Abstract
A unique characteristic of the hepatitis B virus is the production of a secreted form (precore or HBeAg) of the structural nucleocapsid (core or HBcAg). By using T cell receptor (TCR) transgenic (Tg) and TCR × HBc/HBeAg double- and triple-Tg pairs, we demonstrate that HBeAg elicits T cell tolerance, whereas HBcAg is nontolerogenic in this system. In fact, TCR × HBc double-Tg mice spontaneously seroconvert to IgG anti-HBc positivity at an early age. However, the presence of HBeAg in the serum of TCR × HBc × HBe triple-Tg mice prevents anti-HBc seroconversion. HBeAg mediates its immunoregulatory effect by eliciting tolerance in HBc/HBeAg-specific T cells. The results suggest that hepadnaviruses have retained a secretory form of the nucleoprotein because it functions as a T cell tolerogen and regulates the immune response to the intracellular nucleocapsid. This HBeAg-mediated immune regulation may predispose to chronicity during perinatal infections and prevent severe liver injury during adult infections.
The hepatitis E antigen (precore or HBeAg) is secreted into the serum as a monomer, whereas the hepatitis core antigen (HBcAg) is cellular, and its particulate structure encapsidates the viral DNA and polymerase (1). These two antigenic forms are crossreactive at the T cell level because of extensive amino acid homology but are not crossreactive at the level of antibody recognition. The function of HBeAg has been the subject of speculation since its discovery >30 years ago (2) because it is not required for viral infection, replication, or assembly (3-5). In the past, we have suggested an immunoregulatory role for HBeAg based on murine experimental studies (6, 7). This issue remains complex because both forms of the nucleoprotein coexist in hepatitis B virus (HBV) natural infection, and the immune responses to HBcAg and HBeAg appear to be regulated independently. For example, HBcAg can behave as a T cell-independent antigen, whereas the immune response to HBeAg is strictly T cell-dependent (8); HBcAg preferentially elicits T helper 1 (Th1) cells, and HBeAg preferentially elicits Th0/Th2 cells (9); and HBcAg and HBeAg differ in terms of their primary antigen-presenting cells (10). Adding to the complexity, we have recently demonstrated that a “split” tolerance between HBeAg and HBcAg occurs because HBeAg is significantly more tolerogenic for T cells. Furthermore, T cell tolerance to HBeAg is clonal and can be mediated by at least three mechanisms: clonal deletion, clonal anergy, and clonal ignorance (unpublished data). High-avidity T cells are likely to be deleted by serum HBeAg, whereas HBeAg-specific T cells of intermediate avidity may not be deleted but nevertheless can be tolerized by means of clonal anergy. Therefore, even HBeAg-specific T cells of lower avidity are vulnerable to HBeAg-induced tolerance.
The current study is focused on a single HBc/HBeAg-specific T cell clonal population with an intermediate avidity for the HBc/HBeAgs represented by a T cell receptor (TCR) transgenic (Tg) lineage. TCR-Tg mice were bred with HBcAg- and/or HBeAg-expressing Tg mice to produce double- and triple-Tg pairs. This approach allowed examination of the effect of endogenous HBeAg and HBcAg, both singularly and combined, on a defined HBc/HBeAg-specific T cell population. The results unambiguously demonstrate that serum HBeAg can function as an efficient T cell tolerogen and can down-regulate the immune response to HBcAg in a Tg system in which HBcAg is a strong immunogen.
Methods
Tg Mice. The 7/16-5 Tg TCR (Vβ11+-Vα5+) is specific for residues 120-140 of HBc/HBeAgs, is restricted by the I-Ab MHC class II molecule, and is expressed on 53% of CD4+ T cells (unpublished data). Tg mice engineered to express relatively high levels of HBeAg (4-10 μg/ml serum) and HBcAg (0.2-2 μg/mg liver protein) exclusively in the liver through the use of the liver-specific major urinary protein promoter have been described (11, 12), as has an HBeAg-Tg lineage that expresses a lower level of HBeAg (10 ng/ml) (13). All Tg mice were bred onto a C57BL/10 background. The mice designated as HbcAg or HBeAg-Tg were hemizygous for the transgenes, as were the 7/16-5 TCR-Tg mice. All animal care was performed according to National Institutes of Health standards as set forth in Guide for the Care and Use of Laboratory Animals (14).
