria R, Chaudhari N, Roper SD. GABA, its receptors, and GABAergic inhibition in mouse taste buds. J. Neurosci. 2011;31:5782–5791. doi: 10.1523/JNEUROSCI.5559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LopezJimenez ND, et al. Two members of the TRPP family of ion channels, Pkd1l3 and Pkd2l1, are co-expressed in a subset of taste receptor cells. J. Neurochem. 2006;98:68–77. doi: 10.1111/j.1471-4159.2006.03842.x. [DOI] [PubMed] [Google Scholar]
- 47.Huang AL, et al. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horio N, et al. Sour taste responses in mice lacking PKD channels. PLoS ONE. 2011;6:e20007. doi: 10.1371/journal.pone.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miura H, Kusakabe Y, Harada S. Cell lineage and differentiation in taste buds. Arch. Histol. Cytol. 2006;69:209–225. doi: 10.1679/aohc.69.209. [DOI] [PubMed] [Google Scholar]
- 50.Oka Y, Butnaru M, von Buchholtz L, Ryba NJP, Zuker CS. High salt recruits aversive taste pathways. Nature. 2013;494:472–475. doi: 10.1038/nature11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castillo D, et al. Induction of ectopic taste buds by SHH reveals the competency and plasticity of adult lingual epithelium. Development. 2014;141:2993–3002. doi: 10.1242/dev.107631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu HX, et al. Multiple Shh signaling centers participate in fungiform papilla and taste bud formation and maintenance. Dev. Biol. 2013;382:82–97. doi: 10.1016/j.ydbio.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perea-Martinez I, Nagai T, Chaudhari N. Functional cell types in taste buds have distinct longevities. PLoS ONE. 2013;8:e53399. doi: 10.1371/journal.pone.0053399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohmoto M, Matsumoto I, Yasuoka A, Yoshihara Y, Abe K. Genetic tracing of the gustatory and trigeminal neural pathways originating from T1R3-expressing taste receptor cells and solitary chemoreceptor cells. Mol. Cell. Neurosci. 2008;38:505–517. doi: 10.1016/j.mcn.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto K, et al. Genetic tracing of the gustatory neural pathway originating from Pkd1l3-expressing type III taste cells in circumvallate and foliate papillae. J. Neurochem. 2011;119:497–506. doi: 10.1111/j.1471-4159.2011.07443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugita M, Shiba Y. Genetic tracing shows segregation of taste neuronal circuitries for bitter and sweet. Science. 2005;309:781–785. doi: 10.1126/science.1110787. [DOI] [PubMed] [Google Scholar]
- 57.Damak S, Mosinger B, Margolskee RF. Transsynaptic transport of wheat germ agglutinin expressed in a subset of type II taste cells of transgenic mice. BMC Neurosci. 2008;9:96. doi: 10.1186/1471-2202-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herness S, Zhao F-L. The neuropeptides CCK and NPY and the changing view of cell-to-cell communication in the taste bud. Physiol. Behav. 2009;97:581–591. doi: 10.1016/j.physbeh.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 59.Hurtado MD, et al. Distribution of Y-receptors in murine lingual epithelia. PLoS ONE. 2012;7:e46358. doi: 10.1371/journal.pone.0046358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang YA, Roper SD. Intracellular Ca2+ and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells. J. Physiol. 2010;588:2343–2350. doi: 10.1113/jphysiol.2010.191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu P, Shah BP, Croasdell S, Gilbertson TA. Transient receptor potential channel type M5 is essential for fat taste. J. Neurosci. 2011;31:8634–8642. doi: 10.1523/JNEUROSCI.6273-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El-Yassimi A, Hichami A, Besnard P, Khan NA. Linoleic acid induces calcium signaling, Src kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. J. Biol. Chem. 2008;283:12949–12959. doi: 10.1074/jbc.M707478200. [DOI] [PubMed] [Google Scholar]