Recombinant Proteins and Synthetic Peptides. Recombinant HBcAg of the ayw subtype was produced in Escherichia coli and purified as described in ref. 15. A recombinant HBeAg corresponding in sequence to serum-derived HBeAg encompassing the 10 precore amino acids remaining after cleavage of the precursor and residues 1-149 of HBcAg was produced as described in ref. 16. The presence of the 10 precore amino acids prevents particle assembly, and HBeAg is recognized efficiently by HBeAg-specific mAbs but displays little HBc antigenicity. Peptides were synthesized by the simultaneous multiple peptide synthesis method (17). The HBe/HBcAg-derived synthetic peptide representing the recognition site for the 7/16-5 TCR was designated by the following amino acid position from the N terminus of HBcAg: 120-140, VSFGVWIRTPPAYRPPNAPIL.
Serology. HBeAg was measured in diluted Tg mouse sera by a commercial ELISA (ETI-EBK PLUS kit, DiaSorin, Stillwater, MN), and rHBeAg was used as a standard. Anti-HBc and -HBe IgG antibodies were measured in murine sera by an indirect solid-phase ELISA with rHBcAg or rHBeAg as the solid-phase ligands as described in ref. 18. The data are expressed as antibody titers representing the reciprocal of the highest dilution of sera required to yield an optical density at 492 nm (OD492), 3 times an equal dilution of preimmunization sera. IgG isotype-specific ELISAs were performed by using IgG1-, IgG2a-, IgG2b-, and IgG3-specific secondary antibodies (Southern Biotechnology Associates). To detect anti-HBc or -HBe antibodies in culture supernatants, the same ELISAs were used, except that undiluted supernatants were used, and data are reported as OD492 values.
Liver HBcAg Content. Liver homogenates were prepared in water by using a tissue homogenizer. The tissue lysates were centrifuged at 20,000 × g for 15 min to remove membranes. The total protein content of the water-soluble supernatant was determined by using a Bradford reagent. The concentration of HBcAg in the supernatant was determined by using a modified commercial anti-HBc assay (ETI-AB-COREK PLUS, DiaSorin). The anti-HBc assay was converted to a solid-phase HBcAg capture assay by replacing the rHBcAg-containing neutralization reagent with dilutions of liver supernatant as the source of HBcAg. By using rHBcAg as a standard, the assay was capable of detecting 0.3 ng/ml HBcAg.
In Vitro Cytokine Analysis. Spleen cells from either unprimed or primed TCR-Tg or -Ag double- or triple-Tg mice were cultured (5 × 106 per ml) with concentrations of a series of antigens. Culture supernatants were harvested at 48 h for IL-2 and at 96 h for IFN-γ determinations. Cytokines were measured by two-site ELISA with pairs of cytokine-specific mAbs. One unlabeled mAb was absorbed to the microtiter plate well and used as a capture antibody, and the other, labeled mAb served as the probe.
Adoptive Transfer. Donor spleen cells (30 × 106) derived from 7/16-5 TCR-Tg or 7/16-5 × HBe double-Tg mice were injected intravenously into CD4/CD8-depleted HBcAg or HBeAg single-Tg or HBc/HBeAg double-Tg recipient mice. The spleen cell inocula contained ≈3 × 106 TCR+CD4+ T cells. Whole spleen inocula were used for convenience and yielded similar results to transferred, purified CD4+ T cell populations. Preactivation of 7/16-5 splenic T cells consisted of 3-day in vitro culture with the HBc/HBeAg-derived core peptide (p)120-140 (1.0 μg/ml).
Results
Serum HBeAg Inhibits Spontaneous IgG Anti-HBc Seroconversion. As models of chronic HBV infection, TCR × HBc/HBeAg Tg pairs were used to examine the effect of persistent antigen exposure on T cells. Six of six double-Tg mice expressing the HBc/HBeAg-specific 7/16-5 TCR and the HBcAg (7/16-5 × HBc) spontaneously seroconverted to IgG anti-HBc positivity between 4 and 6 weeks of age (Fig. 1). Anti-HBc antibodies representing all IgG isotypes were produced; however, IgG2b predominated, and IgG2a was more plentiful than IgG1, which was more plentiful than IgG3. Spontaneous anti-HBc seroconversion in 7/16-5 × HBc double-Tg mice indicates that HBcAg is not tolerogenic and, to the contrary, acts as an immunogen for 7/16-5 T cells in vivo. Because particulate HBcAg is extremely immunogenic at the B cell level (10), the relatively small amount of this intracellular antigen that apparently “leaks” from hepatocytes is sufficient to elicit spontaneous anti-HBc seroconversion. In contrast, serum HBeAg does not elicit spontaneous seroconversion to IgG anti-HBe in 7/16-5 × HBe double-Tg mice (data not shown) because 7/16-5 T cells are tolerized by serum HBeAg (see Figs. 4 and 5 and also Fig. 6, which is published as supporting information on the PNAS web site). By breeding triple-Tg mice that express the 7/16-5 TCR, HBcAg, and HBeAg, we were able to determine whether the immunogenic response to the HBcAg or the tolerogenic response to HBeAg would predominate. The presence of HBeAg in the serum (4-10 μg/ml) prevented spontaneous IgG anti-HBc seroconversion in two of six 7/16-5 × HBc × HBe triple-Tg mice and severely inhibited IgG anti-HBc production in the remaining four mice (Fig. 1). The low-level anti-HBc antibody that was produced in the triple-Tg mice was delayed in onset and was IgG-isotype-restricted. Therefore, serum HBeAg can function as an immunoregulatory protein and exert a negative effect on the humoral response to HBcAg in vivo. Furthermore, spleen cells derived from 7/16-5 × HBc and 7/16-5 × HBe double-Tg and 7/16-5 × HBc × HBe triple-Tg mice cultured with HBcAg or HBeAg in vitro revealed that 7/16-5 × HBc spleen cells produced abundant IgG anti-HBc antibodies in primary spleen cultures, whereas 7/16-5 × HBe double-Tg spleen cells produced no IgG anti-HBe, and, more importantly, triple-Tg spleen cells produced no IgG anti-HBc (Fig. 2A). In vitro IgG antibody production in primary spleen cultures paralleled in vivo IgG anti-HBc/e production in these strains (Fig. 1). The absence of in vitro IgG anti-HBe production by 7/16-5 × HBe double-Tg spleen cells confirms that the inability to detect spontaneous IgG anti-HBe antibodies in vivo was not due to immune complexing or masking of anti-HBe by serum HBeAg. Spleen culture supernatants also were examined for IgM anti-HBc production (Fig. 2B). Although spleen cells from all 7/16-5 TCR+ double- and triple-Tg mice produce IgM anti-HBc upon primary culture with HBcAg (7/16-5 TCR single-Tg spleen cells also produce IgM anti-HBc in primary culture with HBcAg; data not shown), it is notable that spleen cells from 7/16-5 × HBc × HBe triple-Tg mice, which produce no IgG anti-HBc in vitro, actually produce elevated levels of IgM anti-HBc. The suppressed IgG and enhanced IgM anti-HBc production observed in 7/16-5 × HBc × HBe triple-Tg spleen cells as compared with 7/16-5 × HBc double-Tg spleen cells suggest that the Th necessary for the switch from IgM to IgG anti-HBc antibody production is curtailed in the presence of HBeAg, and in the absence of sufficient Th function HBcAg induces enhanced IgM anti-HBc antibody production by default. This finding may be relevant to the cyclic reoccurrence of IgM anti-HBc antibody often observed in chronic HBV patients.
Fig. 1.
Serum HBeAg inhibits anti-HBc spontaneous seroconversion. Groups of six 7/16-5 × HBc double (dbl)-Tg mice and 7/16-5 × HBc × HBe triple (tri)-Tg mice were bled at intervals between 3 and 28 weeks of age. Sera were collected, and total IgG and IgG isotypes of anti-HBc antibodies were determined by ELISA. Anti-HBc antibody was quantitated by endpoint dilution of sera. The symbols represent individual mice. The 7/16-5 Tg TCR (Vβ11-Vα5+) is specific for residues 120-140 of the HBc/HBeAgs, restricted by the I-Ab MHC class II molecule, and expressed on 53% of CD4+ T cells. Control HBcAg single-Tg mice did not spontaneously produce anti-HBc.
Fig. 2.
In vitro anti-HBc/e antibody production in 7/16-5 TCR+ double- and triple-Tg mice. Spleen cells derived from 7/16-5 × HBc and 7/16-5 × HBe double-Tg mice and 7/16-5 × HBc × HBe triple-Tg mice were cultured with varying concentrations of recombinant HBcAg or HBeAg for 5 days. Culture supernatants were collected, and IgG (A) and IgM (B) anti-HBc/e antibody production was determined by ELISA. Undiluted culture supernatants were assayed, and the results are expressed as OD492 values corrected by subtracting background OD492 values obtained from media control wells lacking antigen. The symbols represent individual mice, and the results are representative of at least five mice per group.