- 63.Gao N, et al. Voltage-gated sodium channels in taste bud cells. BMC Neurosci. 2009;10:20. doi: 10.1186/1471-2202-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taruno A, et al. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 2013;495:223–226. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang YA, Pereira E, Roper SD. Acid stimulation (sour taste) elicits GABA and serotonin release from mouse taste cells. PLoS ONE. 2011;6:e25471. doi: 10.1371/journal.pone.0025471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, et al. Coding of sweet, bitter, and umami tastes. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 67.Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. Fat and carbohydrate preferences in mice: the contribution of alpha-gustducin and Trpm5 taste-signaling proteins. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1504–R1513. doi: 10.1152/ajpregu.00364.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc. Natl Acad. Sci. USA. 2003;100:15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vandenbeuch A, et al. Role of the ectonucleotidase NTPDase2 in taste bud function. Proc. Natl Acad. Sci. USA. 2013;110:14789–14794. doi: 10.1073/pnas.1309468110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dando R, Roper SD. Acetylcholine is released from taste cells, enhancing taste signalling. J. Physiol. 2012;590:3009–3017. doi: 10.1113/jphysiol.2012.232009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chang RB, Waters H, Liman ER. A proton current drives action potentials in genetically identified sour taste cells. Proc. Natl Acad. Sci. USA. 2010;107:22320–22325. doi: 10.1073/pnas.1013664107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herness S, Zhao F, Lu S, Kaya N, Shen T. Expression and physiological actions of cholecystokinin in rat taste receptor cells. J. Neurosci. 2002;22:10018–10029. doi: 10.1523/JNEUROSCI.22-22-10018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hajnal A, Covasa M, Bello NT. Altered taste sensitivity in obese, prediabetic OLETF rats lacking CCK-1 receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R1675–R1686. doi: 10.1152/ajpregu.00412.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Michel MCXVI, et al. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY and pancreatic polypeptide receptors. Pharmacol. Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- 75.Shen T, et al. Co-expression patterns of the neuropeptides vasoactive intestinal peptide and cholecystokinin with the transduction molecules α-gustducin and T1R2 in rat taste receptor cells. NeuroScience. 2005;130:229–238. doi: 10.1016/j.neuroscience.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 76.Armelagos GJ. Brain evolution, the determinates of food choice, and the omnivore’s dilemma. Crit. Rev. Food Sci. Nutr. 2014;54:1330–1341. doi: 10.1080/10408398.2011.635817. [DOI] [PubMed] [Google Scholar]
- 77.Kinnamon SC, Reynolds SD. Cell biology. Using taste to clear the air(ways) Science. 2009;325:1081–1082. doi: 10.1126/science.1179180. [DOI] [PubMed] [Google Scholar]
- 78.Eberlein GA, et al. A new molecular form of PYY: structural characterization of human PYY(3–36) and PYY(1–36) Peptides. 1989;10:797–803. doi: 10.1016/0196-9781(89)90116-2. [DOI] [PubMed] [Google Scholar]
- 79.Kashyap SR, et al. Lipid-induced insulin resistance is associated with increased monocyte expression of scavenger receptor CD36 and internalization of oxidized LDL. Obesity (Silver Spring) 2009;17:2142–2148. doi: 10.1038/oby.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baillie AG, Coburn CT, Abumrad NA. Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homolog. J. Membr. Biol. 1996;153:75–81. doi: 10.1007/s002329900111. [DOI] [PubMed] [Google Scholar]
- 81.Laugerette F, et al. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Invest. 2005;115:3177–3184. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol. Rev. 2008;60:470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fiori JL, et al. Resveratrol prevents β-cell dedifferentiation in nonhuman primates given a high-fat/high-sugar diet. Diabetes. 2013;62:3500–3513. doi: 10.2337/db13-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Drucker DJ, Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu. Rev. Physiol. 2014;76:561–583. doi: 10.1146/annurev-physiol-021113-170317. [DOI] [PubMed] [Google Scholar]