A trivial explanation for the negative effect of HBeAg on anti-HBc seroconversion could be the masking of the detection of anti-HBc antibodies by serum HBeAg. Addition of serum from HBeAg-Tg mice to dilutions of anti-HBc antibodies did not affect detection of the anti-HBc antibodies (Fig. 3A). A second explanation for the reduced anti-HBc production in 7/16-5 × HBc × HBe triple-Tg mice could be lower amounts of HBcAg in the livers of mice expressing both transgenes. Although HBc/HBe double-Tg mice tend to express ≈2-fold less intracellular HBcAg than HBc single-Tg mice, the absence of a correlation between anti-HBc seroconversion and the amount of HBcAg in the liver indicates that HBcAg concentration, which ranged from 0.2 to 2.0 μg/mg liver protein, was not a limiting factor for anti-HBc antibody production (Fig. 3B).
Fig. 3.
Inhibition of anti-HBc seroconversion by serum HBeAg is not due to immune complexes or limiting HBcAg content in the liver. (A) Dilutions of anti-HBc antibody produced spontaneously by 7/16-5 × HBc double-Tg mice were mixed 1:1 with either serum derived from HBeAg-Tg mice (4-10 μg/ml HBeAg) or normal mouse serum (NMS). The mixtures were analyzed by ELISA for anti-HBc activity. (B) Liver HBcAg concentration (0.2-2.0 μg/mg) was determined by ELISA on soluble protein derived from homogenized liver tissue of Tg mice expressing HBcAg alone or HBcAg plus either high (4-10 μg/ml; HBc/HBe-Tg) or low [10 ng/ml; HBc/HBe (lo)-Tg] concentrations of serum HBeAg. The HBcAg-Tg and HBc/HBeAg double-Tg mice were also either transgenic for the 7/16-5 TCR (circles) or recipients of adoptively transferred 7/16-5 T cells (triangles). Before the harvesting of liver tissue, mice were bled at multiple intervals, and sera were monitored by ELISA for anti-HBc seroconversion. The anti-HBc antibody titer is expressed as an endpoint dilution. There was no correlation between anti-HBc seroconversion and the concentration of HBcAg in the liver.
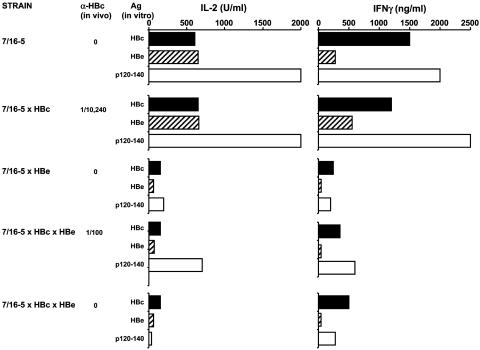
Serum HBeAg Elicits T Cell Tolerance in 7/16-5 TCR-Tg T Cells. Because of the high frequency of HBc/HBeAg-specific T cells in 7/16-5 TCR-Tg mice (53% of CD4+), unprimed splenic T cells cultured with HBc/HBeAgs in vitro produce significant quantities of IL-2 and IFN-γ (Fig. 4). Splenic T cells from 7/16-5 × HBc double-Tg mice produce equivalent amounts of IL-2 and IFN-γ as T cells from single TCR-Tg mice when cultured with HBcAg, HBeAg, or the p120-140, which is recognized by 7/16-5 T cells. This lack of T cell tolerance to HBcAg is consistent with the spontaneous production of IgG anti-HBc antibody in 7/16-5 × HBc double-Tg mice in vivo. In contrast, splenic T cells from 7/16-5 × HBe double-Tg mice are significantly tolerant at the T cell level and produce minimal T cell cytokines during in vitro culture with the HBc/HBeAg panel (Fig. 4). In addition to reduced cytokine production, T cells from 7/16-5 × HBe double-Tg mice also demonstrate HBc/HBeAg-specific proliferation defects relative to T cells from single TCR-Tg mice. Furthermore, the T cell tolerance elicited by serum HBeAg in 7/16-5 × HBe double-Tg mice is not due to clonal deletion of 7/16-5 T cells in the thymus or spleen and instead represents a form of in vivo anergy (unpublished data). Examination of T cell cytokine production in 7/16-5 × HBc × HBe triple-Tg mice revealed that the presence of serum HBeAg induced HBc/HBeAg-specific tolerance in 7/16-5 T cells to a degree equivalent to that observed in double-Tg 7/16-5 × HBe mice (Fig. 4). Therefore, the negative effect of serum HBeAg on spontaneous anti-HBc seroconversion observed in 7/16-5 × HBc × HBe triple-Tg mice is mediated at the T cell level by means of the ability of secreted HBeAg, in contrast to intracellular HBcAg, to elicit tolerance/anergy in 7/16-5 T cells. Furthermore, in vivo-activated 7/16-5 T cells mediate liver injury in 7/16-5 × HBc double-Tg mice, but liver injury is reduced significantly by the presence of serum HBeAg in 7/16-5 × HBc × HBe triple-Tg mice (data not shown). Therefore, the immunoregulatory effects of serum HBeAg are not limited to the inhibition of anti-HBc antibody production and also influence HBcAg-specific CD4+ T cell-mediated liver injury. These results suggest that a function of the secreted form of the nucleoprotein in the virus life cycle is to down-regulate the immune response to the intracellular HBcAg by functioning as an efficient T cell tolerogen.
Fig. 4.
Serum HBeAg elicits in vivo tolerance/anergy in 7/16-5 T cells. Unprimed spleen cells (5 × 106 per milliliter) of single 7/16-5 TCR-Tg, 7/16-5 × HBc double-Tg, 7/16-5 × HBe double-Tg, or triple-Tg mice were cultured with the indicated panel of HBc/HBeAgs (1.0 μg/ml), and IL-2 and IFN-γ cytokine production was measured in 2- and 4-day culture supernatants, respectively. In vivo IgG anti-HBc serum titers are shown for each mouse. Data are from individual mice and representative of at least three independent experiments.
Induction of T Cell Tolerance/Anergy in 7/16-5 TCR-Tg T Cells Depends on the Activation State and Is Reversible. To assess the ability of serum HBeAg to regulate the immune response to HBcAg in another model system, adoptive transfer experiments of 7/16-5 donor T cells into single HBcAg- or HBeAg-Tg or double HBc/HBe-Tg recipients were performed. Adoptive transfer of naive spleen cells (30 × 106) from 7/16-5 TCR-Tg donors into T cell-depleted HBeAg-Tg recipients yielded no anti-HBe antibody production, indicating that serum HBeAg was not immunogenic for 7/16-5 T cells in this setting (Fig. 5). Adoptive transfer of an identical number of naive 7/16-5 donor spleen cells into T cell-depleted HBcAg-Tg recipients resulted in high-titer (1:100,000 endpoint dilution) anti-HBc antibody production comprised of all IgG isotypes in recipient mice within 2-4 weeks. In contrast, adoptive transfer of naive 7/16-5 donor spleen cells into HBc/HBeAg double-Tg recipients resulted in minimal anti-HBc antibody production that was 0.2% of that produced in single HBcAg-Tg recipients (Fig. 5). Therefore, in addition to being nonimmunogenic, serum HBeAg tolerized the transferred 7/16-5 T cells to the extent that they no longer mediated anti-HBc seroconversion in HBc/HBeAg-Tg recipients. This result is consistent with the absence of spontaneous anti-HBc seroconversion in 7/16-5 × HBc × HBe triple-Tg mice (Fig. 1). The presence of even low levels of serum HBeAg (10 ng/ml) in HBc/HBe double-Tg recipient mice also curtailed anti-HBc antibody production after adoptive transfer of 7/16-5 T cells (Fig. 5). The inhibitory effect of serum HBeAg on anti-HBc seroconversion is mediated at the T cell level because the transferred 7/16-5 T cells failed to produce antigen-specific IL-2 in HBe single-Tg and in HBc/HBe double-Tg recipient splenic cultures as opposed to positive cytokine production in HBc single-Tg recipient spleen cultures (Fig. 6A). Similarly, in vitro IgG anti-HBc/e antibody production was detected only in the HBc single-Tg recipient spleen culture. In contrast, all Tg recipient spleen cultures produced IgM anti-HBc. However, elevated IgM anti-HBc was produced in HBc × HBe double-Tg recipient spleen cells. This result is consistent with the elevated levels of in vitro IgM anti-HBc produced by spleen cells derived from 7/16-5 × HBc × HBe triple-Tg mice (Fig. 6 B and C).