- 85.Martin B, et al. Modulation of taste sensitivity by GLP-1 signaling in taste buds. Ann. N.Y. Acad. Sci. 2009;1170:98–101. doi: 10.1111/j.1749-6632.2009.03920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kokrashvili Z, Mosinger B, Margolskee RF. T1r3 and α-gustducin in gut regulate secretion of glucagon-like peptide-1. Ann. N.Y. Acad. Sci. 2009;1170:91–94. doi: 10.1111/j.1749-6632.2009.04485.x. [DOI] [PubMed] [Google Scholar]
- 87.Geraedts MCP, Munger SD. Gustatory stimuli representing different perceptual qualities elicit distinct patterns of neuropeptide secretion from taste buds. J. Neurosci. 2013;33:7559–7564. doi: 10.1523/JNEUROSCI.0372-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88.Martin C, et al. Lipid-mediated release of GLP-1 by mouse taste buds from circumvallate papillae: putative involvement of GPR120 and impact on taste sensitivity. J. Lipid Res. 2012;53:2256–2265. doi: 10.1194/jlr.M025874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hirasawa A, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 90.Shin Y-K, et al. Age-related changes in mouse taste bud morphology, hormone expression, and taste responsivity. J. Gerontol. A. Biol. Sci. Med. Sci. 2012;67:336–344. doi: 10.1093/gerona/glr192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aydin S, et al. Examination of the tissue ghrelin expression of rats with diet-induced obesity using radioimmunoassay and immunohistochemical methods. Mol. Cell. Biochem. 2012;365:165–173. doi: 10.1007/s11010-012-1256-4. [DOI] [PubMed] [Google Scholar]
- 92.Lin L, et al. Ablation of ghrelin receptor reduces adiposity and improves insulin sensitivity during aging by regulating fat metabolism in white and brown adipose tissues. Aging Cell. 2011;10:996–1010. doi: 10.1111/j.1474-9726.2011.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kang K, Zmuda E, Sleeman MW. Physiological role of ghrelin as revealed by the ghrelin and GOAT knockout mice. Peptides. 2011;32:2236–2241. doi: 10.1016/j.peptides.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 94.Sinclair MS, et al. Oxytocin signaling in mouse taste buds. PLoS ONE. 2010;5:e11980. doi: 10.1371/journal.pone.0011980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stricker EM, Verbalis JG. Central inhibition of salt appetite by oxytocin in rats. Regul. Pept. 1996;66:83–85. doi: 10.1016/0167-0115(96)00058-4. [DOI] [PubMed] [Google Scholar]
- 96.Kaplan LM, Spindel ER, Isselbacher KJ, Chin WW. Tissue-specific expression of the rat galanin gene. Proc. Natl Acad. Sci. USA. 1988;85:1065–1069. doi: 10.1073/pnas.85.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koegler FH, Ritter S. Galanin injection into the nucleus of the solitary tract stimulates feeding in rats with lesions of the paraventricular nucleus of the hypothalamus. Physiol. Behav. 1998;63:521–527. doi: 10.1016/s0031-9384(97)00480-0. [DOI] [PubMed] [Google Scholar]
- 98.Seta Y, Kataoka S, Toyono T, Toyoshima K. Expression of galanin and the galanin receptor in rat taste buds. Arch. Histol. Cytol. 2006;69:273–280. doi: 10.1679/aohc.69.273. [DOI] [PubMed] [Google Scholar]
- 99.Karatayev O, Baylan J, Leibowitz SF. Increased intake of ethanol and dietary fat in galanin overexpressing mice. Alcohol. 2009;43:571–580. doi: 10.1016/j.alcohol.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adams AC, Clapham JC, Wynick D, Speakman JR. Feeding behaviour in galanin knockout mice supports a role of galanin in fat intake and preference. J. Neuroendocrinol. 2008;20:199–206. doi: 10.1111/j.1365-2826.2007.01638.x. [DOI] [PubMed] [Google Scholar]
- 101.Chevrot M, et al. Obesity alters the gustatory perception of lipids in the mouse: plausible involvement of lingual CD36. J. Lipid Res. 2013;54:2485–2494. doi: 10.1194/jlr.M039446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc. Natl Acad. Sci. USA. 2000;97:11044–11049. doi: 10.1073/pnas.190066697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lu B, Breza JM, Nikonov AA, Paedae AB, Contreras RJ. Leptin increases temperature-dependent chorda tympani nerve responses to sucrose in mice. Physiol. Behav. 2012;107:533–539. doi: 10.1016/j.physbeh.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 104.Talavera K, et al. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022–1025. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- 105.Hellekant G. The blood circulation of the tongue. Front. Oral Physiol. 1976;2:130–145. doi: 10.1159/000393319. [DOI] [PubMed] [Google Scholar]