Fig. 5.
Induction of 7/16-5 T cell tolerance/anergy depends on the T cell activation state and is reversible. Donor spleen cells (30 × 106) derived from 7/16-5 TCR-Tg mice containing ≈3 × 106 TCR+CD4+ T cells were adoptively transferred into CD4/CD8-depleted HBcAg or HBeAg single-Tg, or HBc/HBeAg double-Tg recipient mice (three recipient mice per group). Donor spleen cells contained naive 7/16-5 T cells, 7/16-5 T cells preactivated in vitro by culture with p120-140 (1.0 μg/ml for 3 days), or 7/16-5 T cells derived from 7/16-5 × HBe double-Tg mice. At 2 and 4 weeks after transfer, recipient mice were bled, and total IgG and IgG isotypes of anti-HBc antibody were determined by ELISA. In the HBeAg-Tg recipients adoptively transferred with naive 7/16-5 T cells (first group), IgG anti-HBe antibody was determined. Total IgG anti-HBc-antibody determinations and IgG isotyping were performed on pooled sera from three recipient mice per group.
The preceding experiments were performed by transferring naive 7/16-5 donor spleen cells. Transfer of preactivated 7/16-5 donor spleen cells (i.e., cultured with p120-140) into HBc/e double-Tg recipients elicited 50-fold more in vivo anti-HBc antibody production (1:10,000 titer) as compared with the transfer of naive spleen cells (Fig. 5). Therefore, activated 7/16-5 T cells were less sensitive to tolerance induction by serum HBeAg in the recipient. To determine whether the anergic status of 7/16-5 T cells present in 7/16-5 × HBe double-Tg mice was reversible, spleen cells derived from double-Tg donors also were transferred into either HBcAg or HBc/HBe double-Tg recipients. Transfer into HBc/HBe double-Tg mice resulted in little or no anti-HBc antibody production, whereas transfer into single HBc-Tg recipients resulted in anti-HBc antibody production (1:10,000 titer) (Fig. 5). The level of anti-HBc antibody production induced by anergic 7/16-5 donor T cells transferred into HBc-Tg recipients was 10% of that induced by naive 7/16-5 donor T cells and of restricted IgG isotype but was nonetheless positive, indicating that the tolerant state of 7/16-5 T cells exposed to serum HBeAg in vivo can be reversed or at least altered in the absence of the tolerogen (i.e., HBeAg). Because the HBeAg-mediated T cell tolerance in this system is nondeletional and reversible in the absence of the tolerogen (HBeAg), it is best described as in vivo anergy or adaptive tolerance (19).
Discussion
The split T cell tolerance between HBeAg and HBcAg and the immunoregulatory function of HBeAg described in this study have important implications for perinatal and adult HBV infection and especially for infection with HBeAg- mutant viruses, which are becoming an increasing clinical problem (20, 21). Many common mutations in the precore (22) and core promoter regions (23) of the HBV genome abolish or reduce HBeAg synthesis, respectively. Assuming an immunoregulatory role for HBeAg in natural HBV infection, as suggested by these murine studies, one would predict that infection with precore or core promoter mutant viruses may result in enhanced antiviral immune responses and decreased rates of chronicity. The clinical evidence is largely consistent with this prediction. Neonates infected with HBeAg+ wild-type HBV predominantly (90%) become chronically infected, whereas neonates infected with HBeAg- mutant viruses experience a transient acute infection often accompanied by fulminant hepatitis indicative of an aggressive immune response (24, 25). Similarly, adult infections with HBeAg- mutant viruses often correlate with acute fulminant hepatitis (26-28). Furthermore, emergence of HBeAg- mutant viruses during chronic HBV infection can, in some cases, lead to exacerbation of liver injury and a poor prognosis, most notably if the HBeAg- mutant becomes predominant during periods of high viral load (29, 30). Therefore, the ability of serum HBeAg to function as a T cell tolerogen and to down-regulate the immune response to HBcAg may result in the moderation of HBcAg-specific liver injury during an acute infection and in the promotion and maintenance of viral persistence during a chronic infection, perhaps explaining the correlation between precore and core promoter region mutations and severe liver disease (20, 21, 24-30).