- 106.Eberhard D, Kragl M, Lammert E. ‘Giving and taking’: endothelial and β-cells in the islets of Langerhans. Trends Endocrinol. Metab. 2010;21:457–463. doi: 10.1016/j.tem.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 107.Amisten S, Salehi A, Rorsman P, Jones PM, Persaud SJ. An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacol. Ther. 2013;139:359–391. doi: 10.1016/j.pharmthera.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 108.Kokrashvili Z, et al. Endocrine taste cells. Br. J. Nutr. 2014;111(Suppl. 1):S23–S29. doi: 10.1017/S0007114513002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Newsholme P, Gaudel C, McClenaghan NH. Nutrient regulation of insulin secretion and β-cell functional integrity. Adv. Exp. Med. Biol. 2010;654:91–114. doi: 10.1007/978-90-481-3271-3_6. [DOI] [PubMed] [Google Scholar]
- 110.Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of ‘taste’ in gastrointestinal chemosensing. Curr. Opin. Endocrinol. Diabetes Obes. 2008;15:73–78. doi: 10.1097/MED.0b013e3282f43a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rozengurt E, Sternini C. Taste receptor signaling in the mammalian gut. Curr. Opin. Pharmacol. 2007;7:557–562. doi: 10.1016/j.coph.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Janssen S, Depoortere I. Nutrient sensing in the gut: new roads to therapeutics? Trends Endocrinol. Metab. 2013;24:92–100. doi: 10.1016/j.tem.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 113.Shirazi-Beechey SP, Daly K, Al-Rammahi M, Moran AW, Bravo D. Role of nutrientsensing taste 1 receptor (T1R) family members in gastrointestinal chemosensing. Br. J. Nutr. 2014;111(Suppl. 1):S8–S15. doi: 10.1017/S0007114513002286. [DOI] [PubMed] [Google Scholar]
- 114.Jang H-J, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl Acad. Sci. USA. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Margolskee RF, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc. Natl Acad. Sci. USA. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Daly K, et al. Expression of sweet receptor components in equine small intestine: relevance to intestinal glucose transport. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;303:R199–R208. doi: 10.1152/ajpregu.00031.2012. [DOI] [PubMed] [Google Scholar]
- 117.Reimann F, et al. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu SV, et al. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc. Natl Acad. Sci. USA. 2002;99:2392–2397. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Prandi S, et al. A subset of mouse colonic goblet cells expresses the bitter taste receptor Tas2r131. PLoS ONE. 2013;8:e82820. doi: 10.1371/journal.pone.0082820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Janssen S, et al. Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc. Natl Acad. Sci. USA. 2011;108:2094–2099. doi: 10.1073/pnas.1011508108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Glendinning JI, Yiin Y-M, Ackroff K, Sclafani A. Intragastric infusion of denatonium conditions flavor aversions and delays gastric emptying in rodents. Physiol. Behav. 2008;93:757–765. doi: 10.1016/j.physbeh.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen M, Yang Y, Braunstein E, Georgeson KE, Harmon CM. Gut expression and regulation of FAT/CD36: possible role in fatty acid transport in rat enterocytes. Am. J. Physiol. Endocrinol. Metab. 2001;281:E916–E923. doi: 10.1152/ajpendo.2001.281.5.E916. [DOI] [PubMed] [Google Scholar]
- 123.Ozdener MH, et al. CD36- and GPR120-mediated Ca2+ signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology. 2014;146:995–1005. doi: 10.1053/j.gastro.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sundaresan S, et al. CD36-dependent signaling mediates fatty acid-induced gut release of secretin and cholecystokinin. FASEB J. 2013;27:1191–1202. doi: 10.1096/fj.12-217703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Matsumura S, et al. GPR expression in the rat taste bud relating to fatty acid sensing. Biomed. Res. 2007;28:49–55. doi: 10.2220/biomedres.28.49. [DOI] [PubMed] [Google Scholar]
- 126.Hirasawa A, Hara T, Katsuma S, Adachi T, Tsujimoto G. Free fatty acid receptors and drug discovery. Biol. Pharm. Bull. 2008;31:1847–1851. doi: 10.1248/bpb.31.1847. [DOI] [PubMed] [Google Scholar]
- 127.Itoh Y, et al. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 128.Tomita T, et al. Expression of the gene for a membrane-bound fatty acid receptor in the pancreas and islet cell tumours in humans: evidence for GPR40 expression in pancreatic β cells and implications for insulin secretion. Diabetologia. 2006;49:962–968. doi: 10.1007/s00125-006-0193-8. [DOI] [PubMed] [Google Scholar]
- 129.Duca FA, Sakar Y, Covasa M. The modulatory role of high fat feeding on gastrointestinal signals in obesity. J. Nutr. Biochem. 2013;24:1663–1677. doi: 10.1016/j.jnutbio.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 130.Cartoni C, et al. Taste preference for fatty acids is mediated by GPR40 and GPR120. J. Neurosci. 2010;30:8376–8382. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Godinot N, et al. Activation of tongue-expressed GPR40 and GPR120 by non caloric agonists is not sufficient to drive preference in mice. NeuroScience. 2013;250:20–30. doi: 10.1016/j.neuroscience.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 132.Galindo MM, et al. G protein-coupled receptors in human fat taste perception. Chem. Senses. 2012;37:123–139. doi: 10.1093/chemse/bjr069. [DOI] [PubMed] [Google Scholar]
- 133.Ichimura A, Hirasawa A, Hara T, Tsujimoto G. Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat. 2009;89:82–88. doi: 10.1016/j.prostaglandins.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 134.Xiong Y, et al. Activation of FFA1 mediates GLP-1 secretion in mice. Evidence for allosterism at FFA1. Mol. Cell. Endocrinol. 2013;369:119–129. doi: 10.1016/j.mce.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 135.Tanaka T, et al. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn Schmiedebergs Arch. Pharmacol. 2008;377:523–527. doi: 10.1007/s00210-007-0200-8. [DOI] [PubMed] [Google Scholar]
- 136.Liou AP, et al. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology. 2011;140:903–912. doi: 10.1053/j.gastro.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yao B, Mackie K. Endocannabinoid receptor pharmacology. Curr. Top. Behav. Neurosci. 2009;1:37–63. doi: 10.1007/978-3-540-88955-7_2. [DOI] [PubMed] [Google Scholar]
- 138.Yoshida R, et al. Endocannabinoids selectively enhance sweet taste. Proc. Natl Acad. Sci. USA. 2010;107:935–939. doi: 10.1073/pnas.0912048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br. J. Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gomez R, et al. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J. Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.DiPatrizio NV, Astarita G, Schwartz G, Li X, Piomelli D. Endocannabinoid signal in the gut controls dietary fat intake. Proc. Natl Acad. Sci. USA. 2011;108:12904–12908. doi: 10.1073/pnas.1104675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.DiPatrizio NV, Joslin A, Jung K-M, Piomelli D. Endocannabinoid signaling in the gut mediates preference for dietary unsaturated fats. FASEB J. 2013;27:2513–2520. doi: 10.1096/fj.13-227587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schicho R, Storr M. A potential role for GPR55 in gastrointestinal functions. Curr. Opin. Pharmacol. 2012;12:653–658. doi: 10.1016/j.coph.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Festi D, et al. Gut microbiota and metabolic syndrome. World J. Gastroenterol. 2014;20:16079–16094. doi: 10.3748/wjg.v20.i43.16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Duca FA, Swartz TD, Sakar Y, Covasa M. Increased oral detection, but decreased intestinal signaling for fats in mice lacking gut microbiota. PLoS ONE. 2012;7:e39748. doi: 10.1371/journal.pone.0039748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Swartz TD, Duca FA, de Wouters T, Sakar Y, Covasa M. Up-regulation of intestinal type 1 taste receptor 3 and sodium glucose luminal transporter-1 expression and increased sucrose intake in mice lacking gut microbiota. Br. J. Nutr. 2012;107:621–630. doi: 10.1017/S0007114511003412. [DOI] [PubMed] [Google Scholar]