Although the 7/16-5 TCR lineage represents a single HBeAg-specific T cell clone that is very susceptible to tolerance/anergy, the potential of serum HBeAg to elicit T cell tolerance through the additional mechanisms of clonal deletion, Fas-mediated apoptosis, and clonal ignorance in two additional TCR-Tg lineages and in HBeAg-Tg mice has been demonstrated previously (refs. 7 and 31 and unpublished data). Therefore, T cell tolerance toward HBeAg is clonal and heterogeneous in terms of mechanisms of induction, and this heterogeneity is likely to be evident even within an individual HBV-infected patient. Because the immunogenic target (HBcAg) and the tolerogen (HBeAg) are both present during a wild-type HBV infection, the balance between tolerized, quiescent, and activated HBc/HBeAg-specific T cell clones during various phases (i.e., so-called “tolerance” and “clearance” phases) of chronic infection is likely to be an important determinant of viral clearance and immune-mediated liver injury. The 7/16-5 TCR-Tg lineage paired with HBc/HBe-Tg mice is a particularly useful model because it does not require experimental manipulation (i.e., spontaneous anti-HBc seroconversion); the split tolerance between HBeAg and HBcAg is very apparent; and HBeAg-induced T cell tolerance is mediated by in vivo anergy as opposed to clonal deletion. If 7/16-5-like T cells exist during a natural chronic HBV infection, they may be susceptible to activation through the reversal of the anergic state. The observation that HBeAg-specific T cell tolerance can be reversible in the absence of the tolerogen (Fig. 5) suggests that antiviral treatments that reduce the HBeAg load as well as the viral load, possibly in combination with HBc/HBeAg-specific immunization, may be effective in the treatment of chronic HBV infection. Furthermore, this TCR/Ag double- and triple-Tg model will be useful in screening immunomodulatory drugs for the ability to reverse HBeAg-specific T cell tolerance in vivo.
Supplementary Material
Acknowledgments
We thank Drs. F. Schödel and D. Peterson for providing recombinant proteins and Ms. Rene Lang for editorial assistance. This work was supported by National Institutes of Health Grants 5 R01 AI-20720-21, 5 R01 AI-49730-03, and 5 R01-CA40489 and grants from the Swedish Cancer Foundation and Swedish Science Council.
Author contributions: D.R.M. designed research; J.-N.B., J.J., and J.H. performed research; M.T.C., J.-N.B., M.S., L.G.G., and F.V.C. contributed new reagents/analytical tools; M.T.C., J.-N.B., M.S., L.G.G., F.V.C., J.J., J.H., and D.R.M. analyzed data; and D.R.M. wrote the paper.
Abbreviations: HBV, hepatitis B virus; HBcAg, hepatitis core antigen; HBeAg, hepatitis E antigen; p, peptide; TCR, T cell receptor; Tg, transgenic; Th, T helper.
References
- 1.Ganem, D. & Schneider, R. J. (2001) in Fields Virology, eds. Knipe, D. M., Howley, P. M., Griffin, D. E., Lamb, R. A., Roizman, B., Martin, M. A. & Straus, S. E. (Lippincott-Raven, Philadelphia), pp. 2923-2970.
- 2.Magnius, L. O. & Espmark, J. A. (1972) J. Immunol. 109, 1017-1024. [PubMed] [Google Scholar]
- 3.Chang, C., Enders, G., Sprengel, R., Peters, N., Varmus, H. E. & Ganem, D. (1987) J. Virol. 61, 3322-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlicht, H. J., Salfeld, J. & Schaller, H. (1987) J. Virol. 61, 3701-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, Y., Kew, M. C., Hornbuckle, W. E., Tennant, B. C., Cote, P. J., Gerin, J. L., Purcell, H. & Miller, R. H. (1992) J. Virol. 66, 5682-5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milich, D. R. (1997) J. Viral Hepatol. 4, 48-59. [DOI] [PubMed] [Google Scholar]
- 7.Milich, D. R., Chen, M. K., Hughes, J. L. & Jones, J. E. (1998) J. Immunol. 160, 2013-2021. [PubMed] [Google Scholar]
- 8.Milich, D. R. & McLachlan, A. (1986) Science 234, 1398-1401. [DOI] [PubMed] [Google Scholar]
- 9.Milich, D. R., Schödel, F., Hughes, J., Jones, J. E. & Peterson, D. L. (1997) J. Virol. 71, 2192-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milich, D. R., Chen, M., Schödel, F., Peterson, D. L., Jones, J. E. & Hughes, J. L. (1997) Proc. Natl. Acad. Sci. USA 94, 14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidotti, L. G., Matzke, B., Pasquinelli, C., Shoenberer, J. M., Rogler, C. E. & Chisari, F. V. (1996) J. Virol. 70, 7056-7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidotti, L. G., Martinez, V., Loh, Y.-T., Rogler, C. E. & Chisari, F. V. (1994) J. Virol. 68, 5469-5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milich, D. R., Jones, J. E., Hughes, J. L., Price, J., Raney, A. K. & McLachlan, A. (1990) Proc. Natl. Acad. Sci. USA 87, 6599-6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institutes of Health (1996) Guide for the Care and Use of Laboratory Animals DHEW Publication No. 85-23 (Office of Science and Health Reports, Division of Research Resources/Natl. Inst. of Health, Bethesda).