- 147.Höfer D, Drenckhahn D. Identification of the taste cell G-protein, α-gustducin, in brush cells of the rat pancreatic duct system. Histochem. Cell Biol. 1998;110:303–309. doi: 10.1007/s004180050292. [DOI] [PubMed] [Google Scholar]
- 148.Straub SG, Mulvaney-Musa J, Yajima H, Weiland GA, Sharp GWG. Stimulation of insulin secretion by denatonium, one of the most bitter-tasting substances known. Diabetes. 2003;52:356–364. doi: 10.2337/diabetes.52.2.356. [DOI] [PubMed] [Google Scholar]
- 149.Nakagawa Y, et al. Sweet taste receptor expressed in pancreatic β-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS ONE. 2009;4:e5106. doi: 10.1371/journal.pone.0005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kyriazis GA, Soundarapandian MM, Tyrberg B. Sweet taste receptor signaling in β cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc. Natl Acad. Sci. USA. 2012;109:E524–E532. doi: 10.1073/pnas.1115183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dunnigan MG, Ford JA. The insulin response to intravenous fructose in relation to blood glucose levels. J. Clin. Endocrinol. Metab. 1975;40:629–635. doi: 10.1210/jcem-40-4-629. [DOI] [PubMed] [Google Scholar]
- 152.Le MT, et al. Effects of high-fructose corn syrup and sucrose on the pharmacokinetics of fructose and acute metabolic and hemodynamic responses in healthy subjects. Metabolism. 2012;61:641–651. doi: 10.1016/j.metabol.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nakagawa Y, et al. Sweet taste receptor expressed in pancreatic β-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS ONE. 2009;4:e5106. doi: 10.1371/journal.pone.0005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kyriazis GA, Smith KR, Tyrberg B, Hussain T, Pratley RE. Sweet taste receptors regulate basal insulin secretion and contribute to compensatory insulin hypersecretion during the development of diabetes in male mice. Endocrinology. 2014;155:2112–2121. doi: 10.1210/en.2013-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nakagawa Y, et al. Multimodal function of the sweet taste receptor expressed in pancreatic β-cells: generation of diverse patterns of intracellular signals by sweet agonists. Endocr. J. 2013;60:1191–1206. doi: 10.1507/endocrj.ej13-0282. [DOI] [PubMed] [Google Scholar]
- 156.Spector AC, Travers SP. The representation of taste quality in the mammalian nervous system. Behav. Cogn. Neurosci. Rev. 2005;4:143–191. doi: 10.1177/1534582305280031. [DOI] [PubMed] [Google Scholar]
- 157.Onoda K, Ikeda M, Sekine H, Ogawa H. Clinical study of central taste disorders and discussion of the central gustatory pathway. J. Neurol. 2012;259:261–266. doi: 10.1007/s00415-011-6165-z. [DOI] [PubMed] [Google Scholar]
- 158.Boucher Y, Simons CT, Faurion A, Azérad J, Carstens E. Trigeminal modulation of gustatory neurons in the nucleus of the solitary tract. Brain Res. 2003;973:265–274. doi: 10.1016/s0006-8993(03)02526-5. [DOI] [PubMed] [Google Scholar]
- 159.Hoshi A, et al. A novel objective sour taste evaluation method based on near-infrared spectroscopy. Chem. Senses. 2014;39:313–322. doi: 10.1093/chemse/bjt118. [DOI] [PubMed] [Google Scholar]
- 160.Voigt N, et al. The role of lipolysis in human orosensory fat perception. J. Lipid Res. 2014;55:870–882. doi: 10.1194/jlr.M046029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jones MW, et al. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat. Neurosci. 2001;4:289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- 162.Sugita M, Yamamoto K, Hirono C, Shiba Y. Information processing in brainstem bitter taste-relaying neurons defined by genetic tracing. NeuroScience. 2013;250:166–180. doi: 10.1016/j.neuroscience.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 163.Ohmoto M, Maeda N, Abe K, Yoshihara Y, Matsumoto I. Genetic tracing of the neural pathway for bitter taste in t2r5-WGA transgenic mice. Biochem. Biophys. Res. Commun. 2010;400:734–738. doi: 10.1016/j.bbrc.2010.08.139. [DOI] [PubMed] [Google Scholar]