- 15.Schödel, F., Moriarty, A. M., Peterson, D. L., Zheng, J., Hughes, J. L., Will, H., Leturcq, D. J., McGee, J. S. & Milich, D. R. (1992) J. Virol. 66, 106-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schödel, F., Peterson, D., Zheng, J., Jones, J. E., Hughes, J. L. & Milich, D. R. (1993) J. Biol. Chem. 268, 1332-1337. [PubMed] [Google Scholar]
- 17.Sällberg, M., Ruden, U., Magnius, L. O., Norrby, E. & Wahren, B. (1991) Immunol. Lett. 30, 59-68. [DOI] [PubMed] [Google Scholar]
- 18.Milich, D. R., McLachlan, A., Stahl, S., Wingfield, P., Thornton, G. B., Hughes, J. L. & Jones, J. E. (1988) J. Immunol. 141, 3617-3624. [PubMed] [Google Scholar]
- 19.Schwartz, R. H. (2003) Annu. Rev. Immunol. 21, 305-334. [DOI] [PubMed] [Google Scholar]
- 20.Milich, D. R. & Liang, T. J. (2003) Hepatology 38, 1075-1081. [DOI] [PubMed] [Google Scholar]
- 21.Hilleman, M. R. (2003) Vaccine 21, 4626-4649. [DOI] [PubMed] [Google Scholar]
- 22.Carman, W. F., Jacyna, M. R., Hadziyannis, S., Karayiannis, P., McGarvey, M. J., Makris, A. & Thomas, H. C. (1989) Lancet 2, 588-590. [DOI] [PubMed] [Google Scholar]
- 23.Okamoto, H., Tsuda, F., Akahane, Y., Sugai, Y., Yoshiba, M., Moriyama, K., Tanaka, T., Miyakawa, Y. & Mayumi, M. (1994) J. Virol. 68, 8102-8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terazawa, S., Kojima, M., Yamanaka, T., Yotsumoto, S., Okamoto, H., Tsuda, F., Miyakawa, Y. & Mayumi, M. (1991) Pediatr. Res. 29, 5-9. [DOI] [PubMed] [Google Scholar]
- 25.Chen, H.-L., Chang, C.-J., Kong, M.-S., Huang, F.-C., Lee, H.-C., Lin, C.-C., Liu, C.-C., Lee, I.-H., Wu, T.-C. & We, S.-F. (2004) Hepatology 39, 58-63. [DOI] [PubMed] [Google Scholar]
- 26.Fagan, E. A., Smith, P. M., Davison, F. & Williams, R. (1986) Lancet 2, 538-540. [DOI] [PubMed] [Google Scholar]
- 27.Carman, W. F., Fagan, E. A., Hadziyannis, S., Karayiannis, P., Tassopoulos, N. C., Williams, R. & Thomas, H. C. (1991) Hepatology 14, 219-222. [PubMed] [Google Scholar]
- 28.Liang, T. J., Hasegawa, K., Rimon, N., Wands, J. R. & Ben-Porath, E. (1991) N. Engl. J. Med. 324, 1705-1709. [DOI] [PubMed] [Google Scholar]
- 29.Brunetto, M. R., Giarin, M., Oliveri, F., Chiaberge, E., Baldi, M., Alfarano, A., Serra, A., Saracco, G., Verme, G., Will, H. & Bonino, F. (1991) Proc. Natl. Acad. Sci. USA 88, 4186-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chu, C. M., Yeh, C. T., Lee, C. S., Sheen, I. S. & Liaw, Y. F. (2002) J. Clin. Microbiol. 40, 16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen, M., Sällberg, M., Thung, S. N., Hughes, J., Jones, J. & Milich, D. R. (2000) J. Virol. 74, 7587-7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.