- 164.Chen X, Gabitto M, Peng Y, Ryba NJP, Zuker CS. A gustotopic map of taste qualities in the mammalian brain. Science. 2011;333:1262–1266. doi: 10.1126/science.1204076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Trivedi BP. Neuroscience: hardwired for taste. Nature. 2012;486:S7–S9. doi: 10.1038/486S7a. [DOI] [PubMed] [Google Scholar]
- 166.Miller G. Neuroscience. Sweet here, salty there: evidence for a taste map in the mammalian brain. Science. 2011;333:1213. doi: 10.1126/science.333.6047.1213. [DOI] [PubMed] [Google Scholar]
- 167.Barretto RPJ, et al. The neural representation of taste quality at the periphery. Nature. 2015;15:373–376. doi: 10.1038/nature13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969;222:282–284. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- 169.Nakano Y, et al. Feeding-related activity of glucose- and morphine-sensitive neurons in the monkey amygdala. Brain Res. 1986;399:167–172. doi: 10.1016/0006-8993(86)90613-x. [DOI] [PubMed] [Google Scholar]
- 170.Anand BK, Chhina GS, Sharma KN, Dua S, Singh B. Activity of single neurons in the hypothalamic feeding centers: effect of glucose. Am. J. Physiol. 1964;207:1146–1154. doi: 10.1152/ajplegacy.1964.207.5.1146. [DOI] [PubMed] [Google Scholar]
- 171.Shoji S. Glucose regulation of synaptic transmission in the dorsolateral septal nucleus of the rat. Synapse. 1992;12:322–332. doi: 10.1002/syn.890120409. [DOI] [PubMed] [Google Scholar]
- 172.Lee K, Dixon AK, Rowe ICM, Ashford MLJ, Richardson PJ. The high- affinity sulphonylurea receptor regulates KATP channels in nerve terminals of the rat motor cortex. J. Neurochem. 2002;66:2562–2571. doi: 10.1046/j.1471-4159.1996.66062562.x. [DOI] [PubMed] [Google Scholar]
- 173.Ren X, Zhou L, Terwilliger R, Newton SS, de Araujo IE. Sweet taste signaling functions as a hypothalamic glucose sensor. Front. Integr. Neurosci. 2009;3:12. doi: 10.3389/neuro.07.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cong W, et al. Long-term artificial sweetener acesulfame potassium treatment alters neurometabolic functions in C57BL/6J mice. PLoS ONE. 2013;8:e70257. doi: 10.1371/journal.pone.0070257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Roberts A, Renwick AG, Sims J, Snodin DJ. Sucralose metabolism and pharmacokinetics in man. Food Chem. Toxicol. 2000;38:31–41. doi: 10.1016/s0278-6915(00)00026-0. [DOI] [PubMed] [Google Scholar]
- 176.Wauson EM, et al. The G protein-coupled taste receptor T1R1/T1R3 regulates mTORC1 and autophagy. Mol. Cell. 2012;47:851–862. doi: 10.1016/j.molcel.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.De Araujo IE, et al. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 178.Sclafani A. Psychobiology of food preferences. Int. J. Obes. Relat. Metab. Disord. 2001;25(Suppl. 5):S13–S16. doi: 10.1038/sj.ijo.0801905. [DOI] [PubMed] [Google Scholar]
- 179.Myers KP, Sclafani A. Conditioned enhancement of flavor evaluation reinforced by intragastric glucose. II. Taste reactivity analysis. Physiol. Behav. 2001;74:495–505. doi: 10.1016/s0031-9384(01)00596-0. [DOI] [PubMed] [Google Scholar]
- 180.Ren X, et al. Nutrient selection in the absence of taste receptor signaling. J. Neurosci. 2010;30:8012–8023. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tellez LA, et al. Glucose utilization rates regulate intake levels of artificial sweeteners. J. Physiol. 2013;591:5727–5744. doi: 10.1113/jphysiol.2013.263103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Domingos AI, et al. Leptin regulates the reward value of nutrient. Nat. Neurosci. 2011;14:1562–1568. doi: 10.1038/nn.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Maliphol AB, Garth DJ, Medler KF. Diet-induced obesity reduces the responsiveness of the peripheral taste receptor cells. PLoS ONE. 2013;8:e79403. doi: 10.1371/journal.pone.0079403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lanfer A, et al. T a s te preferences in association with dietary habits and weight status in European children: results from the IDEFICS study. Int. J. Obes. (Lond.) 2012;36:27–34. doi: 10.1038/ijo.2011.164. [DOI] [PubMed] [Google Scholar]
- 185.Ichimura A, et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012;483:350–354. doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- 186.Esposito K, Maiorino MI, Petrizzo M, Bellastella G, Giugliano D. Remission of type 2 diabetes: is bariatric surgery ready for prime time- Endocrine. doi: 10.1007/s12020-014-0463-z. http://dx.doi.org/10.1007/s12020-014-0463-z. [DOI] [PubMed] [Google Scholar]
- 187.Courcoulas AP, et al. Long-term outcomes of bariatric surgery: A National Institutes of Health symposium. JAMA Surg. 2014;149:1323–1329. doi: 10.1001/jamasurg.2014.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Habegger KM, et al. GLP-1R responsiveness predicts individual gastric bypass efficacy on glucose tolerance in rats. Diabetes. 2014;63:505–513. doi: 10.2337/db13-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tichansky DS, et al. Decrease in sweet taste in rats after gastric bypass surgery. Surg. Endosc. 2011;25:1176–1181. doi: 10.1007/s00464-010-1335-0. [DOI] [PubMed] [Google Scholar]
- 190.Bueter M, et al. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol. Behav. 2011;104:709–721. doi: 10.1016/j.physbeh.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 191.Parker HE, et al. Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. Br. J. Pharmacol. 2012;165:414–423. doi: 10.1111/j.1476-5381.2011.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mokadem M, Zechner JF, Margolskee RF, Drucker DJ, Aguirre V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol. Metab. 2014;3:191–201. doi: 10.1016/j.molmet.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ye J, et al. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;306:R352–R362. doi: 10.1152/ajpregu.00491.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Miras AD, et al. Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. Am. J. Clin. Nutr. 2012;96:467–473. doi: 10.3945/ajcn.112.036921. [DOI] [PubMed] [Google Scholar]
- 195.Pepino MY, et al. Changes in taste perception and eating behavior after bariatric surgery- nduced weight loss in women. Obesity (Silver Spring) 2014;22:E13–E20. doi: 10.1002/oby.20649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tichansky DS, Boughter JD, Madan AK. Taste change after laparoscopic Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding. Surg. Obes. Relat Dis. 2006;2:440–444. doi: 10.1016/j.soard.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 197.Hao S, Sternini C, Raybould HE. Role of CCK1 and Y2 receptors in activation of hindbrain neurons induced by intragastric administration of bitter taste receptor ligands. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R33–R38. doi: 10.1152/ajpregu.00675.2007. [DOI] [PubMed] [Google Scholar]
- 198.Jiang E, Yu D, Feng Z. Subdiaphragmatic vagotomy reduces intake of sweet-tasting solutions in rats. Neural Regen. Res. 2013;8:1560–1567. doi: 10.3969/j.issn.1673-5374.2013.17.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mordes JP, el Lozy M, Herrera MG, Silen W. Effects of vagotomy with and without pyloroplasty on weight and food intake in rats. Am. J. Physiol. 1979;236:R61–R66. doi: 10.1152/ajpregu.1979.236.1.R61. [DOI] [PubMed] [Google Scholar]
- 200.Furness JB, et al. Effects of vagal and splanchnic section on food intake, weight, serum leptin and hypothalamic neuropeptide Y in rat. Auton. Neurosci. 2001;92:28–36. doi: 10.1016/S1566-0702(01)00311-3. [DOI] [PubMed] [Google Scholar]
- 201.Drewnowski A, Brunzell JD, Sande K, Iverius PH, Greenwood MR. Sweet tooth reconsidered: taste responsiveness in human obesity. Physiol. Behav. 1985;35:617–622. doi: 10.1016/0031-9384(85)90150-7. [DOI] [PubMed] [Google Scholar]
- 202.Deglaire A, et al. Associations between weight status and liking scores for sweet, salt and fat according to the gender in adults (The Nutrinet-Santé study) Eur. J. Clin. Nutr. 2015;69:40–46. doi: 10.1038/ejcn.2014.139. [DOI] [PubMed] [Google Scholar]